Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, characterized by irreversible memory decline, concerning no rarely spatial memory and orientation, alterations of the mood and personality, gradual loss of motor skills, and substantial loss of capacities obtained by previous long education. We attempted to describe the morphological findings of the mammillary bodies in early cases of AD. Samples were processed for electron microscopy and silver impregnation techniques. The nuclei of the mammillary bodies demonstrated a substantial decrease in the neuronal population and marked abbreviation of dendritic arbors. Decrease in spine density and morphological abnormalities of dendritic spines was also seen. Synaptic alterations were prominent. Alzheimer’s pathology, such as deposits of amyloid-β peptide and neurofibrillary degeneration, was minimal. Electron microscopy revealed mitochondrial alterations and fragmentation of Golgi apparatus, associated frequently with synaptic pathology.
Keywords: Alzheimer’s disease, mammillary bodies, Golgi staining, electron microscopy, dendritic pathology
Introduction
Alzheimer’s disease (AD) is the most common cause of memory decline in elderly people. In addition to accumulations of β-amyloid and tau pathology, which have been established as the morphological hall marks of the disease, 1 the extensive synaptic alterations in various areas of the brain may also contribute substantially in plotting the clinical profile of the disease, which is mostly characterized by gradual deterioration of the cognitive functions, alterations of the mood and personality, and progressive loss of skills and capacities obtained by education during the previous years of the life.
Neuronal loss, which is a prominent finding in the early stages of AD affecting cognition, 2 is associated with lesions of the tracts of the white matter (WM) 3 including the fornix, 4 a fact that has been characterized as “sensitive predictor” of early cognitive decline, 5 in mild cognitive impairment and in early stages of AD. 6
A substantial body of evidence suggest that the mammillary bodies may play a strategic role in memory processes, 7,8 concerning mostly spatial memory and orientation. 9 The fornix and mammillary bodies are essential components of the limbic circuit, 10 which had undergone substantial volume reduction in AD, associated with memory deficits affecting primarily the episodic and spatial memory. The fornix is mostly composed of myelinated nerve fibers, 11 originating from the subiculum of the hippocampus and terminating in the mammillary bodies. 12 The fornix together with hippocampus and mammillary bodies are parts of the memory or “Papez circuit” (hippocampus, mammillary bodies, anterior thalamus, cingulate cortex) or in a broader sense of the Delay–Brion circuit, which includes the hippocampus, the fornix, the mammillary bodies, the anterior thalamus, and the retrosplenial cortex. 13 In addition, the mammillary bodies are also involved in spatial memory via their connections with the neuronal circuits of the medial prefrontal cortex. 14
On experimental level, it is also supported that mammillary bodies play an important role in spatial working memory. 15
It is reported that decreased fractional anisotropy of the fornix on diffusion tensor imaging is one of the earliest magnetic resonance imaging findings observed in normal persons who are at increased risk for cognitive decline or at the very initial stage of AD, 16 which might be associated with hippocampal pathology. 17,18
The aim of this study is to proceed in morphological estimation of the alterations of the mammillary bodies in light and electron microscope in early cases of AD, in an attempt to elucidate the reason of the frequent deterioration of the spatial memory and orientation even in the initial clinical stages of AD.
Material and Methods
Material
This morphological study is based on the examination of 12 brains of patients having AD excised at autopsy 4 to 8 hours after death at temperature of 4°C. All brains were obtained from patients with a definite history of dementia, aged 55 to 82 years, who fulfilled the clinical, neurological, neuropsychological, neuroimaging, and neuropsychiatric criteria for AD. All of them died accidentally 24 to 46 months following the clinical diagnosis of the disease (Table 1). 19
Table 1.
List of the brains of patients with AD included in the study.a
| Gender | Age at Death, y | Duration of the Disease | Length of Brain Fixation | Braak and Braak Stage |
|---|---|---|---|---|
| M | 55 | 3 y | 1 m | II/III |
| F | 62 | 28 m | 1 m | II/III |
| M | 63 | 37 m | 1 m | II |
| F | 66 | 40 m | 1 m | II/III |
| M | 72 | 3 y | 1 m | III |
| M | 74 | 38 m | 1 m | II/III |
| F | 75 | 42 m | 1 m | II/III |
| F | 76 | 46 m | 1 m | III |
| M | 78 | 42 m | 1 m | II/III |
| F | 80 | 2 y | 1 m | II/III |
| M | 82 | 26 m | 1 m | II/III |
Abbreviations: AD, Alzheimer’s disease; F, female; M, male; m, months; y, year.
aThe mammillary bodies were excised and studied from 1974 to 2011.
Additional 12 brains, macroscopically intact of apparently healthy individuals who died accidentally, were used as normal controls. The definite diagnosis of AD was based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Association (NINCDS-ADRDA) criteria. 20
Methods
Samples from the mammillary bodies were excised and processed for electron microscopy and silver impregnation techniques, including rapid Golgi method, Rio Hortega, and Bodian techniques.
Electron microscopy
For electron microscopy, the specimens were immediately immersed in Sotelo 21 fixing solution, composed of 1% paraformaldehyde and 2.5% glutaraldehyde in cacodylate buffer 0.1 mol/L, adjusted at pH 7.35. Then, they were postfixed by immersion in 1% osmium tetroxide for 30 minutes at room temperature and dehydrated in graded alcohol solutions and propylene oxide. Thin sections were cut in a Reichert ultramicrotome (C. Reichert AG, Wien, Austria), contrasted with uranyl acetate and lead citrate, and studied in a Zeiss 9aS electron microscope (Carl Zeiss MicroImaging GmbH Oberkochen).
Light microscope
Silver impregnation techniques
The remaining parts of the mammillary bodies were processed for silver impregnation techniques, according to rapid Golgi staining. Thus, after a 4-week fixation in formalin, they were immersed in a solution of potassium dichromate (7 g potassium dichromate in 300 mL water), including few grains of copper, for 10 days. Then, they were immersed in 1% silver nitrate for 10 days, under continuous agitation in a dark glass jar at a temperature of 16°C.
Following a rapid dehydration in graded alcohol solutions, the specimens were embedded in paraffin and cut, some of them at 100 mm and some at 25 mm, alternatively. Many sections of 25 mm were stained also with methylene blue, according to Golgi–Nissl method. 22 -24 All the sections were mounted in Entellan (Merck Millipore, Darmstadt, Germany) between 2 cover slips and studied in a Zeiss Axiolab Photomicroscope (Carl Zeiss AG, Oberkochen) equipped with a digital camera and a computer.
In the study, the Cavalieri principle, 25,26 which is an unbiased principle for estimating the volume of any structure, 27 was used in order to calculate the volume of the nuclei of the mammillary bodies and the number of neurons per volume unit. 28 In the neurons, we estimated the dendritic arbors, the morphology and the number of the dendritic branches, from the primary to the last one, and the morphology of the dendritic spines in light microscope on sections impregnated by silver nitrate, according to rapid Golgi method and Golgi–Nissl staining. 24
Morphometry
Morphometric studies were performed with an image analyzer using the Neuro J plug-in in ImageJ application (Java). The surface area of the neurons as well as the dendritic arborization was calculated in the mammillary bodies in silver staining at light microscopy. 29 The neurons were estimated from the morphological and morphometric point of view, with much emphasis on the dendritic morphology, accepting the criteria posed by Jacobs, 30 which concern (1) the quality of staining of dendrites and (2) the sufficient contrast between neurons and background. Dendritic arbores were quantitatively estimated in a centrifugal way for the apical dendrite and the basal dendrites. 31
We proceeded in estimation of the diameter and the surface of the neuronal soma, the total dendritic length, the number of dendritic bifurcations, the length and number of dendritic segments per dendritic order, as well as the spinal density per dendritic branch. Thus, the dendrites, which arise from the soma of neurons up to their first symmetrical bifurcation, are considered as first-order branches. The dendritic branches that arise from the first-order branches up to their symmetrical bifurcation are considered as second-order segments or secondary dendrites, and so on.
For the quantitative analysis, we applied ImageJ program after a calibration for the specific types of microscope (Carl Zeiss Axiolab Photomicroscope), and we estimated the dendritic arborization according to Sholl method of concentric circles. 32 Thus, concentric circles were drawn, at intervals of 15 mm centered on the soma of the neuron, and the dendritic intersections within each circle were counted. The dendritic spines were counted on 3 segments of the dendritic field. The first segment, 20 to 30 mm in length, was located on primary dendrite, the second segment, 20 to 30 mm in length, on the secondary dendrite, and the third one, 40 to 50 mm, on the tertiary dendrite.
In electron microscopy, we applied the stereological analysis according to Nyengaard and Gundersen, 33 Gundersen et al, 34 and West 35 -38 on sections of standard thickness, measured by Small’s smallest fold. 39 The quantities comprise number, length, surface area, volume, and spatial distribution for mitochondria, cisternae, and vesicles of the Golgi complex. The volume of the mitochondria and the other organelles was estimated mainly on the basis of the rotator principle. 40 We estimated also the mean nuclear area, the dendritic profiles, 41 the total number of the dendritic spines per dendritic branch, the presynaptic and postsynaptic terminals, 42 -44 and the number of synaptic vesicles per presynaptic component. 44 The surface density of the dendritic spines has been estimated by the use of isotropic test lines 45 combined with image analysis. 46 The number of the synapses was calculated by application of the Horvitz and Thompson estimator, 47 with a physical fractionator of varying sampling fractions according to Widgen et al. 48
The statistical analysis of the data was evaluated by Student’s t tests. The P values less than .05 were considered statistically significant and those less than .01 as highly significant.
Results
Silver Impregnation Technique
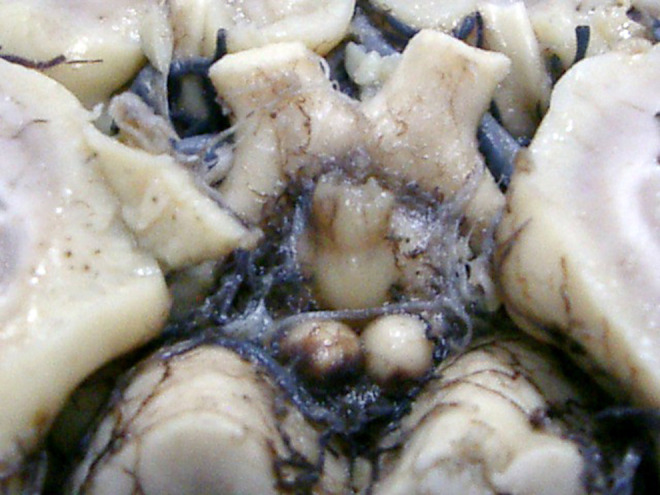
The mammillary bodies of the human brain are situated in the posterior hypothalamus below the infundibulum of the third ventricle, seen on the base of the brain as round hemispheres of a smooth surface, having a mean diameter of 2 to 4 mm (Figures 1 and 2). They are located posterior to the tuber cinereum and anterior to the posterior perforate substance at the ends of the anterior cruces of the fornix (Figure 2B), demonstrating no rarely anatomical variability. 49
Figure 1.
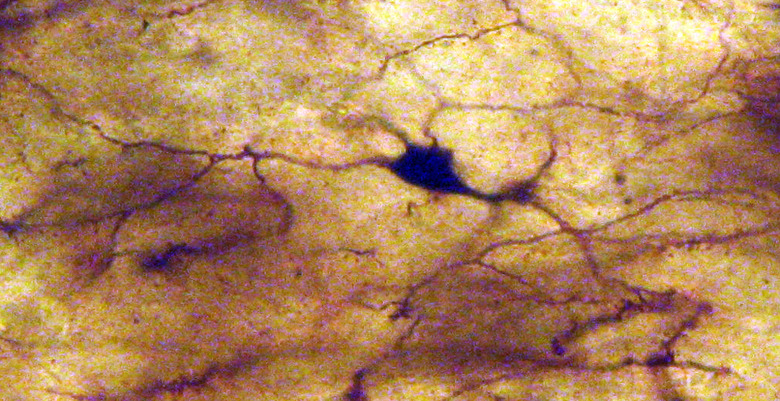
Mammillary bodies of a case of Alzheimer’s disease (62-year-old female).
Figure 2.
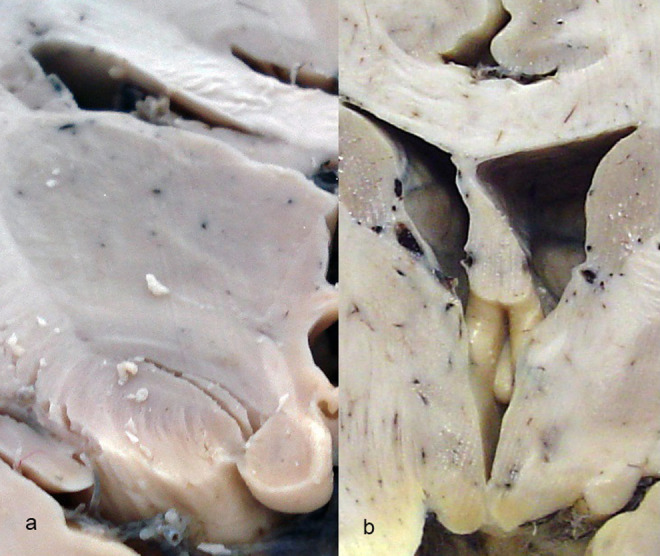
Mammillary bodies in frontal section of (A) the normal control (66-year-old female) and (B) a case of Alzheimer’s disease (AD; 66-year-old female).
By application of the silver impregnation technique in association with Golgi–Nissl method, we could visualize the nuclei of the mammillary bodies, which may be distinguished in medial and lateral ones. In normal controls, the neurons in the lateral nuclei are larger than in the medial ones and compose thick networks of triangular, round, or elongated nerve cells, which have large number of dendrites, bifurcated in numerous branches studded with spines. Groups of 20 or more neurons may be characterized as subdivisions of the main nuclei, although the borders of the various groups are not very distinct and clearly delineated. The majority of the neurons are Golgi type II, with short axons composing short neuronal circuits. The medial group of nuclei, in normal controls, is characterized mostly by small round, small polyhedral cells, and spiny neurons, which form dense neuronal networks hardly distinguished in subgroups. The majority of neurons have large number of dendrites forming thick arbores distributed around the cell body. The majority of neurons in the medial nuclei of the mammillary bodies are also Golgi type II neurons.
In AD, the neuronal population was decreased in comparison with normal controls. The majority of neurons in the lateral and medial nuclei may be characterized as spiny neurons (Figures 3 and 4). Small polyhedral cells were rarely observed. Impressively, the number of dendritic spines was dramatically reduced, as well as the number of tertiary dendritic branches (Figures 5 and 6). Some of the dendrites were totally depleted of spines (Figure 7). In the majority of the brains of patients with AD, a marked astrocytic proliferation around the capillaries and in the neuropil space was noticed in both of the nuclei of the mammillary bodies. It is worth to underline that neuritic plaques and neurofibrillary tangles were not observed in the mammillary bodies in our cases, although they were clearly observed in other areas of the same brains.
Figure 3.
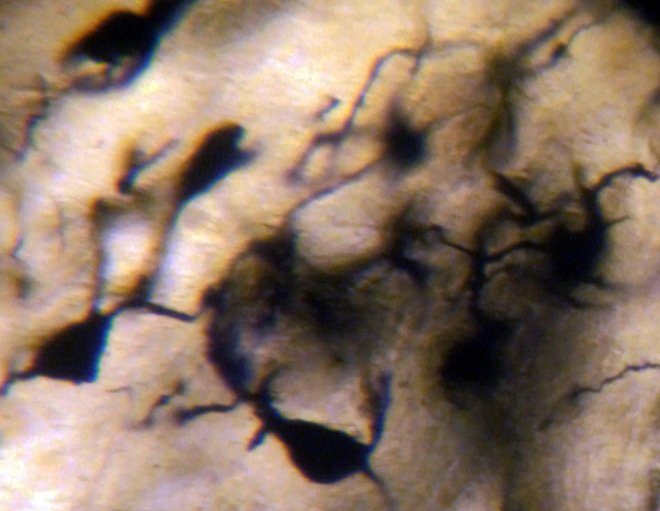
Spiny neurons of the lateral mammillary nucleus of a case of Alzheimer’s disease (AD; 63-year-old male). Golgi silver impregnation technique (magnification ×1200).
Figure 4.
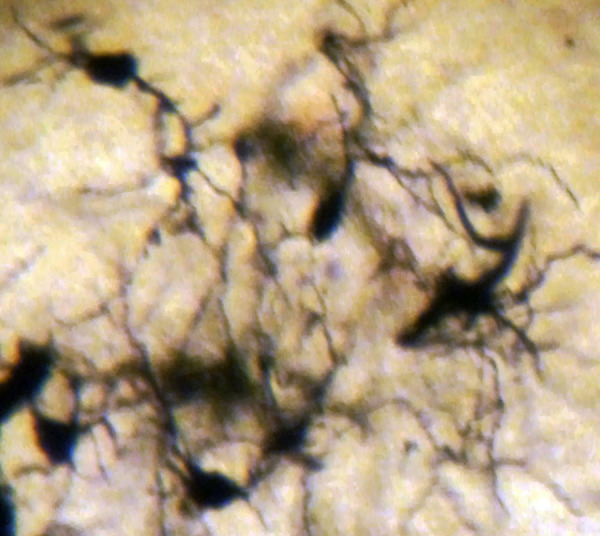
Spiny neurons of the medial mammillary nucleus of a case of Alzheimer’s disease (AD; 63-year-old male). Golgi silver impregnation technique (magnification ×1200).
Figure 5.
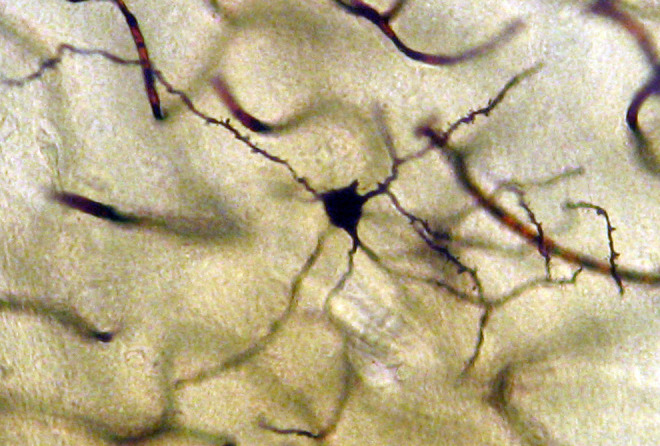
Neuron from the medial mammillary nucleus of a case of Alzheimer’s disease (AD; 72-year-old male). The number of the tertiary dendritic branches and the dendritic spines are dramatically reduced. Golgi silver impregnation technique (magnification ×1200).
Figure 6.
Neuron from the lateral mammillary nucleus of a case of Alzheimer’s disease (AD; 76-year-old female). The number of the tertiary dendritic branches and the dendritic spines are dramatically reduced. Golgi silver impregnation technique (magnification ×1200).
Figure 7.
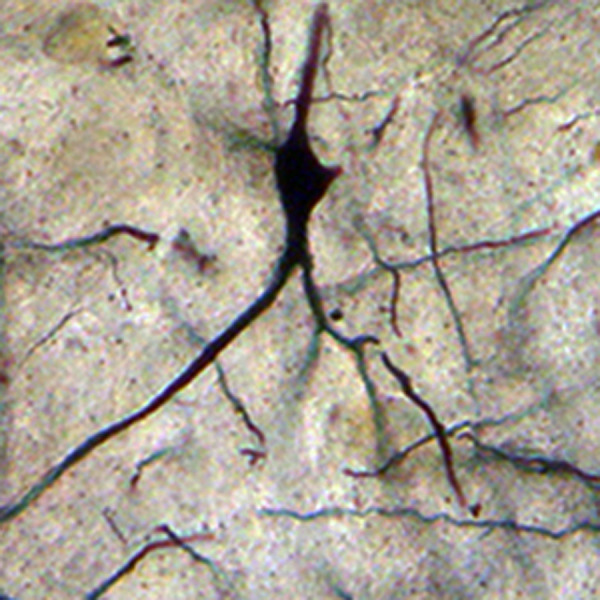
Neuron from the lateral mammillary nucleus of a case of Alzheimer’s disease (AD; 62-year-old female). The secondary dendritic branches are almost depleted of spines. Golgi silver impregnation technique (magnification ×1200).
Electron Microscopy
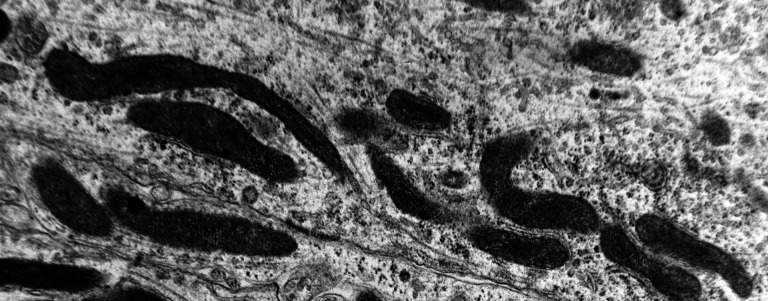
Electron microscopy revealed marked decrease in the density of the spines in the dendritic branches in the main nuclei of the mammillary bodies in brains of patients with AD (Figure 8) as compared to normal controls. The morphological alterations of the dendritic spines consisted of a change in shape and size, distortion, increased thickness of the postsynaptic membrane, and marked alteration of the organelles of the spine (Figure 9). Small dense spines were frequently intermixed with giant spines in the secondary dendritic branches (Figure 10) in a substantial number of neurons. In addition, the presynaptic terminals were characterized by a considerable poverty of synaptic vesicles (Figure 11). In both the presynaptic and postsynaptic terminals, small abnormal mitochondria of a mean radius of 220 nm were observed. A considerable number of mitochondria in the soma and the dendritic profiles demonstrated disruption of the cristae (Figure 12).
Figure 8.
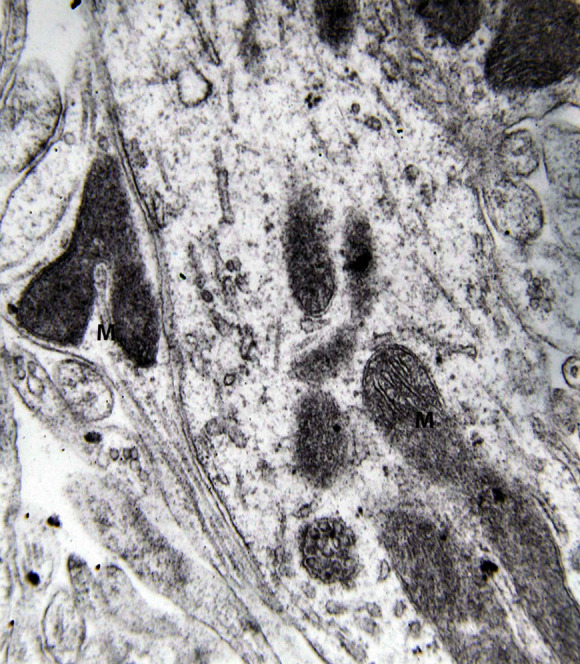
Dendritic profile from the lateral mammillary nucleus of a case of Alzheimer’s disease (AD; 62-year-old female). Marked decrease of dendritic spines is observed. Mitochondrial alterations (M) are obvious (electron micrograph, magnification ×128 000).
Figure 9.
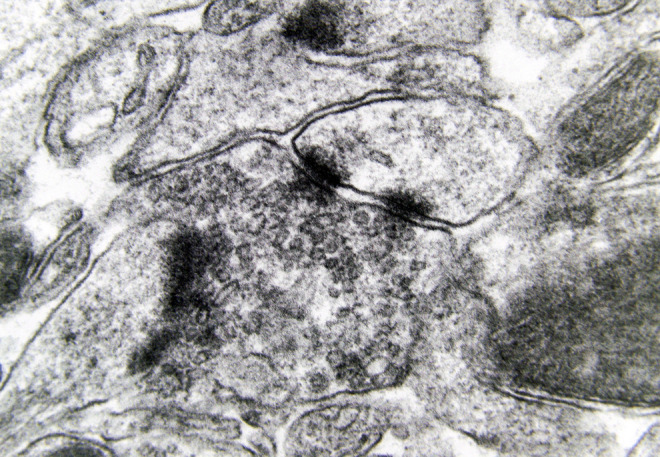
Morphological alterations of the dendritic spines of a case of Alzheimer’s disease (AD; 80-year-old female). The increased thickness of the postsynaptic membrane and the synaptic polymorphism of the presynaptic terminal are obvious (electron micrograph, magnification ×128 000).
Figure 10.
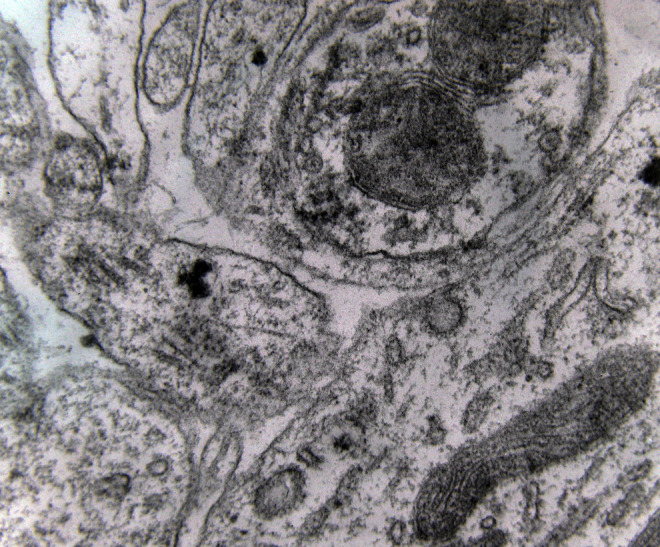
Small dense spines with marked mitochondrial alterations intermixed with giant spines are seen in the lateral mammillary nucleus of a case of Alzheimer’s disease (AD; 78-year-old male) (electron micrograph, magnification ×250 000).
Figure 11.
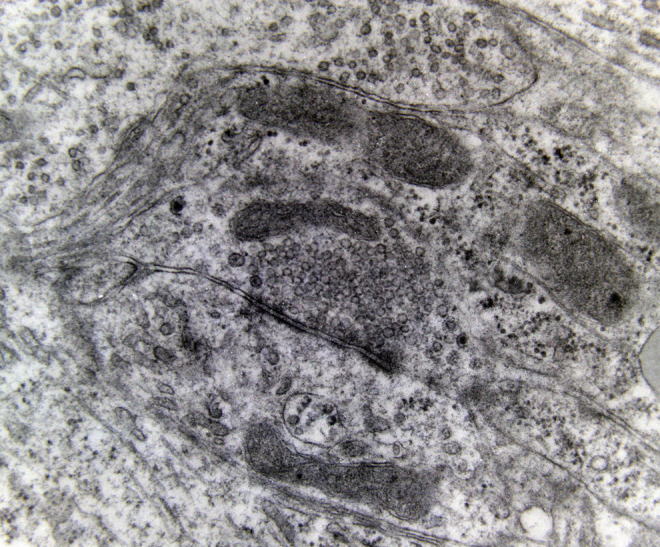
Synaptic profiles of the lateral mammillary nucleus of a case of Alzheimer’s disease (AD; 78-year-old male). The presynaptic terminal is characterized by a considerable poverty of synaptic vesicles (electron micrograph, magnification ×250 000).
Figure 12.
Abnormal mitochondria, very elongated with disruption of the cristae, are seen in the primary dendritic branch of a neuron of the medial mammillary nucleus of a case of Alzheimer’s disease (AD; 74-year-old male) (electron micrograph, magnification ×110 000).
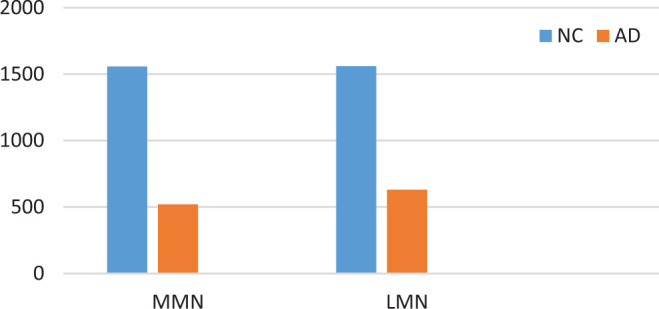
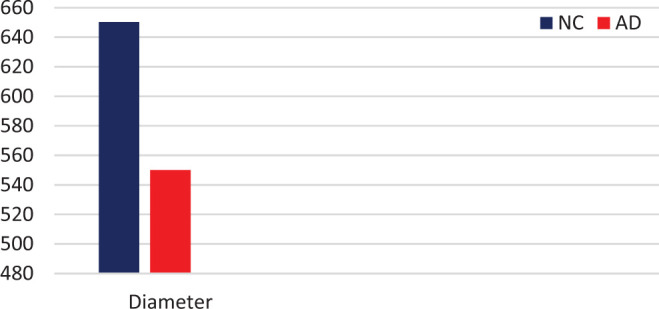
A marked decrease in the number of the secondary dendritic branches was also observed in the nuclei of the mammillary bodies in brains of patients with AD in comparison with the control brains of the same age. The number of the dendritic spines was dramatically decreased in the medial and lateral mammillary nuclei in cases of AD in comparison with the normal controls (Table 2). Very elongated mitochondria, with disruption of the cristae, were observed in the primary dendritic branches in a considerable number of neurons in the mammillary bodies of brains of patients with AD. In the soma of a substantial number of neurons, large number of cisternae of Golgi apparatus appeared to be fragmented (Figure 13). Morphological alterations of the mitochondria were also seen in a considerable number of astrocytes in brains of patients with AD in comparison with the normal controls. Following a detailed morphometric estimation of the mitochondria in the soma, the dendrites, and the spines of neurons of the mammillary bodies in normal controls, we concluded that the ellipsoid mitochondria of the dendritic spines have an average diameter of 650 ± 250 nm and a mean axial ratio of 1.9 ± 0.2. In addition, the round mitochondria appeared to have a mean radius of 350 nm. In brains of patients with AD, mitochondria in the neurons of the mammillary bodies appear to have an average diameter of 440 ± 250 nm and a mean axial ratio of 1.7 ± 0.2 (Table 3). It was noticed that the atrophy or the fragmentation of Golgi apparatus and the mitochondrial alterations coexisted with dendritic and spinal pathology in the majority of neurons.
Table 2.
Average Dendritic Spines per Dendritic Arbor in Neurons of the MMN and LMN, based on Measurement of 100 Neurons (P < .005).
|
Abbreviations: AD, Alzheimer’s disease; LMN, lateral mammillary nuclei; MMN, medial mammillary nuclei; NC, normal control.
Figure 13.
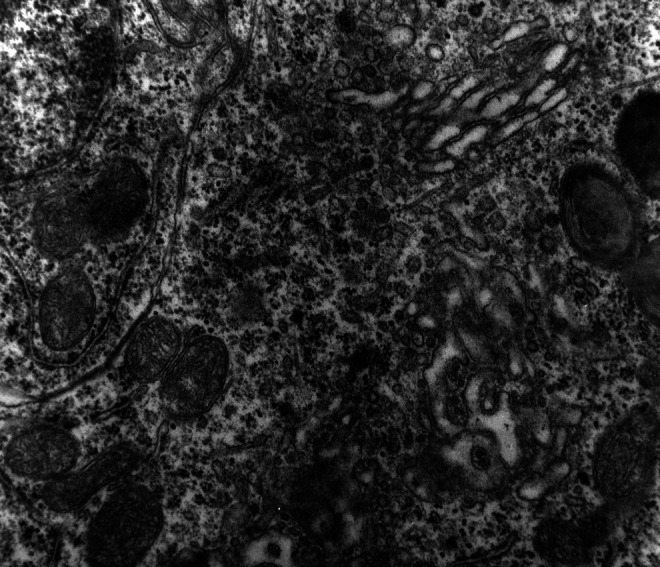
Neuron of the medial mammillary nucleus of a case of Alzheimer’s disease (AD; 80-year-old female). The fragmentation of Golgi apparatus and the alterations of the mitochondria are obvious (electron micrograph, magnification ×128 000).
Table 3.
Mean Diameter (in nm) of Mitochondria in Neurons of Mammillary Bodies, Based on Estimation of 500 Mitochondria (P < .05).
|
Abbreviations: AD, Alzheimer’s disease; NC, normal control.
Discussion
The mammillary bodies and their main projections play a substantial role in memory, particularly in spatial memory 50 -52 and in delayed memory recalls, 53,54 since their projections to the anterior thalamus seem to be of substantial importance for recollective memory. 55,56 As parts of an “extended hippocampal system,” 57 the mammillary bodies receive information from distinct neuronal networks of hippocampal formation, projecting them ipsilaterally to the anterior medial and the anterior ventral thalamic nuclei and bilaterally to the anterior dorsal thalamic nucleus, 58 seen therefore as a relaying information group of nuclei. 59 -61
Given that mammillary bodies and mammillothalamic tract have a substantial contribution in encoding of spatial location, it is reasonable that their lesion induces, as a rule, serious disturbances of visuospatial memory in humans and experimental animals and causes deficits of directional heading movements. 62 -64
In the spectrum of the memory circuits, the mammillary bodies receive information originated from the subiculum of hippocampus via fornix, which are subsequently projected via the mammillothalamic tract to the anterior thalamic nuclei, which are involved in anterograde amnesia. 65,66 The majority of the neurons in mammillary bodies are rather projection cells than interneurons sending excitatory outputs to anterior thalamic nuclei, 67 which may play an important role in the learning-induced plasticity of the neuronal networks of the anterior thalamus. It is worth to mention that the mammillary bodies and the hippocampus are well preserved in the semantic variant of primary progressive aphasia, whereas they become atrophic in behavioral variant of frontotemporal dementia. 68
Fornix, parahippocampal cingulum, and precuneus are also involved in memory organization, in connection with mammillary bodies, since lesions of the fornix may cause loss of neuropil resulting in shrinkage of the mammillary bodies 69 inducing substantial anterograde amnesia. 70,71 It is also well documented that the loss of fornix WM volume is associated with cognitive decline, 71 and lesions of the mammillary bodies and the mammillothalamic tract may disrupt spatial performance and can induce Korsakoff’s syndrome 72 or recollective memory impairment 73,74 or even loss of verbal long-term memory. 75 It is also worth to mention that in addition to memory deficits, lesions of the connections of the mammillary bodies with the dorsal and ventral tegmental nuclei of Gudden may cause impairments in place learning and in proper function and well navigation of the head direction system. The ventral tegmental nucleus of Gudden is interconnected mostly with the medial mammillary nucleus and is mostly involved in spatial learning, via its interactions with the medial mammillary nucleus, whereas the dorsal tegmental nucleus of Gudden is connected with the lateral one, which is part of the head direction system. 76 -79
Numerous neuroanatomical studies of the mammillary bodies in humans and animals revealed that they are mainly comprised lateral and medial nuclei, and each one of them is further distinguished in a number of subnuclei, including neurons with thick dendritic arborization and numerous symmetric synaptic junctions. 80 In humans, the majority of neurons in the mammillary body nuclei are medium-sized spiny neurons, with many dendrites of dense arborization, studded with numerous dendritic spines.
Hippocampal atrophy in AD 81,82 is reasonably associated with degeneration of the fornix 83,84 and other limbic regions with a differentiation among men and women, 85 since the involvement of mammillary bodies is predominant in men as well as the anterior thalamic nuclei in women, a fact that may play an additional role in the tragedy of the cognitive decline by degrading signal transmissions. 86
It is well documented that neurodegeneration and synaptic alterations are considered as principal factors of cognitive decline in patients with AD, 87,88 associated with alterations of mitochondria and Golgi apparatus 89 -91 in various areas of the brain, including the vestibulocerebellar system. 92,93
Although there is considerable morphological variability of mammillary bodies in healthy controls, 94 marked reductions in their volume associated with significant reduction in neuronal numbers and neuropil have been described in a number of neurological conditions, associated with cognitive decline such as Wernicke–Korsakoff syndrome, 95 Wernicke’s encephalopathy, chronic alcohol abuse, 96 and chronic thiamin deficiency. 97 -99
It is important that in our cases, neuritic plaques and neurofibrillary tangles were rarely observed in contrast to previous studies. 100 However, the neuronal population was decreased in comparison with the normal controls, and marked decrease in the number of the secondary dendritic branches was observed, associated with dramatic decrease in spine density, a fact that pleads in favor of a substantial involvement of mammillary bodies in AD, which may explain the disturbances of visuospatial memory and orientation, frequently described in patients even in the initial stages of AD.
Footnotes
This article was accepted under the editorship of the former Editor-in-Chief, Carol F. Lippa.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. [DOI] [PubMed] [Google Scholar]
- 2. Giannakopoulos P, Herrmann FR, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60(9):1495–1500. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Copenhaver BR, Rabin LA, Saykin AJ, et al. The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res. 2006;147(2-3):93–103. [DOI] [PubMed] [Google Scholar]
- 5. Oishi K, Mielke MM, Albert M, Lyketsos CG, Mori S. The fornix sign: a potential sign for Alzheimer’s disease based on diffusion tensor imaging. J Neuroimaging. 2012;22(4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Spulber G, Lehtimäki KK, et al. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2011;32(9):1558–1571. [DOI] [PubMed] [Google Scholar]
- 7. Dunnet SB, Martel FL. Proactive interference effects on short term memory in rats: I. Basic parameters and drug effects. Behav Neurosci. 1990;104(5):655–665. [DOI] [PubMed] [Google Scholar]
- 8. Sziklas V, Petrides M. Memory impairments following lesions to the mammillary region of the rat. Eur J Neurosci. 1993;5(5):525–540. [DOI] [PubMed] [Google Scholar]
- 9. Mair WPG, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis: a neurological and neuropsychological investigation of two cases. Brain. 1979;102(4):749–783. [DOI] [PubMed] [Google Scholar]
- 10. Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;4(3):343–351. [DOI] [PubMed] [Google Scholar]
- 11. Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics. 2011;31(4):1107–1121. [DOI] [PubMed] [Google Scholar]
- 12. Saunders R, Aggleton J. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17(5):396–411. [DOI] [PubMed] [Google Scholar]
- 13. Delay J, Brion S. Le Syndrome de Korsakoff [Korsakoff syndrome]. Paris, France: Masson and Cie; 1969. [Google Scholar]
- 14. Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinas RR. Afferent projections to the mammillary complex of the rat, with special reference to those from surrounding hypothalamic regions. J Comp Neurol. 1992;321(2):277–299. [DOI] [PubMed] [Google Scholar]
- 15. Santín LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102(1-2):137–150. [DOI] [PubMed] [Google Scholar]
- 16. Kantarci K. Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer’s disease. Front Aging Neurosci. 2014;6:316. doi:10.3389/fnagi.2014.00316. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pievani M, Agosta F, Pagani E, et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31(12):1862–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mielke MM, Okonkwo OC, Oishi K, et al. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimers Dement. 2012;8(2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baloyannis SJ, Mavroudis I, Mitilineos D, Baloyannis IS, Costa VG. The Hypothalamus in Alzheimer’s disease: a Golgi and electron microscope study. Am J Alzheimers Dis Other Demen. 2015;30(5):478–487. pii: 1533317514556876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 21. Sotelo JR. Technical improvements in specimen preparation for electron microscopy. Exp Cell Res. 1957;13(3):599–601. [DOI] [PubMed] [Google Scholar]
- 22. Leonard C. Silver degeneration methods. In: Johnson JE, Jr, ed. Current Trends in Morphological Techniques. Boca Raton, FL: CRC Press; 1981:93–140. [Google Scholar]
- 23. Baloyannis SJ. Recent progress of the Golgi technique and electron microscopy to examine dendritic pathology in Alzheimer’s disease. Future Neurol. 2013;8(3):239–242. [Google Scholar]
- 24. Baloyannis SJ. Staining of dead neurons by the Golgi method in autopsy material. Methods Mol Biol. 2015;1254:167–179. [DOI] [PubMed] [Google Scholar]
- 25. Cavalieri B. Geometria Indivisibilibus Continuorum. Bologna, Italy: Typis Clementis Ferronij; 1635 (reprinted 1966 as Geometria Degli Indivisibili. Unione Tipografico-Editrice Torinese, Torino). [Google Scholar]
- 26. Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(3):229–263. [DOI] [PubMed] [Google Scholar]
- 27. Nyengaard JR, Gundersen HJG. Direct and efficient stereological estimation of total cell quantities using electron microscopy. J Microsc. 2006;222(3):182–187. [DOI] [PubMed] [Google Scholar]
- 28. Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136(3):757–767. [DOI] [PubMed] [Google Scholar]
- 29. Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. [DOI] [PubMed] [Google Scholar]
- 30. Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386(4):661–680. [PubMed] [Google Scholar]
- 31. Uylings HBM, Van Eden CG, Parnavelas JG, Kalsbeek A. The prenatal and postnatal development of rat cerebral cortex. In: Kolb E, Tees RC, eds. The Cerebral Cortex of the Rat. Cambridge, MA: MIT Press; 1990:35–76. [Google Scholar]
- 32. Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- 33. Nyengaard JR, Gundersen HJ. Direct and efficient stereological estimation of total cell quantities using electron microscopy. J Microsc. 2006;222(pt 3):182–187. [DOI] [PubMed] [Google Scholar]
- 34. Gundersen HJG, Jensen EBV, KIE K, Nielsen J. The efficiency of systematic sampling in stereology—reconsidered. J Microsc. 1999;193(3):199–211. [DOI] [PubMed] [Google Scholar]
- 35. West MJ. The precision of estimates in stereological analyses. Cold Spring Harb Protoc. 2012;2012(9):937–949. [DOI] [PubMed] [Google Scholar]
- 36. West MJ. Estimating volume in biological structures. Cold Spring Harb Protoc. 2012;2012(11):1129–1139. [DOI] [PubMed] [Google Scholar]
- 37. West MJ. Estimating surface area in biological structures. Cold Spring Harb Protoc. 2013;2013(2):77–82. [DOI] [PubMed] [Google Scholar]
- 38. West MJ. Counting and measuring ultrastructural features of biological samples. Cold Spring Harb Protoc. 2014;2013(7):593–605. [DOI] [PubMed] [Google Scholar]
- 39. Small JV. Measurement of Section Thickness. Fourth European Conference on Electron Microscopy, 1968:609–610. [Google Scholar]
- 40. Jensen EB, Gundersen HJG. Stereological ratio estimation based on counts from integral test systems. J Microsc. 1982;125(1):51–66. [Google Scholar]
- 41. Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the dissector. J Microsc. 1984;134(pt 2):127–136. [DOI] [PubMed] [Google Scholar]
- 42. Geinisman Y, Gundersen HJ, van der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996;25(12):805–881. [DOI] [PubMed] [Google Scholar]
- 43. Fiala JC, Harris KM. Cylindrical diameters method for calibrating section thickness in serial electron microscopy. J Microsc. 2001;202(pt 3):468–472. [DOI] [PubMed] [Google Scholar]
- 44. Feuerverger A, Menzinger M, Atwood HL, Cooper RL. Statistical methods for assessing the dimensions of synaptic vesicles in nerve terminals. J Neurosci Methods. 2000;103(2):181–190. [DOI] [PubMed] [Google Scholar]
- 45. Baddeley AJ, Gundersen HJG, CruzOrive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142(3):259–276. [DOI] [PubMed] [Google Scholar]
- 46. Gardi JE, Nyengaard JR, Gundersen HJG. Using biased image analysis for improving unbiased stereological number estimation—a pilot simulation study of the smooth fractionator. J Microsc. 2006;222(3):242–250. [DOI] [PubMed] [Google Scholar]
- 47. Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. J Am Stat Assoc. 1952;47(260):663–685. [Google Scholar]
- 48. Witgen BM, Grady MS, Nyengaard JR, Gundersen HJG. A new fractionator principle with varying sampling fractions: exemplified by estimation of synapse number using electron microscopy. J Microsc. 2006;222(3):251–255. [DOI] [PubMed] [Google Scholar]
- 49. Tagliamonte M, Sestieri C, Romani GL, Gallucci M, Caulo M. MRI anatomical variants of mammillary bodies. Brain Struct Funct. 2015;220(1):85–90. [DOI] [PubMed] [Google Scholar]
- 50. Neave N, Nagle S, Aggleton JP. Evidence for the involvement of the mammillary bodies and cingulum bundle in allocentric spatial processing by rats. Eur J Neurosci. 1997;9(5):941–955. [DOI] [PubMed] [Google Scholar]
- 51. Sziklas V, Petrides M, Leri F. The effects of lesions to the mammillary region and the hippocampus on conditional associative learning by rats. Eur J Neurosci. 1996;8(1):106–115. [DOI] [PubMed] [Google Scholar]
- 52. Vann SD. A role for the head-direction system in geometric learning. Behav Brain Res. 2011;224(1):201–216. [DOI] [PubMed] [Google Scholar]
- 53. Tanaka Y, Miyazawa Y, Akaoka F, Yamada T. Amnesia following damage to the mammillary bodies. Neurology. 1997;48(1):160–165. [DOI] [PubMed] [Google Scholar]
- 54. Vann SD. Re-evaluating the role of the mammillary bodies in memory Neuropsychologia. 2010;48(8):2316–2327. [DOI] [PubMed] [Google Scholar]
- 55. Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22(3):425–444. [PubMed] [Google Scholar]
- 56. Seki M, Zyo K. Anterior thalamic afferents from the mammillary body and the limbic cortex in the rat. J Comp Neurol. 1984;229(2):242–256. [DOI] [PubMed] [Google Scholar]
- 57. Aggleton JP, Pearce JM. Neural systems underlying episodic memory: insights from animal research. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoder RM, Taube JS. Projections to the anterodorsal thalamus and lateral mammillary nuclei arise from different cell populations within the postsubiculum: implications for the control of head direction cells. Hippocampus. 2011;21(10):1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park K-C, Yoon S-S, Chang DI, et al. Amnesic syndrome in a mammillothalamic tract infarction. J Korean Med Sci. 2007;22(6):1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mayes AR. Memory and amnesia. Behav Brain Res. 1995;66(1-2):29–36. [DOI] [PubMed] [Google Scholar]
- 61. Nelson AJ, Vann SD. Mammillothalamic tract lesions disrupt tests of visuo-spatial memory. Behav Neurosci. 2014;128(4):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vann SD, Aggleton JP. Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic. Tract J Neurosci. 2003;23(8):3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aggleton JP, Sahgal A. The contribution of the anterior thalamic nuclei to anterograde amnesia. Neuropsychologia. 1993;31(10):1001–1019. [DOI] [PubMed] [Google Scholar]
- 64. Vann SD, Honey RC, Aggleton JP. Lesions of the mammillothalamic tract impair the acquisition of spatial but not non spatial contextual discriminations. Eur J Neurosci. 2003;18(8):2413–2416. [DOI] [PubMed] [Google Scholar]
- 65. Veazey RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). 2. Efferent connections. J Comp Neurol. 1982;207(2):135–156. [DOI] [PubMed] [Google Scholar]
- 66. Loftus M, Knight RT, Amaral DG. An analysis of atrophy in the medial mammillary nucleus following hippocampal and fornix lesions in humans and nonhuman primates. Exp Neurol. 2000;163(1):180–190. [DOI] [PubMed] [Google Scholar]
- 67. Dillingham CM, Frizzati A, Nelson AJD, Vann SD. How do mammillary body inputs contribute to anterior thalamic function? Neurosci Biobehav Rev. 2015;54:108–119. pii: S0149-7634(14)00190-0. doi:10.1016/j.neubiorev.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tan RH, Wong S, Kril JJ, et al. Beyond the temporal pole: limbic memory circuit in the semantic variant of primary progressive aphasia. Brain. 2014;137(7):2065–2076. [DOI] [PubMed] [Google Scholar]
- 69. D’Esposito M, Verfaellie M, Alexander MP, Katz DI. Amnesia following traumatic bilateral fornix transection. Neurology. 1995;45(8):1546–1550. [DOI] [PubMed] [Google Scholar]
- 70. Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology. 2012;79(8):748–754. [DOI] [PubMed] [Google Scholar]
- 71. Fletcher E, Raman M, Huebner P, et al. Loss of fornix white matter volume as a predictor of cognitive impairment in cognitively normal elderly individuals. JAMA Neurol. 2013;70(11):1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kril JJ, Harper CG. Neuroanatomy and neuropathology associated with Korsakoff’s syndrome. Neuropsychol Rev. 2012;22(2):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: a reappraisal. Neuropsychologia. 2011;49(5):777–789. [DOI] [PubMed] [Google Scholar]
- 74. Tsivilis D, Vann SD, Denby C, et al. A disproportionate role for the fornix and mammillary bodies in recall vs recognition memory. Nat Neurosci. 2008;11(7):834–842. [DOI] [PubMed] [Google Scholar]
- 75. Teuber HL, Milner B, Vaughan HG. Persistent anterograde amnesia after stab wound of the basal brain. Neuropsychologia. 1968;6(3):267–282. [Google Scholar]
- 76. Gibson B, Butler WN, Taube JS. The head-direction signal is critical for navigation requiring a cognitive map but not for learning a spatial habit. Curr Biol. 2013;23(16):536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5(1):35–44. [DOI] [PubMed] [Google Scholar]
- 78. Vann S D. Gudden’s ventral tegmental nucleus is vital for memory: re-evaluating diencephalic inputs for amnesia. Brain. 2009;132(pt 9):2372–2384. [DOI] [PubMed] [Google Scholar]
- 79. Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J Neurosci. 2007;27(28):7564–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Allen GV1, Hopkins DA. Mammillary body in the rat: a cytoarchitectonic, Golgi, and ultrastructural study. J Comp Neurol. 1988;275(1):39–64. [DOI] [PubMed] [Google Scholar]
- 81. de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer’s disease: the atrophic hippocampus. Lancet. 1989;2(8664):672–673. [DOI] [PubMed] [Google Scholar]
- 82. de Leon MJ, George AE, Golomb J, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol Aging. 1997;18(1):1–11. [DOI] [PubMed] [Google Scholar]
- 83. Copenhaver BR, Rabin LA, Saykin AJ, et al. The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res Neuroimaging. 2006;147(2-3):93–103. [DOI] [PubMed] [Google Scholar]
- 84. Lee DY, Fletcher E, Carmichael OT, et al. Sub-regional hippocampal injury is associated with fornix degeneration in Alzheimer’s disease. Front Aging Neurosci. 2012;4:1. doi:10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Callen DJ, Black SE, Caldwell CB, Grady CL. The influence of sex on limbic volume and perfusion in AD. Neurobiol Aging. 2004;25(6):761–770. [DOI] [PubMed] [Google Scholar]
- 86. Fletcher E. Could the fornix be used to predict cognitive impairment in the elderly. Future Neurol. 2014;9(1):1–4. [Google Scholar]
- 87. Bitner RS, Bunnelle WH, Anderson DJ, et al. Broad spectrum efficacy across cognitive domains by 7 nicotinic acetylcholine receptor agonist correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27(39):10578–10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Baloyannis S, Costa V, Arnaoutoglou A, Arnaoutoglou H. Synaptic alterations in the molecular layer of the cerebellum in Alzheimer’s disease. Neuropath Appl Neurobiol. 1996;22(1):78–79. [Google Scholar]
- 89. Baloyannis SJ, Costa V, Michmizos D. Mitochondrial alterations in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2004;19(2):89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Baloyannis SJ. The Golgi apparatus of Purkinje cells in Alzheimer’s disease. In: Bohl J. ed. Neuropathology Back to the Roots. Aachen, Germany: Shaker Vertag; 2002:1–10. [Google Scholar]
- 91. Baloyannis SJ. Mitochondria are related to synaptic pathology in Alzheimer’s disease. Intern J Alzh Dis. 2011;2011:305395. doi:10.4061/2011/305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Baloyannis SJ, Manolidis SL, Manolidis LS. Synaptic alterations in the vestibulocerebellar system in Alzheimer’s disease—a Golgi and electron microscope study. Acta Otolaryngol. 2000;120(2):247–250. [DOI] [PubMed] [Google Scholar]
- 93. Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):119–126. [DOI] [PubMed] [Google Scholar]
- 94. Tagliamonte M, Sestieri C, Romani GL, Gallucci M, Caulo M. MRI anatomical variants of mammillary bodies. Brain Struct Funct. 2015;220(1):85–90. [DOI] [PubMed] [Google Scholar]
- 95. Kopelman MD. What does a comparison of the alcoholic Korsakoff syndrome and thalamic infarction tell us about thalamic amnesia? Neurosci Biobehav Rev. 2015;54:46–56. pii: S0149-7634(14)00212-7. doi: 10.1016/j.neubiorev.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 96. Belzunegui T, Insausti R, Ibáñez J, Gonzalo LM. Effect of chronic alcoholism on neuronal nuclear size and neuronal population in the mammillary body and the anterior thalamic complex of man. Histol Histopathol. 1995;10(3):633–638. [PubMed] [Google Scholar]
- 97. Brion S, Mikol J, Plas J. Neuropathology of amnesic syndromes in man. Rev Neurol (Paris). 1985;141(10):627–643. [PubMed] [Google Scholar]
- 98. Sullivan EV, Lane B, Deshmukh A, et al. In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res. 1999;23(10):1629–1636. [PubMed] [Google Scholar]
- 99. Sheedy D, Lara A, Garrick T, Harper C. Size of mammillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcohol Clin Exp Res. 1999;23(10):1624–1628. [PMC free article] [PubMed] [Google Scholar]
- 100. Grossi D, Lopez OL, Martinez AJ. Mammillary bodies in Alzheimer’s disease Acta Neurol Scand. 1989;80(1):41–45. [DOI] [PubMed] [Google Scholar]