Abstract
Amyloid β25-35 (Aβ25-35) represents a neurotoxic fragment of Aβ1 - 40 or Aβ1 - 42, and is implicated in the progressive neurodegeneration in cases of the Alzheimer disease (AD). Amyloid β25-35 was shown to lyse rat erythrocytes (RBCs) of all ages, and the extent of the RBC toxicity is directly correlated with Aβ25-35 concentration and cell age. Activities of glycolytic, antioxidant, and Na+/K+-adenosine triphosphatase (ATPase) enzymes, in vivo, are significantly decreased in older RBCs as compared to the young RBCs. In vitro, Aβ25-35 reduced activities of hexokinase, phosphofructokinase, pyruvate kinase, glutathione peroxidase, and glutathione transferase and increased Na+/K+-ATPase activity; these effects are significantly greater in aged RBCs as compared to those of the younger cells. The diminution in activity of certain enzymes may determine the life span of the RBCs in vivo and may be relevant to the human AD; higher sensitivity of older RBCs to Aβ25-35 toxicity may contribute to the ultimate death of the RBCs in patients with AD.
Keywords: Alzheimer disease, membrane stability, energy metabolism, antioxidant enzymes, erythrocytes
Introduction
Amyloid β (Aβ) is a low-molecular-weight peptide and is a structural component of extracellular senile plaques that initiate progressive neurodegeneration in the brains of patients with Alzheimer disease (AD) and a number of other disorders. 1,2 The monomeric Aβ is a soluble metabolite 2 -4 and exists in plasma of both patients with AD and healthy persons. 5 It also binds to the walls of blood vessels 6,7 and directly interacts on erythrocytes (RBCs) in vivo.
Amyloid β25-35 represents a neurotoxic fragment of Aβ1-40 or Aβ1-42 and retains the toxicity of the full-length peptide. 8 Amyloid β25-35 is often selected as a model for full-length peptide because it retains both its physical and biological properties, and additionally, its short length readily allows its derivatives to be synthesized and studied as reviewed by Kaminsky and coworkers. 9 Due to the smaller size, it is relatively easier for the peptide to permeate through the cell membrane, while at the same time its toxicity is similar with the Aβ1 - 40 and Aβ1 - 42. Amyloid β25-35 is widely used instead of the full-length Aβ peptide, and it has replaced other smaller fragments of Aβ peptide in the literature. Upon incubation with Aβ25-35, RBCs are lysed. 10 -12 However, underlying mechanisms are not clear cut to date. The cytotoxic effects may include inhibition of RBC glycolysis or alteration in the activities of Na+/K+-adenosine triphosphatase (ATPase, EC 3.6.3.9) and glutathione peroxidase (GPx, EC 1.11.1.9), 11 which have important roles in energy metabolism and attenuation of oxidative stress.
Life span of rat RBCs is typically 37 days 13 to 66 days, 14 so cells of different ages are always present in the circulation. During the cell life, aging RBCs alter their biological and physical properties permanently and irreversibly. Age-related death and removal of RBCs from the bloodstream occur as a result of the defects in energy metabolism and cell sensitization to the damage in metabolic stress. 15 In various types of mammals, the RBC life span correlates with the activities of intracellular enzymes such as superoxide dismutase (SOD, EC 1.15.1.1; r = .887, our calculation) and GPx (r = .721), as well as with glutathione (GSH) levels (r = .944). 16 These results were interpreted as due to the regulation of RBC lifetime by oxygen radical generation and efficiency of the intrinsic antioxidant defense system.
In order to understand the correlation of aging to the onset of AD, it would be informative to investigate the effects of the Aβ peptides on RBCs of various ages, and such studies are lacking in the literature. Related recent studies suggest that Aβ potentiates phosphate- and calcium ion-induced opening of the mitochondrial channels, 17 and glial and neuronal hemichannels, 18 with the resultant swelling of mitochondria, accompanied by the elevated levels of the lipid peroxidation products. 17 The Aβ25-35 was also shown to induce cyclooxygenase 2 (COX2) expressions and consequent accumulation of prostaglandin E2 in the primary midbrain astrocytes. 19 The elevated levels of prostaglandins, in turn is linked to neuroinflammatory aspects of AD, and various studies suggest that the nonsteroidal anti-inflammatory drugs that block COX enzymes attenuate the incidence of AD through epidemiological studies. 19
We hypothesized that aged RBCs are relatively more sensitive to Aβ toxicity than the younger RBCs. Accordingly, in this study, we have investigated the impacts of the Aβ25-35 in vitro on membrane stability, and its effects on the glycolytic- and antioxidant-related enzymes in rat RBCs of varying ages.
Materials and Methods
We used male Wistar rats that weighed 250 to 300 g. The experimental procedures were approved by the institutional committee of the Institute of Theoretical and Experimental Biophysics and met the guidelines of the European Union for treatment and use of experimental animals (1986) stated by the Decree of Russian Health Ministry of June 19, 2003, number 267 “Guidelines of laboratory practice in Russia.”
Erythrocyte Preparation and Fractionation
The washed RBCs were treated as recommended by the International Committee for Standardization in Haematology. 20 All preparative procedures were performed at 4°C.
A 0.025 mL of 130 mmol/L trisodium citrate, pH 7.4, was added to 0.25 mL of blood (2:1) and mixed with 10 mmol/L potassium phosphate buffer, pH 7.4, containing 10 mmol/L EDTA, 150 mmol/L NaCl, 0.05 mmol/L EDTA, and 0.02 mmol/L phenylmethylsulfonyl fluoride (PhCH2SO2F). The suspension was loaded onto a column prepacked with α-cellulose/hemicrystalline cellulose type 50 (1:1); the filtrate was collected into a centrifuge tube with 1/5 volume of the ice-cold 10 mmol/L potassium phosphate buffer, pH 7.4, containing 10 mmol/L EDTA and 150 mmol/L NaCl, and centrifuged at 1000g for 10 minutes. Then RBCs were washed 3 times for 10 minutes at 1000g, 1300g, and 1600g.
Erythrocytes were separated into 3 fractions of young, middle-age, and old cells as described by Seaman et al 15 using centrifugation in percoll (Pharmacia Fine Chemicals, Sweden) gradient. The washed whole RBC population packed to a hematocrit of approximately 80% was resuspended in percoll solution (114 mmol/L NaCl, 10 mmol/L KH2PO4, pH 7.4, 0.5 mmol/L EDTA, 0.5% glucose, 0.2 mmol/L PhCH2SO2F, and 0.76 mL/mL of percoll with a density of 1.13 g/mL) to give hematocrit of 15%. 21 The suspension contained less than 2% of reticulocytes and leukocytes and no platelets. It was centrifuged at 33 000g for 20 minutes. The upper 1 mm layer in the tube consists of young RBCs, the middle layer of about 80 mm is for the middle-age cells, and the bottom 1.5 mm layer corresponds to old cells. Each age fraction was washed 3 times for 10 minutes at 1000g, 1300g, and 1600g with 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.5 mmol/L EDTA and 140 mmol/L NaCl, 5 mmol/L KCl, and 2.8 mmol/L glucose. Then each age fraction was suspended in the same buffer (1:5) and used in assays.
Mean corpuscular volume (MCV), mean corpuscular hemoglobin (Hb; MCH), and mean corpuscular Hb concentration (MCHC) were determined using Beckman Coulter ACT8 (Beckman Coulter, Brea, CA).
Erythrocytes Incubation With Aβ25-35
The Aβ25-35 solution was prepared as described earlier by Solomadin and coworkers. 12 Rat RBCs were incubated for different times (5-60 minutes) at 25°C in the medium containing nontoxic Aβ35-25 10 mmol/L KH2PO4, pH 7.4, 140 mmol/L NaCl, 5 mmol/L KCl and Aβ25-35 at 5, 10, or 25 μmol/L. In control experiments, nontoxic Aβ35-25 was used at the same concentrations. After incubation, RBCs were precipitated at 300g (5 minutes, 4°C), and the supernatant assayed for Hb absorption as a measure of RBC lysis. Cell lysis was determined spectrophotometrically at 540 nm and expressed as percentage. The RBC lysis and supernatant absorption after cell incubation in distilled water was taken as 100%.
Preparation of RBC Hemolysate
Washed RBCs of each age were hemolysed in 40 volumes of 5 mmol/L Tris–HCl buffer, pH 8, containing 0.5 mmol/L EDTA, 0.2 mmol/L PhCH2SO2F, and 0.02% saponin at 4°C for 10 minutes.
Enzyme Assay
We determined the activities of hexokinase (HK), phosphofructokinase (PFK, EC 2.7.1.11), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK), lactate dehydrogenase (LDH), glucose 6-phosphate dehydrogenase (G6PDH), GPx, glutathione transferase (GT), catalase, SOD, and Na+/K+-ATPase in RBC hemolysate. Activities of HK, GAPDH, PK, LDH, and G6PDH were measured essentially using the Beutler procedures. 22a
Phosphofructokinase activity was estimated spectrophotometrically by the method of Bosshard. 23 The reaction mixture contained 100 mmol/L Tris–HCl, pH 8, 5 mmol/L MgCl2, 1 mmol/L adenosine triphosphate (ATP), 0.2 mmol/L NADH, 100 mmol/L KCl, 1 mmol/L KCN, 2 mmol/L adenosine monophosphate, 5 U/mL of aldolase, 5 U/mL of triosephosphate isomerase, 5 U/mL of GAPDH, and the hemolysate. The reaction was initiated by adding 3 mmol/L fructose-6-phosphate.
Activity of Na+/K+-ATPase (EC 3.6.3.9) was determined by a modified method described earlier. 24 Briefly, 15 µL of the hemolysate was added to 0.5 mL of a buffer solution (30 mmol/L histidine, pH 7.4, 130 mmol/L NaCl, 20 mmol/L KCl, 4 mmol/L MgCl2, and 3 mmol/L ATP) containing 1 mmol/L ouabain (3-[(6-deoxy-α-l-mannopyranosyl)oxy]-1,5,11,14,19-pentahydroxy-, (1β,3β,5β,11α)card-20(22)-enolide) or no ouabain, and was preliminarily heated to 37°C. The mixture was incubated for 20 minutes at 37°C. The reaction was terminated by adding 2 mL of a mixture of 10% trichloroacetic acid and 2% ascorbic acid. Then, the reaction mixture was centrifuged for 5 minutes at 3000g, and 1 mL of the supernatant was added into a solution of 0.5 mL of 1% ammonium molybdate and 1 mL of a mixture consisting of 2% sodium arsenite, 2% sodium citrate, and 2% acetic acid. After 15 minutes, the absorption at 700 nm was measured. The enzyme activity was calculated from the difference in the rates of the formation of inorganic phosphate, in the absence and in the presence of ouabain.
Assays were carried out at 37°C and 340 nm in a Kontron (Uvikon 923) (Firstenberg Machinery Co., Inc, Richmond, CA) double-beam recording spectrophotometer and Varian Cary Eclipse fluorescence spectrophotometer (Agilent Technologies, CA). Enzymatic activities were determined by measuring the rate of NADPH or NAD+ formation.
Protein content was determined with bovine serum albumin as standard.22b The activities of SOD (EC 1.15.1.1), catalase (EC 1.11.1.6), and GPx in the hemolysate were determined by methods cited elsewhere. 25 The gluthatione transferase (GT, EC 2.5.1.18) activity was measured by the Kozer et al method. 26 The hemolysate was added to 100 mmol/L sodium phosphate buffer, pH 7, containing 1 mmol/L EDTA, 1 mmol/L GSH, and 1 mmol/L 1-chloro-2,4-dinitrobenzene, and absorbance at 340 nm was monitored.
Statistical Analysis
Data were presented as mean values ± standard error. Statistical comparisons were performed by Student’s t test. One-way analysis of variance with Bonferroni’s posttests for multiple comparisons was performed using GraphPad Prism version 5.02 for Windows, GraphPad Software (San Diego, California). Bonferroni’s-corrected P values are given in figures and tables.
Results
Rat RBC Indices
The MCV of the whole rat RBC population is much lower than that of human RBCs, approximately 60 versus 100 μm3, respectively, 27 as detected in earlier studies. 28,29 Sutera and coworkers 30 have earlier reported that in human RBCs, MCV was significantly lower (P < .025) in 10% bottom fraction of the whole RBC population than that in 10% top fraction. In this study, we tested whether MCV changes with rat RBC aging. Table 1 shows that the MCVs of middle-age and old RBCs are significantly lower than that of young RBCs, by 6.3% and 7.5% (both P < .0001, Student’s t test), respectively. The mean MCV, as calculated from all young, middle-age, and old RBCs, is 57.7 ± 0.3 (n = 143) and corresponds to the reported value. 28,29
Table 1.
Rat RBC Indices of Cells of Different Ages.
| Index | Young | Middle Age | Old |
|---|---|---|---|
| MCV, fL | 60.6 ± 0.6 (52.3-69.7), n = 42 | 56.8 ± 0.4a(47.8-64.3), n = 56 | 56.1 ± 0.6a(46.8-63.9), n = 45 |
| MCH, pg | 17.9 ± 0.21 (14.9-20.2), n = 38 | 18.4 ± 0.17 (15.1-21.0), n = 53 | 19.8 ± 0.24a,b(16.9-22.9), n = 46 |
| MCHC, % | 29.3 ± 0.22 (26.0-31.6), n = 38 | 32.3 ± 0.17a(30.7-34.8), n = 53 | 35.3 ± 0.23a,b(32.2-39.6), n = 46 |
Abbreviations: MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RBC, erythrocyte.
a P < .001 compared to young cells.
b P < .001 compared to middle-age cells (one-way ANOVA with Bonferroni’s multiple comparison test).
In contrast, the MCH and MCHC of middle-age and old RBCs are significantly higher than those of young RBCs (Table 1). The MCH of old RBCs is 10.6% and 7.6% higher than that of young and middle-age cells, respectively (both P < .0001, Student’s t test).
The MCHC of old and middle-age RBCs is 20.5% and 10.2% higher as compared with that of young cells, respectively, and it is 9.3% higher in old RBCs than in middle-age cells (all P < .0001, Student’s t test). Similar findings were obtained on human RBCs: MCHC was significantly higher (P < .001) in 10% oldest human RBCs than that in 10% most young cells. 30 The results obtained on middle-age RBCs are in accordance with data reported by Kazennov et al 31 using the whole RBC population from adult rats. Interestingly, the MCH of rat RBCs of 3 age groups is significantly lower than that of human values (18-20 vs 27-31 pg), while MCHC is similar in 2 species. 27 Thus, MCV decreases, whereas MCH and MCHC increase with the aging of the rat RBCs.
Amyloid β25-35-Induced Toxicity on Rat RBC Indices
We have chosen Aβ25-35 as the trigger for cellular toxicity and its reverse peptide Aβ35-25 (with reverse amino acid sequence) as a control, based on the prior literature evidence by Yankner et al 32 . These authors have reported that the toxic properties of Aβ peptides were due to Aβ peptide amino acids 25 to 35, whereas the reverse peptide Aβ35-25 and scrambled Aβ25-35 (with scrambled amino acid sequence) are not cytotoxic. 33 It has been shown that in the cell culture assays, Aβ25-35 forms reactive oxygen species (ROS), whereas Aβ35-25 and scrambled Aβ25-35 do not form ROS and are nontoxic. 34 Based on this, it is now generally accepted practice to use nontoxic reversed peptide or scrambled peptide in control experiments, both in vivo 35,36 and in vitro. 34
Rat RBCs are sensitive to the hemolytic effects of Aβ25-35 in vitro as do human RBCs. 10 -12 However, there has been no evidence in the literature to infer whether Aβ25-35 affects rat RBC indices. In this study, we have tested this hypothesis. Exposure of rat RBCs to high concentrations of Aβ25-35 resulted in 10% increase in MCV of young cells (Table 2). Lower MCV of old RBCs was not affected by Aβ25-35 treatment (Table 2).
Table 2.
The Effects of Aβ25-35 on RBC Indices for Young and Old Cells.a
| Index | Young | Old | ||
|---|---|---|---|---|
| Control | Aβ25-35 | Control | Aβ25-35 | |
| MCV, fL | 61.0 ± 2.1 (6) | 67.3 ± 1.5 (7)b | 53.5 ± 1.7 (6) | 54.5 ± 1.2 (7)c |
| MCH, pg | 17.6 ± 0.67 (5) | 17.4 ± 1.05 (6) | 19.2 ± 0.33 (5) | 16.4 ± 0.9 (6) |
| MCHC, % | 28.1 ± 0.1.0 (5) | 25.4 ± 1.61 (6) | 34.9 ± 0.57 (5)d | 29.6 ± 1.86 (6)b |
Abbreviations: Aβ, amyloid β; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RBC, erythrocyte.
aRBC were incubated for 30 minutes at 25°C in the presence of 25 μmol/L Aβ25-35 and then analyzed for MCV, MCH, and MCHC. The number of experiments is indicated in parentheses.
b P < .05 compared to control.
c P < .001 compared to young Aβ25-35.
d P < .001 compared to young control (one-way ANOVA with Bonferroni’s multiple comparison test).
The MCH of both young and old RBCs is unchanged after incubation with 25 μmol/L Aβ25-35. The MCHC of young RBCs is resistant to Aβ25-35 treatment, while MCHC of old RBCs falls by 15% (P < .05) after Aβ25-35 (Table 2).
Concentration Dependence of Aβ25-35 on RBCs
To assess the sensitivity of RBCs to Aβ25-35 toxicity, cells were incubated at various Aβ25-35 concentrations, and then the RBC lysis was estimated for the Hb release from the lysed cells, by monitoring at 540 nm. Incubation of the whole RBC population (not separated into age fractions) with Aβ25-35 caused the cell lysis as shown by spectrophotometry.
The relative number of lysed cells is dependent on the Aβ25-35 concentration (Figure 1). Lysis was 15.1% ± 2.0% at 10 μmol/L Aβ25-35 (n = 4, P < .0001 compared to control, 3.81 ± 0.10, n = 7; Student’s t test) and 32.5% ± 3.0% at 25 μmol/L Aβ25-35 (n = 6, P < .0001). There was a significant difference between effects of 10 and 25 μmol/L Aβ25-35 (P = .0029). These results are comparable with those reported earlier by Kosenko and coworkers. 11,12
Figure 1.

Dependence of the rat RBCs (whole population) lysis on Aβ25-35 concentration. Cells were incubated at 10 μmol/L Aβ25-35 (n = 4) or 25 μmol/L Aβ25-35 (n = 6) for 30 minutes. In the control experiment, cells were incubated with 10 μmol/L Aβ35-25 (n = 7). The same RBC preparation was used under various experimental conditions. Significant differences are indicated by P values (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β; RBC, erythrocyte.
Impact of Amyloid β25-35 on Lysis of RBCs of Different Ages
Sensitivity of RBCs of different ages to Aβ25-35 toxicity was tested as mentioned earlier. Figure 2 shows the effects of 25 μmol/L Aβ25-35 on lysis of young and old RBCs. There was 26% of young and 37% of old cells lysed after 30 minutes incubation, significantly higher than control levels of 3.8% to 4% (P < .0001). There was significant difference between lysis of young and old RBCs (P = .0107).
Figure 2.
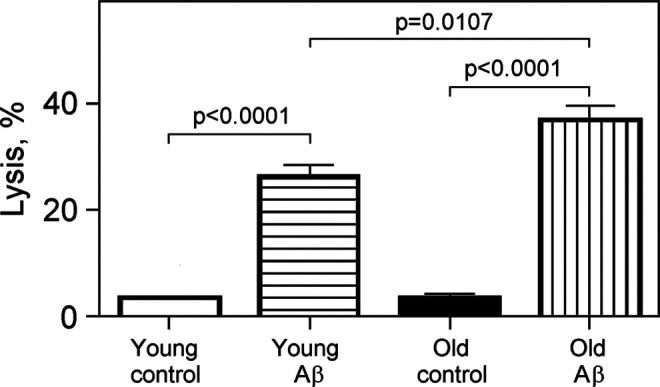
The effect of Aβ25-35 on lysis of young and old erythrocytes. Cells were incubated at 25°C in 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, 25 μmol/L Aβ25-35 (Aβ), or 25 μmol/L Aβ35-25 (control) for 30 minutes; n = 4 to 5. Significant differences are indicated by P values (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β.
In additional experiments, the effects of Aβ25-35 on lysis of young, middle-age, and old RBCs were studied using relatively lower Aβ25-35 concentrations. There are increments of 136% (from 5.72%-13.66%, P > .05) with lysis of young RBCs, 422% (from 4.13%-21.57%, P < .01) with middle-age RBCs, and 370% (from 5.09%-23.93%, P < .001) with old cells after 30 minutes incubation with 10 μmol/L Aβ25-35 (Figure 3). There was also significant difference between lysis of young and old RBCs (P < .05, Bonferroni’s test). Thus, the RBC lysis is increased with cell aging.
Figure 3.

Effects of Aβ25-35 on lysis of young, middle-age, and old RBCs. Cells were incubated for 30 minutes at 25°C in 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, and 10 μmol/L Aβ25-35. Controls were incubated with 10 μmol/L Aβ35-25 The same RBC preparation was used in both control and Aβ25-35 incubations; n = 6 to 14. Significant differences are indicated by P values (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β; RBC, erythrocyte.
Concentration Dependence of Aβ25-35 on Young and Old RBCs
We have investigated whether concentration dependence of Aβ25-35-induced lysis exists in RBCs of varying ages. Our results are summarized in Figure 4; Figure 4 demonstrates a linear correlation between Aβ25-35 concentration and lysis of both young and old RBCs. In the presence of 5 μmol/L Aβ25-35, 7% to 10% of young and old RBCs were lysed in 30 minutes at 25°C. When the Aβ25-35 concentration increased to 10 and 25 μmol/L, about 10% and 25% of young RBCs and 17% and 37% of old RBCs were lysed, respectively. These results show evidently that the old RBCs are more susceptible to Aβ25-35 toxicity than the young RBCs.
Figure 4.

Dependence of lysis of young (2) and old (3) RBCs on Aβ25-35 concentration. Cells were incubated for 30 minutes at 25°C in the potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl and Aβ25-35. In control 1, the cells were incubated with Aβ35-25. Concentrations of Aβ25-35 and Aβ35-25 are indicated on X-axis. The same RBC preparation was used under different experimental conditions; n = 4 to 5. Significant differences are indicated: *P < .05 (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). All differences between Aβ-treated cells and control are highly significant (P < .0001, Student’s t-test). Aβ indicates amyloid β; RBC, erythrocyte.
Young and old RBCs account each for about 1.2% of the whole RBC population, other 97.6% being referred as the middle-age RBCs (see Materials and Method section). So sensitivity of the middle-age RBCs to Aβ25-35 toxicity may be between these 2 extremes. We have observed that the middle-age RBCs are as highly sensitive as the old RBCs to Aβ25-35 toxicity (Figure 3). After 30 minutes of incubation with 10 μmol/L Aβ25-35, 10.5% ± 0.6%, 21.6% ± 4.8%, and 21.2% ± 3.6% of young, middle-age, and old RBCs, respectively, were lysed. Differences between young and middle-age RBCs (P = .0064, Student’s t-test) and young and old RBCs (P = .0004, Student’s t-test) are statistically highly significant, while there was no difference between middle-age and old cells.
Time Dependent Effects of Aβ25-35 on Young and Old RBCs
Incubation of the whole human RBC population with Aβ25-35 resulted in cell lysis, the lysis increasing with the incubation time. The maximum lysis was achieved after 2 hours incubation. 11,12 We therefore studied the time dependence of the Aβ25-35-treatment effect in rat RBCs of different ages during 2 hours incubation, and our results are summarized in Figure 5; Figure 5 shows that lysis of young and old RBCs increases nearly in a linear manner with the time of incubation with Aβ25-35. At any time, lysis of old RBCs is higher than those of the young cells. There are significant differences between young and old RBCs in lysis after 30 minutes (73%, P = .0047 by Student’s t test) and 120 minutes (87%, P = .046 by Student’s t test).
Figure 5.

Dependence of lysis of young (1) and old (2) rat RBCs on the time of cell incubation with Aβ25-35. Cells were incubated for 0 to 120 minutes at 25°C in the potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, and 10 μmol/L Aβ25-35. Then RBCs were precipitated, and the supernatant assayed for hemoglobin (Hb) absorption spectrophotometrically. The Y-axis corresponds to absorbance at 540 nm; n = 4 to 12. Significant differences are indicated: *P < .05 and **P < .01 (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β; RBC, erythrocyte.
Glycolytic Enzyme Activities in RBCs of Different Ages
To assess the effects of cell age on energy metabolism of RBCs, we measured activities of the key glycolytic enzymes. Figure 4 and 6 shows that HK and PK activities in middle-age and old RBCs are 1.5-fold and 2-fold lower, respectively, than in young cells. The differences are highly significant (P < .0001). Both activities are lower in old cells than in middle-age RBCs (P < .05).
The activity of PFK is significantly lower in old RBCs than in young and middle-age cells (P < .001), whereas LDH activity is higher in middle-age RBCs than in young cells, and GAPDH activity is indistinguishable among these 3 cell ages (Figure 6). These results are similar to those of human RBCs in which differences between age cell fractions in HK and PK activities achieved 2- to 4-fold with no differences in the activity of GAPDH. 15,37
Figure 6.
The effects of Aβ25-35 on hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) activities in young and old erythrocytes (RBCs). Cells were incubated at 25°C for 30 minutes in 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, and 10 μmol/L Aβ25-35. Control was incubated with nontoxic Aβ35-25. Enzyme activities are expressed as IU/g hemoglobin (Hb); n = 8 to 18. Significant differences are indicated: *P < .05, **P < .01, and ***P < .001 as compared to young cells; P < .05 as compared to the old control (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β.
Impact of Amyloid β25-35 on Glycolytic Enzymes in RBCs of Different Ages
To assess the effects of Aβ25-35 on glycolytic enzyme activities of RBCs, we have incubated cells of varying ages with Aβ25-35, and the enzymes were assayed in the cell lysates. After 30 minutes incubation with 10 μmol/L Aβ25-35, HK and LDH activities in young RBCs did not change, but PK and PFK activities decreased significantly by 16% (P = .0295) and 32% (P = .0075), respectively (Figure 7). Similar results were obtained earlier on the whole human RBC population; 5 μmol/L Aβ25-35 induced a insignificant reduction in LDH activity and a 3-fold elevation in PFK activity. 24 In old RBCs, 10 μmol/L Aβ25-35 increased paradoxically HK activity (P = .035, Student’s t-test), decreased PFK (P = .0074) and PK (P = .0444) activities with no change in LDH (Figure 7).
Figure 7.
Hexokinase (HK), phosphofructokinase (PFK), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK), and lactate dehydrogenase (LDH) activities in young, middle-age, and old erythrocytes (RBCs). Enzyme activities are expressed as U/g hemoglobin (Hb); n = 8-18. Significant differences are indicated: *P < .05 and ***P < .001 as compared to young cells; + P < .05 and +++ P < .001 as compared to middle-age cells (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test).
Impact of Amyloid β25-35 on Na+/K+-ATPase in RBCs of Different Ages
The major RBC enzyme providing conservation of its form and deformability for penetration into capillaries of any small size is Na+/K+-ATPase. The increase in Aβ25-35-induced RBC lysis by ouabain supported the involvement of Na+/K+-ATPase in the toxic activity of Aβ25-35. 11 We therefore studied the effect of Aβ25-35 on Na+/K+-ATPase in RBCs of varying ages, and the results are summarized in Figure 8. As can be seen from Figure 8, there are dramatic differences between young and old RBCs in their Na+/K+-ATPase activity, and the Aβ25-35 has significantly divergent neurotoxicity in these cell types. The Na+/K+-ATPase activity appears to be 5.8-fold lower in old RBCs than in young cells (P < .0001, Student’s t-test). After 30 minutes incubation with 10 μmol/L Aβ25-35, the enzyme activity remains unchanged in young RBCs but increases by 61% (P = .0266) in old RBCs. Exposure to 25 μmol/L Aβ25-35 causes the increase in Na+/K+-ATPase activity by 30% (P = .0129) in young RBCs and by 124% (P = .0241) in old RBCs, as compared with controls. Under all conditions, Na+/K+-ATPase activity in old RBCs remains much lower, by about 3 to 6 times, than that in young RBCs. Thus, exposure to Aβ25-35 leads to an increase in Na+/K+-ATPase activity in young and old RBCs.
Figure 8.

The effects of Aβ25-35 on Na+/K+-adenosine triphosphatase (ATPase) activity in young and old erythrocytes (RBCs). Cells were incubated at 25°C for 30 minutes in 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, and 10 or 25 μmol/L Aβ25-35. Controls were incubated with nontoxic Aβ35-25. Then enzyme activity was determined in the hemolysate; n = 8 to 18. Significant differences are indicated: **P < 0.01, ***P < 0.001 as compared with young control; +p < 0.05 as compared with old control (One-way ANOVA with Bonferroni's multiple comparison test). Aβ indicates amyloid β.
Impact of Amyloid β25-35 on Antioxidant Enzymes in RBCs of Varying Ages
The main enzymes providing cellular protection against oxidative damage to the biological cell are SOD, GPx, GT, and catalase. The SOD transforms superoxide radical into H2O2, and GPx and catalase metabolize H2O2 with the formation of H2O and O2. Glutathione transferase directly detoxifies 4-hydroxyalkenals and other cytotoxic products of radical and peroxidation reactions through sequestration of GSH. Two other enzymes, glutathione reductase (GR) and G6PDH, although not antioxidants, ensure antioxidant enzyme reaction to proceed constantly by regenerating glutathione and NADPH.
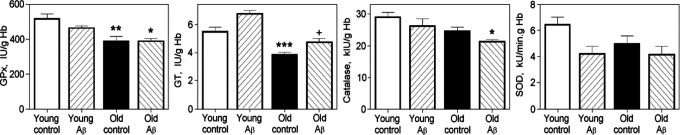
Activities of catalase, GPx, and G6PDH are known to be lower in old human RBCs than in young cells, 38 and the protection against oxidative and peroxidative injury is diminished with RBC aging. 39 In our experiments, the GPx activity in middle-age rat RBCs was 19% lower (P = .0066), and GPx and GT activities in old RBCs were 26% lower (P = .0003 and P = .0026, respectively) than those in young cells. However, there were no differences between RBCs of 3 ages in catalase and SOD activities (Figure 9). We have found that the G6PDH activity is significantly lower in middle-age (P = .0207) and old RBCs (P < .0001) than in young cells (Figure 10).
Figure 9.
Activities of glutathione peroxidase (GPx), glutathione transferase (GT), catalase, and superoxide dismutase (SOD) in young, middle-age, and old erythrocytes (RBCs); n = 7 to 12. Significant differences are indicated: *P < .05 and **P < .01 as compared with young cells (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test).
Figure 10.

Activities of glucose 6-phosphate dehydrogenase (G6PDH) in young, middle-age, and old erythrocytes; n = 13 to 14. Significant differences are indicated: *P < .05, **P < .001 as compared with young cells; +p < 0.05 as compared with middle-age cells (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test).
Kosenko and coworkers showed earlier that Aβ25-35-induced lysis of the whole RBC population increased upon GPx inhibition by mercaptosuccinate, 11 which suggests the dependence of Aβ25-35 erythrotoxicity on the activity of the cellular antioxidant system. In this study, accordingly, we studied sensitivity of RBCs of different ages to Aβ25-35 toxicity using GPx, GT, catalase, and SOD activities as measure. The results are shown in Figure 11.
Figure 11.
The effects of Aβ25-35 on glutathione peroxidase (GPx), glutathione transferase (GT), catalase, and superoxide dismutase (SOD) activities in young and old RBCs. Cells were incubated at 25°C for 30 minutes in 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, and 10 μmol/L Aβ25-35. Controls were incubated with nontoxic Aβ35-25. Then enzyme activity was determined in the hemolysate; n = 4 to 13. Significant differences are indicated: *P < .05, **P < .01, and ***P < .001 as compared with young RBCs; + P < .05 as compared with Aβ-treated young cells (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β; RBC, erythrocyte.
After incubation of young RBCs with 10 μmol/L Aβ25-35, none of the 4 antioxidant enzymes, GPx, GT, catalase, and SOD, changed their activity (Figure 11). In old RBCs under the same conditions, only GT activity increased slightly (by 23%, P = .0152, Student’s t-test).
Amyloid β25-35 at 25 μmol/L induces a significant decrease in GPx activity in both young and old RBCs (Figure 12). Again, old cells are more susceptible to the inhibitory effect of Aβ25-35. The GPx activity is decreased by 31% (P = .0013, Student’s t test) and 15% (P = .0015) in old and young RBCs, respectively.
Figure 12.

The effects of Aβ25-35 on glutathione peroxidase (GPx) activity in young and old erythrocytes (RBCs). Cells were incubated at 25°C for 30 minutes in 10 mmol/L potassium phosphate buffer, pH 7.4, containing 0.9% NaCl, 5 mmol/L KCl, and 10 or 25 μmol/L Aβ25-35. Controls were incubated with nontoxic Aβ35-25, and the enzyme activity was determined in the hemolysate; n = 4. Significant differences are indicated by the P value (one-way analysis of variance [ANOVA] with Bonferroni’s multiple comparison test). Aβ indicates amyloid β.
Discussion
Adult human and rat RBCs do not have the cell nucleus, mitochondria, and ribosomes. Therefore, concepts of protein and enzyme synthesis and gene regulation do not apply to these cells. Erythrocyte enzyme activities as measured under optimal conditions reflect the maximal ability of each enzyme to catalyze the enzymatic reaction.
It is known for a long time that glycolytic and antioxidant enzyme activities in human RBCs change with cell aging in vivo 15,39,40 Marks et al (1958) have claimed 55 years ago: “the aging of erythrocytes and their eventual disintegration may result from a progressive diminution in the activity of certain metabolic systems essential to the maintenance of the integrity of these cells.” 46 They found that G6PDH, 6-phosphogluconic dehydrogenase, and phosphohexose isomerase activities are much lower in old RBCs than in young RBCs and suggested that the diminution in activity of critical enzymes may determine the life span of the RBCs in vivo. Maximal activities of a number of glycolytic enzymes declined at an exponential rate with human RBC aging. The oldest circulating cells retain more than half of the maximal enzyme activity. Hexokinase, aldolase, and PK manifested a faster rate of decline in activity, with a t1/2 significantly shorter than the RBC life span itself. 15
In our study, similar changes were seen in rat RBCs of varying ages. We studied the key glycolytic enzymes, which are responsible for energy supply for the cell functioning (HK, PFK, GAPDH, PK, and LDH), the key antioxidant enzymes responsible for cell protection against oxidative damage (GPx, GT, GR, catalase, SOD, and G6PDH), and Na+/K+-ATPase providing the maintenance of RBC shape and ability for deformation to penetrate capillaries of any small diameter. These results suggest that the aging of RBCs in vivo is accompanied by a decrease in the activities of HK, PFK, PK, G6PDH, GPx, and GT, with no changes in other enzymes. The activity of Na+/K+-ATPase is about 6-fold lower in old RBCs than in young cells. Thus, glycolytic energy metabolism, the ionic balance across the erythrocytic membrane, and the antioxidant defense system are disturbed in RBCs aging in vivo.
Disorders of energy metabolism and enhanced RBC sensitivity to metabolic stress can lead to irreversible damage to the cell and its removal from the circulation. 15 Underlying mechanisms of these processes are not clear. Erythrocytes can be injured by exogenous toxins such as Aβ, which are accumulated in the plasma and vascular endothelium in neurodegenerative diseases. The toxicity of Aβ25-35 to human and rat RBCs was investigated earlier by Mattson and coworkers. 10 -12 However, it is not clear from these results as to whether or not old RBCs are more susceptive to Aβ25-35 toxicity than are the young cells. In the present study, we have found that Aβ25-35-induced hematolysis in all young, middle-age, and old rat RBCs, and sensibility to Aβ25-35 and hemolysis were in proportion to both cell age and Aβ concentration.
There are only a few studies concerning the effects of Aβ25-35 on glycolytic enzymes in the whole RBC population from rats, 11,12 and no such studies on the impacts of Aβ on RBC energy and oxidative metabolism in RBCs of different ages have been carried out to date. Here, we showed for the first time that Aβ25-35 affected glycolytic, antioxidant, and Na+/K+-ATPase enzymes in young, middle-age, and old RBCs and their age-dependant cell survival. After incubation with Aβ25-35, cellular PFK, PK, and GPx activities decreased relatively more in old RBCs than in young cells. The Na+/K+-ATPase activity in young and old RBCs and HK and GPx activities in old RBCs were reduced following the Aβ25-35 exposure. These findings indicate that glycolytic energy metabolism, the ionic balance across the erythrocytic membrane, and the antioxidant defense system are disturbed in RBC aging and may be additionally impaired by Aβ25-35 in vivo.
It is of interest to compare data on enzyme activities in Aβ25-35-treated rat RBCs with those in RBCs from patients with AD. In the whole human RBC population, activities of HK 41,42 and Na+/K+-ATPase are higher, whereas the GPx activity is lower 43 in patients with AD than that in controls. Similar modifications occurred in rat RBCs of varying ages. However, activities of PFK, PK, and LDH in the whole human RBC population from patients with AD were significantly higher than those in control individuals, 44 in contrast to the Aβ25-35 effect on rat RBCs; in order to clarify this inconsistency, further experiments on human RBCs are needed.
As rat RBCs are much more resistant than human RBCs to Aβ25-35 toxicity, 11 our present data suggest that the diminution in activity of certain enzymes may determine the life span of the RBCs in vivo and may be relevant to the mechanisms underlying the onset of human AD. 45
Conclusions
In summary, our data indicate for the first time that the sensitivity of rat RBCs to Aβ25-35 toxicity is directly correlated with cellular age. Further, the glycolytic, antioxidant, and Na+/K+-ATPase enzyme activities are adversely affected by Aβ25-35, especially those from the relatively older cells. This high sensitivity of old RBCs to Aβ25-35 toxicity may contribute to the eventual disintegration of the RBCs in patients with AD.
Footnotes
Authors’ Note: Elena A. Kosenko and Gjumrakch Aliev equally contributed to this work.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Gjumrakch Aliev's work was supported by the GALLY International Biomedical Research Consulting LLC, San Antonio, TX, USA.
References
- 1. Robakis NK. b-Amyloid and amyloid precursor protein, chemistry, molecular biology, and neuropathology, In: Terry RD, Katzman R, Bick KL, eds. Alzheimer Disease. New York, NY: Raven Press; 1994:317–326. [Google Scholar]
- 2. Shoji M, Golde TE, Ghiso J, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258(5079):126–129. [DOI] [PubMed] [Google Scholar]
- 3. Haass C, Schlossmacher MG, Hung AY, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–325. [DOI] [PubMed] [Google Scholar]
- 4. Seubert P, Oltersdorf T, Lee MG, et al. Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature. 1993;361(6409):260–263. [DOI] [PubMed] [Google Scholar]
- 5. Lewczuk P, Esselmann H, Bibl M, et al. Electrophoretic separation of amyloid beta peptides in plasma. Electrophoresis. 2004;25(20):3336–3343. [DOI] [PubMed] [Google Scholar]
- 6. Aliev G. The Role of Oxidative Stress, Mitochondria Failure, and Cellular Hypoperfusion in the Context of Alzheimer Disease: Past, Present and Future. New York: Nova Science Publishers, Inc.; 2013. [Google Scholar]
- 7. Ravi LB, Poosala S, Ahn D, et al. Red cell interactions with amyloid-beta(1-40) fibrils in a murine model. Neurobiol Dis. 2005;19(1-2):28–37. [DOI] [PubMed] [Google Scholar]
- 8. Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure–activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995;64(1):253–265. [DOI] [PubMed] [Google Scholar]
- 9. Kaminsky YG, Marlatt MW, Smith MA, Kosenko EA. Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: evidence for Abeta(25-35). Exp Neurol. 2010;221(1):26–37. [DOI] [PubMed] [Google Scholar]
- 10. Mattson MP, Begley JG, Mark RJ, Furukawa K. Abeta25-35 induces rapid lysis of red blood cells: contrast with Aβ1-42 and examination of underlying mechanisms. Brain Res. 1997;771(1):147–153. [DOI] [PubMed] [Google Scholar]
- 11. Kosenko EA, Solomadin IN, Marov NV, Venediktova NI, Pogosian AS, Kaminskiĭ IuG. Role of glycolysis and antioxidant enzymes in the toxicity of amyloid beta peptide Aβ25-35 to erythrocytes. Bioorg Khim. 2008;34(5):654–660. [DOI] [PubMed] [Google Scholar]
- 12. Solomadin IN, Marov NV, Venediktova NI, Kosenko EA, Kaminskiĭ IuG. Toxic effect of Abeta25-35 and fullerene C60 on erythrocytes. Izv Akad Nauk Ser Biol. 2008;(4):507–512. [PubMed] [Google Scholar]
- 13. Lurie S, Danon D. Life span of erythrocytes in late pregnancy. Obstet Gynecol. 1992;80(1):123–126. [PubMed] [Google Scholar]
- 14. Derelanko MJ. Determination of erythrocyte life span in F-344, Wistar, and Sprague-Dawley rats using a modification of the [3H]diisopropylfluorophosphate ([3H]DFP) method. Fundam Appl Toxicol. 1987;9(2):271–276. [PubMed] [Google Scholar]
- 15. Seaman C, Wyss S, Piomelli S. The decline in energetic metabolism with aging of the erythrocyte and its relationship to cell death. Am J Hematol. 1980;8(1):31–42. [DOI] [PubMed] [Google Scholar]
- 16. Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte life-span in mammals. Comp Biochem Physiol B. 1993;106(3):477–487. [DOI] [PubMed] [Google Scholar]
- 17. Shevtzova EF, Kireeva EG, Bachurin SO. Effect of beta-amyloid peptide fragment 25-35 on nonselective permeability of mitochondria. Bull Exp Biol Med. 2001;132(6):1173–1176. [DOI] [PubMed] [Google Scholar]
- 18. Orellana JA, Shoji KF, Abudara V, et al. Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 2011;31(13):4962–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hüll M, Müksch B, Akundi RS, et al. Amyloid β peptide (25-35) activates protein kinase C leading to cyclooxygenase-2 induction and prostaglandin E2 release in primary midbrain astrocytes. Neurochem Int. 2006;48(8):663–672. [DOI] [PubMed] [Google Scholar]
- 20. Beutler E, Blume KG, Kaplan JC, Löhr GW, Ramot B, Valentine WN. International committee for standardization in haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977;35(2):331–340. [DOI] [PubMed] [Google Scholar]
- 21. Lutz HU, Stammler P, Fasler S, Ingold M, Fehr J. Density separation of human red blood cells on self forming Percoll gradients: correlation with cell age. Biochim Biophys Acta. 1992;1116(1):1–10. [DOI] [PubMed] [Google Scholar]
- 22a. Beutler E. Red Cell Metabolism. Edinburgh: Churchill Livingstone; 1986. [Google Scholar]
- 22b. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23. Bosshard NU, Steinmann B. Enzymes and metabolites of carbohydrate metabolism, In: Blau, N, Duran, M, Gibson, KM, eds. Laboratory Guide to the methods in Biochemical Genetics. Berlin: Springer; 2008. [Google Scholar]
- 24. Kosenko EA, Solomadin IN, Kaminskiĭ IuG. Effect of the β-amyloid peptide Aβ25 35 and fullerene C60 on the activity of enzymes in erythrocytes. Bioorg Khim. 2009;35(2):172–177. [DOI] [PubMed] [Google Scholar]
- 25. Kosenko E, Kaminsky Y, Kaminsky A, et al. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res. 1997;27(6):637–644. [DOI] [PubMed] [Google Scholar]
- 26. Kozer E, Evans S, Barr J, et al. Glutathione, glutathione-dependent enzymes and antioxidant status in erythrocytes from children treated with high-dose paracetamol. Br J Clin Pharmacol. 2003;55(3):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunn HF. Approach to the anemias. In: Goldman L, Schafer AI, eds. Cecil Medicine. 24th ed. Philadelphia, PA: Saunders Elsevier; 2011:Ch.161. [Google Scholar]
- 28. Norton JM. The effect of macrocytosis on rat erythrocyte deformability during recovery from phenylhydrazine-induced anemia. Biorheology. 1990;27(1):21–37. [DOI] [PubMed] [Google Scholar]
- 29. Udden MM. In vitro sub-hemolytic effects of butoxyacetic acid on human and rat erythrocytes. Toxicol Sci. 2002;69(1):258–264. [DOI] [PubMed] [Google Scholar]
- 30. Sutera SP, Gardner RA, Boylan CW, et al. Age-related changes in deformability of human erythrocytes. Blood. 1985;65(2):275–282. [PubMed] [Google Scholar]
- 31. Kazennov AM, Katiukhin LN, Maslova MN, Barvitenko NN, Rustamov FA, Tavrovskaia TV. Changes in erythrocyte properties in early postnatal ontogenesis in rats. Zh Evol Biokhim Fiziol. 2001;37(2):154–156. [PubMed] [Google Scholar]
- 32. Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250(4978):279–282. [DOI] [PubMed] [Google Scholar]
- 33. Mattson MP, Tomaselli KJ, Rydel RE. Calcium-destabilizing and neurodegenerative effects of aggregated β-amyloid peptide are attenuated by basic FGF. Brain Res. 1993;621(1):35–49. [DOI] [PubMed] [Google Scholar]
- 34. Iversen LL, Mortishire-Smith RJ, Pollack SJ, Shearman MS. The toxicity in vitro of beta-amyloid protein. Biochem J. 1995;311(pt 1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kowall NW, McKee AC, Yankner BA, Beal MF. In vivo neurotoxicity of beta-amyloid [Aβ(1-40)] and the Aβ(25-35) fragment. Neurobiol Aging. 1992;13(5):537–542. [DOI] [PubMed] [Google Scholar]
- 36. Games D, Khan KM, Soriano FG, et al. Lack of Alzheimer pathology after beta-amyloid protein injections in rat brain. Neurobiol Aging. 1992;13(5):569–576. [DOI] [PubMed] [Google Scholar]
- 37. Chapman RG, Schaumburg L. Glycolysis and glycolytic enzyme activity of aging red cells in man. Changes in hexokinase, aldolase, glyceraldehyde-3-phosphate dehydrogenase, pyruvate kinase and glutamic-oxalacetic transaminase. Br J Haematol. 1967;13(5):665–678. [DOI] [PubMed] [Google Scholar]
- 38. Bunn HF. Erythocyte destruction and hemoglobin catabolism. Semin Hematol. 1972;9(1):3–17. [PubMed] [Google Scholar]
- 39. Glass GA, Gershon D. Decreased enzymic protection and increased sensitivity to oxidative damage in erythrocytes as a function of cell and donor aging. Biochem J. 1984;218(2):531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fazi A, Accorsi A, Piatti E, Magnani M. Cell age dependent decay of human erythrocytes glutathione S-transferase. Mech Ageing Dev. 1991;58(2-3):255–266. [DOI] [PubMed] [Google Scholar]
- 41. Kaminsky Y, Poghosyan A, Tikhonova L, et al. Glycolytic and proteolytic metabolism in erythrocytes from elderly and demented patients. Am J Neuroprot Neuroregener. 2012;4(1):73–77. [Google Scholar]
- 42. Kaminsky YG, Reddy VP, Ashraf GM, et al. Age-related defects in erythrocyte 2,3-diphosphoglycerate metabolism in dementia. Aging Dis. 2013;4(5):244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kosenko EA, Aliev G, Tikhonova LA, Li Y, Poghosyan AC, Kaminsky YG. Antioxidant status and energy state of erythrocytes in Alzheimer dementia: probing for markers. CNS Neurol Disord Drug Tsargets. 2012;11(7):926–932. [DOI] [PubMed] [Google Scholar]
- 44. Kaminsky Y, Suslikov A, Kosenko E. Specific and pronounced impacts of lisinopril and lisinopril plus simvastatin on erythrocyte antioxidant enzymes. J Clin Pharmacol. 2010;50(2):180–187. [DOI] [PubMed] [Google Scholar]
- 45. Kosenko EA, Solomadin IN, Tikhonova LA, Reddy VP, Aliev G, Kaminsky YG. Pathogenesis of Alzheimer disease: role of oxidative stress, Amyloid beta peptides, systemic ammonia and erythrocyte energy metabolism. CNS Neurol Disord Drug Targets. 2014;13(1):112–119. [DOI] [PubMed] [Google Scholar]
- 46. Marks PA, Johnson AB, Hirschberg E. Effect of age on the enzyme activity in erythrocytes. Proc. Natl. Acad. Sci. 1958;44:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]