Abstract
The d-mannuronic acid (M2000) is a novel nonsteroidal anti-inflammatory drug that has immunosuppressive effects together with antioxidant property. M2000 has shown a notable efficacy in experimental models of multiple sclerosis, rheumatoid arthritis, and nephrotic syndrome. In this work, the effect of M2000 on the treatment of Alzheimer’s disease (AD) was performed by Morris water maze experiment, and the immunological assessments were carried out by Western blot, apoptosis (procaspase-3, Bax/Bcl2, P53), enzymatic (superoxide dismutase [SOD]), and nonenzymatic oxidative stress (malondialdehyde [MDA]) tests. We found that pretreatment of AD in the rat model by M2000 had a potent efficacy on rat behavior and also it led to a significant inhibition of amyloid plaque production. Moreover, our data showed that M2000 can reduce the amount of Bax/Bcl2, P53, MDA, and SOD, as well as it normalized the level of procaspase-3. Our results suggest M2000 is a potential therapeutic agent for the treatment of AD.
Keywords: M2000, d-mannuronic acid, antioxidant, Alzheimer’s disease, MWM model
Introduction
Alzheimer’s disease (AD) not only is the most predominant cause of progressive memory impairment, cognitive deficits, and functional alterations, but it can also lead to some neuropsychiatric disorders such as agitation, aggression, hallucinations, and delusions among elderly population. 1 Globally, in 2006, it has been estimated that about 26.6 million of aged people had AD; this population will become quadruple by the end of 2050. 2 Current treatments and drugs have a small but relative effect on AD, whereas the astonished properties of alginate on neurons survivability make many researchers eager to determine alginate and its component (d-mannuronic acid [M2000]) effects on AD treatments. 3 In our previous studies, the anti-inflammatory and immunosuppressive efficacy of M2000 has been proved in animal models of multiple sclerosis, nephrotic syndrome, rheumatoid arthritis, and immune complex glomerulonephritis. 4 -6 The characterized molecular mechanism of this novel drug is based on its inhibitory effects on matrix metalloproteinase 2 activity, inhibition of immune cells infiltration, reduction of the interleukin 6 (IL-6), and antibody production. 6 In the present study, for the first time, we evaluate the effect of M2000 on AD.
In developed countries, AD is one of the most costly diseases that have 3 different types with a variety of symptoms. 7 Early onset is one form of AD that presents among people younger than age 65; older than age 65 is known as late onset. The late onset is the most common form, and different risk factors are involved in its initiation and progressions. However, a number of genetic risk factors have been identified; only a few AD cases can be clarified by particular gene mutation. Amyloid precursor proteins (APPs), presenilin 1 and 2, are the most important antigens that involved in the AD. Moreover, specific environmental risk factors could contribute to its progression. Finally, the last type of AD is familial, which is linked to certain genes in at least 2 generations of family members. 7,8
Dementia as one of the most important symptom of AD is associated with neurodegeneration, which is started by synaptic injury and then followed by neuronal loss especially in the neocortex and limbic system. Significantly, neurodegeneration is accompanied by astrogliosis, microglial cell proliferation, and the presence of neurofibrillary tangles (NFT), which is composed of dystrophic neurites and hyperphosphorylated tau. 9 -11 Proteolysis of amyloid β (Aβ) precursor protein causes Aβ accumulation, so that if the abnormal accumulations happen, an imbalance level of Aβ production, aggregation, and clearance will appear. The clearance can occur by neprilysin enzyme, chaperone molecules such as apoE, lysosomal, and nonlysosomal pathways. 12,13 It has been suggested that the failure of the clearance mechanisms may have the key role in the plaque and NFT formation, which is responsible for the synaptotoxic effects. 14,15 The plaque and NFT are mostly localized in the hippocampus and cortex regions, which are responsible for learning and memory function. 16
Aβ1-40 and Aβ1-42 are the most forms of Aβ in brain, while in vivo and in vitro studies have reported the higher neurotoxicity of Aβ1-42 with respect to its methionine residue in 35 position. 17,18 The Aβ1-42 induces free radical oxidative stress in the neurodegeneration and neurotoxicity process. This induction could take place by invasive production of reactive oxygen species (ROS), reactive nitrogen species, a loss of function of many antioxidant defense enzymes, cytokines, and chemokine such as IL-4 and IL-10. 19 -21 Age is the main risk factor for AD since oxidative stress increases with aging and its role has been approved in the pathogenesis of AD. 20
The brain is too sensitive to oxidative stress because it has a high level of polyunsaturated fatty acid with high oxygen consumption. Therefore, detoxification of free radical in the brain is so important because lipid and protein oxidation can destruct structure and function of the brain. Endogenous antioxidants such as reduced glutathione, antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase are utilized in the brain. 22 The D-mannuronic acid is known as a uronic acid and since the antioxidant activity of uronic acid and its analog like glucuronic acid and ascorbic acid 23 – 25 has been proven, in this research one of the objectives was to investigate the antioxidant activity of D-mannuronic acid on AD.
The other detriment of Aβ oligomer is increased following the activation of apoptosis in neurons by intrinsic and extrinsic pathways. The intrinsic pathway is mediated by the mitochondrial release of cytochrome c and resultant activation of caspase-9, and the extrinsic pathway originating from the activation of cell surface death receptors such as Fas, resulting in the activation of caspase-8 or -10. Finally, completion of both pathways can be performed by activation of caspase-3. 14,26
Measuring the proapoptotic proteins (Bax, Bad) and antiapoptotic protein (Bcl2) can illustrate the activating and inactivating stages of the intrinsic pathway. The Aβ can induce proapoptotic protein such as Bax and downregulate antiapoptotic protein such as Bcl2. The increased level of Bax is related to senile plaque and NFT in the hippocampus of patients with AD. However, caspase-3 has shown increasing proteolytic cleavage of APP, which leads to form Aβ peptide. Many studies have shown an increased transcription level of caspase-1 to -9, which can increase the expression of Bax and decrease the expression of Bcl2. 26 Therefore, evaluating the effect of antioxidant and antiapoptotic agents on the reduction of oxidative stress and apoptosis in nervous system attracts many researchers to decrease the pathological mechanisms and improve memory and learning.
Materials and Methods
Animals, Housing, and Test Material
Forty Wistar male rats (weight: 180-220 g) were used in this experiment, which was purchased from the Faculty of Pharmacy, Tehran University of Medical Science. Each rat was kept in 1 cage, and cages were kept under standard laboratory conditions (temperature: 23°C ± 2°C; relative humidity: 30%-70%; light–dark cycle: 12/12 hours). All of the rats had enough accession to the ad libitum food and water unlimitedly. All the experiments were approved by Ethical Committee for the Care and Use of Laboratory Animals at Tehran University of Medical Science (code: 9021324001) and Council of Graduate Students of Iran University of Medical Science (code: 24403).
Rats were divided into 4 groups—normal control group (C), sham operation group (S) in which phosphate-buffered saline (PBS) was injected to the intrahippocampus, Alzheimer’s group (Aβ) in which 50 ng/µL per side of Aβ was injected to the intrahippocampus by Hamilton syringe, and M2000 group (Aβ + M) that in addition to Aβ injection, they received M2000 daily via water utility. The M2000 was given to the rats for 6 weeks (from 2 weeks before Aβ injection to 4 weeks after Aβ injection).
β Amyloid1-42 Preparation
The Aβ1-42 was purchased from Sigma-Aldrich (St. Louis, Missouri). It was dissolved in PBS. The solution was then incubated for 5 days at 37°C and finally was diluted into the 50 ng/µL by PBS on the test day.
Stereotaxic Surgery
Rats were anesthetized with the intraperitoneal injection of ketamine (90 mg/kg) and xylazine (5 mg/kg) and then they were put into the stereotaxic apparatus (Stoelting, Wood Dale, Illinois). The stereotaxic surgery for intrahippocampal injection of Aβ was taken from the atlas of Paxinos and Watson (anterior–posterior, 3/08 mm; lateral, ± 2.2 mm; dorsal–ventral 2.2 mm from bregma). The CA1 area of the hippocampus was detected and then injection was performed by a Hamilton syringe (50 ng/µL per side). Every injection lasted 1 minute, and to facilitate diffusion of Aβ, the injection in the left side was performed after 60 seconds. Finally, head of rats was darned.
d-Mannuronic Acid Preparation and Intake
The d-mannuronic acid (M2000) as a small molecule (C6H10O7) with the molecular weight of 194.139 Da was prepared from sodium alginate (Sigma-Aldrich) based on the method of Fattahi et al. 27 Briefly, alginic acid sodium salt (100 g) was dissolved gently in 20% H2SO4 at 0°C, and the mixture was completely stirred at room temperature. This solution was heated at 80°C until its color was changed from cream color to light brown. The hydrolysate solution was cooled to room temperature and was precipitated by centrifugation (3700g). Subsequently, the precipitate was redissolved by neutralization using 1 M Na2CO3. The solution pH was adjusted at 2.85 by 0.1 M HCl. The precipitate was collected and washed once with distilled water. The final precipitate (l-guluronic acid) was spread over and dried out in petri dishes. Accordingly, the supernatant of l-guluronic acid was collected and again adjusted to pH 1.0 by 0.1 M HCl. The precipitate was collected and washed once with distilled water. The final precipitate (d-mannuronic acid) was spread over and dried out in Petri dishes. The monomeric form of d-mannuronic acid and its purity were validated by characterizing the hydrolytic products using Fourier transform infrared spectroscopy and carbon-13 nuclear magnetic resonance spectroscopy, based on the above-described method. 27 Finally, 50 g of M2000 was dissolved in the 350 mL water, then 35 mL of this solution was diluted in daily drinking water of each rat for 6 weeks, based on the dosage of 25 mg/kg/d.
Behavioral Test
The behavioral test was performed with Morris water maze (MWM) apparatus. This test performed a month after surgery. This apparatus is a black circular pool with 136 cm diameter and 35 cm depth, filled with water, and its temperature should maintain at 25°C ± 2°C. The pool was divided into 4 quadrants, and an invisible plate made of Plexiglas was submersed 1 cm under the water surface in one of the quadrants (target quadrant). This test includes training process for 4 days and probe test process for 1 day. Each training day includes 1 block of 4 trials. In each trial, a rat was randomly dropped in the starting point of a pool quadrant. The camera was mounted above the pool that connected to a computer, which included EthoVision software (Noldus Information Technology, Wageningen, the Netherlands), and recorded the rat swimming pathway. In each trial, the rat had 90 seconds time for a free swim, and if they cannot find the plate during this period, they were manually trained by the researcher to find the target plate. After this period, the rat was allowed to rest for 20 seconds on the plate and again was released from the starting point of another quadrant. In other trials and rest days, these processes were repeated. After that the software calculated 3 parameters including escape latency (the time required to find the platform), distance travelled (the path length travelled to reach the platform), and velocity (swimming speed) for 4 days. Finally, on the 5th day (test day), rats were tested for probe trial. In this day, the plate was removed from pool and rat was randomly released into the pool of one of the free quadrants and was allowed to swim for 90 seconds. The purpose of this test was to find out how long rat can swim in the target quadrant. It should be noted that all trials were done at the same time (8 am-12 pm). In this study, the platform was covered with aluminum foil and was submersed 1 cm above the water surface in opposite to the target quadrant, and also rat’s visual ability was confirmed by visibility test. 28
Western Blot Analysis
Rats were decapitated and their brain was quickly removed, and hippocampus was isolated on the ice and immediately frozen in liquid nitrogen and then stored at −80°C. In the test day, the lysis buffer (including Tris–HCl; pH 7.4, NaCl, EDTA; 0.5 M, sodium dodecyl sulfate [SDS], Triton 100X, NaF, NaVO4, NP40%1, and protease inhibitor cocktail) was added to each hippocampus, then it was homogenized. Microtubes including homogenized tissue were centrifuged at 13 000 rpm for 20 minutes at 4°C. Finally, the supernatant was separated for further analysis.
Bovine serum albumin was used for calibration process as a standard reference, and protein concentration was measured by Bradford method. 29 Protein assay for Bradford method was purchased from BioRad Biotechnology (Hercules, California). The same amount of protein (concentration 60 mg/mL) was run on the 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel. Then, the resolved protein was transferred to nitrocellulose membrane (GE Healthcare, Sweden), after that the membrane was incubated in blocking buffer for 1 hour and was probed overnight with proper antibodies at 4°C. The Bax, Bcl2, and P53 are the specific antibodies that were used in the Western blotting of this experiment. The block was washed 3 times, and then it was incubated with secondary antibody for 75 minutes. All the antibodies of this experiment were purchased from Santa Crus (Dallas, Texas). Finally, electrochemiluminescence reagents (GE Healthcare) and radiography film were used for the detection of immunoreactive polypeptides. Band densities were measured by densitometry scan of films with ImageJ software (NIH, Bethesda, Maryland, USA). β-Actin was used as a positive control for Western blotting, which was supplied from Cell Signaling Technology (Beverly, Massachusetts).
Amyloid Plaque Detection
One rat was chosen randomly for brain homogenization. The brain was isolated and put into 15% formalin for 48 hours. The paraffin sections were cut at 6 μm and then were stained by Congo red solution (Merck, Darmstadt, Germany). Finally, plaques were observed by the optical microscope with the magnification of ×400. 30
Measuring SOD, CAT, and Malondialdehyde in Hippocampus Tissue
One ml diluted protease inhibitor was added to the hippocampus tissue and after that 60 ml PBS was added. Then samples were homogenized for 1 minute and centrifuged at 4000 rpm for 10 minutes. Finally, the supernatant was taken for the determination of SOD and CAT activity and malondialdehyde (MDA) level by kit instruction (Zellbio GmbH, Ulm, Germany). Bovine serum albumin was used for calibration process as a standard reference, and protein concentration was measured by Bradford method, which was purchased from BioRad Biotechnology.
Statistical Analysis
Data were analyzed by IBM SPSS Statistics version 23 (IBM Corp, Armonk, New York), and all results were shown as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed for variance, and behavioral variables between groups were compared using Bonferroni post hoc test. Also, 2-way ANOVA followed by Bonferroni post hoc was used to compare the results of behavioral tests for different days. Statistical significance was achieved when the P value was less than .05.
Results
Behavioral Results
Behavioral results of all training days
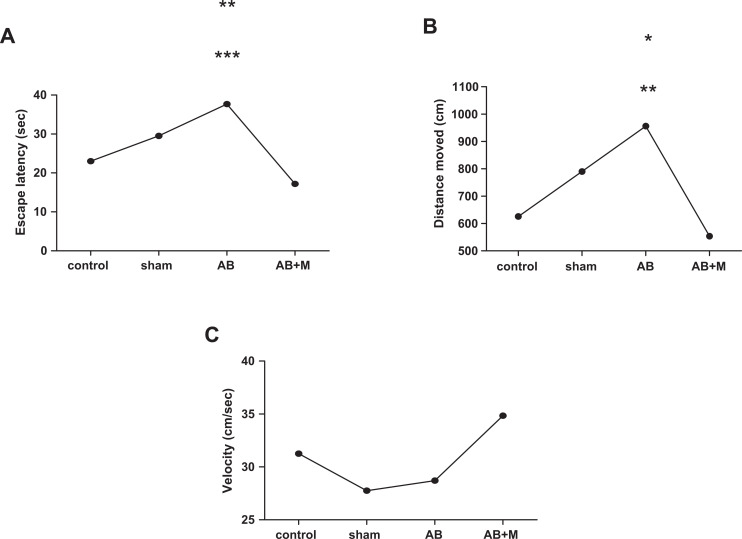
In this study, there was no significant difference between the control and sham operation groups according to all variables. So, all analyses of groups have been compared to the control group. Figure 1 shows the results of behavioral test including escape latency, distance traveled, and velocity of different rat groups. As can be seen, the Aβ group had a significant increase in escape latency (P < .001; Figure 1A) and also distance traveled (P = .016; Figure 1B) in comparison with the control group. In contrast, the Aβ + M group had a significant decrease in the escape latency (P < .0001) and distance traveled (P = .004) compared to the Aβ group, which means that M2000 intake could effectively improve these parameters. Also, M2000 intake plus Aβ injection did not show significant difference compared to the control group. Moreover, there was no significant difference between groups according to swimming speed or velocity (Figure 1C).
Figure 1.
Effect of intrahippocampal (IH) of amyloid β (Aβ), Aβ plus d-mannuronic acid (M2000) intake on (A) escape latency, (B) distance traveled, and (C) swimming speed in Morris water maze (MWM). All results have shown as mean ± standard deviation (SD). **P < .001 versus control group and ***P < .0001 versus Aβ + M (A), *P = .016 versus control group, and **P = .004 versus Aβ + M (B). N = 10 rats for each group.
Behavioral results in each training day
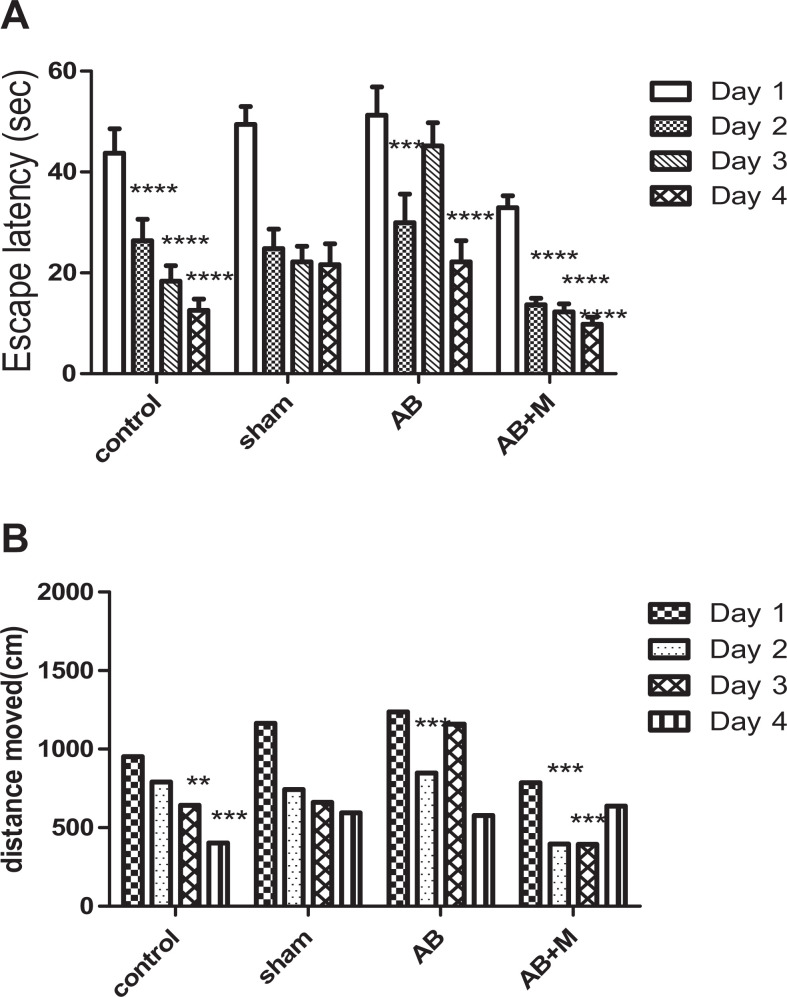
Figure 2 illustrates behavioral test results for different days in all groups. In control group, escape latency (Figure 2A) had a significant improvement from the first day (P < .0001) to the second day, and then there were no significant differences in other days (third and fourth) for the time of escape latency with respect to the fact of the learning process. There was no significant difference between distance moved (Figure 2B) on the second and third day compared to the first day. However, there was a significant reduction on the third day (P < .01) and fourth day (P < .001) compared to the first day.
Figure 2.
Effect of intrahippocampal (IH) of amyloid-β (Aβ), Aβ plus d-mannuronic acid (M2000) intake on (A) escape latency and (B) distance traveled in Morris water maze (MWM) for all training days. All results have shown as mean ± standard deviation (SD). *Significant difference between the first and other training days, **P < .01, ***P < .001, ****P < .0001. N = 10 rats in each group.
In the Aβ group, escape latency (Figure 2A) decreased on the second day (P < .001) and fourth day (P < .0001) compared to the first day, while a significant increase can be seen on the third day. Furthermore, there was a statistical difference between the second and third day (P < .01) and third compared to the fourth day (P < .001). Unlike other groups, the distance moved (Figure 2B) significantly increases on the third day, and as a consequence, there was a significant difference only between the first and fourth day (P < .001) and the third day compared to the fourth day (P < .01).
As can be seen in the Aβ + M group (Figure 2B), the distance moved had an improvement from the begging to the final day. There was a significant difference on the second day and the third day (P < .001) compared to the first day. Also, a significant improvement in escape latency (Figure 2A) on the second, third, and fourth day (P < .0001) in comparison with the first day was detected. Swimming speed (velocity) in all groups between and during different days did not show a significant difference (velocity results have not been shown).
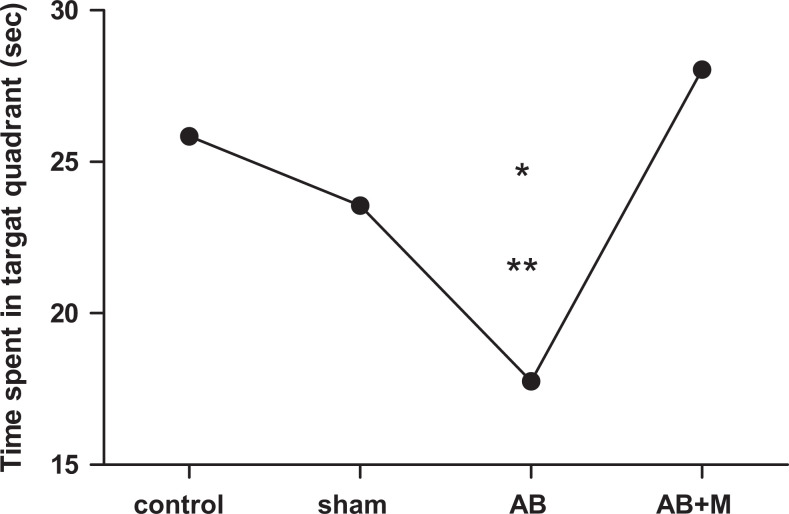
Probe trial test result
Figure 3 reveals the probe test results that its purpose is to find out how long rat can swim in the target guardant after removing platform during the 90 seconds. Figure 3 shows that Aβ injection caused a significant decrease in this parameter (P < .001) compared to the control group. However, in the group that received M2000, time of swimming in target quadrant increased compared to the Aβ group (P < .0001) and did not show a significant difference compared to the control group.
Figure 3.
Probe trial test was performed after removing the platform for all groups. All results have shown as mean ± standard deviation (SD).*P < .001 versus control group and **P < .0001 versus amyloid-β + M2000 group (Aβ + M). N = 10 rats for each group.
Congo red staining results
Amyloid plaques are the most important sign in AD. Therefore, to confirm amyloid plaques formation, hippocampus section was stained by Congo red solution (Figure 4). As can be seen in Figure 4, in Aβ group, amyloid plaque formed and the cell shape had been changed abnormally. Although amyloid plaque was detected in the Aβ + M2000 group, the cell statue was improved and the amyloid plaque size significantly decreased compared to the Aβ group.
Figure 4.
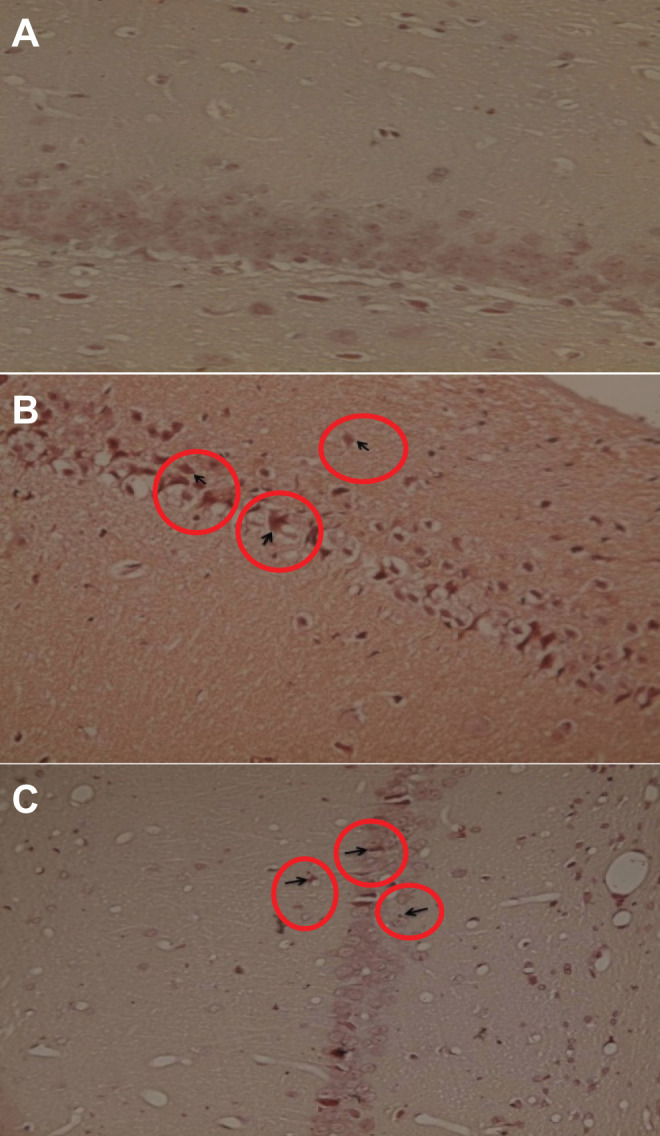
Effect of intrahippocampal (IH) of amyloid β (Aβ), Aβ plus d-mannuronic acid (M2000) intake on the amyloid plaque. A, Hippocampus in the control group that there was no plaque. B, Hippocampus in Aβ group that plaques have been shown as the row. C, Hippocampus Aβ plus M2000 intake group that plaques have decreased and are smaller compared to the Aβ group. N = 3 rats in each group.
Effects of Aβ and M2000 on Bax/Bcl2 Ratio
The Bax/Bcl2 ratio analysis is shown in Figure 5. Bax is one of the proteins that have an important role in developing apoptosis and antiapoptotic protein such as Bcl2, which has a vital role in controlling apoptosis pathway. In this experiment, Aβ injection significantly increased Bax expression (P < .0001) and decreased Bcl2 expression (P < .01).Therefore, the Bax/Bcl2 ratio significantly increased compared to the control group (P < .0001). In group that received M2000, this ratio decreased (P < .0001) in comparison with the Aβ group and did not show a significant difference compared to the control group.
Figure 5.
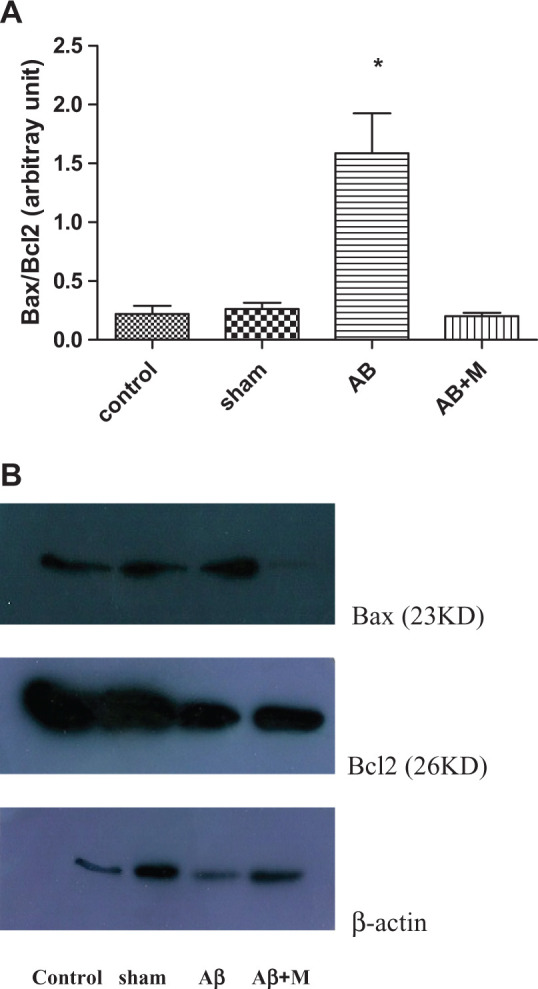
Western blot analysis for evaluating the effect of intrahippocampal injection of amyloid-β (Aβ) and Aβ plus d-mannuronic acid (M2000) intake on Bax, Bcl2, and Bax/Bcl2 ratio as apoptosis markers. The results have shown as mean ± standard deviation (SD). A, Bax/Bcl2 ratio for all groups. B, The density of Bax and Bcl2 bands for all groups. *P < .0001 compared to the control and Aβ + M groups. N = 10 rats in each group.
Effects of Aβ and M2000 on Caspase-3
Caspase-3 plays an important role in final processes of apoptosis. Therefore, to confirm apoptosis process in AD, we measured caspase-3 expression by Western blotting. In fact, procaspase-3 (32 kD) activated and was transformed to the cleaved caspase-3 (17 kD). Figure 6 shows procaspase-3 density in all groups, and unfortunately, the active, cleaved form did not see that possibility due to the fact that its expression is weak. Although the expression level of procaspase-3 in the Aβ group had a significant decrease compared to the control group, procaspase-3 expression increased in the group that takes M2000 (Figure 6; P < .01). This might be due to the activation of procaspase-3 and consequently transformed to its cleaved form.
Figure 6.
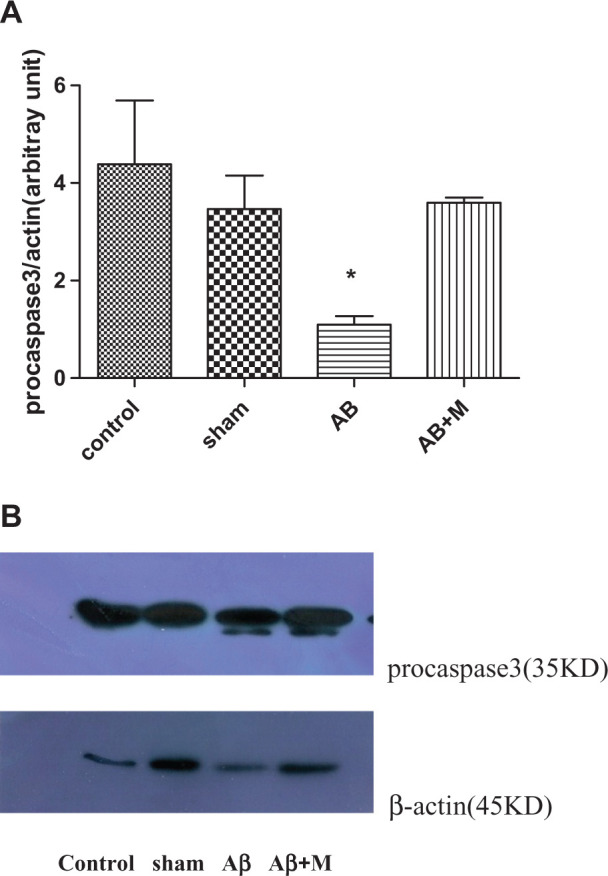
Western blot analysis for evaluating the effect of intrahippocampal injection of amyloid β (Aβ) and Aβ plus d-mannuronic acid (M2000) intake on procaspase-3 as apoptosis marker. The results have shown mean ± standard deviation (SD). A, Procaspase-3 level for all groups and (B) the density of procaspase-3 bands for all groups.*P < .01 compared to the control and Aβ + M groups. N = 10 rats in each group.
Effects of Aβ and M2000 on P53
P53 is one of the proteins that play a key role in apoptosis pathway. Figure 7 highlights P53 expression level between groups. The P53 level was significantly increased (P < .001) in group that had Aβ injection compared to the control group. In contrast, P53 level decreased in the group received M2000 and did not show a significant difference compared to the control group (P < .001).
Figure 7.
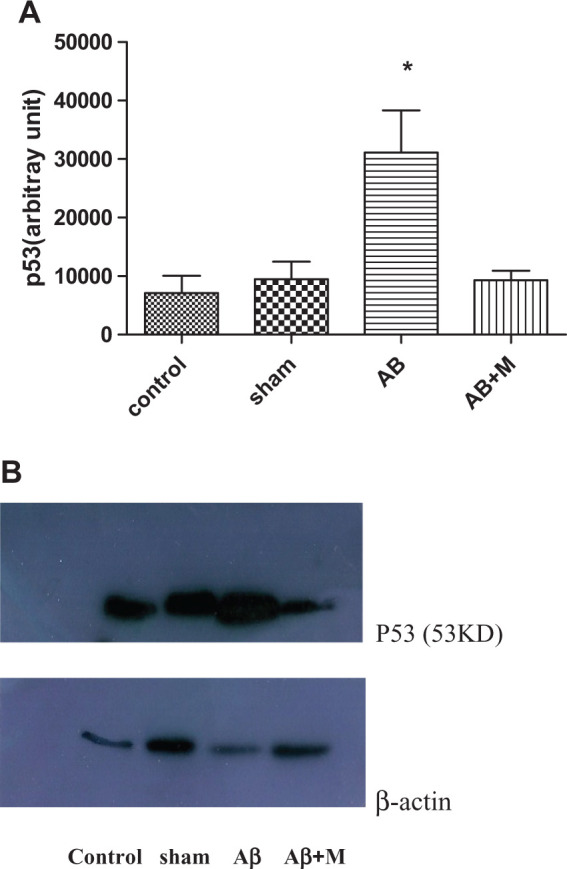
Western blot analysis for evaluating the effect of intrahippocampal injection of amyloid β (Aβ) and Aβ plus d-mannuronic acid (M2000) intake on P53 as apoptosis marker. The results have shown mean ± standard deviation (SD). (A) p53 level for all groups and (B) the density of p53 bands for all groups.*P < .01 compared to the control and Aβ + M groups. N = 10 rats in each group.
Stress Oxidative Test Results
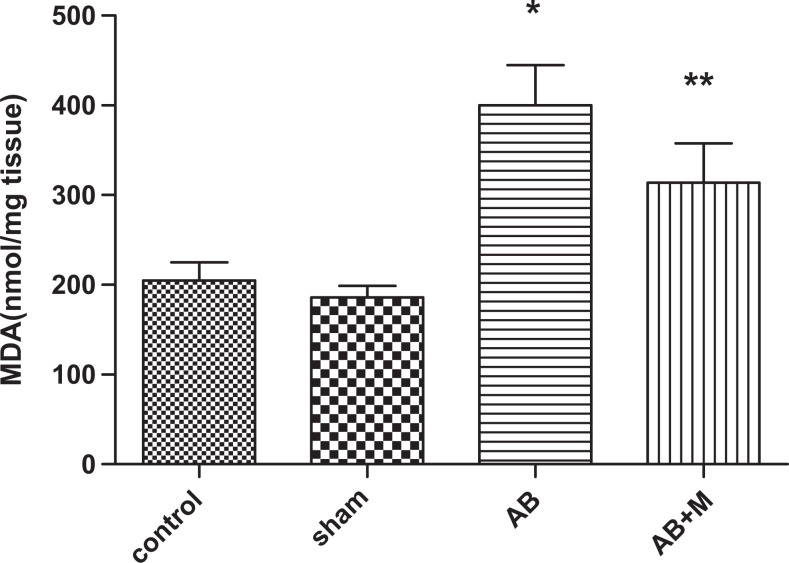
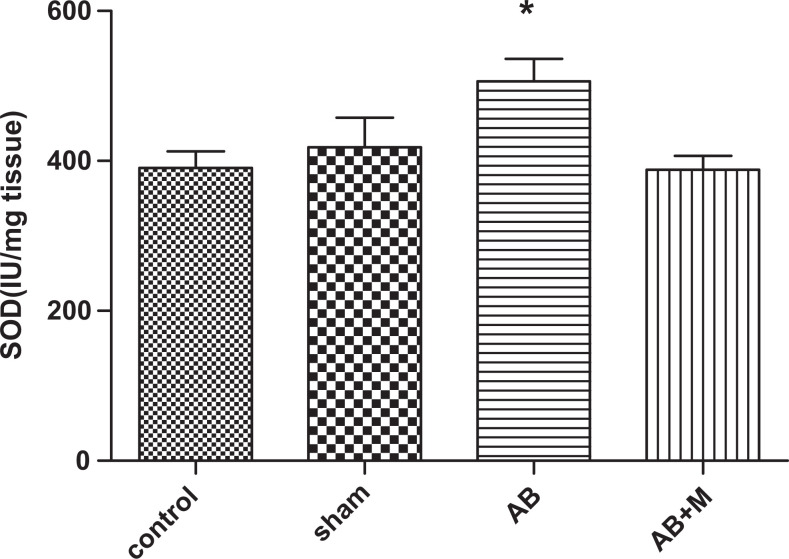
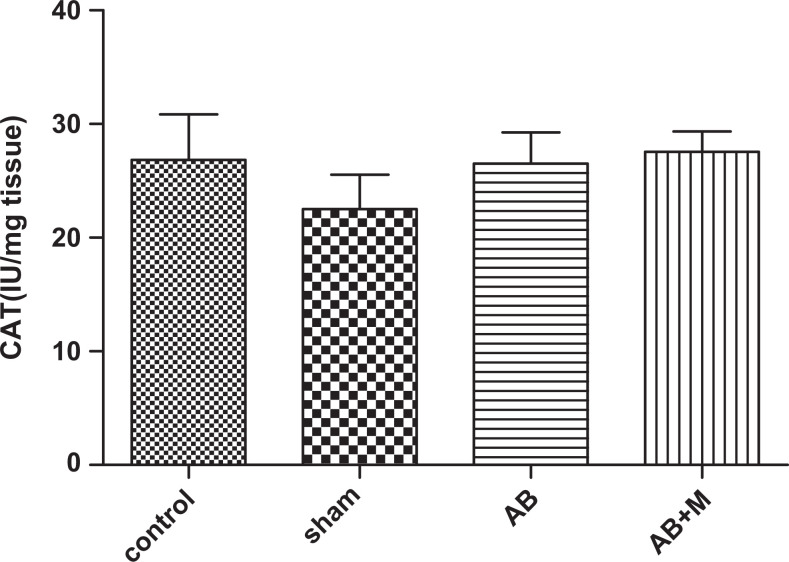
Stress oxidative plays an important role in AD; therefore, we measured some of the markers in oxidative stress pathway including SOD, CAT enzymes as an antioxidant agent, and MDA as an oxidant marker. Figure 8 shows MDA level in hippocampus tissue of all groups. As it has been showed (Figure 8), Aβ injection significantly increased MDA level (P < .0001) compared to the control group. In group received M2000, the MDA level decreased, but it was not significant (P = .05), and also MDA level was significantly higher than the control group (P < .01). Figure 9 highlights SOD activity in the hippocampus tissue. The Aβ injection caused a significant increase (P < .01) compared to the control group, and in the group received M2000, SOD activity significantly decreased (P < .01) and did not show a statistical difference compared to the control group. The level of CAT activity in the hippocampus tissue can be seen in Figure 10, and as it was reported, there was no significant difference in CAT activity of all groups.
Figure 8.
The effect of intrahippocampal injection of amyloid β (Aβ) and Aβ plus d-mannuronic acid (M2000) intake on malondialdehyde (MDA) level as peroxidation lipid in oxidative stress in the hippocampus. The results are shown as mean ± standard deviation (SD). *P < .0001 for Aβ compared to the control and **P < .01 for Aβ + M compared to the control. N = 10 rats in each group.
Figure 9.
The effect of intrahippocampal injection of amyloid β (Aβ) and Aβ plus d-mannuronic acid (M2000) intake on superoxide dismutase (SOD) enzyme activity in the hippocampus. The results have shown mean ± standard error of the mean (SEM). *P < .01 compared to the control and Aβ + M groups. N = 10 rats in each group.
Figure 10.
The effect of intra hippocampal injection of Aβ and Aβ plus M2000 intake on catalase enzyme activity in the hippocampus. The results have shown Mean±SEM. There was no significant difference between groups (p > 0.05). N = 10 rat in each group.
Discussion
Several studies have reported that natural products could have positive effects on treating or slowing the progression of the neurodegenerative disease by attenuating microglial activation and enhancing microglial clearance of Aβ. 30 -33 Alginate as a natural polysaccharide has a remarkable neuroprotective and antineuroinflammatory activity. 33 Previous studies have reported that the alginate oligosaccharides (1300 Da) prepared by enzymatic depolymerization could easily cross the blood–brain barrier and can be applied its efficiency on brain nerve cells. 34,35 In this study, the alginate was depolymerized by chemical reactions, and the d-Mannuronic acid (M2000) was purified to test how it could have an effect on AD. In our previous experiment, a significant effect on the proteinuria, BUN, serum creatinine, and cholesterol level, as well as glomerular lesion was seen. 36 Moreover, it has been indicated that M2000 is relatively safe when administered orally in animals. 27 Therefore, several experiments were done to show M2000 effects on AD, and the results showed that this natural product could have an important key role in AD treatment.
The MWM experiment gives an accurate outcome for spatial learning, memory, and working and can also detect the damage level of cortical regions in the brain, which are the main key factors in AD. 37 Learning manner could be analyzed by escape latency, which is the time it takes to find the platform. 38 In this experiment, escape latency had a significant reduction (Figure 1A) in the Aβ + M group, which means that the intake of M2000 might develop learning procedure in AD. The other variable measures are travel distance and probe trial (Figures 1B and 3) 39 in which both had a significant decrease in the Aβ + M group compared to the Aβ group. These results of improvement in behavioral procedure lead to this conclusion that M2000 could have an effective influence on the AD treatment. The other potent evidence for the efficacy of M2000 on Alzheimer’s treatment was obtained by observing amyloid plaque size (Figure 4). Stained hippocampus section by Congo red showed that the Aβ + M group had a remarkable decrease in plaque size compared to the Aβ group.
Mitochondria-mediated apoptosis can be operated by intrinsic and extrinsic pathways in the brain neuron cells. Mostly, impaired mitochondrial function is associated with the aging process and prevalent age-related diseases including AD. 40,41 Bax is a proapoptotic protein that is accumulated when the cells are engaged with the cellular stress by DNA damage. Meantime, antiapoptotic proteins such as Bcl-2 and Bcl-xL prevent apoptosis by inhibiting the action of proapoptotic proteins. 42 Therefore, the Bax/Bcl2 ratio is an important factor because higher ratio causes a destruction of balance, which could release cytochrome c from mitochondrial membrane to the cytosol and then activate procaspases to induce apoptosis. 43 In this experiment, the expression of procaspase-3 had been detected in all groups. The control group had the highest expression level followed by the Aβ + M group, whereas the Aβ group had the lowest level of procaspase-3 gene expression (Figure 5). From this finding it can be concluded that in the Aβ group, the procaspase has been consumed in the apoptosis process pathway because its level had been dropped, whereas in the Aβ + M group, procaspase had increased up to the control group level. Unfortunately, in our study, we did not observe the active form of caspase-3, and only the procaspase-3 was detected that its expression was different between the groups. This finding could be probably due to its low level of expression of the active form of caspase-3. 44 However, the expression of procaspase-3 was detected at low level in the Alzheimer’s group; it seems that procaspase-3 enters to activate the process.
In this study, the level of P53 was screened because of its role in the cell apoptosis, especially those who exposed to the noxious stresses. 45 It has been shown in Figure 7 that the level of p53 in the Aβ + M group had been dropped near to the level of the control group, whereas there was a significant increase in the Aβ group, which was considered as Alzheimer’s model. Therefore, the usage of M2000 could have a prevention effect on the P53 expression, which can reduce the potency of apoptosis pathway.
The recent data revealed that Aβ-induced free radical and oxidative stress has an important role in the pathogenesis of AD. The ROS cause the lipid and protein oxidation with increasing the level of MDA as a product of lipid peroxidation. In many studies, the level of MDA has been increased in the hippocampus tissue, plasma, and cerebrospinal fluid in patients with AD. 18 On the other hand, harmful effects of ROS can be neutralized by antioxidant systems such as SOD, CAT, and glutathione. The SOD and CAT are the first-line items against toxicity of ROS. Various studies have shown the increased or decreased levels of these enzymes in hippocampus and plasma of patients with AD. 46 As can be seen in Figures 8 and 9, the levels of both SOD and MDA have increased in the Aβ group, indicating the defense mechanism against the oxidative stress in hippocampus tissue. However, the group treated by M2000 showed a considerable reduction in the level of SOD and MDA, which means that the M2000 has a successful impact on the reduction of oxidative stress and free radical side effects.
At present, we are at the end stage of 2 clinical trials (phase 1/2) for this drug in rheumatoid arthritis (IRCT2013062213739N1) and ankylosing spondylitis (IRCT2013062213739N1). Regarding the positive effects of M2000 in these current clinical trial studies in humans, our next aim is to run the other clinical trial study for AD in Iranian patients.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Iran University of Medical Sciences (IUMS; grant number: 24403).
Supplemental Material: The online supplemental table is available at http://journals.sagepub.com/doi/suppl/10.1177/1533317516678086.
References
- 1. Wragg RE, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry. 1989;146(5):577–587. [DOI] [PubMed] [Google Scholar]
- 2. Hampel H, Shen Y, Walsh DM, et al. Biological markers of amyloid β-related mechanisms in Alzheimer’s disease. Exp Neurol. 2010;223(2):334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eftekharzadeh B, Khodagholi F, Abdi A, Maghsoudi N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr Polym. 2010;79(4):1063–1072. [Google Scholar]
- 4. Mirshafiey A, Cuzzocrea S, Rehm B, Mazzon E, Saadat F, Sotoude M. Treatment of experimental arthritis with M2000, a novel designed non-steroidal anti-inflammatory drug. Scand J Immunol. 2005;61(5):435–441. [DOI] [PubMed] [Google Scholar]
- 5. Mirshafiey A, Matsuo H, Nakane S, Rehm BH, Koh CS, Miyoshi S. Novel immunosuppressive therapy by M2000 in experimental multiple sclerosis. Immunopharm Immunot. 2005;27(2):255–265. [DOI] [PubMed] [Google Scholar]
- 6. Mirshafiey A, Rehm B, Sotoude M, Razavi A, Abhari RS, Borzooy Z. Therapeutic approach by a novel designed anti-inflammatory drug, M2000, in experimental immune complex glomerulonephritis. Immunopharm Immunot. 2007;29(1):49–61. [DOI] [PubMed] [Google Scholar]
- 7. Hendrie HC. Epidemiology of dementia and Alzheimer’s disease. Am J Geriatr Psychiatry. 1998;6(2):S3–S18. [DOI] [PubMed] [Google Scholar]
- 8. Bird TD. Genetic factors in Alzheimer’s disease. N Engl J Med. 2005;352(9):862–864. [DOI] [PubMed] [Google Scholar]
- 9. Trojanowski JQ, Lee VM. “Fatal attractions” of proteins: a comprehensive hypothetical mechanism underlying Alzheimer’s disease and other neurodegenerative disorders. Ann N Y Acad Sci. 2000;924(1):62–67. [DOI] [PubMed] [Google Scholar]
- 10. Iqbal K, Grundke-Iqbal I. Neurofibrillary pathology leads to synaptic loss and not the other way around in Alzheimer disease. J Alzheimers Dis. 2002;4(3):235–238. [DOI] [PubMed] [Google Scholar]
- 11. Crews L, Rockenstein E, Masliah E. APP transgenic modeling of Alzheimer’s disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Stuct Funct. 2010;214(2-3):111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selkoe DJ. Amyloid protein precursor and the pathogenesis of Alzheimer’s disease. Cell. 1989;58(4):611–612. [DOI] [PubMed] [Google Scholar]
- 13. Sisodia SS, Price D. Role of the beta-amyloid protein in Alzheimer’s disease. FASEB J. 1995;9(5):366–370. [DOI] [PubMed] [Google Scholar]
- 14. Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24(4):219–224. [DOI] [PubMed] [Google Scholar]
- 15. Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11(3):213–228. [DOI] [PubMed] [Google Scholar]
- 16. Resende R, Moreira PI, Proença T, et al. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44(12):2051–2057. [DOI] [PubMed] [Google Scholar]
- 17. Sjögren M, Andreasen N, Blennow K. Advances in the detection of Alzheimer’s disease—use of cerebrospinal fluid biomarkers. Clin Chim Acta. 2003;332(1):1–10. [DOI] [PubMed] [Google Scholar]
- 18. Mangialasche F, Mangialasche F, Polidori MC, et al. Biomarkers of oxidative and nitrosative damage in Alzheimer’s disease and mild cognitive impairment. Ageing Res Rev. 2009;8(4):285–305. [DOI] [PubMed] [Google Scholar]
- 19. Butterfield DA, Kanski J. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzheimer’s amyloid β-peptide 1–42. Peptides. 2002;23(7):1299–1309. [DOI] [PubMed] [Google Scholar]
- 20. Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59(2):278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holmes C, Butchart J. Systemic inflammation and Alzheimer’s disease. Biochem Soc Trans. 2011;39(4):898–901. [DOI] [PubMed] [Google Scholar]
- 22. Ishrat T, Parveen K, Khan MM, et al. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2009;1281(24):117–127. [DOI] [PubMed] [Google Scholar]
- 23. Kim YM, Kim HJ, Song EJ, Lee KJ. Glucuronic acid is a novel inducer of heat shock response. Mol Cell Biochem. 2004;259(1-2):23–33. [DOI] [PubMed] [Google Scholar]
- 24. Sato H, Takahashi T, Ide H, et al. Antioxidant activity of synovial fluid, hyaluronic acid, and two subcomponents of hyaluronic acid. Synovial fluid scavenging effect is enhanced in rheumatoid arthritis patients. Arthritis Rheum. 1988;31(1):63–71. [DOI] [PubMed] [Google Scholar]
- 25. Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. [DOI] [PubMed] [Google Scholar]
- 26. Castro RE, Santos MM, Glória PM, et al. Cell death targets and potential modulators in Alzheimer’s disease. Curr Pharm Des. 2010;16(25):2851–2864. [DOI] [PubMed] [Google Scholar]
- 27. Fattahi MJ, Abdollahi M, Agha Mohammadi A, et al. Preclinical assessment of β-d-mannuronic acid (M2000) as a non-steroidal anti-inflammatory drug. Immunopharm Immunot. 2015;37(6):535–540. [DOI] [PubMed] [Google Scholar]
- 28. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. [DOI] [PubMed] [Google Scholar]
- 30. Frank S, Copanaki E, Burbach GJ, Müller UC, Deller T. Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice. Neurosci Res. 2009;453(1):41–44. [DOI] [PubMed] [Google Scholar]
- 31. Zhu Y, Bickford PC, Sanberg P, Giunta B, Tan J. Blueberry opposes β-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activation protein kinase. Rejuvenation Res. 2008;11(25):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem Res. 2012;37(9):1829–1842. [DOI] [PubMed] [Google Scholar]
- 33. Teng P, Li Y, Cheng W, Zhou L, Shen Y, Wang Y. Neuroprotective effects of Lycium barbarum polysaccharides in lipopolysaccharide-induced BV2 microglial cells. Mol Med Rep. 2013;7(6):1977–1981. [DOI] [PubMed] [Google Scholar]
- 34. Fan Y, Hu J, Li J, et al. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374(3):222–226. [DOI] [PubMed] [Google Scholar]
- 35. Guo X, Xin X, Gan L, Nie Q, Geng M. Determination of the accessibility of acidic oligosaccharide sugar chain to blood-brain barrier using surface plasmon resonance. Biol Pharm Bull. 2006;29(1):60–63. [DOI] [PubMed] [Google Scholar]
- 36. Mirshafiey A, Cuzzocrea S, Rehm B, Matsuo H. M2000: a revolution in pharmacology. Med Sci Monit Basic Res. 2005;11(8):PI53–PI63. [PubMed] [Google Scholar]
- 37. D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36(1):60–90. [DOI] [PubMed] [Google Scholar]
- 38. Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial memory and learning in transgenic mice: fact or artifact? Physiology. 1998;13(3):118–123. [DOI] [PubMed] [Google Scholar]
- 39. Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the most sensitive measure of water maze probe test performance? Front Integr Neurosci. 2009;9(3):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Douillard H, Koutroumanis M, Goodyer C, LeBlanc A. Amyloid β peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates Bax expression in human neurons. J Neurosci. 1996;16(23):7533–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kitamura Y, Shimohama S, Kamoshima W, et al. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 1998;780(2):260–269. [DOI] [PubMed] [Google Scholar]
- 42. Atabakhsh E, Bryce DM, Lefebvre KJ, Schild-Poulter C. RanBPM has proapoptotic activities that regulate cell death pathways in response to DNA damage. Mol Cancer Res. 2009;7(12):1962–1972. [DOI] [PubMed] [Google Scholar]
- 43. Mayer B, Oberbauer R. Mitochondrial regulation of apoptosis. Physiology. 2003;18(3):89–94. [DOI] [PubMed] [Google Scholar]
- 44. Hwang DY, Chae KR, Kang TS, et al. Alterations in behavior, amyloid β-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer’s disease. FASEB J. 2002;16(8):805–813. [DOI] [PubMed] [Google Scholar]
- 45. Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–1058. [DOI] [PubMed] [Google Scholar]
- 46. Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free radical-mediated damage to brain in Alzheimer’s disease and its transgenic mouse models. Free Radic Biol Med. 2008;45(3):219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]