Abstract
Alzheimer’s disease (AD) is a common neurodegenerative disease in the elderly individuals and its effective therapies are still unavailable. This study was designed to investigate the neuroprotection of sulforaphane (SFN) in AD-lesion mice induced by combined administration of d-galactose and aluminium. Results showed that SFN ameliorated spatial cognitive impairment and locomotor activity decrease in Morris water maze and open field test, respectively. And attenuated numbers of amyloid β (Aβ) plaques in both hippocampus and cerebral cortex of AD-lesion mice were detected by immunohistochemistry. According to spectrophotometry and quantitative reverse-transcriptase polymerase chain reaction results, a significant increase in carbonyl group level and obvious decreases in both activity and messenger RNA expression of glutathione peroxidase were found in brain of AD-lesion mice compared with control, but not in SFN-treated AD-lesion mice. In conclusion, SFN ameliorates neurobehavioral deficits and protects the brain from Aβ deposits and peroxidation in mice with Alzheimer-like lesions, suggesting SFN is likely a potential phytochemical to be used in AD therapeutics.
Keywords: Alzheimer’s disease, sulforaphane, phytochemical, neurobehavior, amyloid β, oxidative stress
Introduction
The World Health Organization estimated that there were 36 million people living with dementia worldwide in 2010, increasing to 66 million by 2030 and 115 million by 2050. 1 Alzheimer’s disease (AD), a neurodegenerative dementia affecting the elderly individuals, accounts for 50% to 70% of patients with dementia. 2 The etiology of AD is multifactorial with genetic, environmental, behavioral, and developmental components playing a role. 3 It is widely reported that the pathological hallmarks of the disease include neuronal loss in regions of the brain involved in learning and memory functions, senile plaques composed of deposits of amyloid β (Aβ) mainly, and neurofibrillary tangles consisting of hyperphosphorylated τ protein. 4 –6 Although the detailed mechanism of AD has still been unclear, compelling evidence supports the central role of Aβ in the pathogenesis of the disease. 7 And the oxidative stress has been proposed to play an important role in neurotoxicity induced by Aβ deposits. 8
The multiple mechanisms involved in the pathogenesis of AD created considerable difficulty in producing an effective treatment. Several cholinesterase inhibitors and memantine (an N-methyl-d-aspartate receptor antagonist), based on the lower levels of the neurotransmitter, acetylcholine, and glutamate-mediated neurotoxicology found in the brain of patients of AD, are the only drugs currently approved by the US Food and Drug Administration for use. 9 However, these drugs provide symptomatic improvement alone but do not halt or delay the disease progression. Given the involvement of Aβ accumulation and oxidative stress in the etiology and pathology of AD, the promising approaches to preventive interventions for AD include attenuating Aβ deposits and antioxidant therapy. Certain naturally occurring dietary phytochemicals such as curcumin, resveratrol, and green tea catechins, owing to the safety and efficacy, have received considerable recent attention as alternative candidates for AD therapy. 10 Sulforaphane (SFN), a compound found in cruciferous vegetables, has been shown to exhibit anticarcinogenic, anti-inflammatory, antioxidative, chemopreventive, and cytoprotective properties. 11,12 Recently, it has been reported that SFN can penetrate blood–brain barrier and exert neuroprotective effects in diverse in vitro cell culture and in vivo animal models of neurological disorders. 13,14 Lee et al found that SFN protected against Aβ-induced oxidative cell death by activation of NF-E2-related factor 2 in SHSY5Y cells. 15 In addition, SFN could ameliorate cognitive impairment and protect the brain from amyloidogenic damages in an Aβ-induced AD acute mouse model, 16 but the therapeutic effect of SFN did not involve inhibition of Aβ aggregation in vitro. As is well known, increased Aβ deposits are not only related to Aβ aggregation but also its formation and degradation or cleaning. Therefore, the exact effect mechanism of SFN in AD has not yet been ascertained. In this study, we examined whether SFN can attenuate cognitive deficits, augment antioxidant defence, and reduce the Aβ plaque burden of brain in AD mice.
Materials and Methods
Regents
d-(+)-Galactose (purity: ≥98%) and aluminum chloride (purity: ≥97.0%) were purchased from Sigma Chemical Corporation (St Louis, Missouri); d,l-SFN (purity: ≥97.0%) was purchased from Toronto Research Chemicals Inc (Toronto, Canada); rabbit anti-Aβ1-42 polyclonal antibody was obtained from Abcam Inc (Cambridge, United Kingdom); and rabbit anti-β-actin polyclonal antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, California). Immunohistochemistry kits were purchased from Beijing Zhongshan Biotechnology (Beijing, China). Test kits for carbonyl group, sulfhydryl (SH) group, superoxide dismutase (SOD), and glutathione peroxidase (GPX) were obtained from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). All other reagents were of the highest purity available and from commercial sources.
Animals and Treatment
Eight-week old C57BL/6 mice (animal code SCXK 2008-1105) were purchased from the Experimental Animal Center at China Medical University (Shenyang, China). The mice were maintained on a 12-hour light–dark cycle with controlled temperature at 22°C ± 2°C, humidity at 55% ± 15%, and food and water ad libitum. The animal experiment was approved by the Animal Care and Use Committee at the China Medical University with a permit number of CMU62043003, which complies with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize suffering and the number of animals used.
According to the body weight, mice were randomly divided into 3 groups (n = 16, equal numbers of male and female mice). Mice in groups having AD lesion (AD) and having AD lesion with SFN intervention (AD + SFN) were administered aluminum-containing water (0.4 g/100 mL) daily and freely and injected subcutaneously with 200 mg/kg d-galactose (dissolved in physiological saline) every other day. Distilled water and equivalent physiological saline were given to mice in the control group by drinking freely and subcutaneous injection, respectively. Additionally, mice in the AD + SFN group were treated with 25 mg/kg SFN (dissolved in distilled water) by a single oral gavage daily, and mice in groups of AD and control were gavaged with equivalent distilled water.
Behavioral tests of mice were performed 80 days after treatment, and then animals were killed 5 days after finishing the tests. Six mice in each group were used for immunohistochemical assay to investigate the number of Aβ immunoreactive plaques. The brains of 10 mice in each group were immediately removed and divided into halves. The cortex of left cerebral was weighed and then homogenated in physiological saline to obtain 10% homogenate. The homogenate was centrifuged at 1500g for 10 minutes at 4°C, and the supernatant was collected for testing parameters of oxidative stress. The cortex of right cerebral was used for real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR).
Behavioral Tests
The behavioral tests of mice were performed in a silent, isolated room at the temperature of 22°C ± 2°C. A video camera viewing the experimental area was positioned on the vertical from the center of the arena and connected to a personal computer.
Open field test
The circular open field (diameter: 120 cm and height: 50 cm) consisted of 25 transparent areas at the peripheral, central, and intermediary of the arena. The mice were placed in the center of the open field and allowed to explore for 3 minutes. Behavioral parameters, including immobility time in the central area (latency), times of cross areas, and times of upright, were measured. After each trial, the floor was cleaned with a damp cloth and dried.
Morris water maze
On the following day after finishing the open field test, a 5-day water maze procedure, as previously described with minor modifications, 17,18 was carried out to assess spatial learning and memory. The test was conducted in a pool (120 cm in diameter) filled with water opacified with milk powder. For analytical purposes, the interior of the pool was virtually divided into 4 quadrants. An escape platform (8 cm in diameter) was hidden in the center of quadrant 2 at 1 cm below the surface level of water.
The procedure included escape trials (4 days) and a probe trial (1 day). In escape trials, each mouse was given 4 trials with 60 seconds intertrial interval per day. At the beginning, the animals were placed in the water from the midpoint of each quadrant’s edge in turn, with the head facing the pool wall. The escape latency to the hidden platform was recorded. If a mouse failed to locate the hidden platform within 60 seconds, it was taken away from water and placed on the platform for 10 seconds. On the fifth day, the platform was removed, and a probe trial was then performed. The mouse was allowed to swim freely, and the times that the mouse crossed the center of quadrant 2 (passing times) within 60 seconds were recorded. After each trial, the mouse was dried with a towel and placed back into its cage. The data of escape latency in escape trials and the passing times in the probe trial were analyzed.
Immunohistochemistry Assays
Mice were deeply anesthetized and transcardially perfused with saline followed by ice-cold 4% paraformaldehyde (pH 7.4). The brains (from bregma −2.5 mm to −3.5 mm) were removed, postfixed with the same fixative overnight at 4°C, and embedded in paraffin. Coronal sections (5 μm) were dried well at 60°C for 2 days and then dewaxed in xylene and dehydrated in an alcohol row, subsequently, washed them 3 times in phosphate-buffered saline (PBS; pH 7.2). Endogenous peroxidase activity was blocked by immersion in 3% hydrogen peroxide for 10 minutes. Nonspecific binding of the primary antibodies was blocked by incubating the sections with diluted normal goat serum for 30 minutes at room temperature. Sections were incubated overnight with primary antibody (1:200) diluted in PBS. After being washed with PBS, sections were incubated with secondary antibody (goat antirabbit immunoglobulin G) for 30 minutes. The sections were washed with PBS and then incubated with horseradish peroxide avidin–biotin complex for another 15 minutes and a repeated washing step with PBS. The reaction products were visualized with diaminobenzidine chromogen solution. Sections were then counterstained with hematoxylin and observed the number of Aβ immunoreactive plaques under a light microscope.
Oxidative Stress Analyses
Levels of carbonyl group and SH group, as well as activities of GPX and SOD in the cerebral cortex, were measured by colorimetric diagnostic kits according to the manufacturer’s instructions. Total protein in supernatant was detected using Coomassie brilliant blue method. Levels of carbonyl group and SH group were expressed as nanomoles per milligram of fresh tissue protein, and activities of GPX and SOD were described in units per milligram of fresh tissue protein.
Quantitative RT-PCR Analyses
According to the manufacturer’s instructions, total messenger RNA (mRNA) from the cerebral cortex of mice was isolated using SV Total RNA Isolation System kit (Promega Corporation, Madison, Wisconsin). Reverse transcription and first-strand synthesis from each sample were carried out using the PrimeScript RT-PCR System kit (TaKaRa Dalian Biotechnology, Dalian, China) according to manufacturer's instructions. Resulting complementary DNAs were used as templates for qRT-PCR in the ABI 7500 Real-Time PCR system (Applied Biosystems, Inc., Carlsbad, CA) using SYBR Premix Ex Taq Mix (TaKaRa Dalian). The primers were synthesized and purified by TaKaRa Dalian with the following sequences: GPX, forward: 5′-TGTGGAAATGGATGAAAGTCCAG-3′, reverse: 5′-CATGGGACCATAGCGCTTCAC-3′ (122 bp product); SOD forward: 5′-ATTGGCCGTACAATGGTGGT-3′, reverse: 5′-AGACTCAGACCACACAGGGA-3′ (150 bp product); β-actin forward: 5′-CATCCGTAAAGACCTCTATGCCAAC-3′, reverse: 5′-ATGGAGCCACCGATCCACA-3′ (171 bp product). The PCR reaction was amplified in the program including an initial denaturation step at 95°C for 30 seconds, followed by 40 cycles for denaturation at 95°C for 5 seconds, annealing at 60°C for 34 seconds, subsequent melting curves for 15 seconds at 95°C, 1 minute at 60°C, and 15 seconds at 95°C to ensure that only a single product was amplified. Absolute values from each sample were normalized to β-actin (constitutive gene) mRNA as a reference standard.
Statistical Analyses
All data were presented as means ± standard error of the means. Statistical analyses were performed using 1-way analysis of variance (ANOVA) followed by post hoc Fisher's least significant difference multiple comparison tests with the SPSS 13.0 software for Windows (version 13.0; SPSS, Chicago, Illinois), except that the escape latency in the Morris water maze was analyzed using 2-way ANOVA with repeated measures. Probability values less than .05 were considered statistically significant.
Results
Signs and Body Weights
During the treatment, no significant signs of toxicity were observed in mice. As shown in Figure 1, there were no statistical difference (P > .05) in body weights of mice among groups each week.
Figure 1.
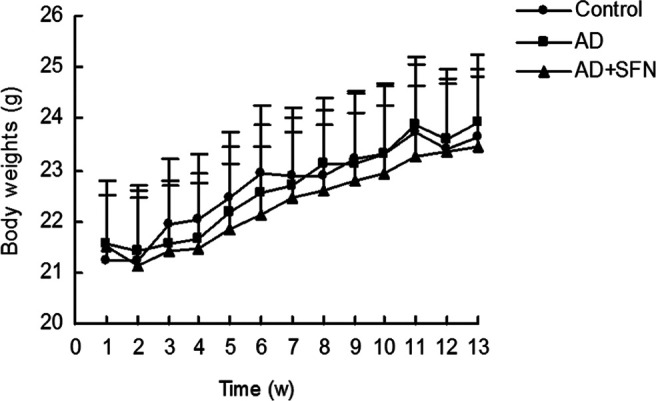
Measurement of mice body weights during treatment. During the experiment, there were no significant difference (P > .05) in the body weights of mice among groups each week (n = 16; mean ± standard error of the mean [SEM]; 1-way analysis of variance [ANOVA] followed by post hoc least significant difference [LSD] multiple comparison tests).
Behavioral Tests
The results from open field test were shown in Figure 2. Compared with control, there was a significant decrease (P < .05) in times of cross areas and a significantly longer latency (P < .05) in the central area in AD mice, but no significant difference (P > .05) in SFN-treated AD mice. And an obvious increase (P < .05) in times of cross areas was found in SFN-treated AD mice compared with AD mice. In addition, no significant difference was observed in times of upright among groups of control, AD, and AD + SFN.
Figure 2.
Analysis of open field test in mice of control, AD, and AD + SFN. Compared with control, there was a significant decrease in times of cross areas and a significantly longer latency in the central area in AD mice, but no significant difference in SFN-treated AD mice (A and B). And an obvious increase in times of cross areas was found in SFN-treated AD mice compared with AD mice (A). In addition, no significant difference was observed in times of upright among groups of control and AD mice with or without SFN treatment (C; n = 16; mean ± standard error of the mean [SEM]; 1-way analysis of variance [ANOVA] with post hoc least significant difference [LSD] tests; *P < .05 vs the control group, # P < .05 vs the AD group). AD indicates Alzheimer’s disease; SFN, sulforaphane.
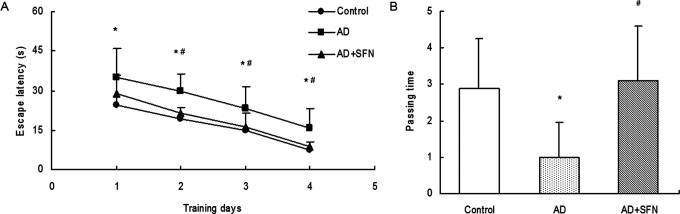
As shown in Figure 3, compared with control, the escape latency from day 1 to day 4 in escape trials of Morris water maze was significantly prolonged (P < .05) in AD mice but not significantly increased (P > .05) in AD mice treated with SFN. And there were significant differences (P < .05) in escape latency from day 2 to day 4 between groups of AD and AD + SFN. In the probe trial, the passing times significantly reduced (P < .05) in AD mice compared with control. However, a remarkable increase (P < .05) in passing times was found in SFN-treated AD mice compared with AD mice.
Figure 3.
Analysis of Morris water maze in mice of control, AD, and AD + SFN. Compared with control, the escape latency from day 1 to day 4 in escape trials was significantly prolonged in AD mice but not significantly increased in AD mice treated with SFN, and there were significant differences in escape latency from day 2 to day 4 between groups of AD and AD + SFN (A). In the probe trial, the passing times significantly reduced in AD mice compared with control; however, a remarkable increase in passing times was found in SFN-treated AD mice compared with AD mice (B) (n = 16; mean ± standard error of the mean [SEM]; 2-way ANOVA with repeated measures for escape latency and 1-way ANOVA for passing time both with post hoc least significant difference [LSD] tests; *P < .05 vs the control group, # P < .05 vs the AD group). AD indicates Alzheimer’s disease; ANOVA, analysis of variance; SFN, sulforaphane.
Immunohistochemistry Assays
Immunohistochemistry results of amyloid β were shown in Figure 4. Brown plaques imply the localization of Aβ immunoreactivity in mice brain. The numbers of Aβ immunoreactive plaques were increased remarkably (P < .05) both in hippocampus and cerebral cortex of mice in the AD group compared with groups of control and AD + SFN. However, there were no significant differences (P > .05) in the numbers of Aβ immunoreactive plaques both in the hippocampus and cerebral cortex between control and AD + SFN mice.
Figure 4.
Amyloid β immunoreactive plaques in hippocampus and cerebral cortex (200×). Brown plaques imply the localization of Aβ immunoreactivity in mice brain (see arrows in A, bars = 100 μm). The number of Aβ immunoreactive plaques of per section was increased remarkably (P < .05) in both hippocampus and cerebral cortex of AD mice compared with those in groups of control and AD + SFN; however, there was no significant difference (P > .05) between control and AD + SFN mice (n = 6; mean ± standard error of the mean [SEM]; 1-way analysis of variance [ANOVA] with post hoc least significant difference [LSD] tests; *P < .05 vs the control group, # P < .05 vs the AD group). Aβ indicates amyloid β; AD, Alzheimer’s disease; SFN, sulforaphane.
Oxidative Stress Analyses
As shown in Figure 5, compared with control, a significant increase (P < .05) in carbonyl group level and an obvious decrease (P < .05) in both GPX activity and relative GPX mRNA expression were found in the cerebral cortex of AD mice, but no significant difference (P > .05) in AD + SFN mice. However, no significant alternations (P > .05) were found in both SOD activity and relative SOD mRNA expression in the cerebral cortex of mice among groups of control, AD, and AD + SFN. Additionally, there were no statistical difference (P > .05), although appeared in a downward trend in control, AD + SFN, and AD mice in turn, in SH group level in the cerebral cortex of mice among groups.
Figure 5.
Analyses of oxidative stress levels in the cerebral cortex of mice. Compared with control, a significant increase in carbonyl group level (A) and obvious decreases in both GPX activity and relative GPX mRNA expression (C and D) were found in brain of AD mice but not in AD+SFN mice. However, there were not statistically different in sulfhydryl group level (B), relative superoxide dismutase (SOD) activity, and mRNA expression (E and F) in mice brain among groups (n = 10; mean ± standard error of the mean [SEM]; one-way analysis of variance [ANOVA] with post hoc least significant difference [LSD] tests; *P < .05 vs the control group, # P < .05 vs the AD group). AD indicates Alzheimer’s disease; GPX, glutathione peroxidase; mRNA, messenger RNA; SFN, sulforaphane.
Discussion
Animal models play a major role in defining critical disease-related mechanisms and the preclinical evaluation of potential therapeutic interventions in AD. Most models are based on amyloid pathology, τ pathology, and both amyloid and τ pathology. 19 Besides transgenic animal models, use of nontransgenic animal models of AD could highlight the potential of exploiting spontaneous and induced animal models for neuropathological, neurochemical, neurobehavioral, and neuroprotective studies of AD. 20 It has been reported that Alzheimer-like lesions were found in mice brain induced by combined administration of d-galactose and aluminium, suggesting combined use of d-galactose and aluminium was an effective way to establish AD animal model for studies of AD pathogenesis and therapeutic evaluation. 21
As is well known that memory loss is prominent and early symptom in AD. 22 Kim et al found that SFN ameliorated cognitive function of Aβ-induced AD acute mouse models in Y-maze and passive avoidance behavior tests. 16 Sulforaphane was also found, in the present study, to be potentially effective in ameliorating cognitive function in Morris water maze in AD mice induced by combined administration of d-galactose and aluminium. Moreover, several studies have reported that SFN may potentially be effective in ameliorating cognitive function in animal model of traumatic brain injury, 14 idiopathic Parkinson’s disease, 23 cortial neurons injury, 24 and spinal cord injury. 25 In a word, previous studies and ours indicated that SFN significantly attenuated cognitive impairment in a variety of diseases of nervous system, AD included. In addition, both patients with AD 26 and AD model animals 27,28 have shown less activity. Interestingly, this study showed an ameliorative effect of SFN on the decreased locomotor activity in open field test in AD mice, suggesting a possible antianxiety effect of SFN.
An increasing body of evidence suggests that Aβ is the key to the profound neuronal and synaptic degeneration in the brain regions, such as hippocampus and cerebral cortex, implicated in learning and memory. 29 Immunohistochemistry analyses in both hippocampus and cerebral cortex from this study showed that obviously increased Aβ immunoactive plaques in AD mice could be significantly diminished by SFN, suggesting that SFN protects the brain from Aβ deposits in AD mice. As is well known, augmented production, increased aggregation, or deficient clearance of Aβ results in the build-up of a variety of pathogenic Aβ assemblies. 30 It has been reported that SFN didn’t impact on Aβ aggregation; 16 therefore, whether SFN affects generation or clearance of Aβ needs to be studied.
Oxidative stress occurs when the generation of reactive oxygen species (ROS), chemically reactive molecules containing oxygen, in a system exceeds that system’s ability to neutralize and to eliminate them. The human brain is responsible for approximately 20% of our body oxygen consumption and thus subjected to a high metabolically derived level of ROS. 31 Amyloid β pathology was found to increase ROS levels and increased ROS levels in turn produced more Aβ. 32 Therefore, many studies have been performed to demonstrate the beneficial effects of antioxidants, particularly dietary components, in slowly developing AD. 10
Sulforaphane has been shown to protect against oxidative stress induced by hypoxia–ischemic injury, oxygen, and glucose deprivation, and so on. In particular, evidence suggested that SFN appeared to be a promising compound with neuroprotective properties that may play an important role in preventing neurodegeneration, which could be mainly ascribed to its peculiar antioxidative ability. 33 Carbonyl formation is an important marker of protein oxidation and can arise from direct free radical attack on some amino acid side chains. In this study, we found that the carbonyl group level could be lowered by SFN in the cerebral cortex of AD mice, suggesting the protective effect of SFN against peroxidation, restored endogenous antioxidants in AD mice brain.
Reactive oxygen species can be restrained by a complex system of endogenous free radical scavengers, including SH groups. Low-molecular-weight donors of SH group are used to regulate redox status of cellular thiols and protect SH-containing proteins from excessive oxidation. 34,35 Several SH groups have been reported to protect against AD. 36 –38 In our study, although the result is not statistically significant, the SH group level of AD mice trended to be lower than others. Furthermore, previous studies showed that the total level of SH group was not significantly altered in hippocampus and cerebellum of patients with AD, while the level of protein-bound SH group was decreased in AD hippocampus compared with controls. 35 Therefore, further study is needed to determine the change in SH group and the effect of SFN in AD, as well as the intervention effect of SFN.
Meanwhile, GPX and SOD are the major enzymes for scavenging ROS. Glutathione peroxidase is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. 39 It catalyzes the oxidation of 2 moles of glutathione in the presence of hydrogen peroxide to yield oxidized glutathione and water. A steady reduction in GPX was observed in patients with AD, and it has also been well documented that SFN could upregulate GPX activity. 40,41 In this study, we also found that GPX activity decreased in AD mice but not obviously altered in SFN-treated AD mice. It suggests that SFN may play an antioxidative role through upregulating GPX activity in AD.
Superoxide dismutase catalyzes the dismutation of the superoxide anion (O2·) into H2O2, which can be transformed into H2O and O2. 42 Some laboratories have reported an increase in SOD activity in some brain regions, 43 –45 while others have reported a slight decrease in the same brain regions compared with age-matched control tissue. 46,47 In our study, however, there was no significant difference in SOD activity of cerebral cortex among groups, which is in coincidence with the SOD mRNA level in AD cerebellum reported by Somerville et al. 48 As discussed previously, the activity of SOD in AD is still divergent, and more information needs to be further studied.
In this study, the hippocampus sample was too limited to assess all the biochemical indices. Consequently, we only detected carbonyl group level in hippocampus, and the result showed that the alteration in carbonyl group level in hippocampus among groups was consistent with that in cerebral cortex (data not shown). In addition, the present study did not include a group of mice treated only with SFN, which is very necessary for illustrating whether the effects or side effects are detected in mice exposed to 25 mg/kg SFN. However, the results from some previous studies, with a group of animals treated only with SFN, suggest that no significant effects or side effects were found in mice exposed to 0.5 to 30 mg/kg SFN. 16,49,50 Furthermore, considering the conclusions that we obtained in regard to mice with Alzheimer-like lesions induced by combined administration of d-galactose and aluminium, it should be prudent to extrapolate it from animals to humans.
Conclusions
Taken together, SFN likely protects against neurobehavioral deficits, Aβ deposits, and oxidative stress in AD progression. These findings suggest that SFN is likely a potential phytochemical used in preventing and curing AD.
Acknowledgments
The authors wish to thank Nan Chen, Yue Zuo, and Shu-Huai Xing for their contributions to the project.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Alzheimer’s Disease International. World Alzheimer Report. London: Alzheimer’s Disease International; 2009. [Google Scholar]
- 2. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whalley LJ, Staff RT, Murray AD. Genetic and environmental factors in late onset dementia: possible role for early parental death. Int J Geriatr Psychiatry. 2013;28(1):75–81. [DOI] [PubMed] [Google Scholar]
- 4. Lippa SM, Lippa CF, Mori H. Alpha-synuclein aggregation in pathological aging and Alzheimer's disease: the impact of beta-amyloid plaque level. Am J Alzheimers Dis Other Demen. 2005;20(5):315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castellani RJ, Lee HG, Perry G, Smith MA. Antioxidant protection and neurodegenerative disease: the role of amyloid-beta and tau. Am J Alzheimers Dis Other Demen. 2006;21(2):126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keage HA, Carare RO, Friedland RP, et al. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 2009;9:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao P, Manczak M, Calkins M, et al. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer’s disease: implications for neuroprotection and lifespan extension. Hum Mol Genet. 2012;21(13):2973–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christensen DD. Higher-dose (23 mg/day) donepezil formulation for the treatment of patients with moderate-to-severe Alzheimer's disease. Postgrad Med. 2012;140(6):110–116. [DOI] [PubMed] [Google Scholar]
- 10. Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer's disease. J Neurochem. 2010;112(6):1514–1530. [DOI] [PubMed] [Google Scholar]
- 11. Garber K. Biochemistry: a radical treatment. Nature. 2012;489(7417):S4–S6. [DOI] [PubMed] [Google Scholar]
- 12. Hampton T. Broccoli extract may help reduce UV skin damage. JAMA. 2007;298(23):2371. [DOI] [PubMed] [Google Scholar]
- 13. Alfieri A, Srivastava S, Siow RC, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood–brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–1022. [DOI] [PubMed] [Google Scholar]
- 14. Dash Pk, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett. 2009;460(2):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee C, Park GH, Lee SR, Jang JH. Attenuation of β-amyloid-induced oxidative cell death by sulforaphane via activation of NF-E2-related factor 2. Oxid Med Cell Longev. 2013;2013:313510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim HV, Kim HY, Ehrlich HY, Choi SY, Kim DJ, Kim Y. Amelioration of Alzheimer’s disease by neuroprotective effect of sulforaphane in animal model. Amyloid. 2013;20(1):7–12. [DOI] [PubMed] [Google Scholar]
- 17. Vandevord PJ, Bolander R, Sajja VS, Hay K, Bir CA. Mild neurotrauma indicates a range-specific pressure response to low level shock wave exposure. Ann Biomed. 2012;40(1):227–236. [DOI] [PubMed] [Google Scholar]
- 18. Wang CY, Xie JW, Wang T, et al. Hypoxia-triggered m-calpain activation evokes endoplasmic reticulum stress and neuropathogenesis in a transgenic mouse model of Alzheimer’s disease. CNS Neurosci Ther. 2013;19(10):820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(11):a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaushal A, Wani WY, Anand R, Gill KD. Spontaneous and induced nontransgenic animal models of AD: modeling AD using combinatorial approach. Am J Alzheimers Dis Other Demen. 2013;28(4):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao F, Li XG, Zhang XY, et al. Combined administration of d-galactose and aluminium induces Alzheimer like lesions in brain. Neurosci Bull. 2011;27(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Musilli M, Nicolia V, Borrelli S, Scarpa S, Diana G. Behavioral effects of Rho GTPase modulation in a model of Alzheimer's disease. Behav Brain Res. 2013;237:223–229. [DOI] [PubMed] [Google Scholar]
- 23. Morroni F, Tarozzi A, Sita G, et al. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology. 2013;36:63–71. [DOI] [PubMed] [Google Scholar]
- 24. Wu X, Zhao J, Yu S, Chen Y, Wu J, Zhao Y. Sulforaphane protects primary cultures of cortical neurons against injury induced by oxygen-glucose deprivation/reoxygenation via antiapoptosis. Neurosci Bull. 2012;28(5):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, de Rivero Vaccari JP, Wang H, et al. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J Neurotrauma. 2012;29(5):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70(3):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blaney CE, Gunn RK, Stover KR, Richard EB. Maternal genotype influences behavioral development of 3×Tg-AD mouse pups. Behav Brain Res. 2013;252:40–48. [DOI] [PubMed] [Google Scholar]
- 28. Chakroborty S, Kim J, Schneider C, et al. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. J Neurosci. 2012;32(24):8341–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lomoio S, López-González I, Aso E, et al. Cerebellar amyloid-β plaques: disturbed cortical circuitry in AβPP/PS1 transgenic mice as a model of familial Alzheimer's disease. J Alzheimers Dis. 2012;31(2):285–300. [DOI] [PubMed] [Google Scholar]
- 30. Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer’s disease. Science. 2006;314(5800):781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pimentel C, Batista-Nascimento L, Rodrigues-Pousada C, Menezes RA. Oxidative stress in Alzheimer's and Parkinson's diseases: insights from the yeast Saccharomyces cerevisiae . Oxid Med Cell Longev. 2012;9:1321–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reddy PH. Mitochondrial dysfunction in aging and Alzheimer's disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9(10):1647–1658. [DOI] [PubMed] [Google Scholar]
- 33. Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev. 2013;2013:415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer's disease. Neurosci Lett. 2001;302(2–3):141–145. [DOI] [PubMed] [Google Scholar]
- 36. Kosuge Y, Koen Y, Ishige K, et al. S-allyl-l-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunicamycin-induced neuronal death. Neuroscience. 2003;122(4):885–895. [DOI] [PubMed] [Google Scholar]
- 37. Pensalfini A, Cecchi C, Zampagni M, et al. Protective effect of new S-acylglutathione derivatives against amyloid-induced oxidative stress. Radic Biol Med. 2008;44(8):1624–1636. [DOI] [PubMed] [Google Scholar]
- 38. West AK, Hidalgo J, Eddins D, Levin ED, Aschner M. Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology. 2008;29(3):489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Niu W, Yang X, Wang Y. Effects of combined acupuncture and eugenol on learning-memory ability and antioxidation system of hippocampus in Alzheimer disease rats via olfactory system stimulation. J Tradit Chin Med. 2013;33(3):399–402. [DOI] [PubMed] [Google Scholar]
- 40. Tarozzi A, Morroni F, Merlicco A, et al. Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line. J Neurochem. 2009;111(5):1161–1171. [DOI] [PubMed] [Google Scholar]
- 41. Tsai SJ, Chiu CP, Yang HT, Yin MC. S-Allyl cysteine, s-ethyl cysteine, and s-propyl cysteine alleviate β-amyloid, glycative, and oxidative injury in brain of mice treated by d-galactose. J Agric Food Chem. 201;59:6319–6326. [DOI] [PubMed] [Google Scholar]
- 42. Zhao HD, Zhang F, Shen G, et al. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16(24):3220–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furuta A, Price DL, Pardo CA, et al. Localization of superoxide dismutases in Alzheimer's disease and Down's syndrome neocortex and hippocampus. Am J Pathol. 1995;146(2):357–367. [PMC free article] [PubMed] [Google Scholar]
- 44. Marcus DL, Thomas C, Rodriguez C, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998;150(1):40–44. [DOI] [PubMed] [Google Scholar]
- 45. Mizuno Y, Ohta K. Regional distributions of thiobarbituric acid-reactive products, activities of enzymes regulating the metabolism of oxygen free radicals, and some of the related enzymes in adult and aged rat brains. J Neurochem. 1986;46(5):1344–1352. [DOI] [PubMed] [Google Scholar]
- 46. Chen L, Richardson JS, Caldwell JE, Ang LC. Regional brain activity of free radical defense enzymes in autopsy samples from patients with Alzheimer's disease and from nondemented controls. Int J Neurosci. 1994;75(1–2):83–90. [DOI] [PubMed] [Google Scholar]
- 47. Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Somerville MJ, Percy ME, Bergeron C, Yoong LK, Grima EA, McLachlan DR. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991;9(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 49. Wang W, Wu Y, Zhang G, et al. Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res. 2014;1544:54–61. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Zhang Z, Sun W, et al. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev. 2014;2014:123963. [DOI] [PMC free article] [PubMed] [Google Scholar]