Abstract
This controlled study examines the efficacy of a comprehensive group program aimed at care partners of patients with mild cognitive impairment (MCI), which comprises elements of psychoeducation, cognitive rehabilitation, and cognitive behavioral therapy. Pre- and posttreatment quantitative and qualitative data were collected in the significant others of 84 patients with MCI, 27 of whom had first been assigned to a waiting list, thus serving as their own control group. Also, the significant others rated their sense of competence, well-being, distress, acceptance, helplessness, and awareness. Quantitative data analysis did not reveal statistically significant differences between the control and the intervention condition, but qualitative results suggest that at program completion the significant others reported gains in knowledge, insight, acceptance, and coping skills. In the present sample of significant others, the group intervention was not proven effective. Suggestions for program adjustments and alternative outcome measures are discussed.
Keywords: mild cognitive impairment, caregivers, psychotherapy, psychoeducation, group therapy, qualitative study
Introduction
Mild cognitive impairment (MCI) is a syndrome characterized by cognitive deficits in the context of normal daily functioning. 1 MCI is a risk factor for the development of dementia, with annual MCI–dementia conversion rates reported to be 2% to 31%. 2 As a result, the progression of their illness is uncertain for many patients, and the diagnosis may even exacerbate feelings of uncertainty. 3 Moreover, having a partner or loved one with MCI may be unsettling, and finding ways to support the changing partner or relative can be difficult. That is, the transition from being a partner to being a carer of a person with MCI (who at the end may even develop dementia) gives rise to ambiguous feelings and behavior, distress, and interpretations of the changes observed. 4 -7 In several studies, both patients with MCI and their partners expressed a need for more information and support, 7 -11 indicating the call for a psychosocial intervention aimed at information, support, and effective coping. Several reviews 12 -15 have investigated the efficacy of cognitive interventions in patients with MCI, with promising, albeit inconclusive results. Interventions aimed at the needs of loved ones of patients with MCI, however, do not exist to our knowledge.
Based on reviews of interventions in early-stage dementia, 16,17 we designed a multicomponent program in which patients with MCI and their partners or other loved ones participate. It combines principles from cognitive behavioral therapy with memory-rehabilitation elements. A (uncontrolled) pilot study showed promising first results in both the patients and the care partners. 18 Also, beneficial effects on the patients with MCI have been reported in a previous article on the outcome of the controlled intervention in patients with MCI. 19 The aim of the current article was to evaluate the effects of our MCI intervention program in significant others of patients with MCI combining a quantitative and qualitative approach. 20 We hypothesized that after program completion, the significant others’ sense of competence would be increased and the level of distress would be reduced and their well-being would be increased. We furthermore expected the degree of acceptance of their partners’ condition and awareness to increase and the level of helplessness to reduce.
Methods
Participants
Eligible patients with MCI and their significant others were recruited from 4 regional outpatient memory clinics in the east of the Netherlands (Radboud University Nijmegen Medical Centre; Maasziekenhuis Pantein, Boxmeer; Rijnstate hospital, Arnhem; Slingeland hospital, Doetinchem). Inclusion criteria were an MCI diagnosis, age over 50 years, and the availability of a partner, spouse, relative, or close friend willing to participate. MCI was diagnosed using a multidisciplinary approach described in detail elsewhere, 18,19 in accordance with generally accepted criteria. 1 Exclusion criteria were absence of informed consent, psychiatric comorbidity, coexisting somatic disorders if dominant to MCI, severe concentration difficulties impeding communication, inability to communicate in Dutch, lack of motivation to share experiences in a group, and evidence of preexisting partner-relationship problems unrelated to the cognitive impairments, by means of an intake interview performed by a psychotherapist (L.W.A.J.W.B.) and by available neuropsychological test results. Ethical approval was obtained from the regional (Arnhem-Nijmegen) medical ethics committee, and informed consents were obtained from the patient and the partner.
Procedure
Patients and their significant others fulfilling the inclusion criteria were informed about the group program by their geriatricians or neurologists. Interested dyads were subsequently invited for an interview with a psychotherapist at their local hospital, who explained the aims and content of the intervention and evaluation. All subsequent assessments were conducted by trained research assistants. During the waiting period and the intervention participants received no other psychosocial or medical intervention for their cognitive complaints.
Demographic data were collected with a general checklist. Educational level was rated using seven categories in accordance with the Dutch educational system, (1 = less than primary school, ie, <6 years of education; 7 = university degree, ie, >14 years of education). Several variables were collected for descriptive purposes from the patients and their loved ones. In the patients, overall cognitive impairment was measured with the Dutch version 21 of the Mini-Mental State Examination (MMSE), 22 and episodic memory function was assessed with the delayed recall measure of the Dutch version of the Rey Auditory Verbal Learning Test. 23 In the care partners, coping was assessed with 2 subscales (active coping and avoidance) of the Utrecht Coping List. 24 Social support was evaluated with the Social Support List-Interaction version (SSL12-I), which consists of the subscales Everyday Social Support, Social Support in Problem Situations, and Esteem Support. 25 Satisfaction with the partner relationship was assessed with the Emotional Satisfaction scale of the Dutch version of the Maudsley Marital Questionnaire. 26
Intervention
The intervention consisted of 10 weekly 2-hour group sessions and was based on cognitive-behavioral therapeutic principles combined with memory rehabilitation elements. Each group consisted of 5 to 8 patients all accompanied by a partner/spouse, adult child, relative, or close friend. In the first 90 minutes of each session, patients and partners participated in separate groups, each with its own therapist, while both groups explored the same theme. In the remaining 30 minutes, both groups came together and key issues were summarized and highlighted to promote mutual support among the care partners and the patients.
The focus of the program was the acquisition of knowledge and skills to adequately cope with MCI-associated symptoms and their consequences, learning to recognize memory problems in everyday life, and to explore explanations and attributions, communication with loved ones, and self-regulation skills. Moreover, topics such as dependency on others, the diagnostic label, and stigmatization were discussed. Sessions started with a discussion of topical questions, problems, or experiences the patients or their care partners had encountered. These topics were then related to past, present, or upcoming themes of the program. Next, the theme of the current session was introduced by the therapist. All participants were instructed to prepare for the sessions with relevant texts selected from a patient handbook on coping with memory impairment that was specifically written for use in this intervention. 27 Patients and care partners were asked to monitor their thoughts, feelings, and behavior in situations of cognitive failure or stress, with the aim to reduce or prevent irrational and stress-inducing cognitions.
The following topics were discussed in the 10 subsequent sessions: memory function in general, MCI as a clinical label, therapeutic possibilities, strategies to enhance memory, ways to recognize strain, learning to relax, the importance of undertaking pleasant everyday activities, and coping with social conflicts and worrying. The participants were encouraged to recognize and cognitively restructure dysfunctional self-evaluations and negative social and unduly anxious cognitions. By sharing their reactions and thoughts, participants experienced that others in similar situations may show different and perhaps more adequate behavior. Subsequently, participants were asked how they felt about their responses and positive sentiments were reinforced. If not, alternative responses were explored and practiced via role playing. The separate sessions ended with an evaluation of the most important issues discussed and a summary of the thoughts and behaviors the participants decided to change. In the plenary part of the session, both therapists itemized the major topics and experiences that were discussed in the separate sessions, after which the patients and care partners were invited to add to this. At the end of the plenary session, the therapists explained the theme of the next session and the associated home assignments and provided the participants with the materials for that assignment. 18,19 The therapists delivering the treatment were all registered psychologists trained and supervised by the first author.
Quantitative Study
The quantitative study had a pragmatic, nonrandomized, waiting list controlled design, with all eligible patient–partner dyads receiving the group treatment either within 8 weeks of their recruitment or after 8 weeks or more on a waiting list (waiting for a new intervention group to begin). The “intervention-only” group (dyads receiving treatment within 8 weeks after intake) was first assessed in 2 weeks prior to the start of the intervention (T1) and within 2 weeks following program completion (T2). When the time between inclusion and the start of the next intervention was more than 8 weeks, dyads were assigned to a waiting list, serving as our control group, at the start of which period they took a baseline assessment (T0). Their pre- and posttreatment (T1 and T2) tests were scheduled as in the intervention-only group. Waiting list intervals (T0-T1) ranged from 8 to 16 weeks. To maximize statistical power, the total intervention group we report on here is composed of significant others having received “immediate” treatment and those having received treatment after having spent at least 8 weeks on a waiting list. 13
Primary Outcome Measures
The primary outcome measure, sense of competence, was assessed with the Sense of Competence Questionnaire reflecting the perceived burden related to the challenges the significant other faces. 28 The inventory has the 3 following subscales: satisfaction with the MCI partner as the recipient of the support, satisfaction with one’s own performance as a significant other/care partner, and the implications of the involvement in the patient’s care for one’s own personal life, and the total score was used in the analysis (higher scores reflect a lower sense of competence). Note that loved ones of patients with MCI are not considered caregivers in the traditional sense, as by definition, the MCI label assumes that the patient is independently functioning at home.
Secondary Outcome Measures
The secondary outcome measures were as follows: Distress was evaluated by means of the Dutch version 29 of the Geriatric Depression Scale—Short Form (GDS-15), 30 well-being was assessed using 4 subscales from the Dutch version of the RAND-36: 31 Social Functioning, Role-Emotional, Mental Health, and Vitality. Acceptance was assessed with the subscale Acceptance from the Illness Cognition Questionnaire (ICQ), 32 and Helplessness was evaluated with the same-named subscale of the ICQ. Awareness of memory failure was evaluated using the Dutch version of the Informant Questionnaire on Cognitive Decline in the Elderly—Short Form, 33 a self-report measure assessing the observer’s perception of memory failure (by asking for examples of everyday life memory failures) and as such can be applied for assessing (changes in) awareness of memory failure. 34 The significant others also completed the frequency scale of the Dutch version 35 of the Revised Memory and Behavior Problems Checklist (RMBPC), 36 reflecting their awareness of the patient’s memory, mood, and behavioral problems. In addition, the Hindrance scale of the RMBPC was used to measure to what extent the significant others were affected by the observed behavioral problems.
Analyses
First, baseline characteristics for the waiting list group and the intervention-only group were statistically compared. To analyze changes in the primary outcome measure following the waiting list and intervention intervals, we applied a linear mixed model (LMM) for repeated measurements. 19 We opted for LMM because, as opposed to repeated measures analysis of variance, this model does not require data to be present at each assessment for a participant to be included in the statistical analysis, while at the same time accommodating the dependency caused by repeated measurements. In the outcome variables, differences between T0 and T1 (change after waiting period) and between T1 and T2 (pre-and posttreatment change) were calculated and used as repeated measures with Interval (2 levels), Group (17 levels), Sex (2 levels), and their first-order interactions as fixed factors. Sex was included as a fixed factor because it might be an important aspect influencing outcome. 13 To correct for treatment location and/or therapist effects, we introduced Group as one fixed factor into the analysis. Interval (T0-T1 vs T1-T2) was entered as a within-subject variable. We used the MIXED procedure from the SPSS package. The power calculation we ran in our pilot study had shown that approximately 70 significant others were required to reach a power of 0.8 on the primary outcome measure, with an effect size of 0.2 and a significance level of 0.05. 18 All primary and secondary outcome measures were administered in the care partners.
Qualitative Study
The qualitative data were analyzed using the interpretative phenomenological analysis method that involves a constant comparative analysis to identify common themes and issues. 37,38 Trained master-level psychology students assisted in and monitored each group, noting the statements the participants made during the sessions. We made use of these anonymous written notes of the final (10th) sessions in which the intervention was evaluated. We adhered to the following interview guide: (1) Which of the program themes was or were the most helpful? (2) What are the main benefits of the group program? (3) Which of the issues we dealt with still need your attention? (4) How did both you and your partner experience the group? (5) Do you have suggestions to improve the program? After the participants had given their personal evaluations, they were specifically encouraged to broach negative aspects or topics they had missed in the program. The notes of their evaluations were independently read and analyzed by 2 researchers (M.V. and L.W.A.J.W.B.), using the open-coding procedure, an interpretive process by which data are broken down analytically and conceptually labeled, generating a comprehensive understanding of themes and patterns in the data. 26 First, codes were assigned which were closely related to the quotations. For example, the quotations: “I have to take care that my alertness to changes in my husband’s memory problems will not stress me out too much” and “I will take care not to worry too much” were both coded as alertness for negative consequences to the self. Codes referring to the same phenomenon were grouped into categories and categories grouped into themes. Consensus among researchers about codes, categories, and themes was reached by peer-group discussion conducted by a clinical neuropsychologist/psychotherapist (L.W.A.J.W.B.), a senior medical sociologist (M.J.F.J.V.D.) experienced in qualitative end-of-life research, and a graduate psychology student (M.V.). Care partner evaluations were included until the saturation point of qualitative data was reached. ATLAS.ti (computer software Berlin, Germany: ATLAS.ti Scientific Software Development GmbH) was used to manage the data set and to allow for systematic searching and cross-referencing.
Results
Baseline Characteristics
Table 1 lists the baseline data. Between-group comparisons revealed no significant differences in demographics and main characteristics, or the primary outcome measure at baseline, apart from a slightly, yet significantly higher level of acceptance and passive coping in the waiting list control group compared to the intervention-only group.
Table 1.
Baseline Characteristics of the Significant Others for the 2 Study Groups (Means + SD or n + Percentages).a
| Waiting list control group (T0; n = 27) | Intervention only group (T1; n = 58) | P value | |
|---|---|---|---|
| Demographics of care partner | |||
| Age | 67.0 (8.2) | 69.4 (8.2) | .22 |
| Education (1 = low;7 = high) | 5.1 (0.9) | 4.9 (1.0) | .98 |
| Sex distribution | 14 M (51.9%)/13 F (48.1%) | 22 M (38%)/36 F (62%) | |
| MMSE of the spouse with MCI | 25.9 (3.9) | 25.4 (3.2) | .50 |
| Type of partnership | |||
| Married/living together | 24 (88.9%) | 53 (91.4%) | .70 |
| Living apart | 1 (3.7%) | 0 | |
| Daughter/sister | 2 (7.4%) | 5 (8.6%) | |
| SSLI2-I | |||
| Everyday support | 10.8 (1.9) | 10.7 (1.7) | .83 |
| Support in problem situations | 9.3 (1.8) | 9.7 (2.6) | .49 |
| Esteem support | 10.5 (2.1) | 10.5 (2.3) | .96 |
| UCL | |||
| Active coping | 19.3 (3.5) | 17.8 (3.5) | .17 |
| Passive coping | 15.2 (2.9) | 16.7 (3.1) | .04 |
| MMQ-Emotional satisfaction | 15.6 (11.3) | 17.3 (13.3) | .59 |
| Sense of Competence Questionnaire | |||
| Total score | 89.7 (20.4) | 87.5 (19.7) | .83 |
| Consequences for personal life | 27.9 (7.7) | 26.5 (8.1) | .49 |
| Satisfaction with patient | 20.4 (7.4) | 19.6 (7.6) | .14 |
| Satisfaction with own performance | 41.2 (11.3) | 41.0 (9.0) | .94 |
| GDS-15 | 1.5 (1.6) | 2.3 (2.4) | .21 |
| GDS-15 > 5 (%) | 3.7 % (1/27) | 10.9 % | |
| RAND-36 | |||
| Well-being | 305.4 (71.5) | 293.6 (66.9) | .47 |
| Acceptance | 18.3 (3.8) | 15.9 (3.1) | .05 |
| Helplessness | 10.0 (3.3) | 10.3 (3.1) | .53 |
| RMBPC | |||
| Frequency | 12.0 (8.1) | 15.0 (9.2) | .14 |
| Hindrance | 22.0 (8.8) | 25.5 (10.0) | .17 |
| IQCODE | 62.6 (7.4) | 62.6 (6.8) | .99 |
Abbreviations: MMSE, Mini Mental State Examination; SSL12-I, Social Support List-Interaction version; UCL, Utrecht Coping List; MMQ, Maudsley Marital Questionnaire; GDS-15, Geriatric Depression Scale; RMBPC, Revised Memory and Behavioral Problems Checklist; IQCODE, Informant Questionnaire Cognitive Decline in the Elderly.
a Differences were tested by student t tests or chi-square tests.
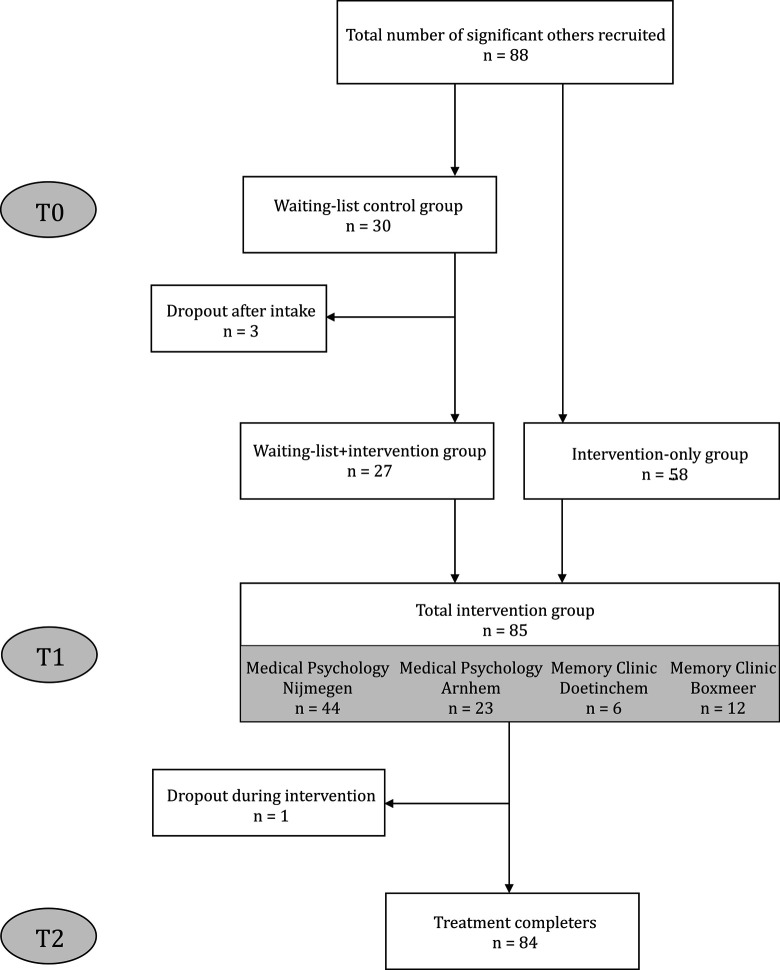
A total of 88 patients with MCI and their significant others expressed an interest to participate in our study and were included (Figure 1). The intake procedure for 30 dyads took place more than 8 weeks before the start of a new group intervention program, and they were initially assigned to our waiting list, thus constituting the control group, with 3 dyads dropping out after the baseline assessment (T0) because of somatic disease unrelated to MCI (n = 1) or lack of time (n = 2). The other 58 dyads started treatment within 8 weeks of their intake interview, accordingly constituting the intervention-only group. Thus, a total of 85 dyads (27 in waiting list control group and 58 in the intervention-only group) were assessed at T1 (pre-treatment). The intervention-only group was twice the size of the control group due to the variability in the flow of referrals in the 4 participating hospitals and the study schedule. 13 For one care partner in the final intervention group, no posttreatment (T2) data were available because of loss of motivation. Thus, the data of 84 significant others were entered into the LMM analyses.
Figure 1.
Flowchart showing the inclusion of significant others of patients with MCI.
Quantitative Results
Table 2 shows the means of the baseline, pre- and posttreatment outcomes and the effect sizes on the significant others for descriptive purposes. The LMM analysis revealed no significant difference between the waiting list period and the intervention period for our primary outcome measure Sense of Competence (F[1,36.4] = 0.30, P = .59), nor for any of the secondary outcome measures (all F values <1.79).
Table 2.
Means With (SDs) for the Primary and Secondary Outcome Measures, and ES (Cohen’s d) for the Waiting List and Intervention Intervals, for Descriptive Purposes.a
| T0 (n = 27) | T1 (n = 85) | T2 (n = 84) | Mean ES waiting list period (n = 27) | Mean ES intervention ieriod (n = 84) | |
|---|---|---|---|---|---|
| Primary outcome measure | |||||
| SCQ total | 89.7 (20.4) | 88.5 (21.0) | 88.0 (21.7) | 0.04 | −.02 |
| SCQ own performance | 41.2 (11.3) | 41.7 (9.8) | 42.7 (10.2) | 0.15 | .10 |
| SCQ patient | 20.4 (7.4) | 20.1 (7.0) | 19.3 (7.4) | 0.12 | −.11 |
| SCQ personal life | 27.9 (7.7) | 26.5 (9.2) | 25.8 (8.6) | -0.11 | −.07 |
| RMBPC hindrance | 12.0 (8.1) | 14.3 (9.5) | 13.7 (9.2) | 0.06 | −.05 |
| Secondary outcome measures | |||||
| GDS-15 distress | 1.5 (1.6) | 2.0 (2.2) | 2.1 (2.8) | 0.00 | .04 |
| RAND-36 well-being | 305.4 (71.5) | 299.4 (67.8) | 288.1 (78.9) | 0.07 | −.17 |
| ICQ acceptance | 18.3 (3.8) | 17.0 (4,7) | 17.1 (4.3) | 0.10 | .04 |
| ICQ helplessness | 10.0 (3.3) | 10.0 (2.9) | 10.2 (3.2) | -0.21 | .06 |
| RMBPC frequency | 22.0 (8.8) | 24.3 (9.9) | 26.1 (10.5) | 0.00 | .18 |
| IQCODE | 62.7 (7.4) | 62.4 (7.4) | 63.0 (8.7) | -0.07 | .06 |
Abbreviations: SCQ, Sense of Competence Questionnaire; RMBPC, Revised Memory and Behavioral Problems Checklist ; GDS-15, Geriatric Depression Scale; ICQ, Illness Cognition Questionnaire; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; ES: effect sizes.
a Linear mixed model analyses did neither show a significant intervention effect on the primary outcome measure (F(1, 36.4) = 0.30, P = 0.59), nor on any of the secondary outcome measures (F values <1.79).
Qualitative Results
The information saturation point for the qualitative analyses was reached after the program evaluations of 70 significant others had been coded and refined. Their sociodemographic characteristics and those of their partners with MCI were similar to the data recorded for the full study sample (mean age 68.8, standard deviation [SD] = 6.8; mean educational level 4.8, SD = 1.0; 61.4% women; 90% lived together as partners; patients’ mean MMSE was 25.3, SD = 2.8).
Analyses of the written notes of the 10th session of 12 intervention groups resulted in 355 quotations from 70 care partners. These were transformed into 33 codes and 17 final categories. Table 3 lists the final 6 common themes: Valuation of the program, Knowledge, Insight, Acceptance, Coping, and Emotional changes. We have added the 17 categories and some representative quotations to elucidate the results of each category.
Table 3.
Overview of Themes and Subthemes in the Qualitative Analysis.
| Themes and categories | Quotations |
|---|---|
| 1. Valuation of the program | |
| 1a: Emotional aspects | “My partner enjoyed the sessions.” “Some sessions were very emotional.” |
| 1b: Social aspects | “I cherished the sympathy the other group members expressed.” |
| 1c: Quality of intervention | “I appreciated the written background information.” “I will miss the group because my partner’s condition will progress and I will need similar support then too.” |
| 2. Knowledge | |
| 2a: MCI and memory | “My understanding of how memory works has increased.” “I still have questions about the precise type of MCI my husband is suffering from.” |
| 2b: Memory-enhancing techniques | “We cannot solve the memory problem itself, but you can make use of memory aids.” |
| 2c: Dealing with own negative responses | “I’ve learned the importance of talking with other people about it.” |
| 2d: Dealing with relational changes | “I’ve learned a lot from the session, about disagreements and social conflicts.” |
| 3. Insight | |
| 3a: Coping with self-relevant implications | “I’m now better aware of the coping process we’re both going through, of which the shifts in denial and defiance and acceptance are part.” “I realize that my husband and I should keep up our leisure activities. Until now we had become too passive in this respect.” |
| 3b: Problems due to MCI-related changes | “I became aware that my wife has difficulties managing some situations. I now think differently about this.” |
| 3c: Keeping alert with respect to changes in memory functioning | “I intend to be more alert that my partner does not become too passive. I will not take over her tasks too quickly but will assist my wife in performing them herself.” |
| 3d: Keeping alert with respect to negative consequences to the self | “I’ll try not to get worried too much about the daily hazards.” “I’ll try to deal with my growing sense of loneliness.” |
| 3e: Keeping alert with respect to relational changes | “I intend to spend one-on-one time with my partner.” “I have to check my criticizing attitude.” “I have to mind not to be devaluing when supporting my partner.” |
| 4. Acceptance | |
| 4a: MCI | “Thanks to the therapy I’ve become more accepting of my wife’s MCI.” “I hope my wife will recover from her memory problems.” |
| 4b: Relational changes | “We can’t discuss it in the way I would like to do, but I’m now more accepting because my husband used to be like this before.” |
| 5. Coping | |
| 5a: Instrumental coping - Applying memory strategies | “I repeatedly refer him to our agenda when he asks me about our appointments.” |
| 5b: Emotional coping | “I feel less guilty now of leaving him on his own when I’m feeling stressed. I know this is a good way to deal with stress” “I’m talking about our situation with others, even though my partner doesn’t want me to do so. It isn’t easy, but it helps me a lot.” |
| 5c: Coping with relational changes | “My partner has changed in that he is more open towards me. The memory problems are a common theme of our talks now.” “I’ve learned to agree with my partner even when I know he’s wrong, but only with unimportant subjects.” “We still have difficulties discussing the problems together.” |
| 6. Emotional changes | “I feel more relaxed and confident now” “I now have more faith in that what I do is right.” “I now am less often annoyed when my husband has forgotten something.” |
Abbreviation: MCI, mild cognitive impairment.
Valuation of the Program
The significant others described positive emotional experiences only. Some sessions had evoked sad emotions, but the beneficial aspect of sharing these feelings was emphasized. The social gains derived from the intervention were expressed as relief from sharing worries and experiences, knowing not to be the only one to feel irritated, and the compassion conveyed by their peers. Also, the comments on the methods employed by the therapists were all positive. The background information and instructions handed out to help the participants prepare for the next session at home or to read over afterward was much appreciated. The views on the number of sessions were diverse, some were positive because they now felt sufficiently equipped to cope better on their own, while many expressed the wish for this support group to be continued given that their partner’s condition would deteriorate, creating new problems.
Knowledge
The significant others described knowledge gains in 4 categories: they were now familiar with MCI as a diagnostic entity and the function of memory had learned techniques to help optimize their partner’s memory functions as well as ways to deal with their own negative reactions and present and future relational changes. These categories largely corresponded to the main program themes. Some significant others reported that the information had generated new questions that added to the uncertainty. We also detected that some had occasionally misinterpreted the information provided. For instance, one participant harbored the thought that MCI was not the same as dementia, on the basis of which he had concluded that his partner’s problems would not progress.
Insight
The care partners reported an increased insight into several domains; they had gained more insight into their own coping and grieving process, into the coping and the emotional responses of their partners with MCI, into their present and future situation, and had gained awareness of the problems that could emerge in the future. They were prepared for problematic changes in their own or their partner’s functioning and for the consequences of their relationship.
Acceptance
Acceptance of MCI-associated changes was often mentioned to have increased, although some reported that the process of acceptance had only just begun. Still, some of the evaluations revealed a lack of acceptance. These participants were, for instance, still looking for ways to cure the memory problems. It was also reported that gains in acceptance in partners with MCI had led to less refusal of assistance from others. Care partners indicated that their acceptance had increased due to the realization that they could not change all problems.
Coping
The significant others’ coping skills had been augmented. Instrumental coping had improved in that they now applied memory-enhancing strategies; they encouraged their partners with MCI to apply these to enhance their memory capacities and reminded them to keep using them. They made use of practical memory aids ranging from calendars to satellite navigation systems (global positioning system). Emotional coping had likewise changed; most significant others reported to have learned to deal with their own negative reactions and with relational changes better. They explained how they had changed their behavior after having reframed beliefs and thoughts. One care partner, for instance, was now sharing her problems with others despite her husband’s explicit wish not to do so. She derived support from sharing her worries and had concluded that she did not harm her husband by doing so, a thought that prevented her from doing so earlier. Finally many concluded that since the intervention, their partners with MCI had also become more open in discussing their memory problems.
Emotional Changes
The significant others described a sense of relief, felt more comfortable in social contexts, and expressed an overall improved sense of relaxation and inner calm. The absence of any mention of mounting stress or negative emotions was most striking.
Discussion
This is the first controlled study investigating the efficacy of a multicomponent group therapy for individuals with MCI and their significant others, focusing on the changes in the significant others. In contrast to our previously reported positive results on acceptance in the patients with MCI themselves, 19 statistically significant beneficial effects of the intervention were neither found on sense of competence, our primary outcome measure, nor on the secondary measures of well-being, distress, and illness cognitions and alertness to memory changes. In a previous uncontrolled pilot study, we reported an increase in alertness to memory changes in the care partners, 18 but the current controlled design could not replicate this finding. It is unlikely that the sample size of 84 was too small to show a statistically significant effect, as a power analysis in the pilot study estimated the optimal sample size at 70. Moreover, a recent study comparing the effects of a group intervention in patients with (early) dementia and their care partners on quality of life (96 dyads) to a waiting list condition (46 dyads) also did not establish significant posttreatment changes in the significant others. 39 In contrast to the outcomes of the quantitative analyses, the qualitative data suggest lowered stress levels, based on the augmented levels of acceptance and alertness to MCI-related problems, while also gains in knowledge, insight, and coping skills were reported
We will discuss several explanations for this discrepancy. First, an explanation may be found in the dual-process theory on coping with loss. 40 As all dyads had received the MCI diagnosis no longer than 16 weeks prior to start of the intervention, all can be assumed to be in the early phase of adaptation. In this stage, partners have to first appraise the stressor before they can formulate adaptations to it. Adaptations are categorized as loss-oriented or restoration-oriented adaptation tasks, with increased levels of distress and anxiety accompanying the early stages of appraisal and adaptation. The dual-process theory proposes that adaptation to a chronic or life-threatening illness is characterized by oscillations between 2 domains, that is, addressing emotional issues within the loss-oriented context and handling practical adaptations within the restoration-oriented framework. This process is characterized by a gradual interplay of accepting, denying, and trivializing. When this process is stimulated, well-being is expected to increase, but the duration of this first adaptation is unclear. The ambiguity of the MCI label 41 may prolong this early phase, and our qualitative data illustrate this early adaptation process. The care partners gave numerous examples illustrating adapted behavior and appraisals/reappraisals of stressors. Possibly, the moment of evaluation may have been too early in the disease process to reliably measure quantitative effects in sense of competence, well-being, distress, acceptance, helplessness, or alertness to memory changes.
A second factor that may explain the absence of beneficial effects on the outcome measures is the heterogeneity of the MCI concept. In our sample, all MCI subtypes were present 1 and different neurobehavioral patterns for amnestic and nonamnestic MCI have been reported. 42 Possibly, the significant others of patients with amnestic MCI may face different problems than the care partners of patients with nonamnestic MCI, although our qualitative analyses did not produce a clear pattern with respect to specific problems related to the type of MCI. Future studies on interventions for patients with MCI and their primary caregivers should include larger samples of the different MCI subtypes to facilitate more detailed subgroup analyses.
Third, the outcome measures may, in retrospect, not have been optimal for determining postintervention changes in our trial, as they may have been too generic (ie, not specific for MCI). The qualitative data provided information that is useful for selecting more appropriate outcome measures. Specifically, in their program evaluations, the significant others gave numerous examples of augmented instrumental and emotional coping skills. The categories in the coping theme reflected improved coping with the main stressors, that is, memory and relational problems and negative reactions like feeling guilty or feeling alone. These descriptions reflect a change in attitude, which has been suggested as one of the most crucial achievements for care partners, enabling them to cope with the MCI-related changes. 41 More specifically, instruments assessing coping skills such as the capacity and the willingness to take care of one’s own health, openness to respite care, and communication skills aimed at a loved one with cognitive problems may be more sensitive and appropriate outcome measures. 5 As far as we know, such a syndrome-specific coping inventory has not yet been developed for MCI. Moreover, all themes highlighted in the qualitative analyses are related to improved self-efficacy, knowledge, and interpersonal communication. Consequently, future studies should include self-efficacy scales, more sensitive stress measures as well as measures related to the knowledge about MCI or the quality of interpersonal relationships for outcome assessment.
Furthermore, we should take into account the limitations of the present study. First, ours was not a fully randomized trial; the patient–care partner dyads were pseudorandomly assigned to the waiting list condition on the basis of the pseudorandom moment they were referred for treatment. However, the criterion for successful randomization was largely met, because the treatment and control groups did not largely differ with respect to baseline outcome measures and patient characteristics. Also, the waiting period itself may have affected the intervention outcome differentially. Furthermore, the participants we enrolled (estimated at approximately 50% of all MCI couples diagnosed in the four participating memory clinics) represent only a subgroup of all newly diagnosed patients with MCI and their significant others. We could not directly compare our sample with the dyads that were not enrolled, but the reasons for not participating were diverse. 12 It is possible that our sample predominantly included highly motivated people, more open to support, with adequate coping skills. Finally, a limitation of the qualitative study is that the written notes were taken in the group sessions and not in individual interviews. This may have promoted socially desirable responses, as some therapists were also involved in the study. Also, the evaluation sessions were not audio recorded, making it not possible to use verbatim transcripts of the evaluation. However, it should be stressed that note-taking during the sessions was done by trained graduate psychology students who did not act as a therapist in the group session.
In conclusion, our psychotherapeutic intervention for individuals with MCI and their primary care partners is feasible but has only limited beneficial effects in some–but not all–significant others. Although no statistically significant effects on outcome variables were found after the intervention relative to the waiting list control condition, qualitative analyses suggest that the program facilitated the learning process of the significant others, enabling them to take better care of their partner with MCI or loved one by helping them to change their insight into and attitude toward the MCI-related problems and discover new ways to cope with these. In the present sample of significant others who experienced relatively low levels of distress, the group intervention aimed at improving the significant others’ sense of competence of patients with MCI has not been proven effective. Program adjustments and alternative MCI-specific outcome measures should be studied, especially in care partners with higher levels of distress. This intervention may be a valuable addition to other interventions aimed at care partners of MCI or early-dementia patients.
Acknowledgments
The authors thank Floor Kraaimaat and Jan Pieter Teunisse for their initial contribution to the study design and development of the program, Sanne van den Berg, Myrthe Dekkers, Ilja Klabbers, and Ria te Winkel for collecting the study data, the psychotherapists Caroline Geleijns, Klaas Jansma, Frank van Raak, and Nelleke van Schuylen-borgh-van Es for their assistance in the trial, and Rogier Donders for his contribution to the statistical analyses.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported in part by grants from Alzheimer Nederland, Innovatiefonds Zorgverzekeraars (RVVZ), and Stichting RCOAK. Trial registration number: NCT00285753 (Clinical Trials.gov).
References
- 1. Petersen RC. Mild cognitive impairment as diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 2. Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129–140. [DOI] [PubMed] [Google Scholar]
- 3. Joosten-Weyn Banningh L, Vernooij-Dassen M, Olde Rikkert MGM, Teunisse JP. Mild cognitive impairment: coping with an uncertain label. Int J Geriatr Psychiatry. 2008;23(2):148–154. [DOI] [PubMed] [Google Scholar]
- 4. Wegierek AM. Taking care of a loved one who has Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27(7);463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blieszner R, Roberto KA, Wilcox KL, Barham EJ, Winston BL. Dimensions of ambigious loss in couples coping with mild cognitive impairment. Fam Relat. 2007;56:196–209. [Google Scholar]
- 6. Bruce JM, McQuiggan M, Williams V, Westervelt H, Tremont G. Burden among spousal and child caregivers of patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25(4):385–390. [DOI] [PubMed] [Google Scholar]
- 7. Garand L, Dew MA, Eazor LR, Dekosky ST, Reynolds CF, 3rd. Caregiving burden and psychiatric morbidity in spouses of persons with mild cognitive impairment. Int J Geriatr Psychiatry. 2005;20(6):512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryan KA, Weldon A, Huby NM, et al. Caregiver support service needs for patients with mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies HD, Newkirk LA, Pitts CB, et al. The impact of dementia and mild memory impairment (MMI) on intimacy and sexuality in spousal relationships. Int Psychogeriatr. 2010;22(4):618–628. [DOI] [PubMed] [Google Scholar]
- 10. McIlvane JM, Popa MA, Robinson B, Houseweart K, Haley WE. Perceptions of illness, coping, and well-being in persons with mild cognitive impairment and their care partners. Alzheimer Dis Assoc Disord. 2008;22(3):284–292. [DOI] [PubMed] [Google Scholar]
- 11. Ryan KA, Weldon A, Huby NM, et al. Caregiver support service needs for patients with mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev. 2013;12(1):263–275. [DOI] [PubMed] [Google Scholar]
- 13. Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev. 2012;36(4):1163–1178. [DOI] [PubMed] [Google Scholar]
- 14. Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18(4):281–296. [DOI] [PubMed] [Google Scholar]
- 15. Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatr. 2011;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acton GJ, Kang J. Interventions to reduce the burden of caregiving for an adult with dementia: a meta-analysis. Res Nurs Health. 2001;24(5):349–360. [DOI] [PubMed] [Google Scholar]
- 17. Brodaty H, Green A, Ko A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc. 2003;51(5):657–664. [DOI] [PubMed] [Google Scholar]
- 18. Joosten-Weyn Banningh LW, Kessels RP, Olde Rikkert MG, Geleijns-Lanting CE, Kraaimaat FW. A cognitive behavioural group therapy for patients diagnosed with mild cognitive impairment and their significant others: feasibility and preliminary results. Clin Rehabil. 2008;22(8):731–740. [DOI] [PubMed] [Google Scholar]
- 19. Joosten-Weyn Banningh LW, Prins JB, Vernooij-Dassen MJ, Wijnen HH, Olde Rikkert MG, Kessels RP. Group therapy for patients with mild cognitive impairment and their significant others: results of a waiting-list controlled trial. Gerontology. 2011;57(5):444–454. [DOI] [PubMed] [Google Scholar]
- 20. O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. Br Med J. 2010;341:c4587. [DOI] [PubMed] [Google Scholar]
- 21. Kempen GI, Brilman EI, Ormel J. The Mini Mental Status examination: normative data and a comparison of a 12-item and 20-item version in a sample survey of community-based elderly [in Dutch]. Tijdschr Gerontol Geriatr. 1995;26(4):163–172. [PubMed] [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 23. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290–302. [DOI] [PubMed] [Google Scholar]
- 24. Scheurs PJG, Van de Willige G, Brosschot JF, Van Tellegen B, Graus GMH. Manual of the Utrecht Coping List [In Dutch]. Lisse, The Netherlands: Swets & Zeitlinger; 1993. [Google Scholar]
- 25. Van Eijk LM, Kempen GI, van Sonderen FL. A short scale to measure social support in the elderly: the SSL12-I [in Dutch]. Tijdschr Gerontol Geriatr 1994;25(5):192–196. [PubMed] [Google Scholar]
- 26. Arrindell W. On the psychometric properties of the Maudsley Marital Questionnaire (MMQ): evaluation of self-ratings in distressed and ‘normal’ volunteer couples based on the Dutch version. Pers Individ Dif. 1983;4:293–306. [Google Scholar]
- 27. Joosten L, Van den Berg S, Teunisse J.P. Help Me Reminding: A Guide for People With Mild Memory Problems and Their Loved Ones [in Dutch]. Houten, the Netherlands: Bohn Stafleu van Loghum; 2008. [Google Scholar]
- 28. Jansen AP, Van Hout HP, Van Marwijk HW, et al. Sense of competence questionnaire among informal caregivers of older adults with dementia symptoms: a psychometric evaluation. Clin Pract Epidemiol Ment Health. 2007;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van de Rest O, van der Zwaluw N, Beekman AT, de Groot LC, Geleijnse JM. The reliability of three depression rating scales in a general population of Dutch older persons. Int J Geriatr Psychiatry. 2010;25(10):998–1005. [DOI] [PubMed] [Google Scholar]
- 30. Herrman N, Mittmann N, Silver IL, et al. A validation study of the geriatric depression scale short form. Int J Ger Psychiatry. 1996;11:457–460. [Google Scholar]
- 31. Van der Zee KI, Sanderman R. Measuring the general health status with the RAND-36: a manual [in Dutch]. Groningen, The Netherlands: Noordelijk Centrum voor Gezondheidsvraagstukken;1993. [Google Scholar]
- 32. Evers AW, Kraaimaat FW, Van Lankveld W, Jongen PH, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: Illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. 2001;69(6):1026–1036. [PubMed] [Google Scholar]
- 33. De Jonghe JF, Schmand B, Ooms ME, Ribbe MW. Abbreviated form of the Informant Questionnaire on COgnitive Decline in the Elderly [in Dutch]. Tijschr Gerontol Geriatrie. 1997;28(5):224–229. [PubMed] [Google Scholar]
- 34. Dekkers M, Joosten-Weyn Banningh EW, Eling PA. (2009). Awareness in patients with mild cognitive impairment (MCI) [in Dutch]. Tijdschr Gerontol Geriatr. 2009;40(1):17–23. [DOI] [PubMed] [Google Scholar]
- 35. Teunisse S, de Haan R, Walstra GJM, de Rooij SEJA, Zwart M. Behavioural Problems in Mild Dementia: Clinical Relevance and Methodological Evaluation of the Revised Memory and Behavioral Problems Checklist. In: Teunisse S. Clinimetrics in Dementia [PhD thesis], University of Amsterdam, 1997. [Google Scholar]
- 36. Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: a revised memory and behavior problems checklist. Psychol Aging. 1992;7(4):622–631. [DOI] [PubMed] [Google Scholar]
- 37. Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 3rd ed. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- 38. Smith JA. Reflecting on the development of interpretative phenomenological analysis and its contribution to qualitative research in psychology. Qual Res Psychol. 2004;1:39–54. [Google Scholar]
- 39. Logsdon RG, Pike KC, McCurry SM, Hunter P, Maher J, Snyder L, Teri L. (2010). Early-stage memory loss support groups: outcomes from a randomized controlled clinical trial. J Gerontol B Psychol Sci Soc Sci. 2010;65(6):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stroebe M, Schut H. The dual process model of coping with bereavement: a decade on. Omega. 2010;61(4):273–289. [DOI] [PubMed] [Google Scholar]
- 41. Blieszner R, Roberto KA. Care partner responses to the onset of mild cognitive impairment. Gerontologist. 2010;50(1):11–22. [DOI] [PubMed] [Google Scholar]
- 42. Rozzini L, Vicini Chilovi B, et al. Neuropsychiatric symptoms in amnestic and nonamnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25(1):32–36. [DOI] [PubMed] [Google Scholar]