Abstract
Retinal nerve fiber layer (RNFL) thickness, ganglion cell layer (GCL) thickness, and macular volume (MV) utilizing spectral domain optical coherence tomography (SD-OCT) were compared among patients with Alzheimer’s disease (AD) dementia, non-Alzheimer’s disease (non-AD) dementia, amnestic mild cognitive impairment (aMCI), Parkinson’s disease (PD), and age- and sex-matched controls in a cross-sectional cohort study. A total of 116 participants were diagnosed and evaluated (21 AD, 20 aMCI, 20 non-AD, 20 PD, and 34 controls) after comprehensive neurological, neuropsychology, and magnetic resonance imaging (MRI) volumetric evaluations. Retinal nerve fiber layer thickness, GCL thickness, and MV were measured. Analysis of variance models were used to compare groups on MRI volumetric measures, cognitive test results, and SD-OCT measures. Associations between SD-OCT measures and other measures were performed using mixed-effect models. Spectral domain optical coherence tomography analysis of retinal markers, including RNFL thickness, GCL thickness, and MV, did not differ between amnestic MCI, AD dementia, PD, non-AD, dementia, and age- and sex-matched controls in a well-characterized patient cohort.
Keywords: optical coherence tomography, retinal nerve fiber thickness, Alzheimer’s disease, dementia, mild cognitive impairment, non-Alzheimer’s dementia, Parkinson’s disease
Introduction
Alzheimer’s disease (AD) is the most common form of dementia worldwide. A major challenge in the field of AD includes finding reliable and cost-effective biomarkers for the diagnosis of AD and the assessment of treatment response for this progressive disease. The current National Institute of Aging/Alzheimer Association (NIA/AA) guidelines for the diagnosis of AD include a combination of clinical history, cognitive tests, and biomarkers. 1 There is still an unmet need for a sensitive and specific biomarker test that can be administered in a physician’s office to aid in early clinical diagnosis of AD. Retinal changes have been reported as a promising biomarker in a number of neurodegenerative diseases. 2,3
Histopathological studies have suggested possible loss of retinal nerve fiber layer (RNFL) thickness in AD from depletion of retinal ganglion cells and optic nerve axons as identified in multiple studies, 4 –6 although some dispute this conclusion. 7,8 Clinical studies of retinal function in AD have demonstrated mixed results. Some studies have noted abnormalities in retinal layer electrophysiology, RNFL thinning, and optic nerve head thinning, 9 –22 whereas other studies have not found supporting evidence that AD and retinal dysfunction are linked. 23,24 The exact etiologies of these proposed retinal changes are still controversial. 2,15,25 The differences in the results have been attributed to differing experimental techniques, varying stages of AD, and small study populations.
The specificity of these findings is also unknown, as retinal changes have also been found in mild cognitive impairment (MCI) 15 –18 and other neurodegenerative conditions including Parkinson’s disease (PD) 26 –28 and dementia with Lewy bodies. 29 Previous optical coherence tomography (OCT) studies (that included both time-domain and spectral domain optical coherence tomography [SD-OCT]) did not compare RNFL thickness across neurodegenerative diseases in a single study to evaluate whether changes reported were specific to AD. Detailed neurocognitive testing was also lacking, as screening cognitive tests, especially the Mini-Mental State Examination, were used to characterize AD and for correlations between clinical severity of AD and RNFL thickness. It is also unclear how retinal changes compare with the current generation of clinically useful MRI biomarkers.
The aim of the present study was to compare retinal changes across multiple neurodegenerative diseases (AD, non-AD, and PD) and different severity of cognitive impairment (normal cognition, MCI, and dementia) utilizing SD-OCT. We hypothesized that participants with AD would have thinner RNFL measures than non-AD, PD, and age- and sex-matched controls, and thinner RNFL will correlate with higher severity of cognitive impairment.
Methods
This was a cross-sectional cohort study approved by the Cleveland Clinic Institutional Review Board. A total of 116 participants evaluated at the Cleveland Clinic Lou Ruvo Center for Brain Health or Center for Neurological Restoration between October 2012 and August 2014 were included in the study. These included participants diagnosed with AD, amnestic MCI, non-AD dementia, or PD and participants with normal cognition who were age- and sex-matched controls. All participants were 50 years or older and were able to consent for participating in the study. Only patients with English proficiency were included in the study. Study participants underwent screening for a history of ophthalmic disease by a clinician. Each participant was screened for uncontrolled diabetes, hypertension, and related vision complaints. Participants with any ophthalmological problems likely to affect retinal layer thickness (glaucoma, macular degeneration, diabetic retinopathy, retinal tears, and vision loss) in their medical history were excluded.
Clinical Diagnostics and Evaluation
The diagnoses of AD (probable AD with evidence of the AD pathophysiological process; evidence of MRI hippocampal and medial temporal atrophy as a biomarker for downstream neuronal degeneration), non-AD dementia (dementia unlikely due to AD), amnestic MCI (MCI due to AD—intermediate likelihood), and normal cognition were established at consensus multidisciplinary case conferences using the NIA/AA-2011 diagnostic criteria. 30,31 The diagnosis of PD was made by an experienced neurologist specializing in movement disorders (H.F.) if participant fulfilled the UK Brain Bank Criteria for PD. 32 Patients with PD completed the motor examination section, part III, of Unified Parkinson’s Disease Rating Scale (UPDRS) in the medication-off state. Patients with PD were within 2 to 5 years of clinical diagnosis. All participants with cognitive impairment completed a neurological evaluation, detailed neurocognitive testing, MRI of the brain, and blood tests to rule out common reversible causes of dementia. Participants with normal cognition also underwent a neurological evaluation, detailed neurocognitive testing, and MRI of the brain.
Neurocognitive Assessment
As part of neurocognitive testing, study participants completed the Montreal Cognitive Assessment (MoCA), 33 Logical Memory subtest of the Wechsler Memory Scale—Fourth Edition (WMS-IV), 34 Hopkins Verbal Learning Test–Revised (HVLT-R), 35 phonemic and semantic verbal fluency, 36 and Trail Making Test (parts A and B). 37
Magnetic Resonance Imaging Volumetric Assessment
Volumetric data from MRI of the brain provided detailed regional volumetric evaluation of the hippocampus and whole brain corrected for intracranial volume, using a standard Alzheimer’s Disease Neuroimaging Initiative sequence featuring a T1-weighted volumetric acquisition (at either 1.5 T or 3 T on Siemens scanners), followed by MRI postprocessing using NeuroQuant software (Cortech Labs Inc, La Jolla, California).
Ophthalmic Imaging Assessment
All participants underwent optic nerve head and macular cube scan using the Cirrus 4000 HD-OCT (Zeiss, Oberkochen, Germany). Spectral domain optical coherence tomography scans were performed by an experienced technician. All scans were reviewed for image quality and analysis artifacts by a blinded physician. Cross-sectional RNFL thickness, ganglion cell layer (GCL) thickness, and macular volume (MV) were measured utilizing the standard analysis algorithms in the Cirrus reader software.
Statistical Analysis
Case–control analysis was undertaken with the RNFL thickness, GCL thickness, and MV collected with age- and sex frequency-matched controls. Approximately 20 participants for each group were chosen based on previous studies in AD and PD where the RNFL thickness measures within each subject group were normally distributed with standard deviations (SDs) from 3 15 to 15 μm. 28 If the true difference in the experimental and control means (with the experimental group having thinner RNFL) is approximately 12 μm with a SD of 15 μm, 2,28 20 participants are needed in each disease group compared to 20 controls to be able to reject the null hypothesis (α = 0.05) that the population means of the experimental and control groups are equal with probability (power) 0.80. Categorical patient characteristics were summarized using frequencies and percentages, whereas continuous measures were described using means and SDs or means and standard errors when repeated-measure methods were used. Normality of the measures was assessed graphically. Analysis of variance models with post hoc Bonferroni multiple comparison procedures were used to compare groups on MRI measures, cognitive test results, and OCT measures by eye, OCT averages across eyes, and OCT differences between eyes. Associations between OCT measures and other measures were performed using mixed-effect models. Analyses were performed using SAS software (version 9.2; Cary, North Carolina [SAS Institute Inc]). An overall significance level of 0.50 was assumed for all comparisons.
Results
Participants
Overall, 116 participants were enrolled in the study, including participants with AD dementia (n = 21), amnestic MCI (n = 21), non-AD dementia (n = 20), PD (n = 20), and 34 age-/sex-matched controls by the frequency matching sampling design. The participants were comparable across all groups on age and sex. Patients with PD were significantly more educated than the patients with MCI (16.4 vs 13.9 years, P = 0.005), with no other significant differences observed across other groups. The mean UPDRS motor examination score when off medications was 23 (SD: 10.3) for the PD group (Table 1).
Table 1.
Demographics, MoCA Cognitive, and UPDRS Motor Scores.a
| Demographics | AD Dementia (n = 21) | Amnestic MCI (n = 21) | Non-AD Dementia (n = 20) | PD (n = 20) | Normal Cognition (n = 34) |
|---|---|---|---|---|---|
| Age | 65.8 ± 11.1 | 68.2 ± 6.7 | 68.7 ± 8.4 | 62.6 ± 9.5 | 65.1 ± 8.3 |
| Education years | 14.9 ± 2.7 | 13.9 ± 2.5b | 14.4 ± 2.5 | 16.4 ± 2.8c | 15.3 ± 2.7 |
| Female % | 62 | 57 | 45 | 45 | 59 |
| MoCA cognitive score | 16.0 ± 5.4b,c,d | 21.2 ± 3.4b,d,e,f | 15.0 ± 6.3b,c,d | 25.8 ± 2.8c,e,f | 26.6 ± 2.4c,e,f |
| UPDRS motor score | 23 (10.6) |
Abbreviations: AD, Alzheimer’s disease; ANOVA, analysis of variance; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
a Values are presented as mean ± SD. P < .05, ANOVA.
b Significantly different from PD.
c Significantly different from MCI.
d Significantly different from normal.
e Significantly different from non-AD.
f Significantly different from AD.
Neurocognitive Results
The mean MoCA cognitive scores for the groups are noted in Table 1, and the detailed neuropsychology scores and statistical differences between the groups are presented in Supplementary Table 1. As expected, the participants with AD and non-AD dementia performed significantly worse on all cognitive measures in comparison to the controls. Similarly, the MCI group had significantly worse performance than the controls on all measures except for trails B and phonemic fluency. The cases with PD performed similarly to controls on all cognitive tests except for trails A.
Magnetic Resonance Imaging Results
On MRI volumetric measures, the amnestic MCI and AD dementia groups had significantly lower hippocampal volumes (corrected for intracranial volume) compared to participants with normal cognition, whereas PD and non-AD dementia had no significant difference in hippocampal volumes (corrected for intracranial volume) compared to the normal cognition group. Total brain volume (corrected for intracranial volume) was significantly lower among AD and non-AD dementia group compared to the PD group and those with normal cognition. Detailed MRI volumetric measures are presented in Supplementary Table 2.
Table 2.
Retinal Measures by Analysis Group.a
| Retinal Measures | AD Dementia (n = 21) | Amnestic MCI (n = 21) | Non-AD Dementia (n = 20) | PD (n = 20) | Normal Cognition (n = 34) | P |
|---|---|---|---|---|---|---|
| Macular cube volume | 9.9 (0.1) | 9.9 (0.1) | 9.9 (0.1) | 9.9 (0.1) | 9.8 (0.1) | 0.89 |
| Macular cube GCL + IPL thickness | 75.7 (1.7) | 78.6 (1.8) | 76.5 (1.8) | 77.5 (1.8) | 73.5 (1.4) | 0.17 |
| Rim area | 1.3 (0.1) | 1.3 (0.1) | 1.3 (0.1) | 1.4 (0.1) | 1.2 (0.0) | 0.26 |
| Disc area | 1.7 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.9 (0.1) | 1.6 (0.1) | 0.11 |
| Average CD ratio | 0.5 (0.0) | 0.5 (0.0) | 0.5 (0.0) | 0.5 (0.0) | 0.4 (0.0) | 0.95 |
| Vertical CD ratio | 0.5 (0.0) | 0.5 (0.0) | 0.5 (0.0) | 0.5 (0.0) | 0.4 (0.0) | 0.99 |
| Cup volume | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | 0.66 |
| RNFL thickness | 88.9 (2.1) | 89.9 (2.1) | 89.9 (2.1) | 88.5 (2.1) | 85.3 (1.6) | 0.35 |
| RNFL quadrant S | 107.2 (3.3) | 109.3 (3.2) | 111.9 (3.4) | 110.1 (3.4) | 106.0 (2.6) | 0.66 |
| RNFL quadrant N | 72.5 (2.5) | 69.8 (2.5) | 73.8 (2.6) | 72.9 (2.5) | 66.9 (2.0) | 0.16 |
| RNFL quadrant I | 114.4 (3.6) | 117.0 (3.6) | 114.3 (3.7) | 111.9 (3.7) | 108.7 (2.8) | 0.44 |
| RNFL quadrant T | 61.8 (2.5) | 63.4 (2.5) | 59.5 (2.6) | 59.2 (2.6) | 59.1 (2.0) | 0.66 |
Abbreviations: AD, Alzheimer’s disease; ANOVA, analysis of variance; CD, cup–disc ratio; GCL, ganglion cell layer; IPL, inner plexiform layer; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale; RNFL, retinal nerve fiber layer; SEM, standard error of the mean.
a Values are presented as mean (SEM). P < .05: mixed effect model adjusting for age and sex. Mean of both eyes for each participant. A significance level of .005 was used for pairwise ad hoc comparisons.
Ophthalmic Imaging Results
Among SD-OCT measures, the RNFL, GCL, and MV were not significantly different across all groups. Using all SD-OCT measures in a mixed-effect model did not identify any significant differences between the groups. The RNFL thickness measures analysis by group and quadrant also did not show any statistically significant difference between the groups (Table 2 and Figures 1 and 2).
Figure 1.
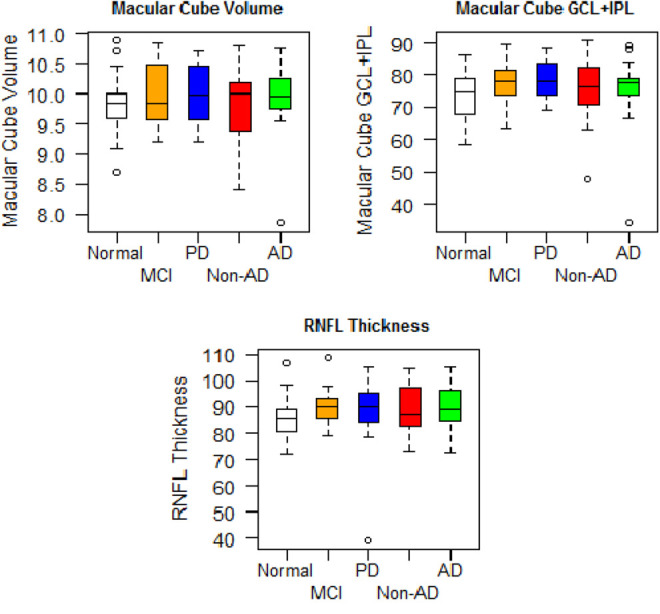
Retinal measures by group mean and standard deviation (SD).
Figure 2.
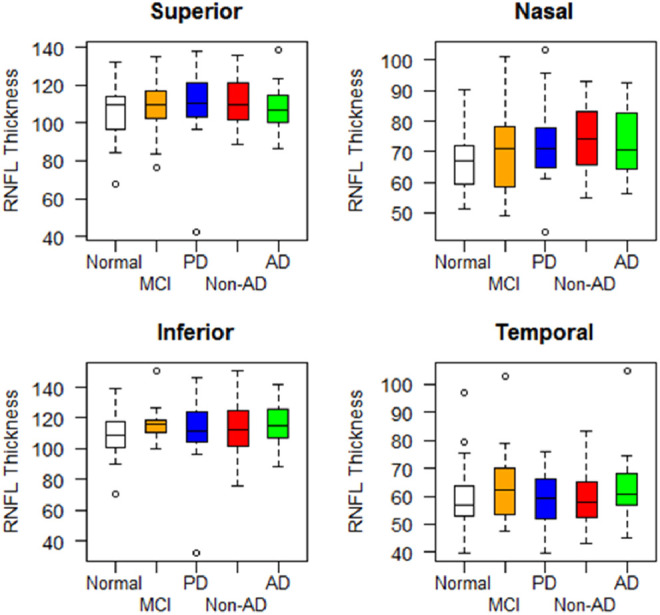
Retinal nerve fiber layer (RNFL) thickness by group and retinal quadrant, mean and standard deviation (SD).
Discussion
Measurement of RNFL thickness using OCT has been generating significant interest, with many studies having reported a significant decrease in the mean overall RNFL thickness in patients with AD. 2 Retinal nerve fiber layer thickness appeared to have a potential utility in the diagnosis of AD. 2,3 Although our study had very well-characterized patients with detailed neurocognitive testing and MRI volumetric analysis that could readily differentiate between participants with normal cognition from MCI and AD, we did not find differences on any of the retinal measures between any of our subject groups as initially hypothesized. No correlation between RNFL thinning and severity of cognitive impairment was noted as initially hypothesized. We found significant overlap between the PD, MCI, AD, non-AD, and age- and sex-matched normal groups in their RNFL thickness, MV, and GCL thickness. It is possible that RNFL changes are noted in more severe stages of dementia than evaluated in our study, which could potentially explain the discrepancy between the histological evaluations reported at autopsy and our clinical study. It has been our experience that patients with MoCA <10 very often could not follow instructions and complete an OCT evaluation successfully, making OCT testing unreliable in this population. Furthermore, if RNFL thickness changes are noted only in the late stages of dementia, its role as a clinical biomarker in differentiating AD from other etiologies at earlier stages of the disease is limited. It is possible that the mean difference in RNFL thickness between AD and normal control groups is smaller than 12 μm for which the study was designed, in which case a larger sample size might be needed to delineate group differences. As MRI and neurocognitive measures noted significant group differences even between the MCI and normal control groups in our sample, the clinical advantage of RNFL thickness measurement compared to clinically useful current biomarkers is not clear.
Our study has some advantages over previous studies. All participants met NIA/AA criteria for MCI due to AD and AD dementia. 30,31 The neurocognitive profiles noted on neuropsychological testing and MRI measures of hippocampal volumes were consistent with the diagnoses of AD and aMCI. Standard multidisciplinary consensus was performed to rule out reversible dementia causes and provide clinically appropriate diagnosis in all cases. Unlike prior studies, participants with normal cognition and PD also underwent detailed neurocognitive testing and MRI volumetric scans to enable accurate clinical characterization. Additionally, unlike most previous studies, the current study had a well-characterized non-AD dementia group (frontotemporal dementia, dementia with Lewy bodies, vascular dementia, corticobasal degeneration, and mixed dementia) in addition to the PD group to distinguish changes specific to AD as a neurodegenerative disorder.
Among most prior studies, estimates of differences were potentially limited by the small size of the studies. Our analysis was powered to differentiate RNFL group differences within 15 μm SD from the mean. The SD of RNFL thickness measures within most quadrants for normal cognition, MCI, and AD groups and mean RNFL thickness for all groups (PD right eye being the exception at 15.5 SD) were powered to reject the null hypothesis at 0.80 probability. Additionally, improving the power of the analysis on increasing the group sample size of possible AD etiology to n = 42 by combining the aMCI and AD dementia groups did not change results nor did a post hoc analysis using 1 randomly chosen eye per patient. The SD-OCT used in this study is a current generation system with a speed of image acquisition around 27 000 axial scans/second. The volumetric data obtained with these SD-OCT systems and the improved resolution must be considered when comparing the results to previous studies utilizing time-domain OCT systems. Longitudinal data on RNFL thinning with normal aging obtained with SD-OCT 38 note significant variance in RNFL measures. 38 Furthermore, the values identified in the above-mentioned longitudinal study were consistent with the data obtained in this current study. 38 It is also possible that the discrepancy between multiple prior studies and the current results could be due to a bias in the literature against reporting a negative finding of lack of RNFL thickness difference between the groups.
The current study has a few limitations. Although all participants met NIA/AA criteria (probable AD dementia with evidence of the AD pathophysiological process, dementia unlikely due to AD, and MCI due to AD, intermediate likelihood), no amyloid markers (cerebrospinal fluid Aβ42 or amyloid imaging) were used to increase the accuracy of the diagnosis. There could be intermixture of AD pathology among AD and non-AD dementia subtypes, and not all participants with amnestic MCI might have AD as the underlying etiology. Further, this study being cross-sectional, we could not draw conclusions regarding changes in RNFL thinning within a single individual over time. Even as no significant retinal thickness changes were noted in our population with AD and non-AD, a role for retinal amyloid imaging approaches to diagnose AD is still open and now being clinically tested as part of the Australian Imaging, Biomarkers and Lifestyle study. 39 Microvascular network alterations in the retina have also been reported as promising. 40 Additionally, this study focused only on the SD-OCT parameters that are available using current generation SD-OCT software. Additional focused retinal layer analysis may also hold promise as a screening tool including outer nuclear layer and ellipsoid zone assessment. This research utilizing a custom SD-OCT analysis algorithm is currently ongoing.
Conclusions
We found that retinal measures of RNFL thickness, GCL thickness, and MV with SD-OCT are unable to distinguish AD dementia from non-AD dementia, PD, amnestic MCI, and normal controls in a clinically well-characterized sample. These results do not support a role for RNFL and GCL thicknesses or for MV for diagnostic purposes in AD and PD.
Footnotes
Authors’ Note: J.A.P. contributed to conception, design of the study, analysis/interpretation of data, and drafting/revising the manuscript for content. R.B. contributed to design of the study, analysis/interpretation of data, and revising the manuscript for content. A.B.J. and H.F. contributed to analysis/interpretation of data and revising the manuscript for content. A.R.G. contributed to conception, interpretation of data, and revising the manuscript for content. J.B. contributed to analysis of data. J.E. and J.B.L. contributed to interpretation of data and revising the manuscript for content. S.E.J. contributed to MRI and image analysis. Statistical analysis was performed by James Bena, MS, Quantitative Health Sciences, CCF.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by RPC Grant 2012-1019. Grant support also for J.P.E.: NIH/NEI K23-EY022947-01A1; Ohio Department of Development TECH-13-059. R.B. has the following disclosures: Biogen, Novartis, Genentech, Genzyme, and Mallinckrodt and participates in clinical research funded by Biogen. J.B.L. has the following disclosures: consulting fees from Axovant, Navidea Biopharmaceutical, and Piramal Healthcare. Grant support from Genzyme/Sanofi. H.F. has the following disclosures: Medical Communications Media, Inc and Pfizer, Inc. J.P.E. has the following relevant disclosures: Bioptigen, Thrombogenics, Leica, Zeiss, and Alcon.
Supplemental Material: The online supplemental tables are available at http://aja.sagepub.com/supplemental
References
- 1. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson KL, Yeo JM, Waddell B, et al. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement Diagn Asses Dis Moni. 2015;1(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikram MK, Cheung CY, Wong TY, Chen CP. Retinal pathology as biomarker for cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2012;83(9):917–922. [DOI] [PubMed] [Google Scholar]
- 4. Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17(3):377–384. [DOI] [PubMed] [Google Scholar]
- 5. Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. 1996;17(3):385–395. [DOI] [PubMed] [Google Scholar]
- 6. Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315(8):485–487. [DOI] [PubMed] [Google Scholar]
- 7. Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann Neurol. 1993;33(3):248–257. [DOI] [PubMed] [Google Scholar]
- 8. Davies DC, McCoubrie P, McDonald B, Jobst KA. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br J Ophthalmol. 1995;79(6):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hedges TR, Perez Galves R, Speigelman D, Barbas NR, Peli E, Yardley CJ. Retinal nerve fiber layer abnormalities in Alzheimer’s disease. Acta Ophthalmol Scand. 1996;74(3):271–275. [DOI] [PubMed] [Google Scholar]
- 10. Tsai CS, Ritch R, Schwartz B, et al. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch Ophthalmol. 1991;109(2):199–204. [DOI] [PubMed] [Google Scholar]
- 11. Katz B, Rimmer S, Iragui V, Katzman R. Abnormal pattern electroretinogram in Alzheimer’s disease: evidence for retinal ganglion cell degeneration? Ann Neurol. 1989;26(2):221–225. [DOI] [PubMed] [Google Scholar]
- 12. Trick GL, Barris MC, Bickler-Bluth M. Abnormal pattern electroretinograms in patients with senile dementia of the Alzheimer’s type. Ann Neurol. 1989;26(2):226–231. [DOI] [PubMed] [Google Scholar]
- 13. Danesh-Meyer HV, Birch H, Ku JY, Carroll S, Gamble G. Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology. 2006;67(10):1852–1854. [DOI] [PubMed] [Google Scholar]
- 14. Krasodomska K, Lubiński W, Potemkowski A, Honczarenko K. Pattern electroretinogram (PERG) and pattern visual evoked potential (PVEP) in the early stages of Alzheimer’s disease. Doc Ophthalmol. 2010;121(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;420(2):97–99. [DOI] [PubMed] [Google Scholar]
- 16. Lu Y, Li Z, Zhang X, Ming B, et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett. 2010;480(1):69–72. [DOI] [PubMed] [Google Scholar]
- 17. Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113(7):523–526. [DOI] [PubMed] [Google Scholar]
- 18. Ascaso FJ, Cruz N, Modrego PJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol. 2014;261(8):1522–1530. [DOI] [PubMed] [Google Scholar]
- 19. Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48(5):2285–2289. [DOI] [PubMed] [Google Scholar]
- 20. Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113(7):523–526. [DOI] [PubMed] [Google Scholar]
- 21. Iseri PK, Altinaş O, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26(1):18–24. [DOI] [PubMed] [Google Scholar]
- 22. Kergoat H, Kergoat MJ, Justino L, Chertkow H, Robillard A, Bergman H. An evaluation of the retinal nerve fiber layer thickness by scanning laser polarimetry in individuals with dementia of the Alzheimer type. Acta Ophthalmol Scand. 2001;79(2):187–191. [DOI] [PubMed] [Google Scholar]
- 23. Justino L, Kergoat M, Bergman H, Chertkow H, Robillard A, Kergoat H. Neuroretinal function is normal in early dementia of the Alzheimer type. Neurobiol Aging. 2001;22(4):691–695. [DOI] [PubMed] [Google Scholar]
- 24. Sadun AA, Borchert M, DeVita E, Hinton DR, Bassi CJ. Assessment of visual impairments in patients with Alzheimer’s disease. Am J Ophthalmol. 1987;104(2):113–120. [DOI] [PubMed] [Google Scholar]
- 25. Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8(1):117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004;44(24):2793–2797. [DOI] [PubMed] [Google Scholar]
- 27. Bodis-Wollner I. Retinopathy in Parkinson disease. J Neural Transm. 2009;116(11):1493–1501. [DOI] [PubMed] [Google Scholar]
- 28. Yu JC, Feng IF, Xiang Y, et al. Retinal fiber nerve layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014;9(1):e85718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis. 2013;34(3):659–664. [DOI] [PubMed] [Google Scholar]
- 30. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 34. Wechsler D. Wechsler Memory Scale–Fourth Edition. San Antonio, TX: Pearson; 2009. [Google Scholar]
- 35. Benedict RHB, Schretlen DG, Groninger L, et al. Hopkins Verbal Learning Test-Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 36. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and Animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 37. Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 38. Alasil T, Wang K, Keane PA, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22(7):532–541. [DOI] [PubMed] [Google Scholar]
- 39. Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(suppl 1):s204–s217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheung CY, Ong YT, Ikram MK, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10(2):135–142. [DOI] [PubMed] [Google Scholar]


