Abstract
Objective:
The present study was designed to evaluate the effect of acetylcholinesterase inhibitor (AchEI) therapy on balance, gait, and orthostatic hypotension (OH) in elderly patients with Alzheimer’s disease (AD).
Methods:
A total of 102 elderly patients with AD have been recently diagnosed and were treated with AchEI and underwent comprehensive geriatric assessment at baseline and at the end of the sixth month.
Results:
Timed Up and Go test and Tinetti Performance-Oriented Mobility Assessment values and the prevalence of OH were not different at the end of the sixth month versus baseline (P > .05). However, it was determined that changes in balance were better in the patients who showed cognitive improvement at the end of the sixth month (P < .05).
Conclusion:
Curative effects of AchEIs, which are used in the treatment of AD, on cognitive performance are reflected also in balance functions. Moreover, it was observed that these drugs do not increase the prevalence of OH.
Keywords: Alzheimer’s disease, elderly, gait, balance, acetylcholine esterase inhibitors, orthostatic hypotension
Introduction
Falls are closely associated with hip fractures, head trauma, death and, in the long term, fear of falling, loss of autonomy, restrictions in daily activities, disability, decrease in quality of life, and admittance to the nursing home in elderly patients. 1 The risk of falls is increased at least by 2 times in the elderly patients with dementia as compared to the elderly patients with normal cognitive functions, and the annual incidence of falls is considered to be 60% to 80%. 2 It is known that the risk increases just beginning from the prodromal stages of Alzheimer’s disease (AD). 3 Cognitive deficit in elderly patients with AD impairs balance and gait functions by influencing executive functions, attention, and visuospatial perception and enhances the risk of falls. 4 -6 Another cause of falls in elderly patients is orthostatic hypotension (OH) 7 with a prevalence rate of 27.5%. 8 Although there is no large-scale study investigating the prevalence of OH in patients with AD, it is known that OH is more prevalent as compared to the individuals without cognitive deficit. 9 For the symptomatic treatment of the cognitive deficit in AD, acetylcholinesterase inhibitors (AchEIs), donepezil, rivastigmine, and galantamine are the first-line drugs. 10 Donepezil hydrochloride is a reversible, noncompetitive, piperidine-type cholinesterase inhibitor (ChEI), 11 and rivastigmine is also a centrally acting ChEI that stops the metabolization of acetylcholine esterase and butyrylcholinesterase. 12 The third one, galantamine, is similar to other centrally acting ChEIs, and it is also associated with allosteric potentiation of the nicotinic receptor N-methyl-d-aspartate and facilitation of synaptic transmission. 13 Although these AchEIs may improve balance and gait performance in the older adult patients with AD by improving attention and executive functions, 14 contradictory results were obtained in a limited number of studies performed on this subject. 15 It is not clear whether AchEIs, which are known to be safe from cardiac aspect, have a role in the development of OH. 10
The aim of the present study is to evaluate the long-term use of AchEIs on balance and gait functions and postural blood pressure changes.
Materials and Methods
Patients
A total of 102 patients with AD, who applied to the geriatrics department as outpatients between June 2013 and May 2015, underwent comprehensive geriatric assessment (CGA), informed about the study and gave consent, and were enrolled in this prospective study. The study was consonant to the Declaration of Helsinki and was approved by the local ethics committee.
Inclusion Criteria
Patients with AD
The patients, who had not been diagnosed with dementia before and have not been receiving antidementia drug, were diagnosed with AD based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association and Diagnostic and Statistical Manual of Mental Disorder (Fifth Edition) diagnostic criteria. 16,17 In addition, each of these patients was evaluated via magnetic resonance imaging. After making the diagnosis, AchEIs (donepezil, galantamine, and rivastigmine) were commenced, and the effective dose was reached by titrating in the first 4 weeks (donepezil 10 mg/d, galantamine 24 mg/d, rivastigmine 10 cm2/d patch). Patients who completed the 6-month follow-up period under this treatment were included in the study.
Exclusion Criteria
Age <65 years.
Patients who developed intolerance, hypersensitivity, or adverse event during treatment with AchEIs.
Cases who have been diagnosed with non-AD dementia.
Patients with serious orthopedic disease (eg, serious osteoarthritis, lumbar disk hernia).
Patients with other neurological diseases such as normal pressure hydrocephaly, Parkinson’s disease, multiple system atrophy, and spinal cord injury, which would influence balance and gait functions.
Patients with a history of stroke who left clinical sequel.
Patients with anemia (hemoglobin <11 g/dL); with acute or chronic renal and hepatic insufficiency, adrenal or pituitary insufficiency, and untreated thyroid disease; with cardiovascular diseases such as serious aortic stenosis or carotid artery stenosis; and frequent alcohol consumption; dehydration, electrolyte imbalance, acute hemorrhage, sepsis, malignancy, paraneoplastic syndrome, and similar serious comorbid diseases.
Patients with vestibular system disease that leads to balance disorder.
Immobile patients.
Patients receiving medications that could influence motor performance (dopamine agonists, benzodiazepine, etc).
Patients’ medications were not changed during the 6-month period.
Comprehensive Geriatric Assessment
Sociodemographic characteristics of the participants including age, gender, education, and living environment were recorded. All patients were examined whether they have experienced falls (based on the information obtained from the patient or patient’s relative, presence of more than 1 fall within 1 year due not to seizure or acute stroke), have cataract, hearing loss, hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, peripheral vascular disease, hyperlipidemia, cerebrovascular disease, depression, osteoporosis, and polypharmacy in their medical history. In addition, comorbid conditions were assessed using Geriatrics Index of Comorbidity. 18 Cognitive functions of the patients were evaluated by Cognitive State Test (COST) 19 and Mini-Mental State Examination (MMSE). In addition, scores of the Geriatric Depression Scale (GDS) and Basic Activities of Daily Living (BADL) and Instrumental Activities of Daily Living (IADL) indexes were also recorded for each patient.
After the patients received their daily drugs in the morning, they were evaluated by another researcher, who was blind for the diagnosis and for the drugs the patient was receiving, using Timed Up and Go (TUG) test and Tinetti Performance Oriented Mobility Assessment (POMA). The TUG measures the time the patient takes to stand up from an armchair, walk 3 m, turn, walk back to the chair, and sit down again. 20 Scoring for POMA-gait, POMA-balance, and POMA-total was done by systematically observing the movements of the patient from sitting to standing and walking for 3 m. 21
Diagnosis of OH
The OH was defined as a drop of at least 20 mm Hg in systolic blood pressure and/or 10 mm Hg in diastolic blood pressure with the change in position. 22
Laboratory Findings
Vitamin B12 and folic acid levels were found using Diagnostic Modular Systems autoanalyzer (Modular E170 and P800, Roche, Basel,. Switzerland).
Follow-Up
The CGA was reperformed at the end of the sixth month in patients with AD. They were reevaluated in terms of gait and balance functions, OH and polypharmacy, and vitamin B12 and folic acid levels. The patients were divided into 3 groups according to the cognitive test scores on the sixth month: improvement (increase in MMSE score was ≥2), no change (change in MMSE score ≤1), and worsening (decrease in MMSE score was ≥2). 23
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. The Kolmogorov-Smirnov test was used to analyze continuous variables in terms of suitability of normal distribution. Continuous variables with normal distribution were analyzed by independent sample t test, whereas continuous variables without normal distribution were analyzed by Mann-Whitney U test. Differences in proportions were analyzed using χ2 test. Probabilities <.05 were considered significant. All statistical analyses were done using SPSS 15.0 (SPSS Inc, Chicago, Illinois). Whether there is a significant change between the visits performed at baseline and at the sixth month was analyzed using Wilcoxon signed rank test. Changes between the visits were calculated by ([after treatment − before treatment]/[before treatment]) formula. The relation between AchEI therapy and the changes between visits were assessed by Kruskal-Wallis test. Changes between the visits in the patient groups that improved, not changed, and worsened at the end of the sixth month, which were determined based on the cognitive test scores, were analyzed by Kruskal-Wallis test. Paired t test was used for the comparison of the values between baseline and the sixth month visit in each AchEI group. The McNemar test was used for changes in OH between the visits.
Results
Baseline characteristics and comorbidities of the patients are demonstrated in Table 1. Baseline and the sixth month MMSE, COST, BADL, and IADL index scores and GDS results were similar in patients with AD (P > .05). Moreover, there was no difference between the visits in terms of POMA-gait, POMA-balance, POMA-total, and TUG values and OH (P > .05; Table 2). Table 3 demonstrates that mean changes in only POMA-balance were significantly different between the visits in those with cognitive improvement, with no change, and with worsening (Table 3).
Table 1.
Baseline Characteristics of the Patients.a
| Characteristics | |
| Age, years | 79.1 ± 8.9 |
| Gender (female/male) | 52/50 |
| Education, years | 5.1 ± 4.4 |
| Falls (%) | 29.4 |
| GIC | 2.1 ± 0.5 |
| MMSE | 18.1 ± 5.2 |
| COST | 20.9 ± 5.1 |
| GDS | 5.3 ± 3.4 |
| BADL | 87.5 ± 15.9 |
| IADL | 6.1 ± 4.4 |
| Comorbidities (%) | |
| Cataract | 24.5 |
| Hearing loss | 18.6 |
| HT | 58.8 |
| DM | 26,4 |
| CAD | 11.7 |
| CHF | 4.9 |
| PVD | 3.9 |
| CVD | 3.9 |
| OP | 17.6 |
| HL | 12.7 |
| Depression | 33.3 |
Abbreviations: BADL, Basic Activities of Daily Living (0: worst-100: best); CAD, coronary artery disease; CHF, congestive heart failure; COST, Cognitive State Test (0: worst to 30: best); CVD, cerebrovascular disease; DM, diabetes mellitus; GDS, Geriatric Depression Scale (0: worst to 15: best); GIC, Geriatrics Index of Comorbidity; HL, hyperlipidemia; HT, hypertension; IADL, Instrumental Activities of Daily Living (0: worst to 17: best); MMSE, Mini-Mental State Examination (0: worst to 30: best); OP, osteoporosis; PVD, peripheral vascular disease.
an = 102.
Table 2.
Comparison of Baseline and Sixth Month Findings in Patients With AD.
| Patients (102) | |||
|---|---|---|---|
| Baseline | Sixth Month | P Value | |
| POMA-balance | 11.8 ± 3.3 | 11.4 ± 4.0 | .42 |
| POMA-gait | 9.7 ± 2.5 | 9.4 ± 2.6 | .08 |
| POMA-total | 21.4 ± 5.4 | 20.8 ± 6.5 | .35 |
| TUG | 19.6 ± 7.7 | 20.1 ± 7.2 | .90 |
| OH | 39.2 | 27.4 | .07 |
| Systolic OH | 24.5 | 17.6 | .40 |
| Diastolic OH | 31.3 | 21.5 | .15 |
| Number of drugs used | 4.7 ± 3.0 | 4.3 ± 1.7 | .46 |
| Vitamin B12, pmol/L | 525.0 ± 418.9 | 475.2 ± 250.1 | .93 |
| Folic acid, ng/mL | 8.2 ± 3.0 | 8.1 ± 5.2 | .77 |
Abbreviations: OH, orthostatic hypotension; POMA, Tinetti Performance Oriented Mobility Assessment (balance score [0: worst to 16: best], gait score [0: worst to 12: best], total score [0: worst to 28: best]); TUG, Timed Up and Go test.
Table 3.
Mean Change in POMA and TUG From Baseline According to Cognitive Changes in Patients With AD.
| Improvement (24) | No Change (40) | Worsening (38) | P Value | |
|---|---|---|---|---|
| POMA-balance | 0.18 ± 0.34 | 0.11 ± 0.31 | −0.03 ± 0.29 | <.05 |
| POMA-gait | 0.00 ± 0.07 | 0.73 ± 0.20 | 0.31 ± 1.65 | .31 |
| POMA-total | 0.04 ± 0.10 | 0.39 ± 0.25 | 0.02 ± 0.41 | .28 |
| TUG | −0.08 ± 0.08 | −0.04 ± 0.34 | 0.01 ± 0.51 | .42 |
Abbreviations: AD, Alzheimer’s disease; POMA, Tinetti Performance-Oriented Mobility Assessment; TUG, Timed Up and Go test.
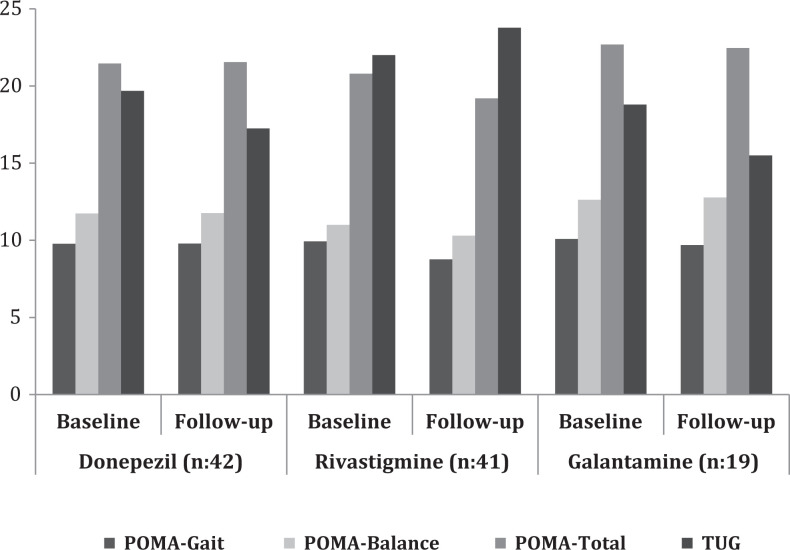
As is seen in Figure 1, when the changes in gait and balance variables of the patients were compared according to the type of AchEI (donepezil n = 42; rivastigmine n = 41; and galantamine n = 19), there are no changes in the POMA and TUG scores within and between the groups at the end of the 6 months (P > .05 for each comparison). Analyzing the relation between using AchEI and development of OH, it was determined that there is no change in OH as compared to the baseline in 76.1% and OH improved in 23.9% of the patients receiving donepezil; there is no change in OH as compared to the baseline in 51.2%, OH improved in 26.8%, and OH was developed in 22.0% of the patients receiving rivastigmine; and there is no change in OH as compared to baseline in 78.9%, OH improved in 5.2%, and OH was developed in 15.9% of the patients receiving galantamine.
Figure 1.
Changes in gait and balance variables of the patients according to acetylcholinesterase inhibitors. When the changes in gait and balance variables of the patients were compared according to the type of AchEI, there are no changes in the POMA and TUG scores within and between the groups at the end of the 6 months (P > .05 for each comparison). AchEI indicates acetylcholinesterase inhibitor; POMA, Tinetti Performance Oriented Mobility Assessment; TUG, Timed Up and Go test.
Discussion
The present study determined that balance and gait functions are impaired in patients with AD, and AchEIs have no unfavorable effect on balance, gait, and OH.
Cognitive deficit and gait and balance impairment are the 2 major geriatric syndromes, and it is being dwelled on in the recent years that these 2 syndromes might be closely associated with each other independent from aging 2 because cognitive function plays a key role in the regulation of balance and gait, particularly in elderly patients. Difficulty in maintaining attention, failure in executive functions, deficits in visuospatial perception, behavioral and psychological symptoms of dementia, loss of motivation, presence of extrapyramidal signs, and regional white matter involvement, which are encountered beginning from the prodromal stages in patients with AD, may influence mobility, and all of these may be worsened along with the progression in AD. 3 -5,7,24 According to the current literature, balance and gait functions may be affected in elderly patients with AD.
Acetylcholinesterase inhibitors have stabilizing effect on memory function and delaying effect on functional decrease, although they are not curative. 25 Although it is not definitely known by which mechanism these drugs delay functional decrease, the hypothesis that they improve not only cognitive functions but also motor functions is being dwelled on in the recent years. According to this hypothesis, these drugs, which increase acetylcholine concentration, may contribute to the initiation and maintenance of gait by improving attention and executive functions via cerebral cortex; to the control of length of step and walking speed via subcortical cortex (basal ganglia); and to the rhythmicity of gait and preservation of balance by enhancing neurogenic transmission in the spinal canal. 11 In a limited number of studies that were performed based on this hypothesis, it was determined that galantamine did not affect balance and gait in 9 patients with AD 26 and AchEI therapy did not affect 43 mild-to-moderate patients with AD 27 ; whereas another study, which evaluated only 6 patients with mild AD, demonstrated that donepezil enhances motor performance. 10 In the present study, it was determined that AchEI therapy has no effect on balance and gait in the long term, and, moreover, each of 3 AchEIs has no superiority to each other concerning this issue. In addition, it was demonstrated that the change in balance parameters is better in the cases in which cognitive improvement was determined at the end of the sixth month, as compared to the cases that displayed cognitive worsening. This supports the conclusion that balance problems in the elderly patients with AD result rather from cognitive functions, such as attention and executive functions, than classical motor disorders, which arise from basal ganglion or cerebellum. 15 This effect is quite important in preventing elderly patients with AD from falls.
The present study is remarkable also because of evaluating the relation between long-term AchEI use and OH, which is among important causes of falls in elderly patients. Although AchEIs are cardiologically safe, whether they lead to OH has not been exposed clearly. 28 -31 In one of the few studies on this subject in which a small case group (39 patients) including non-AD dementia was followed for short term (12.2 ± 4.8 weeks), it was reported that AchEIs have no effect on the development of OH. 31 In the present study, it was demonstrated that 6-month AchEI therapy had no negative effect on OH in 102 elderly patients with AD. These data support also the results of our previous studies. 28 -31
The present study is also remarkable for being one of the largest studies that evaluate the effect of 6-month AchEI therapy both on balance and gait and on OH in patients with AD. Nevertheless, the present study has some limitations. First, patients treated with AchEIs could not be compared to the untreated patients since there was no patient with AD receiving AchEI therapy. Although a placebo-controlled study is the ideal way to assess the effects of AchEIs on balance and gait functions and OH in older adults, such a study is difficult to perform because of ethical reasons. Second, the elderly patients with AD could not be evaluated according to the stages. Third, limited number of patients receiving galantamine therapy made the comparison among AchEIs difficult. Fourth, cognitive functions have been evaluated as a whole but not separately as attention, which affect balance and gait, frontal lobe functions, and executive functions.
In conclusion, evaluation of balance and gait functions and OH should be included in dementia practice in order to assess the risk of falls in elderly patients with AD and to take preventive measures in time. It seems that positive effects on cognitive performance of AchEIs are reflected in balance functions in patients with AD. Although these drugs have been demonstrated not to be effected in the development of OH, elderly patients should be followed closely due to the multiple comorbidities and polypharmacy.
Acknowledgments
The authors thank Hulya Ellidokuz for statistical analysis.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bailly S, Haesebaert J, Decullier E, et al. Mortality and profiles of community-dwelling fallers. Results from the EPIDOS cohort. Maturitas. 2014;79(3):334–339. [DOI] [PubMed] [Google Scholar]
- 2. Shaw FE. Prevention of falls in older people with dementia. J. Neural Transm. 2007;114(10):1259–1264. [DOI] [PubMed] [Google Scholar]
- 3. Ogama N, Sakurai T, Shimizu A, Toba K. Regional white matter lesions predict falls in patients with amnestic mild cognitive impairment and Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(1):36–41. [DOI] [PubMed] [Google Scholar]
- 4. Martin K, Thomson R, Blizzard L, Wood A, Garry M, Srikanth V. Visuospatial ability and memory are associated with falls risk in older people: a population-based study. Dement Geriatr Cogn Disord. 2009;27(5):451–457. [DOI] [PubMed] [Google Scholar]
- 5. Tangen GG, Engedal K, Bergland A, Moger TA, Mengshoel AM. Relationships between balance and cognition in patients with subjective cognitive impairment, mild cognitive impairment, and Alzheimer disease. Phys Ther. 2014;94(8):1123–1134. [DOI] [PubMed] [Google Scholar]
- 6. Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. [DOI] [PubMed] [Google Scholar]
- 7. Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One. 2009;4(5):e5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soysal P, Yay A, Isik AT. Does vitamin D deficiency increase orthostatic hypotension risk in the elderly patients? Arch Gerontol Geriatr. 2014;59(1):74–77. [DOI] [PubMed] [Google Scholar]
- 9. Mehrabian S, Duron E, Labouree F, et al. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci. 2010;299(1-2):45–48. [DOI] [PubMed] [Google Scholar]
- 10. Isik AT, Bozoglu E, Yay A, Soysal P, Ateskan U. Which cholinesterase inhibitor is the safest for the heart in elderly patients with Alzheimer’s disease? Am J Alzheimers Dis Other Demen. 2012;27(3):171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shintani EY, Uchida KM. Donepezil: an anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54(24):2805–2810. [DOI] [PubMed] [Google Scholar]
- 12. Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible study cholinesterase inhibitor. Clin Ther. 2003;25(6):1631–1653. [DOI] [PubMed] [Google Scholar]
- 13. Schilström B, Ivanov VB, Wiker C, Svensson TH. Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology. 2007;32(1):43–53. [DOI] [PubMed] [Google Scholar]
- 14. Montero-Odasso M, Wells J, Borrie M. Can cognitive enhancers reduce the risk of falls in people with dementia? An open-label study with controls. J Am Geriatr Soc. 2009;57(2):359–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beauchet O, Launay CP, Allali G, Annweiler C. Changes in gait variability with anti-dementia drugs: a systematic review and meta-analysis. CNS Drugs. 2014;28(6):513–518. [DOI] [PubMed] [Google Scholar]
- 16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision: . Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 18. Zekry D, Loures Valle BH, Lardi C, et al. Geriatrics Index of Comorbidity was the most accurate predictor of death in geriatric hospital among six comorbidity scores. J Clin Epidemiol. 2010;63(9):1036–1044. [DOI] [PubMed] [Google Scholar]
- 19. Babacan-Yildiz G, Isik AT, Ur E, et al. COST: Cognitive State Test, a brief screening battery for Alzheimer disease in illiterate and literate patients. Int Psychogeriatr. 2013;25(3):403–412. [DOI] [PubMed] [Google Scholar]
- 20. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 21. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429–434. [DOI] [PubMed] [Google Scholar]
- 22. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46(5):1470. [DOI] [PubMed] [Google Scholar]
- 23. Miranda LF, Barbosa MA, Regina P, et al. Good rate of clinical response to cholinesterase inhibitors in mild and moderate Alzheimer’s disease after three months of treatment. Dement Neuropsychol. 2013;7(2):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perrault A, Wolfson C, Egan M, Rockwood K, Hogan DB. Prognostic factors for functional independence in older adults with mild dementia: results from the Canadian study of health and aging. Alzheimer Dis Assoc Disord. 2002;16(4):239–247. [DOI] [PubMed] [Google Scholar]
- 25. Herrmann N, Lanctôt KL, Hogan DB. Pharmacological recommendations for the symptomatic treatment of dementia: the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 2012. Alzheimers Res Ther. 2013;5(suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Assal F, Allali G, Kressig RW, Herrmann FR, Beauchet O. Galantamine improves gait performance in patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56(5):946–947. [DOI] [PubMed] [Google Scholar]
- 27. Beauchet O, Launay CP, Allali G, et al. Anti-dementia drugs and changes in gait: a pre-post quasi-experimental pilot study. BMC Neurol. 2013;13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Isik AT, Yildiz GB, Bozoglu E, Yay A, Aydemir E. Cardiac safety of donepezil in elderly patients with Alzheimer disease. Intern Med. 2012;51(6):575–578. [DOI] [PubMed] [Google Scholar]
- 29. Isik AT, Bozoglu E, Naharci MI, Kilic S. Evaluation of the effects of galantamine on cardiac function in elderly patients with Alzheimer’s disease. Am J Geriatr Pharmacother. 2010;8(5):454–459. [DOI] [PubMed] [Google Scholar]
- 30. Isik AT, Soysal P, Yay A. Which rivastigmine formula is better for heart in elderly patients with Alzheimer’s disease: oral or patch? Am J Alzheimers Dis Other Demen. 2014;29(8):735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. da Costa Dias FL, Ferreira Lisboa da Silva RM, de Moraes EN, Caramelli P. Cholinesterase inhibitors modulate autonomic function in patients with Alzheimer's disease and mixed dementia. Curr Alzheimer Res. 2013;10(5):476–481. [DOI] [PubMed] [Google Scholar]