Abstract
Patients who have dementia with Lewy bodies (DLB) and undergo surgery may develop aggravated postoperative cognitive dysfunction or postoperative delirium. Many patients with DLB respond poorly to surgery and anesthesia, and their conditions may worsen if they have other medical complications along with dementia. They may also face high risk of prolonged hospital stay, increased medical problems and/or mortality, causing significant physical, psychosocial, and financial burdens on individuals, family members, and society. Anesthesia, pain medications, old age, and surgery-related stresses are usually held responsible for the complications; however, the exact causes are still not fully understood. Literature on surgery-related complications for patients with DLB appears to be inadequate, and hence the topic merits detailed and systematic research. This article reviews postoperative complications and various surgery-related risk factors for DLB in light of other dementias such as Alzheimer’s disease, as their neuropathologic features overlap with those of DLB.
Keywords: Lewy body dementia, Alzheimer’s disease, delirium, postoperative cognitive dysfunction, surgery, anesthesia
Introduction
Dementia with Lewy bodies (DLB) is a neurodegenerative dementing disorder. It is the second most common form of dementia after Alzheimer’s disease (AD) that affects the elderly population. The number of patients with dementia will be more than 70 million globally by 2030, 1 while in the United States about one-fifth of total patients with dementia are patients with DLB. 2 Patients with DLB are 3.5 times more prone to experience delirium than AD, 3 and the recurrence of delirium is also more common in DLB than other dementia diseases. Although the disease modifying treatment options for DLB are not established, the overall treatment expense for DLB can be much higher than patients with other dementia. 4
Dementia with Lewy bodies is primarily characterized as cognitive decline, fluctuation of attention, intermittent visual hallucinations, and spontaneous motor features such as akinesia, rigidity, and tremor. 5,6 Other clinical symptoms include dysautonomia and sleep disorders such as rapid eye movement (REM) sleep behavior disorder (RBD). 7,8
Dementia with Lewy bodies is caused by aggregation of an abnormal protein (α synuclein) in neurons in the form of Lewy bodies (LBs) and Lewy neuritis (LNs) resulting in damage, mainly cholinergic and dopaminergic neurons that diminish the levels of neurotransmitter (acetylcholine and dopamine) 9 and thereby impact the neurons’ ability to communicate with each other causing decline in attention, memory, and learning new skills. When patients undergo surgery, the cholinergic system is depressed by the anesthesia to generate anesthetic effects. These effects are even more pronounced in elderly patients with DLB because of their marked baseline cholinergic losses.
Various anesthetic agents and other surgery-related stresses such as pain, anxiety also have a significant impact on brain that alter brain function and result in cognitive dysfunction. For the patients with dementia, due to their fluctuation of cognition and memory problems, changes in environment (hospital, operation theatre, light, noise, etc), daily routine, medications, and so forth also contribute to anxiety and stress that affect their cognition and behavior in the postoperative stage. Age-related physiologic changes, other comorbidities, and use of multiple medications further deteriorate patient response to surgery.
Patients with DLB respond poorly to surgery and anesthesia, causing significant physical, psychosocial, and financial burdens on individuals and society. 10,11 Characterizing the full spectrum of surgical complications in patients with DLB could help improve outcomes and reduce costs. There has been a large scope to analyze the preoperative comorbidities, contributing factors, and characteristics of major surgical complications among patients with DLB. 12
Numerous studies have been performed to understand the impact of surgery on AD and Parkinson’s disease (PD) and delirium. To the best of authors’ knowledge, no systematic study has been performed to understand the impact of surgery on delirium or postoperative cognitive dysfunction (POCD) in patients with DLB. In other words, the molecular mechanisms of the surgery-related disorders are largely unknown. The lack of research basically precludes drawing reliable conclusions regarding the impact of surgery-related stresses in patients with DLB. In this article, we will mainly review the risk factors and probable cause of cognitive impairments in the patient with DLB due to surgery in the light of typical surgical complications found in patients with dementia having AD and PD.
Clinical Features
Clinical features of DLB include cognitive impairment, motor, neuropsychiatric, motor, sleep, and autonomic disorders as summarized in Figure 1. 13
Figure 1.
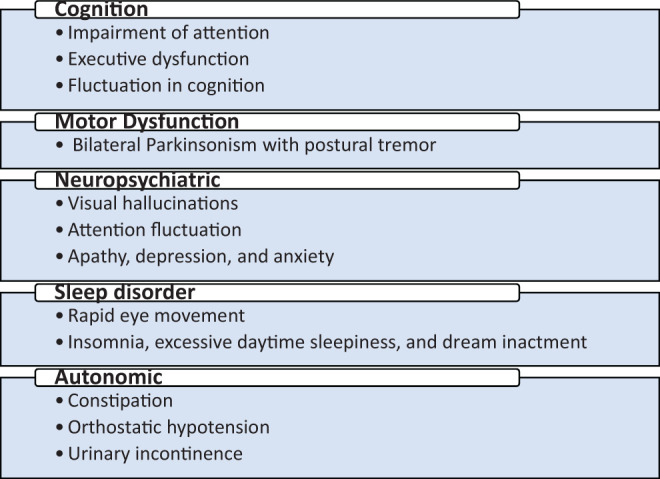
Common clinical features of dementia with Lewy bodies (DLB). 7,13
Cognition
The most common clinical feature in patient with DLB is cognitive decline. Patients exhibit impairment in attention, executive dysfunction (eg, planning, prioritizing, and sequencing), and visuospatial deficit. 14 -16 Fluctuations in cognition are considered to be the hallmarks of DLB. 13,17 Fluctuations in cognition are expressed as unresponsiveness or staring with a fixed look at times, excessive daytime sleepiness or hypersomnolence, and limited awareness about surrounding or unclear speech alternating with mentally sound, rational behavior and normal function. 7,14
Motor
Patients with DLB also develop motor functional disability with extrapyramidal signs such as tremor, rigidity, bradykinesia, postural disability, gait difficulty, and facial immobility. 13,14,18 These symptoms are common in idiopathic PD too; however, they occur bilaterally instead of unilaterally in patients with DLB. 13 Tremor in patients with DLB commonly occurs with a complex mixed tremor pattern showing a significant response to acute and chronic dopaminergic treatments. 19
Neuropsychiatric Features
Neuropsychiatric features such as visual hallucinations, depression, apathy, and delusions are common in patients with DLB. 7,20 This involves detailed appearance of people, animals, body parts, machines, and so forth, 7 which patients are not typically frightened of seeing. 13 Auditory hallucinations and extracampine hallucinations (a vague feeling of human presence) may also be experienced by some patients with DLB. 21 Delusions may be associated with misidentification such as Paranoid, Capgras (friends and relatives are replaced by identical poster), and Phantom boarder (unseen individuals living in one’s room). 20 Depression and apathy are also common in patients with DLB having more severe dementia. 7
Sleep Disorder
Sleep symptoms such as insomnia, excessive daytime sleepiness, and dream enactment behavior are common in patients with DLB. The REM is another sleep disorder that may occur in a subset of patients with DLB. 14 Dream enactment as a result of REM causes the patients to take action (vocalization or violent behavior) and may result in injuries to patients or bed partners. 7,13 The REM is considered to be a significant risk factor for cognitive deficit and Parkinsonism, and the patients with DLB having REM sleep behavior show earlier onset of other symptoms than those who do not have REM sleep disorder. 8
Autonomic Symptoms
Patients with DLB exhibit autonomic features such as constipation, urinary inconsistence, and orthostatic hypotension. 22-23 Constipation is a common early complaint that usually leads to cognitive decline or motor symptoms in the long term. 7 However, the orthostatic hypotension appears to be the most serious symptom causing autonomic dysfunction and increases the risk of falls. Other common features include decreased sweating, heat in tolerance, urinary dysfunction, diarrhea, seborrhea, impotence, and carotid hypersensitivity. 7
Overall, the most common presenting symptom is cognition (memory impairment) followed by visual hallucinations, depression, problem-solving difficulty, and motor dysfunction while memory impairment together with visual hallucination is considered to be the major differentiating feature for DLB than other dementias. 24
Pathogenesis and Pathophysiology
Lewy Body and Lewy Neuritis
Dementia with Lewy bodies is one of the neurodegenerative (synucleinopathy) diseases, caused by abnormal accumulation of α-synuclein protein in neurons. The α-synuclein protein is found in presynaptic region of the neuron presumably affecting regulation of synaptic activity or neurotransmission. 25 Abnormal aggregation, accumulation, and deposition of α-synuclein proteins form LBs (10-20 μm round eosinophilic hyaline inclusions) or LNs (elongated thread-like dystrophic axons and dendrites), causing the loss of neurons mainly cholinergic and dopaminergic neurons. 26
Location, Distribution, and Density of LB
The locations in the brain are affected by LBs or LNs include cerebral cortex, limbic system areas, basal ganglia (putamen, caudate nuclei), and brain stem. The temporal lobe areas of cerebral cortex (superior temporal gyrus and parahippocampal gyrus) are affected together with limbic system areas (hippocampus and amygdala). Other cortical regions involve superior frontal cortex, occipital cortex, cingulated gyrus, insula and claustrum. 27,28 The densities of LB in the brains of patients with DLB are the greatest in temporal lobe and limbic regions, especially in the parahippocampal gyrus, amygdala, and the superior temporal gyrus. 29 However, a fewer LBs have also been found in the frontal, parietal, and occipital regions. 30
Genetic Factors for LB
Genetic factors for DLB have not been well studied yet. Woodruff et al 31 proposed that the genetic factors are important in patients with DLB as they are important in patients with AD. They studied family history in patients from an autopsy series of AD and DLB and in living healthy controls. They found that the family history of dementia was more common in AD and DLB compared with controls. Harding et al 32 argued that DLB is more likely to run in families in the form of autosomal dominant inheritance pattern compared with PD. The risk of developing DLB is believed to be affected by gene mutations in synuclein proteins. 33 The mutations in β-synuclein (V70M and P123H mutations) have been found to be associated with the DLB phenotype. 34 Mutations in α-synuclein gene is typically associated with the PD; however, it has also been found in the patients with DLB in some isolated cases. 33 The research in identifying genetic factors for DLB is at a very early stage, and more research need to be done to obtain precise connection with family inheritance.
As there is overlap in disorders of DLB with AD and PD with dementia, the gene mutation in AD and PD may also be associated with DLB. It has been reported that the gene mutation in amyloid precursor protein (APP) and both presenilin genes (PSEN1 and PSEN2) are also identified to be the possible causes of DLB. 35,36 The apolipoprotein E4 allele is considered to be a genetic risk factor in AD; however, an overexpression of this allele has been seen in those with the DLB phenotype. 37
There might also be a link between GBA gene mutation and DLB. Although several studies had failed to clarify the relationship between DLB and GBA gene, 38 Goker-Alpan et al 39 identified that GBA mutation in 23% patients with different synucleinopathies (75 autopsy specimens). They suggested that the mutation in the GBA (lysosomal protein) may interfere with the clearance or promote aggregation of LB. Polymorphisms in the serotonin transporter gene may also be linked with the symptom of DLB like hallucinations and delusions. 40
Pathologic Association of DLB With PD and AD
The coexistence of DLB pathologies with AD and PD occurs both at the regional and cellular levels. The aggregates of α-synuclein in the form of LBs and LNs are the pathologic hallmarks of DLB. The DLB and PD with dementia are both α-synucleinopathies and progressive neurodegenerative disorders sharing similar pathology and clinical features. 41 On the other hand, the formation of extracellular β-amyloid (Aβ) plaques and intraneuronal neurofibrillary tangles (NFTs) is the characteristic pathology of AD. 42 However, up to 80% of patients with DLB show coexistent Alzheimer disease (AD) pathology in the form of extracellular Aβ plaques and intracellular aggregates of the microtubule-associated protein τ (MAPT) in NFTs and neuropil threads. 43
The complex interactions at the cellular level between τ, α-synuclein, and Aβ in DLB pathology have been studied elsewhere. An investigation in animal models of neurodegenerative diseases suggests possible interactions between τ, α-synuclein, and Aβ that may cause the aggregation of each other. 44 -48 Another recent study with patients with DLB has showed that there is a simultaneous existence between τ and synuclein in DLB. This study also proposed that Aβ deposition and the MAPT gene (MAPT H1 haplotype) are the possible causes in DLB pathogenesis. 49
Diagnostic Criteria
Both clinical and pathological criteria are used for the diagnosis of DLB. The clinical diagnostic features of DLB are provided in Table 1. The diagnosis of DLB is categorized in 2 distinct groups:
Table 1.
| Central feature |
|
| Core features |
|
| Suggestive features |
|
| Supportive features |
|
Abbreviation: DLB, dementia with Lewy bodies.
Clinically probable DLB: If at least 2 core features or dementia with at least 1 core feature and 1 suggestive feature are diagnosed.
Clinically possible DLB: If 1 or more suggestive features are diagnosed.
Patients with DLB usually demonstrate clear pathological features. However, some of the neuropathology may coincide with that of AD. The likelihood of DLB is determined by pathological criteria such as location of LBs and the extent of AD pathology. The locations considered for pathological diagnosis include brain stem, limbic system, and neocortex. 50
The likelihoods of DLB based on pathological features are summarized in Table 2. It is reported that the patients showing high likelihood of DLB (based on the pathological features) also demonstrated core clinical features of DLB.
Table 2.
Likelihoods of DLB Based on Pathological Features. 13
| High likelihood | Diffuse neocortical Lewy body pathology with either low or intermediate AD pathology, or limbic Lewy body pathology with low AD pathology |
| Intermediate likelihood | Limbic-predominant Lewy body pathology and intermediate AD pathology, or diffuse neocortical Lewy body pathology with high AD pathology |
| Low likelihood | Brain stem-predominant Lewy body pathology and any AD pathology category, or limbic-predominant Lewy body pathology with high AD pathology |
Abbreviations: AD, Alzheimer’s Disease; DLB, dementia with Lewy bodies.
Treatment of DLB
The treatment of DLB remains still challenging. This is because there is no disease modifying treatment and the adverse effects of medication often make the treatment difficult. There are a number of reports in the literature regarding the use of medications that may be considered for the symptomatic treatment of DLB as discussed in the sections subsequently and also summarized in Table 3. Caution must be exercised with the symptomatic treatment as this may worsen the symptoms in other domains.
Table 3.
| Symptoms | Pharmacological Therapy |
|---|---|
| Cognitive symptoms |
|
| Motor symptoms |
|
| Behavioral symptoms |
|
| Autonomic dysfunction |
|
| Sleep disorder | Low doses of clonazepam are recommended for REM sleep behavior disorder |
Abbreviations: DLB, dementia with Lewy bodies; REM, rapid eye movement.
Cognitive
The cholinergic loss in the central nervous system (CNS) starts earlier and more prominent in patients with DLB than in patients with AD. Deficits in attention and cognition and the presentation of neuropsychiatric symptoms presumably occur as a result of cholinergic loss. Hence, it has been suggested that acetylcholinesterase inhibitors (AChEIs) are more effective for the treatment of patients with DLB than for patients with AD. 53 Several clinical studies of AChEIs treatment using donepezil, galantamine, and rivastigmine in patients with DLB suggest that AChEIs improve on cognitive and behavioral symptoms. 54 -58 Another clinical trial suggest that rivastigmine improved attentional and behavioral symptoms. 59 However, none of the studies investigated the comparative performance of those AchEI drugs in patients with DLB.
The clinical trials of memantine, a drug that acts on the glutamatergic system by blocking NMDA receptors, have not been found to be conclusive in patients with DLB. 60,61 However, an early treatment with this medicine has been reported to provide longer survival in a small group of patients with DLB and PD. 41
Motor
The controlled clinical trials for the treatment of motor features in patients with DLB have not been evaluated yet. Levodopa, the main drug of choice for the motor treatment in patients with PD, has been found to be not as effective in patients with DLB as in PD. 62 -65 This drug has also been reported to cause psychosis as a significant side effect in patients with DLB. 63,64 If the motor syndrome is tolerable in patients with DLB, the treatment with Levodopa should be avoided. 66 Nevertheless, clinicians may use low dose of Levodopa if required. 51 Other anti-Parkinson medications such as MAO inhibitors, COMT inhibitors, amantadine, and anticholinergics are likely to worsen cognitive impairments and should be avoided. 67
Neuropsychiatric Symptoms
Neuropsychiatric symptoms in DLB are characterized as hallucination, agitation, anxiety, depression, behavioral changes, and so forth. There is no approved medication for the treatment of neuropsychiatric symptoms in DLB. Medications for mild symptoms are not usually recommended; however, if treatment is necessary, AChEIs are usually recommended as a drug of choice. They are reported to decrease psychiatric symptoms such as apathy, anxiety, hallucinations, and delusions in patients with DLB. 68
Some patients with DLB may also be treated with antipsychotic drugs. Typical antipsychotic drugs such as haloperidol are not recommended because of their neuroleptic sensitivity in patients with DLB. Low doses of atypical antipsychotic drugs such as risperidone and olanzapine are usually well tolerated and do not result in motor deterioration. 69 However, they can cause motor deterioration in advanced patients with DLB. 70 Atypical neuroleptics (such as clozapine, quetiapine, etc) are also recommended for patients with DLB, 71 especially when cholinesterase inhibitors are ineffective.
Sleep
Education on sleep hygiene and healthy lifestyle can improve sleep problems in patients with DLB. Patients should maintain a daily routine, avoid day time naps, and afternoon caffeine intake. 72 The REM sleep behavior disorder (RDB) of patients with DLB can be treated with melatonin or clonazepam or quetiapine. 72 Nonpharmacological treatment of RBD involves increasing safety for the patient like placing mattress on the floor, padding the corners of furniture, and removing potentially dangerous objects from the bedroom. 73
Autonomic
Treatment of orthostatic hypotension should start with the nonpharmacological measures such as educating patients on sitting or postural positions, leg elevation, using elastic stockings, increasing salt intake, and so forth. 66 Medications such as midodrine (vasoconstrictor) and fludrocortisone (mineralocorticoid) may be recommended if required.
Urinary urgency, frequency, and urge incontinence can be treated with anticholinergic drugs like oxybutynin, tolterodine tartrate, berthanechol chloride, and propantheline. However, since anticholinergic drugs can aggravate cognitive problems, these medications should be used cautiously.
For the treatment of constipation, nonpharmacological interventions such as taking high-fiber diet, adequate fluid intake, physical exercise, and so forth should be used first. Various pharmacological agents like psyllium, polyethylene glycol, methylcellulose, misoprostol, magnesium hydroxide, and chloride channel activating agent lubiprostone can be used. 74 -77
Common Postsurgical Complications in Patients With DLB
Elderly patients are more susceptible to develop postoperative complications including POCD and postoperative delirium (POD) who undergo surgery. 52 While the neurological deficits are obvious in elderly patients with DLB, because of having the preexisting synucleinopathy such as DLB, they are prone to experience ever further cognitive deterioration after surgery in the form of POCD and POD. 78,79 Although both POCD and POD are very common in elderly surgical patients, delirium is considered as a major postoperative complication in them. 80
Postoperative Cognitive Dysfunction
Postoperative cognitive dysfunction is characterized by the impairment of memory, concentration, language comprehension, and social integration. 81 The diagnosis of POCD is difficult because there is no recognized diagnostic criteria of POCD. However, it is usually diagnosed based on the performance of neuropsychological testing administered before and after the surgery. 82 It can also be diagnosed when the cognitive impairments do not meet the criteria of delirium, dementia, or amnestic disorder. 83 -85
Possible causes of POCD are the type and duration of anesthesia/surgery, inflammation, hypoxemia, hypotension, neurodegeneration, and patient vulnerability 82 including old age, severity of coexisting illness, postoperative infections, respiratory complications, and a second operation. 86,87 The POCD is the most frequently reported following cardiac surgery and is also known to occur in other surgical procedures 81
The molecular mechanism underlying POCD is not clearly understood yet. Research has shown that the POCD is primarily associated with synaptic dysfunction and loss. 6,88 The causes of the synaptic dysfunction and loss may be due to the effect of anesthesia and other perioperative factors. 89 Anesthetics have also been reported to affect synaptic function by acting on the ion channels, as discussed later in this article.
A recent literature review has shown that along with anesthesia other perioperative factors such as hypoxia and hypocapnia can cause POCD by advancing AD neuropathogenesis, 89 especially for the patients with AD having previous history of ischemic stroke 90 or with coexisting evidence of cerebral ischemia. 91,92 Hypoxia has been suggested to increase AD neuropathology by increasing Aβ formation, 93 decreasing APP processing, 94 inducing apoptosis, 95 -97 and producing reactive oxygen species 93 resulting in POCD. Hypercapnia may induce apoptosis and increase Aβ production and thus may contribute to AD neuropathogenesis and POCD. 98
Excessive iron accumulation is also claimed to be responsible for triggering postsurgical transient neurocognitive decline. This is because appropriate level of iron is required for normal neuronal metabolism, that is, transportation of oxygen, electron transfer, synthesis of neurotransmitters, and the production of myelin. 99,100 Hence, the balancing of iron is suggested to be a key strategy for preventing POCD. 81
There is no research that confirms the mechanism of POCD in DLB but the above-mentioned mechanisms may prompt POCD in patients with DLB as AD and DLB share same pathology to some extent.
Postoperative Delirium
Delirium is an acute mental condition characterized by transient disturbance of consciousness and cognition, with rapid onset and a fluctuating course. 80,101 It is well described in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) as summarized in Table 4. The patients may exhibit hypoactive, hyperactive, or mixed delirium states. Severity of delirium can be rated and may have prognostic value. 103 More than 40% hospitalized elderly patients experience delirium. 104 Recognizing delirium is important because it can lead to increased morbidity and mortality in elderly patients. Confusion Assessment Method (CAM) is commonly used method for diagnosing the presence and severity of delirium. The assessment is made based on the onset of symptoms, inattention, disorganized thinking, and altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation/retardation, and altered sleep wake cycle. 105 There are 2 versions of CAMs; the short form (4 items) is used for the hospitalized patients and the long form (10 items) is used for research. 106,107 The short form is scored with increasing severity from 0 to 7 and the long form is scored with increasing severity from 0 to 19. The points in median to separate delirious from nondelirious patients for short and long forms are 3 and 6, respectively. However, there are several methods available to identify delirium namely CAM, Delirium Symptom Interview, Confusion Assessment Method for the Intensive Care Unit, Intensive Care Delirium Screening Checklist.
Table 4.
Diagnostic Criteria of Delirium (DSM-5). 102
|
Abbreviation: DSM-5, Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition).
The pathophysiology of POD is not clearly understood. However, it is hypothesized that delirium may be due to imbalance in neurotransmitters mainly for reduced acetylcholine. Inflammatory mediators, various stressors, and serum anticholinergic activity may also be associated with development of POD. 108,109
Risk factors for delirium can be associated with predisposing and/or immediate precipitating factors as outlined in Table 5. Dementia is one of the strongest risk factors for delirium and it also determines its severity. 110 -112 In addition severity of cognitive impairment, old age, severe comorbidities, use of psychoactive and narcotic drugs, and metabolic abnormalities are factors most often found associated with delirium among elderly patients. 109 Environmental risk factors are also associated with the severity of the delirium in hospital. Research has shown that number of room changes, hospital units, absence of timepiece, absence of reading glasses, use of physical restraints, and presence of family members may precipitate delirium severity. 113
Table 5.
Risk Factors for Delirium. 109
| Precipitating Factors in Delirium | Predisposing Factors in Delirium | Drug-Related Factors | |
|---|---|---|---|
| Drug With High Anticholinergic Activity | Other Drugs | ||
| Narcotics Severe acute illness Urinary tract infection Hyponatremia Hypoxemia Shock Anemia Pain Physical restraint Bladder catheter use Iatrogenic event Orthopedic surgery Cardiac surgery Noncardiac surgery Intensive care unit admission High number of hospital procedures | Older age Male sex Visual impairment presence of dementia severity of dementia depression functional dependence immobility Hip fracture dehydration alcoholism severity of physical illness Stroke Metabolic abnormalities | Antipsychotics (eg, chlorpromazine) Antibiotics Codeine Cimetidine Captopril Dipyridamole Dyazide Digoxin Furosemide Isosorbide dinitrate Nifedipine Prednisolone Theophylline Tricyclic antidepressants | Antiparkinsonian agents (eg, l-DOPA) Antidiarrheal agents (containing belladonna) Benzodiazepines Chlorpheniramine Diphenhydramine Haloperidol Laxatives Narcotics Nonsteroidal anti-inflammatory drugs Ranitidine Warfarin |
Patients with DLB have several risk factors for delirium such as dementia, cognitive deficit, old age, and cholinergic deficiency. In patients who have a previous history of delirium, the chance of future experience of delirium will be more. 114 The recurrence of delirium is more in patients with DLB when compared to patients with AD. 114 Consequently, the chance of POD in patients with DLB may also be higher than those without DLB.
Treatment of POD is very important as it is a medical emergency. Identifying the underlying causes, change in environment and behavioral support are important measures for the treatment of POD. Pharmacological treatment is mainly recommended for noncompliant cases. Haloperidol is primarily used for POD with agitation; however, this drug is contraindicated for patients with DLB. 109,115 Atypical antipsychotic drugs like olanzapine and risperidone have also been found to be successful in POD patients.
Both POCD and POD affect a significant number of patients causing serious clinical and economical sufferings. 79,81 They include poor functional recovery, longer hospital stay, increase morbidity and mortality, higher treatment cost and discharge to skilled facilities. 116 -118 They may also bring enormous distress to the patients, family members, and care givers. 119
Common Risk Factors for Surgical Complications in Patients With DLB
There are several known and possible risk factors that are responsible for postoperative complications in the form of POCD and POD for patients with DLB. The major risk factors that affect various regions of the brain are perioperative anxiety, pain, anesthesia, and surgery, and so forth. Age, comorbidity, and use of various medications may further complicate the postoperative outcome. Any of these risk factors contribute significant stresses on the brain resulting in alteration of brain function. Here we will discuss how these risk factors may affect the patient’s brain.
Anxiety
Elderly patients who undergo surgery may experience acute psychological stress during the perioperative period. 120,121 Patients with dementia can be at various stages of cognitive dysfunction. The environment of the operating theater (lighting, noises, and unfamiliar voices) can be confusing and frightening to the elderly patients with dementia. 122 Changes in daily routine, environment (hospital and operation theatre), and medications are the common stressors for elderly patients with dementia. 123 These stressors may not only trigger challenging behavior but also become the source of anxiety for patients with dementia. 122 Anxiety causes significant stress on the brain and may alter brain function by sensitizing the brain to subsequent exposure of stressors. In the brain, there may be reduced synaptic strength, apoptosis, and or phenotypic changes due to anxiety. 124
Surgery
Surgical injury activates endocrine, metabolic, and inflammatory responses that ultimately induce stress response. A very important stress hormone such as cortisol is released in response to stressful conditions like surgery. 124,125 Because of their liposoluble characteristics, they can pass through the blood–brain barrier causing cognition impairment and POD, especially to elderly patients with CNS disease like dementia. 125
Surgical injury result in inflammatory response at the site of wound and also triggers systemic inflammation. An exaggerated systemic inflammatory response is very harmful for the brain. 126,127 Proinflammatory cytokines like interleukin 6 and tumor necrosis factor α may alter brain function. 124 An animal study has shown that proinflammatory cytokines may be involved in change in memory and hippocampal function. 128
Anesthetic Agents
Numerous studies have been performed to understand the effects of anesthetic agents on patients with AD and PD; however, their impacts on DLB have not been studied yet. Since the neuropsychological and neuropathologic features of DLB overlap with AD and PD, and amyloid pathology have been also found in the brains of people with DLB, 129,130 it is assumed that anesthetic agents may also impact patients with DLB in a similar fashion as they do in AD and PD. Impacts of anesthetic agents on various neural systems are summarized in Table 6.
Table 6.
| Anesthetic Agents | Impacted Area/Neuronal Functionality |
|---|---|
| Desflurane, isoflurane and sevoflurane, barbiturates, opioids, morphine, and fentanyl (most of the general anesthetics) | Interacting with cholinergic receptors and suppresses acetylcholine release; thus inhibit cholinergic system resulting in significant deterioration of cognitive functions (learning, memorization) |
| Midazolam, Isoflurane, nitrous oxide, ketamine, diazepam, pentobarbital, thiopental, propofol, and ethanol | Anesthetic agents trigger widespread apoptotic neurodegeneration, hippocampal synaptic dysfunction, and causing memory loss |
| Morphine | Akinesia |
| Fentanyl | Muscle rigidity |
| Inhaled anesthetics such as isoflurane nitrous oxide and IV propofol at high concentration | Enhance β-amyloid oligomerization and affect dopamine release |
Abbreviations: DLB, dementia with Lewy bodies.
The cholinergic system (Ach producing neurotransmitter) is one of the most important neurotransmitter system of the brain that regulates high cognitive functions such as memory and learning. It is also responsible for dendrite arborization, neuronal development, and differentiation. General anesthetic agents decrease Ach release and inhibit cholinergic transmission to produce the effect of general anesthesia such as loss of consciousness, pain, memory, and voluntary movement. 134 Most of the general anesthetics such as desflurane, isoflurane, sevoflurane, barbiturate, opioids, morphine, fentanyl, and so forth interact with cholinergic receptors to produce inhibitory effect of cholinergic system, which is already compromised due to age- and dementia-related changes, 135 resulting in pathogenesis of further POCD and delirium. This causes challenges for patients with DLB who already have compromised neuronal transmission in the choice of anesthetic drugs.
Certain type of anesthetics like anesthetic cocktail (midazolam, isoflurane, and nitrous oxide) have been found to produce widespread apoptotic changes, deficits in hippocampal synaptic function, and persistent memory and learning impairments in rats. 131 Ketamine, diazepam, pentobarbital, thiopental, propofol, and ethanol may cause the similar neurotoxic effect. 124 Opioid at high concentration may also have a harmful effect on the nervous system. 136 Anesthesia induced neurodegeneration have also been reported to cause persistent hippocampal dysfunction and cognitive dysfunction. 137
Volatile anesthetics mainly isoflurane, as researched on animal, was found to produce AD like pathology like Aβ oligomerization and cytotoxicity and aberrant τ phosphorylation and aggregation. IV propofol may also produce same AD like pathology. 138
Anesthetic agents may interact with antiparkinsonian medications. Inhalational anesthetics at their normal clinical doses may inhibit synaptic reuptake of dopamine resulting in high extracellular dopamine concentration 139 and finally affect release of dopamine. 140 Hence, halothane in not recommended for patients taking l-DOPA. Those who are taking l-DOPA should continue their therapy during perioperative period. 141 Thiopental may produce parkinsonian symptoms 142 ; however there is no clear evidence supporting this effect. Fentanyl can cause muscle rigidity 132 and Morphine can cause akinesia at higher doses in patients with PD. 143
Pain
International association for the study of pain define pain as an unpleasant sensory and emotional experience associated with actual and potential tissue damage or described in terms of such damage. 144 It means patient’s perception of pain is important to understand the magnitude of pain. For patient with dementia, it is very important to recognize patient’s pain and further management of it as pain itself may impair cognitive function. 145 Poor management may contribute to delirium. Painful stimuli such as nociceptive input during the perioperative period are likely to alter gene expression and rapid neuronal sensitization. 146 This may damage the CNS damage 147 and thus it may cause a significant negative impact to patient with dementia. Moreover, it is important to note that dementia impairs patients’ perception of pain and ability to report it to the caregivers or physicians. Patients may not be able to recall pain sensation to evaluate and rate their pain. In such cases, health care professionals can evaluate the pain by using nonverbal techniques such as facial expression, moaning, body movement, and change in activity patterns.
For patient with dementia, treatment of pain is challenging as pain medications such as both opioid and NSAID can impair cognitive function and balance. 148 Morphine is significantly associated with POD. 146
Age-Related Factors
Aging results in several structural, biochemical, and functional changes to central and peripheral nervous systems in elderly patients. 129 Neuronal axon loss, neuronal cytoskeleton changes (eg, neuritic plaques), loss of dendrites, decrease in synaptic activity, and accumulation of amyloid protein are the common features of structural changes in nervous system. The biochemical changes include neurotransmitter imbalance, circulatory changes, and metabolic disturbances while the functional changes are gait changes, cognitive impairment, sleep disorder, and loss of physical equilibrium. The age-related deficits in nervous system and their impact on neurological dysfunction, postoperative complications, and response to medication are discussed in Table 7.
Table 7.
Age-Related Changes in Nervous System and Their Impacts. 129
| Affected Nervous System | Deficits With Aging | Effects on Patient’s Daily Activity, Medication, and Surgery |
|---|---|---|
| Central nervous system (CNS, brain and spinal cord) |
|
|
| Peripheral nervous system |
|
|
| Autonomic nervous system |
|
|
Elderly patients also experience age-related pharmacokinetic changes causing poor response to anesthetic agents and/or medications during surgery. The common pharmacokinetic changes and their impacts are as follows:
Decrease in hepatic blood flow: reduction of first pass elimination.
Reduction in renal blood flow: decrease in both creatinine clearance, glomerular filtration rate and tubular secretion activities.
Decrease in albumin or other binding proteins: requires higher fraction of plasma-free drug.
Decrease in total body water and muscle: smaller effective dose and longer duration of drug effect, especially for lipophilic drugs.
Summary
Dementia with LBs is a neurodegenerative dementing disorder. It is primarily characterized as cognitive decline, fluctuation of attention, intermittent visual hallucinations, spontaneous motor features, dysautonomia, and sleep disorders. Patients with DLB undergoing surgery may develop aggravated POCD and/or POD. Literature suggests that recurrence of delirium is more in patients with DLB when compared to patients with AD. Therefore, the chance of POD in patients with DLB may also be higher than other patients with dementia.
The exact causes of surgery-related complications in patients with DLB are still not fully understood and the literature on this topic appears to be inadequate. However, several research have been published on the surgery-related complications and risk factors in patients with AD and PD. To the best of authors’ knowledge, no clinical studies have been published on perioperative risk factors and postsurgical complications in patients with DLB. However, the existing literature suggests that DLB shares common neuropathology with AD and PD. This review article discusses the postoperative complications and various surgery-related risk factors for DLB in the light of AD and PD.
Patients with DLB are inherently sensitive to stresses, including changes in environment, daily routines, medications, and surgery, which can affect their cognition and behavior. These effects are more pronounced in elderly patients with DLB because of their marked baseline cholinergic losses. Many patients with DLB respond poorly to surgery and anesthesia, and their conditions may worsen if they have other medical complications along with dementia. Anesthetic agents can further impact the neurons in patients with DLB. The unfamiliar environment of the operating room and hospital may trigger challenging behavior and changes in cognition; the physical stresses on their body from the surgery and pain medications can also alter their cognition. Other age-related changes and health conditions unrelated to DLB can impair their ability to respond to stresses as well. As a result, patients with DLB are likely to face high risk of prolonged hospital stay, increased medical problems and/or mortality, causing significant physical, psychosocial, and financial burdens on individuals, family members, and society. Overall, this article highlights the need for clinical research to understand impacts of perioperative risk factors and postsurgical complications in patients with DLB.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. [DOI] [PubMed] [Google Scholar]
- 2. Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20(10):1255–1263. [DOI] [PubMed] [Google Scholar]
- 3. Colette W, Nancy E, Rosanne G, Karen Y. Differentiating Alzheimer disease, Lewy body, and Parkinson dementia using DSM-5. J Nurse Pract. 2014;10(4):262–270. [Google Scholar]
- 4. Carolyn WZ, Nikolaos S, Karina S, et al. Comparison of costs of care between patients with Alzheimer’s disease and dementia with Lewy bodies. Alzheimers Dement. 2008;4(4):280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richard A. Visual signs and symptoms of dementia with Lewy bodies. Clin Exp Optom. 2012;95(6):621–630. [DOI] [PubMed] [Google Scholar]
- 6. Pao WC, Boeve BF, Ferman TJ, et al. Polysomnographic findings in dementia with Lewy bodies. Neurologist. 2013;19(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stella K, James EG. Update on dementia with Lewy bodies, current translational geriatrics and experimental gerontology reports. 2013;2(3):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dugger BN, Boeve BF, Murray ME, et al. Rapid eye movement sleep behavior disorder and subtypes in autopsy-confirmed dementia with Lewy bodies. Mov Disord. 2012;27(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greicius MD, Geschwind MD, Miller BL. Presenile dementia syndromes: an update on taxonomy and diagnosis. J Neurol Neurosurg Psychiatry. 2002;72(6):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract. 2008;20:423–428. [DOI] [PubMed] [Google Scholar]
- 11. Jonsson L, Wimo A. The cost of dementia in Europe: a review of the evidence, and methodological considerations. Pharmacoeconomics. 2009;27:391–403. [DOI] [PubMed] [Google Scholar]
- 12. Hu CJ, Liao CC, Chang CC, Wu CH, Chen TL. Postoperative adverse outcomes in surgical patients with dementia: a retrospective cohort study. World J Surg. 2012;36(9):2051–2058. [DOI] [PubMed] [Google Scholar]
- 13. Jennifer RVM. Dementia with Lewy bodies. Semin Neurol. 2013;33:330–335. [DOI] [PubMed] [Google Scholar]
- 14. Huang Y, Halliday G. Can we clinically diagnose dementia with Lewy bodies yet? Transl Neurodegener. 2013;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Auning E, Rongve A, Fladby T, et al. Early and presenting symptoms of dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2011;32(3):202–208. [DOI] [PubMed] [Google Scholar]
- 16. Hamilton JM, Landy KM, Salmon DP, Hansen LA, Masliah E, Galasko D. Early visuospatial deficits predict the occurrence of visual hallucinations in autopsy-confirmed dementia with Lewy bodies. Am J Geriatr Psychiatry. 2012; 20(9): 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ballard CG, Aarsland D, McKeith I, et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology. 2002;59(11):1714–1720. [DOI] [PubMed] [Google Scholar]
- 18. McKeith I. Dementia with Lewy bodies and Parkinson’s disease with dementia: where two worlds collide. Pract Neurol. 2007;7(6):374–382. [DOI] [PubMed] [Google Scholar]
- 19. Onofrj M, Varanese S, Bonanni L, et al. Cohort study of prevalence and phenomenology of tremor in dementia with Lewy bodies. J Neurol. 2013;260(7):1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging. 2013;30(8):603–611. [DOI] [PubMed] [Google Scholar]
- 21. Sato Y, Berrios GE. Extracampine hallucinations. Lancet. 2002;361(9367):1479–1480. [DOI] [PubMed] [Google Scholar]
- 22. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245–250. [DOI] [PubMed] [Google Scholar]
- 23. Horimoto Y, Matsumoto M, Akatsu H. Autonomic dysfunctions in dementia with Lewy bodies. J Neurol. 2003;250(5):530–533. [DOI] [PubMed] [Google Scholar]
- 24. Auning E, Rongve A, Fladby T, et al. Early and presenting symptoms of dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2011;32(3):202–208. [DOI] [PubMed] [Google Scholar]
- 25. Koehler NKU, Stransky E, Shing M, Gaertner S, Meyer M. Altered serum IgG levels to α-synuclein in dementia with Lewy bodies and Alzheimer’s disease. PLoS One. 2013;8(5):e64649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eva N, Alex K, Monica E, Valentina SS, Lars L, Mats H. Protofibril-binding antibodies for treatment and diagnostics of Parkinson’s Disease, Dementia with Lewy Bodies and other Alpha-Synucleinopathies, US patent no. 8632776 B2.
- 27. Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson’s disease with dementia from dementia with Lewy bodies? Acta Neuropathol. 2006;112:253–260. [DOI] [PubMed] [Google Scholar]
- 28. Perneczky R, Haussermann P, Diehl-Schmid J, et al. Metabolic correlates of brain reserve power in dementia with Lewy bodies: an FDG PET study. Dement Geriatr Cogn Disord. 2007;23(6):416–422. [DOI] [PubMed] [Google Scholar]
- 29. Armstrong RA, Cairns NJ, Lantos PL. The spatial patterns of Lewy bodies, senile plaques and neurofibrillary tangles in dementia with Lewy bodies. Exp Neurol. 1998;150(1):122–127. [DOI] [PubMed] [Google Scholar]
- 30. Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125(pt 2):391–403. [DOI] [PubMed] [Google Scholar]
- 31. Woodruff BK, Graff-Radford, Ferm TJ, et al. Family history of dementia is a risk factor for Lewy body disease. Neurology. 2006;66(12):1949–1950. [DOI] [PubMed] [Google Scholar]
- 32. Harding AJ, Das A, Kril JJ, Brooks WS, Duffy D, Halliday GM. Identification of families with cortical Lewy body disease. Am J Med Genet B Neuropsychiatr Genet. 2004;128B(1):118–122. [DOI] [PubMed] [Google Scholar]
- 33. Nishioka K, Wider C, Vilariño-Güell C, et al. Association of alpha-, beta-, and gamma-synuclein with diffuse Lewy body disease. Arch Neurol. 2010;67(8):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohtake H, Limprasert P, Fan Y, et al. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63(5):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meeus B, Theuns J, Van Broeckhoven C. The genetics of dementia with Lewy bodies: what are we missing? Arch Neurol. 2012;69(9):1113–1118. [DOI] [PubMed] [Google Scholar]
- 36. Bonifati V. Recent advances in the genetics of dementia with Lewy bodies. Curr Neurol Neurosci Rep. 2008;8(3):187–189. [DOI] [PubMed] [Google Scholar]
- 37. Tsuang D, Leverenz JB, Lopez OL, et al. APOE ∊4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Debby T, James LB, Oscar LL, Doug G, Cyrus Z. Genetic Variant Increases Risk for Dementia in Lewy Body Diseases; 2012. Web site. http://www.lbda.org/content/genetic-mutation-increases-risk-dementia-lewy-body-diseases.
- 39. Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67(5):908–910. [DOI] [PubMed] [Google Scholar]
- 40. Creese B, Ballard C, Aarsland D, Londos E, Sharp S, Jones E. Determining the association of the 5HTTLPR polymorphism with delusions and hallucinations in Lewy body dementia. Am J Geriatr Psychiatry. 2013;22(6):580–586. [DOI] [PubMed] [Google Scholar]
- 41. Stubendorff K, Larsson V, Ballard C, Minthon L, Aarsland D, Londos E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson’s disease with dementia: a prospective study. BMJ Open. 2014;4(7):e005158. doi:10.1136/ bmjopen-2014-005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie Z, Tanzi RE. Alzheimer’s disease and post-operative cognitive dysfunction. Exp Gerontol. 2006;41(4):346–359. [DOI] [PubMed] [Google Scholar]
- 43. Marsh SE, Blurton-Jones M. Examining the mechanisms that link beta-amyloid and alpha-synuclein pathologies. Alzheimers Res Ther. 2012;4(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kotzbauer PT, Giasson BI, Kravitz AV, et al. Fibrillization of alpha-synuclein and tau in familial Parkinson’s disease caused by the A53T alpha-synuclein mutation. Exp Neurol. 2004;187(2):279–288 [DOI] [PubMed] [Google Scholar]
- 45. Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–640. [DOI] [PubMed] [Google Scholar]
- 46. Badiola N, de Oliveira RM, Herrera F, et al. Tau enhances alpha-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS One. 2011;6(10):e26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uversky VN. Neuropathology, biochemistry, and biophysics of alphasynuclein aggregation. J Neurochem. 2007;103(1):17–37. [DOI] [PubMed] [Google Scholar]
- 48. Ciaccioli G, Martins A, Rodrigues C, Vieira H, Calado P. A powerful yeast model to investigate the synergistic interaction of alpha-synuclein and tau in neurodegeneration. PLoS One. 2013;8(2):e55848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Colom-Cadena M, Gelpi E, et al. Confluence of α-synuclein, tau, and β-amyloid pathologies in dementia with Lewy Bodies. J Neuropathol Exp Neurol Vol. 2013;72(12):1203–1212. [DOI] [PubMed] [Google Scholar]
- 50. Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74(7):852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burn DJ, McKeith IG. Current treatment of dementia with Lewy bodies and dementia associated with Parkinson’s disease. Mov Disord. 2003;18(suppl 6):S72–S79. [DOI] [PubMed] [Google Scholar]
- 52. Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies. Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev. 2012;3:CD006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer’s patients with symptoms suggestive of concomitant Lewy body pathology. Curr Med Res Opin. 2006;22(1):49–59. [DOI] [PubMed] [Google Scholar]
- 54. Thomas AJ, Burn DJ, Rowan EN, et al. A comparison of the efficacy of donepezil in Parkinson’s disease with dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry. 2005;20(10):938–944. [DOI] [PubMed] [Google Scholar]
- 55. Mori S, Mori E, Iseki E, Kosaka K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: preliminary findings from an open-label study. Psychiatry Clin Neurosci. 2006;60(2):190–195. [DOI] [PubMed] [Google Scholar]
- 56. Rowan E, McKeith IG, Saxby BK, et al. Effects of donepezil on central processing speed and attentional measures in Parkinson’s disease with dementia and dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2007;23(3):161–167. [DOI] [PubMed] [Google Scholar]
- 57. Grace J, Daniel S, Stevens T, et al. Long-term use of rivastigmine in patients with dementia with Lewy bodies: an open-label trial. Int Psychogeriatr. 2001;13(2):199–205. [DOI] [PubMed] [Google Scholar]
- 58. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401–405. [DOI] [PubMed] [Google Scholar]
- 59. McKeith IG, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(924):2031–2036. [DOI] [PubMed] [Google Scholar]
- 60. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613–618. [DOI] [PubMed] [Google Scholar]
- 61. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969–977. [DOI] [PubMed] [Google Scholar]
- 62. Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76(9):1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76(9):1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lucetti C, Logi C, Del Dotto P, et al. Levodopa response in dementia with Lewy bodies: a 1-year follow-up study. Parkinsonism Relat Disord. 2010;16(8):522–526. [DOI] [PubMed] [Google Scholar]
- 65. Molloy S, McKeith IG, O’Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76(9):1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boot BP, McDade EM, McGinnis SM, Boeve BF. Treatment of dementia with Lewy bodies. Curr Treat Options Neurol. 2013;15(6):738–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nelson PT, Kryscio RJ, Jicha GA, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73(14):1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–2036. [DOI] [PubMed] [Google Scholar]
- 69. Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry. 1999;60(2):107–115. [DOI] [PubMed] [Google Scholar]
- 70. Walker Z, Grace J, Overshot R, et al. Olanzapine in dementia with Lewy bodies: a clinical study. Int J Geriatr Psychiatry. 1999;14(6):459–466. [PubMed] [Google Scholar]
- 71. Friedman JH, Fernandez HH. A typical antipsychotics in Parkinson sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156–170. [DOI] [PubMed] [Google Scholar]
- 72. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 73. Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. 2010;6(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- 74. Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EM. Constipation in Parkinson’s disease: objective assessment and response to psyllium. Mov Disord. 1997;12(6):946–951. [DOI] [PubMed] [Google Scholar]
- 75. Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov Disord. 2007;22(9):1239–1244. [DOI] [PubMed] [Google Scholar]
- 76. Wood LD, Neumiller JJ, Setter SM, Dobbins EK. Clinical review of treatment options for select nonmotor symptoms of Parkinson’s disease. Am J Geriatr Pharmacother. 2010;8(4):294–315. [DOI] [PubMed] [Google Scholar]
- 77. Ondo WG, Kenney C, Sullivan K, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology. 2012;78(21):1650–1654. [DOI] [PubMed] [Google Scholar]
- 78. Lawrence VA, Hilsenbeck SG, Mulrow CD, Dhanda R, Sapp J, Page CP. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1999;10(12):671–678. [DOI] [PubMed] [Google Scholar]
- 79. Tognoni P, Simonato A, Robutti N, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch Gerontol Geriatr. 2011;52(3):166–169. [DOI] [PubMed] [Google Scholar]
- 80. Ramaiah R, Lam AR. Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin. 2009;27(3):485–496. [DOI] [PubMed] [Google Scholar]
- 81. An LN, Yue Y, Guo WZ, et al. Surgical trauma induces iron accumulation and oxidative stress in a rodent model of postoperative cognitive dysfunction. Biol Trace Elem Res. 2013;151(2):277–283. [DOI] [PubMed] [Google Scholar]
- 82. Lisbeth E. Dissecting the possible influences of anesthesia and surgery on Alzheimer’s disease. Neurodegenerative Dis Manag. 2013;3(2):115–122. [Google Scholar]
- 83. American Psychiatric Association. Diagnostic Criteria From DSM-IV-TR. Vol xii. Washington, DC: American Psychiatric Association; 2000:370. [Google Scholar]
- 84. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(suppl 1):i41–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112(3):440–451. [DOI] [PubMed] [Google Scholar]
- 86. Ramaiah R, Lam AM. Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin. 2009;27(3):485–496. [DOI] [PubMed] [Google Scholar]
- 87. Sauër AM, Kalkman C, Van DD. Postoperative cognitive decline. J Anesthesiol. 2009;23(2):256–259. [DOI] [PubMed] [Google Scholar]
- 88. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. [DOI] [PubMed] [Google Scholar]
- 89. Bilotta F, Doronzio A, Stazi E, et al. Postoperative cognitive dysfunction: toward the Alzheimer’s disease pathomechanism hypothesis. J Alzheimers Dis. 2010;22(3):81–89. [DOI] [PubMed] [Google Scholar]
- 90. Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984). Neurology. 1996;46(1):154–159. [DOI] [PubMed] [Google Scholar]
- 91. Nagy A. Formation of mouse chimeric embryos from ES cells. In: Houdebine L-M, ed. Transgenic Animals: Generation and Use., Amsterdam: Harwood Academic Publishers; 1997:167–172. [Google Scholar]
- 92. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The nun study. JAMA. 1997;277(10):813–817. [PubMed] [Google Scholar]
- 93. Green KN, Boyle JP, Peers C. Hypoxia potentiates exocytosis and Ca2+ channels in PC12 cells via increased amyloid beta peptide formation and reactive oxygen species generation. J Physiol. 2002;541(3):1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Webster NJ, Green KN, Settle VJ, Peers C, Vaughan PF. Altered processing of the amyloid precursor protein and decreased expression of ADAM 10 by chronic hypoxia in SH-SY5Y: no role for the stress-activated JNK and p38 signalling pathways. Mol Brain Res. 2004;130(1-2):161–169. [DOI] [PubMed] [Google Scholar]
- 95. Daval JL, Pourié G, Grojean S, et al. Neonatal hypoxia triggers transient apoptosis followed by neurogenesis in the rat CA hippocampus, pediatric research. Pediatric Res. 2004;55(4):561–567. [DOI] [PubMed] [Google Scholar]
- 96. Namura S, Zhu J, Fink K, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18(10):3659–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8(2):207–219. [DOI] [PubMed] [Google Scholar]
- 98. Xie Z, Moir RD, Romano DM, Tesco G, Kovacs DM, Tanzi RE. Hypocapnia induces caspase-3 activation and increases Abeta production. Neurodegener Dis. 2004;1(1):29–37. [DOI] [PubMed] [Google Scholar]
- 99. Stankiewicz JM, Brass SD. Role of iron in neurotoxicity: a cause for concern in the elderly? Curr Opin Clin Nutr Metab Care. 2009;12(1):22–29. [DOI] [PubMed] [Google Scholar]
- 100. Ma L, Wang W, Zhao M, Li M. Foot-shock stress-induced regional iron accumulation and altered iron homeostatic mechanisms in rat brain. Biol Trace Element Res. 2008;126(1-3):204–213. [DOI] [PubMed] [Google Scholar]
- 101. Voyer P, Richard S, Doucet L, Carmichael PH. Factors associated with delirium severity among older persons with dementia. J Neurosci Nurs. 2011;43(2):62–69. [DOI] [PubMed] [Google Scholar]
- 102. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 103. Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50(3):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cole MG, McCusker J, Voyer P, et al. Subsyndromal delirium in older long-term care residents. Incidence, risk factors, and outcomes. J Am Geriatr Soc. 2011;59(10):1829–1836. [DOI] [PubMed] [Google Scholar]
- 105. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brooks M. New scoring tool gauges severity of delirium. Medscape Medical News [serial online]. Ann Intern Med. 2014;160:526–533, 574–575.24733193 [Google Scholar]
- 108. Zaal IJ, Slooter AJ. Light levels of sedation and DSM-5 criteria for delirium. Eur J Intens Care Med. 2014;40(2):300–301. [DOI] [PubMed] [Google Scholar]
- 109. Burns A, Gallagley A, Byrne J. Delirium. J Neurol Neurosurg Psychiatry. 2004;75(3):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. McCusker J, Cole M, Bellavance F, Primeau F. Reliability and validity of a new measure of severity of delirium. Int Psychogeriatr. 1998;10(4):421–433. [DOI] [PubMed] [Google Scholar]
- 112. Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age Ageing. 2009;38(1):19–26. [DOI] [PubMed] [Google Scholar]
- 113. Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099–1105. [DOI] [PubMed] [Google Scholar]
- 114. Vardy E, Holt R, Gerhard A, Richardson A, Snowden J, Neary D. History of a suspected delirium is more common in dementia with Lewy bodies than Alzheimer’s disease: a retrospective study. Int J Geriatr Psychiatry. 2014;29(2):178–181. [DOI] [PubMed] [Google Scholar]
- 115. Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s—delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365. [DOI] [PubMed] [Google Scholar]
- 116. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27(1):44–49. [DOI] [PubMed] [Google Scholar]
- 117. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(1):i41–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Minden SL, Carbone LA, Barsky A, et al. Predictors and outcomes of delirium. Gen Hosp Psychiatry. 2005;27(3):209–214. [DOI] [PubMed] [Google Scholar]
- 119. Rade MC, Yadeau JT, Ford C, Reid MC. Postoperative delirium in elderly patients after elective hip or knee arthroplasty performed under regional anesthesia. HSS J. 2011;7(2):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Johnston M. Anxiety in surgical patients. Psychol Med. 1980;10(1):145–152. [DOI] [PubMed] [Google Scholar]
- 121. Mathews A, Ridgeway V. Personality and surgical recovery: a review. Br J Clin Psychol. 1981;20(4):243–260. [DOI] [PubMed] [Google Scholar]
- 122. Alice C. Older people with dementia in the perioperative care environment: key issues for perioperative nursing. Br J Anaesth Recovery Nurs. 2011;12(3-4):45–49. [Google Scholar]
- 123. Gerdner L, Buckwalter K, Reed D. Impact of a psychoeducational intervention on caregiver response to behavioral problems. Nurs Res. 2002;51(6):363–374. [DOI] [PubMed] [Google Scholar]
- 124. Borsook D, George E, Kussman B, Becerra L. Anesthesia and perioperative stress: consequences on neural networks and postoperative behaviors. Prog Neurobiol. 2010;92(4):601–612. [DOI] [PubMed] [Google Scholar]
- 125. Zaal IJ, Slooter AJ. Delirium in critically ill patients: epidemiology, pathophysiology, diagnosis and management. Drugs. 2012;72(11):1457–1471. [DOI] [PubMed] [Google Scholar]
- 126. Angele MK, Chaudry IH. Surgical trauma and immunosuppression: pathophysiology and potential immunomodulatory approaches. Langenbecks Arch Surg. 2005;390(4):333–341. [DOI] [PubMed] [Google Scholar]
- 127. Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38(12):1336–1345. [DOI] [PubMed] [Google Scholar]
- 128. Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106(3):436–443. [DOI] [PubMed] [Google Scholar]
- 129. Enrique N, Karen M, Karen LB. Comparison of dementia with Lewy bodies to Alzheimer’s disease and Parkinson’s disease with dementia. Mov Disord. 2004;19(1):60–67. [DOI] [PubMed] [Google Scholar]
- 130. Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Klausner JM, Caspi J, Lelcuk S, et al. Delayed muscle rigidity and respiratory depression following fentanyl anesthesia. Arch Surg. 1988;123(1):66–67. [DOI] [PubMed] [Google Scholar]
- 133. Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB. Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth. 2006;97(4):445–452. [DOI] [PubMed] [Google Scholar]
- 134. Ma J, Shen B, Stewart LS, Herrick IA, Leung LS. The septohippocampal system participates in general anesthesia. J Neurosci. 2002;22(2):RC200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pratico C, Quattrone D, Lucanto T, Amato A, Penna O, Fodale V. Drugs of anesthesia acting on central cholinergic system may cause post-operative cognitive dysfunction and delirium. Med Hypotheses. 2005;65(5):972–982. [DOI] [PubMed] [Google Scholar]
- 136. Keogh CF, Andrews GT, Spacey SD, Forkheim KE, Graeb DA. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon”. Am J Roentgenol. 2003;180(3):847–850. [DOI] [PubMed] [Google Scholar]
- 137. Stratmann G, Sall JW, May LD, Loepke AW, Lee MT. Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function. Anesth Analg. 2010;110(2):431–437. [DOI] [PubMed] [Google Scholar]
- 138. Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101(3):703–709. [DOI] [PubMed] [Google Scholar]
- 139. El-Maghrabi EA, Eckenhoff RG. Inhibition of dopamine transport in rat brain synaptosomes by volatile anesthetics. Anesthesiology. 1993;78(4):750–756. [DOI] [PubMed] [Google Scholar]
- 140. Mantz J, Varlet C, Lecharny JB, Henzel D, Lenot P, Desmonts JM. Effects of volatile anaesthetics, thiopental and ketamine on spontaneous and depolarization-evoked dopamine release from striatal synaptosomes in the rat. Anesthesiology. 1994;80(2):352–363. [DOI] [PubMed] [Google Scholar]
- 141. Safiya IS, Himanshu V. Parkinson’s disease and anaesthesia. Indian J Anaesth. 2011;55(3):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Easdown LJ, Tessler MJ, Minuk J. Upper airway involvement in Parkinson’s disease resulting in postoperative respiratory failure. Can J Anaesth. 1995;42(4):344–347. [DOI] [PubMed] [Google Scholar]
- 143. Berg D, Becker G, Reiners K. Reduction of dyskinesia and induction of akinesia induced by morphine in two parkinsonian patients with severe sciatica. J Neural Transm. 1999;106(7-8):725–728. [DOI] [PubMed] [Google Scholar]
- 144. Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle: IASP Press; 1986. [Google Scholar]
- 145. Martha DB, Evelyn H, Victor TC, Michael HC, Lynn S. Cognitive impairment and pain management: review of issues and challenges. J Rehabil Res Dev. 2007;44(2):315–330. [DOI] [PubMed] [Google Scholar]
- 146. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3):S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Colette W, Nancy EE, Rosanne G, Karen Y. Differentiating Alzheimer disease, Lewy body, and Parkinson dementia using DSM-5. J Nurse Pract. 2014;10(4):262–270. [Google Scholar]


