Abstract
Objective:
A meta-analysis was performed to better clarify the association between hemochromatosis (HFE) gene and the risk of Parkinson’s disease (PD).
Methods:
Pooled odds ratio (OR) with 95% confidence interval (CI) was calculated from fixed- and random-effect models. Heterogeneity among studies was evaluated using the I2 and Q test. Egger’s test was used to estimate the publication bias.
Results:
We identified 8 articles with 9 independent studies for this meta-analysis. The present meta-analysis showed no significant association of Y allele with the risk of PD in dominant (OR = 0.87, 95% CI = 0.70-1.09), recessive (OR = 1.58, 95% CI = 0.61-4.10), and codominant (OR = 0.88, 95% CI = 0.72-1.09) models for C282Y. There were also no significant associations of D allele with the risk of PD in dominant (OR = 1.04, 95% CI = 0.87-1.24), recessive (OR = 1.23, 95% CI = 0.70-2.18), and codominant (OR = 1.04, 95% CI = 0.89-1.22) genetic models for H63D. No publication bias was detected.
Conclusion:
The meta-analysis indicated that C282Y and H63D polymorphisms in the HFE gene might not be associated with PD.
Keywords: hemochromatosis, gene, Parkinson’s disease, meta-analysis
Introduction
Parkinson’s disease (PD) is the second most common form of neurodegenerative disease, clinically characterized by resting tremor, muscular rigidity, bradykinesia, and postural instability. Parkinson’s disease affects 2% of the population older than 65 years. 1,2 It is considered a multifactorial disease, in which genetic factors play an important role. Some Mendelian genes linked to inherited forms of PD have been identified, and a number of common genetic variants have been described in many association studies as risk factors for the sporadic form of the disease. 3 Several reports showed an involvement of iron-mediated oxidative stress in the pathogenesis of PD. 4
The gene of hemochromatosis (HFE) encodes the HFE protein whose function is to regulate iron absorption. The role of HFE as an important regulator of cellular iron homeostasis determines its potential involvement in the pathogenesis of PD. Homozygosity or compound heterozygosity for the variants C282Y and H63D of the HFE gene can lead to iron overload and to the disorder known as hereditary HFE. 4,5 The C282Y mutation is highly penetrant and leads to intracellular sequestration of iron. 6 The H63D mutation is less penetrant and is a susceptibility factor for HFE. 7 Its role in iron metabolism makes HFE a potential candidate gene for PD. Studies have been conducted to evaluate the effect of C282Y and H63D genetic polymorphisms in the HFE gene and the risk of PD, however the results were conflicting. Hence, we perform the current meta-analysis to identify the association of HFE gene with the risk of PD.
Materials and Methods
Literature Search
The available articles published in English (up to January 2015) were identified by extended computer-based searches from the following databases: (1) PubMed and (2) Web of Science. The following key words were used: (“HFE” or “hemochromatosis” or “C282Y”or “H63D”) and (“polymorphism” or “mutation” or “genes”) and (“Parkinson’s disease” or “Parkinson” or “PD”). We also reviewed the references cited in the studies and review articles to identify additional studies not captured by our database searches.
Inclusion Criteria
The inclusion criteria were as follows: (1) case–control or cohort study published as original study to evaluate the association between C282Y and H63D polymorphisms in the HFE gene and risk of PD; (2) numbers for the genotype were reported in case and control groups for case–control studies, exposed and unexposed groups for cohort studies for each genotype, or data provided to calculate them; and (3) participants in each study should come from the same ethnicity and period. The most recent and complete articles were chosen if data from the same population had been published more than once. Two investigators carefully reviewed all identified studies independently to determine whether an individual study was eligible for inclusion in this meta-analysis.
Data Extraction
Two investigators collected the data independently and reached a consensus on all items. The following basic information was extracted from the eligible studies: first author, journal, year of publication, country, ethnicity of studied population, sample size, mean age, and distributions of allele and genotype. When it came to conflicting evaluations, it was resolved by the third reviewer.
Statistical Analysis
Departure from Hardy-Weinberg equilibrium (HWE) for the C282Y and H63D and genotype distribution of HFE gene in cases and controls was tested by χ2 analysis with exact probability (HWE: P > .05). Odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of the association of the C282Y and H63D polymorphisms in HFE gene with the risk of PD. We conducted analysis for 2 polymorphisms considering dominant (YY + CY vs CC) and (DD + HD vs HH), recessive (YY vs CY + CC) and (DD vs HD + HH), and codominant (Y vs C) and (D vs H) models for C282Y and H63D, respectively. The I2 of Higgins and Thompson 8 and Q test were used to assess heterogeneity among studies. The DerSimonian and Laird random effect model (REM) was adopted as the pooling method if substantial heterogeneity is present (I 2 > 50%) 9 ; otherwise, the fixed-effect model (FEM) was used as the pooling method. Influence analysis 10 was conducted to describe how robust the pooled estimator is to the removal of individual studies. If the main estimate of an individual study’s omitted analysis lies outside the 95% CI of the combined analysis, it is suspected of excessive influence. Egger’s test 11 was used to estimate the publication bias. Besides, both theoretical and empirical evidence suggest that general genetic variants causally associated with common diseases will have small effects (risk ratios mostly <3.0), 12,13 considering the fact that original studies with relatively small participants might be underpowered to detect the effect. Thus, for sensitivity analysis, we further excluded the studies with OR > 3.0 as the criteria to control the impact within each single study on the pooled effect. STATA version 10.0 (Stata Corporation, College Station, Texas) was used to perform statistical analyses. All reported probabilities (P values) were 2 sided, with P value less than .05 considered statistically significant.
Results
Characteristics of Studies
We identified 8 articles 14 -21 with 9 eligible outcomes for this meta-analysis, including 9 outcomes for the C282Y polymorphism and 8 outcomes for the H63D polymorphism. All included articles were case–control designs. General characteristics and genotype distributions of the above-mentioned polymorphisms are summarized in Tables 1 and 2. Figure 1 presents the flowchart for exclusion/inclusion process.
Table 1.
Characteristics of the C282Y Polymorphism in the HFE Gene Genotype Distributions in Studies Included in This Meta-Analysis.
| Author | Year | Country | Ethnicity | Number (Case/Control) | Genotype CC/CY/YY | P for HWE in control | P for HWE in case | References | |
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| Borie et al14 | 2002 | Paris | Europe | 71/57 | 66/5/0 | 52/5/0 | .73 | .76 | 14 |
| Buchanan et al21 | 2002 | Australia | Oceania | 438/485 | 391/46/1 | 405/76/4 | .84 | .77 | 21 |
| Dekker et al16 | 2003 | Netherlands | Europe | 101/2136 | 89/10/2 | 1838/290/8 | .34 | .06 | 16 |
| Dekker et al16 | 2003 | Netherlands | Europe | 44/2136 | 38/6/0 | 1838/290/8 | .34 | .63 | 16 |
| Guerreiro et al18 | 2006 | Portugal | Europe | 132/115 | 114/18/0 | 110/5/0 | .81 | .40 | 18 |
| Aamodt et al15 | 2007 | Norway | Europe | 315/396 | 263/50/2 | 330/64/2 | .56 | .82 | 15 |
| Halling et al20 | 2008 | Denmark | Europe | 54/110 | 44/10/0 | 87/21/2 | .58 | .45 | 20 |
| Greco et al17 | 2011 | Italy | Europe | 181/180 | 178/3/0 | 177/3/0 | .91 | .91 | 17 |
| Mariani et al19 | 2013 | Italy | Europe | 73/139 | 70/3/0 | 137/2/0 | .93 | .93 | 19 |
Abbreviations: HFE, hemochromatosis; HWE, Hardy-Weinberg equilibrium.
Table 2.
Characteristics of the H63D Polymorphism in the HFE Gene Genotype Distributions in Studies Included in This Meta-Analysis.
| Author | Year | Country | Ethnicity | Number (Case/Control) | Genotype HH/HD/DD | P for HWE in Control | P for HWE in Case | References | |
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||
| Borie et al14 | 2002 | Paris | Europe | 66/59 | 42/23/1 | 39/20/0 | .12 | .27 | 14 |
| Dekker et al16 | 2003 | Netherlands | Europe | 122/2567 | 89/31/2 | 1838/661/68 | .36 | .71 | 16 |
| Dekker et al16 | 2003 | Netherlands | Europe | 54/2567 | 38/16/0 | 1838/661/68 | .36 | .20 | 16 |
| Guerreiro et al18 | 2006 | Portugal | Europe | 132/115 | 89/38/5 | 74/39/2 | .22 | .71 | 18 |
| Aamodt et al15 | 2007 | Norway | Europe | 330/428 | 263/60/7 | 330/91/7 | .80 | .11 | 15 |
| Halling et al20 | 2008 | Denmark | Europe | 67/125 | 44/20/3 | 87/33/5 | .41 | .71 | 20 |
| Greco et al17 | 2011 | Italy | Europe | 181/180 | 125/49/7 | 141/36/3 | .69 | .43 | 17 |
| Mariani et al19 | 2013 | Italy | Europe | 77/139 | 53/24/0 | 99/38/2 | .44 | .11 | 19 |
Abbreviations: HFE, hemochromatosis; HWE, Hardy-Weinberg equilibrium.
Figure 1.
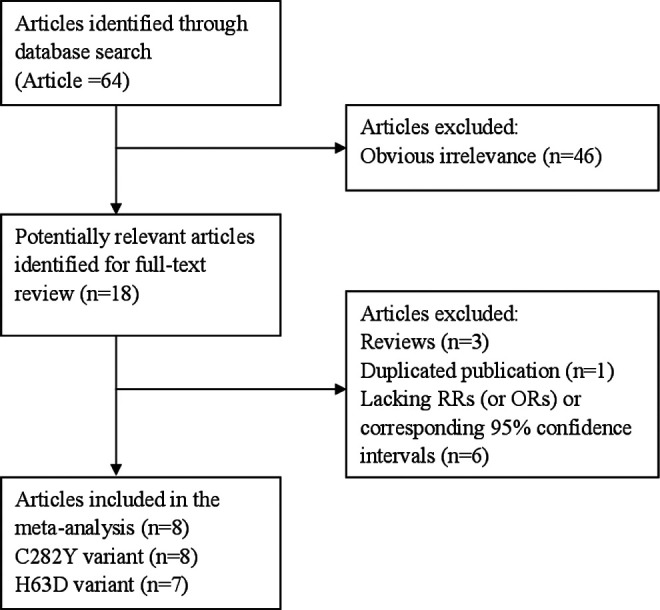
Flowchart of meta-analysis for exclusion/inclusion of studies.
Quantitative Synthesis
The results of the pooled analysis are summarized in Table 3.
Table 3.
Pooled Measures on the Relationship of C282Y and H63D polymorphisms in the HFE Gene With Risk of Parkinson’s Disease.a,b,c
| Polymorphism | Inherited Model | All Included Articles | Q Value | I2 (%) | P for Heterogeneity | After Excluding Articles With OR > 3.0 | I2 (%) | P for Heterogeneity | Articles Excluded | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | Pooled OR (95% CI) | |||||||||||
| FEM | REM | FEM | REM | |||||||||
| All data | C282Y | Dominant | 0.87 (0.70-1.09) | 0.95 (0.69-1.30) | 12.56 | 36.3 | .13 | 0.82 (0.65-1.02) | 0.82 (0.65-1.02) | 0.0 | .63 | 18 |
| Recessived | 1.58 (0.61-4.10) | 1.40 (0.43-4.58) | 11.56 | 30.8 | .22 | 0.77 (0.23-2.56) | 0.77 (0.23-2.56) | 0.0 | .56 | 14,16-19 | ||
| Codominant | 0.88 (0.72-1.09) | 0.95 (0.71-1.29) | 12.74 | 37.2 | .12 | 0.83 (0.67-1.03) | 0.83 (0.67-1.03) | 0.0 | .55 | 18 | ||
| H63D | Dominant | 1.04 (0.87-1.24) | 1.04 (0.87-1.24) | 7.00 | 0.0 | .60 | – | – | – | – | – | |
| Recessive | 1.23 (0.70-2.18) | 1.23 (0.70-2.18) | 7.00 | 0.0 | .78 | – | – | – | – | – | ||
| Codominant | 1.04 (0.89-1.22) | 1.04 (0.89-1.22) | 7.00 | 0.0 | .58 | – | – | – | – | – | ||
| Europe | C282Y | Dominant | 1.04 (0.80-1.36) | 1.05 (0.79-1.41) | 8.64 | 7.4 | .37 | 0.95 (0.72-1.26) | 0.95 (0.72-1.3) | 0.0 | .93 | 18 |
| Recessived | 2.37 (0.83-6.82) | 2.37 (0.83-6.82) | 8.00 | 0.0 | .43 | 1.19 (0.29-5.00) | 1.19 (0.29-5.0) | 0.0 | .66 | 16 | ||
| Codominant | 1.05 (0.82-1.35) | 1.05 (0.82-1.36) | 8.07 | 0.9 | .42 | 0.97 (0.75-1.26) | 0.97 (0.75-1.3) | 0.0 | .94 | 18 | ||
Abbreviations: CI, confidence interval; FEM, fixed-effect model; HFE, hemochromatosis; OR, odds ratio; REM, random-effect model.
aDominant model: YY + CY versus CC for C282Y and DD + HD versus HH for H63D.
bRecessive model: YY versus CY + CC for C282Y and DD versus HD + HH for H63D.
cCodominant model: Y versus C for C282Y and for D versus H for H63D.
dFour articles (Borie et al, Guerreiro et al, Greco et al, and Mariani et al) in C282Y were not sufficient to calculated pooled OR.
The C282Y Polymorphism
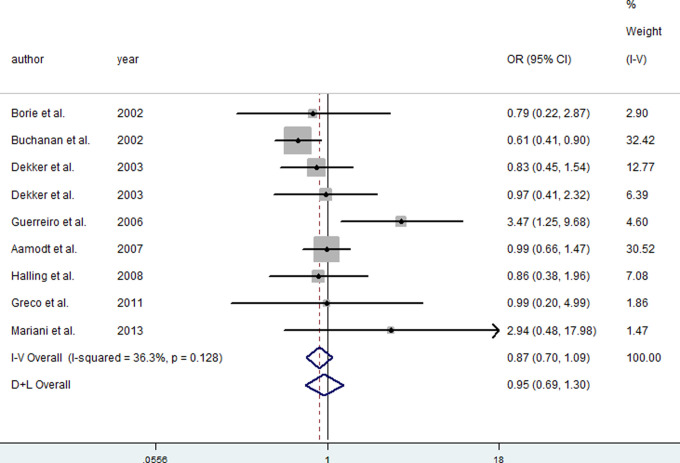
For the C282Y polymorphism, overall, no significant association was found between the Y allele and the risk of PD in dominant (FEM: OR = 0.87, 95% CI = 0.70-1.09), recessive (FEM: OR = 1.58, 95% CI = 0.61-4.10), and codominant (FEM: OR = 0.88, 95% CI = 0.72-1.09) models. Considering 8 of the 9 studies come from Europe, the subgroup analysis for European population was conducted. The Y allele also found no association with risk of PD in dominant (FEM: OR = 1.04, 95% CI = 0.80-1.36), recessive (FEM: OR = 2.37, 95% CI = 0.83-6.82), and codominant (FEM: OR = 1.05, 95% CI = 0.82-1.35) models. All included articles were in HWE for cases and controls. The associations were not altered significantly after excluding articles with OR > 3.0. 16,18 Figure 2 presents the forest plot of OR for PD in dominant model of the C282Y polymorphism in the HFE gene.
Figure 2.
Meta-analysis for the risk of Parkinson’s disease (PD) depending on the C282Y polymorphism in the hemochromatosis (HFE) gene. Forest plots of relationship between the C282Y polymorphism in the HFE gene and risk of PD in dominant model. White diamond denotes the pooled odds ratio (OR). Black squares indicate the OR in each study, with square sizes inversely proportional to the standard error of the OR. Horizontal lines represent 95% confidence intervals (CIs).
Note: Figure is available in full colour in the online version at aja.sagepub.com
The H63D Polymorphism
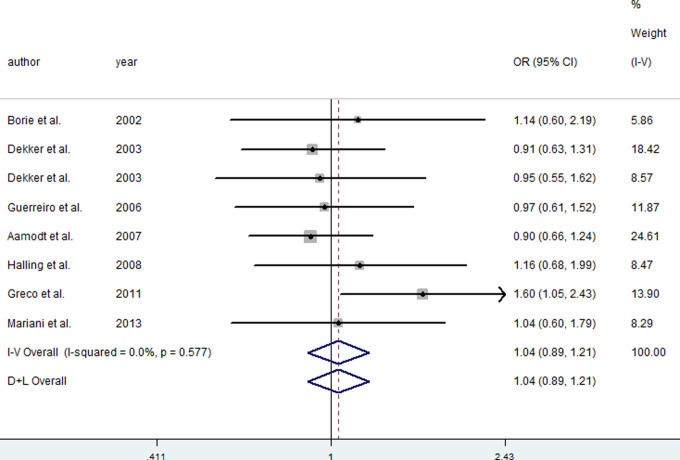
For the H63D polymorphism, this meta-analysis showed no significant effect of the D allele on risk of PD in dominant (FEM: OR = 1.04, 95% CI = 0.87-1.24), recessive (REM: OR = 1.23, 95% CI = 0.70-2.18), and codominant (FEM: OR = 1.04, 95% CI = 0.89-1.22) models. The included studies about the H63D polymorphism were all conducted in Europe, so the subgroup analysis about H63D was not further investigated. All included articles were in HWE for cases and controls, and all included articles were with OR < 3.0. Figure 3 presents the forest plot of OR for PD in codominant model of the H63D polymorphism in the HFE gene.
Figure 3.
Meta-analysis for the risk of Parkinson’s disease (PD) depending on the H63D polymorphism in the hemochromatosis (HFE) gene. Forest plots for relationship between the H63D polymorphism in the HFE gene and risk of PD in codominant model. White diamond denotes the pooled odds ratio (OR). Black squares indicate the OR in each study, with square sizes inversely proportional to the standard error of the OR. Horizontal lines represent 95% confidence intervals (CIs).
Note: Figure is available in full colour in the online version at aja.sagepub.com
Sensitivity Analysis After Excluding Articles With OR > 3.0
If the frequency of mutation homozygous was 0 in the case group, then it was not sufficient to calculate pooled OR for recessive model. For this reason, 4 articles 14,17 -19 about C282Y polymorphism were not sufficient to calculate the pooled OR in the recessive model. For the overall risk of PD, after excluding the articles with OR > 3.0, 16,18 the risk effects of the Y allele showed no significant effect in dominant (FEM: OR = 0.82, 95% CI = 0.65-1.02), recessive (REM: OR = 0.77, 95% CI = 0.23-2.56), and codominant (FEM: OR = 0.83, 95% CI = 0.67-1.03) models. No studies with OR > 3.0 existed in the H63D polymorphism.
About Heterogeneity
For the 2 polymorphisms, moderate evidence of heterogeneity (I 2 < 50%) was found in 3 genetic models. For the C282Y polymorphism, only 1 study was conducted in Europe, hence subgroup analyses were performed only on European population and the heterogeneity was effectively decreased or removed after the subgroup analyses.
Influence Analysis
Before and after exclusion of articles with OR > 3.0, no individual study was found having excessive influence on the pooled effect in any of the dominant, recessive, and codominant models considering all the polymorphisms.
Publication Bias
Egger’s test was used to assess the publication bias. No significant publication bias was detected in any of the above-mentioned inherited models considering the 2 polymorphisms. Figure 4 presents the result of the publication bias for PD in recessive model of the H63D polymorphism in the HFE gene.
Figure 4.
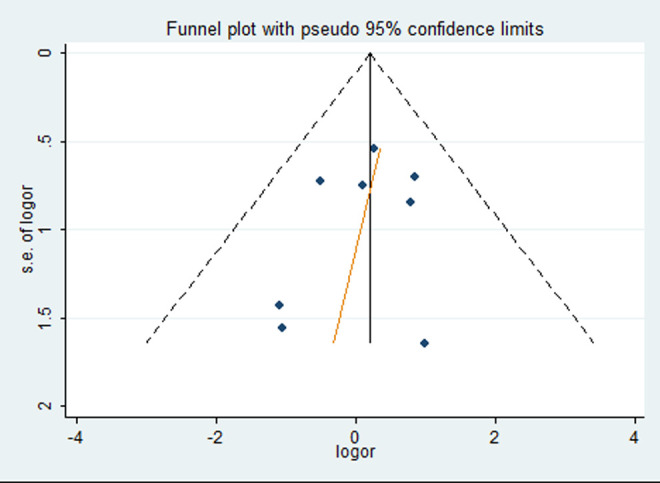
Egger’s test of publication bias for Parkinson’s disease (PD) in recessive model of the H63D polymorphism in the hemochromatosis (HFE) gene.
Discussion
Parkinson’s disease is a progressive neurodegenerative disease. Many studies had suggested that altered iron homeostasis may be an initial trigger for neuronal cell death in neurodegenerative diseases such as PD. 22,23 Hemochromatosis gene is an important regulator of cellular iron homeostasis. The C282Y and H63D single-nucleotide polymorphisms within the HFE gene can give rise to phenotypes with altered iron parameters. Recently, studies have been performed to evaluate the correlation between C282Y and H63D polymorphisms in the HFE gene and the risk of PD. However, the results remained controversial. Since an individual study has a relatively small number of participants with low power to detect the effect, a meta-analysis may be the appropriate approach to obtain a more definitive conclusion.
In this meta-analysis, we summarize the results of 9 case–control studies published so far on the association between C282Y and H63D polymorphisms in the HFE gene and risk of PD. No significant association of Y allele of C282Y and D allele of H63D was found in all of the genetic models and stratified analysis. The meta-analysis indicated that C282Y and H63D polymorphisms in the HFE gene might not be associated with PD.
For a simple genetic variant with 2 alleles, the classical models of inheritance (dominant, recessive, and codominant model) are typically assumed for complex traits when the inheritance model is unknown. 24,25 Besides, maximal power is achieved, when the inherited model is unknown, with codominant model alone or all 3 genetic models tested together. Thus, we tested all 3 models simultaneously. 26
Moderate heterogeneity was found among all the studies for the C282Y polymorphism in the HFE gene. As the differences exist in different genetic backgrounds populations and only 1 study in Europe, subgroup analysis was performed only including European population. The results did not change significantly, and the heterogeneity was effectively decreased or removed after the subgroup analyses by ethnicity. Moreover, considering general genetic variants associated with common diseases always have small effects, we further excluded the studies with OR > 3.0 to control the impact within each single study on the pooled effect. The results did not change significantly, and the heterogeneity was 0 in all inherited models after excluding articles with OR > 3.0. Besides, in this meta-analysis, for C282Y and H63D polymorphisms, no individual study was found having excessive influence on the pooled effect, and no significant publication bias were found in any of the above-mentioned inherited models, suggesting the associations observed should be stable.
This current meta-analysis, to the best of our knowledge, is the first study to integrate the association between C282Y and H63D polymorphisms in the HFE gene and risk of PD, and we interpreted our findings with full caution. Nonetheless, there are some limitations that should be addressed in this meta-analysis. For example, lack of original information for included studies made it impracticable to stratify by other variables, such as smoking status, drinking status, family history, or other relevant diseases, which may affect PD.
Conclusion
In conclusion, our meta-analysis suggested that C282Y and H63D polymorphisms in the HFE gene might not be associated with PD. With regard to PD with multifactorial etiology, our study also warrants for well-designed, large-scale studies with consideration of gene–gene and gene–environment interactions to further evaluate this possible association.
Footnotes
This article was accepted under the editorship of the former Editor-in-Chief, Carol F. Lippa.
Authors’ Note: Chunhong Duan and Meiyun Wang equally share the first authorship.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. de Rijk MC, Tzourio C, Breteler MM. et al. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(suppl 3):S26–S36; discussion S36-S38. [DOI] [PubMed] [Google Scholar]
- 3. Annesi G, Nicoletti G, Tarantino P. et al. FRAXE intermediate alleles are associated with Parkinson’s disease. Neurosci Lett. 2004;368(1):21–24. [DOI] [PubMed] [Google Scholar]
- 4. Snyder AM, Connor JR. Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta. 2009;1790(7):606–614. [DOI] [PubMed] [Google Scholar]
- 5. Rhodes SL, Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis. 2008;32(2):183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waheed A, Parkkila S, Zhou XY. et al. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A. 1997;94(23):12384–12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Risch N. Haemochromatosis, HFE and genetic complexity. Nat Genet. 1997;17(4):375–376. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47(8):15–17. [Google Scholar]
- 11. Begg CB. A comparison of methods to detect publication bias in meta-analysis by P. Macaskill, S. D. Walter and L. Irwig, Statistics in Medicine. 2001;20:641–654. Stat Med. 2002;21(12): 1803; author reply 1804. [DOI] [PubMed] [Google Scholar]
- 12. Ioannidis JP. Commentary: grading the credibility of molecular evidence for complex diseases. Int J Epidemiol. 2006;35(3):572–578; discussion 593-596. [DOI] [PubMed] [Google Scholar]
- 13. Khoury MJ, Little J, Gwinn M, Ioannidis JP. On the synthesis and interpretation of consistent but weak gene-disease associations in the era of genome-wide association studies. Int J Epidemiol. 2007;36(2):439–445. [DOI] [PubMed] [Google Scholar]
- 14. Borie C, Gasparini F, Verpillat P. et al. Association study between iron-related genes polymorphisms and Parkinson’s disease. J Neurol. 2002;249(7):801–804. [DOI] [PubMed] [Google Scholar]
- 15. Aamodt AH, Stovner LJ, Thorstensen K, Lydersen S, White LR, Aasly JO. Prevalence of haemochromatosis gene mutations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(3):315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dekker MC, Giesbergen PC, Njajou OT, van Swieten JC, Hofman A, Breteler MM, van Duijn CM. Mutations in the hemochromatosis gene (HFE), Parkinson’s disease and parkinsonism. Neurosci Lett. 2003;348(2):117–119. [DOI] [PubMed] [Google Scholar]
- 17. Greco V, De Marco EV, Rocca FE. et al. Association study between four polymorphisms in the HFE, TF and TFR genes and Parkinson’s disease in southern Italy. Neurol Sci. 2011;32(3):525–527. [DOI] [PubMed] [Google Scholar]
- 18. Guerreiro RJ, Bras JM, Santana I. et al. Association of HFE common mutations with Parkinson’s disease, Alzheimer’s disease and mild cognitive impairment in a Portuguese cohort. BMC Neurol. 2006;6:24. [Google Scholar]
- 19. Mariani S, Ventriglia M, Simonelli I. et al. Effects of hemochromatosis and transferrin gene mutations on peripheral iron dyshomeostasis in mild cognitive impairment and Alzheimer’s and Parkinson’s diseases. Front Aging Neurosci. 2013;5:37. [Google Scholar]
- 20. Halling J, Petersen MS, Grandjean P, Weihe P, Brosen K. Genetic predisposition to Parkinson’s disease: CYP2D6 and HFE in the Faroe Islands. Pharmacogenet Genomics. 2008;18(3):209–212. [DOI] [PubMed] [Google Scholar]
- 21. Buchanan DD, Silburn PA, Chalk JB, Le Couteur DG, Mellick GD. The Cys282Tyr polymorphism in the HFE gene in Australian Parkinson’s disease patients. Neurosci Lett. 2002;327(2):91–94. [DOI] [PubMed] [Google Scholar]
- 22. Hirsch EC, Faucheux BA. Iron metabolism and Parkinson’s disease. Mov Disord. 1998;13(suppl 1):39–45. [PubMed] [Google Scholar]
- 23. Ioannidis JP, Ntzani EE, Trikalinos TA. et al. Replication validity of genetic association studies. Nat Genet. 2001;29(3):306–309. [DOI] [PubMed] [Google Scholar]
- 24. Salanti G, Higgins JP. Meta-analysis of genetic association studies under different inheritance models using data reported as merged genotypes. Stat Med. 2008;27(5):764–777. [DOI] [PubMed] [Google Scholar]
- 25. Salanti G, Southam L, Altshuler D, et al. Underlying genetic models of inheritance in established type 2 diabetes associations. Am J Epidemiol. 2009;170(5):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lettre G, Lange C, Hirschhorn JN. Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet Epidemiol. 2007;31(4):358–362. [DOI] [PubMed] [Google Scholar]