Abstract
Background:
Clock-watching activity in patients with dementia has not been documented in detail.
Methods:
A male patient having semantic dementia was monitored at a dementia care unit in a general hospital in Japan. We used an integrated circuit monitoring system to record the distance and location of ambulation and the total number of movements that occurred outside the patient’s room.
Results:
The patient was reported to clock watch a couple of years prior to monitoring. In 2011, when monitoring started, he regularly came out of his room saying, “8 o’clock” about 40 minutes into every hour. It seemed as if he could only recognize the minute hand. The median number of sensor detections increased by 4-fold at this clock-watching phase. Behavior consistent with his clock-watching patterns was also detected during the night. In 2012, clock-watching activity disappeared.
Conclusions:
This study documented the progression of clock-watching and subsequent disappearance with worsening cognitive function.
Keywords: clock-watching, objective measurement, semantic dementia, sleep disturbances
Introduction
Second to Alzheimer’s disease (AD), frontotemporal lobar degeneration (FTLD) is the most common type of early-onset degenerative dementia and is primarily a disease of behavior and language dysfunction. 1 Frontotemporal lobar degeneration has 3 clinical types: semantic dementia (SD), behavioral variant frontotemporal dementia (bvFTD), and progressive nonfluent aphasia. 2,3
Although clock-watching is a typical behavior reported in patients with SD, 3 its definition differs among studies. Clock-watching has been described as a compulsion to be somewhere in time or to do things at the same time 4 as well as constantly watching and worrying about the time. 5 Despite being seen in most types of common dementia, 5,6 detailed descriptions of clock-watching have not been reported.
Reports indicate that the prevalence of clock-watching is higher in people with FTLD than those with AD. 6,7 However, among the different types of FTLD, the relative prevalence is less clear. Using a semistructured interview, Snowden et al found that the prevalence of clock-watching in people with SD was twice that of people with bvFTD. 3 In contrast, in a study assessing stereotypic and ritualistic behaviors, Nyatsanza et al reported no significant differences in the prevalence of clock-watching between these types of dementia. 7
These conflicting results may reflect inconsistencies in the definitions of clock-watching used in different experiments. A detailed description of clock-watching activity would thus contribute to a delineation of this clinical phenotype of dementia. In our series of integrated circuit (IC)-tag monitoring studies, we have documented the temporal and spatial movements of nearly 100 patients. 8,9 Here, we present data from nursing records and objective movement indicators for 1 patient with SD who exhibited clock-watching behavior over the course of a year while being monitored.
Methods
Setting
A 60-bed care unit specializing in dementia at a general hospital in Japan cooperated with the study in 2011 and 2012. The Integrated Circuit Tag Monitoring System (Matrix Inc, Osaka, Japan) was used to monitor the distance moved and the location of the movements in patients with dementia. Thirty-nine antennas (sensors) were placed on the ceiling of the unit to capture patient movements in the corridor and day rooms (Figure 1). Integrated circuit tags (4.6 × 3.0 × 1.15 cm3) were attached to patients’ shirts with adhesive tape. Descriptions of the system have been published previously. 10,11
Figure 1.
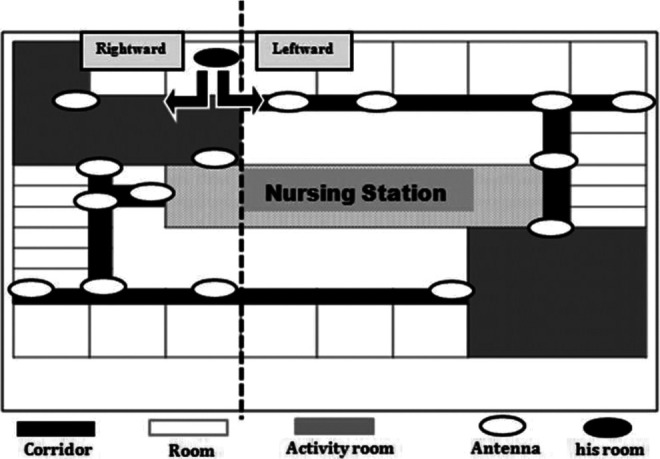
Layout of the study unit.
NEMURI SCAN 12 (Paramount Bed, Co, Ltd, Tokyo, Japan) was used to monitor quality of sleep. NEMURI SCAN (77 × 28.6 × 2.5 cm3) is an actigraphy device that is placed under a mattress to measure sleep duration, duration of time in bed, sleep efficiency, the number of times one gets out of bed, and the time at which one gets out of bed.
Diagnostic Criteria
A consensus guideline of FTLD 13,14 was used for the diagnosis of SD. The clinical profile, electroencephalogram, functional brain imaging based on single-photon emission computed tomography (CT), magnetic resonance imaging of the brain, and blood test results were used for a differential diagnosis.
Data Collection
Monitoring was conducted between September and December 2011 and the same period during 2012. Of the 56 patients monitored, 1 male patient had SD. He was monitored around the clock. Demographic data were abstracted from medical records, and descriptions of wandering and sleep were abstracted from nursing records. The Neuropsychiatric Inventory—Nursing Home version (NPI-NH) 15 was used by the staff at a regular staff conference to evaluate behavioral and psychological symptoms of dementia. The Clinical Dementia Rating (CDR) 16 was determined by trained nurses to determine the severity of dementia.
Data Analysis
Microsoft Excel 2010 and JMP software ver 9.0.2 (SAS Institute Inc, Tokyo, Japan) were used for data analysis. The monitoring software generated the number of detections by each antenna and calculated the distance the patient moved per day. NEMURI SCAN Viewer was used to analyze the sleep indicators. Daytime was defined as between 6:00 am and 9:00 pm, and nighttime was defined as between 9:00 pm and 6:00 am. These times corresponded to the time schedule for lights on and lights out.
A Kruskal-Wallis test was used to test for difference in number of IC-system detections between clock-watching and nonclock-watching phases. The JMP ver 9.0.2 was used for statistical analysis. A Kruskal-Wallis test was also used to test the differences in quality of sleep indicators between 2011 and 2012. The JMP ver 9.0.2 was used for statistical analysis.
Ethical Considerations
This study was approved by the Medical Ethics Committee of the Osaka University School of Allied Health and the ethics committee of the hospital. Written informed consent for this case study was obtained from the patient’s sibling, who went to see the patient on weekly basis and was the major decision maker regarding the patient’s care.
Patient
A 62-year-old male, right-handled, and having SD was monitored for this study. He had no family history of dementia and psychiatric illness. In his 40s, he quit his long-held stable job as a manager and began an approximately 10-year career as a temporary agency worker. He then stopped working entirely and lived with his elderly mother. Approximately a year half prior to the initial evaluation by the neurologist, he started to go to the railroad crossing by the park to watch the train in the afternoon. Then, he started to hand out cigarettes to people in the park who tried to decline the offer. He was arrested for shoplifting once. His attitude toward the sibling became more like an attitude toward a stranger, and the patient stopped calling his sibling by the sibling’s first name. By the time he was taken to a neurological clinic, he could not speak in sentences and was forgetful. He was suspected to have SD and was referred to the study hospital for further evaluation in 2008. A brain scan using CT showed pronounced atrophy of the right anterior temporal pole. Blood tests showed no abnormality of thyroid function and no deficiency in vitamin B1, B12, or folic acid. Neurological examination showed that the grasp reflex for both sides was positive, and no other abnormalities were noted. Semantic aphasia was too advanced to conduct the Standard Language Test of Aphasia. He had advanced prosopagnosia and could not recognize the faces of his sibling, mother, or famous people. The patient had 2-way anomia, that is, he experienced difficulty both in retrieving and in recognizing certain words, including “clock,” “pencil,” and “hammer.”
Semantic dementia progressed over the course of a year, and it became difficult for him to converse with the physician. He constantly watched the clock on the display of his mobile phone. Because of the increased care burden, he was admitted to the study unit in 2010. Progression of clock-watching and SD is summarized in Table 1.
Table 1.
Progression of Clock-Watching Activity and Semantic Aphasia in a Patient With Semantic Dementia.
| Year(s) | Clock-Watching | Semantic Aphasia | Other Measurements or Information | |
|---|---|---|---|---|
| 2008-2009 | Outpatient | Daily outing to the same park at the same time in the afternoon. Constantly checking the clock on patient’s mobile phone. | Advanced prosopagnosia Two-way anomia for common words | Arrested for shoplifting |
| 2010 | Inpatient | Unable to distinguish the hour hand from the minute hand on a clock. Unable to distinguish daytime from nighttime. | Spoke few words, always related to time (8, 1, 4 o’clock) | Independent with basic activities of daily living |
| 2011-study period | Patient left his room about 40 minutes every hour to look for food. Patient kept the ceiling light on at night to watch the wall clock. | Spoke words related to 8 o’clock only | CDR score = 0.5 NPI-NH (disinhibition = 1; apathy = 0; sleep disorder = 4; eating disorder = 4) Median distance moved/day (interquartile) = 1681 m (2464 m) | |
| 2012-study period | Disappearance of clock-watching activity | Rarely spoke autonomously. | CDR score = 3 NPI-NH (disinhibition = 8; apathy = 8; sleep disorder = 4; eating disorder = 4) Median distance moved/day (interquartile) = 1106 m (419 m) |
Abbreviations: CDR, Clinical Dementia Rating Scale; NPI-NH, Neuropsychiatric Inventory–Nursing Home Version.
Results
Progression of SD From Admission Until the End of Monitoring (January 2010 to October 2012)
At admission in January 2010, the patient was independent with the ability to perform activities of daily living. The Mini-Mental State Examination could not be administered because of SD. He rarely spoke autonomously, and his conversation with the staff was very limited. Analog clocks were hung in his room and the activity rooms he shared with other patients, and his concerns with them were occasionally documented in the nursing record. For example, a nurse once noted that he said, “It’s still one o’clock. That’s three hours before 4 o’clock. I’ll sleep till then.” From March 2010, his preference for food changed, and he only ate rice and would not eat any side dishes.
In September 2011, when IC-tag monitoring began, his CDR score was 0.5, which worsened to 3 within a year. Other notable changes included increased disinhibition and apathy as measured by the NPI-NH (Table 1). With increased apathy, he started to urinate in bed. Despite a plan that prompted him to refrain from doing so, the frequency of urinary incontinence in bed increased over time.
Clock-Watching Activity Documented in the Nursing Records
In October 2011, the staff noted that he could only recognize the minute hand of a clock. He spent most of the day in bed and often came out during the daytime around 40 minutes into every hour (when the minute hand was on the 8), saying phrases such as “already, 8 o’clock, isn’t it.” His conversation was limited to 8 o’clock. In the same month, staff twice recorded that he left the ceiling light on at night, and although staff turned it off, the light was on again when they did their next rounds an hour later. In November, while resting in bed, he was repeatedly observed checking the wall clock by opening the curtain surrounding his bed. He frequently came out of his room when the minute hand was on the 8, saying phrases such as “8 o’clock. Why no rice?” He started spitting cooked rice and began eating less.
In the following year, semantic aphasia advanced further and the only word he ever spoke was “strange,” and he did not talk to the staff about time. He no longer left the light on at night.
Further atrophy of the temporal region was confirmed by CT scan (Figure 2). His movement was mostly limited to his room and the activity room. He remained hospitalized because no nursing home was found to which he could be referred.
Figure 2.
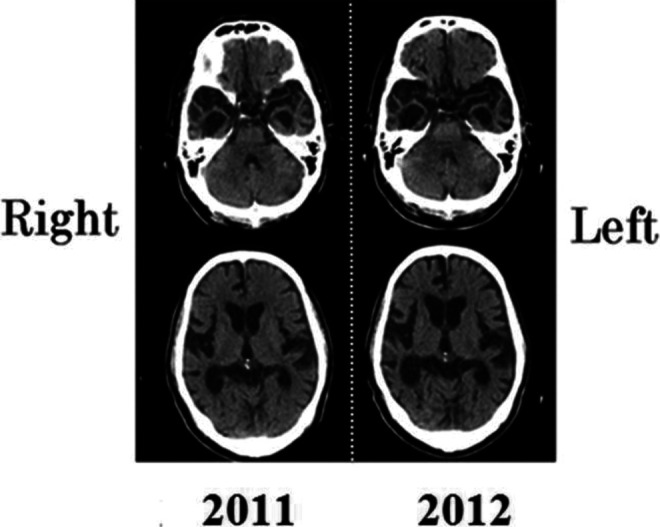
Brain computed tomography (CT) scans of the patient in 2011 and 2012.
Disappearance of Clock-Watching Activity Between 2011 and 2012 as Measured by Objective Indicators
Changes in activity level according to the IC-tag monitoring system
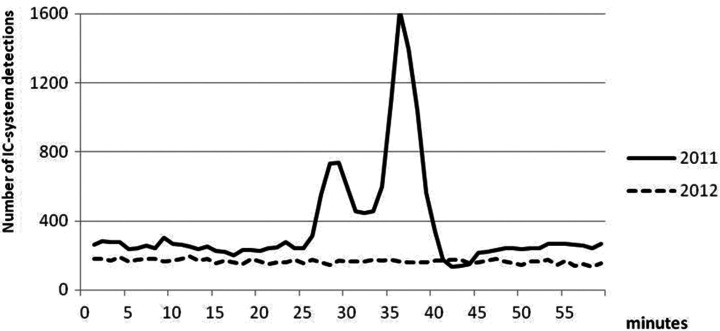
The number of detections by the IC sensor antennas showed a bimodal distribution peaking around 27 minutes (between the 25- and 30-minute points) and 37 minutes (between the 35- and 40-minute points) into every hour during the study period in 2011 (Figure 3).
Figure 3.
Changes in activity levels by the minute (2011 and 2012).
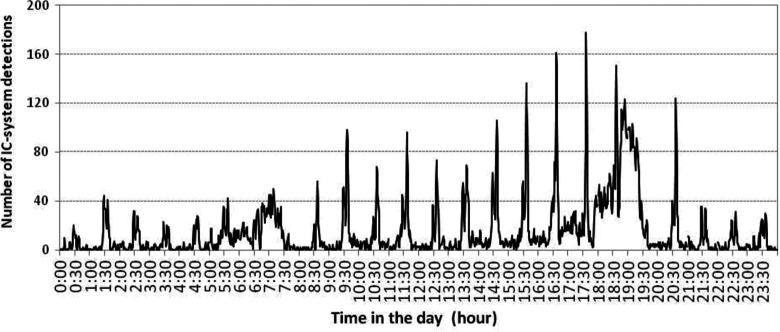
When the number of detections was examined by conventional time, a bimodal distribution was observed every hour except during breakfast and dinner (Figure 4). We defined 2 clock-watching phases for each hour that centered around the peaks of the bimodal distribution. The first clock-watching phase was between 25 and 30 minutes into every hour, and the second was between 35 and 40 minutes into every hour. Compared with time outside the clock-watching phases, the median number of detections in the first phase was 2.3 times higher (P < .01) and that of the second phase was 4.1 times higher (P < .01) than the nonclock-watching phase. The bimodal distribution of the number of detections was also seen during the night (Figure 4). The number of detections by the IC system was approximately 70% of that during the daytime. During the night, compared with time outside the clock-watching phase, the median number of detections in the first phase was 6.9 times higher (P < .01) and that of the second phase was 7.6 times higher (P < .01). There was no mention of clock-watching activity during the night in the nursing record.
Figure 4.
Activity levels measured by the number of integrated circuit (IC)-system detections by military hour in 2011.
In 2012, the total number of IC-system detections as viewed by conventional hours became flat, and the bimodal distribution was no longer evident (Figure 3). There was no description of wandering activity by the patient in the nursing record.
Places of clock-watching activity
The IC-detection system showed that the patient’s location during clock-watching and nonclock-watching phases differed in 2011 (Figure 5). When he came out of his room, he took a leftward route 57% of the time during the first clock-watching phase and a rightward route 91% of the time during the second clock-watching phase. He took a rightward route 74% of the time during the nonclock-watching phase. However, in 2012, there were no differences in the routes between clock-watching and nonclock-watching phases (Figure 5).
Figure 5.
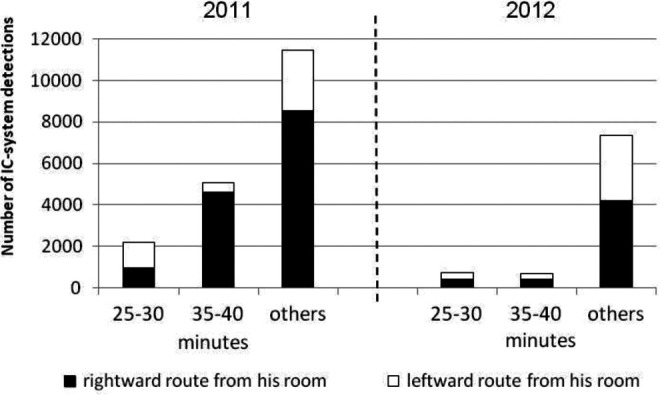
Difference in walking locations during clock-watching and nonclock-watching phases of the hour in 2011 and 2012.
Worsening quality of sleep
Quality of sleep worsened from 2011 to 2012 as assessed by NEMURI SCAN. The NEMURI SCAN was used 11 days in 2011 and 30 days in 2012. The median proportion of sleep efficiency significantly decreased, while the median number of awakenings significantly increased (Table 2). However, decrease in the duration of sleep in minutes did not reach statistical significance.
Table 2.
Changes in Quality of Sleep Indicators Measured by NEMURI Scan From 2011 to 2012.
| 2011, Median ± Interquartile Range | 2012, Median ± Interquartile Range | P Value | |
|---|---|---|---|
| Duration of sleep, minutes | 422.0 ± 69.5 | 371.0 ± 96.8 | >.05 |
| Sleep efficiency, % | 74.0 ± 15.0 | 65.0 ± 4.8 | <.05 |
| Number of awakenings | 1.0 ± 1.0 | 3.0 ± 3.0 | <.01 |
Changes in quality of sleep were not evident in the nursing record, and the sleep-disorder score measured by the NPI-NH was 4 for both 2011 and 2012.
Changes in CT scan images
Computed tomography scan images were interpreted by the fourth author. In 2011, the right temporal pole showed pronounced atrophy, and minor atrophy was noted in the left temporal pole. The frontal lobe and other adjacent areas, such as orbitofrontal area, were spared. In a CT scan conducted a year later, the right temporal pole had shrunk further and atrophy of the left temporal pole had spread. In addition, we observed minor atrophy of the orbitofrontal area and frontal convexity. The parietal lobe and occipital lobe were spared.
Discussion
This study used objective indicators and found pronounced clock-watching and subsequent disappearance of clock-watching activity in a patient with SD. The patient’s preoccupation with time manifested in a sharp rise in activity at certain specific and repeated times each hour, even during the night.
The current study clearly shows that the clock-watching activity first worsened and then ceased within a year. Upon admission, the patient could recognize day and night. Eight months later when the IC-tag monitoring began, he could no longer recognize the hour hand on a clock. Astute observation by the nurse revealed that the patient could only recognize the minute hand of the clock, as he used to come out from his room around 40 minutes into every hour thinking it was 8 o’clock. This seemingly obsessive activity might indicate that an hour became half a day for him, and he could no longer integrate information from other source such as daylight to recognize the time of the day.
The pathophysiological mechanisms of clock-watching are unknown. Snowden et al suggest that the progression of semantic loss might lead to a “shrinking world view” in contrast to the relative preservation of numerical concepts, including those related to time. 3 Thus, temporally bound routines may become a sole framework upon which affected individuals rely. However, Thompson et al examined an age-matched cohort of individuals with right and left SD with comparable backgrounds, such as duration of dementia (n = 47), and found that the prevalence of clock-watching was much higher in individuals with right dominant SD. 17 This suggests that specific brain regions may account for clock-watching activity. Indeed, Rosen et al found that the 4 behavioral abnormalities measured by the Neuropsychiatric Inventory were correlated with cell loss in a specific region of the brain, as measured by voxel-based morphometry. 18 For example, aberrant motor behavior was associated with tissue loss in the anterior cingulate cortex and the premotor cortex.
Clock-watching activity tends to be based on information that is familiar to patients with SD. We speculate that the second phase of clock-watching activity, which occurred around 40 minutes into every hour, was related to food, as the patient was heard to have said “8 o’clock. Why no rice?” on 1 occasion. He may have come out to look for a food trolley, which could be seen down the corridor to the right of his room. We could not find any clear motivation for clock-watching behavior in the first phase, although the nursing records stated that the patient mentioned that it was “almost 6 o’clock” at 17:25. The patient did not appear to be waiting for food. This case suggests that clock-watching may have multiple causes.
This study is probably the first study to document the occurrence of nighttime clock-watching activity, which reflects poor sleep quality. Nighttime clock-watching was difficult to observe for the staff because the patient’s activity only lasted several minutes, and his room was on the opposite side of the nurse station. Difficulty in observing and documenting nighttime patient activity has been reported in a previous IC-tag study, 19 which showed poor agreement between the hourly sleep record kept by the staff and the movement detected by the IC tag. If obsessive clock-watching is found in a patient with dementia, existence of nighttime clock-watching should be checked as part of general monitoring of sleep quality.
To the best of our knowledge, the complete disappearance of clock-watching activity has not been previously reported in patients with FTLD. However, we observed the termination of clock-watching activity in our patient. There are 2 main possible explanations for this phenomenon. First, increasing apathy may have contributed to a loss of interest in clock-watching. Spreading atrophy was depicted in the most recent CT scan, accompanied by a decrease in autonomous activities. Bedwetting was documented, and the NPI-NH apathy score of the patient increased. Second, the spreading atrophy may have diminished the ability of the patient to interpret or recognize the minute hand of the clock. Previous studies have shown that patients with SD retain many aspects of number knowledge in spite of severe comprehension problems in other domains. This is likely because the parietal lobe, which plays a critical role in number processing, is spared. 20 Our patient might have been able to recognize the numbers on the clock but could have lost the ability to recognize the minute hand, as he had previously lost the ability to recognize the hour hand.
In order to document the progression of clock-watching activity, there is a need to refine the definition of clock-watching. One of the definitions in the previous studies is “does he/she clock watch or seem pre-occupied by time?” (p. 1401). 7 This item is probably insufficient for capturing a variety and severity of the activity. Some other scales to measure stereotypic or ritualistic behavior questionnaire may be more suitable for assessing clock-watching in patients with SD. The Stereotypy Rating Inventory 21 contains a subdomain assess daily rhythm related to clock-watching; questions include the times and durations for activities such as sleeping, waking, watching television, taking walks, and eating. These items are assessed by frequency and severity. In our study, scales specific to FTLD were not used because the number of patients was small, and details of clock-watching activity were revealed after analysis of the movement data.
Assessing clock-watching activity and lifestyle can contribute to planning person-centered care and improve quality of life for patients. Patients with FTLD tend to become agitated when their stereotypic behaviors are disturbed. 22 Yamakawa et al stress the importance of assessing preadmission lifestyle and time-bound behavior and taking these into consideration when devising a plan for patient care. 23 Setting up simple activities when time-bound behaviors are expected to occur will facilitate the adaptation of patient with SD to the new environment.
One limitation of our study is that the available documentation showing the progression of SD symptoms in our patient was limited to the period of hospitalization. As the sibling informant did not live with the patient, we were unable to gather information about the onset and progression of SD. We speculate that the onset of SD occurred shortly before the patient discontinued stable employment. Noticeable clock-watching activity, such as engaging in a precisely timed daily outing to the park, started approximately 10 years after SD onset.
As the initial neurological evaluation was conducted when the patient was in an advanced stage of SD, we were not able to document the progression of SD. However, the loss of a job, clock-watching, and prosopagnosia observed in this patient are consistent with the findings of a study by Thompson et al, which reported that these symptoms were more prevalent in people with right dominant SD than in those with left dominant SD. 17
The major limitation of this study was that our sample consisted of a single case, making it difficult to generalize using our findings. Further studies are needed involving larger populations of people with dementia. A prospective design would be helpful for examining the onset of clock-watching and other symptoms, as well as associated neurological changes with specific brain regions.
Conclusion
This study documented the progression of clock-watching activity and its subsequent disappearance in a patient with SD. Clock-watching occurred even during the night, suggesting poor quality of sleep. Detailed documentation of clock-watching can lead to a better understanding of clinical dementia phenotypes.
Acknowledgments
The authors acknowledge the cooperation and contribution of the hospital’s unit staff. We also extend appreciation to the patient and his sibling for their cooperation.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by a Grant-in-Aid for Scientific Research (B, No. 23390516, 2011-2013) and Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare (Research on dementia; H25-Dementia-General-001) to K. Shigenobu (Chief researcher: M. Ikeda).
References
- 1. Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Brit J Psychiat. 2002;180:140–143. [DOI] [PubMed] [Google Scholar]
- 2. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kertesz A, Nadkarni N, Davidson W, Thomas AW. The frontal behavioral inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsych Soc. 2000;6(4):460–468. [DOI] [PubMed] [Google Scholar]
- 5. Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 2001;103(6):367–378. [DOI] [PubMed] [Google Scholar]
- 6. Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyatsanza S, Shetty T, Gregory C, Lough S, Dawson K, Hodges JR. A study of stereotypic behaviours in Alzheimer’s disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2003;74(10):1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakaoka A, Suto S, Makimoto K, Yamakawa M, Shigenobu K, Tabushi K. Pacing and lapping movements among institutionalized patients with dementia. Am J Alzheimers Dis. 2010;25(2):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishikata S, Yamakawa M, Suto S, Shigenobu K, Makimoto M. Degree of ambulation and factors associated with the median distance moved per day in Alzheimer’s disease patients. Int J Nurs Pract. 2013;19(suppl 3):56–63. [DOI] [PubMed] [Google Scholar]
- 10. Greiner C, Makimoto K, Suzuki M, Yamakawa M, Ashida N. Feasibility study of the integrated circuit tag monitoring system for dementia residents in Japan. Am J Alzheimers Dis. 2007;22(2):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makimoto K, Lee EA, Kang Y, Yamakawa M, Ashida N, Shin KR. Temporal patterns of movements in institutionalized elderly with dementia during 12 consecutive days of observation in Seoul, Korea. Am J Alzheimers Dis. 2008;23 (2):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kogure T, Shirakawa S, Shimokawa M, Hosokawa Y. Automatic sleep/wake scoring from body motion in bed: validation of a newly developed sensor placed under a mattress. J Physiol Anthropol. 2011;30(3):103–109. [DOI] [PubMed] [Google Scholar]
- 13. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 14. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shigenobu K, Hirono N, Tabushi K, Ikeda M. Validity and reliability of the Japanese version of the neuropsychiatric inventory-nursing home version (NPI-NH). Brain Nerve. 2008;60(12):1463–1469. [PubMed] [Google Scholar]
- 16. Morris JC. The clinical dementia rating (CDR): current vision and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 17. Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral–cognitive implications. Neurology. 2003;61(9):1196–1203. [DOI] [PubMed] [Google Scholar]
- 18. Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(pt 11):2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamakawa M, Suto S, Shigenobu K, Kunimoto K, Makimoto K. Comparing dementia patients’ nighttime objective movement indicators with staff observation. Psychogeriatrics. 2012;12(1):18–26. [DOI] [PubMed] [Google Scholar]
- 20. Jefferies E, Bateman D, Lambon Ralph MA. The role of the temporal lobe semantic system in number knowledge: evidence from late-stage semantic dementia. Neuropsychologia. 2005;43(6):887–905. [DOI] [PubMed] [Google Scholar]
- 21. Shigenobu K, Ikeda M, Fukuhara R, et al. The Stereotypy Rating Inventory for frontotemporal lobar degeneration. Psychiat Res. 2002;110(2):175–187. [DOI] [PubMed] [Google Scholar]
- 22. Kumamoto K, Arai Y, Hashimoto N, Ikeda M, Mizuno Y, Washio M. Problems family caregivers encounter in home care of patients with frontotemporal lobar degeneration. Psychogeriatrics. 2004;4(2):33–39. [Google Scholar]
- 23. Yamakawa M, Shigenobu K, Makimoto K, Zhu C, Ashida N, Tabushi K. Environmental control interventions for frontotemporal dementia with reversed sleep–wake cycles. Am J Alzheimers Dis. 2008;23(5):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]