Abstract
Herbal medicines for the treatment of Alzheimer’s disease (AD) have attracted considerable attention nowadays. Alzheimer’s disease is described in traditional Persian medicine (TPM) by the term Nesyān. In this study, 5 main medicinal medieval Persian manuscripts were reviewed to filter plants reported for the treatment of Nesyān. Databases were searched for related possible mechanisms of action of these medicinal plants. Each herb was searched for along with these keywords: “acetyl and butyryl cholinesterase inhibition,” “antioxidant,” “anti-inflammatory,” and “anti-amyloidogenic.” In Total, 44 herbs were used for the treatment of Nesyān; 40 of those were authenticated. Also, 30 plants had at least one of the mechanisms of action that were searched for or related pharmacological functions known for the treatment of AD. In this work, we introduce promising candidates in TPM that could undergo further investigation for identification of their active compounds and clinical validation in the treatment of AD.
Keywords: Alzheimer’s disease, traditional medicine, drug discovery.
Introduction
With the aging of the population nowadays, we can see an increase in the prevalence of dementia. 1 The most common cause of irreversible dementia is known to be Alzheimer’s disease (AD). 2 There are many genetic as well as environmental factors that trigger the related pathological decline. 3 Over the past few years, numerous studies have been conducted on this disease. Many of these have resulted in discoveries that revealed the complex pathophysiological processes responsible for its progression. Despite these advances, there is no effective medication for AD yet. Many agents that showed effects in primary researches failed to demonstrate efficacy in the improvement of AD when studied in clinical trials. 4 The reason for this is the complex nature of this disease. It is a syndrome and the result of multiple molecular abnormalities. Any effective treatment should target the neurodegenerative cascade responsible. 2 Its main pathological feature is the presence of β-amyloid peptide brain plaques. 5
However, research today suggests that the pathogenesis of this disease is not restricted to the neuronal factors but includes immunological reactions in the brain. Inflammatory reactions are responsible for the progression and severity of the disease. 6 Neuroinflammation is observed in the brain in affected cases. As a result, increasing inflammatory cytokines and free radical attack could be seen in them. 7 Oxidative stress is another factor that contributes to the progression and initiation of this disease. 8 As regards its treatment—although therapeutic effectiveness still remains uncertain—treatments of choice for AD are cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists. 9 One of the therapeutic approaches today is the multi-target-directed ligand strategy. The aim is to hit different targets of AD. 2 A multicomponent and, therefore, multi-target approach could also be seen in the treatments of traditional systems of medicine. 10
In recent years, traditional herbal medicines for the treatment of AD have attracted considerable attention among researchers. 11 These medications have been used for thousands of years by humankind. It is unlikely that their side effects have been neglected during these years. 12 Iran has a rich history of traditional medicine. This system of medicine has its roots in ancient Persia and has been influenced by medical systems of great ancient civilizations. Iranian scholars were engaged in the study of cognitive disorders as well. 13 Their findings have been recorded in their books, which are still available today. 14
Alzheimer’s disease is discussed in traditional Persian (TP) books under the term Nesyān. The clinical manifestation of Nesyān was defined in TP books as difficulty in remembering recent events, while recollections of happenings of the past are easier in the early stages. 15 We studied Nesyān from famous Persian medieval medical books and collected information about herbs used in the treatment of Nesyān. These herbs could be an interesting subject for further research and in clinical trials.
Materials and Methods
To collect the necessary data, 5 main medicinal medieval Persian manuscripts were studied for plants used in Nesyān. The books that were studied were Kit ¯ ab al-h ¯ awı¯ fı¯ al-t.ibb (The Comprehensive Book on Medicine) by Rhazes (9th-10th centuries), Canon of Medicine by Avicenna (10th-11th centuries), Ikhtiy ¯ ar ¯ ati Badı¯’ı¯ (Selections for Badı¯’ı¯) by H¯ ajjı¯ Zayn al-’At.t.¯ ar (14th century), Tuhfat al-mu’minı¯n (Present for the Faithful) by Daylamı¯ Tunak ¯ abunı¯ (17th century), and Makhzan al-adviyah (The Storehouse of Medicaments) by Alavı¯ Shı¯r ¯ azı¯ (18th century). These books are listed in Table 1.
Table 1.
List of the Persian Medical Books Studied for Herbal Remedies of Nesyān.
| Book Title/ Reference (National Library of Medicine) | Century | Author |
|---|---|---|
| Kit ¯ ab al-h ¯ awı¯ fı ¯ al-t.ibb (The Comprehensive Book on Medicine or Liber Continens)/MS A 17-NLM, NLM Microfilm Reel: FILM 48-115 no. 3 | 9th-10th | Rhazes |
| Kitāb al-Qānūn fī al-Tibb (Canon of Medicine)/ MS P 21, 22-NLM, NLM Microfilm Reel: FILM 48-136 no. 2 | 10th-11th | Avicenna |
| Ikhtiyārāt-i Badī ‘ī (Selections for Bad ı¯ ’ ı¯ )/NLM Microfilm Reel: FILM 48-132 no. 4 | 14th | Zayn al-Dīn ‘Alī ibn al-Ḥusayn al-Anṣārī |
| Tuhfat al-mu’minı¯n (Present for the Faithful)/MS P 21, 22-NLM, NLM Microfilm Reel: FILM 48-136 no. 2 | 17th | by Daylamı¯ Tunak ¯ abunı¯ |
| Makhzan al-adviyah (The Storehouse of Medicaments)/MS P 12-NLM, NLM Microfilm Reel: FILM 48-133 no. 2 | 18th | Aqīlī al-‘Alavī al-Khurasānī al-Shīrāzī |
The plants used in the treatment of Nesyān, according to these manuscripts, were listed. Authentications of the plants were confirmed in accordance with botanical books, such as the Dictionary of Medicinal Plants, Matching the Old Medicinal Plant Names with Scientific Terminology, Indian Medicinal Plants, and Dictionary of Iranian Plant Names. Databases such as PubMed and ScienceDirect were searched for related possible mechanisms of action of these medicinal plants. Hence, each herb was searched for along with these keywords: “Acetyl and butyrylcholinesterase inhibition,” “antioxidant,” “anti-inflammatory,” and “anti-amyloidogenic.” Each herb was searched for in the PubMed database for clinical trials and AD.
Results
In the Persian manuscripts studied, a total number of 44 herbs used for the treatment of Nesyān was found; 40 of them could be identified botanically (Table 2). From this number, 30 herbs had at least one of the mechanisms of action that were searched for or related pharmacological functions known for the treatment of AD (Table 3). We introduced 30 herbs derived from TP medicine, the suitable properties of which in the treatment of AD have been proven by modern medicine. Modern research had proven that all these herbs have antioxidant activity. From the medicinal herbs found for the treatment of Nesyān, 15 had proven acetyl cholinesterase and/or butyrylcholinesterase inhibition effects. The method of determination in most cases was by the Ellman assay. From the plants searched for, Persian walnut, garlic, and ginger had proven antiamyloidogenic effects. Twenty-four of these plants showed anti-inflammatory effects proven by experimental analysis. In the next step, we highlighted the herbs that had all the mechanisms of action searched for and that are related to the treatment of AD. In this respect, we found Zingiber officinale Roscoe and Allium sativum L., which have proven acetyl and butyrylcholinesterase inhibition, antioxidant, anti-inflammatory, and antiamyloidogenic properties. The effect of these 30 herbs on AD was not studied in clinical trials.
Table 2.
Medicinal Herbs for Treatment of Nesyān From Reports of Traditional Persian Medicine.
| Scientific Name | Family | Traditional Name | Part(s) Used | Rout(s)/Dose |
|---|---|---|---|---|
| Iris florentina L. | Iridaceae | Irsā | Root | Oral (9 g) |
| Piper betle L. | Piperaceae | Tānbool | Leaf | Oral (9 g) |
| Zingiber officinale Roscoe | Zingiberaceae | Zangabil | Root | Oral (4.8 g) |
| Santalum album L. | Santalaceae | Sandal | Wood | Oral (4.5 g) |
| Piper nigrum L. | Piperaceae | Felfel | Fruit | Oral (4.5 g) |
| Acorus calamus L. | Acoraceae | Vaj | Latex, root | Oral (4.5 g) |
| Cymbopogon schoenanthus (L.) Spreng | Poaceae | Ezkhar | Flower | Oral (2.25-4.5 g) |
| Nepeta menthoides Boiss | Lamiaceae | Ostokhodos | Flower, aerial part | Oral (4.8-12 g), topical |
| Drimia maritima (L.) Stearn | Asparagaceae | Esghil | Root | Topical |
| Melilotus officinalis (L.) Pall | Fabaceae | Eklilol malek | Fruit, leaf | Nasal |
| Inula conyza (Griess.) DC | Asteraceae | Barnoof | Latex | Nasal |
| Allium sativum L. | Amaryllidaceae | Soom | Bulb | Oral |
| Teucrium polium L. | Lamiaceae | Jo’dah | Aerial part | Oral |
| Citrullus colocynthis (L.) Schrad | Cucurbitaceae | Hanzal | Fruit | Oral (1.2-2.4 g) |
| Brassica nigra (L.) K.Koch | Brassicaceae | Khardal | Oil, seed | Oral (7.2 g), topical |
| Costus acanthocephalus K.Schum | Costaceae | Ghost | Root | Topical |
| Boswellia sacra Flueck | Burseraceae | Kondor | Gum | Oral (1.2 g) |
| Lodoicea maldivica (J.F.Gmel.) Pers. | Arecaceae | Nārjil bahri | Fruit | Oral (0.25-0.5 g) |
| Bryonia alba L. | Cucurbitaceae | Fāsharā | Oral (2.4-4.5 g) | |
| Phyllanthus emblica L. | Phyllanthaceae | Amlaj | Fruit | Oral (7.2-24 g) |
| Ferula assa-foetida L. | Apiaceae | Anjodān | Seed | Oral (9 g) |
| Scolopendrium vulgare Sm. | Aspleniaceae | Sqoolufandariun | Oral (4.8 g) | |
| Zingiber zerumbet (L.) Rescoe ex Sm | Zingiberaceae | Zoronbād | Root | Oral (2.4 g) |
| Thymus serpyllum L. | Lamiaceae | Namām | Leaf | Oral (9-12 g) |
| Pistacia lentiscus L. | Anacardiaceae | Mastaki | Gum | Oral (200 g) |
| Pistacia vera L. | Anacardiaceae | Fostogh | Fruit | Oral (4.5 g) |
| Vitis vinifera L. | Vitaceae | Zabib | Fruit | Oral (72 g) |
| Asarum europaeum L. | Aristolochiaceae | Asāroon | Root | Oral (4.5 g) |
| Anacardium occidentale L. | Anacardiaceae | Balādor | Fruit | Oral (1.2 g) |
| Cupressus sempervirens L. | Cupressaceae | Joozol sarv | Fruit | Oral (1.2-2.35 g) |
| Cinnamomum verum J.Presl | Lauraceae | Dārchini | Bark | Oral (2.4 g) |
| Terminalia chebula Retz | Combretaceae | Ehlilaj kāboli | Fruit | Oral (4.8-12 g) |
| Alhagi maurorum Medik. | Fabaceae | Taranjabin | Latex | Oral (24-90 g) |
| Juglans regia L. | Juglandaceae | Jouz | Bark of root | Topical |
| Peganum harmala L. | Nitrariaceae | Hormal | Oral (4.5-9 g) | |
| Vitex agnus-castus L. | Verbenaceae | Aslagh | Leaf, fruit | Oral (4.5 g), topical |
| Pimpinella anisum L. | Apiaceae | Anisoon | Seed | Oral (1.2 g) |
| Peucedanum officinale L. | Apiaceae | Bokhor akrād | Gum | Oral (2.25 g) |
| Veratrum album L. | Melanthiaceae | Kharbagh | Root | Oral (4.5 g) |
| Heracleum persicum Desf. ex Fisch | Apiaceae | Safidolioon | Fruit, seed | Nasal |
Table 3.
AD-Related Pharmacological Activities for Selected Plants.
| Scientific Name | Related Activities | Reference | |
|---|---|---|---|
| Mechanism of Action | Assay/Extract or Fractions of the Plants/Outcomes | ||
| Iris florentina L. | Cholinesterase inhibition Antioxidant | Ellman/high phenolic content fractions/active (IC50 < 100 µg/mL) DPPH, H2O2, ABTS/80.52% inhibition | 16 |
| Piper betle L. | Cholinesterase inhibition Antioxidant activity | Ellman/aqueous lyophilized/positive DPPH, LP/aqueous extract/active | 17,18 |
| Zingiber officinale Roscoe | Cholinesterase inhibition Anti-amyloidogenic Antioxidant Anti-inflammatory | Rat brain/aqueous/positive Ellman/methanol/IC50 = 41, 52 µg/mL Methanol/increased in cell survival against amyloid β (Aβ)-induced toxicity (rat hippocampal cell culture), formation of Aβ oligomers prevention DPPH, FRAP/methanol/IC50 = 70 ± 0.30 µg/mL (DPPH), 845.4 ± 56.62 μM Fe (II) equivalents/g dry weight (FRAP) Lipopolysaccharide (LPS)-stimulated raw cells/12-dehydrogingerdione/positive | 19 –21 |
| Santalum album L. | Antioxidant Anti-inflammatory | DPPH, FRAP, OH radical, LP, TEAC, NO radical/dichloromethane-methanol extract/comparable to quercetin Proliferation assay/aqueous/positive | 22,23 |
| Piper nigrum L. | Antioxidant Analgesic and anti-inflammatory Cholinesterase inhibition | Tissue lipid peroxidation, TBARS, SOD (in vivo)/supplementation with black pepper or piperine/positive Tail immersion, hot plate, and acetic acid induced writhing, carrageenan-induced paw inflammation (in vivo)/piperine, hexane and ethanol/potent effects Ellman/ethanol/73% (inhibition) | 24 –26 |
| Acorus calamus L. | Cholinesterase inhibition Antioxidant Anti-inflammatory | Ellman/hydroalcoholic, essential oil (asarone)/potent activity DPPH, FRAP/methanol/positive Vincristine-induced painful neuropathy/hydroalcoholic/side effects attenuation | 27 –29 |
| Cymbopogon schoenanthus (L.) Spreng. | Cholinesterase inhibition Antioxidant | Ellman/ethyl acetate and methanol extracts/ (IC50 = 0.23 mg/mL) DPPH, β-carotene bleaching/proanthocyanidins extract/0.164, 0.11 mg/mL | 30 |
| Nepeta menthoides Boiss. & Buhse | Cholinesterase inhibition Antioxidant | Ellman/essential oil/IC50 = 64.87 µg/mL DPPH, FRAP/oil/ 28.36 µg/mL, 68.90 µmol Fe2+/g dry plant | 31 |
| Allium sativum L. | Cholinesterase inhibition Antioxidant Anti-inflammatory Anti-amyloidogenic | Ellman/allicin/active DPPH, lipid peroxidation/polar fraction/active Modulation of leukocyte cell proliferation and cytokine production Transgenic Alzheimer’s mouse/dietary garlic/positive | 32 –36 |
| Melilotus officinalis (L.) Pall | Antioxidant Anti-inflammatory | ABTS, OH radical, SOD/methanol extract/effective Extract ≈ 0.25% coumarin/active | 37,38 |
| Teucrium polium L. | Antiamnesic activity Cholinesterase inhibition Anti-inflammatory Antioxidant | Scopolamine-induced antiamnesic/hydroalcoholic Ellman/hydroalcoholic/68.5% inhibition Carrageenan-induced paw edema/ethanol/positive DPPH/methanol extract/IC50 =20.1 μg/mL | 39 –41 |
| Citrullus colocynthis (L.) Schrad | Cholinesterase inhibition Antioxidant Anti-inflammatory | Aqueous methanol/83.54% inhibition DPPH/methanol/inhibition at 2500 μg/mL Carrageenan-induced paw edema/aqueous/effective | 42 –44 |
| Brassica nigra (L.) K.Koch | Antioxidant Anti-inflammatory | DPPH, OH radical/methanol, ethanol/IC50 = 63.09 µg/mL Protease inhibition/ethanol/42.57% inhibition (250 µg/mL) | 45,46 |
| Boswellia sacra Flueck | Antioxidant Anti-inflammatory | Antiglycation, DPPH/methanol/positive Different related assays/boswellic acids | 47,48 |
| Phyllanthus emblica L. | Cholinesterase inhibition Antioxidant Anti-inflammatory Cholinesterase inhibition | Passive avoidance, rewarded alternation (in vivo colorimetric)/ethanol/positive DPPH, SOD, TBARS/ethanol/effective Against human PMN and platelet/polar extracts/90% inhibition Ellman/methanol/IC50 (53.88, 65.12 μg/mL) | 49 –51 |
| Ferula assa-foetida L. | Antioxidant Anti-inflammatory | NO, H2O2, TBARS/essential oil/IC50 (0.012-0.035 μg/mL) Hot plate, paw edema/gum resin/positive | 52,53 |
| Zingiber zerumbet (L.) Rescoe ex Sm | Antioxidant Anti-inflammatory | DPPH/ethanol/effective Against NO production from lipopolysaccharide-induced macrophages/zerumbone, 3-O-methyl kaempferol/IC50 (4.37 and 24.35 μM) | 54,55 |
| Thymus serpyllum L. | Cholinesterase inhibition Radical scavenging | Ellman/ethanol/positive DPPH/ethanol/ IC50 < 50 μg/mL | 56,57 |
| Pistacia lentiscus L. | Antioxidant Anti-inflammatory | DPPH/methanol/positive Carrageenan-induced paw edema/essential oil/positive | 58,59 |
| Pistacia vera L. | Antioxidant Anti-inflammatory | DPPH, SOD, TEAC/seeds and skin/positive Carrageenan-induced edema/oleoresin/positive (500 mg/kg) | 60,61 |
| Vitis vinifera L. | Antioxidant Anti-inflammatory | DPPH, lipid peroxidation/astringin, astringinin/effective IL-8, nuclear factor-κB pathway/raisin/inhibition | 62,63 |
| Anacardium occidentale L. | Cholinesterase inhibition Anti-inflammatory | In vitro/effective Carrageenan induced paw edema/ tannins/ Effective | 64 –66 |
| Cinnamomum verum J.Presl | Antioxidant Anti-inflammatory | DPPH, ABTS, OH, SOD/ Methanol/effective Carrageenan-induced paw edema/procyanidine polyphenols/inhibition | 67,68 |
| Terminalia chebula Retz | Cholinesterase inhibition Antioxidant Anti-inflammatory | Ellman/methanol/ dose-dependent SOD, lipid peroxidation/chebulinic acid, chebolanin, casuarinin/effective COX and 5-LOX/ethanol, chebulagic acid/effective | 69 –71 |
| Alhagi maurorum Medik | Cholinesterase inhibition Antioxidant Anti-inflammatory | Ellman/methanol/ >50% inhibition DPPH/phenolic fraction/effective Carrageenan-induced paw edema/ethanol/effective | 72 –74 |
| Juglans regia L. | Antioxidant Anti-inflammatory Anti-amyloidogenic | DPPH/dichloromethane and water extracts/effective TNF-α-induced endothelial expression of VCAM-1 and ICAM-1/methanol, ellagic acid/reduction in the procedure Methanol/Aβ fibril formation inhibition (90%) | 75 –77 |
| Peganum harmala L. | Cholinesterase inhibition Antioxidant Anti-inflammatory | Ellman/methanol, dichloromethane, harmine, harmaline/41.2, 95.5, 8.4, 10.9 μg/mL DPPH, β-carotene/hydroalcoholic/effective THP-1-derived macrophages/methanol/ ↑ (release and expression of IL-10 mRNA), ↓ (IL-1, IL-6 and TNF-α mRNA expression) | 13,78 |
| Vitex agnus-castus L. | Antioxidant Anti-inflammatory | DPPH, rat brain homogenate auto-oxidation/ethyl acetate/IC50 = 68.14 µg/mL Cell-based (in vitro)/metabolites/effective | 79,80 |
| Pimpinella anisum L. | Antioxidant Anti-inflammatory | SOD, OH, metal chelating/methanol/positive Carrageenan-induced paw edema/fixed oil/strong | 81,82 |
| Heracleum persicum Desf. ex Fisch | Antioxidant Anti-inflammatory | Linoleic acid/ethyl acetate, furanocoumarins/effective Carrageenan-induced paw edema/essential oil, hydroalcoholic/effective | 83,84 |
Abbreviations: AD, Alzheimer’s disease; COX, cyclooxygenase; DPPH, 2, 2-diphenyl-1-picrylhydrazyl; FRAP, ferric reducing ability of plasma; ICAM, intercellular adhesion molecule; IL, interleukin; LOX, lipoxygenase; LP, lipid peroxidation; NO, nitric oxide; OH, hydroxyl radical; ORAC, oxygen radical absorbance capacity; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TEAC, trolox equivalent antioxidant capacity; THP-1, human monocytic cell line derived from an acute monocytic leukemia patient; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule.
Discussion and Conclusion
Alzheimer’s disease is a chronic condition and the most common cause of irreversible dementia. It increasingly leads to mental disability and death. The available treatments only aim to relive the symptoms and not its progression. 85 Despite tremendous efforts invested in drug discovery research to identify an effective medication for AD, there is no successful cure yet. Currently, there are many compounds being investigated for the treatment of this disease. Although the etiology of AD remains unknown, research has suggested several pathological and biological factors that contribute to AD. Acetylcholinesterase (AChE) and butyrylcholinesterase have been found to be important compounds in cognitive impairment associated with AD. 86 High activity of AChE enzyme in patients with AD leads to the presence of acetylcholine in the synapses of the cerebral cortex and its deficiency in the cerebral cortex. Acetylcholinesterase inhibitors are commonly prescribed drugs for the treatment of AD. Their use has limitations, such as adverse side effects. 15 Three drugs approved by the Food and Drug Administration for the treatment of AD possess acetyl cholinesterase inhibition properties. These are donepezil, galantamine, and rivastigmine. These drugs can slow down the process of progression of the symptoms of AD for 6 to 12 months. These drugs are nowadays prescribed to patients with mild to severe symptoms related to AD. They inhibit AChE from hydrolyzing acetylcholine. Owing to its heavy hepatic toxicity, tacrine—which is also an AChE inhibitor—is hardly prescribed today. 85
In recent years, researchers have evinced interest in the compounds derived from traditional medicines as sources of medicinal phytochemicals. 86 Traditional Persian medicine is a system of medicine mainly based on herbal therapies. What makes this system significant is that all records of physicians and pharmacists are available in the extant manuscripts of these scholars. 87 Cognitive disorder was known to these physicians; they described a clinical condition that had similar clinical manifestations as AD. 15 Herbal treatments were recommended in TP books. 88 If these herbs were effective in the treatment of AD, they should have related mechanisms of action as well. In this review, we have introduced 40 herbs from traditional Persian medicine (TPM) for the treatment of Nesyān, which has the same clinical manifestations as AD, according to TPM books. We found that 30 of these herbs had at least 1 mechanism89,90 ,of action related to AD treatment (Table 3). 13,16 -77,78 , 85-90 From the found herbs of TPM, 15 had proven AChE and/or butyrylcholinesterase inhibition effects. Recent research into drug discovery has focused on phytochemicals such as AChE inhibitors with the least side effects. 19 From the list of found herbs recommended by Persian scholars, Iris florentina L., 16 Piper betle L., 17 Z officinale Roscoe, 19 Piper nigrum L., 26 Acorus calamus L., 27 Cymbopogon schoenanthus (L.) Spreng., 30 Nepeta menthoides Boiss. & Buhse, 31 A sativum L., 32 Teucrium polium L., 40 Citrullus colocynthis (L.) Schrad., 42 Phyllanthus emblica L., 49 Thymus serpyllum L., 56 Terminalia chebula Retz., 69 Alhagi maurorum Medik., 72 and Peganum harmala L. 13 have acetyl and/or butyrylcholinesterase inhibition properties. Possession of this property was confirmed by Ellman method in the modern literature that was searched. Even though it was not a mechanism known to the ancient Persians, it does indicate that the use of these herbs in the treatment of AD can be explained by modern pharmacology as well.
Evidence has suggested that inflammation, too, plays a role in the pathogenesis of this disease. 6 As a result, another group of compounds suggested for the treatment of AD are of anti-inflammatory agents. The chronic use of nonsteroidal anti-inflammatory drugs is today recognized as a factor that delays the onset of AD and reduces its risk. 89
Twenty-four of the herbs that were recommended by ancient Persians to treat Nesyān also have proven anti-inflammatory activity. Their anti-inflammatory activity has been proven by experimental analysis in modern research. These herbs include Z officinale Roscoe, 21 Santalum album L., 23 P nigrum L., 25 A calamus L., 29 A sativum L., 35 Melilotus officinalis (L.) Pall, 38 T polium L., 40 C colocynthis (L.) Schrad., 44 Brassica nigra (L.) K. Koch, 46 Boswellia sacra Flueck., 48 P emblica L., 50 Ferula assa-foetida L., 53 Zingiber zerumbet (L.), Rescoe ex Sm, 55 Pistacia lentiscus L., Pistacia vera L., 59 Vitis vinifera L., 61 Anacardium occidentale L., 63 Cinnamomum verum J. Presl, 66 T chebula Retz., 68 A maurorum Medik., 74 Juglans regia L., 76 P harmala L., 78 Vitex agnus-castus L., 80 Pimpinella anisum L., 82 and Heracleum persicum Desf. ex Fisch. 84
Another biochemical factor associated with AD is oxidative damage. It occurs as an early event of AD. Increasing administration of antioxidants has been shown to be helpful in lowering conversion of mild cognitive impairment to dementia. 90 From the herbs prescribed by TPM for AD treatment, all had proven antioxidant property. Their antioxidant activity was confirmed in the research mainly by DPPH assay.
Neuritic or senile plaques could be observed pathologically in the brains of patients with AD. The major constituent of these plaques is amyloid β-protein (Ab). 77 Although soluble Ab is a normal constituent of blood and cerebral fluid in AD, it assembles into insoluble fibrils. Studies on synthetic Ab have shown that this compound is neurotoxic; this effect is correlated with the extent of fibril formation. The drugs that could convert the fibrillar form of Ab to its nonfibrillar form and have antiamyloidogenic effects are considered potential drugs in treating and preventing AD. 77 Three of the recommended herbs used by TPM for the treatment of AD had proven effects on β-amyloid peptide brain plaques. Z officinale Roscoe, 20 A sativum L., 36 and Juglans regia L. 77 showed Ab fibril formation inhibition in animal studies.
Although the feasibility of applying TPM in AD therapy was demonstrated here, further preclinical and clinical studies should be conducted to investigate the therapeutic function in patients with AD.
Taken as a whole, 3 of the recommended medicinal plants used by TPM for the treatment of AD had possessed all activities (cholinesterase inhibition, antioxidant, anti-inflammatory, and antiamyloidogenic activities, based on Table 3; Figure 1). These medicaments could be suggested as lead natural medicines for further clinical and pharmaceutical aspects. On the other side, remedies such as P nigrum L., A calamus L., C colocynthis (L.) Schrad., P emblica L., T chebula Retz., A maurorum Melik, and P harmala L. have exerted 3 out of all suggested activities including cholinesterase inhibition, antioxidant, and anti-inflammatory effects (Figure 2).
Figure 1.
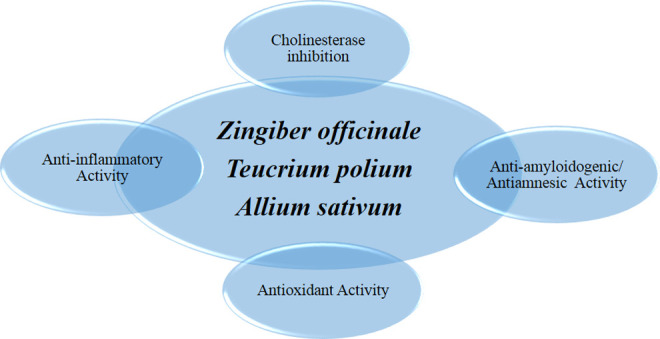
Schematic view of lead natural medicament possessing all suggested activities.
Figure 2.
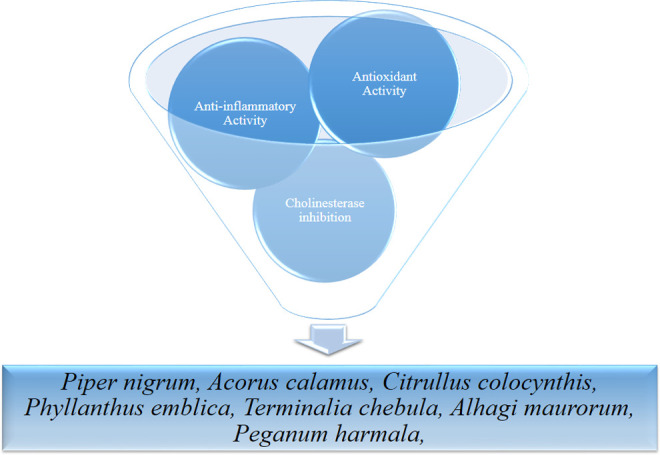
Schematic view of lead natural medicament possessing 3 out of all suggested.
Despite much effort being invested by scientists to discover new drugs for the treatment of AD, there is no effective medicine for this disease yet. Even though many compounds are undergoing investigation, only a few show significant effects in clinical trials. Traditional systems of medicine contain valuable information on medication used by our ancestors. In this work, we introduced promising candidates in TPM that could undergo an investigation for the identification of their active compounds and clinical validation in the treatment of AD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Shah H, Albanese E, Duggan C, et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15(12):1285–1294. [DOI] [PubMed] [Google Scholar]
- 2. Rosini M, Simoni E, Minarini A, Melchiorre C. Multi-target design strategies in the context of Alzheimer’s disease: acetylcholinesterase inhibition and NMDA receptor antagonism as the driving forces. Neurochem Res. 2014;39(10):1914–1923. [DOI] [PubMed] [Google Scholar]
- 3. Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron. 2014;83(1):11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. New Engl J Med. 2013;368(13):1169–1171. [DOI] [PubMed] [Google Scholar]
- 5. Näslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA. 2000;283(12):1571–1577. [DOI] [PubMed] [Google Scholar]
- 6. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meraz-Ríos MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernández J, Campos-Peña V. Inflammatory process in Alzheimer’s disease. Front Integr Neurosci. 2013;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santos-Neto LLd, de Vilhena Toledo MA, Medeiros-Souza P, de Souza GA. The use of herbal medicine in Alzheimer’s disease—a systematic review. Evid Based Complement Alternat Med. 2006;3(4):441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HU, Ryu JY, Lee JO, Lee SY. A systems approach to traditional oriental medicine. Nat Biotech. 2015;33(3):264–268. [DOI] [PubMed] [Google Scholar]
- 11. Liu P, Kong M, Yuan S, Liu J, Wang P. History and experience: a survey of traditional Chinese medicine treatment for Alzheimer’s disease. Evid Based Complement Alternat Med. 2014;2014:642128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(suppl 1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adhami HR, Farsam H, Krenn L. Screening of medicinal plants from Iranian traditional medicine for acetylcholinesterase inhibition. Phytother Res. 2011;25(8):1148–1152. [DOI] [PubMed] [Google Scholar]
- 14. Hosseinkhani A, Zargaran A, Zarshenas MM, Mehdizadeh A. Abkama, the first reported antibiotic in gastritis and infections throughout history. Pharm Hist (Lond). 2013;43(2):39–41. [PubMed] [Google Scholar]
- 15. Saifadini R, Tajadini H, Choopani R, Mehrabani M, Kamalinegad M, Haghdoost A. Perception of Alzheimer disease in Iranian traditional medicine. Iran Red Crescent Med J. 2016;18(3):e22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ullah F, Ayaz M, Sadiq A, et al. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina. Nat Prod Res. 2016;30(12):1440–1444. [DOI] [PubMed] [Google Scholar]
- 17. Valentao P, Goncalves RF, Belo C, de Pinho PG, Andrade PB, Ferreres F. Improving the knowledge on Piper betle: targeted metabolite analysis and effect on acetylcholinesterase. J Sep Sci. 2010;33(20):3168–3176. [DOI] [PubMed] [Google Scholar]
- 18. Dasgupta N, De B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004;88(2):219–224. [Google Scholar]
- 19. Oboh G, Ademiluyi AO, Akinyemi AJ. Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp Toxicol Pathol. 2012;64(4):315–319. [DOI] [PubMed] [Google Scholar]
- 20. Han YA, Song CW, Koh WS, et al. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother Res. 2013;27(8):1200–1005. [DOI] [PubMed] [Google Scholar]
- 21. Mathew M, Subramanian S. In vitro evaluation of anti-Alzheimer effects of dry ginger (Zingiber officinale Roscoe) extract. Indian J Exp Biol. 2014;52(6):606–612. [PubMed] [Google Scholar]
- 22. Misra BB, Dey S. Phytochemical analyses and evaluation of antioxidant efficacy of in vitro callus extract of East Indian Sandalwood Tree (Santalum album L.). Lett Appl Microbiol. 2012;55:476–486. [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, Chaphalkar S. Immunopharmacological screening of aqueous root extract of Santalum album. J Herbmed Pharmacol. 2016;5(1):7–11. [Google Scholar]
- 24. Vijayakumar R, Surya D, Nalini N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9(2):105–110. [DOI] [PubMed] [Google Scholar]
- 25. Tasleem F, Azhar I, Ali SN, Perveen S, Mahmood ZA. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac J Trop Med. 2014;7S1:S461–S468. [DOI] [PubMed] [Google Scholar]
- 26. Kumar S, Brijeshlata DS. Screening of traditional Indian spices for inhibitory activity of acetylcholinesterase and butyrylcholinesterase enzymes. Int J Pharma Bio Sci. 2012;3(1):59–65. [Google Scholar]
- 27. Mukherjee PK, Kumar V, Mal M, Houghton PJ. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007;73(3):283–285. [DOI] [PubMed] [Google Scholar]
- 28. Devi SA, Ganjewala D. Antioxidant activities of methanolic extracts of sweet-flag (Acorus calamus) leaves and rhizomes. J Herbs Spices Med Plants. 2011;17(1):1–11. [Google Scholar]
- 29. Muthuraman A, Singh N, Jaggi AS. Protective effect of Acorus calamus L. in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem Toxicol. 2011;49(10):2557–2563. [DOI] [PubMed] [Google Scholar]
- 30. Khadri A, Neffati M, Smiti S, et al. Araújo ME. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT Food Sci Technol. 2010;43(2):331–336. [Google Scholar]
- 31. Kahkeshani N, Razzaghirad Y, Ostad SN, et al. Cytotoxic, acetylcholinesterase inhibitor and antioxidant activity of Nepeta menthoides Boiss & Buhse essential oil. J Essent Oil Bear Pl. 2014;17(4):544–552. [Google Scholar]
- 32. Singh V, Singh D. Enzyme inhibition by allicin, the molluscicidal agent of Allium sativum L. (garlic). Phytother Res. 1996;10(5):383–386. [Google Scholar]
- 33. Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008;111(4):925–929. [Google Scholar]
- 34. Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48(4):209–215. [DOI] [PubMed] [Google Scholar]
- 35. Kumar S. Dual inhibition of acetylcholinesterase and butyrylcholinesterase enzymes by allicin. Indian J Pharmacol. 2015;47(4):444–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chauhan NB. Anti-amyloidogenic effect of Allium sativum in Alzheimer’s transgenic model Tg2576. J Herb Pharmacother. 2003;3(1):95–107. [PubMed] [Google Scholar]
- 37. Braga PC, Dal Sasso M, Lattuada N, et al. Antioxidant activity of Melilotus officinalis extract investigated by means of the radical scavenging activity, the chemiluminescence of human neutrophil bursts and lipoperoxidation assay. J Med Plant Res. 2013;7(7):358–365. [Google Scholar]
- 38. Pleşca-Manea L, Pârvu AE, Parvu M, Taǎmaş M, Buia R, Puia M. Effects of Melilotus officinalis on acute inflammation. Phytother Res. 2002;16(4):316–319. [DOI] [PubMed] [Google Scholar]
- 39. Orhan I, Aslan M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J Ethnopharmacol. 2009;122(2):327–332. [DOI] [PubMed] [Google Scholar]
- 40. Capasso F, Cerri R, Morrica P, Senatore F. Chemical composition and anti-inflammatory activity of an alcoholic extract of Teucrium polium L. Boll Soc Ital Biol Sper. 1983;59(11):1639–1643. [PubMed] [Google Scholar]
- 41. Sharififar F, Dehghn-Nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009;112(4):885–888. [Google Scholar]
- 42. Shahat AA, Ibrahim AY, Ezzeldin E, Alsaid MS. Acetylcholinesterase inhibition and antioxidant activity of some medicinal plants for treating neurodegenerative disease. Afr J Tradit Complement Altern Med. 2015;12(3):97–103. [Google Scholar]
- 43. Kumar S, Kumar D, Jusha M, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharmaceutica. 2008;58(2):215–220. [DOI] [PubMed] [Google Scholar]
- 44. Marzouk B, Marzouk Z, Haloui E, Fenina N, Bouraoui A, Aouni M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J Ethnopharmacol. 2010;128(1):15–19. [DOI] [PubMed] [Google Scholar]
- 45. Chung SK, Osawa T, Kawakishi S. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Biosci Biotechnol Biochem. 1997;61(1):118–123. [Google Scholar]
- 46. Alam MB, Hossain MS, Haque ME. Antioxidant and anti-inflammatory activities of the leaf extract of Brassica nigra . Int J Pharm Sci Res. 2011;2(2):303. [Google Scholar]
- 47. Al-Harrasi A, Ali L, Ceniviva E, et al. Antiglycation and antioxidant activities and HPTLC analysis of Boswellia sacra Oleogum resin: the sacred frankincense. Trop J Pharm Res. 2013;12(4):597–602. [Google Scholar]
- 48. Ammon HP. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006;72(12):1100–1116. [DOI] [PubMed] [Google Scholar]
- 49. Uddin MS, Mamun A, Hossain MS, Akter F, Iqbal MA, Asaduzzaman M. Exploring the effect of Phyllanthus emblica L. on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: promising natural gift for the mitigation of Alzheimer’s disease. Ann Neurosci. 2016;23(4):218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi ZM, Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica . Planta Med. 1997;63(6):518–524. [DOI] [PubMed] [Google Scholar]
- 51. Biswas K, Islam MA, Sharmin T, Biswas PK. In-vitro cholinesterase inhibitory activity of dry fruit extract of Phyllanthus emblica relevant to the treatment of Alzheimer’s disease. Phytomedicine. 2015;4:5–8. [Google Scholar]
- 52. Kavoosi G, Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: effect of collection time. Food Chem. 2013;138(4):2180–2187. [DOI] [PubMed] [Google Scholar]
- 53. Bagheri SM, Hedesh ST, Mirjalili A, Dashti-R MH. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of Ferula assa-foetida oleo gum resin. J Evid Based Complementary Altern Med. 2015;21(4):271–276. [DOI] [PubMed] [Google Scholar]
- 54. Nag A, Bandyopadhyay M, Mukherjee A. Antioxidant activities and cytotoxicity of Zingiber zerumbet (L.) Smith rhizome. J Pharmacogn Phytochem. 2013;2(3):102–108. [Google Scholar]
- 55. Chien T, Chen L, Lee CJ, Lee F, Wang CC. Anti-inflammatory constituents of Zingiber zerumbet . Food Chem. 2008;110(3):584–589. [Google Scholar]
- 56. Kindl M, Blažeković B, Bucar F, Vladimir-Knežević S. Antioxidant and anticholinesterase potential of six thymus species. Evid Based Complement Altern Med. 2015;2015:403950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mata A, Proença C, Ferreira A, Serralheiro M, Nogueira J, Araújo M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007;103(3):778–786. [Google Scholar]
- 58. Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107(3):1120–1130. [Google Scholar]
- 59. Maxia A, Sanna C, Frau MA, Piras A, Karchuli MS, Kasture V. Anti-inflammatory activity of Pistacia lentiscus essential oil: involvement of IL-6 and TNF-alpha. Nat Prod Commun. 2011;6(10):1543–1544. [PubMed] [Google Scholar]
- 60. Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie. 2010;92(9):1115–1122. [DOI] [PubMed] [Google Scholar]
- 61. Orhan I, Küpeli E, Aslan M, Kartal M, Yesilada E. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L. J Ethnopharmacol. 2006;105(1-2):235–240. [DOI] [PubMed] [Google Scholar]
- 62. Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61(21):2103–2110. [DOI] [PubMed] [Google Scholar]
- 63. Di Lorenzo C, Sangiovanni E, Fumagalli M, et al. Evaluation of the anti-inflammatory activity of raisins (Vitis vinifera L.) in human gastric epithelial cells: a comparative study. Int J Mol Sci. 2016;17(7):pii:E1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Lima S, Feitosa C, Citó AM, et al. Effects of immature cashew nut-shell liquid (Anacardium occidentale) against oxidative damage in Saccharomyces cerevisiae and inhibition of acetylcholinesterase activity. Genet Mol Res. 2008;7(3):806–818. [DOI] [PubMed] [Google Scholar]
- 65. Trevisan M, Pfundstein B, Haubner R, et al. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem Toxicol. 2006;44(2):188–197. [DOI] [PubMed] [Google Scholar]
- 66. Mota ML, Thomas G, Barbosa Filho JM. Anti-inflammatory actions of tannins isolated from the bark of Anacardium occidentale L. J Ethnopharmacol. 1985;13(3):289–300. [DOI] [PubMed] [Google Scholar]
- 67. Mathew S, Abraham TE. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006;94(4):520–528. [Google Scholar]
- 68. Vetal S, Bodhankar SL, Mohan V, Thakurdesai PA. Anti-inflammatory and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats. Food Science and Human Wellness. 2013;2(2):59–67. [Google Scholar]
- 69. Nag G, De B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int J Pharm Pharm Sci. 2011;3(3):121–124. [Google Scholar]
- 70. Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC. Antioxidant and free radical scavenging activities of Terminalia chebula . Biol Pharm Bull. 2003;26(9):1331–1335. [DOI] [PubMed] [Google Scholar]
- 71. Reddy DB, Reddy T, Jyotsna G, et al. Chebulagic acid, a COX–LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009;124(3):506–512. [DOI] [PubMed] [Google Scholar]
- 72. Ashraf M, Ahmad K, Ahmad I, Ahmad S, Arshad S, Shah SMA. Acetylcholinesterase and NADH oxidase inhibitory activity of some medicinal plants. J Med Plant Res. 2011;5(10):2086–2089. [Google Scholar]
- 73. Ahmad S, Riaz N, Saleem M, Jabbar A, Nisar-Ur-Rehman, Ashraf M. Antioxidant flavonoids from Alhagi maurorum . J Asian Nat Prod Res. 2010;12(2):138–143. [DOI] [PubMed] [Google Scholar]
- 74. Awaad AS, El-Meligy R, Qenawy S, Atta A, Soliman GA. Anti-inflammatory, antinociceptive and antipyretic effects of some desert plants. J Saudi Chem Soc. 2011;15(4):367–373. [Google Scholar]
- 75. Orhan IE, Suntar IP, Akkol EK. In vitro neuroprotective effects of the leaf and fruit extracts of Juglans regia L. (walnut) through enzymes linked to Alzheimer’s disease and antioxidant activity. Int J Food Sci Nutr. 2011;62(8):781–786. [DOI] [PubMed] [Google Scholar]
- 76. Papoutsi Z, Kassi E, Chinou I, Halabalaki M, Skaltsounis L, Moutsatsou P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br J Nutr. 2008;99(4):715–722. [DOI] [PubMed] [Google Scholar]
- 77. Chauhan N, Wang K, Wegiel J, Malik MN. Walnut extract inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Curr Alzheimer Res. 2004;1(3):183–188. [DOI] [PubMed] [Google Scholar]
- 78. Abolhasani L, Salehi EA, Kenari RE. Study of antioxidant capacity and stability of phenolic compounds from the seeds of Peganum harmala . J Appl Environ Biol Sci. 2015;4(11):218–222. [Google Scholar]
- 79. Hajdú Z, Hohmann J, Forgo P, et al. Diterpenoids and flavonoids from the fruits of Vitex agnus-castus and antioxidant activity of the fruit extracts and their constituents. Phytother Res. 2007;21(4):391–394. [DOI] [PubMed] [Google Scholar]
- 80. Choudhary MI, Jalil S, Nawaz SA, Khan KM, Tareen RB. Antiinflammatory and lipoxygenase inhibitory compounds from Vitex agnus-castus . Phytother Res. 2009;23(9):1336–1339. [DOI] [PubMed] [Google Scholar]
- 81. Gülçın İ, Oktay M, Kıreçcı E, Küfrevıoǧlu Öİ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003;83(3):371–382. [Google Scholar]
- 82. Tas A, Ozbek H, Atasoy N, Enes Altug M, Ceylan E. Evaluation of analgesic and anti inflammatory activity of Pimpinella anisum fixed oil extract. Indian Vet J. 2006;83(8):840–843. [Google Scholar]
- 83. Hajhashemi V, Sajjadi SE, Heshmati M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J Ethnopharmacol. 2009;124(3):475–480. [DOI] [PubMed] [Google Scholar]
- 84. Souri E, Farsam H, Sarkheil P, Ebadi F. Antioxidant activity of some furanocoumarins isolated from Heracleum persicum . Pharm Biol. 2004;42(6):396–399. [Google Scholar]
- 85. Cheung TS, Song TH, Ng TB, et al. Therapeutic effects of herbal chemicals in traditional Chinese medicine on Alzheimer’s disease. Curr Med Chem. 2015;22(19):2392–2403. [DOI] [PubMed] [Google Scholar]
- 86. Hard to swallow. Nature. 2007;448(7150):105–106. [DOI] [PubMed] [Google Scholar]
- 87. Zarshenas M, Hosseinkhani A, Zargaran A, Kordafshari G, Mohagheghzadeh A. Ophthalmic dosage forms in medieval Persia. Pharm Hist (Lond). 2013;43(1):6–8. [PubMed] [Google Scholar]
- 88. Zargaran A, Zarshenas MM, Hosseinkhani A, Mehdizadeh A. Jawarish, a Persian traditional gastrointestinal dosage form. Pharm Hist (Lond). 2012;42(2):24–25. [PubMed] [Google Scholar]
- 89. McGeer PL, Rogers J, McGeer EG. Inflammation, antiinflammatory agents, and Alzheimer’s disease: the last 22 years. J Alzheim Dis. 2016;54(3):853–857. [DOI] [PubMed] [Google Scholar]
- 90. Rinaldi P, Polidori MC, Metastasio A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24(7):915–919. [DOI] [PubMed] [Google Scholar]


