Abstract
Synthetic biology has developed sophisticated cellular biosensors to detect and respond to human disease. However, biosensors have not yet been engineered to detect specific extracellular DNA sequences and mutations. Here, we engineered naturally competent Acinetobacter baylyi to detect donor DNA from the genomes of colorectal cancer (CRC) cells, organoids, and tumors. We characterized the functionality of the biosensors in vitro with co-culture assays and then validate in vivo with sensor bacteria delivered to mice harboring colorectal tumors. We observe horizontal gene transfer from the tumor to the sensor bacteria in our mouse model of CRC. This Cellular Assay for Targeted, CRISPR-discriminated Horizontal gene transfer (CATCH) enables the biodetection of specific cell-free DNA.
Bacterial engineering has allowed the development of living cell diagnostics and therapeutics (1–3), including microbes that respond to gut inflammation (4), intestinal bleeding (5), pathogens (6) and hypoxic tumors (7). Bacteria can access the entire gastrointestinal tract to produce outputs measured in stool (4) or urine (7). Cellular memory, such as bistable switches (4, 8, 9) or genomic rearrangements (10), enables bacteria to store information over time. Some bacteria are naturally competent for transformation and can sample extracellular DNA directly from their environment (11). Natural competence is one mechanism of horizontal gene transfer (HGT), the exchange of genetic material between organisms outside vertical, “parent to offspring”, transmission (12). HGT is common between microbes (12). It may also occur from microbes into animals and plants (13) and, in the opposite direction, from eukaryotes to prokaryotes (14). The forward engineering of bacteria to detect and respond to mammalian DNA via HGT, however, has not been explored.
Acinetobacter baylyi is a highly competent and well-studied bacterium (15, 16) that is largely non-pathogenic in healthy humans (17), can colonize the murine gastrointestinal tract (18), and acquires unpurified, environmental DNA from lysed cells (19). Our CATCH strategy delivers bacterial biosensors to sample and genomically integrate target DNA (Fig. 1). To demonstrate this concept, we use the biosensor to detect engineered tumor cells. We then develop genetic circuits to detect natural, non-engineered tumor DNA sequences, discriminating oncogenic mutations at the single base level. Since the target sequence and output gene are modular, our approach can be generalized to detect arbitrary DNA sequences and respond in a programmable manner.
Figure 1. Engineered bacteria to detect tumor DNA.

Engineered A. baylyi bacteria are delivered rectally in an orthotopic mouse model of CRC. The naturally competent A. baylyi take up tumor DNA shed into the colorectal lumen. The tumor donor DNA is engineered with a kanR cassette flanked by KRAS homology arms. The sensor bacteria are engineered with matching KRAS homology arms that promote homologous recombination. Sensor bacteria that undergo HGT from tumor DNA acquire kanamycin resistance and are quantified from luminal contents by serial dilution on antibiotic selection plates.
Results
Engineering cancer cell lines, organoids and sensor bacteria
To test the hypothesis that bacteria could detect specific mammalian DNA, we generated transgenic donor human cancer cells with a kanamycin resistance gene (kanR) inside KRAS homology arms (Figs. 1, 2A–C, S1, S2). KRAS is an important oncogene in human cancer, and a driver mutation in KRAS often accompanies the progression of simple into advanced colorectal adenomas (20). Our technology is currently confined to the detection of specific sequences and thus for cancer detection is limited to hotspot mutations, such as KRASG12D. We stably transduced this donor cassette into 3 conventional human CRC cell lines with differing background genetic alterations (RKO is microsatellite instability high, MSI-H, BRAFV600E; LS174T is MSI-H, KRASG12D; SW620 is microsatellite stable, MSS, KRASG12V) and two human CRC organoid lines (RAH057T is MSS, KRASG12D; RAH038T is MSI-H, BRAFV600E) using a lentiviral vector. To construct the sensor bacteria, we inserted a complementary landing pad with KRAS homology arms into A. baylyi. We tested both a “large insert” design, where 2 kb of donor cassette must transfer (Fig. 2A & B, S2A, data file S1), and a “small insert” design where only 8 bp must transfer to repair 2 stop codons (Fig. 2C, S2B, supplementary materials and methods)(21). The initial biosensor output was growth on kanamycin plates (Fig. 2 and Fig S2).
Figure 2: Sensing KRASG12D DNA in vitro.

A, Donor DNA was derived from plasmid, purified cancer cell genomic DNA, or raw lysate (top) that recombined into biosensor A. baylyi cells (bottom). Horizontal gene transfer included either a large, 2 kb insert B, or a small, 8 bp insert to repair 2 stop codons C, in both cases conferring kanamycin resistance. D-G) A. baylyi biosensors were incubated with plasmid DNA, purified RKO-KRAS or LS174T-KRAS genomic DNA, or raw RKO-KRAS lysate, all containing the donor cassette, or purified RKO or LS174T genomic DNA as controls. Biosensor cells included either “large insert” (B, D & E) or “small insert” (C, F & G) designs, and transformations were performed in liquid culture (D & F) or on solid agar surfaces (E & G). Two sample t-tests compared data to RKO and LS174T genomic DNA controls under the same conditions. H, CRISPR spacers targeting the KRAS G12D mutation (boxed), using the underlined PAMs. Fraction of total biosensor cells expressing the indicated CRISPR spacers that were transformed by plasmid donor DNA with wild type (I) or mutant G12D (J) KRAS. Statistics were obtained using two sample, one-sided t-tests, with p-values displayed on the figures. Data points below detection are shown along the x-axis, at the limit of detection.
Detection of cell-free DNA from cancer cell lines.
We tested these designs using various donor DNA sources, both in liquid culture and on solid agar (Fig. 2A). The “large insert” biosensors detected donor DNA from purified plasmids and genomic DNA both in liquid (Fig. 2D) and on agar (Fig. 2E). On agar, they also detected raw, unpurified lysate, albeit at just above the limit of detection (Fig. 2E). As expected (22), the “small insert” design improved detection efficiency approximately 10-fold, reliably detecting even raw lysate (Fig. 2F–G, Movie S1). Across conditions, detection on solid agar was more efficient than in liquid culture. Importantly, these experiments confirmed that the biosensors did not require DNA purification (19).
Mutations in codon 12 of KRAS occur in 27% of CRC (23), accounting for 72% of all CRC KRAS mutations (24), and are common in solid tumors generally (25). To test whether sensor bacteria could discriminate between wild-type and mutant KRAS (KRASG12D), we utilized A. baylyi’s endogenous Type I-F CRISPR-Cas system (26). We stably transduced a donor RKO cell line with the kanR-GFP donor cassette flanked by wild-type KRAS, and a second line with KRASG12D flanking sequences. Next, we designed three CRISPR spacers targeting the wild-type KRAS sequence at the location of the KRASG12D mutation, using the A. baylyi protospacer-adjacent motif (PAM) (Fig. 2H). We inserted these as single-spacer arrays into a neutral locus in the “large insert” A. baylyi sensor genome.
The sensor bacteria should reject wild-type KRAS through CRISPR-mediated DNA degradation but allow integration of the KRASG12D sequence. Two of the three spacers blocked transformation by both wild-type and mutant DNA (Fig. 2I–J). However, spacer 2, for which the KRASG12D mutation eliminated the PAM site, selectively permitted only KRASG12D donor DNA (Fig. 2I–J). The other common mutations in codon 12 of KRAS all eliminate this PAM as well (23). Thus, sensor A. baylyi can be engineered to detect a mutational hotspot in the KRAS gene with single-base specificity.
Detection of cell-free DNA from tumorigenic organoid lines.
Next, we evaluated our sensor and donor constructs in organoid culture (Fig. 3A). We previously used CRISPR/Cas9 genome engineering to generate compound BrafV600E; Tgfbr2Δ/Δ; Rnf43Δ/Δ; Znrf3Δ/Δ; p16Ink4aΔ/Δ (BTRZI) mouse organoids that recapitulate serrated CRC when injected into the mouse colon (27). We transduced BTRZI organoids with the donor DNA construct to generate donor CRC organoids and incubated their lysate with the more efficient “small insert” A. baylyi biosensors. Using qPCR we confirmed that the BTRZI organoids we generated contained only 2 copies of the target donor DNA (Fig. S3). As with the CRC cell lines, the sensor A. baylyi incorporated DNA from donor organoid lysate, but not from control lysates from the parental organoids (Figs. 3B, S4 & S5A). Next, we co-cultured GFP-expressing sensor A. baylyi with parental or donor organoids for 24 hours on Matrigel. The GFP-expressing sensor bacteria enveloped the organoids (Fig. 3C). Following co-culture with donor, but not parental, organoids, the A. baylyi sensor bacteria acquired donor DNA via HGT (Figs. 3D, S5B & C). Finally, we estimated the detection limit of our biosensor for target DNA in stool. To achieve this, we added increasing amounts of donor plasmid to a defined mixture of biosensor and stool (5 ×107 biosensor mixed with 0.017 g/100 μl stool slurry). The detection limit was 3 pg of plasmid or 2.7×105 copies of target DNA, for a given incubation volume and time (Fig. S6).
Figure 3: Detection of donor DNA from BTRZI-KRAS-kanR organoids in both an in vitro and an in vivo model of colorectal cancer.

A, Schema depicting in vitro co-culture of A. baylyi sensor bacteria with BTRZI-KRAS-kanR (CRC donor) organoid lysates or viable organoids to assess HGT repair of kanamycin resistance gene (kanR). B, Recombination with DNA from crude lysates enables growth of A. baylyi sensor on kanamycin. C, Representative images of GFP-tagged A. baylyi biosensor surrounding parental BTRZI (control) and BTRZI-KRAS-kanR donor organoids at 24h. Scale bar 100 μm. D, Co-culture of established CRC BTRZI-KRAS-kanR donor organoids with A. baylyi sensor enables growth of A. baylyi sensor on kanamycin. In B & D, n = 5 independent experiments each with 5 technical replicates, one sample t-test on transformed data was used for statistical analysis with p-values as indicated. E, Schema depicting in vivo HGT experiments: generation of BTRZI-KRAS-kanR (CRC donor) tumors in mice via colonoscopic injection, with tumor pathology validated by H&E histology, administration of biosensors, and analysis of luminal contents. Scale bars 200μm. F, rectal delivery of A. baylyi biosensor to mice bearing CRC donor tumors results in kanamycin resistant A. baylyi biosensor in luminal contents via HGT with transformation efficiency of 1.5×10−9 (limit of detection 1.25×10−10). HGT rate calculated from CFU on kanamycin/chloramphenicol/vancomycin (transformants) and chloramphenicol/vancomycin (total A. baylyi) selection plates, n=3–5 mice/group. One-way ANOVA with Tukey’s post-hoc on log10 transformed data was used for statistical analysis. G, ROC curve analysis of HGT CFU following enema, AUC = 1, p = 0.009.
Detection of cell-free tumor DNA in an orthotopic mouse model of colorectal cancer.
Given that cancer-to-bacterial HGT occurred in vitro, and in the presence of stool, we sought to test the CATCH system in vivo. We first confirmed that our BTRZI, orthotopic CRC model released tumoral DNA into the colorectal lumen. Engineered CRC organoids were injected orthotopically, by mouse colonoscopy, into the mouse colon to form colonic tumors, as previously described (27). Using digital droplet PCR, we measured Braf mutant tumor DNA in stools collected from tumor-bearing and control mice. The BTRZI model reliably released tumor DNA into the colorectal lumen (Fig. S7).
We next conducted an orthotopic CRC experiment (Fig. 3E). NSG mice were injected with donor or non-donor organoids, or neither. At week 5, once the tumors had grown into the lumen, sensor (or parental) A. baylyi bacteria were delivered twice via rectal enema. The mice were subsequently euthanized and the colorectum harvested with the luminal effluent plated for analysis. Serial dilutions were then plated on agar with different antibiotic combinations (Fig 3F).
HGT from tumors to biosensors was only detected in donor tumor-bearing mice that were administered sensor bacteria. There was no HGT detected in any control group (Fig. 3F). The resistant colonies were confirmed to be the engineered biosensor strain by antibiotic resistance, green fluorescence, 16S sequencing, and HGT-mediated kanR repair of individual colonies (Fig. S8). Thus, CATCH discriminated mice with and without CRC in our experimental model (Fig. 3G).
Detection of non-engineered DNA.
Finally, we designed living biosensors to detect and analyze non-engineered cancer DNA. The tetR repressor gene was inserted between the KRAS homology arms in the biosensor, and in a second locus, we placed an output gene under control of the P_LtetO-1 promoter (28) (Fig. 4A). Here, the output gene was kanamycin resistance for ease of measurement, but the output gene is arbitrary and exchangeable.
Figure 4: Detection of non-engineered DNA.
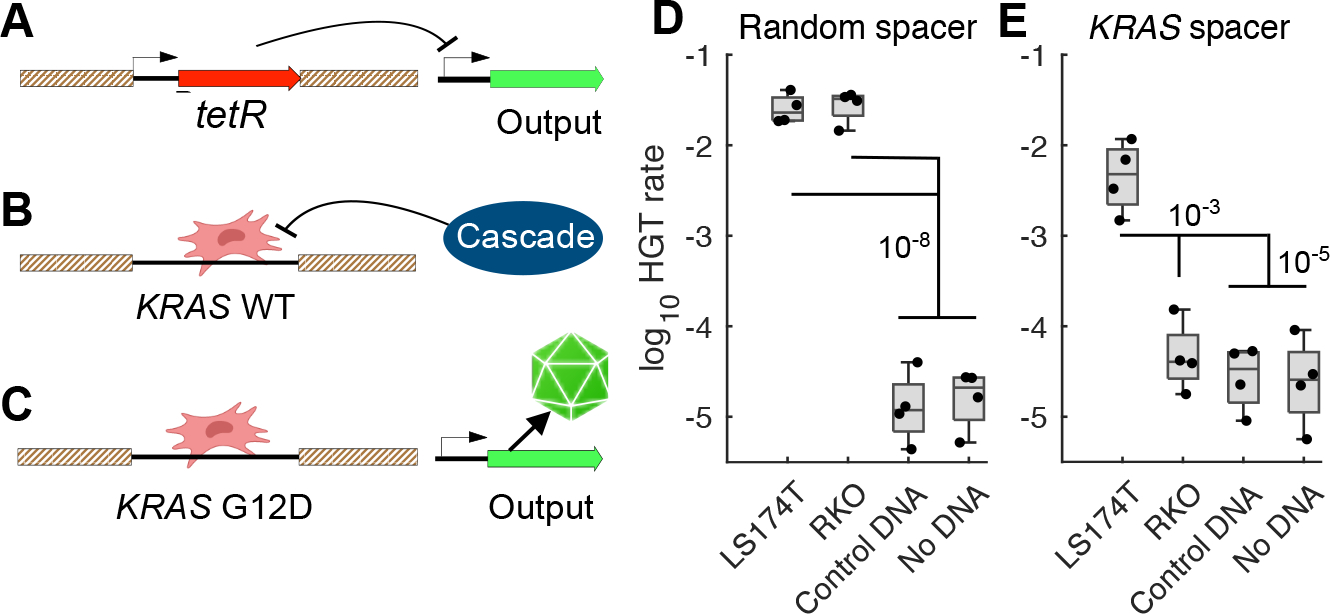
A, tetR located between the homology arms on the A. baylyi genome represses expression of the output gene. B, Target DNA with wild-type KRAS sequence is recognized and degraded by the Type I-F CRISPR-Cas effector complex, Cascade. C, Target DNA with the KRASG12D mutation avoids degradation, replaces tetR in the biosensor genome, and relieves repression of the output gene. Fraction of biosensors with either a random CRISPR spacer D, or a spacer targeting wild type KRAS E, that detected donor DNA. Statistics were obtained via two sample t-tests and are displayed on the figure.
In this design, expression of the output gene is constitutively repressed (Fig. 4A). Upon recombination with the KRAS target DNA, the repressor tetR is deleted from the genome. If the incoming KRAS sequence is wild type at the G12 locus, Cascade, the Type I-F CRISPR-Cas effector complex, detects and degrades it (Fig. 4B). However, if the G12 locus is mutated, the PAM site and therefore CRISPR-Cas targeting are eliminated, and expression from the output gene turns on (Fig. 4C).
We tested this natural DNA sensor design in vitro using PCR products from LS174T and RKO genomes as donor DNA. Natural DNA biosensors with a random CRISPR spacer detected DNA sequences from both cell lines (Fig. 4D), and biosensors with the KRAS spacer accurately detected only DNA sequence from LS174T cells, which contain the KRASG12D mutation (Fig. 4E), demonstrating biosensor detection and discrimination of natural target DNA.
Discussion
The sensor bacteria described here demonstrate that a living biosensor can detect specific mammalian DNA shed from CRC in vivo in the gut, with no sample preparation or processing. Engineered donor cassettes are not required for CATCH biosensors to detect, discriminate, and report on target sequences, although the final natural DNA biosensors will need an improved signal-to-background ratio to reliably detect sequences within whole genomic DNA. The homology arms and CRISPR spacers are modular, so this strategy could be readily adapted to detect and analyze arbitrary target sequences of interest.
Our technology is not yet ready for clinical application. This approach requires further development to ensure that future versions, at least those designed for gastrointestinal use, may be delivered orally and achieve sufficient luminal density to allow reliable detection by non-invasive sampling such as in stool or blood. As the technology advances towards clinical care, we will also need to more critically evaluate the performance of CATCH compared to other relevant disease-specific tests such as, in this case, colonoscopy and in vitro nucleic acid assays (29, 30). There is also further bioengineering required to limit the risk of biosensors escaping circuit-mediated cell death and to improve the efficiency of natural DNA detection. Finally, as our technology progresses, careful analysis is essential to ensure patient safety, to minimize the risk of spreading antibiotic resistance and to satisfy biocontainment concerns. These necessary next steps are being actively pursued and are important as CATCH is applied to additional preclinical models and before it is trialed in humans.
In vitro DNA analysis helps detect and manage important human diseases, including cancer and infection (31). However, in vitro sensing requires potentially invasive removal of samples, and many DNA diagnostics do not achieve clinically relevant sequence resolution, with more advanced techniques remaining too expensive for routine use in all settings (32). Direct sampling of the gut in vivo may offer important advantages. The gastrointestinal tract contains marked DNase activity (33), which limits the lifetime of free DNA in both rodents and humans (18, 34, 35), and may thus reduce the information content of downstream fecal samples. Bacterial biosensors located in situ could capture and preserve DNA shortly after its release before degradation by local DNases. Perhaps the most exciting aspect of CATCH, however, is that unlike in vitro diagnostics, once target DNA is captured, it could be coupled to direct and genotype-complementary delivery of nanobodies, peptides, or other small molecules for the treatment of cancer or infection (36, 37). CATCH allows for the cellular detection of cell-free DNA and thus may prove useful in future synthetic biology applications, wherever, and whenever, DNA detection and analysis is important.
Supplementary Material
Acknowledgements:
P Winning, Winning Media, for design assistance with Figure 1. Figure 2A and figure 3A created with BioRender.com. B Leggett and V Whitehall, Queensland Institute of Medical Research, for the original gift of the parental RKO, SW620 and LS174T human CRC cell lines used in this study.
Funding:
This work was supported by NIH grant R01CA241728 (JH) and NHMRC ideas grant 2020555 (DW).
Footnotes
Competing interests: JH is a co-founder and board member of, and JH, DW and SW have equity in, GenCirq Inc, which focuses on cancer therapeutics. D.W., J.H., R.C., S.W., and J.W. are inventors on a provisional patent application, “Detecting disease-associated target nucleic acids in a mammal and treatment thereof,” filed by the University of California San Diego with the US Patent and Trademark Office (application no. 63/528,234). All other authors declare that they have no competing interests.
Data and materials availability: All data are available in the manuscript or the supplementary materials.
References and notes
- 1.Slomovic S, Pardee K, Collins JJ, Synthetic biology devices for in vitro and in vivo diagnostics. Proc National Acad Sci. 112, 14429–14435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedlmayer F, Aubel D, Fussenegger M, Synthetic gene circuits for the detection, elimination and prevention of disease. Nat Biomed Eng. 2, 399–415 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Lim WA, June CH, The Principles of Engineering Immune Cells to Treat Cancer. Cell. 168, 724–740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riglar DT, Giessen TW, Baym M, Kerns SJ, Niederhuber MJ, Bronson RT, Kotula JW, Gerber GK, Way JC, Silver PA, Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol. 35, 653–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mark M, Phillip N, Alison H, Sean C, Sarah F, Logan J, Joy C, Shane M, Richard S, Robert CJ, Vladimir B, Robert L, Giovanni T, Anantha CP, Timothy LK, An ingestible bacterial-electronic system to monitor gastrointestinal health. Science. 360, 915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao N, Cubillos-Ruiz A, Cameron DE, Collins JJ, Probiotic strains detect and suppress cholera in mice. Sci Transl Med. 10, eaao2586 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danino T, Prindle A, Kwong GA, Skalak M, Li H, Allen K, Hasty J, Bhatia SN, Programmable probiotics for detection of cancer in urine. Sci Transl Med. 7, 289ra84–289ra84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotula JW, Kerns SJ, Shaket LA, Siraj L, Collins JJ, Way JC, Silver PA, Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc National Acad Sci. 111, 4838–4843 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner TS, Cantor CR, Collins JJ, Construction of a genetic toggle switch in Escherichia coli. Nature. 403, 339–342 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Courbet A, Endy D, Renard E, Molina F, Bonnet J, Detection of pathological biomarkers in human clinical samples via amplifying genetic switches and logic gates. Sci Transl Med. 7, 289ra83 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Chang Joshua M, J Rosemary R, Natural competence and the evolution of DNA uptake specificity. J Bacteriol. 196, 1471–1483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soucy SM, Huang J, Gogarten JP, Horizontal gene transfer: building the web of life. Nature Reviews Genetics. 16, 472–482 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Robinson KM, Sieber KB, Hotopp JCD, A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer. Plos Genet. 9, e1003877 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotopp JCD, Horizontal gene transfer between bacteria and animals. Trends Genet. 27, 157–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young DM, Parke D, Ornston LN, Opportunities for genetic investigation afforded by Acinetobacter baylyi, a nutritionally versatile bacterial species that is highly competent for natural transformation. Annu Rev Microbiol. 59, 519–551 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Palmen R, Vosman B, Buijsman P, Breek CKD, Hellingwerf KJ, Physiological characterization of natural transformation in Acinetobacter calcoaceticus. Microbiology+. 139, 295–305 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Chen T-L, Siu L-K, Lee Y-T, Chen C-P, Huang L-Y, Wu RC-C, Cho W-L, Fung C-P, Acinetobacter baylyi as a Pathogen for Opportunistic Infection▿. J Clin Microbiol. 46, 2938–2944 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordgård L, Nguyen T, Midtvedt T, Benno Y, Traavik T, Nielsen KM, Lack of detectable DNA uptake by bacterial gut isolates grown in vitro and by Acinetobacter baylyi colonizing rodents in vivo. Environmental Biosafety Research. 6, 149–160 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Cooper RM, Tsimring L, Hasty J, Inter-species population dynamics enhance microbial horizontal gene transfer and spread of antibiotic resistance. eLife. 6, 8053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL, Genetic Alterations during Colorectal-Tumor Development. New Engl J Medicine. 319, 525–532 (1988). [DOI] [PubMed] [Google Scholar]
- 21.See supplementary materials.
- 22.Simpson DJ, Dawson LF, Fry JC, Rogers HJ, Day MJ, Influence of flanking homology and insert size on the transformation frequency of Acinetobacter baylyi BD413. Environmental Biosafety Research. 6, 55–69 (2007). [DOI] [PubMed] [Google Scholar]
- 23.AACR Project GENIE Consortium, AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 7, 818–831 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Liu Y, Cai S, Yang C, Lin Z, Zhou L, Liu L, Cheng X, Zeng W, Not all mutations of KRAS predict poor prognosis in patients with colorectal cancer. Int J Clin Exp Patho. 12, 957–967 (2018). [PMC free article] [PubMed] [Google Scholar]
- 25.Priestley P, Baber J, Lolkema MP, Steeghs N, de Bruijn E, Shale C, Duyvesteyn K, Haidari S, van Hoeck A, Onstenk W, Roepman P, Voda M, Bloemendal HJ, Tjan-Heijnen VCG, van Herpen CML, Labots M, Witteveen PO, Smit EF, Sleijfer S, Voest EE, Cuppen E, Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 575, 210–216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper RM, Hasty J, One-Day Construction of Multiplex Arrays to Harness Natural CRISPR-Cas Systems. Acs Synth Biol. 9, 1129–1137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lannagan TRM, Lee YK, Wang T, Roper J, Bettington ML, Fennell L, rbanac L, Jonavicius L, Somashekar R, Gieniec K, Yang M, Ng JQ, Suzuki N, Ichinose M, Wright JA, Kobayashi H, Putoczki TL, Hayakawa Y, Leedham SJ, Abud HE, Yilmaz ÖH, Marker J, Klebe S, Wirapati P, Mukherjee S, Tejpar S, Leggett BA, Whitehall VLJ, Worthley DL, Woods SL, Genetic editing of colonic organoids provides a molecularly distinct and orthotopic preclinical model of serrated carcinogenesis. Gut. 68, 684–692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz R, Bujard H, Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Research. 25, 1203–1210 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myhrvold C, Freije CA, Gootenberg JS, Abudayyeh OO, Metsky HC, Durbin AF, Kellner MJ, Tan AL, Paul LM, Parham LA, Garcia KF, Barnes KG, Chak B, Mondini A, Nogueira ML, Isern S, Michael SF, Lorenzana I, Yozwiak NL, MacInnis BL, Bosch I, Gehrke L, Zhang F, Sabeti PC, Field-deployable viral diagnostics using CRISPR-Cas13. Science. 360, 444–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JS, Ma E, Harrington LB, Costa MD, Tian X, Palefsky JM, Doudna JA, CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 546, eaar6245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong Y, Xu F, Wu J, Schubert J, Li MM, Application of Next Generation Sequencing in Laboratory Medicine. Ann Lab Med. 41, 25–43 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwamoto M, Huang JY, Cronquist AB, Medus C, Hurd S, Zansky S, Dunn J, Woron AM, Oosmanally N, Griffin PM, Besser J, Henao OL, C. for D. C. and P. (CDC), Bacterial enteric infections detected by culture-independent diagnostic tests--FoodNet, United States, 2012–2014. Mmwr Morbidity Mortal Wkly Rep. 64, 252–7 (2015). [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada O, Ishikawa H, Tosaka-Shimada H, Yasuda T, Kishi K, Suzuki S, Detection of Deoxyribonuclease I Along the Secretory Pathway in Paneth Cells of Human Small Intestine. J Histochem Cytochem. 46, 833–840 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Wilcks A, van Hoek AHAM, Joosten RG, Jacobsen BBL, Aarts HJM, Persistence of DNA studied in different ex vivo and in vivo rat models simulating the human gut situation. Food Chem Toxicol. 42, 493–502 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Netherwood T, Martín-Orúe SM, O’Donnell AG, Gockling S, Graham J, Mathers JC, Gilbert HJ, Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol. 22, 204–209 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Din MO, Danino T, Prindle A, Skalak M, Selimkhanov J, Allen K, Julio E, Atolia E, Tsimring LS, Bhatia SN, Hasty J, Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 536, 81–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R, The microbiome and human cancer. Science. 371, eabc4552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


