Abstract
Background:
Cancer and cardiovascular diseases are the two leading causes of death in industrialized countries. Optimal management of life-threatening presentations of both of their diseases can pose significant challenges. The current study aimed to address the incidence, management, and outcome of acute coronary syndromes (ACS) in patients with active hematological malignancies.
Methods:
This retrospective registry-based cohort study included adults with active leukemia or lymphoma who were hospitalized at Mayo Clinic Rochester from 01/01/2004 to 12/31/2014. The diagnosis of ST-segment elevation MI (STEMI) or non-ST-segment elevation MI (NSTEMI) was made based on the 3rd Universal Definition for MI, or of unstable angina (UA) in the absence of cardiac troponin elevation. Main outcome measures included all-cause, cardiac, and non-cardiac death in-hospital and at one year.
Results:
Of 5300 adult patients with active hematological malignancies, 73 (1.4%) were diagnosed with an ACS (78.1% NSTEMI and 13.7% STEMI). 17.5% and 40% of NSTEMI and STEMI patients underwent coronary angiography, with percutaneous coronary intervention in 5.3% and 30%, respectively. While >80% of patients received β-blocker therapy, only half of all and <50% of patients managed “medically” received antiplatelet, anticoagulant, and/or statin therapy. The in-hospital and 1-year mortality was 21.9% and 58.9%, respectively, of which 25% and 15% were cardiac in etiology. Aspirin, beta-blocker, statins, and angiotensin-converting enzyme inhibitor/angiotensin-II receptor blocker were associated with better mortality outcomes.
Conclusions:
In a large, contemporary study of adults with active hematologic malignancies, ACS was uncommon, but commonly managed not in keeping with societal guideline recommendations.
Keywords: Acute coronary syndrome, Hematologic malignancy, Prognosis
1. Introduction
Heart disease and cancer remain the two leading causes of morbidity and mortality in the United States [1]. While those patients confronted with both disease entities experience a particular predicament, heart disease and cancer are commonly viewed in separation rather than integration [2]. With the improved survival outcomes in both disciplines, recognition and optimal management of both disease processes has been gaining increasing importance [3,4]. Though the focus has been on cardiomyopathy [5], vascular diseases may also pose serious challenges [6,7].
Patients who develop an acute coronary syndrome (ACS) while hospitalized for treatment of acute leukemia or lymphoma are a prime example for the significant challenges encountered when trying to optimally manage life-threatening presentations of both, malignancies and cardiovascular diseases at the same time. Comorbidities ranging from leukocytosis to leukopenia, thrombocytosis to thrombocytopenia, erythrocytosis to anemia, infection, renal and hepatic dysfunction, in addition to frailty and debility may oppose guideline-recommended ACS therapies, which are based on trials in non-cancer patients and have not been validated in cancer cohorts. The Society for Cardiovascular Angiography and Interventions (SCAI) has published an expert consensus to amend some of the uncertainties in this area [8]. However, only one study so far has addressed the question of how ACS is managed in patients on active cancer therapy. [9]
The current retrospective study was performed to address the knowledge gaps in this area. We focused our efforts on hospitalized patients with active hematologic malignancies to avoid widespread inhomogeneity across the admittedly very diverse cancer population, which is seen in various clinical practice settings by various subspecialty providers. The current study thus defines the incidence, presentation, management strategies, and outcome of ACS in in-patients with active hematological malignancies.
2. Methods
2.1. Study population
Consecutive adult patients (age ≥ 18 years) with active hematologic malignancies (leukemia and lymphoma; n = 5300) who were hospitalized at our institution from 2004 to 2014 were retrospectively identified from the Mayo Clinic Leukemia and Lymphoma database. Active hematologic malignancy was defined as those undergoing concurrent evaluation by hematology without a diagnosis of remission. Those with a clinical diagnosis of ACS were independently identified via chart review. The study protocol was approved by the Mayo Clinic institutional board review.
2.2. Patient assessment
All patient charts were reviewed and the following baseline clinical data were collected: demographics, hematologic malignancy diagnosis, cancer treatment therapies, medication list, and medical co-morbidities. Subsequently, patients with ACS were independently reviewed for clinical symptoms, electrocardiogram (ECG) findings, management strategies, coronary angiography, complications, and mortality. ST-segment elevation myocardial infarction (STEMI) was defined as ST-segment elevation in 2 contiguous leads ≥2 mm in the precordial leads or ≥1 mm in the limb leads. All other ECGs were considered non-ST segment elevation ACS (including unstable angina and NSTEMI). The diagnosis of MI was in keeping with the Universal Definition for MI [10], and those not meeting the definition were classified as unstable angina (UA). The Adult Comorbidity Evaluation-27 (ACE-27) score, the Charlson comorbidity index (CI) and the National Cardiovascular Data Registry (NCDR) percutaneous coronary intervention (PCI) risk index were calculated as described before. [11–13]
2.3. Statistical analysis
Results are presented as mean (standard deviation) for continuous measures or median (range) for skewed distributions. Categorical measures are presented as frequency (percentage). Paired Student’s t-test, and χ2-test were used for group comparisons with continuous and categorical data, respectively. Multivariable logistic models were conducted for in-hospital and 1 year death using variables who’s univariate logistic models resulted in a Wald’s χ2 significance value of p < 0.1.
Long-term survival comparisons for treatment were made by Kaplan-Meier (log-rank test) and Cox proportional hazards analyses (reported as hazard ratio (HR) and 95% confidence interval (CI)). Survival was measured from the date of index ACS to the date of death or last follow-up. A logistic regression using age, hemoglobin, and platelet count as covariates was conducted to generate probabilities of receiving a treatment. The inverses of these probabilities were then used as weights to adjust the Kaplan Meier curves and the Cox proportional hazards models. This inverse probability weighting method was necessary to insure each treatment was being compared independently of possible cofounders that affect treatment assignment by increasing the weights for patients who were unlikely to receive the treatment that was given.
Unadjusted cumulative incidence curves were used for cardiac vs. non-cardiac death applying competing risks methods. A p-value to compare cardiac vs. non-cardiac cumulative incidence at one year was determined by simulation. If the risk for cause specific death is equal, then the probability of cardiac vs. non-cardiac death is 50/50. The simulated data were generated using this fact to permute a random event for each patient who experienced mortality. The cumulative incidence curves were fitted again, and the distance between the curves at one year was found. This distance was compared to the distance found in the data to determine if it was more extreme than what was observed. The number of times it was more extreme was divided by the number of permutations to calculate the p-value for the distance between the curves at 1 year. 100,000 permutations were used in this simulation.
For all analyses a two-sided p-value <0.05 was considered to represent statistical significance. Analyses were conducted using SAS version 9 software (SAS Institute, Cary NC) and R version 3.4.2 (R Core Team 2017).
3. Results
3.1. Clinical characteristics
Of 5300 patients with active hematologic malignancies, 73 (1.4%) were identified as having ACS, and the clinical characteristics of these patients are shown in Table 1. These patients were predominantly men (two thirds) and of older age (70 ± 10 years, range 43–98 years). Acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) accounted for nearly all myeloid malignancies, and non-Hodgkin lymphoma (NHL) for nearly all lymphoid malignancies. More than half of patients with myeloid malignancies had not received chemotherapy whereas this was the case for <1 in 5 patients with lymphoid malignancies. Half of the patients had a previous history of either CAD or cerebrovascular disease. Cardiovascular risk factors were common including smoking in approximately 70%, hypertension in 60%, hyperlipidemia in 50%, and diabetes in 25% of the patients. Beta-blocker was the most common baseline cardiovascular medication. Baseline ejection fraction prior to ACS presentation (available in 67 patients) was 58.0 ± 12.2% (range 20–76%).
Table 1.
Baseline comorbidity characteristics.
| Overall (N = 73) | Lymphoid (N = 29) | Myeloid (N = 44) | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, yrs | 70 ± 10 | 68 ± 12 | 71 ± 9 |
| Female, n (%) | 23 (31.5) | 11 (37.9) | 12 (27.3) |
| Cancer characteristics | |||
| Acute myeloid leukemia, n (%) | 23 (31.5) | – | 23 (52.2) |
| Chronic myelogenous leukemia, n (%) | 19 (26.0) | – | 19 (43.2) |
| Acute lymphoblastic leukemia, n (%) | 1 (1.4) | – | 1 (2.3) |
| Non-Hodgkin’s lymphoma, n (%) | 28 (38.3) | 27 (93.1) | – |
| Other, n (%) | 2 (2.7) | 2 (6.9) | 1 (2.3) |
| Cancer treatment history | |||
| Chemotherapy initiated within 1 month of presentation, n (%) | 22 (30.1) | 9 (31.0) | 13 (29.6) |
| Most common chemotherapies | |||
| Hydroxyurea | 3 (4.1) | 3 (23.1) | |
| Cytarabine | 2 (2.7) | 2 (15.4) | |
| ATRA | 2 (2.7) | 2 (15.4) | |
| Decitabine | 2 (2.7) | 2 (15.4) | |
| Anthracyclines | 16 (21.9) | 9 (100) | 7 (53.9) |
| Vinca alkaloids | 9 (12.3) | 9 (100) | |
| Dexamethasone | 9 (12.3) | 9 (100) | |
| Cyclophosphamide | 8 (11.0) | 8 (88.9) | |
| Rituxamab | 8 (11.0) | 8 (88.9) | |
| Methotrexate | 3 (4.1) | 3 (33.3) | |
| Bleomycin | 2 (2.7) | 2 (22.2) | |
| Ifosfamide | 2 (2.7) | 2 (22.2) | |
| Etoposide | 2 (2.7) | 2 (22.2) | |
| Prior chemotherapy | 28 (38.4) | 20 (69.1) | 8 (18.2)* |
| Prior radiation therapy, n (%) | 5 (6.8) | 5 (17.2) | 0 |
| Prior chest radiation therapy, n (%) | 1 (1.4) | 1 (3.4) | 0 |
| Prior hematopoietic stem cell transplantation, n (%) | 10 (13.7) | 9 (31.0) | 1 (2.3)* |
| Cardiovascular history | |||
| Body mass index, kg/m2 | 27.9 ± 6.3 | 28.2 ± 6.7 | 27.7 ± 6.1 |
| Smoking, n (%) | 50 (68.5) | 19 (65.5) | 31 (70.5) |
| Hypertension, n (%) | 43 (58.9) | 15 (51.7) | 28 (63.6) |
| Hyperlipidemia, n (%) | 36 (49.3) | 14 (48.3) | 22 (50.0) |
| Diabetes, n (%) | 18 (24.7) | 7 (24.1) | 11 (25.0) |
| Chronic kidney disease, n (%) | 12 (16.4) | 4 (13.8) | 8 (18.2) |
| Coronary artery disease, n (%) | 31 (42.5) | 10 (34.5) | 21 (47.7) |
| History of MI | 18 (24.7) | 6 (20.7) | 12 (27.3) |
| History of CABG | 11 (15.1) | 3 (10.3) | 8 (18.2) |
| History of PTCA/PCI | 15 (20.5) | 4 (13.8) | 11 (25.0) |
| Cerebrovascular disease, n (%) | 6 (8.2) | 4 (13.8) | 2 (4.5) |
| Congestive heart failure, n (%) | 6 (8.2) | 2 (6.9) | 4 (9.1) |
| Baseline Ejection fraction, % | 58.0 ± 12.2 | 57.0 ± 12.0 | 58.7 ± 12.5 |
| Baseline medical therapy | |||
| Antiplatelet, n (%) | 27 (37.0) | 12 (41.3) | 15 (34.1) |
| Beta-blocker, n (%) | 40 (54.8) | 14 (48.3) | 26 (59.1) |
| ACEI/ARB, n (%) | 27 (37.0) | 11 (37.9) | 16 (36.4) |
| Statin, n (%) | 27 (37.0) | 10 (34.5) | 17 (38.6) |
| Anticoagulation, n (%) | 7 (9.6) | 5 (17.2) | 2 (4.5) |
| Code status | |||
| DNR | 4 (5.5) | 1 (3.4) | 3 (6.8) |
| DNR/DNI | 10 (13.7) | 5 (17.2) | 5 (11.4) |
| Full code | 52 (71.2) | 21 (72.4) | 31 (70.5) |
| Unknown | 7 (9.6) | 2 (6.9) | 5 (11.4) |
p < 0.05 for myeloid vs. lymphoid.
3.2. ACS presentation
As shown in Table 2, of 73 patients with ACS, 6 had UA (8.2%); 57 had NSTEMI (78.1%) and 10 had STEMI (13.7%). Presenting symptoms varied especially among patients with lymphoid malignancies whereas >90% of patients with myeloid malignancies experienced chest pain or dyspnea. In both groups, post-ACS EF was 50% on average. Initial cardiac troponin T (cTnT) was 0.34 ± 0.86 ng/dL while peak cTnT was 0.58 ± 1.01 ng/dL (p < 0.0001). As for other laboratory parameters, WBC count was higher whereas hemoglobin was lower in myeloid malignancy patients.
Table 2.
Acute coronary syndrome characteristics.
| Overall (N = 73) | Lymphoid (N = 29) | Myeloid (N = 44) | |
|---|---|---|---|
| Presentation characteristics | |||
| Type of ACS | |||
| Unstable angina, n (%) | 6 (8.2) | 5 (17.2) | 1 (2.3)* |
| NSTEMI, n (%) | 57 (78.1) | 19 (65.5) | 38 (86.4)* |
| STEMI, n (%) | 10 (13.7) | 5 (17.2) | 5 (11.4) |
| Presenting symptoms | |||
| Asymptomatic, n (%) | 7 (9.6) | 5 (17.2) | 2 (4.5) |
| Chest pain, n (%) | 38 (52.1) | 14 (48.3) | 24 (54.5) |
| Dyspnea, n (%) | 20 (27.4) | 4 (13.8) | 16 (36.4)* |
| Heart failure, n (%) | 16 (21.9) | 6 (20.7) | 10 (22.7) |
| Hypotension, n (%) | 4 (5.5) | 3 (10.3) | 1 (2.3) |
| Others, n (%) | 10 (13.7) | 6 (20.7) | 4 (9.1) |
| Laboratory parameters | |||
| Hemoglobin, g/dL | 9.4 ± 1.7 | 10.2 ± 1.7 | 8.8 ± 1.5* |
| WBC count, /μL | 25,200 ± 50,200 | 13,400 ± 16,400 | 33,100 ± 62,300 |
| Platelet count, /μL | 117,500 ± 158,200 | 121,100 ± 91,600 | 115,100 ± 190,920 |
| Creatinine, mg/dL | 1.2 ± 0.6 | 1.1 ± 0.5 | 1.2 ± 0.6 |
| Peak troponin T (ng/mL) | 0.58 ± 1.01 | 0.44 ± 0.68 | 0.68 ± 1.18 |
| Post ACS ejection fraction, % | 49.3 ± 15.2 | 50.3 ± 13.2 | 48.6 ± 16.3 |
| Management characteristics | |||
| Medical, n (%) | 55 (75.3) | 18 (62.1) | 37 (84.1)* |
| Unstable angina, n (%) | 2 (3.6) | 1 (5.6) | 1 (2.7) |
| NSTEMI, n (%) | 47 (64.4) | 14 (77.8) | 33 (89.2) |
| STEMI, n (%) | 6 (10.9) | 3 (16.7) | 3 (8.1) |
| Invasive, n (%) | 18 (24.7) | 11 (37.9) | 7 (15.9)* |
| Unstable angina, n (%) | 4 (22.2) | 4 (36.4) | 0 (0) |
| PCI, n (%) | 4 (100) | 4 (100) | – |
| NSTEMI, n (%) | 10 (55.6) | 5 (45.5) | 5 (71.4) |
| PCI, n (%) | 3 (30.0) | 2 (40.0) | 1 (25.0) |
| STEMI, n (%) | 4 (22.2) | 2 (18.2) | 2 (28.6) |
| PCI, n (%) | 3 (75.0) | 1 (50.0) | 2 (100) |
| Coronary angiography | 18 (24.7) | 11 (37.9) | 7 (15.9)* |
| Type 1 MI | 6 (33.3) | 3 (27.3) | 3 (42.9) |
| Type 2 MI | 12 (66.7) | 8 (72.7) | 4 (57.1) |
| Medications | |||
| Aspirin, n (%) | 40 (54.8) | 19 (65.5) | 21 (47.7) |
| P2Y12RI, n (%) | 15 (20.5) | 9 (31.0) | 6 (13.6) |
| Heparin, n (%) | 31 (42.5) | 17 (58.6) | 14 (31.8)* |
| Beta-blockers, n (%) | 62 (84.9) | 25 (86.2) | 37 (84.1) |
| ACEI/ARB, n (%) | 24 (32.9) | 10 (34.5) | 14 (31.8) |
| Statin, n (%) | 42 (57.5) | 18 (62.1) | 24 (54.5) |
p < 0.05 for myeloid vs. lymphoid.
3.3. ACS management
Cardiology was involved in the care of the majority the patients (N = 68,93.2%) either in a consultative role (N = 45, 61.6%) or as the subsequent primary service during the hospitalization (N = 23, 31.5%). Echocardiograms were obtained in 63 patients (86.3%), of whom 26 patients (41.3%) had a drop in ejection fraction greater than or equal to 10% compared to a prior echocardiogram or had an ejection fraction <50%. In addition, new wall motion abnormalities were noted in 40 patients (63.5%). Of the 73 patients with ACS (Table 2), the majority of patients (n = 55; 75.3%) were managed medically whereas 18 patients (24.7%) underwent coronary angiography. Those referred to catheterization had a higher hemoglobin level (10.3 vs. 9.0 g/dL; p = 0.0089) and platelet count (232,722 vs. 79,745/μL; p = 0.0202), and lower white blood cell count (11,767 vs. 29,636/μL; p = 0.0298) (Supplemental Table 1).
Coronary angiography showed intracoronary thrombus in six of the 18 patients (33.3%), severe coronary artery disease (three vessel and left main disease) in nine patients (50.0%), and mild or moderate coronary artery disease in three patients (16.7%). Ultimately, 10 patients (55.6%) underwent percutaneous coronary intervention. Five of these 10 patients had complications (2 major bleeding requiring transfusion or further surgical therapies, one transient complete heart block, one acute kidney injury not requiring dialysis, and one small arteriovenous fistula in access site not requiring further interventions).
In terms of medical therapy, while >80% of patients received beta-blocker, approximately half received antiplatelet, anticoagulant, and/or statin therapy with extremely low utilization in those not referred to the catheterization laboratory (Supplemental Table 1). Anemia and thrombocytopenia were associated with lower antiplatelet and/or anticoagulant therapy utilization. The utilization of the aforementioned antiplatelet and anticoagulant drugs categorized by the American College of Physician (ACP) defined hemoglobin cutoff for blood transfusions is outlined in Supplemental Fig. 1A [14]. Across the categories of platelet counts of 10,000–49,000/μL, 50,000–99,000/μL and ≥100,000/μL, only 28%, 61%, and 85% of patients received aspirin, 0%, 17%, and 46% received a P2Y12 receptor inhibitor, and 28%, 44%, and 62% unfractionated heparin (UFH). The stratification based on the cutoff for dual-antiplatelet therapy (DAPT) defined by SCAI is presented in Supplemental Fig. 1B. Applying the SCAI cutoff of 10,000/μL for aspirin and 30,000/μL for DAPT, only 58% and 27% of eligible patients received these therapies. Only 58% of patients received statin therapy, and this percentage was even lower in patients managed non-invasively (47%). Similarly, a significantly lower percentage of patients received angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II receptor blockers (ARBs) (n = 24; 32.9%), with stated reasons being renal insufficiency and/or low blood pressure.
3.4. Outcomes
A total of 7 patients (9.6%) had an in-hospital bleeding event, and the incidence was numerically but not statistically higher among those with platelet counts <50,000/μL (12.3% vs. 4.2%, p=0.27), hemoglobin levels <8 g/dL(13.3% vs. 8.6%, p = 0.58), on aspirin (15.1% vs. 5.0%, p=0.15), DAPT (10.4% vs. 6.67% p = 0.67), or UFH (12.9% vs. 7.1%, p = 0.41). There was no increased bleeding rate in patients on DAPT+UFH versus UFH alone (6.7% vs. 18.8%, p = 0.32).
As shown in Supplemental Table 2, the in-hospital and 1-year mortality was 21.9% and 58.9%, respectively, of which 75.0% and 70.2% were non-cardiac in etiology. Non-cardiac in-hospital causes of death included malignancy (n = 4), septic shock (n = 3), non-cardiac respiratory failure (n = 3), intracranial hemorrhage (n=1), and gastrointestinal bleed (n = 1). In-hospital cardiac causes of death were cardiac arrest (n = 2), cardiogenic shock (n = 1), and recurrent NSTEMI (n = 1). On the contrary, at one-year follow-up, non-cardiac causes of death were most commonly due to malignancy (n = 17) with other causes being septic shock (n = 2), and non-cardiac respiratory failure (n=2). Cardiac causes of death were heart failure (n=2) and valvular heart disease (n=1). In addition, at five-year follow-up, non-cardiac causes of death were most commonly due to malignancy (n=8) with other causes being unknown (n = 3) (Fig. 1).
Fig. 1.
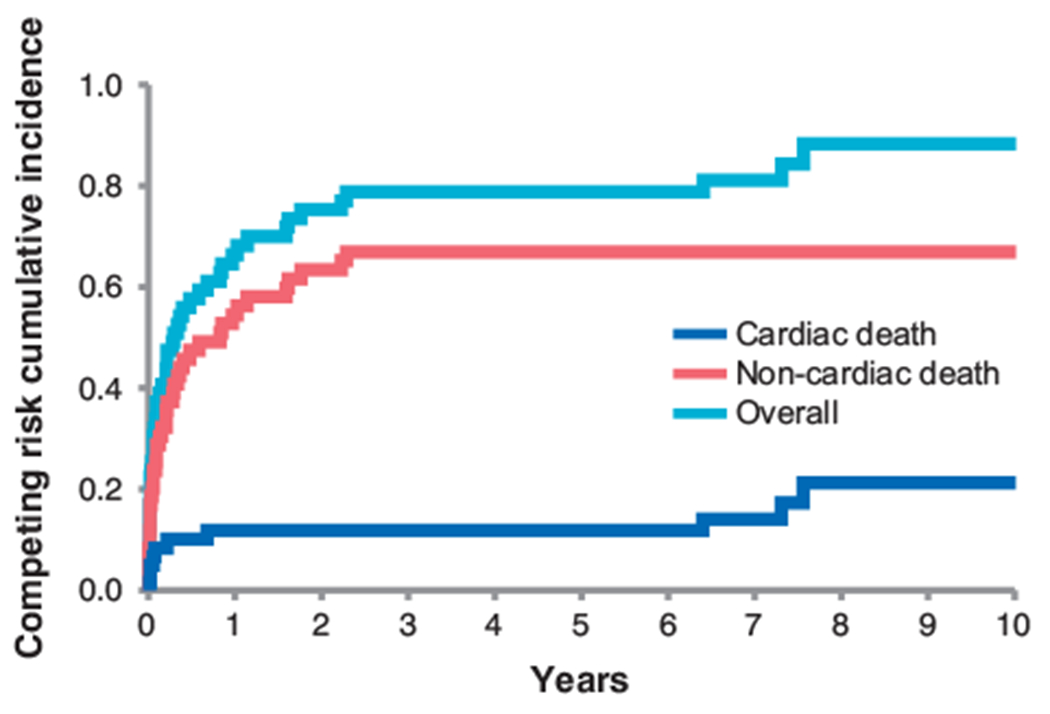
Competitive risk analysis of cardiac and non-cardiac mortality over time (p < 0.001 between cardiac and non-cardiac).
All guideline-recommended therapies except for UFH were associated with better survival outcomes in the non-adjusted analyses (Supplemental Table 3). After using the inverse probability weighting to adjust for age, hemoglobin, and platelet count, however, Cox proportional hazard analyses identified only the following as significant negative predictors of mortality: aspirin use (HR 0.345 (95% CI 0.237–0.502), p < 0.0001), beta-blocker use (HR 0.571 (95% CI 0.396–0.824), p = 0.003), statin use (HR 0.676 (95% CI 0.470–0.972), p = 0.035), and ACE-I/ARBs therapy (HR 0.565 (95% CI 0.391–0.817), p = 0.002) (Fig. 2). DAPT (HR 1.053 (95% CI 0.744–1.490), p = 0.77), UFH (HR 1.142 (95% CI 0.792–1.649), p = 0.48), coronary angiography (HR 0.757 (95% CI 0.522–1.098), p = 0.14), and PCI (HR 0.904 (95% CI 0.454–1.798), p = 0.77) were not associated with improved mortality and remained neutral in effect when combined with the listed medical therapies of proven mortality benefit.
Fig. 2.
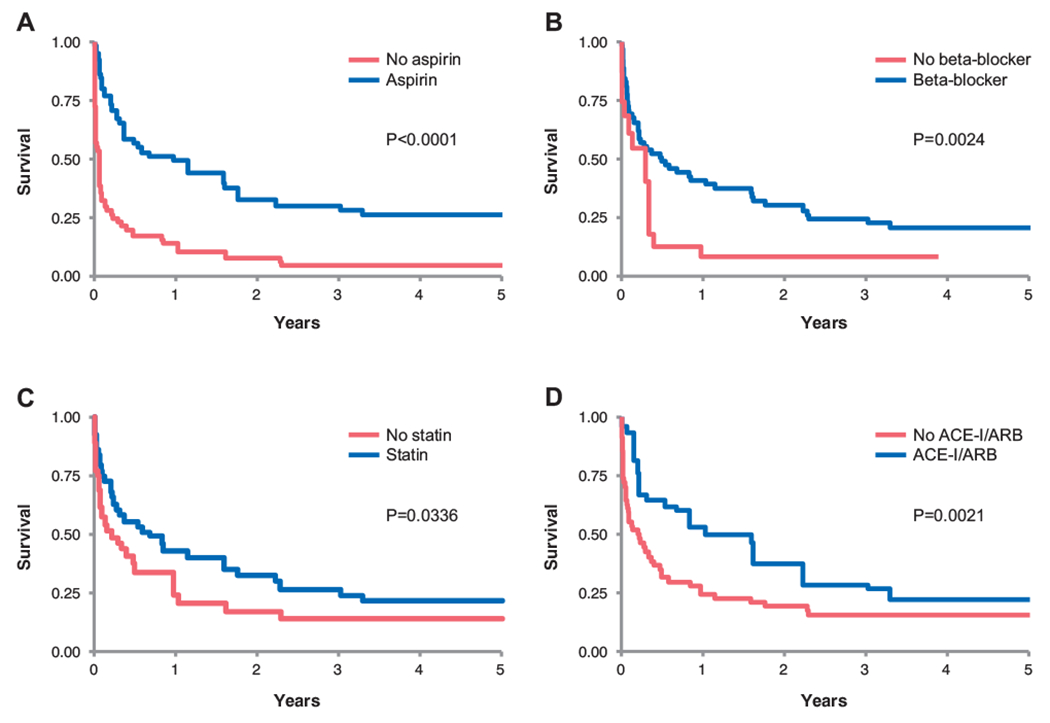
Kaplan-Meier survival curves for therapies identified by inverse probability weighing to be associated with a survival benefit: aspirin (A), beta-blocker (B), statin (C), and ACE inhibitor/ARB (D).
4. Discussion
This is the first study to evaluate the current management and referral practice of ACS in patients with active hematologic malignancies. This study demonstrated that [1] ACS is not common but commonly managed non-aggressively in patients with active hematologic malignancies due to their comorbidities, [2] general cardiology services were involved in >90% of cases, [3] the majority of patients did not receive American College of Cardiology/American Heart Association Class I recommendations of anti-platelet/anticoagulant and statin therapy and [4] deaths in this patients cohort were mainly non-cardiac in nature.
4.1. ACS in patients with active hematologic malignancy
In this selected cohort of hospitalized patients with active hematologic malignancies, ACS occurred in approximately 1.4% of the patients. This compares to a wide range of reported rates of in-hospital MI [15]. In a study by the Department of Veterans Affairs Health System an incidence of 11.2% was reported, of which 9.5% were STEMIs [16]. Other studies, however, indicated that the incidence may not be as high, at least not for STEMIs, occurring more at a rate of 0.02–0.03% [15,17]. Most of these were noted in patients presenting initially with respiratory symptoms and least commonly when the admitting diagnosis was cancer. In our cohort of hematological cancer patients, approximately 10% of the MIs were STEMIs. Patient numbers were too low to allow for any conclusive statement on differences in presentations of MI between hematological malignancies. While in general less common for inpatient MIs, about half of our patients presented with chest pain. Dyspnea, hypotension, and other symptoms of circulatory/heart failure, however, were still relatively frequent and need to alert practitioners to evaluate (hospitalized) hematology patients for MI. As testing is usually driven by symptoms, the current data are reflective largely of symptomatic MIs, although 1 in 10 patients in this series did not report symptoms. Importantly, half of the patients in this series had a history of atherosclerotic cardiovascular disease and the majority had at least one cardiovascular risk factor (mainly older age, male gender, and smoking history). Accordingly, this is a patient population at risk, yet in view of this risk, the incidence is not exceedingly high.
4.2. Management of ACS in hematologic malignancy
The management strategies of ACS in patients with active hematological malignancies are not well described. Prior studies reported significant under-treatment of patients with in-hospital MIs in terms of guideline-recommended therapies, esp. antiplatelet drugs, statins, and PCI. [18] The same was noted in our cohort, and despite the fact that >90% of the patients were evaluated by a cardiology team. In two cases the critical care team did not feel the need for specialty consultation and the other three cases were patients on hospice/near end-of-life care. These observations clearly outline that the reason for lack of use of guideline-recommended therapies is not due to the lack of access to expert care or the decision for comfort care. In fact, the great majority of patients wished to be full code, indicating a patient preference to maximum treatment including those relating to life-threatening cardiac conditions. Why care providers deviated from standard practice may be answered, at least in part, by some correlative observations.
First, presentation type seems to influence referral patterns for an invasive approach. Along these lines, patients with UA, although only a few in this cohort, were the most likely to be taken for coronary angiography (4 in 6 patients) unless felt to have a low clinical probability of acute plaque rupture, and indeed, all of those referred underwent PCI. Patients with STEMI were the second most likely group to be referred to the catheterization laboratory, but still only 40% underwent coronary angiography; PCI was performed in only 3 out of 4 cases. The stated reasons for medical management of the remaining STEMI patients were a) end-of-life care in three patients, of which two died in-hospital, one death being cardiac in nature, and b) low platelet counts (range from 6000 to 30,000/μL) with varying co-morbidities. In patients with NSTEMI, only 10 patients (17.5%) were taken to the cardiac catheterization laboratory, and only 3 patients (5.3%) had PCI. The reasons for non-invasive management of the majority of NSTEMI patients included a) presumed type 2 MI (in 59.6%) due to hypoxia, tachycardia, anemia with reassuring echocardiographic studies, b) low platelet counts (range 3000–51,000/μL), and c) terminal status, patient wishes or code status.
Otherwise, predictive factors for pursuit of an invasive strategy included higher hemoglobin level, higher platelet count, and lower white blood cell count. Low hemoglobin and/or platelet count also correlated with avoidance of antiplatelet therapy and/or anticoagulant therapy, and it is intuitive that patients would not be referred to the catheterization laboratory unless they are able to tolerate these medications. Aspirin was usually avoided unless platelet counts were >10,000/μL and its use remained restricted to 28% in those with platelet counts in the range of 10,000–50,000/μL. The same was true for the use of UFH. No patient with a platelet count in this range received a P2Y12 receptor inhibitor, and even of those with counts of 50,000–100,000/μL only 17% were given a P2Y12 receptor inhibitor. The utilization was especially low in those not referred to the catheterization laboratory. The same is true for statin medications, which were generally not started due to the acuity of care or clinical complexities. In addition, in setting of low blood pressure and/or acute kidney injury, ACE-I/ARBs were deferred. However, beta-blockers were usually administered without significant limitation in this cohort. These observations echo those in patients with in-hospital MI in general [18]. This is important as one may argue that cancer patients, historically and generally, have been excluded from national registries and randomized clinical trials, limiting the evidence base of care in these patients. As a consequence, management strategy of ACS in cancer patients with multiple co-morbidities are usually “individualized”, but the question is whether a deviation from guideline recommendations is “justified”, e.g. by evidence of acceptable outcomes.
4.3. Outcomes of ACS in patients with hematologic malignancies
Compared with outpatient MIs, patients who are suffering an MI while hospitalized for other reasons have a worse prognosis with in-hospital mortalities as high as 19% [18]. This has been related in part to the lack of the application of guideline recommended therapies including an invasive approach in those patients qualifying by presenting with a STEMI or high-risk score non-ST segment elevation-ACS. This being said, in patients with an in-hospital MI, those with a lower perceived mortality risk seemingly benefit the most from PCI [19]. This is intuitive, as with increasing burden and degree of comorbidities even the most optimal cardiac care will become less and less impactful. For obvious reasons this is very relevant for patients with active hematological malignancies with a high non-cardiac morality rate.
In this context, it is pertinent though that aspirin and beta-blocker use was associated with better overall survival as was statin and ACE-I/ARB therapy while DAPT, UFH, cardiac catheterization, and PCI were not. These findings are in general agreement with the results from a prior study from MD Anderson on a less selected cancer cohort that found aspirin and beta-blocker use to be independently associated with a 23% and 36% mortality risk reduction [9]. Importantly, neither this prior nor our current study could not constitute a survival benefit of an invasive approach. In distinction though, the current study was able to confirm other guideline-recommended therapies to be of benefit in the studied hematological malignancy population, including statins and ACE-I/ARB. In fact, the benefit of these medications emerged much sooner than that of beta-blocker. No intervention, however, made as fast and as profound of a difference as aspirin. This observation is consistent with a prior study that found the benefit of aspirin to be even more striking and without an increased risk of severe bleeding complications even in the presence of thrombocytopenia [20]. It is of interest that in our study 1 in 5 patients with platelet counts of 10,000–30,000/μL received aspirin, pointing out that providers are not opposed to treating patients with this degree of thrombocytopenia with aspirin. Indeed, the SCAI expert consensus document supports the use of aspirin for patients with ACS and platelet counts of at least 10,000/μL (Supplemental Fig. 1) [8]. However, still only 58% of eligible patients in the current study cohort received aspirin therapy, pointing out opportunities for education, practice change, and improvement of care in this area. The same might be true for the use of statins and ACE-I/ARBs, whereas providers seem to be generally in-tuned to the benefits of beta-blocker.
The benefit-risk ratio of P2Y12 receptor inhibitors in cancer patients is less well defined. Based on clinical experiences at larger cancer centers such as MD Anderson in Houston, TX, the SCAI expert consensus stated that DAPT and PCI are safe in patients with platelet counts of 30,000/μL or more [ 8]. In the current series only 27% of eligible patients received DAPT. No increase in bleeding events was observed, but, we also did not find a significantly better long-term outcome with DAPT either, thus remaining of uncertain benefit in hematological malignancy patients with ACS despite the class I recommendations in the general population [21,22].
The same holds true for an invasive approach. One of its key advantages is to define culprit lesions and to direct care. For instance, type 1 MIs with rupture or erosion and thrombus formation may benefit more from an antiplatelet and invasive approach. As shown here though, type I MIs accounted for only one third of ACS cases, possibly explaining why DAPT and an invasive approach did not yield a mortality benefit in the overall cohort. Also the prognosis is not necessarily better for patients with NSTEMI or type 2 MI [23,24]. In fact, some reports suggest that the opposite is true, but largely related to the burden of comorbidities [25,26]. In this particular comorbid cohort, the very high mortality rate of 64% at 1 year is to be considered. This being said, cardiac mortality accounts for 17.5% of defined causes of death and with optimal cardiovascular therapy there might still be opportunities for improving overall outcomes.
4.4. Limitations
The current study, although on 5300 patients, still represents a single center study with its inherent limitations. Practice patterns differ and the results of this study may thus not apply to other institutions. Furthermore, the current analysis was focused on specific hematological malignancies and is therefore not generalizable to all cancer patients, especially not to those with non-hematological malignancies. Studies in these patient groups are to be encouraged. An important aspect of these studies is to identify shortcomings in management and outcome that are of interest for the medical community at large and encourage refinement of practice patterns and improvement of outcomes. It was not the goal and therefore not the setup of this study to provide conclusive links between any given chemotherapeutic and its ACS risk, last but not least because most cancer therapeutics were administered as part of a combination chemotherapy regimen rather than in isolation. Of further note, less than half of the patients in the myeloid group had received chemotherapy, arguing for the fact that ACS risk in these patients may not be related to chemotherapy exposure but may rather be inherent to these patients and/or potentially modulated by their malignancy burden. Finally, as with all retrospective studies, limitations and biases associated with the nature of such studies have to be recognized. To address these concerns we did take an analytic approach to adjust for selection bias using inverse probability weighting methods.
5. Conclusion
ACS in patients with active hematologic malignancies is not common but commonly managed non-aggressively in terms of medical therapy and catheterization referral. The leading cause of death in-hospital and within 1-year of ACS remains non-cardiac in nature, but even so, 1 in 7 to 1 in 4 patients experience cardiac death, pointing out the significance of cardiovascular care in these patients.
Supplementary Material
Footnotes
Author disclosures/conflicts of interest: none.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2018.10.008.
References
- [1].Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA, Centers for Disease C, et al. , CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors–United States, 2005–2013, MMWR Suppl. 63 (4) (2014) 3–27. [PubMed] [Google Scholar]
- [2].Herrmann J, Lerman A, An update on cardio-oncology, Trends Cardiovasc. Med 24 (7) (2014) 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. , Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level, J. Am. Coll. Cardiol 65 (25) (2015) 2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M, Evaluation and management of patients with heart disease and cancer: cardio-oncology, Mayo Clin. Proc 89 (9) (2014) 1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Herrmann J, Tyrosine kinase inhibitors and vascular toxicity: impetus for a classification system? Curr. Oncol. Rep 18 (6) (2016) 33. [DOI] [PubMed] [Google Scholar]
- [6].Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. , Vascular toxicities of Cancer therapies: the old and the new–an evolving avenue, Circulation 133 (13) (2016) 1272–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang EH, Watson KE, Herrmann J, Should vascular effects of newer treatments be addressed more completely? Future Oncol. 11 (14) (2015) 1995–1998. [DOI] [PubMed] [Google Scholar]
- [8].Iliescu C, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. , SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista), Catheter. Cardiovasc. Interv 87 (5) (2016) 895–899. [DOI] [PubMed] [Google Scholar]
- [9].Yusuf SW, Daraban N, Abbasi N, Lei X, Durand JB, Daher IN, Treatment and outcomes of acute coronary syndrome in the cancer population, Clin. Cardiol 35 (7) (2012) 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. , Third universal definition of myocardial infarction, J. Am. Coll. Cardiol 60 (16) (2012) 1581–1598. [DOI] [PubMed] [Google Scholar]
- [11].Wass M, Hitz F, Schaffrath J, Muller-Tidow C, Muller LP, Value of different comorbidity indices for predicting outcome in patients with acute myeloid leukemia, PLoS One 11 (10) (2016), e0164587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE, The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma - a Swedish population-based study, BMC Cancer 15 (2015) 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, et al. , Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry, J. Am. Coll. Cardiol 55 (18) (2010) 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of P, Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians, Ann. Intern. Med 159 (11) (2013) 770–779. [DOI] [PubMed] [Google Scholar]
- [15].Levine GN, Dai X, Henry TD, Calfon Press M, Denktas AE, Garberich RF, et al. , In-hospital ST-segment elevation myocardial infarction: improving diagnosis, triage, and treatment, JAMA Cardiol. 3 (6) (2018. Jun 1) 527–531. [DOI] [PubMed] [Google Scholar]
- [16].Maynard C, Lowy E, Rumsfeld J, Sales AE, Sun H, Kopjar B, et al. , The prevalence and outcomes of in-hospital acute myocardial infarction in the Department of Veterans Affairs Health System, Arch. Intern. Med 166 (13) (2006) 1410–1416. [DOI] [PubMed] [Google Scholar]
- [17].Dai X, Bumgarner J, Spangler A, Meredith D, Smith SC, Stouffer GA, Acute ST-elevation myocardial infarction in patients hospitalized for noncardiac conditions, J. Am. Heart Assoc 2 (2) (2013), e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Erne P, Bertel O, Urban P, Pedrazzini G, Luscher TF, Radovanovic D, et al. , Inpatient versus outpatient onsets of acute myocardial infarction, Eur. J. Intern. Med 26 (6) (2015) 414–419. [DOI] [PubMed] [Google Scholar]
- [19].Kaul P, Federspiel JJ, Dai X, Stearns SC, Smith SC Jr., Yeung M, et al. , Association of inpatient vs outpatient onset of ST-elevation myocardial infarction with treatment and clinical outcomes, JAMA 312 (19) (2014) 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sarkiss MG, Yusuf SW, Warneke CL, Botz G, Lakkis N, Hirch-Ginsburg C, et al. , Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes, Cancer 109 (3) (2007) 621–627. [DOI] [PubMed] [Google Scholar]
- [21].Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. , AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Circulation 130 (25) (2014) e344–e426 (2014). [DOI] [PubMed] [Google Scholar]
- [22].O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, et al. , ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, Circulation 127 (4) (2013) e362–e425 (2013). [DOI] [PubMed] [Google Scholar]
- [23].Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, et al. , Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI Program (French registry of acute ST-elevation or non-ST-elevation myocardial infarction) 1995 to 2015, Circulation 136 (20) (2017) 1908–1919. [DOI] [PubMed] [Google Scholar]
- [24].Chapman AR, Shah ASV, Lee KK, Anand A, Francis O, Adamson P, et al. , Long term outcomes in patients with type 2 myocardial infarction and myocardial injury, Circulation 137 (12) (2018. Mar 20) 1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baron T, Hambraeus K,Sundstrom J, Erlinge D, Jernberg T, Lindahl B, et al. , Type 2 myocardial infarction in clinical practice, Heart 101 (2) (2015) 101–106. [DOI] [PubMed] [Google Scholar]
- [26].Vora AN, Wang TY, Hellkamp AS, Thomas L, Henry TD, Goyal A, et al. , Differences in short- and long-term outcomes among older patients with ST-elevation versus non-ST-elevation myocardial infarction with angiographically proven coronary artery disease, Circ. Cardiovasc. Qual. Outcomes 9 (5) (2016) 513–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


