Abstract
Purpose:
This phase Ib/2 trial investigated pembrolizumab-containing trimodality therapy in patients with gastroesophageal junction (GEJ) adenocarcinoma.
Patients and Methods:
Patients with GEJ adenocarcinoma (cT1–3NanyM0) received neoadjuvant pembrolizumab-containing chemoradiation (CROSS regimen) followed by surgical resection and adjuvant pembrolizumab. The primary endpoints were tolerability in the first 16 patients and pathologic complete response [pCR (ypT0N0)]. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). An independent propensity-score-matched cohort (treated with CROSS without immunotherapy) was used for comparison. Exploratory analyses included immune biomarkers in the tumor microenvironment (TME) and plasma.
Results:
We enrolled 31 eligible patients, of whom 29 received all expected doses of neoadjuvant pembrolizumab and 28 underwent R0 resection. Safety endpoints were met. The primary efficacy endpoint was not met [7/31 (22.6%) achieved pCR]. Patients with high [i.e., combined positive score (CPS) ≥ 10] baseline expression of programmed death (PD)-L1 in the TME had a significantly higher pCR rate than those with low expression [50.0% (4/8) vs. 13.6% (3/22); P = 0.046]. Patients with high PD-L1 expression also experienced longer PFS and OS than propensity-score-matched patients. Among trial patients with PD-L1 CPS < 10, unprespecified analysis explored whether extracellular vesicles (EV) could identify further responders: an elevated plasma level of PD-L1–expressing EVs was significantly associated with higher pCR.
Conclusions:
Adding pembrolizumab to trimodality therapy showed acceptable tolerability but did not meet the pre-specified pCR endpoint. Exploratory analyses suggested that high PD-L1 expression in the TME and/or on EVs may identify patients most likely to achieve tumor response.
Introduction
Immune checkpoint inhibitors targeting programmed death (PD)-1 (anti–PD-1) in combination with chemotherapy have become standard first-line therapy for the treatment of metastatic esophageal, gastroesophageal junction (GEJ), and gastric adenocarcinoma (1, 2). In patients with nonmetastatic esophageal/GEJ carcinoma who had suboptimal response to chemoradiation, adjuvant nivolumab (vs. placebo) was found to significantly increase disease-free survival (DFS; ref. 3). Outcomes from the addition of immune checkpoint inhibitors to neoadjuvant chemoradiation in this disease have not been extensively examined. Almost half the patients with GEJ adenocarcinoma present with locally advanced disease that is amenable to surgical resection. A current standard of care for these patients is based on the CROSS trial and consists of weekly carboplatin and paclitaxel with concurrent radiotherapy followed by surgery (4). However, the 5-year overall survival (OS) rate among adenocarcinomas is only 47% with most relapses occurring in distant anatomic sites (4), underscoring the need for novel systemic therapies.
There is compelling preclinical evidence to support combining radiotherapy with immune checkpoint inhibitors (5, 6) as radiotherapy not only improves local tumor control but also promotes systemic immune response by stimulating cytokine production (7) and release of neoantigens (8). Furthermore, radiotherapy was shown to upregulate tumor-cell PD-L1 expression and the addition of anti–PD-1/-L1 to radiotherapy delayed tumor growth and improved survival in animal models (9-11). The improvement in DFS in CheckMate-577 from the addition of nivolumab after trimodality therapy suggests that anti–PD-1 may cooperate with radiotherapy that is sequentially administered prior to PD-1 blockade to eradicate micrometastases (3).
PD-L1 protein expression in the tumor microenvironment (TME) has been implicated as a predictive biomarker for the efficacy of anti–PD-1 therapy in this disease (1, 2, 12). PD-L1 is scored immunohistochemically according to its expression in both immune and tumor cells [combined positive score (CPS)] or in tumor cells alone [tumor proportion score (TPS)]. PD-L1 expression in immune versus tumor cells may be driven by different mechanisms (13-16). In addition to cell-bound PD-L1, circulating PD-L1 in extracellular vesicles (EV) comprising exosomes and microvesicles can suppress T-cell function and has been associated with poor prognosis of patients with cancer (17-20).
We performed a phase Ib/2 study (MC1541) that investigated the clinical activity of pembrolizumab (anti–PD-1) in combination with trimodality therapy in patients with resectable GEJ adenocarcinoma. We explored the roles of PD-L1 expression in the TME by CPS and TPS and in circulating EVs to predict treatment response.
Patients and Methods
Study design and participants
MC1541 is an open-label, single-arm phase Ib/2 study (NCT02730546) investigating the safety and efficacy of pembrolizumab in combination with neoadjuvant chemoradiation and adjuvant pembrolizumab monotherapy after surgical resection of primary cancer at two Mayo Clinic sites (Minnesota, Arizona). The phase Ib portion of the study determined the safety and tolerability of pembrolizumab in combination with neoadjuvant chemoradiation. A minimum of 15 evaluable patients were required for analysis of dose-limiting toxicities (DLT) before activation of the phase II portion that evaluated the pathologic complete response (pCR) rate of pembrolizumab in combination with neoadjuvant chemoradiation.
Inclusion criteria were: age 18-year–old and above; both sexes; histologically or cytologically confirmed adenocarcinoma of the GEJ (cT1–3NanyM0) amenable to surgical resection as determined by endoscopic ultrasound (EUS) ≤35 days prior to registration; Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and adequate nutritional status and organ function. Major exclusion criteria were: cT1N0 or T4Nany tumors, tumors that extend ≥5 cm into the stomach, disease involvement of supraclavicular lymph nodes, prior treatment for this malignancy, and prior radiation therapy to chest or abdomen. All patients provided written, informed consent for study participation. The study protocol and amendments were approved by the institutional review board and conducted in accordance with the Declaration of Helsinki.
Study procedures and treatment
Patients were staged by EUS and PET-CT, CT, or MRI before start of treatment. Neoadjuvant chemoradiation consisted of weekly carboplatin (AUC 2 mg/mL/minute) and paclitaxel (50 mg/m2) on day 1, 8, 15, 22, and 29 administered with concurrent daily radiotherapy (41.4 Gy in 23 fractions) according to the CROSS regimen. At the beginning of the phase Ib portion, neoadjuvant pembrolizumab was administered on day 1, 22, and 43. After enrolling the first 8 subjects, the protocol was amended to have pembrolizumab initiated 1 week before the initiation of chemoradiation and the third dose omitted (day 7 and 15) to avoid potential delays in surgery resulting from toxicities unrelated to pembrolizumab while also maintaining the protocol-specified duration of at least 24 days between the date of last pembrolizumab administration and surgery. In the subsequent phase II portion, pembrolizumab was initiated 2 weeks before the initiation of chemoradiation (day 14 and 8) to explore dynamic changes in future research in circulating cells (see Supplementary Methods). Since outcomes did not significantly differ between these schedules, data from the phase Ib and phase II portions were pooled (Supplementary Methods). Pembrolizumab-containing chemoradiation was followed by repeat PET-CT and, if no disease progression was observed, surgical resection was performed. After surgery, adjuvant pembrolizumab 200 mg was administered once every 3 weeks for a total of six doses. CT scans (chest, abdomen, pelvis) were obtained postsurgery, then every 3 months for 1 year, and then every 4 months for 2 more years.
Endpoints
The primary objective of the phase Ib portion was to assess safety with dose limiting toxicities (DLT) defined as the following:
-
Type 1 DLT (neoadjuvant):
Grade 4 nonhematologic adverse event (AE) or a delay in initiating neoadjuvant therapy by more than 14 days; or a delay in surgery such that surgery occurs more than 12 weeks after the last dose of radiotherapy–all at least possibly due to pembrolizumab–or any grade 5 AE.
-
Type 2 DLT (postoperative):
Grade 5 AE within 30 days after surgery; or grade 4 nonhematologic AE within 30 days after surgery at least possibly related to pembrolizumab, excluding AEs that resolve to grade 2 or less within 7 days.
-
Type 3 DLT (adjuvant):
Grade 4 nonhematologic AE at least possibly related to pembrolizumab; or any grade 5 AE during adjuvant pembrolizumab.
DLTs will be analyzed, with consideration to proceed to the phase II portion if all of the following criteria are met out of 15 evaluable patients:
≤4 patients with type 1 DLT
≤2 patients with grade 5 type 2 DLT
≤6 patients with grade 4 nonhematologic type 2 DLT
≤3 patients with grade 4 nonhematologic type 3 DLT
≤1 patient with grade 5 type 3 DLT
Dose delays or interruptions of chemoradiation at least possibly related to toxicity of the treatment regimen would be considered in the safety evaluation. Neoadjuvant AEs were defined as the start of neoadjuvant therapy until 90 days after the last dose of neoadjuvant therapy or until surgery, whichever occurred first. Postoperative AEs were defined within 30 days after surgery. Adjuvant AEs were defined as the start of adjuvant therapy until 90 days after the last dose of adjuvant therapy.
The primary objective of the phase II portion was to evaluate the pCR rate of pembrolizumab in combination with neoadjuvant chemoradiation defined as number of patients with pCR divided by total evaluable patients, with data pooled from the phase Ib and II portions. pCR was defined as the absence of viable tumor cells at the primary tumor site and all resected lymph nodes in the surgical specimens (ypT0N0). Secondary endpoints included completion rate of chemoradiation, delay or withdrawal in surgery, postoperative complications, R0 resection rate, progression-free survival (PFS), and OS. Exploratory endpoints were based on IHC and serum studies to identify tissue and circulating biomarkers that are associated pCR, PFS, and OS. Toxicity was assessed in all evaluable patients who started neoadjuvant therapy per protocol according to the Common Terminology Criteria for Adverse Events v4.03. Adverse events of special interest were not predefined, but all AEs were prospectively collected with grade and attribution. PFS was defined as the time from the date of study registration to the date of death due to all causes, recurrence if R0 resection was achieved, R1/R2 resection at surgery, or disease progression before undergoing surgery, which ever occurred first among evaluable patients. OS was defined as the time from the date of study registration to the date of death due to all causes among evaluable patients.
Propensity-score matching
To compare the outcomes of this study with conventional neoadjuvant chemoradiation, a cohort of 93 patients with newly diagnosed resectable adenocarcinoma of the esophagus or GEJ treated with standard neoadjuvant weekly carboplatin and paclitaxel with concurrent daily radiation (41.4–50.4 Gy), with planned surgical resection, at Mayo Clinic was selected for propensity score matching. A logistic regression model was used to match on baseline variables (age, cT stage, cN stage, and signet-ring cell histology); a 1:2 to 1:4 ratio matching ratio was used with a caliper of 0.15.
Exploratory studies
Baseline and surgical specimens were stored in formalin-fixed, paraffin-embedded blocks. IHC staining was performed for PD-L1 CPS and TPS with the 22C3 antibody (Dako). Using standard criteria, CPS was defined as the percent of PD-L1–positive tumor and immune cells divided by the number of tumor cells multiplied by 100, and TPS as the percent of PD-L1–positive tumor cells divided by the number of tumor cells. PD-L1–expressing EVs were measured by nanoscale flow cytometry, as we previously described (21). Briefly, plasma samples were centrifuged at 2,000 x g and incubated with H1A antibody (Dong Lab) conjugated with Alexa-647 at room temperature. EVs were measured with the A60 Micro-Plus Nanoscale Flow Cytometer (Apogee Flow Systems) and data was analyzed with FlowJo v10.6.1.
Statistical analysis
The pCR rate was defined as the number of patients with pCR divided by total evaluable patients. All eligible patients who signed consent and began protocol treatment were considered evaluable. With at least 15 patients in the phase Ib portion, 15 patients in the phase II portion would provide 81% power to detect an improvement in the pCR rate of 27% compared with historic control of 21% (i.e., achieving pCR rate of at least 48% with the denominator being eligible patients who did not withdraw consent; ref. 4). A total of 30 evaluable patients from phase Ib and II portions provide 83% power to claim the proposed regimen warrants further study at one-sided significance level of 0.08, if at least 10 patients achieve pCR (Supplementary Methods). Analysis of tissue-based PD-L1 expression and circulating biomarkers was preplanned, but the analytic approach for tissue-based PD-L1 was not predefined, and analysis of extracellular vesicles was not prespecified. As biomarker analyses were exploratory, power calculations were not indicated. Descriptive statistics including median, range, frequency, and statistical graphs were used to summarize patient characteristics, tumor response, and biomarker data. The Kaplan–Meier method and Cox proportional hazards models including HR and 95% confidence intervals (CI) were used to assess PFS and OS along with the log–rank test for statistical significance. Data cut-off for PFS and OS was Nov 12, 2021. Wilcoxon rank–sum tests were used to compare continuous data between groups of interest. Associations of categorical variables were done via χ2 or Fisher exact tests. Two-sided tests were performed and a P < 0.05 was considered statistically significant. JMP v14.1 and SAS v9.4 were used for statistical analysis.
Data availability statement
The data generated in this study are available within the article and its supplementary data files.
Results
Patients
From July 2016 to February 2021, we enrolled 31 eligible patients at two Mayo Clinic sites (Supplementary Fig. S1). 16 patients were enrolled in the phase Ib portion. All safety endpoints in the phase Ib portion were met (one DLT during neoadjuvant therapy, one DLT postoperatively, and no DLTs during adjuvant therapy). Accordingly, the study proceeded to the phase II portion during which an additional 15 patients were enrolled. Baseline characteristics are shown in Table 1. Most patients had adenocarcinoma arising from the GEJ (93.5%); were male (96.8%), 29 had cT3 (93.5%), 27 were cN-positive (87.1%), and 6 had signet ring cell histology (19.4%). DNA mismatch repair (MMR)/microsatellite instability status was available in 67.7% (21/31) patients, and all had intact MMR or microsatellite-stable tumors.
Table 1.
Patient characteristics.
| Baseline variable | Total (n = 31) |
|---|---|
| Age, years | |
| Median | 62 |
| Range | 44.0-76.0 |
| Gender, n (%) | |
| Female | 1 (3.2%) |
| Male | 30 (96.8%) |
| Race, n (%) | |
| White | 31 (100.0%) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 31 (100.0%) |
| Tumor location, n (%) | |
| Gastroesophageal junction | 29 (93.5%) |
| Gastric cardia | 2 (6.5%) |
| Histologic grade (differentiation), n (%) | |
| G2 (moderately differentiated) | 18 (58.1%) |
| G3 (poorly differentiated) | 12 (38.7%) |
| GX (grade cannot be assessed) | 1 (3.2%) |
| Clinical tumor stage, n (%) | |
| T2 | 2 (6.5%) |
| T3 | 29 (93.5%) |
| Clinical nodal stage, n (%) | |
| N0 | 4 (12.9%) |
| N1 | 13 (41.9%) |
| N2 | 12 (38.7%) |
| N3 | 2 (6.5%) |
| Signet ring cell histology, n (%) | |
| Yes | 6 (19.4%) |
| No | 25 (80.6%) |
| PD-L1 CPS score, n (%) | |
| <1 | 11 (35.5%) |
| 1–9 | 11 (35.5%) |
| ≥10 | 8 (25.8%) |
| Unknown | 1 (3.2%) |
Completion of treatment
Of 31 patients, 29 (93.5%) received all expected doses of neoadjuvant pembrolizumab. 2 patients in the phase Ib portion missed the third dose of pembrolizumab after completing chemoradiation: 1 due to immune-related myocarditis who did not undergo surgery; 1 due to neutropenia and pulmonary embolism who continued protocol treatment and underwent planned surgical resection without delay. All patients in the phase II portion received the assigned doses of pembrolizumab. 26 of 31 (83.9%) patients received all doses of chemotherapy. 5 patients missed a single dose of carboplatin/paclitaxel due to neutropenia (n = 4) and severe allergic reaction (n = 1). All patients completed the full course of radiotherapy. 21 of 29 (72.4%) patients who underwent surgical resection started adjuvant pembrolizumab. Reasons for not starting adjuvant treatment include preoperative disease progression (n = 1), insufficient recovery from surgery (n = 3), postoperative disease progression (n = 2), and patient preference (n = 2). Among patients who started adjuvant pembrolizumab, median doses administered was 6 (range 1–6); reasons for missing a dose include disease progression (n = 6) and toxicity (n = 1, grade 2 palpitations).
Primary safety endpoint
Overall toxicity
During neoadjuvant therapy, 17 of 31 patients (54.8%) experienced grade 3 to 4 AEs regardless of attribution (Table 2) mostly commonly leukopenia (19.4%), neutropenia (16.1%), and hypertension (12.9%). No grade 5 AE was observed. During adjuvant therapy, 10 of 21 patients (47.6%) who started treatment had grade 3 to 5 AEs regardless of attribution (Table 2) mostly commonly hypertension (14.3%), pain (14.3%), and elevated aspartate aminotransferase (AST) or alanine aminotransaminase (ALT; 14.3%). One patient had grade 5 disseminated intravascular coagulation in the setting of disease progression.
Table 2.
Grade 3 to 5 AEs regardless of attribution.
| Neoadjuvant (n = 31) | Postoperative (n = 29) | Adjuvant (n = 21)d | ||||||
|---|---|---|---|---|---|---|---|---|
| AE | Grade 3 | Grade 4 | AE | Grade 3 | Grade 4 | AE | Grade 3 | Grade 4 |
| Leukopenia | 6 (19.4%) | 0 | Infectiousa | 3 (10.3%) | 3 (10.3%) | Hypertension | 3 (14.3%) | 0 |
| Neutropenia | 5 (16.1%) | 0 | Cardiacb | 5 (17.2%) | 0 | Pain | 3 (14.3%) | 0 |
| Hypertension | 4 (12.9%) | 0 | Pulmonaryc | 3 (10.3%) | 0 | AST/ALT increased | 3 (14.3%) | 0 |
| Thrombocytopenia | 1 (3.2%) | 1 (3.2%) | Ileus | 3 (10.3%) | 0 | Alkaline phosphatase increased | 2 (9.5%) | 0 |
| Hypotension | 1 (3.2%) | 1 (3.2%) | Anastomotic leak | 3 (10.3%) | 0 | Dysphagia | 2 (9.5%) | 0 |
| Febrile neutropenia | 1 (3.2%) | 0 | Hypertension | 2 (6.9%) | 0 | Infections | 2 (9.5%) | 0 |
| Hemolysis | 1 (3.2%) | 0 | Hemorrhage | 2 (6.9%) | 0 | Weight loss | 2 (9.5%) | 0 |
| Dysphagia | 1 (3.2%) | 0 | Anemia | 2 (6.9%) | 0 | Anemia | 1 (4.8%) | 0 |
| Allergic reaction | 0 | 1 (3.2%) | Thrombocytopenia | 1 (3.4%) | 0 | Aspiration | 1 (4.8%) | 0 |
| Syncope | 1 (3.2%) | 0 | Leukocytosis | 1 (3.4%) | 0 | Nausea | 1 (4.8%) | 0 |
| Pulmonary embolism | 1 (3.2%) | 0 | Pulmonary embolism | 1 (3.4%) | 0 | Cholecystitis | 1 (4.8%) | 0 |
| Sinus tachycardia | 1 (3.2%) | 0 | Gastric conduit leak | 1 (3.4%) | 1 (3.4%) | Ascites | 1 (4.8%) | 0 |
| Oral mucositis | 1 (3.2%) | 0 | Gastric fistula | 0 | 1 (3.4%) | Enterocolitis | 1 (4.8%) | 0 |
| Sepsis | 0 | 1 (3.2%) | Duodenal stump leak | 0 | 1 (3.4%) | Hyperglycemia | 1 (4.8%) | 0 |
| Dehydration | 1 (3.2%) | 0 | Diarrhea | 1 (3.4%) | 0 | |||
| Hypophysitis | 1 (3.2%) | 0 | Gastric obstruction | 1 (3.4%) | 0 | |||
| Fatigue | 1 (3.2%) | 0 | Small bowel dilatation | 1 (3.4%) | 0 | |||
| Diarrhea | 1 (3.2%) | 0 | Creatinine increased | 1 (3.4%) | 0 | |||
| Myocarditis | 0 | 1 (3.2%) | Hypernatremia | 0 | 1 (3.4%) | |||
| Anorexia | 1 (3.4%) | 0 | ||||||
Infectious complications include sepsis, bacteremia, pelvic infection, and empyema.
Cardiac complications include atrial fibrillation, sinus tachycardia, and pericardial effusion.
Pulmonary complications include aspiration, hypoxia, and pleural effusion.
1 patient had grade 5 disseminated intravascular coagulation in the setting of disease progression.
Surgery
Of 31 patients, 29 (93.5%) proceeded to surgery. 2 patients did not undergo surgery for immune-related grade 4 myocarditis (n = 1) and disease progression (n = 1). 1 patient experienced a 1 week delay in surgery due to grade 2 pericarditis considered related to radiotherapy. As shown in Table 2, the most common grade 3 to 4 surgical complications were infectious (20.7%) and cardiac (17.2%). Anastomotic leakage occurred in 10.3% (3/29) of patients. No deaths occurred within 30 days after surgery.
Immune-related toxicity
Grade 2 or higher immune-related AEs (irAE) occurred in 5 patients during neoadjuvant therapy and 5 during adjuvant therapy (Table 3). During neoadjuvant therapy, 1 patient (described above) had grade 4 myocarditis, grade 3 hypophysitis, and grade 2 cranial nerve palsy; 2 patients had grade 2 rash; 1 patient had grade 3 diarrhea; and 1 had grade 3 hemolysis. During adjuvant therapy, 1 patient had grade 2 rash; 1 had grade 2 chest palpitations; 1 had grade 2 increased ALT; 1 had grade 3 increased AST; and 1 had grade 3 increased ALT and AST and grade 3 cholecystitis. All patients recovered without severe sequelae.
Table 3.
irAEs.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Neoadjuvant (n = 31) | ||||
| Diarrhea/colitis | 6 (19.4%) | 0 | 1 (3.2%) | 0 |
| Rash | 3 (9.7%) | 2 (6.5%) | 0 | 0 |
| AST/ALT increased | 1 (3.2%) | 0 | 0 | 0 |
| Endocrinopathya | 1 (3.2%) | 0 | 1 (3.2%) | 0 |
| Hemolysis | 0 | 0 | 1 (3.2%) | 0 |
| Myocarditisb | 0 | 0 | 0 | 1 (3.2%) |
| CNS palsy | 0 | 1 (3.2%) | 0 | 0 |
| Adjuvant (n = 21) | ||||
| Diarrhea/colitis | 10 (47.6%) | 1 (4.8%) | 0 | 0 |
| Rash | 3 (14.3%) | 1 (4.8%) | 0 | 0 |
| AST/ALT increased | 1 (4.8%) | 1 (4.8%) | 3 (14.3%) | 0 |
| Endocrinopathyc | 1 (4.8%) | 0 | 0 | 0 |
| Palpitations | 0 | 1 (4.8%) | 0 | 0 |
| Cholecystitis | 0 | 0 | 1 (4.8%) | 0 |
Hypophysitis.
The patient who developed grade 4 myocarditis presented with a complete heart block and sustained ventricular tachycardia after receiving two doses of neoadjuvant pembrolizumab. His symptoms persisted despite high-dose steroid, amiodarone and flecainide drip treatment, and dual chamber pacer, which finally and immediately resolved after plasma exchange. His left ventricular ejection fraction was maintained at about 50% throughout.
Hyperglycemia.
Notably, the patient who developed grade 4 myocarditis (after two doses of neoadjuvant pembrolizumab) discontinued protocol treatment and did not undergo surgical resection. PET-CT and esophagogastroduodenoscopy (EGD) with biopsy after completing chemoradiation were negative for cancer. Local recurrence was identified 5 months after study enrollment, and systemic anticancer treatment without immunotherapy was resumed. He remains alive 5 years later without radiographic evidence of disease.
Primary efficacy endpoint
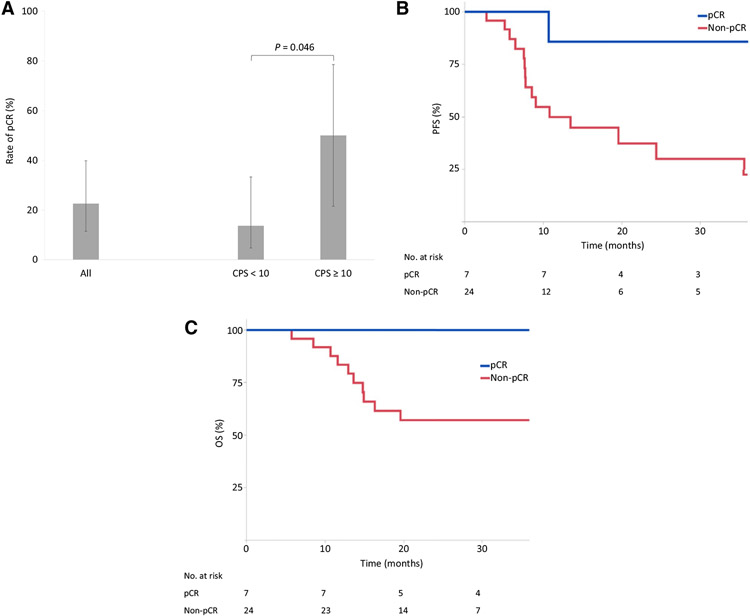
The R0 resection rate was 90.3% (28/31). A pCR (ypT0N0) was observed in 22.6% of patients (7/31; 95% CI, 11.4–39.8; Fig. 1A). pCR was significantly associated with longer PFS (P = 0.031; Fig. 1B) and OS (P = 0.063; Fig. 1C). In total, 93 patients from the propensity-score-matched cohort (Supplementary Table S1) who received standard carboplatin/paclitaxel-based chemoradiation were compared with 31 patients in this study. No statistically significant difference in the pCR rate was observed between the cohorts [22.6% (7/31) vs. 12.9% (12/93), P = 0.21]. Median follow-up for PFS was 24.7 months in MC1541 and 22.5 months in the propensity-score-matched cohort (24.2 vs. 22.0, respectively, for OS). Median PFS was 19.6 months in MC1541 versus 14.6 months in the propensity-score-matched cohort (log–rank P = 0.409; 2-year rates 48.5% vs. 32.2%; HR = 0.79; 95% CI, 0.40–1.57; Supplementary Fig. S2A). Median OS was not reached for either group (log–rank P = 0.950; 2-year rates 66.3% vs. 64.5%; HR = 0.98; 95% CI, 0.39–2.44; Supplementary Fig. S2B).
Figure 1.
Rate of pCR in MC1541 overall and according to baseline expression of PD-L1 in the TME (CPS ≥ 10 vs. <10; A). PFS (B) and OS (C) by pCR status for patients in MC1541.
Exploratory analyses
PD-L1 expression in the TME
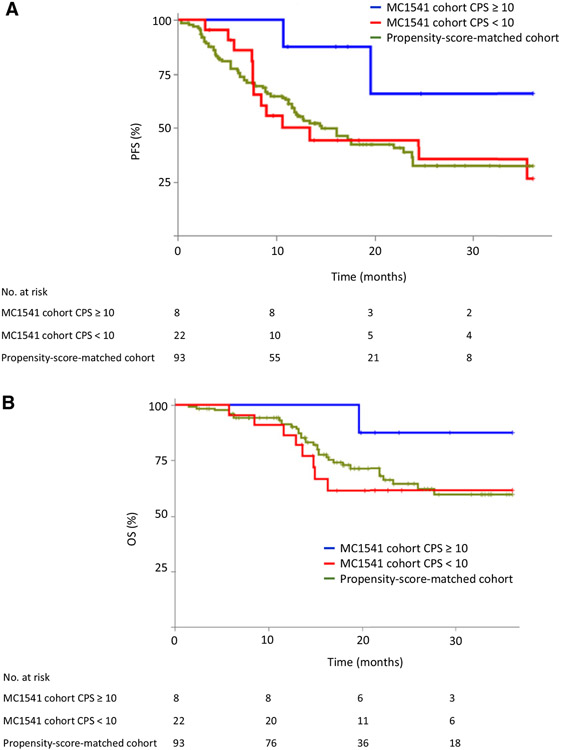
Data on baseline PD-L1 expression in the TME was available in 96.8% (30/31) of patients. Patients whose TME showed positive PD-L1 expression in immune and tumor cells had a significantly higher pCR rate [50.0% (4/8; 95% CI, 21.5–78.5) in CPS ≥ 10 vs. 13.6% (3/22; 95% CI, 4.7–33.3) in CPS < 10; P = 0.046; Fig. 1A). CPS ≥ 10 (vs. <10) was numerically associated with longer PFS (log–rank P = 0.061; 2-year rates 65.6% vs. 44.3%; HR = 0.26; 95% CI, 0.06–1.18; Fig. 2A) and OS (log–rank P = 0.162; 2-year rates 87.5% vs. 61.6%; HR = 0.25; 95% CI, 0.03–2.03; Fig. 2B). Patients with CPS ≥ 10 also had numerically longer PFS (log–rank P = 0.16; 2-year rates 65.6% vs. 32.2%; HR = 0.28; 95% CI, 0.06–1.22; Fig. 2A) and OS (log–rank P = 0.36; 2-year rates 87.5% vs. 64.5%; HR = 0.29; 95% CI, 0.04–2.32; Fig. 2B) than the propensity-score-matched cohort.
Figure 2.
PFS (A) and OS (B) in MC1541 patients according to baseline expression of PD-L1 in the TME (CPS ≥ 10 vs. <10) and in a PD-L1-unselected propensity-score-matched cohort.
PD-L1 expression in tumor cells (i.e., TPS ≥ 1%) was observed in 16.7% (5/30) of patients, which was numerically associated with a higher pCR rate [40.0% (2/5) in TPS ≥ 1% vs. 20.0% (5/25) in TPS < 1%; P = 0.358). TPS ≥ 1% (vs. <1%) was also numerically associated with a longer PFS (log–rank P = 0.154; 2-year rates 75.0% vs. 44.5%; HR = 0.25; 95% CI, 0.03–1.94; Supplementary Fig. S3A) and OS (log–rank P = 0.126; 2-year rates 100.0% vs. 61.8%; HR = inestimable; P = 0.995; Supplementary Fig. S3B). In addition, we observed that 24.0% (6/25) of patients with TPS < 1% contained some PD-L1–expressing tumor cells, rendering their TPS >0 but <1% (Supplementary Fig. S4A). Stepwise increases in PD-L1 expression in tumor cells (TPS = 0 vs. >0 to <1% vs. ≥1%) were significantly associated with a higher level of PD-L1 expression in immune cells in the TME (Supplementary Fig. S4A). TPS > 0 (vs. 0) was statistically significantly associated with longer PFS (log–rank P = 0.023; 2-year rates 78.8% vs. 33.0%; HR = 0.25; 95% CI, 0.07–0.91; Supplementary Fig. S4B) and was numerically associated with longer OS (log–rank P = 0.052; 2-year rates 90.9% vs. 54.0%; HR = 0.16; 95% CI, 0.02–1.31; Supplementary Fig. S4C).
Baseline PD-L1 expression in the TME was available in 19 patients in the propensity-score-matched cohort who did not receive anti–PD-1 containing chemoradiation and was not significantly associated with pCR, PFS, or OS (Supplementary Table S2; Supplementary Fig. S5).
PD-L1–expressing extracellular vesicles
We explored PD-L1–expressing EV levels measured in patient plasma. Baseline EV data were available in 100% (31/31) of patients and showed significant heterogeneity between patients [median 2.4, mean 44.2 (range 0.8–654.9) EV/nL; Fig. 3A]. Circulating PD-L1–expressing EV levels did not differ significantly in patients whose TME exhibited high (vs. low) PD-L1 expression [median 1.8 (interquartile range 1.3–3.8) EV/nL in CPS ≥ 10 vs. 2.5 (interquartile range 1.9–8.0) EV/nL in CPS < 10, respectively; Supplementary Fig. S6], and was not significantly associated with pCR, PFS, or OS.
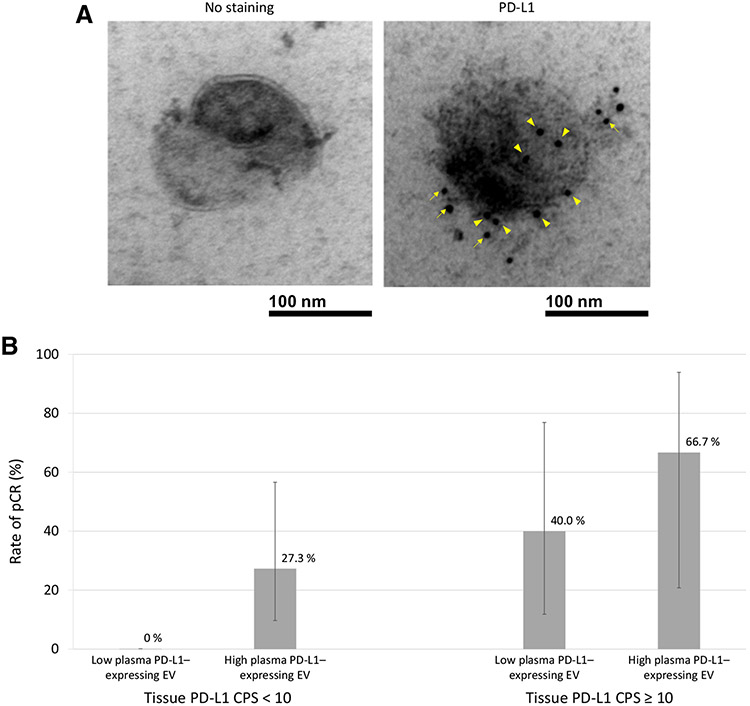
Figure 3.
A, Transmission electron microscopy images of a single EV isolated at baseline from a patient treated on MC1541 with PD-L1 CPS = 2 and TPS = 0 in the TME. Right, A single EV expresses multiple PD-L1 molecules shown by immunogold as intense black dots on the membrane surface (arrowheads) and in surrounding peri-membrane glycocalyx matrix (arrows). Left, A single EV without staining for PD-L1. B, pCR rate after chemoradiation in MC1541 patients according to baseline PD-L1 expression (CPS < 10 and ≥10) and baseline level of PD-L1-expressing EVs.
Since high PD-L1 CPS expression in the TME already identified responders to pembrolizumab-containing chemoradiation, we explored whether PD-L1–expressing EVs among patients with low PD-L1 CPS in the TME could identify further responders. Among patients whose TME demonstrated low PD-L1 expression (CPS < 10), those with a high (≥median 2.4 EV/nL) versus low (<median) level of PD-L1–expressing EVs had a significantly higher pCR rate [27.3% (3/11) vs. 0 (0/11), P = 0.031; Fig. 3B]. To rule out potential confounding by tissue expression of PD-L1 in tumor cells, we then restricted analysis to patients whose TME had a complete absence of PD-L1 in tumor cells (i.e., TPS 0) and found that the association remained significant. Among patients with both CPS < 10 and TPS = 0, high versus low levels of PD-L1–expressing EVs were associated with pCR rates of 33.3% (3/9) vsversus 0 (0/8), respectively (P = 0.036). In the small subgroup of patients whose TME had CPS ≥ 10, a high (vs. low) level of PD-L1–expressing EVs was associated with a numerically higher pCR rate [66.7% (2/3) vs. 40.0% (2/5); Fig. 3B].
Discussion
We examined the combination of PD-1 blockade (pembrolizumab) with concurrent neoadjuvant carboplatin/paclitaxel-based chemoradiation followed by surgery in patients with locally advanced adenocarcinoma of GEJ. This treatment met the primary safety endpoint. Neoadjuvant treatment-related toxicity and postoperative toxicity reported in this study were comparable with the CROSS study, except for additional irAEs associated with anti–PD-1 therapy. 5 patients (16%) experienced irAEs with neoadjuvant pembrolizumab, of whom 4 underwent surgery without delay. The rates of irAEs with neoadjuvant pembrolizumab in our study appear similar to prior trials examining anti–PD-1/-L1–containing chemoradiation in esophageal/GEJ adenocarcinoma (22, 23). Notably, there were no instances of pneumonitis.
In the overall MC1541 cohort of PD-L1–unselected patients, the combination of pembrolizumab with the CROSS chemoradiation regimen did not meet the primary efficacy endpoint of pCR (current 23% vs. historic 21%). This may have been due to a high prevalence of adverse tumor characteristics in our population, such as signet-ring cell histology (19%) and clinical node positivity (87%) at baseline, both of which have been associated with adverse prognosis in this disease (24, 25). However, our pCR rates in patients who started neoadjuvant chemoradiation are similar to those reported in other recent studies including PERFECT [25% (10/40), ref. 23] and Shah and colleagues [21% (4/19), ref. 22]. Taken together, these findings suggest that the addition of anti–PD-1/-L1 therapy to neoadjuvant chemoradiation in esophageal/GEJ adenocarcinoma may not significantly impact pCR in PD-L1–unselected patients. In our cohort and other studies, pCR was significantly associated with longer PFS and OS, but the degree to which pCR or other endpoints of primary tumor response accurately forecasts long-term clinical benefit remains controversial in PD-L1–unselected patients (26, 27). Of note, our study was not powered for PFS or OS.
We performed exploratory analyses to evaluate whether immune biomarkers could identify patients who might benefit from this therapy. We focused on PD-L1 expression, a marker of a T-cell–inflamed TME that has been associated with immune signatures indicative of T-cell activation (28). A practical advantage of PD-L1 CPS or TPS over genomic signatures of T-cell inflammation is its immediate availability in most clinical pathology laboratories. Analysis of tissue-based PD-L1 expression was preplanned, with more precise definitions and cut-off points of CPS and TPS delineated posthoc based on currently used clinical cut-off points in this disease.
We found that the subset of patients with high tissue expression of PD-L1 in tumor and/or immune cells (i.e., CPS ≥ 10 vs. <10) experienced significantly higher pCR rates (50% vs. 14%, respectively; P = 0.046). Patients with a CPS ≥ 10, who comprised 27% (8/30) of the cohort, also had numerically longer PFS and OS compared with patients with lower PD-L1 levels (2-year rates 65.6% vs. 44.3% for PFS and 87.5% vs. 61.6% for OS). These outcomes among PD-L1–high patients who received pembrolizumab-containing trimodality therapy compare favorably with those observed in the CROSS study in PD-L1–unselected patients with adenocarcinoma histology (pCR rate 21%, 2-year PFS rate 57%, 2-year OS rate 65%). In addition, this group had outcomes that were favorable compared with a propensity-score-matched PD-L1–unselected cohort treated with carboplatin/paclitaxel-containing chemoradiation (pCR rate 12.9%, 2-year PFS rate 32.2%, 2-year OS rate 64.5%). To our knowledge, these are the first data in this disease to indicate that high expression of PD-L1 in the TME at baseline is significantly associated with improved outcomes from anti–PD-1/-L1–containing trimodality therapy and suggest its potential utility for patient selection and analysis.
Although PD-L1 CPS has been implicated as a predictive marker in metastatic gastroesophageal cancer, its predictive value in patients with nonmetastatic disease treated with curative intent has not been extensively examined. PD-L1 CPS was significantly associated with pCR in patients with resectable esophageal squamous cell carcinoma who received anti–PD-1 (camrelizumab) in combination with chemotherapy prior to surgical resection (29). Of three trials in gastroesophageal adenocarcinoma that have reported results from the addition of PD-1/-L1 blockade to neoadjuvant chemoradiation (22, 23, 30), only the PERFECT study [which examined atezolizumab (anti–PD-L1)] reported data on baseline PD-L1 expression in the TME (23), to our knowledge. In PERFECT, higher (vs. lower) CPS was numerically, but not statistically significantly, associated with increased PFS, OS, and tumor response, consistent with our study. Interestingly, positive PD-L1 expression in the TME appeared to be associated with longer DFS from adjuvant nivolumab (vs. placebo) following trimodality therapy in an exploratory analysis of CheckMate-577 (3). We focused on a PD-L1 CPS cut-off point of 10 because this was identified as the ideal cut-off point for identifying patients with esophageal cancer who benefit from pembrolizumab in the metastatic setting (1, 31) and is the only cut-off point for pembrolizumab with a category 1 or 2A recommendation by National Comprehensive Cancer Network and European Medicines Agency.
Notably, due to the lack of a randomized control arm in our study, it is possible that PD-L1 expression may simply be a marker for improved prognosis. Existing literature on the prognostic impact of PD-L1 expression in patients who have not received anti–PD-1/-L1–containing therapy has not yielded uniform results, to date, but suggests that PD-L1 expression may be associated with adverse or null prognosis. In metastatic or trimodality-treated gastric/esophageal cancer, increasing expression levels of PD-L1 appear to correlate with slightly shorter median OS and PFS in the control arms of randomized phase III trials (1-3). In nonmetastatic disease, the prognostic impact of PD-L1 expression has been reported to be adverse (32, 33), favorable (34-36), and null (37, 38). One meta-analysis comprising 3291 patients showed that PD-L1 overexpression in tumor and/or immune cells in the TME was associated with worse OS (HR = 1.46; 95% CI, 1.08–1.98; P = 0.01; random-effect) consistent with results from 11 of 15 included studies (39). In our propensity-score-matched cohort, a limited analysis suggested that PD-L1 expression was not associated with favorable prognosis in patients treated with chemoradiation that did not contain anti–PD-1/-L1.
We also explored tumor-cell expression of PD-L1 and its association with response to pembrolizumab-containing chemoradiation. Although tumor-cell expression of PD-L1 is common in other tumor types [e.g., frequency ~50% in non–small cell lung cancer (NSCLC); ref. 40], its frequency in gastroesophageal adenocarcinomas appears to be lower at approximately 2% to 18% (2, 3, 41). PD-L1 expression in tumor vs. surrounding immune cells in the TME may be regulated by distinct mechanisms that differ over time and with regard to their adaptive immune response and sensitivity to anti–PD-1/-L1 therapy (13-16, 42). We first examined the TPS cut-off point of ≥1% for PD-L1 expression because it is the only TPS cut-off point identified to date that predicts clinical benefit from PD-1 blockade in esophageal cancer (43). Only 16.7% (5/30) of patients in our study had PD-L1 TPS ≥ 1%, consistent with prior data (3). PD-L1 TPS ≥ 1% (vs. <1%) was numerically, but not statistically, associated with a higher pCR rate and longer PFS (2-year rates 75.0% vs. 44.5%; log–rank P = 0.154) and OS (2-year rates 100.0% vs. 61.8%; log–rank P = 0.126). In further exploratory analysis, TPS at the cut-off point of 0 identified more patients with PD-L1–expressing tumor cells and was significantly associated with a higher frequency of PD-L1 expression in surrounding immune cells; TPS > 0 was significantly associated with longer PFS. Small increments in PD-L1 TPS ranging from 0 to 100% were associated with incrementally enhanced response rates in patients with advanced NSCLC treated with pembrolizumab monotherapy (44). Taken together, these findings suggest further research of PD-L1 expression on tumor cells may be warranted.
We further explored PD-L1–expressing EVs in plasma and their association with expression of tissue PD-L1 and tumor response. To our knowledge, these are the first data evaluating PD-L1–expressing EVs in association with efficacy in solid tumor patients treated with PD-1/-L1 blockade in a prospective trial. We did not observe a significant difference in levels of PD-L1–expressing EVs in TMEs with high vs. low levels of PD-L1 expression (CPS ≥ 10 vs. <10). Prior studies indicate that exosomal PD-L1 levels are typically consistent with the levels of PD-L1 expressed in their parental tumor cells (17, 20, 45). Yet emerging evidence suggests that tumor cells from some malignancies produce high levels of PD-L1–containing exosomes, but are devoid of PD-L1 on the tumor cell surface, despite expressing constitutively high levels of PD-L1 mRNA (19). Recent data indicate that PD-L1–expressing EVs may behave similarly as PD-L1–expressing tumor cells in suppressing the activity of tumor-reactive CD8+ T cells, suggesting the therapeutic potential for targeting high PD-L1–expressing EVs with anti–PD-1 therapy (17). Since high PD-L1 CPS in the TME already identified responders, we explored whether PD-L1–expressing EVs among patients with low PD-L1 CPS in the TME could identify further responders. We found that, among tumors with low PD-L1 expression (CPS < 10), a higher level of PD-L1–expressing EVs was significantly associated with a higher pCR rate. The positive association of PD-L1–expressing EVs with response in tumors with CPS < 10 did not result from confounding by tumor-cell expression of PD-L1, since the association remained significant when limited to CPS < 10 tumors that had low (TPS <1%) or absent expression (TPS 0) of PD-L1 in tumor cells. These data suggest that measurement of PD-L1–expressing EVs may identify additional responders to anti–PD-1–containing chemoradiation despite low expression of PD-L1 in the TME, but further research is needed in independent cohorts to confirm these findings.
In conclusion, this trial met the primary safety endpoint but not the primary pCR endpoint. In patients with high PD-L1 expression in the TME, our data suggest that incorporating anti–PD-1 therapy into neoadjuvant chemoradiation and adjuvant treatment of GEJ adenocarcinoma may improve pCR and survival. Further understanding primary immune resistance is an important area of ongoing research, as response has been associated with low expression of co-inhibitory checkpoints (23). In addition, PD-L1 expression on EVs may identify further responders and warrants further study. These data suggest the importance of examining baseline expression of PD-L1 in the TME in ongoing and future curative-intent studies of this approach.
Supplementary Material
Translational Relevance.
In this study, we prospectively evaluated the safety and efficacy of adding anti-programmed death (PD)-1 therapy (pembrolizumab) to standard neoadjuvant chemoradiation and surgery in patients with locally advanced gastroesophageal adenocarcinoma. We report promising activity using this approach in the subset of patients with high baseline tissue expression of PD-L1. These results suggest the importance of measuring tissue PD-L1, a readily measurable marker of T-cell inflammation, for patient stratification and/or efficacy analysis in larger randomized trials. In addition, we show that elevated plasma levels of PD-L1–expressing extracellular vesicles may identify responders to immunotherapy in patients with low/absent tissue expression of PD-L1. This observation supports further investigation of vesicle-bound PD-L1 as a therapeutic target, as well as a novel biomarker to enhance patient selection. Together, these unique findings demonstrate that anti–PD-1–containing trimodality therapy can lead to favorable tumor response in biomarker-selected patients.
Acknowledgments
This investigator-initiated study was supported by funds from Merck and Mayo Clinic.
Footnotes
Authors’ Disclosures
C.T. Sanhueza reports personal fees from MSD, BMS, Tecnofarma, Pfizer, AstraZeneca, Janssen, and Roche outside the submitted work. J. Herrmann reports grants from NCI and SHL and personal fees from Elsevier outside the submitted work. R.R. McWilliams reports personal fees from Zentalis Pharmaceuticals outside the submitted work. W.W. Ma reports grants from Merck during the conduct of the study. T.S. Bekaii-Saab reports research funding (to institution) from Agios, Aris, Arcus, Atreca, Boston Biomedical, Bayer HealthCare, Eisai, Celgene, Eli Lilly and Company, Ipsen, Clovis, Seattle Genetics, Genentech, Novartis, Mirati, Merus, AbGenomics, Incyte, Pfizer, and BMS; consulting for Ipsen, Arcus, Pfizer, Seattle Genetics, Bayer HealthCare, Genentech, Incyte, Eisai, and Merck; consulting (to self) from Stemline, AbbVie, Boehringer Ingelheim, Janssen, Daichii Sankyo, Natera, Treos Bio, Celularity, Exact Sciences, Sobi, BeiGene, Kanaph, AstraZeneca, Deciphera, MJH Life Sciences, Aptitude Health, Illumina, and Foundation Medicine; Independent Data Monitoring Committee/Data Safety Monitoring Board at Fibrogen, Suzhou Kintor, AstraZeneca, Exelixis, Merck/Eisai, PanCAN and 1Globe; and part of the Scientific Advisory Board of Imugene, Immuneering, Xilis, Replimune, and Sun Biopharma. In addition, T.S. Bekaii-Saab reports royalties from Uptodate; inventions/patents for WO/2018/183488: HUMAN PD1 PEPTIDE VACCINES AND USES THEREOF licensed to Imugene, and WO/2019/055687: METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER CACHEXIA licensed to Recursion. D.H. Ahn reports personal fees from Genentech, Eisai, Advanced Accelerator Applications, Exelixis and Incyte outside the submitted work. H.H. Yoon reports grants and other support from Merck during the conduct of the study; other support from OncXerna, Zymeworks, MacroGenics, BMS, BeiGene, AstraZeneca, Boston Biomedical, Elevar, and CARsgen outside the submitted work. No disclosures were reported by the other authors.
Additional information: There was no writing support from a company-paid writer for this manuscript.
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Sun J-M, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759–71. [DOI] [PubMed] [Google Scholar]
- 2.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet North Am Ed 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly RJ, Ajani JA, Kuzdzal J, Zander T,Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021;384:1191–203. [DOI] [PubMed] [Google Scholar]
- 4.Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 2021;39:1995–2004. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795–803. [DOI] [PubMed] [Google Scholar]
- 7.Andarawewa KL, Erickson AC, Chou WS, Costes SV, Gascard P, Mott JD, et al. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor β–induced epithelial to mesenchymal transition. Cancer Res 2007;67:8662–70. [DOI] [PubMed] [Google Scholar]
- 8.Baschnagel AM, Williams L, Hanna A, Chen PY, Krauss DJ, Pruetz BL, et al. c-Met expression is a marker of poor prognosis in patients with locally advanced head and neck squamous cell carcinoma treated with chemoradiation. Int J Radiat Oncol Biol Phys 2014;88:701–7. [DOI] [PubMed] [Google Scholar]
- 9.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–68. [DOI] [PubMed] [Google Scholar]
- 10.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res 2015;3: 610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Caliò A, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 2015; 10:e0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer 2019;7:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguchi T, Ward JP, Gubin MM, Arthur CD, Lee SH, Hundal J, et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res 2017;5:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847–56. [DOI] [PubMed] [Google Scholar]
- 16.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res 2015;21:3969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res 2018;24:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019;177:414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Che X, Qu J, Hou K, Wen T, Li Z, et al. Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol 2019;26:3745–55. [DOI] [PubMed] [Google Scholar]
- 21.Gomes J, Lucien F, Cooper TT, Kim Y, Williams KC, Liao X, et al. Analytical considerations in nanoscale flow cytometry of extracellular vesicles to achieve data linearity. Thromb Haemost 2018;118:1612–24. [DOI] [PubMed] [Google Scholar]
- 22.Shah MA, Almhanna K, Iqbal S, Thakkar P, Schneider BJ, Yantiss R, et al. Multicenter, randomized phase II study of neoadjuvant pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal adenocarcinoma (EAC). J Clin Oncol 2021;39:4005. [Google Scholar]
- 23.van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a single-arm phase II feasibility trial (PERFECT). Clin Cancer Res 2021;27:3351–9. [DOI] [PubMed] [Google Scholar]
- 24.Enlow JM, Denlinger CE, Stroud MR, Ralston JS, Reed CE. Adenocarcinoma of the esophagus with signet ring cell features portends a poor prognosis. Ann Thorac Surg 2013;96:1927–32. [DOI] [PubMed] [Google Scholar]
- 25.Anderegg MC, Lagarde SM, Jagadesham VP, Gisbertz SS, Immanuel A, Meijer SL, et al. Prognostic significance of the location of lymph node metastases in patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Ann Surg 2016;264:847–53. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HH, Ou FS, Soori GS, Shi Q, Wigle DA, Sticca RP, et al. Induction versus no induction chemotherapy before neoadjuvant chemoradiotherapy and surgery in oesophageal adenocarcinoma: a multicentre randomised phase II trial (NCCTG N0849 [Alliance]). Eur J Cancer 2021;150:214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields RC, Strong VE, Gönen M, Goodman KA, Rizk NP, Kelsen DP, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer 2011;104:1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayers M, Nebozhyn M, Cristescu R, McClanahan TK, Perini R, Rubin E, et al. Molecular profiling of cohorts of tumor samples to guide clinical development of pembrolizumab as monotherapy. Clin Cancer Res 2019;25:1564–73. [DOI] [PubMed] [Google Scholar]
- 29.Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10: e003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly RJ, Smith KN, Anagnostou V, Thompson E, Hales RK, Battafarano RJJ, et al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J Clin Oncol 2019;37:142. [Google Scholar]
- 31.Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020;38:4138–48. [DOI] [PubMed] [Google Scholar]
- 32.Eto S, Yoshikawa K, Nishi M, Higashijima J, Tokunaga T, Nakao T, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 2016;19:466–71. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19–24. [DOI] [PubMed] [Google Scholar]
- 34.Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016;7:24269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai C, Geng R, Wang C, Wong A, Qing M, Hu J, et al. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol 2016;10:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016;19:42–52. [DOI] [PubMed] [Google Scholar]
- 37.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 2017;20:407–15. [DOI] [PubMed] [Google Scholar]
- 38.Soeratram TT, Creemers A, Meijer SL, de Boer OJ, Vos W, Hooijer GK, et al. Tumor-immune landscape patterns before and after chemoradiation in resectable esophageal adenocarcinomas. J Pathol 2022;256:282–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One 2017;12:e0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol 2016;11:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, et al. Epithelial PD-L2 expression marks Barrett’s esophagus and esophageal adenocarcinoma. Cancer Immunol Res 2015;3:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med 2022;386:449–62. [DOI] [PubMed] [Google Scholar]
- 44.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 28–372:2018;2015. [DOI] [PubMed] [Google Scholar]
- 45.Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 2018;4:eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.