Abstract
Introduction
The purpose of this study was to explore the effects of repeated low-level red-light (RLRL) therapy on the structure and vasculature of the choroid and retina in Chinese children with premyopia.
Methods
This study was a single-center randomized clinical trial. A total of 94 children with premyopia (− 0.50 D < spherical equivalent [SE] ≤ + 0.75 D) were randomly assigned to either the RLRL therapy or control group. Follow-up visits were planned at 1, 3, 6, 9, and 12 months. Optical coherence biometry was used to measure axial length (AL) and anterior segment parameters. Choroidal thickness (CT), retinal thickness (RT), superficial retinal vascular density (SRVD), deep retinal vascular density (DRVD), choriocapillaris perfusion area (CCPA), and choroidal vessel volume (CVV) were measured by optical coherence tomography angiography, centered on the foveal, parafoveal (ParaF), and perifoveal (PeriF) regions.
Results
The thickening of the choroid was observed across the entire macular region at different time points in the RLRL therapy group. Relative to the baseline measurement, foveal CT significantly increased at the 1-month follow-up with RLRL therapy, with a mean (± standard deviation [SD]) adjusted change of 16.96 ± 19.87 μm. The greatest magnitude of foveal CT changes was observed at the 3-month visit (an increase of 19.58 ± 20.59 μm), with a slight reduction in the extent of foveal CT increase at the 6-month visit (an increase of 15.85 ± 23.77 μm). The second greatest CT increase was observed at the 9-month visit (an increase of 19.57 ± 35.51 μm), after which the extent of CT increase gradually decreased until the end of the study at the 12-month visit (an increase of 11.99 ± 32.66 μm). We also observed a significant increase in CT in the ParaF and PeriF areas in the RLRL group over 12 months. In contrast, CT across the entire macular region in the control group significantly decreased throughout the follow-up visits (all P < 0.05). Regarding the vascular parameters of the choroid, significant increases in CVV were observed primarily in the ParaF and PeriF regions of the choroid in the RLRL group. In comparison, the control group exhibited decreases in CVV throughout the entire area. Furthermore, notable elevations in CCPA were detected in the PeriF area of the choroid in the RLRL group during the 1-month (an increase of 0.40 mm2), 3-month (an increase of 0.25 mm2), and 12-month visits (an increase of 0.42 mm2) (all P < 0.05). In addition, no notable differences were observed between the groups regarding foveal RT and retinal vascular parameters throughout the 12 months (P > 0.05). Notably, RLRL therapy achieved a notable reduction in SE shift by 73.8%, a substantial decrease in AL change by 67.9%, and a significant reduction in myopia incidence by 45.1% within 1 year.
Conclusion
Our study demonstrated a significant increase in CT and flow in the RLRL-treated eyes throughout the 12-months of the study. Combined with its reduction in spherical equivalent progression and axial elongation, RLRL could be used as an effective therapy for preventing progression in premyopes.
Trial Registration
ChiCTR2200062028.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-023-00875-x.
Keywords: Repeated low-level red light, Children, Premyopia, Foveal thickness, Choroid, Retina vasculature, Myopic shift, Optical coherence tomography angiography
Key Summary Points
| Why carry out this study? |
| In recent decades, myopia has emerged as a significant global public health concern. While repeated low-level red-light (RLRL) therapy has recently been recognized as a safe and effective approach for managing myopia, scientific reports on medical interventions aimed at preventing the onset of myopia are scarce. |
| There is also a lack of understanding regarding the impact of RLRL therapy on the structural and vascular characteristics of the retina and choroid in children with premyopia. |
| What was learned from the study? |
| Eyes treated with RLRL therapy showed a significant increase in choroidal thickness and flow throughout the 12 months, as well as a reduction in spherical equivalent progression and axial elongation, suggesting that RLRL could be used as an effective therapy for the control of the progression of axial myopia in children with premyopia susceptible to myopic progression. |
| The effectiveness of RLRL therapy in controlling myopia may be partially mediated through choroidal thickening. |
| The findings once again highlight the specific role of choroidal signaling pathways in inducing modifications that mitigate scleral hypoxia and remodeling, ultimately aiding in the effective prevention of myopia among populations with premyopia. |
Introduction
Myopia has become a significant global public health concern. Studies have shown that myopia progresses more rapidly when it occurs at a younger age [1–3] and that early-onset myopia is a risk factor for high myopia [4], which can lead to serious ocular complications, including retinal detachment, retinal break, choroidal neovascularization, and myopic maculopathy. Such complications can significantly impair vision and are often irreversible [5–7]. Premyopes represent a critical population characterized by a reduction in physiological hyperopia and more positive near retinoscopy [8]. After the onset of myopia in children, its progression is difficult to control; therefore, it is crucial to keep children in a premyopia state to delay or prevent the onset of myopia.
Recent evidence has suggested that increased outdoor activity and reduced long-term near-work could potentially aid in preventing myopia onset [9–14]. However, limited information is available in the literature regarding medical interventions to prevent myopia. Repeated low-level red-light (RLRL) therapy has recently emerged as a safe and effective therapy for myopia control, offering an alternative to increasing bright-light exposure [15, 16]. A clinical study has demonstrated that RLRL therapy significantly reduced the rate of myopia progression and axial elongation over a period of 6 months compared to orthokeratology and single-vision spectacles (SVS) [15]. In a 12-month, multicenter, randomized clinical trial involving 264 children aged 8 to 13 years, RLRL therapy was shown to be an effective treatment for myopia control compared to SVS, and no functional or structural damage was reported [16].
Despite the demonstrated effectiveness of RLRL therapy in myopia control, the mechanism underlying its suppressive effect remains unclear. Recent evidence has identified scleral hypoxia as a key promoter of scleral remodeling and myopia development [17, 18]. To address this issue, He et al. hypothesized that RLRL treatment might improve blood flow and metabolism of the fundus, leading to amelioration of scleral hypoxia and restoration of scleral collagen levels [19]. In recent years, there has been increasing interest in the role of the choroid and retina in myopia etiology, as the choroid has been found to react to optical defocus and the retina is expected to process the sign of optical defocus and initiate a multilayered signaling cascade from the retina to the choroid and then to the sclera [20].
To date, few studies have explored the effects of RLRL therapy on the structural and vascular characteristics of the retina and choroid in children with premyopia. In this study, we investigated the impact of RLRL therapy on premyopes and evaluated the short-term influence of RLRL therapy on the structural and vascular parameters of the choroid and retina to further improve our knowledge on the effects of RLRL therapy and identify potential biomarkers for predicting therapeutic response.
Methods
Study Design
This was a single-center, single-masked, randomized controlled trial in which 94 children were enrolled between 12 August 2021 and 3 September 2022, at Tianjin Medical University Eye Hospital, Tianjin, China. Children were randomly assigned to the RLRL therapy group or the control group at a ratio of 1:1 using stratified block randomization with a block length of eight. Independent investigators conducted outcome measurements for masking purposes, and the statistician was also blinded to allocation. Written informed consent and verbal assent were obtained from a parent/legal guardian, and the study adhered to the principles of the Declaration of Helsinki. The institutional review board of Tianjin Medical University Eye Hospital approved the study (No. 2021KY-11). Investigators and key personnel at each site involved in the present study were trained and certified prior to study commencement. There were no changes in the protocol and methods after trial commencement. The trial was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/) with the registration number ChiCTR2200062028.
Inclusion and Exclusion Criteria
A total of 94 children (94 eyes from 94 subjects) with premyopia were enrolled in the study, with the diagnosis of premyopia based on cycloplegic optometry results (− 0.50 D < spherical equivalent [SE] ≤ + 0.75 D). Children aged 7–12 years with regular follow-ups for at least 12 months were included. Exclusion criteria included astigmatism of ≥ 2 D, other ocular diseases (such as amblyopia, strabismus, congenital cataract, glaucoma, corneal scar, optic neuropathy, traumatic ocular injury, uveitis, and ocular tumor), history of any ocular surgery, and any systemic diseases or conditions that may affect visual function and development (including diabetes mellitus and chromosome anomaly).
Intervention and Study Procedures
Children in the RLRL therapy group received RLRL treatment twice daily, with each session lasting 3 min and at least 4 h between treatments, while those in the control group did not. Given that the treatment time for the two daily sessions needed to be separated by more than 4 h, one treatment session was planned to occur prior to the child going to school in the morning and the second session was planned that same afternoon after school. The RLRL intervention was provided by a desktop device (Eyerising, Suzhou Xuanjia Optoelectronics Technology, Kunshan city, China). This technology incorporates semiconductor laser diodes that emit low-intensity, single-wavelength 650 (10)-nm red-light laser beam at an illuminance level of approximately 1600 lx through the pupil to the fundus. This device was certified as a Class IIa medical device by the State Administration for Market Regulation of China. The light power of this RLRL device entering a 4-mm pupil (the maximum pupil size under the condition of bright-light exposure over 10 s) is 0.29 mW. These power levels are classified as Class 1, which is at a level considered safe for direct ocular exposure. Guardians/parents of the children received training to oversee the intervention program at home. To ensure accurate tracking and recording of treatment history, the device was integrated with an automated diary function and connected to the internet. In addition, two investigators were tasked with managing intervention compliance. To enhance treatment compliance, parents or legal guardians received weekly reminders about the intervention program in an attempt to maintain consistency and effectiveness in the intervention process.
All participants underwent a standardized examination at the baseline visit, followed by five scheduled follow-up visits at 1, 3, 6, 9, and 12 months. A cycloplegic refraction test was conducted both before enrollment and at every 6 months during the follow-up period. To achieve cycloplegia, four drops of compound tropicamide eye drops were administered at intervals of approximately 5 min. At 30 min after the administration of the final drop, cycloplegic autorefraction was assessed using the KR-8800 desktop autorefractor (Topcon Corp., Tokyo, Japan). This autorefractor obtained three readings, each separated by 0.25 D, for both spherical and cylindrical components which were then averaged. Subsequently, an experienced optometrist performed cycloplegic retinoscopy. Best-corrected visual acuity (BCVA) was measured at a distance of 5 m and converted to the logarithm of the minimum angle of resolution (logMAR) scale. Prior to initiating cycloplegia, several ocular parameters were measured using the IOL Master 700 swept-source optical coherence tomography (OCT)-based biometer (Carl Zeiss AG, Jena, Germany), including axial length (AL), anterior chamber depth (ACD), lens thickness (LT), and keratometry values (flat keratometry [Kf]). The Swept-Source Optical Coherence Tomography/Optical Coherence Tomography Angiography (SS-OCT/OCT-A) system (VG200S; SVision Imaging, Henan, China) was employed to evaluate macular structures and vascular characteristics in all eyes. To minimize the potential confounding effects of diurnal variations, all measurements were conducted between 12 a.m. and 3 p.m. The macular images were classified into three concentric circles according to the grid of the Early Treatment of Diabetic Retinopathy Study (ETDRS), centered on the foveal region (within central 1-mm diameter), the parafoveal (ParaF) region (from an inner diameter of 1 mm to an outer diameter of 3 mm), and the perifoveal (PeriF) region (between the 3 and 6 mm concentric ring). The major automated thickness outcomes recorded included ganglion cell complex (GCC) thickness (perpendicularly from the inner edge of the nerve fiber layer [NFL] to the outer edge of the inner plexiform layer [IPL]; see Electronic Supplementary Material [ESM] Fig. S2A); the inner nuclear layer (INL) thickness (perpendicularly from the inner to the outer edge of the INL; see ESM Fig. S2B); the outer retinal layer (ORL) thickness (measured from the inner edge of the outer plexiform layer [OPL] to the outer edge of the retinal pigment epithelium (RPE)/Bruch’s membrane complex; see ESM Fig. S2C); retinal thickness (RT) (measured from the inner edge of the nerve fiber layer [NFL] to the outer edge of the RPE/Bruch’s membrane complex; see ESM Fig. S2D); and choroid thickness (CT) (defined as the vertical distance between the outer edge of the retinal pigment epithelium [RPE] and the outer edge of the choroid; see ESM Fig. S2E). Retinal microvascular parameters included superficial retinal vascular density (SRVD) (ESM Fig. S3A) and deep retinal vascular density (DRVD) (ESM Fig. S3B). The SRVD was automatically defined by the system as microvessels located 5 μm above the inner limiting membrane to the one-third interface of the ganglion cell layer and inner plexiform layer (GCL + IPL). Likewise, the DRVD was defined as the microvessels located from the one-third interface of GCL + IPL to 25 μm below the lower border of the INL. For choroidal microvascular parameters, choriocapillaris perfusion area (CCPA) was measured, which involved maximum projection 20 μm deep to Bruch’s membrane (ESM Fig. S3C). Choroidal vessel volume (CVV), also known as choroidal luminal volume, was defined as the volume of the large and medium choroidal vessels (ESM Fig. S4).
Statistical Analysis
The minimum sample size required for the analysis was conservatively determined to be N = 42 per cohort of participants who completed the trial. This sample size was considered sufficient to detect a small treatment effect, specifically a difference of 0.25 D in refraction, with a standard deviation (SD) of 0.35 D. The type I error rate was set at 0.05, and the statistical power was set at 80%. Balanced recruitment by age between 7 and 12 years (inclusive) was assumed for this sample size estimation. Only the right eye of each participant was analyzed in this study. During the treatment phase, both endpoints were summarized with mean value ± SD or median with interquartile range at every visit. To ensure the accuracy of the results, only SE data with complete cycloplegia were included in this analysis. A change in a parameter was determined as the difference between the baseline value and the corresponding follow-up value. Changes in AL, SE, retinal, and choroidal parameters at each follow-up time point were compared using a generalized linear mixed model (GLMM). Baseline age, gender, group, visit, group-by-visit interaction, and corresponding baseline parameters were fixed factors in the models, and subjects were included as random factors. The estimated mean differences, 95% confidence intervals, and two-sided P values were calculated.
Linear regression models were utilized to evaluate the factors associated with changes in AL and SE over a period of 12 months in the RLRL group. The analysis encompassed baseline age, gender, CT, AL/SE, and change in fovea CT over 12 months into the models. To facilitate visualization of the results, a 1-unit change in the regression models was deemed equivalent to a 10-mm change in fovea CT. A statistical significance level of 0.05 was consistently applied throughout the analysis. All statistical tests were performed with SPSS software (version 26.0; SPSS IBM, Armonk, NY, USA).
Results
Baseline Characteristics
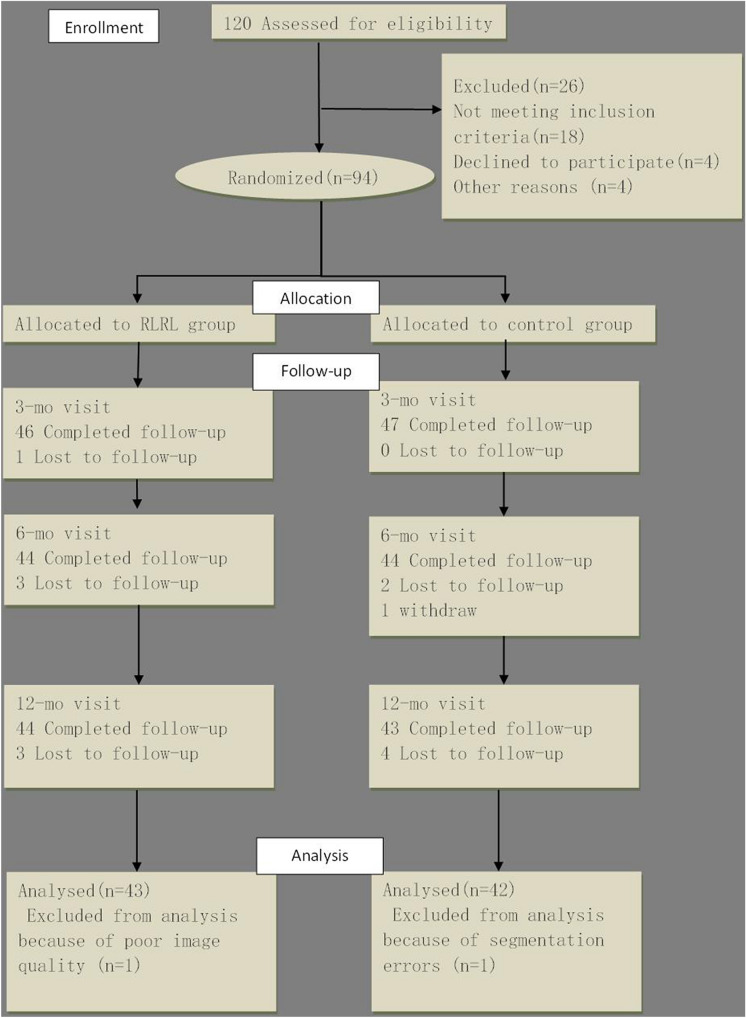
A total of 94 consecutive children met the prescribed criteria and were consequently enrolled in the study. Among these children, seven did not have a completed follow-up dataset, one had segmentation errors, and one had poor image quality. These children were excluded from the analysis, leaving 85 children (90.4%) in the final analysis (43 in RLRL group; 42 in control group) (Fig. 1). The distribution of baseline age, gender, AL, and SER was well balanced between the RLRL group and control group (P > 0.05; Table 1).
Fig. 1.
Flowchart of the study. RLRL Repeated low-level red-light (therapy)
Table 1.
Demographics and baseline ocular characteristics between the RLRL and the control group
| Characteristics | All patients were randomly assigned to: | P value | |
|---|---|---|---|
| RLRL group (N = 47) | Control group (N = 47) | ||
| Age, years | |||
| Mean ± SD | 8.98 ± 1.31 | 8.95 ± 1.52 | 0.817 |
| Median (P25, P75) | 9.00 (8.00, 10.00) | 8.50 (8.00, 10.00) | |
| Gender | |||
| Boy | 24 (55.81%) | 22 (52.38%) | 0.763 |
| Girl | 19 (44.19%) | 20 (47.62%) | |
| AL, mm | |||
| Mean ± SD | 23.57 ± 0.78 | 23.30 ± 0.73 | 0.116 |
| Median (P25, P75) | 23.59 (22.98, 24.08) | 23.32 (22.68, 23.92) | |
| SE, D | |||
| Mean ± SD | 0.17 ± 0.35 | 0.30 ± 0.35 | 0.104 |
| Median (P25, P75) | 0.25 (0.00, 0.50) | 0.25 (0.00, 0.50) | |
Data are presented as the mean ± standard deviation (SD), as a number with the percentage in parentheses, or as the median with the interquartile range (P25, P75) in parentheses, as appropriate
AL Axial length, D diopter, P25, P75 25th percentile, 75th percentile, SE spherical equivalence, RLRL repeated low-level red-light
Safety
During the initiation phase of treatment, two children reported intolerance to bright light and dry eye symptoms. However, these side effects were transient and resolved either spontaneously or with minimal intervention. Over the subsequent 12 months of treatment, no participants withdrew from the study due to intolerability or discomfort. Moreover, there were no recorded side effects in terms of complaints, functional loss (BCVA), or anatomical changes (OCT/OCTA scans) during the 12-month follow-up period.
Changes in Structural and Vascular Parameters of the Choroid at Follow-Up Visits
As presented in Table 2 and Fig. 2, the thickening of the choroid was observed across the entire macular region against different time points in the RLRL group. Foveal CT relative to baseline CT significantly increased at the 1-month follow-up with RLRL therapy, with a mean (± SD) adjusted change of 16.96 ± 19.87 μm. The greatest magnitude in foveal CT change was observed at the 3-month visit (an increase of 19.58 ± 20.59 μm), with a slight reduction in the extent of foveal CT increase at the 6-month visit (an increase of 15.85 ± 23.77 μm). The second greatest CT increase was observed at the 9-month visit (an increase of 19.57 ± 35.51 μm), after which the extent of CT increase gradually decreased until the end of the study at the 12-month visit (an increase of 11.99 ± 32.66 μm). We also observed a significant increase in choroidal thickness (CT) in the ParaF and PeriF areas in the RLRL group over 12 months. In contrast, CT across the entire macular region in the control group significantly decreased throughout the follow-up visits (all P < 0.05).
Table 2.
Cumulative adjusted changes in choroidal thickness across the entire macular region at different time points in the repeated low-level red-light therapy and control groups
| Time/visit | Cumulative adjusted mean changes | P value | |
|---|---|---|---|
| RLRL group | Control group | ||
| Change in CT-fovea, μm | |||
| 1 Month | |||
| Mean ± SD | 16.96 ± 19.87# | − 2.16 ± 16.74 | < 0.001* |
| Median (P25, P75) | 17.92 (5.91, 32.17)# | − 0.01 (− 9.14, 8.82) | |
| 3 Months | |||
| Mean ± SD | 19.58 ± 20.59# | − 8.57 ± 26.45# | < 0.001* |
| Median (P25, P75) | 20.55 (6.99, 29.29)# | − 4.14 (− 21.95, 6.36)# | |
| 6 Months | |||
| Mean ± SD | 15.85 ± 23.77# | − 7.62 ± 25.29# | < 0.001* |
| Median (P25, P75) | 15.72 (− 5.11, 28.65)# | 0.51 (− 23.60, 9.96)# | |
| 9 Months | |||
| Mean ± SD | 19.57 ± 35.51# | − 22.50 ± 24.21# | < 0.001* |
| Median (P25, P75) | 19.06 (− 9.52, 41.02)# | − 22.41 (− 43.71, − 0.98)# | |
| 12 Months | |||
| Mean ± SD | 11.99 ± 32.66# | − 28.74 ± 26.89# | < 0.001* |
| Median( P25, P75) | 12.75 (− 5.94, 24.14)# | − 30.46 (− 48.44, − 4.07)# | |
| Change in CT-ParaF, μm | |||
| 1 Month | |||
| Mean ± SD | 17.16 ± 19.57# | − 1.82 ± 16.01 | < 0.001* |
| Median (P25, P75) | 17.48 (6.38, 28.97)# | − 0.75 (− 10.20, 9.66) | |
| 3 Months | |||
| Mean ± SD | 18.49 ± 19.45# | − 7.44 ± 24.03# | < 0.001* |
| Median(P25, P75) | 18.10 (7.99, 27.15)# | − 4.07 (− 20.10, 7.56)# | |
| 6 Months | |||
| Mean ± SD | 15.64 ± 24.93# | − 6.51 ± 22.77# | < 0.001* |
| Median (PD25, P75) | 13.64 (− 0.92, 30.36)# | − 1.05 (− 22.38, 9.86)# | |
| 9 Months | |||
| Mean ± SD | 18.21 ± 33.47# | − 20.84 ± 21.69# | < 0.001* |
| Median (P25, P75) | 16.58 (− 8.84, 40.79)# | − 18.50 (− 40.82, − 3.39)# | |
| 12 Months | |||
| Mean ± SD | 10.69 ± 31.77# | − 25.83 ± 25.40# | < 0.001* |
| Median (P25, P75) | 12.13 (− 7.08, 24.04)# | − 27.42 (− 42.86, − 5.60)# | |
| Change of CT-PeriF, μm | |||
| 1 Month | |||
| Mean ± SD | 15.53 ± 20.59# | − 1.75 ± 14.45 | < 0.001* |
| Median (P25, P75) | 16.96 (3.21, 29.00)# | 1.00 (− 10.95, 6.89) | |
| 3 Months | |||
| Mean ± SD | 16.79 ± 19.17# | − 7.08 ± 21.80# | < 0.001* |
| Median (P25, P75) | 15.76 (7.84, 28.61)# | − 5.21 (− 20.55, 2.31)# | |
| 6 Months | |||
| Mean ± SD | 13.10 ± 25.20# | − 6.07 ± 21.14# | < 0.001* |
| Median (P25, P75) | 10.27 (− 3.77, 25.61)# | − 3.87 (− 17.31, 8.41)# | |
| 9 Months | |||
| Mean ± SD | 14.15 ± 30.69# | − 20.75 ± 20.74# | < 0.001* |
| Median (P25, P75) | 11.82 (− 5.49, 38.14)# | − 22.17 (− 37.79, − 3.64)# | |
| 12 Months | |||
| Mean ± SD | 3.89 ± 26.80# | − 24.75 ± 24.21# | < 0.001* |
| Median (P25, P75) | 0.60 (− 7.16, 16.86)# | − 30.03 (− 40.79, − 8.14)# | |
Data are presented as the mean ± standard deviation (SD) and as the median with the interquartile range (P25, P75) in parentheses, as appropriate
Adjusted estimates at each time point were calculated on the basis of generalized linear mixed model, adjusted for baseline age, gender, group, visit, group* visit, and corresponding baseline parameters as fixed factors, and subjects as the random factor
CT Choroidal thickness, P25, P75 25th percentile, 75th percentile, ParaF parafoveal, PeriF perifoveal, RLRL repeated low-level red-light
*Between-group significant differences at P < 0.05
#Within-group significant differences with baseline at P < 0.05
Fig. 2.
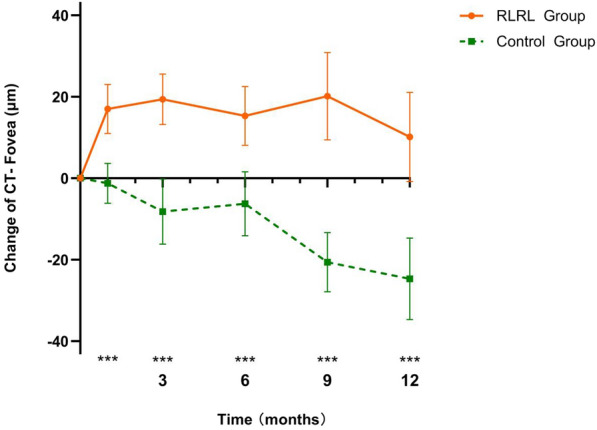
Adjusted mean changes in foveal CT at different time points in the control and RLRL groups. Asterisks indicate significant differences between groups at ***P < 0.05. CT Choroidal thickness, RLRL repeated low-level red-light
Regarding the vascular parameters of the choroid, significant increases in CVV were observed primarily in the ParaF and PeriF regions of the choroid in the RLRL group. In contrast, the control group exhibited a decrease in CVV in the PeriF region over 12 months (P < 0.05). Furthermore, notable elevations in CCPA were detected in the PeriF area of the choroid in the RLRL group at the 1-month (an increase of 0.40 mm2), 3-month (an increase of 0.25 mm2), and 12-month visits (an increase of 0.42 mm2) (all P < 0.05) (Table 3).
Table 3.
Cumulative adjusted changes in the choroidal vascular parameters across the entire macular region at different time points in the repeated low-level red-light therapy and control groups
| Time point/visit | Cumulative adjusted mean changes | P value | |
|---|---|---|---|
| RLRL group | Control group | ||
| Change in CVV-Fovea, mm3 | |||
| 1 Month | |||
| Mean ± SD | 0.00 ± 0.01 | − 0.00 ± 0.01 | 0.137 |
| Median (P25, P75) | 0.00 (− 0.00, 0.01) | − 0.00 (− 0.00, 0.01) | |
| 3 Months | |||
| Mean ± SD | 0.00 ± 0.01 | − 0.00 ± 0.01 | 0.072 |
| Median (P25, P75) | 0.01 (− 0.00, 0.01) | 0.00 (− 0.01, 0.01) | |
| 6 Months | |||
| Mean ± SD | 0.00 ± 0.01 | − 0.00 ± 0.01 | 0.013* |
| Median (P25, P75) | 0.00 (− 0.00, 0.01) | − 0.00 (− 0.01, 0.00) | |
| 9 Months | |||
| Mean ± SD | 0.00 ± 0.01 | − 0.01 ± 0.01 | 0.002* |
| Median (P25, P75) | 0.00 (− 0.00, 0.01) | − 0.00 (− 0.02, 0.00) | |
| 12 Months | |||
| Mean ± SD | − 0.01 ± 0.01 | − 0.01 ± 0.01 | 0.116 |
| Median (P25, P75) | − 0.00 (− 0.01, 0.01) | − 0.01 (− 0.02, − 0.00) | |
| Change in CVV-ParaF, mm3 | |||
| 1 Month | |||
| Mean ± SD | 0.02 ± 0.03 | − 0.02 ± 0.04 | < 0.001* |
| Median (P25, P75) | 0.02 (0.00, 0.04) | − 0.01(− 0.04, 0.00) | |
| 3 Months | |||
| Mean ± SD | 0.07 ± 0.06# | − 0.01 ± 0.07 | < 0.001* |
| Median (P25, P75) | 0.05 (0.03, 0.09)# | − 0.01(− 0.05, 0.03) | |
| 6 Months | |||
| Mean ± SD | 0.14 ± 0.08# | 0.03 ± 0.11 | < 0.001* |
| Median (P25, P75) | 0.13 (0.07, 0.20)# | 0.02 (− 0.03, 0.09) | |
| 9 Months | |||
| Mean ± SD | 0.21 ± 0.09# | 0.06 ± 0.14 | 0.001* |
| Median (P25, P75) | 0.20 (0.14, 0.31)# | 0.05 (− 0.01, 0.16) | |
| 12 Months | |||
| Mean ± SD | 0.28 ± 0.12# | 0.09 ± 0.21 | < 0.001* |
| Median (P25, P75) | 0.32 (0.16, 0.39)# | 0.09 (− 0.01, 0.19) | |
| Change in CVV-PeriF, mm3 | |||
| 1 Month | |||
| Mean ± SD | 0.15 ± 0.34# | − 0.02 ± 0.27 | 0.062 |
| Median (P25, P75) | 0.06 (− 0.07, 0.34)# | 0.01 (− 0.11, 0.08) | |
| 3 Months | |||
| Mean ± SD | 0.17 ± 0.28# | − 0.00 ± 0.25 | 0.003* |
| Median (P25, P75) | 0.13 (− 0.04, 0.33)# | 0.01 (− 0.20, 0.20) | |
| 6 Months | |||
| Mean ± SD | 0.17 ± 0.33# | − 0.03 ± 0.33 | 0.012* |
| Median (P25, P75) | 0.15 (− 0.07, 0.27)# | − 0.02 (− 0.24, 0.15) | |
| 9 Months | |||
| Mean ± SD | 0.16 ± 0.41# | − 0.13 ± 0.32# | < 0.001* |
| Median (P25, P75) | 0.09 (− 0.09, 0.31)# | − 0.08 (− 0.38, 0.08)# | |
| 12 Months | |||
| Mean ± SD | 0.00 ± 0.35 | − 0.19 ± 0.33# | 0.011* |
| Median (P25, P75) | 0.01 (− 0.20, 0.17) | − 0.24 (− 0.44, 0.03)# | |
| Change in CCPA-Fovea, mm2 | |||
| 1 Month | |||
| Mean ± SD | − 0.00 ± 0.02 | − 0.00 ± 0.02 | 0.240 |
| Median (P25, P75) | − 0.00 (− 0.01, 0.00) | 0.00 (− 0.01, 0.01) | |
| 3 Months | |||
| Mean ± SD | − 0.00 ± 0.02 | − 0.00 ± 0.01 | 0.628 |
| Median (P25, P75) | − 0.00 (− 0.01, 0.01) | − 0.00 (− 0.01, 0.01) | |
| 6 Months | |||
| Mean ± SD | − 0.00 ± 0.02 | − 0.00 ± 0.03 | 0.395 |
| Median (P25, P75) | 0.00 (− 0.01, 0.01) | − 0.00 (− 0.01, 0.01) | |
| 9 Months | |||
| Mean ± SD | − 0.00 ± 0.02 | 0.00 ± 0.04 | 0.430 |
| Median (P25, P75) | − 0.01 (− 0.01, 0.01) | − 0.00 (− 0.01, 0.01) | |
| 12 Months | |||
| Mean ± SD | 0.00 ± 0.01 | 0.01 ± 0.03 | 0.194 |
| Median (P25, P75) | 0.00 (− 0.01, 0.01) | 0.01 (− 0.01, 0.02) | |
| Change in CCPA-ParaF, mm2 | |||
| 1 Month | |||
| Mean ± SD | 0.04 ± 0.15 | − 0.03 ± 0.15 | 0.052 |
| Median (P25, P75) | 0.04 (− 0.05, 0.11) | − 0.02 (− 0.07, 0.02) | |
| 3 Months | |||
| Mean ± SD | − 0.00 ± 0.14 | − 0.02 ± 0.12 | 0.249 |
| Median (P25, P75) | − 0.00 (− 0.06, 0.08) | − 0.02 (− 0.07, 0.02) | |
| 6 Months | |||
| Mean ± SD | 0.00 ± 0.12 | − 0.02 ± 0.16 | 0.554 |
| Median (P25, P75) | 0.01 (− 0.07, 0.07) | − 0.01 (− 0.10, 0.05) | |
| 9 Months | |||
| Mean ± SD | 0.01 ± 0.13 | − 0.02 ± 0.17 | 0.349 |
| Median (P25, P75) | − 0.01 (− 0.09, 0.07) | − 0.04 (− 0.12, 0.08) | |
| 12 Months | |||
| Mean ± SD | − 0.01 ± 0.12 | − 0.01 ± 0.14 | 0.862 |
| Median (P25, P75) | − 0.03 (− 0.10, 0.07) | 0.01 (− 0.09, 0.08) | |
| Change in CCPA-PeriF, mm2 | |||
| 1 Month | |||
| Mean ± SD | 0.40 ± 1.21# | − 0.18 ± 0.99 | 0.002* |
| Median (P25, P75) | 0.48 (− 0.18, 1.17)# | − 0.13 (− 0.41, 0.21) | |
| 3 Months | |||
| Mean ± SD | 0.25 ± 0.92# | − 0.09 ± 0.75 | 0.061 |
| Median (P25, P75) | 0.27 (− 0.19, 0.80)# | − 0.12 (− 0.45, 0.27) | |
| 6 Months | |||
| Mean ± SD | 0.24 ± 0.78 | − 0.08 ± 0.85 | 0.011* |
| Median (P25, P75) | 0.21 (− 0.43, 0.77) | − 0.17 (− 0.62, 0.18) | |
| 9 Months | |||
| Mean ± SD | 0.20 ± 0.98 | − 0.02 ± 0.84 | 0.259 |
| Median (P25, P75) | 0.15 (− 0.27, 0.75) | − 0.10 (− 0.54, 0.41) | |
| 12 Months | |||
| Mean ± SD | 0.42 ± 0.81# | 0.01 ± 0.73 | 0.055 |
| Median (P25, P75) | 0.37 (− 0.07, 0.82)# | 0.03 (− 0.29, 0.53) | |
Data are presented as the mean ± standard deviation (SD) and as the median with the interquartile range (P25, P75) in parentheses, as appropriate
Adjusted estimates at each time point were calculated on the basis of generalized linear mixed model, adjusted for baseline age, gender, group, visit, group* visit, and corresponding baseline parameters as fixed factors, and subjects as the random factor
CCPA Choriocapillaris perfusion area, CVV choroidal vessel volume, P25, P75 25th percentile, 75th percentile, ParaF parafoveal region, PeriF perifoveal region, RLRL Repeated low-level red-light;
*Between-group significant difference at P < 0.05
#Within-group significant difference with baseline at P < 0.05
Changes in Structural and Vascular Parameters of the Retina at Follow-Up Visits
As presented in ESM Tables S1 and S2, throughout the 12-month duration of RLRL therapy, no differences were observed between the RLRL group and the control group regarding foveal RT and retinal vascular parameters (P > 0.05). In the perifoveal region, however, a notable reduction in retinal thickness, particularly in the outer RT, was evident in the control group over 12 months (P < 0.05), while such alterations were minimal or absent in the RLRL group.
Effect of RLRL Intervention on Myopia Incidence
Comparison of myopia incidence rates between the RLRL group and the control group over 12 months based on cycloplegic retinoscopy in shown in ESM Fig. S1. The 6-month incidence rate of myopia was notably lower in the RLRL group than in the control group (9.3% [4/43 children] vs. 21.4% [9/42 children], respectively), resulting in a substantial 57.9% reduction in incidence. Furthermore, at the 12-month mark, the children in RLRL group maintained a lower myopia incidence rate (20.9% [9/43 children] vs. 38.1% [16/42 children], respectively). The absolute mean difference between the two groups was calculated to be 17.2%, representing a 45.1% reduction in incidence.
Changes in SE, AL and Other Ophthalmic Parameters
In the RLRL group, the mean (SD) change in SE over a period of 12 months was – 0.11 (0.44) D. In contrast, the control group exhibited a mean (SD) change of – 0.42 (0.36) D. The absolute mean difference in SE change between the two groups was found to be – 0.31 D (P < 0.001) (Fig. 3), indicating a notable 73.8% reduction in SER shift. The 12-month SE changes for each group, as well as the mean difference between the RLRL and control groups, are presented in ESM Table S3. Furthermore, the mean (SD) increase in AL over 12 months was 0.09 (0.21) mm for the RLRL group, compared to 0.28 (0.12) mm for the control group. This resulted in an absolute mean difference in AL change of 0.19 mm (P < 0.001), representing a substantial 67.9% reduction in AL change (Fig. 4). The AL change at the 1-, 3-, 6-, 9-, and 12-month visits for each group, as well as the mean difference between the two groups, are also presented in ESM Table S3. Regarding the anterior segment parameters, no significant differences were observed between the control and RLRL group in terms of anterior chamber depth, flat keratometry, and lens thickness at different time points.
Fig. 3.
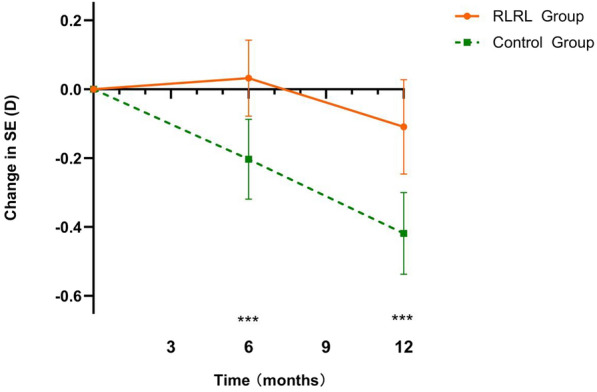
Adjusted mean changes in cycloplegic SE refractions at different time points in the control and RLRL therapy groups. Asterisks indicate significant differences between groups at ***P < 0.05. RLRL Repeated low-level red-light, SE spherical equivalent
Fig. 4.
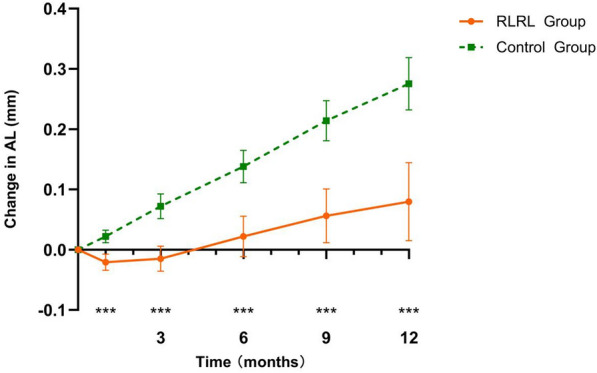
Adjusted mean changes in AL at different time points in the control and RLRL groups. Asterisks indicate significant differences between groups at ***P < 0.05. AL Axial length, RLRL repeated low-level red-light
Prediction of Longitudinal Changes in AL and SE
Multiple regression analysis was performed to explore the factors associated with changes in AL and SE over the 12 months following RLRL therapy. The statistical analysis demonstrated that the changes in AL (standardized coefficient β = − 0.571, P < 0.001) and SE (standardized coefficient β = 0.467, P = 0.008) were significantly correlated with the thickening of the foveal choroid over 12 months but not with baseline AL/SE, baseline fovea CT, age, or gender (Table 4).
Table 4.
Multiple regression analysis for factors associated with changes in axial length and spherical equivalent over 12 months in repeated low-level red-light therapy group
| Factors | Regression coefficient β | Standardized coefficient β | P value |
|---|---|---|---|
| Change in AL | |||
| Change in fovea CT, per 10 mm | − 0.03 | − 0.571 | < 0.001* |
| Baseline FCT, per 10 mm | 0.01 | 0.154 | 0.271 |
| Age, per 1 year | − 0.023 | − 0.152 | 0.279 |
| Baseline AL, per 1 mm | 0.010 | 0.043 | 0.800 |
| Male vs. female | 0.075 | 0.182 | 0.299 |
| Change in SE | |||
| Change in fovea CT, per 10 mm | 0.060 | 0.467 | 0.008* |
| Baseline FCT, per 10 mm | − 0.01 | − 0.116 | 0.447 |
| Age, per 1 year | 0.020 | 0.065 | 0.667 |
| Male vs. female | − 0.111 | − 0.127 | 0.443 |
| Baseline SE, per 1 D | − 0.060 | − 0.046 | 0.778 |
The coefficient of determination (R2) was 0.40 for change in AL and 0.26 for change in SE
*P < 0.05 after adjusted for age, gender, baseline AL/SE, baseline fovea CT and change in fovea CT over 12 months
AL Axial length, CT choroidal thickness, D Diopter, RLRL repeated low-level red-light, SE spherical equivalent, FCT foveal choroidal thickness
Discussion
Evaluating the effects of myopia on the retinal and choroidal structure and vasculature in children is crucial as myopia often appears and advances during childhood. To our knowledge, this was the first trial to date to assess the impact of the RLRL therapy on the retinal and choroidal structure and vasculature in children with premyopia.
The mechanism underlying how RLRL controls myopia progression is not fully understood, although several previous studies have shown a negative correlation between the CT and the severity of axial myopia [21–25]. A growing body of evidence suggests that RLRL exposure increases CT, potentially improving choroidal blood flow and controlling myopia. However, only four studies have analyzed changes in CT after RLRL exposure [15, 26–28], and previous studies were characterized by a number of limitations such as short follow-up times, single measuring point, lack of control groups, and subjective bias. In the present study, the automatic segmentation and measurement of subjects’ macular retinal and choroidal thicknesses and vascular parameters across the entire macular region were performed using the built-in software of an OCT instrument to evaluate the effect of using RLRL on children with premyopia.
In the present study, we observed thickening of the choroid across the entire wide-field region at all five follow-up visits for the RLRL group. The greatest magnitude of foveal CT changes was observed at the 3-month visit (an increase of 19.58 μm) with a slight reduction in the extent of foveal CT increase at the 6-month visit (an increase of 15.85 μm). The second greatest CT increase was observed at the 9-month visit (an increase of 19.57 μm), after which the extent of CT increase gradually decreased until the end of the study at the 12-month visit (an increase of 11.99 μm). In comparison, the authors of a clinical trial of 120 children with myopia aged 8 to 13 years with a SE of 2.30 ± 0.85 D observed that macular CT increased to its maximum at 1 month of RLRL therapy (an increase of 14.755 μm), followed by a reduction in the magnitude of thickening at 3 months (an increase of 5.286 μm) and 6 months (an increase of 1.543 μm, then a steady increase at 12 months (an increase of 9.089 μm) [19]. These findings suggest that the magnitude of CT changes observed in children with premyopia was greater than those previously reported in children with myopia and that this population may experience a more accelerated phase of choroidal thickening after RLRL therapy, rather than maintenance of thickness at a steady level. Notably, measuring foveal CT alone is insufficient to comprehensively reflect changes in the choroid during myopia progression. Jin’s study indicated that peripheral choroidal thinning may precede the development of peripheral hyperopia and serve as a sensitive predictor of myopia progression [29]. Moreover, decreases in CT in the ParaF and PeriF regions have been associated with myopia development. Results from previous studies have also suggested that the PeriF choroid is more susceptible to attenuation compared to the ParaF and central choroid, implying that choroidal thinning may initiate peripherally during the early stages of myopia development [30]. In our study, we observed a significant increase in CT in the ParaF and PeriF areas in the RLRL group over 12 months. This thickening of the choroid in these areas may result in subtle relative peripheral myopic defocus, which in an earlier study was recognized as a protective factor against myopia progression [30].
Since the AL was measured as the distance from the cornea to the RPE using an IOL-Master device, a thickened choroid may decrease the AL by pushing the retina forward. In the present study, we observed a slight hyperopic shift and AL shortening at the early stage of RLRL therapy accompanied by a steady thickening of the foveal CT during subsequent RLRL therapy sessions. These findings indicated that the changes in foveal CT and AL or SE were not uniform throughout the follow-up period. Moreover, no significant differences in other anterior segment parameters were observed between pre-treatment and post-treatment, suggesting that the attenuation of AL and SE was not attributable to alterations in the structure of the anterior segment comprising the cornea, anterior chamber and lens. This irregularity implies that, in addition to the attenuation of axial elongation resulting from choroidal thickening, other physiological modifications in the choroid associated with myopia may also contribute to the observed differences in axial elongation between the two groups.
Findings from clinical studies are increasingly suggesting that the choroid plays an important role in the regulation of eye growth and the development of myopia. Located between the retina and sclera, the choroid is a highly vascularized layer that is instrumental in modulating scleral metabolism during ocular development influenced by visual cues and also plays an active role in emmetropization or the pathogenesis of myopia [31, 32]. Given that hypoxia has been linked to myopia and that choroidal blood flow serves as the primary source of oxygen and nourishment [33], it is conceivable that RLRL therapy could alleviate scleral ischemia and hypoxia by augmenting choroidal blood flow, leading to increased scleral thickness and hardness. This, in turn, may prevent the progression of myopia and inhibit excessive axial elongation.
To investigate this hypothesis, we conducted an assessment of choroidal vasculature parameters prior to and following RLRL treatment. Our findings revealed a consistent elevation in ParaF and PeriF choroidal vascular volume (CVV), indicating that augmented blood flow within the choroidal luminal, a primary constituent of the choroid, may contribute to choroidal thickening. Moreover, we observed an increase in choriocapillaris blood perfusion during follow-up visits, with the most pronounced enhancement occurring at the 1-month visit, specifically in the PeriF region; subsequent increments were less prominent at later follow-up visits, suggesting that the augmentation of choriocapillaris blood perfusion in peripheral areas primarily occurred during early therapy. We hypothesize that this may be attributed to the heightened sensitivity of the small-diameter choroidal vessels within the choriocapillaris to the warm effects of red light, particularly in peripheral regions, during the initial stages of treatment.
In addition to modifications in choroid structure and function, accumulating evidence indicates that the retina plays a crucial role in regulating ocular growth and the development of myopia. However, the relationship between retinal thickness (RT) and the progression of myopia in children remains unclear, as previous studies have reported conflicting findings. In a separate study of adults, individuals aged > 40 years with longer AL (> 24 mm) were associated with increased RT in the foveal, PeriF, and posterior pole regions [34]. Conversely, another investigation of Chinese children aged 11 to 12 years reported an increase in foveal RT but a decrease in ParaF and PeriF RT with increasing AL [35]. The absence of a significant correlation between foveal RT and AL was also observed in adults aged between 16 and 35 years [36]. While findings in the foveal region remain open to discussion, consistent retinal thinning in the ParaF and PeriF areas as a function of AL may result from decreased resistance to mechanical stretching of the retina, a reduction in the number of photoreceptors, or even chorioretinal atrophy in highly myopic eyes [35, 37, 38]. In this study, we noted that although no differences in changes of foveal RT and foveal vascular parameters were evident between the RLRL group and the control group over 12 months, the control group exhibited significant decreases in the PeriF area specifically in the outer RT during myopia onset and progression, while such changes were less pronounced or absent in the RLRL group during follow-up visits. We hypothesize that RLRL therapy maintains the structural and functional integrity of the PeriF retina against mechanical stretch induced by excessive axial elongation, potentially by enhancing choroidal blood perfusion, which serves as the primary blood supply for the outer retina.
In addition to assessing the impact of RLRL on the structural and vascular characteristics of the choroid and retina, we also investigated the factors associated with axial elongation and myopia progression in children with premyopia. Recent evidence suggests that RLRL therapy may be an effective and promising alternative treatment for myopia control [16]. However, the literature on medical interventions to prevent the onset of myopia is still limited. While high concentrations of atropine (0.025% or 0.05%) have been observed to prevent myopia onset and myopic shift in premyopic school children, these treatments are often accompanied by such side effects as photophobia, blurred near vision and concerns about long-term ocular or systemic diseases [39, 40]. In addition, a recent study indicated that low-concentration atropine (0.01%) with slight side effects did not significantly differ from placebo in a premyopic pediatric population [40]. Increased outdoor time has also been consistently shown to have a preventive impact on myopia onset, with an efficacy rate ranging from 11.0% to 54.3% within 1 year [41–43]. However, it is worth noting that our study population differed from those in these outdoor trials, as we enrolled children with premyopia with a higher risk of developing myopia. Our results demonstrated that RLRL therapy achieved a notable reduction in SE shift by 73.8%, a substantial decrease in AL changes by 67.9% and a significant reduction in myopia incidence by 45.1% within 1 year, suggesting that this therapy could be initiated in children without myopia who are at high risk of developing myopia as a strategy to potentially delay its onset. In this study, the results from a multiple linear regression analysis showed a significant correlation between the progression of myopia or axial elongation and the thickening of the foveal choroid over 12 months in children with premyopia aged between 7 and 12 years receiving RLRL therapy. However, factors such as age, gender, AL, and foveal CT prior to treatment did not exhibit a notable correlation, suggesting that the primary mechanism underlying the effectiveness of RLRL therapy in controlling myopia may be partially mediated through choroidal thickening (adjusted R2 in linear regression models was 40% and 26% for AL and SE changes over 12 months, respectively). These observations once again highlight the specific role of choroidal signaling pathways in inducing modifications that mitigate scleral hypoxia and remodeling, ultimately aiding in the effective prevention of myopia among populations with premyopia.
Our study has a few limitations. First, the study was conducted in a single center with a relatively small sample size, which may limit the generalizability of the findings. Secondly, the 12-month follow-up period may not be sufficiently long for a comprehensive evaluation of the long-term effects of RLRL therapy on choroidal and retinal parameters. Thirdly, it is essential to conduct additional validation studies in order to ascertain the predictive efficacy of our model in many demographics and contexts. This will provide valuable clinical information that may effectively inform the treatment of myopia in susceptible individuals. Finally, a rebound in AL growth has been reported in children. Chen et al. reported an increase in AL by 0.16 mm 3 months after cessation of RLRL therapy in children with myopia [27]. However, the fluctuations in measured choroidal parameters after discontinuation of RLRL remain unknown. It would be advisable to conduct studies to investigate this potential rebound effect and to establish appropriate management strategies in the future.
Conclusion
In summary, our study demonstrated that a significant increase in CT and flow in eyes receiving RLRL treatment at the 12-month visit. Combined with the reduction in SE progression and axial elongation, RLRL could be used as an effective therapy for preventing progression in premyopes. However, further studies with a larger sample size and a long-term follow-up are needed to establish its use in this population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the children and their parents or guardians who participated in the study.
Authorship
All named authors meet the International Committee of Medical Journal. Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Zhuzhu Liu made leading contributions to the acquisition, analysis, and interpretation of data. Ziwen Sun made supporting contributions to the analysis and interpretation of data. Bei Du made leading contributions to the provision of the equipment used in the work. Huaixue Gou made leading contributions to the provision of the software used in the work. Biying Wang made supporting contributions to the provision of the equipment used in the work. Zeya Lin and Nuo Ren made supporting contributions to the provision of the software used in the work. Emmanuel Eric Pazo made supporting contributions to the revision of manuscript. Lin Liu made supporting contributions to the conception and design of the work. Ruihua Wei made leading contributions to the conception and design of the work. All authors read and approved the final manuscript.
Funding
This study and its publication, including the journal’s Rapid Service Fee was supported by the National Natural Science Foundation of China, Grant/Award Number: 82070929.
Data Availability
The datasets generated/analyzed during the current study are available from the first author on reasonable request.
Declarations
Conflict of Interest
Zhuzhu Liu, Ziwen Sun, Bei Du, Huaixue Gou, Biying Wang, Zeya Lin, Nuo Ren, Emmanuel Eric Pazo, Lin Liu, Ruihua Wei declare no competing interests.
Ethical Approval
This study was reviewed and approved by The Ethics Committee of Tianjin Medical University Eye Hospital (NO.2021KY-11). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin, and the study adhered to the principles of the Declaration of Helsinki.
Footnotes
Zhuzhu Liu and Ziwen Sun contributed equally to this work and share first authorship.
Contributor Information
Lin Liu, Email: liulin_117@126.com.
Ruihua Wei, Email: rwei@tmu.edu.cn.
References
- 1.Teasdale T, Goldschmidt E. Myopia and its relationship to education, intelligence and height. Preliminary results from an on-going study of Danish draftees. Acta Ophthalmol. 1988;185:41–43. doi: 10.1111/j.1755-3768.1988.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 2.Fledelius H. Myopia prevalence in Scandinavia. A survey, with emphasis on factors of relevance for epidemiological refraction studies in general. Acta Ophthalmol. 1988;185:44–50. doi: 10.1111/j.1755-3768.1988.tb02661.x. [DOI] [PubMed] [Google Scholar]
- 3.Fredrick D. Myopia. BMJ. 2002;324(7347):1195–1199. doi: 10.1136/bmj.324.7347.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang C, Yen E, Su J, et al. Impact of family history of high myopia on level and onset of myopia. Investig Ophthalmol Vis Sci. 2004;45(10):3446–3452. doi: 10.1167/iovs.03-1058. [DOI] [PubMed] [Google Scholar]
- 5.Hsu W, Cheng C, Liu J, et al. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111(1):62–69. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Iwase A, Araie M, Tomidokoro A, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113(8):1354–1362. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113(7):1134.e1131–1111. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Drobe B, de Saint-André R. The pre-myopic syndrome. Ophthalmic Physiol Optics. 1995;15(5):375–378. doi: 10.1046/j.1475-1313.1995.9500075o.x. [DOI] [PubMed] [Google Scholar]
- 9.Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93(8):997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- 10.Guggenheim J, Pong-Wong R, Haley C, et al. Correlations in refractive errors between siblings in the Singapore cohort study of risk factors for myopia. Br J Ophthalmol. 2007;91(6):781–784. doi: 10.1136/bjo.2006.107441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones L, Sinnott L, Mutti D, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Investig Ophthalmol Vis Sci. 2007;48(8):3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutti D, Mitchell G, Moeschberger M, et al. Parental myopia, near work, school achievement, and children's refractive error. Investig Ophthalmol Vis Sci. 2002;43(12):3633–3640. [PubMed] [Google Scholar]
- 13.Rose K, Morgan I, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Saw S, Chua W, Hong C, et al. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. 2002;43(2):332–339. [PubMed] [Google Scholar]
- 15.Xiong F, Mao T, Liao H, et al. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. BioMed Res Int. 2021;2021:8915867. doi: 10.1155/2021/8915867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129(5):509–519. doi: 10.1016/j.ophtha.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Chen W, Zhao F, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci USA. 2018;115(30):E7091–E7100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metlapally R, Wildsoet C. Scleral mechanisms underlying ocular growth and myopia. Prog Mol Biol Transl Sci. 2015;134:241–248. doi: 10.1016/bs.pmbts.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong R, Zhu Z, Jiang Y, et al. Longitudinal changes and predictive value of choroidal thickness for myopia control after repeated low-level red-light therapy. Ophthalmology. 2023;130(3):286–296. doi: 10.1016/j.ophtha.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Nickla D, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin P, Zou H, Zhu J, et al. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol. 2016;168:164–176. doi: 10.1016/j.ajo.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Vincent S, Collins M, Read S, et al. Retinal and choroidal thickness in myopic anisometropia. Invest Ophthalmol Vis Sci. 2013;54(4):2445–2456. doi: 10.1167/iovs.12-11434. [DOI] [PubMed] [Google Scholar]
- 23.Wei W, Xu L, Jonas J, et al. Subfoveal choroidal thickness: the Beijing eye study. Ophthalmology. 2013;120(1):175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 24.Read S, Collins M, Vincent S, et al. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(12):7578–7586. doi: 10.1167/iovs.13-12772. [DOI] [PubMed] [Google Scholar]
- 25.Tan C, Cheong K. Macular choroidal thicknesses in healthy adults–relationship with ocular and demographic factors. Invest Ophthalmol Vis Sci. 2014;55(10):6452–6458. doi: 10.1167/iovs.13-13771. [DOI] [PubMed] [Google Scholar]
- 26.Tian L, Cao K, Ma D, et al. Investigation of the efficacy and safety of 650 nm low-level red light for myopia control in children: a randomized controlled Trial. Ophthalmol Ther. 2022;11(6):2259–2270. doi: 10.1007/s40123-022-00585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Wang W, Liao Y, et al. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: a randomized controlled trial. Graefe's Arch Clin Ophthalmol. 2023;261(2):575–584. doi: 10.1007/s00417-022-05794-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, Xing C, Qiang W, et al. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic Physiol Opt. 2022;42(2):335–344. doi: 10.1111/opo.12939. [DOI] [PubMed] [Google Scholar]
- 29.Jin P, Zou H, Xu X, et al. Longitudinal changes in choroidal and retinal thicknesses in children with myopic shift. Retina. 2019;39(6):1091–1099. doi: 10.1097/iae.0000000000002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charman WN, Radhakrishnan H. Peripheral refraction and the development of refractive error: a review. Ophthalmic Physiol Opt. 2010;30(4):321–338. doi: 10.1111/j.1475-1313.2010.00746.x. [DOI] [PubMed] [Google Scholar]
- 31.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 32.Summers J. The choroid as a sclera growth regulator. Exp Eye Res. 2013;114:120–127. doi: 10.1016/j.exer.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Zhang S, Zhang G, et al. Increased choroidal blood perfusion can inhibit form deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2020;61(13):25. doi: 10.1167/iovs.61.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan C, Yu J, Chen L, et al. Posterior pole retinal thickness measurements by the retinal thickness analyzer in healthy Chinese subjects. Retina. 2006;26(2):176–181. doi: 10.1097/00006982-200602000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Luo H, Gazzard G, Fong A, et al. Myopia, axial length, and OCT characteristics of the macula in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47(7):2773–2781. doi: 10.1167/iovs.05-1380. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z, Zhou X, Jiang C, et al. Effects of myopia on different areas and layers of the macula: a Fourier-domain optical coherence tomography study of a Chinese cohort. BMC Ophthalmol. 2015;15:90. doi: 10.1186/s12886-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, He X, Zhu J, et al. Macular measurements using optical coherence tomography in healthy Chinese school age children. Invest Ophthalmol Vis Sci. 2011;52(9):6377–6383. doi: 10.1167/iovs.11-7477. [DOI] [PubMed] [Google Scholar]
- 38.Wu P, Chen Y, Chen C, et al. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye (Lond) 2008;22(4):551–555. doi: 10.1038/sj.eye.6702789. [DOI] [PubMed] [Google Scholar]
- 39.Fang P, Chung M, Yu H, et al. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocular Pharmacol Ther. 2010;26(4):341–345. doi: 10.1089/jop.2009.0135. [DOI] [PubMed] [Google Scholar]
- 40.Yam J, Zhang X, Zhang Y, et al. Effect of low-concentration atropine eyedrops vs placebo on myopia incidence in children: the LAMP2 randomized clinical trial. JAMA. 2023;329(6):472–481. doi: 10.1001/jama.2022.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in china: a randomized clinical trial. Jama J Am Med Assoc. 2015;314(11):1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 42.Jin J, Hua W, Jiang X, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun eye care study. BMC ophthalmol. 2015;15:73. doi: 10.1186/s12886-015-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X, Sankaridurg P, Wang J, et al. Time outdoors in reducing myopia: a school-based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology. 2022;129(11):1245–1254. doi: 10.1016/j.ophtha.2022.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated/analyzed during the current study are available from the first author on reasonable request.