Abstract
Inbred strains of mice vary in their susceptibility to Coccidioides immitis. We infected resistant DBA/2 (D2) mice and three susceptible strains of mice (C57BL/6 [B6], BALB/c, and CAST/Ei) by intraperitoneal injection of arthroconidia and determined the severity of infection based on colony counts of fungus in the spleens and lungs 14 days after infection. We used quantitative reverse transcription-PCR to measure the amounts of cytokines made in the spleens and lungs of infected mice. Susceptible mice made 1,000-fold more interleukin-10 (IL-10) than resistant D2 mice and about 10-fold more IL-4. In contrast, D2 mice had more IL-12 p40 in their lungs than did B6 mice. Resistant and susceptible mice made equivalent amounts of gamma interferon, IL-6, and IL-2. In order to determine whether IL-10 adversely affected the response to C. immitis, we infected IL-10-deficient mice, and they were found to be as resistant as D2 mice. This result indicates that IL-10 plays a crucial role in determining susceptibility to C. immitis in inbred mice. Because IL-4 mRNA levels were higher in most strains of susceptible mice, we also infected IL-4-deficient B6 mice. They were more resistant than B6 controls but not as resistant as IL-10-deficient mice. Thus, both IL-10 and IL-4 adversely affect the ability of C57BL mice to resist infection with C. immitis, but IL-10 has a larger effect and is the cytokine that is consistently associated with susceptibility in all strains of inbred mice.
Coccidioidomycosis is endemic in the southwestern United States in areas where Coccidioides immitis, the etiologic agent, grows in the desert soil. It is estimated that 75% of long-term residents in the area where the disease is highly endemic are infected (13). The vast majority of those infections are either asymptomatic or self-limited, but between 5 and 10% of infections result in progressive pulmonary or disseminated disease (5). People who have self-limited infections make little or no antibody to the fungus, but they develop delayed hypersensitivity as manifested by a positive skin test reaction to fungal antigens. The more antibody patients make, the worse their prognosis (33). This suggests that susceptibility to C. immitis is associated with a Th2 immune response to infection.
Not all people infected have the same risk of dissemination; progressive infection is about 100-fold more likely in Filipinos and African Americans than in Caucasians, implying that there is a genetic basis for susceptibility in people (reviewed in reference 8). We found a similar variation in susceptibility to C. immitis infection among inbred strains of mice (17), a finding that was confirmed by Cox et al. (3). DBA/2 (D2) is the most resistant mouse strain, and resistance is the dominant phenotype. Analysis of a backcross between D2 and BALB/c mice suggested that a single gene accounted for the difference in resistance (18).
We undertook this study in order to determine whether there was a correlation between resistance to C. immitis and the type of cytokine response that was made. In this study, we found that susceptible strains (BALB/c, C57BL/6 [B6], and CAST/Ei) made more interleukin-10 (IL-10) and IL-4 mRNA than did resistant strains. Mice with a mutated IL-10 gene were highly resistant to C. immitis, and IL-4-deficient B6 mice were moderately resistant compared to susceptible B6 controls.
MATERIALS AND METHODS
Mice.
All mice were purchased from Jackson Laboratory, Bar Harbor, Maine, including breeding pairs of the IL-10 knockout (KO) mice, which were fifth generation bred onto a C57BL/10 background (21). The IL-10 KO mice were bred in our own facilities under pathogen-free conditions. IL-4 KO mice were ninth generation backcrossed onto B6 (19). Only female mice were infected because male mice are more susceptible to C. immitis (7a). Mice were allowed free access to food and water and were housed in a HEPA-filtered isolation unit after they were infected.
Infection.
The RS strain of C. immitis was used in all experiments (17). The fungus was grown at room temperature on Mycophil agar (BBL, Cockeysville, Md.) to full maturity, and then the mold was washed with saline to release arthroconidia. The hyphal suspension was gently disrupted with glass beads until almost entirely individual arthroconidia were obtained, and the suspension was stored at 4°C. Mice were injected intraperitoneally (i.p.) with 500 to 1,000 arthroconidia in 0.2 ml of saline. The inoculum size was confirmed by culture.
At necropsy, the spleen and right lung were removed and homogenized as previously described (6). The spleen was divided in half before homogenization. Serial dilutions of the homogenized organs were plated on Mycosel agar (BBL) and incubated at room temperature. Colonies of mold were counted before they sporulated. The lower limit of detection was 5 × 101 CFU/spleen and 1 × 102 CFU/lung. We assigned dead mice the value of 5 × 106 CFU/lung, the mean value found in moribund mice.
mRNA quantification.
Organs were removed expeditiously, and the left lung and a portion of the spleen were immediately frozen in liquid nitrogen for later processing. Frozen tissue was homogenized in Ultraspec RNA reagent (Biotex Laboratories Inc., Houston, Tex.). Following homogenization, RNA was extracted according to the manufacturer’s instructions (Biotex bulletin 27). The pellets of RNA were resuspended in RNase-free Tris-buffered (10 mM) EDTA (1 mM) at pH 8.0, extracted once with an equal volume of acid phenol (pH 4.3), reprecipitated, and resuspended in RNase-free water. Extracted RNA was intact as assessed by agarose gel electrophoresis and ethidium bromide staining. The amount of cytokine-specific mRNA in the extracted RNA was determined by quantitative reverse transcription-PCR (RT-PCR) as previously reported (6). mRNA for β-actin was also amplified to control for the efficiency of extraction of RNA from different mouse strains. Equal amounts of RNA from three or four mice in each group were pooled to obtain mean values.
IL-10 measurement.
Spleens and lungs were removed from mice 14 days after i.p. infection. Single-cell suspensions were prepared and placed in Costar wells at a density of 2 × 107/ml for spleen cells and 1 × 107/ml for lung cells in 0.5 ml of RPMI with 5% fetal calf serum added. After 6 h of incubation at 37°C, the supernatants were removed and stored at −70°C for later use. IL-4 and IL-10 in the supernatants were measured by a capture enzyme-linked immunosorbent assay with a sensitivity of 20 pg/ml (R&D, Minneapolis, Minn.).
Statistical analysis.
Differences between groups of mice were compared by the Mann-Whitney two-tailed U test and considered significantly different at P ≤ 0.05. Analysis was performed with SPSS version 7.5.1 software.
RESULTS
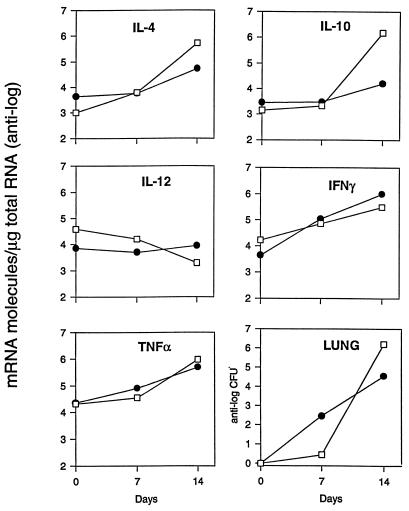
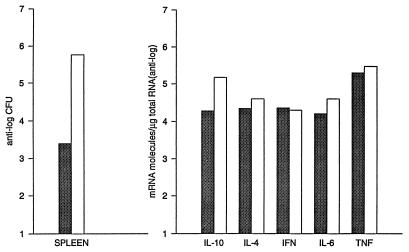
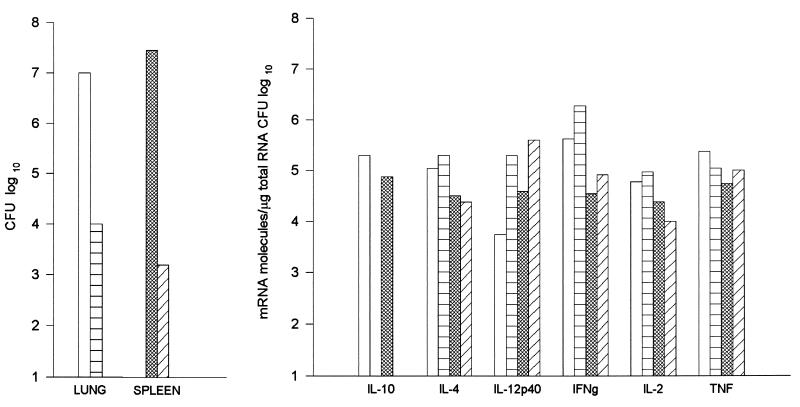
We first determined the time course of infection and compared it to the time course of cytokine induction in B6 and D2 mice. Mice were infected i.p. with arthroconidia and sacrificed 7 and 14 days later. On day 7 after infection, all the mice had normal-appearing lungs and only one of four mice of each strain had detectable fungus in their lungs (>102 CFU). As shown in Fig. 1, on day 14 B6 mice had a mean of 1.6 × 106 CFU/lung, whereas D2 mice had a mean of only 3.6 × 104 CFU/lung. On day 7, infection was detectable in the spleens of all four B6 mice and two of four D2 mice, but the numbers of CFU were very low in all mice (<103 CFU/spleen) and there was not a significant difference between the two mouse strains (data not shown). On day 14, the geometric mean number of CFU in spleens of B6 mice was 6.7 × 105, but the value for D2 mice was only 3 × 103 (Fig. 2).
FIG. 1.
Comparison of the cytokine mRNA levels and colony counts in the lungs of B6 and D2 mice infected i.p. with C. immitis. Four mice from each strain were sacrificed on days 7 and 14 after infection. On day 7, only one of four mice in each group had detectable amounts of fungus in their lungs (>102 CFU). We pooled equal amounts of RNA from the four infected mice in each experimental group to assay the cytokine mRNA levels. Thus, the values shown are the means for four mice. Closed circles, D2; open squares, B6.
FIG. 2.
Comparison of cytokine-specific mRNA levels and colony counts from spleens of B6 and D2 mice infected 14 days before with C. immitis. The CFU in spleens are the geometric means for 10 mice. The cytokine levels were determined from a mixture of equal amounts of RNA from four mice in each group. The mice that were selected all had approximately the same severity of infection (CFU differed by ± 1 standard deviation from the geometric mean for the group). Shaded bars, D2; open bars, B6.
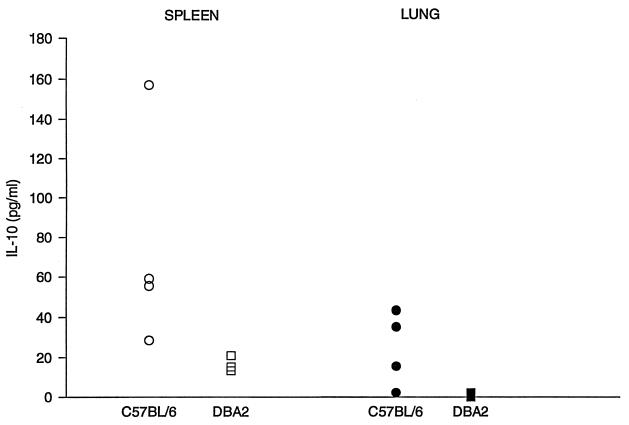
We measured mRNA levels in the lungs on days 7 and 14 after infection (Fig. 1). On day 7, when the gross appearance of the lungs was normal, there was very little increase over background in the amount of mRNA for IL-10, IL-4, or IL-12 p40 in either mouse strain. There was a small increase in the amounts of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) mRNAs in both strains of mice, but levels were nearly identical in the two strains. On day 14, however, there were striking differences between the cytokine responses in the resistant and susceptible strains. Susceptible B6 mice had approximately 1,000 times more IL-10 and 10 times more IL-4 mRNA than D2 mice. For both those cytokines, the day 14 levels in B6 mice were at least 100 times higher than they were on day 7. In D2 mice, the IL-10 mRNA level increased only about 3-fold and the IL-4 mRNA level increased about 20-fold from day 7 to day 14. Levels of IL-12 p40 mRNA were about five times higher in D2 mice than in B6 mice on day 14, but the disparity between the IL-12 p40 levels in the two strains was due primarily to a decrease from baseline levels in B6 mice; on day 14, IL-12 p40 levels were 10-fold lower than they were in uninfected lungs. The strains had equivalent amounts of TNF-α and IFN-γ. There was no difference in the amounts of β-actin mRNA from the two strains (data not shown). In the spleen, an organ that is also infected hematogenously, B6 mice had 10 times more IL-10 mRNA than D2 mice on day 14. However, there was no more than a twofold difference in the amounts of IL-2, IL-4, IL-6, IFN-γ, or TNF-α in the spleens of the two strains (Fig. 2). We also measured the amount of IL-10 and IL-4 that was secreted by lung and spleen cells from infected mice, using a sensitive capture enzyme-linked immunosorbent assay. As shown in Fig. 3, we found more IL-10 to be made by spleen and lungs cells from B6 mice than by cells from D2 mice; IL-4 was not detected from cells of either strain.
FIG. 3.
Comparison of amounts of IL-10 made by B6 and D2 mice. The spleen and one lung were removed from mice 14 days after infection and made into single-cell suspensions. Lung cells (1 × 107) and spleen cells (2 × 107) were placed in 12- and 24-well Costar plates, respectively, and incubated at 37°C for 6 h in RPMI 3containing 5% endotoxin-free fetal calf serum. Supernatants were frozen at −70°C until assayed with a kit from R&D Systems.
In order to be sure that the association between high levels of IL-10 and IL-4 and susceptibility to infection was not unique to B6 mice, we infected two additional susceptible strains (BALB/c and CAST/Ei) (Table 1). The BALB/c mice had approximately the same 2-week mortality, and survivors had numbers of CFU per lung that were similar to those for B6 mice, while the CAST/Ei strain was even more susceptible than the B6 strain (9 of 12 deaths versus 2 of 8 deaths in B6 mice by day 14). We did not obtain exact numbers of CFU per lung from the three surviving CAST/Ei mice because we did not make enough dilutions to reach an end point, but we can say they had >2 × 106 CFU/lung. As shown in Table 1, all the susceptible strains also had 20 to 100 times more IL-10 and 4 to 20 times more IL-4 than the D2 mice. However, while all three susceptible strains had >10 times the level of IL-10 mRNA that D2 mice had, for IL-4 mRNA that degree of disparity was found only in the BALB/c strain, which was not the most susceptible strain. The CAST/Ei strain was the most susceptible strain, and these mice had only four times as much IL-4 mRNA relative to D2 mice. In this experiment there was no significant difference in the amounts of IL-6, IL-12 p40, or IFN-γ mRNAs made by the different strains.
TABLE 1.
Cytokine mRNA levels in the lungs of four strains of inbred mice 14 days after infection with C. immitisa
| Cytokine | Valueb for mouse
strain:
|
|||
|---|---|---|---|---|
| D2 | B6 | BALB/c | CAST/Ei | |
| IL-10 | 5.2 × 103 | 4.2 × 105 | 1.1 × 105 | 1.9 × 105 |
| IL-4 | 1.7 × 104 | 6.2 × 104 | 3.5 × 105 | 9.0 × 104 |
| IL-6 | 1.6 × 105 | 3.2 × 105 | NDc | ND |
| IL-2 | 5.0 × 103 | 4.0 × 104 | ND | ND |
| IL-12 p40 | 6.0 × 104 | 1.4 × 104 | 5 × 104 | 6.2 × 104 |
| IFN-γ | 1.7 × 105 | 1.1 × 105 | 9.0 × 105 | 8.5 × 105 |
| TNF-α | 1.5 × 105 | 1.0 × 106 | 5.6 × 105 | 6.4 × 105 |
CFU in lungs were as follows: D2, 8.6 × 103; B6, 2 × 106; BALB/c, 8.1 × 105, CAST/Ei, >2 × 106. Values are geometric means for surviving mice; there were 10 mice in each group initially.
Number of mRNA molecules per microgram of RNA from four mice.
ND, not determined.
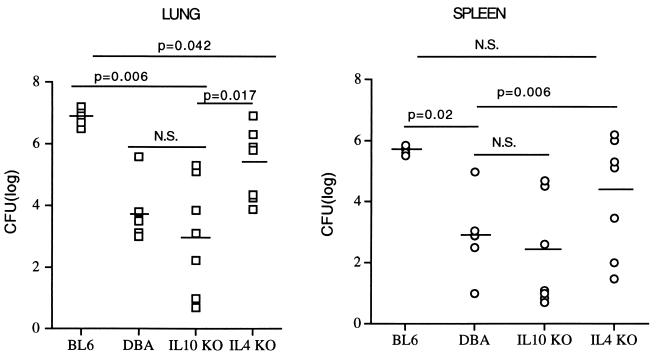
We then infected C57BL mice that had a KO mutation in either the IL-10 or the IL-4 gene, which renders them IL-10 or IL-4 deficient (19, 21). Although both B6 and B10 mice are highly susceptible to C. immitis (17), the IL-10 KO mice were highly resistant and the IL-4 KO mice were moderately resistant to infection. The control B6 mice had extensive pneumonia, and in this experiment 5 of 14 (36%) died before day 14. In contrast, 0 of 17 IL-10 KO mice died and 3 of 10 IL-4 KO mice died. The differences in mortality were also reflected in the extent of the pneumonia in surviving mice (Fig. 4). There was a mean of 1.0 × 107 CFU/lung in B6 mice compared to 2.3 × 105 CFU/lung in IL-4 KO mice and only 1.0 × 104 CFU/lung in the IL-10 KO mice. The numbers of CFU per lung in the IL-10 KO mice were significantly lower than levels for both the B6 and IL-4 KO groups and not significantly different from those for the D2 mice (Fig. 4). The lung CFU counts in the IL-4 KO mice were significantly lower than those in the B6 mice but also higher than those in the D2 mice. The differences in spleen CFU counts between the groups were similar to what was found with lung CFU counts, except that the IL KO spleen CFU were not significantly lower than the CFU in the B6 mice (P = 0.5).
FIG. 4.
Colony counts in spleens and lungs of B6, D2, and IL-4 and IL-10 KO mice. Each datum point represents the CFU from an individual mouse. The geometric means are shown as bars. The values for the IL-4 KO mice are from the seven surviving animals. The values shown for the B6 mice include those for the one survivor (699 CFU/lung and 5.58 CFU/spleen); the others are based on mean values for moribund B6 mice. The P values shown were determined with the two-tailed Mann-Whitney U test (see Materials and Methods). N.S., not significant; DBA, D2 mice.
Of interest, the cytokine mRNA levels in the IL-10 KO mice were also different from the levels in B6 mice (Fig. 5). The IL-10 KO mice had 40 times as much IL-12 p40 and 5 times as much IFN-γ in their lungs as the B6 controls. In their spleens they had 10 times the amount of IL-12 p40 but approximately the same amount of IFN-γ (twofold more). Interestingly, there was almost no difference in the amounts of IL-4 made by the two strains. Because IL-10 KO mice develop inflammatory colitis as they age (21), which could have changed their baseline levels of cytokine production, we analyzed the mRNA levels in uninfected mice and found no significant difference from levels in uninfected B6 mice (data not shown).
FIG. 5.
Comparison of cytokine-specific mRNA levels and colony counts in the lungs and spleens of normal B6 and IL-10-deficient B6 mice 14 days after infection. Cytokine levels were assayed as described in the legend to Fig. 2. □, B6 lung; ▩, B6 spleen; ▤, IL-10-deficient lung; ▨, IL-10-deficient spleen.
DISCUSSION
In this paper, we show that there was a direct correlation between susceptibility to C. immitis and increased levels of IL-10 and IL-4 mRNAs in the lungs of inbred mice 14 days after infection (Fig. 1 and Table 1). Furthermore, we show that IL-10 KO mice were highly resistant to C. immitis (Fig. 4). Surviving IL-4 KO mice had less severe pneumonia than surviving B6 mice, but they had higher numbers of CFU in their lungs and spleens than did the IL-10 KO mice. These findings show that high levels of IL-10 are the principle reason that C57BL mice are susceptible to C. immitis, though IL-4 also has an adverse affect on resistance. Because all groups of mice were infected with the same inoculum of arthroconidia, we are able to conclude that the high levels of IL-10 and IL-4 are not simply the consequence of a severe infection in a susceptible animal and that these cytokines are somehow responsible for susceptibility to infection.
We have not yet established how IL-10 affects resistance to C. immitis in these mice. IL-10 was originally described as a T-cell product that was one of the prototypic cytokines of the Th2 response (25). However, it is now known that IL-10 is also made by macrophages, CD8+ T cells, B cells, and epithelial cells (7, 15). Furthermore, since IL-10-deficient mice (21) develop inflammatory colitis, it is clear that IL-10 plays an important role in down-regulating inflammation (28). IL-10 also down-regulates proinflammatory cytokines made by polymorphonuclear leukocytes (1) and macrophages and stimulates synthesis of IL-1ra (reviewed in reference 25). In our system, IL-10 may be preventing macrophage activation by IFN-γ or down-regulating synthesis of IFN-γ or IL-12 (10), as in the absence of IL-10, infected mice had higher levels of IL-12 p40 mRNA and IFN-γ mRNA in their lungs and spleens (Fig. 5). Since IL-12 is needed to up-regulate IFN-γ secretion from both T cells and NK cells (31) and IFN-γ is a counterregulatory cytokine for IL-4, the end result of excessive IL-10 production would be to shut off the Th1 pathway, which would allow the Th2 (IL-4-driven) pathway to predominate. IL-10 also down-regulates major histocompatibility complex class II and B7 expression on macrophages (9) and inhibits IL-12 secretion by dendritic cells, thereby preventing the development of antigen-specific IFN-γ-secreting T cells (4).
The effects of IL-10 were seen early in infection and probably reflect the response of the innate immune system, rather than the acquired immune response which involves CD4+ T cells. However, this early IL-10 response may direct CD4+ cells into the Th2 pathway in susceptible strains of mice (25). The importance of IL-12 and IFN-γ in resistance to C. immitis was demonstrated by Magee and Cox, who administered these cytokines to susceptible BALB/c mice and showed some improvement in outcome (22, 23). We did not observe IL-10 KO mice past day 15, and so we cannot say with certainty whether they would continue to do well or eventually would succumb to infection or be unable to shut off their immune response and become ill from that.
Of great interest are the recent reports that for two diseases, leishmaniasis and leprosy, the extent of the IL-10 response in humans is directly proportional to the severity of the infection. Three groups have shown that patients with progressive leishmaniasis make more IL-10 than patients with asymptomatic, self-limited infection (14, 16, 24). Also, Yamamura et al. found higher levels of IL-10 in skin lesions of patients with lepromatous leprosy than in patients with tuberculoid leprosy (34), and anti-IL-10 increases the proliferative response of peripheral blood mononuclear cells (PBMC) stimulated with Mycobacterium leprae (32). This suggests that in humans as well as mice, too much IL-10 can direct the immune response toward the Th2 type.
High levels of IL-4 may also contribute to susceptibility to C. immitis, as originally reported by Magee and Cox (23). They studied BALB/c mice, and in our experiments this was the strain with the highest level of IL-4 among three susceptible strains (Table 1). IL-4 is made by CD4+ Th2 lymphocytes, and it plays a crucial role in the differentiation of CD4+ Th0 cells to Th2 cells (20). Magee and Cox (23) first reported that cells from infected BALB/c mice secreted more IL-4 than cells from D2 mice. They proposed that this drove the mice toward a Th2 response. They attempted to make BALB/c mice more resistant by treating them with a neutralizing monoclonal antibody to IL-4, but that resulted in only a modest decrease in lung CFU and no difference in CFU in the spleen. We found that IL-4 KO B6 mice were even more resistant than BALB/c mice treated with antibody to IL-4. Although Magee and Cox infected mice by the intranasal route and we used the i.p. route, our experiments confirm their findings. We conclude that IL-10 plays a more important role than IL-4 because (i) IL-10 levels in the lungs and spleens were more highly associated with susceptibility than IL-4 levels in several mouse strains; (ii) IL-10 mRNA levels increased more than IL-4 levels in B6 mice; (iii) IL-10 KO mice were more resistant than IL-4 KO mice; and (iv) IL-4 levels were not lower in IL-10 KO mice, even though those mice were highly resistant to C. immitis.
There are several murine models of genetic susceptibility to infection due to excessive production of either IL-4 or IL-10 by the susceptible strains. The best-studied example is the adverse effect of IL-4 on the response to Leishmania major infection (12, 26, 30). In contrast, IL-10 appears to be responsible for the susceptibility of BALB/c mice to Chlamydia trachomatis pneumonia, and neutralizing antibody to IL-10 reduces chlamydia titers in the lung by about 1 log unit and elevates IFN-γ levels (35). There is also good evidence for genetic susceptibility of inbred mice to Candida albicans, and this too is due to IL-10 secretion by susceptible mice (29). In that system, D2 mice are susceptible and B6 mice are resistant, the converse of what we find with C. immitis, even though for both fungal pathogens a high level of IL-10 is deleterious. This clearly illustrates that in inbred mice, the cytokine response to infection is driven by the specific pathogen and does not reflect a global regulatory defect in the host.
Very little is known about the pattern of cytokines made by people with coccidioidomycosis. Recently Corry et al. stimulated PBMC from patients with disseminated infection (with coccidioidal antigen), and they made significantly less IFN-γ than did PBMC from healthy infected controls (2). However, there was no difference in IL-4 or IL-10 levels. There are several possible reasons why the results obtained by Corry et al. differ from ours; others have found that circulating CD4+ T cells in humans are largely Th0 rather than either Th1 or Th2 and that Th0 cells make both types of cytokines (11). CD4+ cells in lymph nodes are more likely to be differentiated into Th1 or Th2 types (27). We studied cytokine response in infected organs, which we believe is more likely to represent the relevant response. It is also possible that the relevant cytokine responses occur acutely during the primary infection, and Corry et al. studied patients at various times after infection. Studies of patients with acute coccidioidomycosis would be of interest.
ACKNOWLEDGMENTS
This study was supported by grants from the Department of Veterans Affairs Research Service and NIAID, NIH grant 5 PO1 AI37232. L. Eckmann is a recipient of a Career Development award from the Crohn’s Colitis Foundation of America.
We thank Richard Haubrich and the UCSD Treatment Center Data and Biostatistics Unit for assistance with statistical analysis.
REFERENCES
- 1.Cassatella M A, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corry D B, Ampel N M, Christian L, Locksley R M, Galgiani J N. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J Infect Dis. 1996;174:440–443. doi: 10.1093/infdis/174.2.440. [DOI] [PubMed] [Google Scholar]
- 3.Cox R A, Kennell W, Boncyk L, Murphy J W. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988;56:13–17. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 5.Drutz D J, Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis. 1978;117:559–583. doi: 10.1164/arrd.1978.117.3.559. [DOI] [PubMed] [Google Scholar]
- 6.Eckmann L, Fierer J, Kagnoff M F. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J Immunol. 1996;156:2894–2900. [PubMed] [Google Scholar]
- 7.Enk A H, Katz S I. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992;149:92–95. [PubMed] [Google Scholar]
- 7a.Fierer, J. Unpublished data.
- 8.Fiese M J. Coccidioidomycosis. Springfield, Ill: Charles C. Thomas; 1958. [Google Scholar]
- 9.Flores Villaneuva P O, Reiser H, Stadecker M J. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10. Effect on expression of B7 and B7-2 costimulatory molecules by macrophages. J Immunol. 1994;153:5190–5199. [PubMed] [Google Scholar]
- 10.Gajewski T F, Fitch F W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 11.Gajewski T F, Lancki D W, Stack R, Fitch F W. “Anergy” of THO helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994;179:481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks M J, Hagaman R M, Barbee R A. The prevalence of cellular immunity to coccidioidomycosis in a highly endemic area. West J Med. 1986;144:425–428. [PMC free article] [PubMed] [Google Scholar]
- 14.Holaday B J, Pompeu M M, Jeronimo S, Texeira M J, Sousa A, Vasconcelos A W, Pearson R D, Abrams J S, Locksley R M. Potential role for interleukin-10 in the immunosuppression associated with kala azar. J Clin Invest. 1993;92:2626–2632. doi: 10.1172/JCI116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankovic D, Sher A. Initiation and regulation of CD4+ T-cell function in host-parasite models. Chem Immunol. 1996;63:51–65. [PubMed] [Google Scholar]
- 16.Karp C L, El-Safi S H, Wynn T A, Satti M M H, Kordofani A M, Hashim F A, Hag-Ali M, Neva F A, Nutman T B, Sacks D L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkland T N, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitisinfection. Infect Immun. 1983;40:912–916. doi: 10.1128/iai.40.3.912-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkland T N, Fierer J. Genetic control of resistance to Coccidioides immitis: a single gene that is expressed in spleen cells determines resistance. J Immunol. 1985;135:548–552. [PubMed] [Google Scholar]
- 19.Kopf M, Le Gros G, Bachmann M, Lamers C, Bluethmanns H, Kohler G. Disruption of the murine IL-4 gene blocks TH-2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 20.Kubo M, Ransom J, Webb D, Hashimoto Y, Tada T, Nakayama T. T-cell subset-specific expression of the IL-4 gene is regulated by a silencer element and STAT6. EMBO J. 1997;16:4007–4020. doi: 10.1093/emboj/16.13.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10 deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 22.Magee D M, Cox R A. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63:3514–3519. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magee D M, Cox R A. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect Immun. 1996;64:3609–3613. doi: 10.1128/iai.64.9.3609-3613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melby P C, Andrade-Narvaez F J, Darnell B J, Valencia-Pacheco G, Tryon V V, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62:837–842. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore K W, O’Garra A, de Waal Malefyt R, Viera P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 26.Morris L, Troutt A B, McLeod K S, Kelso A, Handman E, Aebischer T. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993;61:3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 28.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/−mice: an overview. J Leukocyte Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 29.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 30.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 32.Sieling P A, Abrams J S, Yamamura M, Salgame P, Gloom B R, Rea T H, Modlin R L. Immunosuppressive roles for IL-10 and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. J Immunol. 1993;150:5501–5510. [PubMed] [Google Scholar]
- 33.Smith C E, Saito M T, Simons S A. Pattern of 39,500 serologic tests in coccidioidomycosis. JAMA. 1956;160:546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- 34.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, HayGlass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatismouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]