Abstract
Introduction
Since many biological drug patents have expired, biosimilar agents (BIOs) have been developed; however, there are still some reservations in their use, especially in childhood. The aim of the current study is to evaluate the efficacy and safety of tumor necrosis factor (TNF) inhibitors BIOs as treatment for pediatric non-infectious uveitis (NIU).
Methods
Data from pediatric patients with NIU treated with TNF inhibitors BIOs were drawn from the international AutoInflammatory Disease Alliance (AIDA) registries dedicated to uveitis and Behçet's disease. The effectiveness and safety of BIOs were assessed in terms of frequency of relapses, risk for developing ocular flares, best-corrected visual acuity (BCVA), glucocorticoids (GCs)-sparing effect, drug survival, frequency of ocular complications, and adverse drug event (AE).
Results
Forty-seven patients (77 affected eyes) were enrolled. The BIOs employed were adalimumab (ADA) (89.4%), etanercept (ETA) (5.3%), and infliximab (IFX) (5.3%). The number of relapses 12 months prior to BIOs and at last follow-up was 282.14 and 52.43 per 100 patients/year. The relative risk of developing ocular flares before BIOs introduction compared to the period following the start of BIOs was 4.49 (95% confidence interval [CI] 3.38–5.98, p = 0.004). The number needed to treat (NNT) for ocular flares was 3.53. Median BCVA was maintained during the whole BIOs treatment (p = 0.92). A significant GCs-sparing effect was observed throughout the treatment period (p = 0.002). The estimated drug retention rate (DRR) at 12-, 24-, and 36-month follow-up were 92.7, 83.3, and 70.8%, respectively. The risk rate for developing structural ocular complications was 89.9/100 patients/year before starting BIOs and 12.7/100 patients/year during BIOs treatment, with a risk ratio of new ocular complications without BIOs of 7.1 (CI 3.4–14.9, p = 0.0003). Three minor AEs were reported.
Conclusions
TNF inhibitors BIOs are effective in reducing the number of ocular uveitis relapses, preserving visual acuity, allowing a significant GCs-sparing effect, and preventing structural ocular complications.
Trial Registration
ClinicalTrials.gov ID NCT05200715.
Keywords: Adverse events, Biosimilars, Drug retention rate, Pediatric uveitis, Steroid-sparing effect, Tumor necrosis factor (TNF)-inhibitors
Key Summary Points
| Why carry out this study? |
| To describe a cohort of patients with pediatric-onset non-infectious uveitis treated with tumor necrosis factor (TNF)-inhibitors biosimilars, examining their response to treatment, the overall drug retention rate, and the safety profile. |
| What were the study outcomes/conclusions? |
| TNF-inhibitors biosimilars showed a significant reduction of the occurrence of ocular flares and maintaining visual acuity, exhibiting a glucocorticoid-sparing effect, along with a favorable overall drug survival and a limited occurrence of mild adverse events. |
| What was learned from the study? |
| TNF-inhibitors biosimilars have demonstrated both effectiveness and safety in the treatment of pediatric non-infectious uveitis. |
| Given their potential cost-effectiveness, the utilization of biosimilars should be thoughtfully contemplated in the context of enhancing accessibility. |
Introduction
Pediatric non-infectious uveitis (NIU) is a rare sight-threatening condition primarily affecting the uveal tract, and often occurring asymptomatically. It may lead to severe structural complications with variable visual loss risk if not timely and properly treated [1, 2]. Despite the existence of ongoing trials [3], the management of NIU continues to rely on the expertise of individual centers, guided by expert opinions and algorithms proposed by multidisciplinary teams. The first-line approach involves the administration of local and/or systemic glucocorticoids (GCs); nevertheless, in the pediatric population, careful consideration should be given to potential ocular complications as well as growth retardation and other metabolic side effects associated with their use. To allow a rapid decrease in the corticosteroid deal and/or in chronic or refractory cases, stepway treatment consists of the early introduction of disease-modifying antirheumatic agents (DMARDs). Biologic treatment is progressively emerging as a pivotal element in the therapeutic strategy for pediatric NIU, also as an early therapeutic approach. The use of biologic therapies is supported by increasing evidence of effective uveitis control, vision stability or improvement, and a steroid-sparing effect, thus preventing and minimizing the occurrence of complications. High-quality evidence suggests the use of these drugs not only in cases of NIU refractory to conventional therapy but also in place of conventional disease-modifying antirheumatic agents (cDMARDs). This could be particularly relevant in the early stages of uveitis, in case of severe inflammation, before ocular structural complications due to uveitis or topical medication occur [4–6].
The anti-tumor necrosis factor (TNF) agent adalimumab (ADA) is the only biologic drug formally approved for pediatric NIU and current evidence therefore supports the preferential use of ADA as a first-line anti-TNF agent, especially in juvenile idiopathic arthritis (JIA) associated anterior uveitis [7–15]. Recent registry-based study from the AutoInflammatory Disease Alliance (AIDA) Network confirmed that ADA has an effective therapeutic role even in pediatric patients affected by sight-threatening non-anterior uveitis, reducing ocular inflammatory activity, and allowing an overall GCs-sparing effect [16].
Current evidence also supports the efficacy of infliximab (IFX) for disease control, vision stability, and steroid-sparing in pediatric NIU as well [17–19].
Biosimilars (BIOs) are produced by recombinant techniques, resulting in biologically analogous properties to the respective biologic originator. Their introduction to the market has paved the way to a higher accessibility to biologic DMARDs through an increased sustainability of pharmaceutical costs [20, 21]. While data on the effectiveness and safety of BIOs are steadily increasing, obstacles to their use persist, particularly in pediatric patients. These barriers encompass concerns related to the nature of the delivery devices, physical characteristics of the product, as well as issues regarding their accessibility and distribution [22–27].
Studies based on real-world data reported from an adult population showed efficacy and safety of BIOs in NIU. Fabiani et al. examined ocular flare rates in NIU pre- and post-switch from a variety of anti-TNF bio-originators to their respective BIOs; their study revealed no statistically significant difference in flare rates during the 12 months after the switch. Moreover, no instances of severe adverse event (AE) were noted after the switch [28]. In a retrospective study of 26 adults affected by uveitis associated with Behçet’s syndrome from AIDA registry, ADA BIO SB5 was effective in significantly reducing uveitis relapses and the occurrence of retinal vasculitis; furthermore, SB5 biosimilar improved visual acuity allowed a significant GC-sparing effect with an excellent drug retention rate (DRR) [29]. On the other hand, limited literature exists regarding the use of BIOs for NIU in the specific pediatric population [30–35]; only one study, carried out by Sözeri et al., focuses exclusively on a pediatric cohort suffering from NIU treated with IFX [34].
Therefore, this study is aimed at describing and analyzing the effectiveness and safety profile of TNF inhibitors BIOs in a multicenter cohort of pediatric patients with NIU enrolled in the AIDA International Registries dedicated to uveitis and Behçet’s disease [36, 37].
Methods
This is a registry-based observational multicenter ambidirectional cohort study, from the AIDA Network, based on real-world data. The cohort was selected among 536 patients with NIU enrolled in the international Uveitis AIDA Registry [36]. Moreover, patients with uveitis included in the international AIDA Registry for Behçet’s disease [37] were enrolled. Inclusion criteria were: (1) onset of NIU at an age below 18 years and (2) start treatment with biosimilar drugs in pediatric age. Figure 1 summarizes the flow leading to the selection of patients on April 1, 2023. Data collected were referred to the time of uveitis onset, the start of treatment with BIOs (baseline) and the 3-, 6- and 12-month assessments from the beginning of treatment or at the last available follow-up assessment. The following demographic, clinical, and therapeutic data were collected: age, sex, any diseases associated, age at uveitis onset, clinical manifestations, disease activity and duration, concomitant and previous treatments, dosages of BIO used and treatment duration, disease course and any possible concomitant drugs during the BIO treatment, ocular complications, AE and/or reasons for BIOs discontinuation when occurred. Patients with insufficient follow-up data or missing baseline values were excluded from the final cohort. The medical records of 47 diagnosed with NIU by ophthalmologists trained in uveitis were reviewed. The primary aims of the study were to: (i) describe the cohort of patients with pediatric-onset uveitis treated with BIOs and evaluate the safety of BIOs; (ii) describe the response to biosimilar molecules in terms of disease control activity. Secondary aims were to: (i) analyze any steroid-sparing effect and (ii) examine the overall persistence of BIOs treatment. The response to biosimilar molecules was evaluated through the following endpoints: (i) evaluation of the relative risk (RR) of developing ocular flares comparing the 12 months preceding the start of BIOs and the whole period of BIOs administration; (ii) computation of the number needed to treat (NNT) of flares (meant as number of flares to treat with BIOs to spare one flare occurrence); (iii) any statistically significant decrease in the number of ocular relapses, defined according to the Standardization of Uveitis Nomenclature (SUN) Working Group grading scheme [38], during the 12 months before the start of BIOs and in the whole BIOs treatment period expressed as number of events/100 patients/year; (iv) change in visual acuity from the baseline to the last follow-up assessment through the best-corrected visual acuity (BVCA) reported on Snellen charts into decimals; (v) any significant decrease in the daily systemic GC doses (prednisone or equivalent) assessed as mg/kg/day at each time-point from baseline; (vi) number of patients who discontinued GCs at the last follow-up or at any time points during the biosimilar treatment; (vii) BIOs DRR during the study period; (viii) analysis of any related reasons to BIOs discontinuation.
Fig. 1.
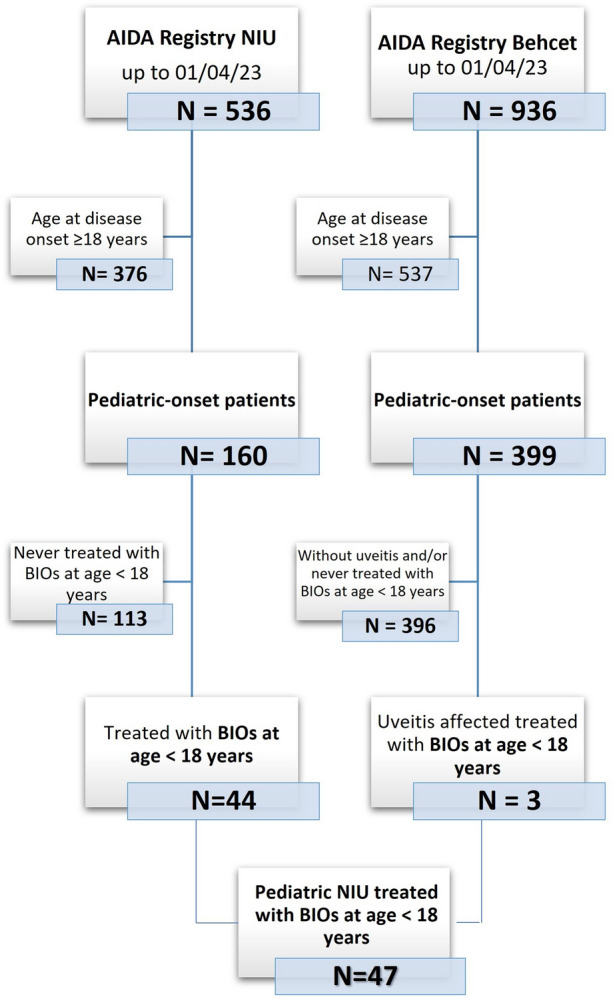
Study flow diagram illustrating the process leading to the selection of patients included in the present study among all patients recruited in the International AutoInflammatory Disease Alliance (AIDA) Registry for uveitis and Behçet’s disease until April 1, 2023. BIOs biosimilars, N number, NIU non-infectious uveitis
The ocular flare was defined as a worsening of inflammatory activity after a period of inactive disease, according to the SUN working group nomenclature and according with the standardization of vitreal inflammatory activity proposed by Nussenblatt et al. [38, 39].
This study has been approved in the field of the AIDA Project by the Ethics Committee of Azienda Ospedaliera Universitaria Senese, Siena, Italy (Ref. No. 14951); it was conducted in accordance with the recommendations by the Declaration of Helsinki and subsequent updates. All patients provided their consent, which was obtained from the parents or by the legal guardians.
Statistical analysis was performed by using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, NY, USA). Descriptive statistics included sample sizes, mean, and standard deviation or median and interquartile range (IQR), as appropriate. Shapiro–Wilk test was used to assess normality distribution of data. Wilcoxon’s signed-rank test was used to compare the mean values between two related groups on the same continuous, dependent variable. Differences between the median values of ≥ 3 paired continuous variables violating the assumption of normality were analyzed by Friedman’s test and Conover’s post hoc test. The cumulative survival rate was studied by Kaplan–Meier plot with the event being the drug discontinuation. The threshold for statistical significance was set to p < 0.05 and all p-values were two-sided.
Results
Forty-seven patients corresponding to 22 males (46.8%) and 25 females (53.2%) were enrolled in this study: their demographic, clinical, and therapeutic features are summarized in Table 1. An associated systemic disease was identified in 27 (57.4%) cases; conversely, 20 patients (42.6%) suffered from idiopathic NIU. The most represented undergoing clinical conditions were JIA (21 patients, 44.7%) and Behçet's disease (three patients, 6.4%). Unilateral and bilateral ocular involvement was described in 17 (36.2%) and 30 (63.8%) cases, respectively. Altogether, 77 eyes were affected by NIU. Table 2 describes features of the intraocular inflammation. Regarding the anatomical pattern of uveitis according to SUN criteria, uveitis was classified as anterior, intermediate, posterior uveitis or panuveitis in 45 (58.4%), 14 (18.2%), 3 (3.9%), or 15 (19.5%) eyes, respectively. In two eyes (one patient) uveitis anatomical classification were not provided. In eight patients (12 eyes), intermediate involvement consisted in a pars planitis. Other than uveitis, inflammatory ocular or orbital involvement was present in 2 eyes (2.6%), consisting of dry eye disease; ocular structural complications had already occurred in 31 eyes (40%) at the time of the enrollment.
Table 1.
Demographic and clinical features of patients enrolled in the study
| Patients, N | 47 |
| Eyes, N | 77 |
| Female/Male, N | 25/22 |
| Mean age at uveitis onset, years (mean ± SD) | 8.70 ± 3.65 |
| Unilateral/bilateral involvement, N | 17/30 |
| Concomitant systemic diseases, N (%) | JIA, 21 (44.7) |
| Behçet’s disease, 3 (6.4) | |
| Sarcoidosis, 1 (2.2) | |
| Scleroderma, 1 (2.2) | |
| Other connectivitis, 1 (2.2) | |
| Mean age at the start of BIOs, years (months ± SD) | 10.73 ± 3.81 |
| Mean duration of uveitis at the start of BIOs, months (median (IQR)) | 12 (22.5) |
BIOs biosimilar agent, IQR interquartile range, JIA juvenile idiopathic arthritis, N number of patients, SD standard deviation
Table 2.
Features of the eyes of patients with uveitis included in the study
| Anatomical classification of the uveitis, N eyes (%) | Anterior, 45 (58.4) |
| Intermediate, 14 (18.2) | |
| Posterior, 3 (3.9) | |
| Panuveitis, 15 (19.5) | |
| Pathogenetic classification at the onset, N eyes (%) | Non-granulomatous, 48 (62.3) |
| Granulomatous, 16 (20.8) | |
| Unknown, 13 (16.9) | |
| Uveitis presentation, N eyes (%) | Sudden, 30 (39) |
| Insidious, 41 (62.3) | |
| Unknown, 6 (20.8) | |
| Uveitis course, N eyes (%) | Acute, 4 (5.2) |
| Recurrent, 25 (32.5) | |
| Chronic, 39 (50.1) | |
| Unknown, 7 (9.1) | |
| Structural ocular complications at the baseline, N eyes | Posterior synechiae, 20 |
| Macular edema, 10 | |
| Ocular hypertension, 8 | |
| Macular ischemia, 3 | |
| Steroid-induced cataract, 3 | |
| Open-angle glaucoma, 2 | |
| Chorioretinal scars, 2 | |
| Band keratopathy, 2 | |
| Iris bombè, 1 | |
| Amblyopia, 1 | |
| Retinal ischemia, 2 |
N number of eyes, Unknown missing value
Thirty-six patients (76.6%) had started biologic agents due to ocular disease activity, one patient (2.1%) had undergone biologic treatment because of extraocular manifestations, while ten patients (21.3%) had started biologic agents for both ocular and systemic disease activity. Forty-five patients (95.7%) used their first biologic agent, while two patients (4.3%) had previously used a different biologic molecule (ADA originator in one case and etanercept (ETA) originator in the other case).
In 42 patients (69 eyes), BIOs were started without switching from the corresponding originators. In five patients, a BIO switch from the corresponding originator was reported: in four cases (six eyes), the switch to the BIOs occurred directly from the originator due to non-medical reasons, while in one patient (two eyes), the BIO was introduced following a temporary suspension due to an AE to the originator. The BIOs employed were ADA (89.4%—biosimilar name molecules: ABP501 26.3%; GP2017 36.8%; SB5 26.3%), ETA (5.3%—biosimilar name molecule: GP2015) and IFX (5.3%—biosimilar name molecules: GP1111 2.7%; SB2 2.7%).
As a whole, 169 ocular flares were recorded prior to BIOs introduction and 76 ocular flares were reported thereafter; the rate of ocular flares was 282.14 flares/100 patients/year during the 12 months preceding the start of BIOs and 52.34 flares/100 patients/year during BIOs treatment. The relative risk of developing ocular flares before BIOs introduction compared to the period following the start of BIOs was 4.49 (95% CI 3.38–5.98, p = 0.004). The NNT for ocular flares was 3.53.
Median BCVA was 1 IQR (IQR = 0.0, range, 0.0–1.0) at baseline and 1 IQR (IQR = 0.0, range, 0.6–1) at 12 months (p = 0.92). Table 3 summarizes data concerning other concomitant treatments (topical steroids, GCs, and cDMARDs) employed at the start of BIOs and at the last follow-up evaluation.
Table 3.
Treatments associated with BIOs, described at the baseline and at the last assessment
| Regional treatment with glucocorticoids preceding baseline, N patients (%) | Peribulbar injection 2 (4.3) |
| Subconjunctival injection 1 (2.1) | |
| Local treatment with steroid drops at the start of BIOs, N eyes (%) | 5 (10.7) |
| Local treatment with steroid drops at the last assessment, N eyes (%) | 4 (8.5) |
| Concomitant GC treatment at the start of BIOs, N patients (%) | 25 (53.2) |
| Concomitant GC treatment at the last assessment, N patients (%) | 12 (15.6) |
| Median GC dose at the start of BIOs (prednisone or equivalent), mg/kg/day (median (IQR)) | 0,1 (0.7) |
| Median GC dose at the last assessment (prednisone or equivalent), mg/kg/day (median (IQR)) | 0 (0) |
| Concomitant cDMARDs at the start of BIOs, N patients (%) | MTX, 21 (44.7) |
| CYC, 4 (8.5) | |
| AZA, 4 (8.5) | |
| MYC, 1 (2.1) | |
| COL, 1(2.1) | |
| Unknown, 3 (6.4) | |
| Concomitant cDMARDs at the last assessment, N patients (%) | MTX, 18 (38.3) |
| CYC, 2 (4.3) | |
| AZA, 2 (4.3) | |
| Unknown, 1 (2,1) |
AZA azathioprine, BIOs biosimilar agent drug, cDMARDs conventional disease-modifying antirheumatic drug, COL colchicine, CYC cyclosporine, GC glucocorticoids, IQR interquartile range, MTX methotrexate, MYC mycophenolate mofetil, N number of patients, Unknown missing value
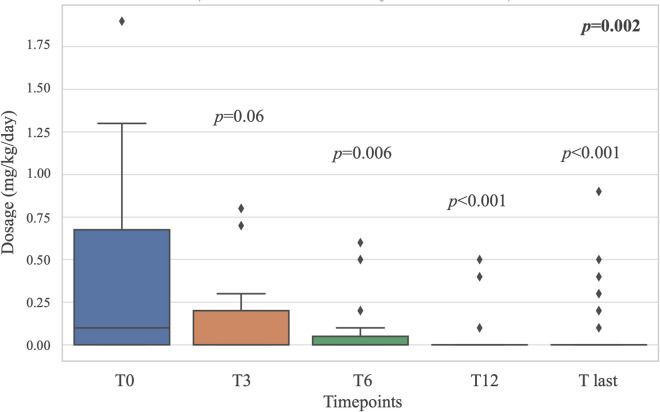
Median daily systemic GC dosage significantly decreased from 0.1 (IQR 0.675, range, 0.07–1.9) mg/kg/day at the start of BIOs to 0 mg/kg/day (IQR 0, range, 0–0.9) at the last assessment (p = 0.002), showing a significant result in steroid-sparing effect starting from the 6-month follow-up assessment (Fig. 2). Systemic GCs had been withdrawn in 13/25 patients (p = 0.02).
Fig. 2.
Boxplots showing glucocorticoids (GCs) reduction over the follow-up period. Both the overall p value obtained through Friedman test (in bold in the top right-hand corner) and p value drawn by post hoc analysis at each time point are provided
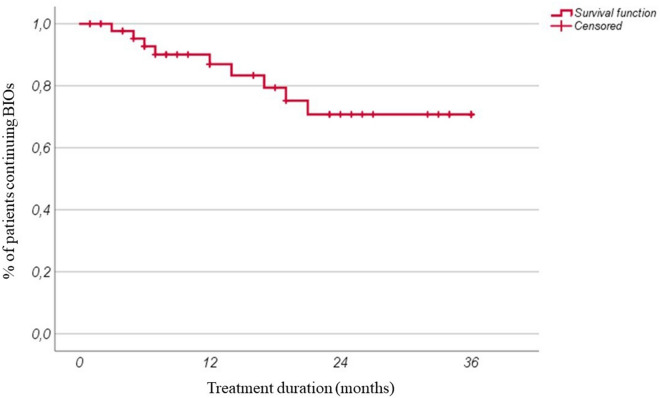
Figure 3 shows the estimated DRR, which is 92.7%, 83.3%, and 70.8% at 12, 24, and 36 months of follow-up, respectively; the median overall duration of follow-up in BIOs was 14 months (IQR 19).
Fig. 3.
Overall biosimilars (BIOs) retention rate of our cohort during follow-up
A total of nine patients (19%) discontinued BIOs; among them, three discontinued their treatment due to either inefficacy (two cases) or partial efficacy (one case) on ocular disease activity. The other reasons for discontinuation included inefficacy (two cases) on extraocular manifestations, the occurrence of an AE (one case, psoriasiform skin reaction), poor compliance (one case) or non-medical reason (one case); one patient suspended due to long-term remission.
Throughout the follow-up duration, six patients (13%) presented new ocular complications, consisting in cataract (two eyes), posterior synechiae (one eye), ocular hypertension (two eyes), macular edema (one eye), band keratopathy (one eye); the development of cataracts and ocular hypertension in one case each were attributed to concurrent steroid usage at ophthalmologic assessment. The risk rate for developing structural ocular complications was 89.9/100 patients/year before starting BIOs and 12.7/100 patients/year during BIOs treatment, with a risk ratio of new ocular complications without BIOs equal to 7.1 (CI 3.4–14.9, p = 0.0003) and a NNT ocular complications of 15.6.
Over the course of BIOs treatment, three patients reported AE. These included an acute infusion reaction, heightened frequency of migraine attacks along with changes in mood tone in one patient, and a case of psoriasiform skin reaction.
Discussion
The present registry-based study describes a subset of pediatric patients with NIU treated with BIOs, shedding further light on their safety and effectiveness in a real-life context. Actually, to the best of our knowledge, this study cohort represents the largest sample of pediatric patients with NIU treated with TNF inhibitors BIOs, thus contributing to the straightening of current evidence on this topic.
According to the current literature, approximately 50% of children with uveitis have an associated systemic disease; in particular, the most common NIU type observed in children is the JIA-associated uveitis, especially anterior uveitis [3–6]. In this study, JIA-associated uveitis includes the 44.7% of all patients with uveitis, all of them suffering from anterior uveitis. Similar to various findings in previous studies, the sex distribution in our patients showed a slight predominance of females (53.2%). These features reflect the epidemiological distribution of pediatric uveitis, aligning therapeutically also with the main indications and consensus regarding therapeutic strategies reported in pediatric uveitis, especially in the context of JIA [1, 3, 5–7, 40, 41].
Regarding effectiveness, the study demonstrates a significant reduction in the frequency of uveitis relapses, which reflects the ability to preserve visual acuity. These findings resemble those observed by Vitale et al., who described a decrease in ocular flare frequency from 3.91 flares per patient per year to 1.1 flares per patient per year in pediatric non-anterior NIU cases treated either with the ADA originator or its BIOs [16]. The effectiveness of BIOs treatment for pediatric NIU is strongly supported also by the risk ratio for developing ocular flares observed in this study, which is 4–5 times higher when patients are not treated with these agents. Interestingly, the NNT observed in our study suggests that BIOs therapy may save one ocular relapse every 3.5 flares occurred. Altogether, these findings support the utility of BIOs in the pediatric setting, reducing the frequency of inflammatory ocular insults and preserving the visual function in pediatric patients.
Indeed, BCVA did not exhibit a significant change between the baseline and the final follow-up visit. The absence of significant statistical differences could potentially be attributed to the initially high visual acuity levels in patients affected by anterior uveitis and in the initiation of biologic therapy at an early stage; this finding underscored the capacity of BIOs to effectively preserve visual acuity over time.
In addition, the risk for ocular complications decreased significantly after BIOs introduction, saving from potentially long-term sight-threatening sequelae. Of note, the risk for structural ocular complications is seven times higher before starting BIOs.
These relevant results were possible despite the noteworthy sparing effect observed on GCs usage. Notably, over half of the patients were able to discontinue GCs, allowing for the reduction of potential structural systemic and eye complications, even more essential in pediatric patients; in fact, at least two of the new ocular complications that occurred in our cohort were directly related to concomitant GC therapy [42].
This study broadens and supports previous literature about the effective role of BIOs for patients with NIU. In particular, a recent real-life study compared originator ADA with biosimilar ADA in pediatric rheumatic diseases, concluding about no significant differences between BIOs and the originator product in terms of safety and efficacy, including the number of uveitis flares [30]. A further study enrolling adults and children (6–17 years old) concluded that ADA biosimilar was effective and well tolerated in Behçet’s uveitis [31]. Zaguia et al. reported the efficacy of IFX-biosimilar in achieving and maintaining uveitis control in a multicenter retrospective case series of 14 patients aged from 7 to 66 years old [32]. Similarly, according to a recent study conducted by Murray et al. on 102 patients (67 children) with NIU undergoing a transition from the originator of ADA to the biosimilar ABP501, non-inferiority of the BIO was found when compared to the originator in relation to post-switch flare rates and GC dosages. However, in that cohort a considerable proportion of patients required reverting to the originator due to AE, in particular injection site reactions [33].
In our cohort, three patients reported AE and only one of them occurred concurrently with drug administration (acute reaction during IFX infusion). Given this context, the choice of BIOs could consider the nature of the buffer and technical issues with the injector, as they are essential to maintain an appropriate balance to prevent undue injection site pain. The continuous development of new formulations and devices dedicated to tackling has led to the emergence of relevant advancements, including citrate- or latex-free products or the use of thinner needles [20, 43].
Our estimated DRR at 12, 24, and 36 months of follow-up is excellent and closely reflects the findings reported by Sota et al., who identified a cumulative SB5 retention rate of 91.8% at both the 12- and 20-month follow-up in 26 successfully treated patients suffering from NIU [44]. Likewise, a comparable DRR has been documented in the literature for patients treated with ADA originator at the 12-month follow-up [45, 46].
Within our cohort, only three out of the nine patients who discontinued BIOs ceased the treatment due to effectiveness issues: two of these patients were on ETA BIOs, initiated for both ocular and extra-ocular disease activity within the context of JIA. The poor ocular response in these patients can likely be attributed to the broader ineffectiveness of ETA in the realm of pediatric NIU, which is in line with existing literature data [47, 48].
Regarding the safety of TNF-inhibitors BIOs, three mild AE were reported, and they did not differ substantially in frequency and severity from those reported in the literature regarding anti-TNF treatment in childhood chronic uveitis [8]. Nevertheless, the few and mild AE reported support the excellent safety profile and do not represent a reason to prefer originators in place of BIOs.
Limitations of this study refer to the relatively small patient cohort and the issues potentially affecting the retrospective phase of data collection. In addition, the frequency of ocular flares was used as endpoint of the study to assess the BIOs effectiveness. In this regard, we assumed that the trend of ocular involvement could not change over time. However, this potential bias may affect all the studies. On the other hand, this is a case-only study, with treated patients accounting for cases and controls; this has excluded the role of any fixed or unmeasurable confounding factors. Moreover, this is an ambidirectional study with a targeted prospective follow-up; therefore, limitations referred to the retrospective data collection are fully overcome during the prospective phase. Finally, data are drawn from international registries capable of reverse geographical-related confounders.
Conclusions
TNF inhibitors BIOs proved an effective therapeutic role in children with NIU, significantly reducing the number of relapses, preserving visual acuity and allowing a significative GC sparing effect. Furthermore, structural ocular complications proved to be prevented by BIOs, supporting their early use to avoid persistent sight damage. The AEs reported were low in number and severity, straightening an excellent BIOs safety.
Author Contributions
Maria Tarsia, Antonio Vitale, and Claudia Fabiani wrote the first draft of the manuscript; Maria Tarsia, Antonio Vitale, Claudia Fabiani, Carla Gaggiano, and Jurgen Sota performed the preliminary data analysis and interpretation; Anna Maselli, Chiara Bellantonio, Silvana Guerriero, Rosanna Dammacco, Francesco La Torre, Gaafar Ragab, Mohamed Tharwat Hegazy, Alex Fonollosa, Maria Pia Paroli, Emanuela Del Giudice, Maria Cristina Maggio, Marco Cattalini, Lampros Fotis, Giovanni Conti, Angela Mauro, Adele Civino, Federico Diomeda, Alejandra de-la-Torre, Carlos Cifuentes-González, Samar Tharwat, José Hernández-Rodríguez, Verónica Gómez-Caverzaschi, Laura Pelegrín, Kalpana Babu, Vishali Gupta, Francesca Minoia, Piero Ruscitti, Stefania Costi, Luciana Breda, Saverio La Bella, Alessandro Conforti, Maria Antonietta Mazzei, Ester Carreño, Rana Hussein Amin, Salvatore Grosso, Bruno Frediani, and Gian Marco Tosi were involved in the study according to their active role in enrolling pediatric patients with uveitis treated with adalimumab biosimilars on April 1, 2023; Alberto Balistreri is the bioengineer involved in the technical management of the platform and registries; Claudia Fabiani and Luca Cantarini took care of the final revision of the manuscript and accounted for AIDA Registries Coordinators.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Maria Tarsia, Antonio Vitale, Carla Gaggiano, Jurgen Sota, Anna Maselli, Chiara Bellantonio, Silvana Guerriero, Rosanna Dammacco, Francesco La Torre, Gaafar Ragab, Mohamed Tharwat Hegazy, Alex Fonollosa, Maria Pia Paroli, Emanuela Del Giudice, Maria Cristina Maggio, Marco Cattalini, Lampros Fotis, Giovanni Conti, Angela Mauro, Adele Civino, Federico Diomeda, Alejandra de-la-Torre, Carlos Cifuentes-González, Samar Tharwat, José Hernández-Rodríguez, Verónica Gómez-Caverzaschi, Laura Pelegrín, Kalpana Babu, Vishali Gupta, Francesca Minoia, Piero Ruscitti, Stefania Costi, Luciana Breda, Saverio La Bella, Alessandro Conforti, Maria Antonietta Mazzei, Ester Carreño, Rana Hussein Amin, Salvatore Grosso, Bruno Frediani, Gian Marco Tosi, Alberto Balistreri, Luca Cantarini, and Claudia Fabiani have nothing to disclose.
Ethical Approval
AIDA Network study and the data collection in Uveitis AIDA registry, have been approved by the Ethics Committee of Azienda Ospedaliera Universitaria Senese, Siena, Italy (ref. no. 14951—ClinicalTrials.gov ID NCT05200715) and was conducted in accordance with the recommendations by the Declaration of Helsinki and subsequent updates. All patients provided their assent; the informed consent was obtained from the parents (or by the legal guardian).
Footnotes
Maria Tarsia and Antonio Vitale contributed equally.
Contributor Information
Luca Cantarini, Email: cantariniluca@hotmail.com.
Claudia Fabiani, Email: claudia.fabiani@aidanetwork.org.
References
- 1.Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112(7):1287–1292. doi: 10.1016/j.ophtha.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 2.Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol. 2003;135(5):676–680. doi: 10.1016/s0002-9394(02)02148-7. [DOI] [PubMed] [Google Scholar]
- 3.Maccora I, Guly C, de Libero C, Caputo R, Ramanan AV, Simonini G. Childhood chronic idiopathic uveitis in a multicentre international cohort [published online ahead of print, 2023 Feb 21] Ocul Immunol Inflamm. 2023 doi: 10.1080/09273948.2023.2169715. [DOI] [PubMed] [Google Scholar]
- 4.Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis-related uveitis. J AAPOS. 2008;12(6):539–545. doi: 10.1016/j.jaapos.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: clinical characteristics and complications. Am J Ophthalmol. 2009;147(4):667–678.e5. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 7.Angeles-Han ST, Lo MS, Henderson LA, et al. Childhood arthritis and rheumatology research alliance consensus treatment plans for juvenile idiopathic arthritis-associated and idiopathic chronic anterior uveitis. Arthritis Care Res (Hoboken) 2019;71(4):482–491. doi: 10.1002/acr.23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Shyamsundar K, Agrawal M, Vichare N, Biswas J. Current knowledge of biologics in treatment of noninfectious uveitis. J Ocul Pharmacol Ther. 2022;38(3):203–222. doi: 10.1089/jop.2021.0098. [DOI] [PubMed] [Google Scholar]
- 9.Maccora I, Fusco E, Marrani E, Ramanan AV, Simonini G. Changing evidence over time: updated meta-analysis regarding anti-TNF efficacy in childhood chronic uveitis. Rheumatology (Oxford) 2021;60(2):568–587. doi: 10.1093/rheumatology/keaa595. [DOI] [PubMed] [Google Scholar]
- 10.Constantin T, Foeldvari I, Anton J, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018;77(8):1107–1117. doi: 10.1136/annrheumdis-2018-213131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Giudice E, Simio C, Scala A, et al. Juvenile idiopathic arthritis-associated uveitis in the era of biological therapy: how the disease changed in more than 20 years of observation in a tertiary referral center in Rome (Italy) Int Ophthalmol. 2022;42(3):775–784. doi: 10.1007/s10792-021-02043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cecchin V, Zannin ME, Ferrari D, et al. Long-term safety and efficacy of adalimumab and infliximab for uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2018;45(8):1167–1172. doi: 10.3899/jrheum.171006. [DOI] [PubMed] [Google Scholar]
- 13.Heiligenhaus A, Minden K, Tappeiner C, et al. Update of the evidence based, interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Semin Arthritis Rheum. 2019;49(1):43–55. doi: 10.1016/j.semarthrit.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. 2017;376(17):1637–1646. doi: 10.1056/NEJMoa1614160. [DOI] [PubMed] [Google Scholar]
- 15.Maccora I, Sen ES, Ramanan AV. Update on noninfectious uveitis in children and its treatment. Curr Opin Rheumatol. 2020;32(5):395–402. doi: 10.1097/BOR.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 16.Vitale A, Casa FD, Guerriero S, et al. Efficacy and safety of adalimumab in pediatric non-infectious non-anterior uveitis: real-life experience from the international AIDA Network Uveitis Registry. Ophthalmol Ther. 2023;12(4):1957–1971. doi: 10.1007/s40123-023-00712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: retrospective case series with long-term follow-up. Am J Ophthalmol. 2007;144(6):844–849. doi: 10.1016/j.ajo.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113(2):308–314. doi: 10.1016/j.ophtha.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Zannin ME, Birolo C, Gerloni VM, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year follow-up data from the Italian Registry [published correction appears in J Rheumatol. 2013 Jan;40(1):106] J Rheumatol. 2013;40(1):74–79. doi: 10.3899/jrheum.120583. [DOI] [PubMed] [Google Scholar]
- 20.US FDA. Biosimilar and interchangeable products. FDA; 2023. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeableproducts.
- 21.Simoens S, Vulto AG. A health economic guide to market access of biosimilars. Expert Opin Biol Ther. 2021;21(1):9–17. doi: 10.1080/14712598.2021.1849132. [DOI] [PubMed] [Google Scholar]
- 22.Aragon Cuevas O, Hedrich CM. Biosimilars in pediatric rheumatology and their introduction into routine care. Clin Immunol. 2020;216:108447. doi: 10.1016/j.clim.2020.108447. [DOI] [PubMed] [Google Scholar]
- 23.Renton WD, Leveret H, Guly C, Smee H, Leveret J, Ramanan AV. Same but different? A thematic analysis on adalimumab biosimilar switching among patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2019;17(1):67. doi: 10.1186/s12969-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JJ, Lee N, Seo YJ, Kim I. Consistency of product quality for SB5, an adalimumab biosimilar. BioDrugs. 2023;37(2):271–277. doi: 10.1007/s40259-023-00581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu T, Jin S, Li C, Chambers JD, Hlávka JP. Factors associated with biosimilar exclusions and step therapy restrictions among US commercial health plans. BioDrugs. 2023;37(4):531–540. doi: 10.1007/s40259-023-00593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megna M, Fornaro L, Potestio L, et al. Efficacy and safety of anti-TNF biosimilars for psoriasis in pediatric and geriatric populations: a 72-week real-life study. Psoriasis (Auckl) 2022;12:199–204. doi: 10.2147/PTT.S365493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maccora I, Lombardi N, Crescioli G, et al. OBSIDIAN—real-world evidence of originator to biosimilar drug switch in juvenile idiopathic arthritis. Rheumatology (Oxford) 2022;61(4):1518–1528. doi: 10.1093/rheumatology/keab572. [DOI] [PubMed] [Google Scholar]
- 28.Fabiani C, Vitale A, Emmi G, et al. The role of biosimilars in uveitis: long-term real-world outcomes of the switch from original to biosimilar TNF-alpha inhibitors. Front Pharmacol. 2019;10:1468. doi: 10.3389/fphar.2019.01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sota J, Gentileschi S, Perfetti MO, et al. Role of adalimumab biosimilar in the treatment of non-anterior uveitis associated with Behçet's syndrome. Ophthalmol Ther. 2021;10(4):1129–1135. doi: 10.1007/s40123-021-00387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulu K, Çakan M, Çağlayan Ş, et al. Real-life data on efficacy and safety of original adalimumab and biosimilar adalimumab (ABP 501) in pediatric rheumatic diseases [published online ahead of print, 2022 Sep 13] Expert Opin Biol Ther. 2022 doi: 10.1080/14712598.2022.2123703. [DOI] [PubMed] [Google Scholar]
- 31.Soheilian M, Ebrahimiadib N, Hedayatfar A, Hosseini M, Zarei M, Anjidani N. Efficacy of biosimilar adalimumab in the treatment of Behçet's uveitis. Ocul Immunol Inflamm. 2022;30(6):1495–1500. doi: 10.1080/09273948.2021.1900276. [DOI] [PubMed] [Google Scholar]
- 32.Zaguia F, Randerson EL, Moorthy RS, Goldstein DA. Efficacy of biosimilar infliximab-dyyb in non-infectious uveitis [published online ahead of print, 2023 Aug 15] Ocul Immunol Inflamm. 2023 doi: 10.1080/09273948.2023.2244071. [DOI] [PubMed] [Google Scholar]
- 33.Murray GM, Griffith N, Sinnappurajar P, et al. Clinical efficacy of biosimilar switch of adalimumab for management of uveitis [published online ahead of print, 2023 Feb 21] Ocul Immunol Inflamm. 2023 doi: 10.1080/09273948.2023.2172591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sözeri B, Kardeş E, Salı E, Çakır D, Demir F. Drug survival of the infliximab biosimilar (CT-P13) in paediatric patients with non-infectious uveitis. Clin Exp Rheumatol. 2021;39(4):907–912. doi: 10.55563/clinexprheumatol/r4gnxm. [DOI] [PubMed] [Google Scholar]
- 35.Demirkan FG, Ulu K, Öztürk K, et al. Toward the integration of biosimilars into pediatric rheumatology: adalimumab ABP 501 experience of PeRA research group. Expert Opin Biol Ther. 2022;22(2):197–202. doi: 10.1080/14712598.2021.2002296. [DOI] [PubMed] [Google Scholar]
- 36.Casa FD, Vitale A, Guerriero S, et al. Development and implementation of the AIDA international registry for patients with non-infectious uveitis. Ophthalmol Ther. 2022;11(2):899–911. doi: 10.1007/s40123-022-00459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitale A, Della Casa F, Ragab G, et al. Development and implementation of the AIDA International Registry for patients with Behçet's disease. Intern Emerg Med. 2022;17(7):1977–1986. doi: 10.1007/s11739-022-03038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 40.Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. 2002;86(1):51–56. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89(4):444–448. doi: 10.1136/bjo.2004.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leal I, Steeples LR, Wong SW, et al. Update on the systemic management of non-infectious uveitis in children and adolescents [published online ahead of print, 2023 Jan 19] Surv Ophthalmol. 2023 doi: 10.1016/j.survophthal.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 43.US FDA. Biological Product Innovation and Competition. FDA; 2023. https://www.fda.gov/drugs/biosimilars/biological-product-innovation-and-competition.
- 44.Sota J, Gentileschi S, Vitale A, et al. Effectiveness of SB5, an adalimumab biosimilar, in patients with noninfectious uveitis: a real-life monocentric experience. Asia Pac J Ophthalmol (Phila) 2021;10(4):360–365. doi: 10.1097/APO.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 45.Llorenç V, Cordero-Coma M, Blanco-Esteban A, et al. Drug retention rate and causes of discontinuation of adalimumab in uveitis: real-world data from the Biotherapies in Uveitis (BioÚvea) Study Group. Ophthalmology. 2020;127(6):814–825. doi: 10.1016/j.ophtha.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Fabiani C, Vitale A, Emmi G, et al. Long-term retention rates of adalimumab and infliximab in non-infectious intermediate, posterior, and panuveitis. Clin Rheumatol. 2019;38(1):63–70. doi: 10.1007/s10067-018-4069-3. [DOI] [PubMed] [Google Scholar]
- 47.Smith JA, Thompson DJ, Whitcup SM, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53(1):18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 48.Schmeling H, Horneff G. Etanercept and uveitis in patients with juvenile idiopathic arthritis. Rheumatology (Oxford) 2005;44(8):1008–1011. doi: 10.1093/rheumatology/keh658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.