Abstract
Background
Uptake of lung cancer screening (LCS) has been slow with less than 20% of eligible people who currently or formerly smoked reported to have undergone a screening CT.
Objective
To determine individual-, health system-, and neighborhood-level factors associated with LCS uptake after a provider order for screening.
Design and Subjects
We conducted an observational cohort study of screening-eligible patients within the Population-based Research to Optimize the Screening Process (PROSPR)–Lung Consortium who received a radiology referral/order for a baseline low-dose screening CT (LDCT) from a healthcare provider between January 1, 2015, and June 30, 2019.
Main Measures
The primary outcome is screening uptake, defined as LCS-LDCT completion within 90 days of the screening order date.
Key Results
During the study period, 18,294 patients received their first order for LCS-LDCT. Orders more than doubled from the beginning to the end of the study period. Overall, 60% of patients completed screening after receiving their first LCS-LDCT order. Across health systems, uptake varied from 41 to 87%. In both univariate and multivariable analyses, older age, male sex, former smoking status, COPD, and receiving care in a centralized LCS program were positively associated with completing LCS-LDCT. Unknown insurance status, other or unknown race, and lower neighborhood socioeconomic status, as measured by the Yost Index, were negatively associated with screening uptake.
Conclusions
Overall, 40% of patients referred for LCS did not complete a LDCT within 90 days, highlighting a substantial gap in the lung screening care pathway, particularly in decentralized screening programs.
Supplementary Information:
The online version contains supplementary material available at 10.1007/s11606-023-08408-9.
KEY WORDS: multilevel, LDCT, centralized, decentralized, disparities, non-hispanic black, Asian, Hispanic
INTRODUCTION
Lung cancer is the leading cause of cancer mortality, accounting for an estimated 1.8 million deaths worldwide in 2021.1 The National Lung Screening Trial (NLST)2 and the Dutch-Belgian Randomized Lung Screening Trial (Nederlands–Leuvens Longkanker Screenings Onderzoek [NELSON])3 demonstrated reductions in lung cancer mortality using multiple rounds of screening with low-dose CT (LDCT). The Affordable Care Act mandates coverage of lung cancer screening (LCS) with LDCT in privately insured patients meeting USPSTF guidelines.4, 5 Centers for Medicare and Medicaid Services (CMS) initiated coverage of LCS in 2015 for those aged 55–77 with 30 + pack-years of smoking6 and recently expanded these criteria to 50–77 years of age with 20 + pack-years of smoking, following an update to the USPSTF guideline in 2021.5 In addition to eligibility assessment which requires detailed smoking history information that is often missing in the medical record, CMS requires documentation of shared decision-making (SDM) including the patient-provider discussion of the harms and benefits of screening, and guidance on smoking cessation.7 The LCS process is therefore complex,8 with several potential barriers to widespread implementation and uptake.
Uptake of screening, or the proportion of eligible individuals that undergo the screening test, is considered an important measure as high rates of participation are generally associated with greater population benefit.9 Reports from the National Health Interview Survey (NHIS) and the Behavioral Risk Factor Surveillance System (BRFSS) indicate low (< 20%) uptake of LCS among screening-eligible adults in the USA.10–13 This estimate of LCS uptake is substantially lower than uptake rates for breast and colorectal cancer screening which range from 60 to 70%.14 Once identified as eligible, patients with a history of smoking may be hesitant to undergo screening because of fear, stigma, or self-blaming for smoking.15, 16 Current tobacco smoking, the primary risk factor for lung cancer, has been associated with lower cancer screening uptake, in general. In addition, tobacco use is associated with lower education and income levels both of which are associated with lower use of cancer screening.17 Furthermore, current tobacco use is highest among the uninsured and those with Medicaid compared to those with other public or private insurance, making access to screening difficult for those at greatest risk for lung cancer.18 Combined with these factors, health systems have taken varying approaches in the implementation of LCS, including SDM and tobacco cessation counseling, which are important components of LCS. These approaches include decentralized models that overwhelmingly rely on PCPs to determine patient eligibility, conduct SDM and tobacco cessation counseling, place LDCT orders, and follow up on abnormal findings and more centralized models that may require patients to attend an additional appointment or telehealth visit but offer staff specially trained to confirm eligibility, conduct LCS SDM and tobacco cessation, order and potentially assist in scheduling LDCTs, and provide follow-up of abnormal findings.19–21 We do not yet know the full impact of these different delivery approaches to LCS uptake and outcomes.
In this study, we aimed to determine if individual-, health-system-, neighborhood-level factors, or a combination of such multilevel factors are associated with the uptake of LCS (completion of LCS-LDCT) after a clinician referral/order for screening in five health systems. Little is known about the uptake of LCS in this highly modifiable window of the care delivery pathway.
METHODS
Study Population
We conducted a retrospective cohort study of adults receiving an order for LCS from a provider at five healthcare systems within the Population-based Research to Optimize the Screening Process (PROSPR)–Lung Consortium. As previously reported, the National Cancer Institute–funded PROSPR-Lung includes diverse community-based healthcare systems from across the USA, including Henry Ford Health (HFH), Kaiser Permanente Colorado (KPCO), Kaiser Permanente Hawaii (KPHI), Marshfield Clinic Health System (MCHS), and the University of Pennsylvania Health System (UPHS).8, 22–24 PROSPR-Lung has established an observational dataset of harmonized EHR data for a cohort of adults aged 35 to 89 years who were affiliated with one of the five health systems from January 1, 2010, to September 30, 2019. The single IRB of record is the KPCO Institutional Review Board which granted a waiver of written informed consent for the study.
Cohort and Data Sources
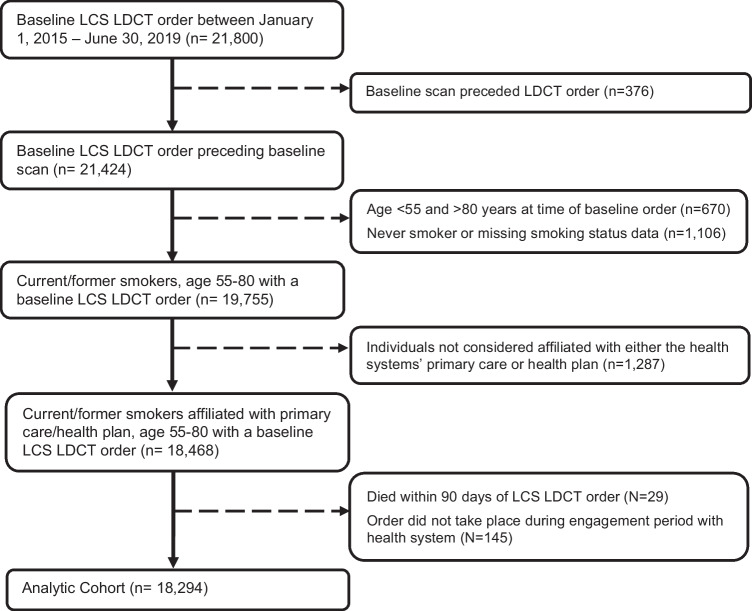
Individuals were included in this study if they were enrolled with one of the health systems’ affiliated health plans or otherwise engaged with the health systems’ primary care practice and had their first EHR order for a baseline screening LDCT between January 1, 2015, and June 30, 2019. This aligns with the start of CMS coverage of LDCT for LCS. Baseline orders were captured using each health system’s internal imaging order codes for LCS. We also included referrals for LDCT from one health system which changed workflow processes during the study period from clinicians placing orders to clinicians placing referrals to the site’s LCS program. We excluded individuals who were never smokers or age ineligible for screening according to 2013 USPSTF guidelines or who died within 90 days of LCS order (Fig. 1).
Figure 1.
Lung cancer screening low-dose CT order—cohort assembly.
The primary outcome, LCS uptake, was defined as the completion of an LCS-LDCT scan within 90 days of the screening order. Completed scans were identified by the presence of a CPT code for LCS (G0297 or S8032) between January 1, 2015, and September 30, 2019. Each patient’s earliest LCS-LDCT completed during this period was considered the baseline scan.
Individual-level covariates extracted from the EHR included date of birth, sex, chronic obstructive pulmonary disease (COPD) diagnosis, self-reported race and ethnicity, smoking status, pack-years smoked, and health insurance at the time of order. The presence of a prescription for a smoking cessation medication within 18 months prior to an LCS-LDCT order was also extracted from the EHR as identified via pharmacy orders and/or prescription fills for nicotine-replacement therapies, bupropion, varenicline, and clonidine. All health systems provided prescription orders and the three systems with affiliated health plans (HFHS, KPCO, KPHI) also provided prescription fills. We captured any prior history of lung cancer from each system’s tumor registry.
In addition to these individual-level covariates, we used the working knowledge of research team members employed by the health systems to categorize each health system’s LCS program structure as either centralized or decentralized.19–21 Decentralized programs (HFH (2015 to mid-2017), MCHS, and UPHS) were those in which primary care providers or their designated clinic staff were responsible for eligibility determination, SDM, placing the order for screening LDCT, and initial follow-up of abnormal findings. Centralized programs, in general, relied on the primary care provider or other outreach to refer eligible or likely eligible patients to the program. Specially trained core staff then delivered comprehensive SDM and scheduled or assisted in scheduling LDCT appointments.19 . Specifically at KPCO and KPHI, after the order or referral was placed by the PCP, a nurse navigator contacted the patient by telephone or the EHR secure messaging system to confirm LCS eligibility, reinforce SDM, and if the patient was eligible then they would assist in scheduling the LDCT. At HFH, the PCP was required to use a radiology LDCT order/referral template which determined a patient’s eligibility before routing the patient to the centralized program. The HFH centralized program would reach out and schedule patients for small group education and SDM sessions held at multiple locations across the health system and led by nurse practitioners. Patient questions are then addressed within the group or in private, based on patient preference. HFH processes allowed most patients, if desired, to complete LCS-LDCT on the same day as the small group education session.
As a neighborhood-level measure, we included the Yost State Index25, 26 calculated for the patient’s census tract of residence. The Yost State Index is a composite score that measures different aspects of socioeconomic status within a census tract based on median household income, median house value, median rent, percent of tract population below 150% of the poverty line, education index,27 percent working class, and percent unemployed. Furthermore, census tracts are categorized into socioeconomic quintiles with equal populations in each quintile within a given state.
Statistical Analysis
Descriptive analyses examined demographic factors (e.g., age, sex, race/ethnicity), smoking status, cessation medication prescription within 18 months prior to order, COPD, prior cancer diagnosis, insurance status, centralized/decentralized LCS program, Yost State Index, LDCT order year, and uptake of LCS by LDCT. LCS-LDCT uptake was defined as completing a screening LDCT within 90 days of order.
Chi-square and t-tests were used to examine the differences in the uptake of screening between levels of study covariates. The Cochran Armitage Test was used to examine the trend over time in uptake proportion. Multilevel models with a logit link, clustered on healthcare system with random intercept effect, were used to investigate the relationship between individual or system characteristics and uptake. Both univariable and multivariable models controlling for other covariates were examined and reported with odds ratios (ORs) with 95% confidence intervals (CIs). Two-sided p-values < 0.05 were considered statistically significant. All analyses were performed using R 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). Sensitivity analysis included restricting to the subset of the cohort with a definitively documented pack-year (30 +) history data.
RESULTS
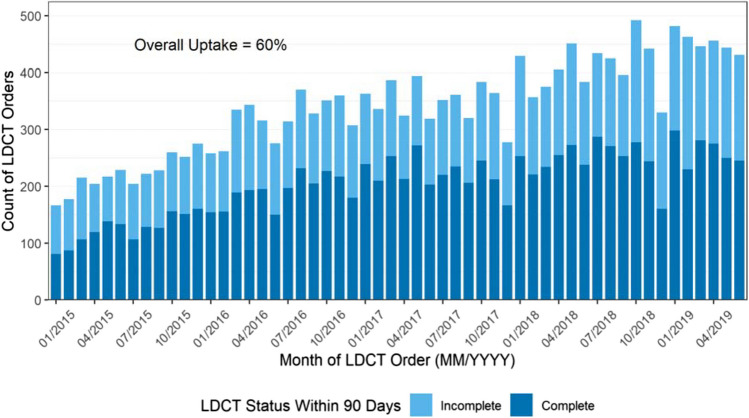
Among the 18,294 study-eligible individuals (Fig. 1), the mean age was 64.4 years (SD 6.0). Fifty-eight percent currently smoked, 48% were female, and 70% were non-Hispanic White (Table 1). Across all sites, monthly orders for LCS more than doubled from 166 in January 2015 to 431 in June 2019 (Fig. 2).
Table 1.
Patients Receiving a Baseline Order/Referral for Lung Cancer Screening by PROSPR-Lung Health System
| Characteristic | Overall N = 18,2942 |
HFH1 N = 58542 |
KPCO1 N = 69152 |
KPHI1 N = 10632 |
MCHS1 N = 12962 |
UPHS1 N = 31662 |
|---|---|---|---|---|---|---|
| Age | ||||||
| 55–59 | 4667 (25.5%) | 1655 (28.3%) | 1664 (24.1%) | 189 (17.8%) | 315 (24.3%) | 844 (26.7%) |
| 60–64 | 4999 (27.3%) | 1635 (27.9%) | 1845 (26.7%) | 260 (24.5%) | 372 (28.7%) | 887 (28.0%) |
| 65–69 | 4663 (25.5%) | 1390 (23.7%) | 1861 (26.9%) | 315 (29.6%) | 327 (25.2%) | 770 (24.3%) |
| 70 + | 3965 (21.7%) | 1174 (20.1%) | 1545 (22.3%) | 299 (28.1%) | 282 (21.8%) | 665 (21.0%) |
| Sex | ||||||
| Female | 8679 (47.4%) | 2841 (48.5%) | 3203 (46.3%) | 413 (38.9%) | 614 (47.4%) | 1608 (50.8%) |
| Male | 9615 (52.6%) | 3013 (51.5%) | 3712 (53.7%) | 650 (61.1%) | 682 (52.6%) | 1558 (49.2%) |
| Race/ethnicity | ||||||
| Asian/Pacific Islander | 780 (4.3%) | 40 (0.7%) | 110 (1.6%) | 597 (56.2%) | 1 (0.1%) | 32 (1.0%) |
| Hispanic | 882 (4.8%) | 58 (1.0%) | 746 (10.8%) | 38 (3.6%) | 14 (1.1%) | 26 (0.8%) |
| Non-Hispanic Black | 2699 (14.8%) | 1387 (23.7%) | 264 (3.8%) | 8 (0.8%) | 1 (0.1%) | 1039 (32.8%) |
| Non-Hispanic White | 12,892 (70.5%) | 3947 (67.4%) | 5386 (77.9%) | 369 (34.7%) | 1251 (96.5%) | 1939 (61.2%) |
| Other | 427 (2.3%) | 75 (1.3%) | 255 (3.7%) | 20 (1.9%) | 16 (1.2%) | 61 (1.9%) |
| Unknown | 614 (3.4%) | 347 (5.9%) | 154 (2.2%) | 31 (2.9%) | 13 (1.0%) | 69 (2.2%) |
| Chronic obstructive pulmonary disease3 | ||||||
| No | 11,683 (63.9%) | 3806 (65.0%) | 4368 (63.2%) | 614 (57.8%) | 684 (52.8%) | 2211 (69.8%) |
| Yes | 6611 (36.1%) | 2048 (35.0%) | 2547 (36.8%) | 449 (42.2%) | 612 (47.2%) | 955 (30.2%) |
| Prior cancer | ||||||
| No | 16,812 (91.9%) | 5403 (92.3%) | 6363 (92.0%) | 938 (88.2%) | 1157 (89.3%) | 2951 (93.2%) |
| Yes | 1482 (8.1%) | 451 (7.7%) | 552 (8.0%) | 125 (11.8%) | 139 (10.7%) | 215 (6.8%) |
| Smoking status | ||||||
| Current | 10,618 (58.0%) | 3626 (61.9%) | 3754 (54.3%) | 602 (56.6%) | 786 (60.6%) | 1850 (58.4%) |
| Former | 7676 (42.0%) | 2228 (38.1%) | 3161 (45.7%) | 461 (43.4%) | 510 (39.4%) | 1316 (41.6%) |
| Prior cessation Rx4 | ||||||
| No | 13,646 (74.6%) | 4453 (76.1%) | 5068 (73.3%) | 869 (81.7%) | 941 (72.6%) | 2315 (73.1%) |
| Yes | 4648 (25.4%) | 1401 (23.9%) | 1847 (26.7%) | 194 (18.3%) | 355 (27.4%) | 851 (26.9%) |
| Insurance | ||||||
| Commercial | 3267 (17.9%) | 1727 (29.5%) | 0 (0.0%) | 0 (0.0%) | 213 (16.4%) | 1327 (41.9%) |
| HMO/Health System Plan | 9344 (51.1%) | 705 (12.0%) | 6856 (99.1%) | 1063 (100.0%) | 720 (55.6%) | 0 (0.0%) |
| Medicaid | 372 (2.0%) | 72 (1.2%) | 0 (0.0%) | 0 (0.0%) | 19 (1.5%) | 281 (8.9%) |
| Medicare | 1828 (10.0%) | 385 (6.6%) | 0 (0.0%) | 0 (0.0%) | 82 (6.3%) | 1361 (43.0%) |
| Unknown | 3483 (19.0%) | 2965 (50.6%) | 59 (0.9%) | 0 (0.0%) | 262 (20.2%) | 197 (6.2%) |
| Centralized LCS program | ||||||
| No | 6925 (37.9%) | 2463 (42.1%) | 0 (0.0%) | 0 (0.0%) | 1296 (100.0%) | 3166 (100.0%) |
| Yes | 11,369 (62.1%) | 3391 (57.9%) | 6915 (100.0%) | 1063 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Yost State Index (quintile) | ||||||
| 5 (high) | 3461 (19.4%) | 1013 (17.7%) | 1330 (19.4%) | 184 (17.4%) | 18 (1.5%) | 916 (30.7%) |
| 4 | 3417 (19.2%) | 1116 (19.5%) | 1483 (21.6%) | 243 (23.0%) | 162 (13.4%) | 413 (13.9%) |
| 3 | 3572 (20.0%) | 1119 (19.6%) | 1530 (22.3%) | 226 (21.4%) | 284 (23.6%) | 413 (13.9%) |
| 2 | 3409 (19.1%) | 1074 (18.8%) | 1266 (18.4%) | 222 (21.0%) | 465 (38.6%) | 382 (12.8%) |
| 1 (low) | 3966 (22.2%) | 1394 (24.4%) | 1257 (18.3%) | 182 (17.2%) | 276 (22.9%) | 857 (28.7%) |
| Unknown | 469 | 138 | 49 | 6 | 91 | 185 |
| Order year | ||||||
| 2015 | 2649 (14.5%) | 247 (4.2%) | 1605 (23.2%) | 278 (26.2%) | 134 (10.3%) | 385 (12.2%) |
| 2016 | 3820 (20.9%) | 978 (16.7%) | 1737 (25.1%) | 294 (27.7%) | 228 (17.6%) | 583 (18.4%) |
| 2017 | 4182 (22.9%) | 1665 (28.4%) | 1355 (19.6%) | 173 (16.3%) | 289 (22.3%) | 700 (22.1%) |
| 2018 | 4921 (26.9%) | 1991 (34.0%) | 1441 (20.8%) | 208 (19.6%) | 390 (30.1%) | 891 (28.1%) |
| 20195 | 2722 (14.9%) | 973 (16.6%) | 777 (11.2%) | 110 (10.3%) | 255 (19.7%) | 607 (19.2%) |
1HFH Henry Ford Health, KPCO Kaiser Permanente Colorado, KPH Kaiser Permanente Hawaii, MCHS Marshfield Clinic Health System, UPHS University of Pennsylvania Health System
2n (%)
3Chronic obstructive pulmonary disease (COPD) in the year prior to the first LDCT order
4Cessation medication prescribed within 18 months prior to order
5Orders only through June 2019
Figure 2.
LDCT status within 90 days from order (01/2015–06/2019).
Overall, 60% of patients with an order completed an LCS-LDCT within 90 days (Fig. 2) with uptake after a PCP order across health systems varying from 41 to 87%. There was an increase in uptake overall from 56% in 2015 to 64% in 2017 (Cochran Armitage test, p < 0.001). In 2018 and 2019, however, uptake waned with 60% and 58% completing LDCT (compared to 2017, p < 0.001), respectively. For those that completed screening, the median time from order to LCS-LDCT overall was 18 days (7 days (1st quartile), 41 days (3rd quartile)) and ranged from a median of 0 to 21 days across the five health systems. (See supplemental Fig. 1 for distribution of days to screen.)
In univariable analyses, patients who were older, male (OR 1.11, 95% CI 1.05–1.18), formerly smoked (OR 1.23, CI 1.16–1.31), or had COPD (OR 1.13, CI 1.06–1.20) were more likely to complete screening, as were those who received an order for screening at a health system with a centralized LCS program. Those with other (OR 0.70, CI 0.57–0.85) or unknown (OR 0.77, CI 0.65–0.91) race and unknown insurance status (OR 0.76, CI 0.66–0.88) were less likely to complete screening than non-Hispanic Whites and those with Medicare (Table 2).
Table 2.
Uptake of Lung Screening CT Within 90 Days and Associated Patient, System, and Neighborhood Factors Among Those with a Provider Order for CT
| CT complete within 90 days | Univariable results | |||||
|---|---|---|---|---|---|---|
| Characteristic | No N = 72821 |
Yes N = 11,0121 |
p-value2 | OR3 | 95% CI3 | p-value |
| Age | < 0.001 | |||||
| 55–59 | 2038 (43.7%) | 2629 (56.3%) | – | – | ||
| 60–64 | 2019 (40.4%) | 2980 (59.6%) | 1.13 | 1.04, 1.23 | 0.004 | |
| 65–69 | 1740 (37.3%) | 2923 (62.7%) | 1.27 | 1.17, 1.38 | < 0.001 | |
| 70 + | 1485 (37.5%) | 2480 (62.5%) | 1.25 | 1.15, 1.37 | < 0.001 | |
| Sex | < 0.001 | |||||
| Female | 3604 (41.5%) | 5075 (58.5%) | – | – | ||
| Male | 3678 (38.3%) | 5937 (61.7%) | 1.11 | 1.05, 1.18 | < 0.001 | |
| Race/ethnicity | < 0.001 | |||||
| Asian/Pacific Islander | 166 (21.3%) | 614 (78.7%) | 1.19 | 0.96, 1.48 | 0.116 | |
| Hispanic | 336 (38.1%) | 546 (61.9%) | 1.03 | 0.89, 1.19 | 0.676 | |
| Non-Hispanic Black | 1252 (46.4%) | 1447 (53.6%) | 1.01 | 0.92, 1.10 | 0.889 | |
| Non-Hispanic White | 5038 (39.1%) | 7854 (60.9%) | – | – | ||
| Other | 208 (48.7%) | 219 (51.3%) | 0.70 | 0.57, 0.85 | < 0.001 | |
| Unknown | 282 (45.9%) | 332 (54.1%) | 0.77 | 0.65, 0.91 | 0.002 | |
| COPD | < 0.001 | |||||
| No | 4853 (41.5%) | 6830 (58.5%) | – | – | ||
| Yes | 2429 (36.7%) | 4182 (63.3%) | 1.13 | 1.06, 1.20 | < 0.001 | |
| Prior cancer | 0.053 | |||||
| No | 6727 (40.0%) | 10,085 (60.0%) | – | – | ||
| Yes | 555 (37.4%) | 927 (62.6%) | – | – | ||
| Smoking status | < 0.001 | |||||
| Current | 4430 (41.7%) | 6188 (58.3%) | – | – | ||
| Former | 2852 (37.2%) | 4824 (62.8%) | 1.23 | 1.16, 1.31 | < 0.001 | |
| Prior cessation Rx | 0.648 | |||||
| No | 5445 (39.9%) | 8201 (60.1%) | – | – | ||
| Yes | 1837 (39.5%) | 2811 (60.5%) | 1.04 | 0.97, 1.12 | 0.244 | |
| Insurance | < 0.001 | |||||
| Commercial | 1453 (44.5%) | 1814 (55.5%) | 0.96 | 0.84, 1.08 | 0.469 | |
| HMO/Health System | 3240 (34.7%) | 6104 (65.3%) | 1.10 | 0.92, 1.32 | 0.299 | |
| Medicaid | 192 (51.6%) | 180 (48.4%) | 0.99 | 0.79, 1.24 | 0.923 | |
| Medicare | 937 (51.3%) | 891 (48.7%) | – | – | ||
| Unknown | 1460 (41.9%) | 2023 (58.1%) | 0.76 | 0.66, 0.88 | < 0.001 | |
| Centralized LCS program | S | < 0.001 | ||||
| No | 3050 (44.0%) | 3875 (56.0%) | – | – | ||
| Yes | 4232 (37.2%) | 7137 (62.8%) | 1.16 | 1.05, 1.29 | 0.005 | |
| Yost State Index4 (quintile) | 0.008 | |||||
| 5 | 1434 (41.4%) | 2027 (58.6%) | – | – | ||
| 4 | 1346 (39.4%) | 2071 (60.6%) | 0.91 | 0.82, 1.00 | 0.053 | |
| 3 | 1429 (40.0%) | 2143 (60.0%) | 0.85 | 0.77, 0.94 | 0.001 | |
| 2 | 1275 (37.4%) | 2134 (62.6%) | 0.89 | 0.80, 0.98 | 0.021 | |
| 1 | 1615 (40.7%) | 2351 (59.3%) | 0.93 | 0.84, 1.02 | 0.112 | |
| Unknown | 183 | 286 | ||||
| Order year | < 0.001 | |||||
| 2015 | 1151 (43.5%) | 1498 (56.5%) | – | – | ||
| 2016 | 1526 (39.9%) | 2294 (60.1%) | 1.20 | 1.08, 1.34 | < 0.001 | |
| 2017 | 1508 (36.1%) | 2674 (63.9%) | 1.50 | 1.35, 1.66 | < 0.001 | |
| 2018 | 1954 (39.7%) | 2967 (60.3%) | 1.27 | 1.15, 1.41 | < 0.001 | |
| 2019 | 1143 (42.0%) | 1579 (58.0%) | 1.17 | 1.04, 1.31 | 0.008 | |
1n (%)
2Pearson’s chi-squared test
3OR odds ratio, CI confidence interval
4N = 17,825
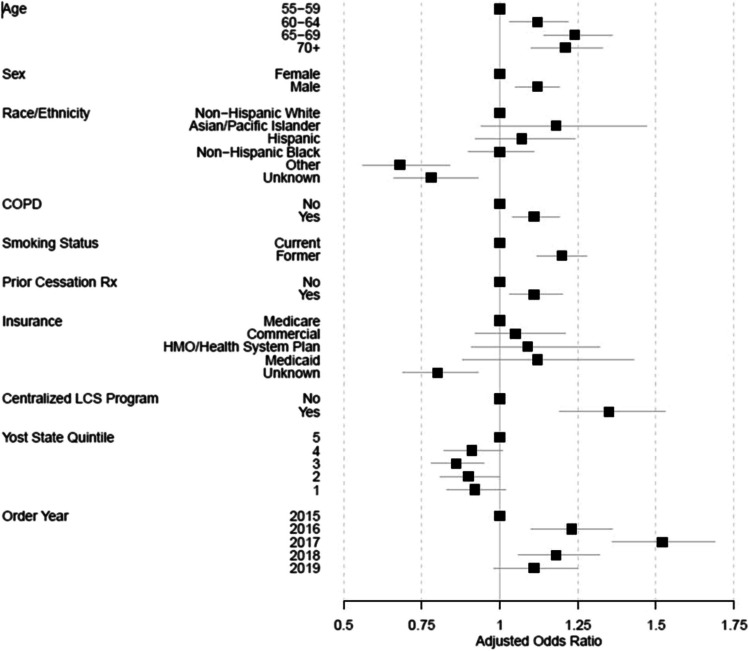
Multivariable results did not differ greatly from the univariable results, although receiving a smoking cessation medication within the 18-month period prior to the screening order was significantly associated with completion of LCS (aOR 1.11, CI 1.03–1.20, p = 0.005) after accounting for other factors (Fig. 3 and Supplemental Table 1). After the year of the LCS-LDCT order, the variables accounting for the greatest variation in screening uptake were smoking status (former), insurance, centralization of the screening program, and age.
Figure 3.
Association of patient, system, and neighborhood factors with completing a screening CT within 90 days of referral/order for screening (adjusted odds ratio, 95% CI).
DISCUSSION
In the USA, LCS rates have remained low. We identified a 40% drop-off between a provider order for LCS and completion of a LDCT among screening-eligible patients within five community-based health systems. The proportion completing LDCT among those with a LDCT order differed substantially across health systems (41–87%) and was associated with factors at the individual- and health-system levels. After controlling for health system, patient factors including older age, male sex, former smoking status, and having COPD were positively associated with uptake (completion of LCS-LDCT). Receiving a cessation medication within 18 months prior to the order (multivariable model) and receiving an order within a centralized LCS program (univariable and multivariable models) were also positively associated with the completion of LCS-LDCT. Other and unknown race and unknown insurance status were negatively associated with uptake. We noted that CT completion rates began to decrease in four of our five systems in 2018 and 2019 after seeing gains between 2015 and 2017. This may have been due to the growth of screening programs and systems’ managing higher volumes of annual screenings along with baseline screenings. Overall smoking status, insurance, centralization, and age explained the greatest variation, after accounting for health system and year of scan.
Only a few previous studies have reported on the uptake of LCS-LDCT after an order for screening, and findings from those studies are consistent with ours regarding both the proportion of those who receive an order who go on to be screened as well as the factors associated with screening uptake. For example, in 2016, Begnaud et al.28 reported that only 63% of 902 orders in a Minnesota-based health system resulted in a completed lung screening CT. From a Texas health system, Gerber et al.29 reported that among those who agreed to participate in a trial testing phone-based LDCT navigation, 81% completed a LCS-LDCT compared to 62% of those who did not enroll and 49% of those who could not be reached. These findings in single site studies, along with our multisite study which found a similar proportion (60%) of patients completing screening overall but substantial differences in uptake across sites, suggests that there are processes under the control of health systems, which can increase uptake of LCS-LDCT.
We found that patients receiving orders for screening within health systems that had centralized LCS programs were more likely to complete screening (63% vs. 56%). In our prior work23 and the work of others,21 centralized programs have also been associated with greater annual adherence to screening. However, our findings between centralized and decentralized programs may in part be explained by differences in processes. Patients who attend centralized programs may have already made up their minds to undergo screening or may have a more positive attitude toward screening. In addition, one health system with a centralized program made it possible for patients to routinely undergo LCS-LDCT on the same day as SDM. Convenience may be an essential component for higher uptake. A greater understanding of the most impactful components of centralized programs with regard to uptake will help health systems determine whether centralization, which is currently implemented in many different ways, is needed permanently to manage the complex lung screening process8 or only needed temporarily to expand LCS. It is likely that some of the most useful processes from centralized programs (e.g., use of established decision aids, same-day LCS-LDCT access) can be integrated into decentralized structures of LCS to help increase uptake.
We also found patients with former smoking status and those with documentation of a cessation medication order prior to screening were more likely to complete screening relative to their counterparts. Most reports on individual-level factors associated with uptake of LCS-LDCT have come from national survey studies, including the BRFSS and NHIS, which use self-report of screening. In general, these studies of screening-eligible adults have not shown a difference in self-reported CT by smoking status.11, 13, 30 Interestingly, two studies, one from the Veterans Administration and the other from a community health system, reported patients who currently smoke were less likely to decline LCS31 and were more likely to receive an order for LCS.32 These findings along with ours, which showed patients who currently smoke are less likely to complete an LCS-LDCT after receiving an order for screening, suggest that these patients may be hesitant to voice their concerns regarding LCS when ordered and/or have greater barriers to screening than patients who formerly smoked. In a meta-analysis, Lopez-Olivo et al.33 found current smoking to also be associated with lower annual adherence to LCS. With regard to our findings for smoking cessation medication orders, Poghosyan et al. using the 2017 BRFSS survey reported that patients who had tried to quit smoking in the past year were more likely to self-report lung screening CT. This would suggest that patients who are trying to quit smoking may be more engaged in their health management, including tobacco cessation and LCS, or that healthcare providers that are discussing and prescribing cessation aides may be more successful at LCS SDM. Health systems may be able to increase uptake of LCS, in part, by targeting patients with evidence of cessation attempts in their records or by identifying and providing training for providers who have low rates of prescribing cessation medications and referring for LCS. For some patients, cessation or readiness for tobacco cessation may be a necessary prior step to screening.
Finally, we found patients that had unknown race or insurance status, which in this study includes both individuals who have undocumented insurance or who may be uninsured and are less likely to undergo LDCT after an order for screening. Racial and ethnic minorities are more likely to be uninsured.34 Interestingly, the patients in our study have received healthcare to the extent that an LCS-LDCT order was placed but lack of insurance creates a barrier to testing and any follow-up that may be needed. It should be noted that all of the participating health systems are located in states that have expanded Medicaid and have systems in place that, if required, assist with insurer authorization, yet a gap appears to still exist. Recent findings noted by Wain et al.35 who used KPCO and KPHI where detailed benefit plan data was available, found that conditional on receiving a LCS order, enrollment in a deductible plan reduces receipt of baseline LCS-LDCT by 7 percentage points. As noted by Rivera et al., programs36 such as the CDC’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP)37 and the Colorectal Cancer Control Program38 help uninsured patients get needed screening exams. As yet, a national program has not been implemented for LCS.
While our study uniquely provides insights associated with LCS-LDCT completion after a clinician order, it is not without some limitations. First, due to the observational design, we were unable to confirm eligibility for screening based on pack-years of smoking. However, we did conduct a sub-analysis including only those patients with complete pack-year data (data not shown) and found no differences in findings. Our follow-up time for assessing completion was only 90 days. As the PROSPR-Lung data matures, we will be able to look at longer follow-up times to confirm our findings and potentially look at patterns of orders and smoking cessation assistance that eventually lead to the completion of LCS-LDCT. Most previous studies have relied on self-report of a CT for screening. We had medical record verification of screening LDCT. A limitation of using medical record data, however, is a void in self-reported individual-level data on socioeconomic factors, like education or income. We did control for insurance and the Yost Index of socioeconomic status, albeit a neighborhood, not individual-level, measure. Others have reported that fear, worry,15, 16 and stigma as well as a lower perceived efficacy of lung screening39 among patients who smoke may reduce uptake. Our observational study did not include these factors. Furthermore, we only captured one health system level factor, centralization, and were not able to capture some workflow process details of centralized programs such as the determination that patients were ineligible or declined screening as many of these details are not easily extracted from the EHR. We also did not have data across sites on distance to screening facilities or ordering provider characteristics. In assessing multilevel factors influencing uptake, factors such as provider discipline, age, sex, and visit history with the patient will be important to evaluate.
In conclusion, addressing the gap between LCS order and LDCT completion could substantially increase currently observed LCS rates. A thorough understanding of the barriers and facilitators to lung screening uptake, many of which are under the control of health systems, is needed in order to maximize the uptake of LCS among eligible individuals. Future studies with diverse patients and a broader spectrum of health systems and delivery models will further help to identify the best practices for increasing LCS uptake and delivering high-quality care.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Number UM1CA221939. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
PROSPR DataShare, or PDS, allows individuals who are not part of the PROSPR initiative, known as external researchers, to request certain data from the PROSPR 2 initiative. PDS also allows external researchers to propose ancillary studies (https://healthcaredelivery.cancer.gov/prospr/datashare/).
Declarations
Conflict of Interest
ABH reports grants from Biodesix, Inc. outside the submitted work. JEL and CND report grant funding from Genentech outside of the submitted work. MJS reports other support from Intuitive, Gongwin Biopharm, and Airiver outside of the submitted work. KR reports grants from AstraZeneca, Pfizer, and personal fees from Merck outside of the submitted work. AV reports funding from MagArray, Inc, Precyte, Inc., Optellum, Ltd, NCCN, Gordon and Betty Moore Foundation, and Lungevity outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 5.Krist AH, Davidson KW, Mangione CM, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 6.Final National Coverage Determination on Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) CAG-00439N (2015).
- 7.Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) CAG-00439R (2022).
- 8.Rendle KA, Burnett-Hartman AN, Neslund-Dudas C, et al. Evaluating Lung Cancer Screening Across Diverse Healthcare Systems: A Process Model from the Lung PROSPR Consortium. Cancer Prev Res (Phila). 2020;13(2):129–136. doi: 10.1158/1940-6207.CAPR-19-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barratt A, Mannes P, Irwig L, Trevena L, Craig J, Rychetnik L. Cancer screening. J Epidemiol Community Health. 2002;56(12):899–902. doi: 10.1136/jech.56.12.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo J, Shen C, Volk RJ, Shih YT. Use of CT and Chest Radiography for Lung Cancer Screening Before and After Publication of Screening Guidelines: Intended and Unintended Uptake. JAMA Intern Med. 2017;177(3):439–441. doi: 10.1001/jamainternmed.2016.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham D, Bhandari S, Pinkston C, Oechsli M, Kloecker G. Lung Cancer Screening Registry Reveals Low-dose CT Screening Remains Heavily Underutilized. Clin Lung Cancer. 2020;21(3):e206–e211. doi: 10.1016/j.cllc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Richards TB, Soman A, Thomas CC, et al. Screening for Lung Cancer - 10 States, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(8):201–206. doi: 10.15585/mmwr.mm6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and Trends in Cancer Screening in the United States. Prev Chronic Dis. 2018;15:E97. doi: 10.5888/pcd15.170465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter-Harris L, Brandzel S, Wernli KJ, Roth JA, Buist DSM. A qualitative study exploring why individuals opt out of lung cancer screening. Family Practice. 2017;34(2):239–244. doi: 10.1093/fampra/cmw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter-Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: what do long-term smokers know and believe? Health Expectations. 2017;20(1):59–68. doi: 10.1111/hex.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasting ML, Haggstrom DA, Lee JL, Dickinson SL, Shields CG, Rawl SM. Financial hardship is associated with lower uptake of colorectal, breast, and cervical cancer screenings. Cancer Causes Control. 2021;32(10):1173–1183. doi: 10.1007/s10552-021-01465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelius ME, Loretan CG, Jamal A, et al. Tobacco Product Use Among Adults - United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(18):475–483. doi: 10.15585/mmwr.mm7218a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alishahi Tabriz A, Neslund-Dudas C, Turner K, Rivera MP, Reuland DS, Elston Lafata J. How Health-Care Organizations Implement Shared Decision-making When It Is Required for Reimbursement: The Case of Lung Cancer Screening. Chest. 2021;159(1):413–425. doi: 10.1016/j.chest.2020.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunez ER, Slatore CG, Tanner NT, et al. National Survey of Lung Cancer Screening Practices in Veterans Health Administration Facilities. Am J Prev Med. 2023;10.1016/j.amepre.2023.05.005 [DOI] [PMC free article] [PubMed]
- 21.Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-Recommended Lung Cancer Screening Adherence Is Superior With a Centralized Approach. Chest. 2022;161(3):818–825. doi: 10.1016/j.chest.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Ritzwoller DP, Meza R, Carroll NM, et al. Evaluation of Population-Level Changes Associated With the 2021 US Preventive Services Task Force Lung Cancer Screening Recommendations in Community-Based Health Care Systems. JAMA Netw Open. 2021;4(10):e2128176. doi: 10.1001/jamanetworkopen.2021.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim RY, Rendle KA, Mitra N, et al. Racial Disparities in Adherence to Annual Lung Cancer Screening and Recommended Follow-up Care: A Multicenter Cohort Study. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202111-1253OC [DOI] [PMC free article] [PubMed]
- 24.Vachani A, Carroll NM, Simoff MJ, et al. Stage Migration and Lung Cancer Incidence After Initiation of Low-Dose Computed Tomography Screening. J Thorac Oncol. 2022;17(12):1355–1364. doi: 10.1016/j.jtho.2022.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. doi: 10.1007/s10552-013-0310-1. [DOI] [PubMed] [Google Scholar]
- 26.Boscoe FP, Liu B, Lee F. A comparison of two neighborhood-level socioeconomic indexes in the United States. Spat Spatiotemporal Epidemiol. 2021;37:100412. doi: 10.1016/j.sste.2021.100412. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States) Cancer Causes Control. 1998;9(4):369–380. doi: 10.1023/a:1008811432436. [DOI] [PubMed] [Google Scholar]
- 28.Begnaud A, Hall T, Allen T. Lung Cancer Screening With Low-Dose CT: Implementation Amid Changing Public Policy at One Health Care System. Am Soc Clin Oncol Educ Book. 2016;35:e468–e475. doi: 10.14694/EDBK_15919510.1200/EDBK_159195. [DOI] [PubMed] [Google Scholar]
- 29.Gerber DE, Hamann HA, Chavez C, et al. Tracking the Nonenrolled: Lung Cancer Screening Patterns Among Individuals not Accrued to a Clinical Trial. Clin Lung Cancer. 2020;21(4):326–332. doi: 10.1016/j.cllc.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayan AK, Gupta Y, Little BP, Shepard JO, Flores EJ. Lung cancer screening eligibility and use with low-dose computed tomography: Results from the 2018 Behavioral Risk Factor Surveillance System cross-sectional survey. Cancer. 2021;127(5):748–756. doi: 10.1002/cncr.33322. [DOI] [PubMed] [Google Scholar]
- 31.Nunez ER, Caverly TJ, Zhang S, et al. Factors Associated With Declining Lung Cancer Screening After Discussion With a Physician in a Cohort of US Veterans. JAMA Netw Open. 2022;5(8):e2227126. doi: 10.1001/jamanetworkopen.2022.27126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Chung S, Wei EK, Luft HS. New recommendation and coverage of low-dose computed tomography for lung cancer screening: uptake has increased but is still low. BMC Health Serv Res. 2018;18(1):525. doi: 10.1186/s12913-018-3338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Olivo MA, Maki KG, Choi NJ, et al. Patient Adherence to Screening for Lung Cancer in the US: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(11):e2025102. doi: 10.1001/jamanetworkopen.2020.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Health Insurance Coverage in the United States: 2016. (U.S. Census Bureau) (2017).
- 35.Wain K, Carroll NM, Honda S, Oshiro C, Ritzwoller DP. Individuals Eligible for Lung Cancer Screening Less Likely to Receive Screening When Enrolled in Health Plans With Deductibles. Med Care. 2023;10.1097/MLR.0000000000001903 [DOI] [PMC free article] [PubMed]
- 36.Raghavan D, Wheeler M, Doege D, et al. Initial Results from Mobile Low-Dose Computerized Tomographic Lung Cancer Screening Unit: Improved Outcomes for Underserved Populations. Oncologist. 2020;25(5):e777–e781. doi: 10.1634/theoncologist.2019-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. National breast and cervical cancer early detection program (NBCCEDP). Centers for Disease Control and Prevention. https://www.cdc.gov/cancer/nbccedp/about.htm
- 38.Joseph DA, Redwood D, DeGroff A, Butler EL. Use of Evidence-Based Interventions to Address Disparities in Colorectal Cancer Screening. MMWR Suppl. 2016;65(1):21–28. doi: 10.15585/mmwr.su6501a5. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62(2):126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
PROSPR DataShare, or PDS, allows individuals who are not part of the PROSPR initiative, known as external researchers, to request certain data from the PROSPR 2 initiative. PDS also allows external researchers to propose ancillary studies (https://healthcaredelivery.cancer.gov/prospr/datashare/).