Abstract
The class I epitope of streptococcal M protein is an epidemiological marker for acute rheumatic fever (ARF)-associated serotypes of group A streptococci and is recognized by anti-M protein monoclonal antibody (MAb) 10B6. Using MAb 10B6, we determined the relationship between the class I epitope of M protein and the α-helical coiled-coil protein myosin. MAb 10B6 reacted by enzyme-linked immunosorbent assay and Western blotting with human cardiac myosin and rabbit skeletal myosin and its heavy meromyosin (HMM) subfragment. Overlapping synthetic peptides of M5 protein were used to identify the region of M5 protein recognized by MAb 10B6. Two C repeat peptides (C2A and C3) containing the amino acid sequence KGLRRDLDASREAK reacted with MAb 10B6. Partial sequence identity, RRDL, was found in the HMM fragment of myosin, which reacted with MAb 10B6. However, not all peptides of M5 protein and myosin containing the RRDL sequence reacted with MAb 10B6. ARF sera and sera from uncomplicated pharyngitis (UNC) reacted with C repeat region peptides of M protein, while acute glomerulonephritis sera were not as reactive. Affinity-purified human antibody to peptide C3 reacted with myosin. The data demonstrate that the class I epitope of M protein is immunologically cross-reactive with myosin and the HMM subfragment, and antibodies to peptide C3 and myosin were present in ARF and UNC sera.
Acute rheumatic fever (ARF) is an inflammatory disease that can follow group A streptococcal pharyngitis. The most serious clinical manifestation is rheumatic carditis; however, arthritis, chorea, erythema marginatum, or subcutaneous nodules may be present (40, 41). The pathogenesis of ARF is thought to be mediated by autoimmune mechanisms activated during a streptococcal infection (40). The autoimmune hypothesis is supported by a number of previous observations, including a time interval of at least 3 weeks between the initial streptococcal throat infection and the onset of ARF (40, 41), the identification of heart-reactive immunoglobulin (Ig) and complement deposits in the myocardium of patients with fatal rheumatic carditis (25–27, 30), and the elevation of heart-reactive antibodies in the sera of patients with ARF (46). Cardiac myosin has been identified as one of the cardiac antigens recognized by these heart-reactive antistreptococcal autoantibodies (13, 29).
Streptococcal M protein, an α-helical coiled-coil protein, structurally and immunologically mimics host tissue antigens, particularly the rod region of myosin (12, 14, 15, 17, 34, 35). Sequence analysis has revealed that streptococcal M proteins contain blocks of internally repeated amino acid sequences referred to as A, B, and C repeat regions (19). The NH2-terminal nonrepeat and A repeat regions contain determinants of type specificity, while epitopes found in the B and more highly conserved C repeat regions may be common to different M serotypes (19). While there are nearly 100 different serological types of group A streptococcal M protein, epidemiological studies indicate that only a limited number of M protein serotypes are associated with ARF outbreaks (6). This finding suggests that certain M protein serotypes may be more rheumatogenic than others. In a previous attempt to classify streptococcal serotypes according to their rheumatogenic capacity, Widdowson identified human antisera directed to a non-type-specific protein moiety of M protein known as M-associated protein (44, 45). However, a more recent classification scheme has been proposed by Bessen and colleagues, in which streptococcal serotypes were grouped based on the expression of a conserved surface-exposed M protein epitope (4). It was demonstrated that the M serotypes associated with the majority of ARF outbreaks possessed an epitope (class I) defined by monoclonal antibody (MAb) probes 10B6 and 10F5. The sequence of the 10B6 and 10F5 epitope was localized to a 15-amino-acid fragment within the C repeat region of the type 6 M protein (23). The remaining serotypes (class II) lack this epitope or the determinant is structurally inaccessible in those strains. There was a close parallel between serotypes designated class I and those serotypes previously classified as M-associated protein I by Widdowson (44, 45). The fact that only certain serotypes within class I streptococci are rheumatogenic implies that these organisms are of a phenotype that is capable of inducing ARF (4). This implication is supported in part by a recent publication in which it was shown that sera of ARF patients contained high levels of antibodies to the class I epitope, suggesting that their disease was the result of an infection by a class I streptococcus (5).
Elevated titers of antibodies to many streptococcal antigens (2), including M protein and the self-antigen myosin (12–15, 17, 29), are associated with ARF. While antibodies to M protein are crucial for the opsonization of streptococci, they have also been implicated in the immunological cross-reactions between streptococci and host tissue antigens such as cardiac myosin (12–15, 17, 29). In earlier studies, many of these cross-reactive epitopes have been localized to the N-terminal, hypervariable A and B repeat regions of the M molecule (12, 15, 17). Myosin-reactive antibodies, found to be elevated in almost all cases of ARF (13), have been shown to bind to human heart tissue and to cross-react with streptococcal M protein (12). Previous studies have demonstrated that immunization of animals with the cell walls of certain strains of group A streptococci resulted in the production of heart-reactive antibodies which could be adsorbed with streptococcal extracts containing streptococcal M protein (16, 24, 28). Human MAbs or myosin affinity-purified antibodies produced from patients with ARF cross-reacted with streptococcal M protein and human cardiac myosin and contributed to the presence of heart-cross-reactive antistreptococcal antibodies in ARF (12, 13, 39). More recent studies have identified cytotoxic antistreptococcal/antimyosin MAbs from rheumatic carditis patients (1). Antimyosin antibody has been shown to deposit in the heart tissues of susceptible mice (31), and a cytotoxic mouse antistreptococcal/antimyosin antibody which binds to the surface of heart cells and to the α-helical coiled coil molecule laminin has been described (10).
Identification of myosin cross-reactive epitopes of M protein recognized in ARF has been reported for the amino-terminal half of the molecule (12, 15, 17), and a study by Vashishtha and Fischetti demonstrated antimyosin antibody responses to the C repeat region. However, the reactivity was directed only to denatured myosin (43). More recently, studies of the C repeat or carboxy-terminal region of M protein have shown T-cell cross-reactions with myosin (38). The goal of the present study was to investigate the possibility that the class I epitope in the C repeat region of M protein cross-reacts immunologically with myosin. In this study we show that MAb 10B6, which recognizes the class I epitope of M protein, reacts with cardiac and skeletal myosin. This study also demonstrates that ARF and UNC sera react with a site in the conserved C repeat region of M protein within the class I epitope of rheumatogenic M protein serotypes. The new data show that in addition to previously described N-terminal epitopes, the class I epitope of streptococcal M protein is immunologically cross-reactive with myosin.
(Portions of this work were presented at the XIII International Lancefield Society Meeting on Streptococci and Streptococcal Diseases at the Pasteur Institute in Paris, France, in September 1996.)
MATERIALS AND METHODS
Human sera and antibodies.
Sera from 12 patients with ARF were from the Oklahoma Children’s Heart Center, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, while poststreptococcal acute glomerulonephritis (AGN) sera were provided by Barry Gray, Department of Pediatrics-Infectious Diseases, University of Alabama at Birmingham, Birmingham, Alabama. Sera from 12 patients with uncomplicated streptococcal pharyngitis (UNC) were provided by Penelope Shackelford, Department of Pediatrics—Infectious Diseases, Washington University School of Medicine, St. Louis, Missouri. Sera from 9 patients with nonrheumatic myocarditis were supplied by Noel Rose and Eyal Taylor, Department of Microbiology and Immunology, Johns Hopkins University, Baltimore, Maryland. Eight normal human serum samples (anti-streptolysin O [ASO], <50 Todd units) were obtained from the Oklahoma Children’s Hospital. The normal human serum pool was obtained from the local blood bank from five healthy individuals. MAb 10B6, a mouse anti-M protein antibody, was previously produced, characterized, and described (23).
Antigens and peptides.
Cardiac myosin was purified from human heart muscle as previously described by Dell et al. and Tobacman and Adelstein (18, 42). Briefly, heart tissue was minced and homogenized on ice in 40 mM KCl, 20 mM imidazole-HCl (pH 7.0), 5 mM EGTA, 5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF) per ml, and 1 mg of leupeptin for 15 s. The washed myofibrils were collected by centrifugation at 16,000 × g for 10 min, resuspended in extraction buffer (0.3 M KCl, 0.15 M K2HPO4 [pH 7.0], 1 mM EGTA, 5 mM dithiothreitol, 0.5 mM PMSF, 1 mg of leupeptin per ml, 5 mg of N-α-tosyl-lysine-l-chloromethyl ketone [TLCK] per ml), and homogenized on ice three times for 30 s each. The homogenate was incubated on ice for 30 min and clarified by centrifugation at 16,000 × g for 10 min. The extracted actomyosin was precipitated from the supernatant by adding 10 volumes of cold distilled water and adjusting the pH to 6.5. Actomyosin was collected by centrifugation at 16,000 × g for 10 min and resuspended in a minimal volume of extraction buffer. With gentle stirring, KCl was added to a final concentration of 0.5 M, and ammonium sulfate was added to 33% to facilitate actin precipitation. Once the suspension was homogeneous, MgCl2 and ATP were added to 5 and 10 mM, respectively, and then actin was removed by immediate centrifugation at 20,000 × g for 15 min. The soluble myosin was stored at 4°C in the presence of the protease inhibitors 0.5 mM PMSF, 1 mg of leupeptin per ml, and 5 mg of TLCK per ml, which were present in all preparations of the myosin. Purified rabbit skeletal myosin, tropomyosin, light meromyosin, and heavy meromyosin were purchased from Sigma Chemical Co. (St. Louis, Mo.). The purity of all proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblotting with antigen-specific antibodies. Synthetic, overlapping peptides corresponding to the sequence of the type 5 streptococcal M protein Streptococcus pyogenes Manfredo (18) were synthesized by Ken Jackson (Molecular Biology Resource Facility, St. Francis Hospital of Tulsa Medical Research Institute, Oklahoma City, Okla.). The M5 peptides were 16 to 19 amino acids long (Table 1) and were chemically synthesized on a DuPont RAMPS manual synthesizer by the fluorenylmethoxycarbonyl strategy (8). The peptides were purified by high-pressure liquid chromatography (32), and the amino acid composition was confirmed by quantitative amino acid analysis.
TABLE 1.
Reactivity of MAb 10B6 with overlapping synthetic peptides of streptococcal M5 protein
| Peptidea | Sequence | Residues | Reactivity of MAb 10B6 (20 μg/ml) |
|---|---|---|---|
| NT1 | AVTRGTINDPQRAKEALD | 1–18 | 0.05 |
| NT2 | KEALDKYELENHDLKTKN | 14–31 | 0.00 |
| NT3 | LKTKNEGLKTENEGLKTE | 27–44 | 0.00 |
| NT4 | GLKTENEGLKTENEGLKTE | 40–58 | 0.00 |
| NT4–5 | GLKTEKKEHEAENDKLK | 54–70 | 0.00 |
| NT5 | KKEHEAENDKLKQQRDTL | 59–76 | 0.00 |
| NT5–6 | DKLKQQRDTLSTQKETLE | 67–84 | 0.00 |
| NT6 | QRDTLSTQKETLEREVQN | 72–89 | 0.00 |
| NT7 | REVQNTQYNNETLKIKNG | 85–102 | 0.00 |
| NT8 | KIKNGDLTKELNKTRQEL | 98–115 | 0.00 |
| B1A | TRQELANKQQESKENEKAL | 111–129 | 0.00 |
| B1B | ENEKALNELLEKTVKDKI | 124–141 | 0.00 |
| B1B2 | VKDKIAKEQENKETIGTL | 137–154 | 0.00 |
| B2 | TIGTLKKILDETVKDKIA | 150–167 | 0.00 |
| B2B3A | KDKIAKEQENKETIGTLK | 163–180 | 0.00 |
| B3A | IGTLKKILDETVKDKLAK | 176–193 | 0.00 |
| B2B3B | DKLAKEQKSKQNIGALKQ | 189–206 | 0.00 |
| B3B | GALKQELAKKDEANKISD | 202–219 | 0.00 |
| C1A | NKISDASRKGLRRDLDAS | 215–232 | 0.06 |
| C1B | DLDASREAKKQLEAEHQK | 228–245 | 0.00 |
| C1C2 | AEHQKLEEQNKISEASRK | 241–258 | 0.00 |
| C2A | EASRKGLRRDLDASREAK | 254–271 | 1.62 |
| C2B | SREAKKQLEAEQQKLEEQ | 267–284 | 0.00 |
| C2C3 | KLEEQNKISEASRKGLRR | 280–297 | 0.00 |
| C3 | KGLRRDLDASREAKKQ | 293–308 | 1.64 |
ELISA.
Assays were performed in Immulon 4 96-well microtiter plates (Dynatech, Chantilly, Va.) coated with antigen (10 μg/ml) overnight at 4°C. Plates were washed three times with phosphate-buffered saline (PBS)–0.05% Tween 20 and then blocked for 1 h with 1% bovine serum albumin in wash buffer. Test antibody was added to wells (serum, 1:100; MAb, 10 μg/ml) and incubated overnight at 4°C. Plates were washed, and goat anti-mouse Ig or goat anti-human Ig conjugated to alkaline phosphatase (Sigma) was added for 2 h at 37°C. Plates were washed with PBS-Tween buffer, and p-nitrophenyl phosphate (Sigma) was added as the enzyme substrate (1 mg/ml) in diethanolamine buffer as described previously (12, 14). After 30 min, the absorbance was read at 410 nM on a Dynatech enzyme-linked immunosorbent assay (ELISA) plate reader.
The competitive inhibition ELISA has been previously described (12, 14). Briefly, antibody was preincubated 1:1 with inhibitor for 1 h at 37°C and then overnight at 4°C. Forty microliters of the antibody-inhibiting mixture was added to the antigen-coated wells (50 μl of 10 μg/ml), and the ELISA was carried out as described. The percentage of inhibition was calculated according to the formula 100 × (A410 of well with MAb plus buffer − A410 of well with MAb plus inhibitor/A410 of well with MAb plus buffer). Antibodies were tested in duplicate, and assays were repeated at least twice.
Affinity-purified anti-C3 peptide antibodies.
M5 peptide affinity columns were prepared by coupling 3 mg of peptide with 0.5 g of 6-aminohexanoic acid N-hydroxysuccinimide ester Sepharose-4B (Sigma). The gel was washed, blocked, and placed in a column (12). Serum (1 to 0.5 ml) was diluted 1:2 with PBS (pH 7.2), applied to the gel column, and incubated overnight at 4°C. Unbound antibody was washed from the column with PBS (pH 7.2). The column was washed until the eluate read zero at 280 nm in a Beckman spectrophotometer. Bound antibody was then eluted from the column with 0.1 M glycine (pH 2.5). Column fractions were immediately neutralized and read at 280 nm in a Beckman spectrophotometer. The fractions containing the antibody were dialyzed overnight against PBS (pH 7.2).
Immunoblotting.
Western immunoblotting was performed as previously described (14). Human cardiac or rabbit skeletal myosin was separated by electrophoresis under reducing conditions in sodium dodecyl sulfate–10% polyacrylamide gels. The protein was electrophoretically transferred overnight from the gel to nitrocellulose membranes with a Bio-Rad blotting apparatus. Membranes were blocked with 2% Tween 20 in PBS (pH 7.2) and reacted with anti-M protein MAb 10B6. Blots were washed in PBS-Tween buffer, reacted with rabbit anti-mouse Ig conjugated to peroxidase, washed again, and reacted with 4-chloronaphthol (Sigma) in buffer and H2O2 substrate (13, 14). Normal mouse sera from BALB/c mice were used as a negative control.
Preparation of antiserum.
Seven-week-old female Lewis rats (Harlan Sprague-Dawley, Indianapolis, Ind.) in groups of three were immunized in the footpad with 500 μg of peptide or PBS emulsified in Freund’s complete adjuvant supplemented with 2 mg of heat-killed mycobacteria (strain H37Ra) per ml and injected intraperitoneally with 2 × 1010 Bordetella pertussis cells (Michigan Department of Public Health, Lansing, Mich.). A group of three animals were boosted on day 7 with 500 μg of peptide in Freund’s incomplete adjuvant. A group of three control animals were given adjuvants alone. Animals were euthanized 21 days after immunization. Three rats from the population were euthanized before immunization to provide the preimmune serum control.
Statistical analysis.
Means with standard deviations were calculated for ARF, UNC, AGN, and normal (NOR) serum groups, and serum groups were compared by the Mann-Whitney test to determine significance calculated as one-tailed P values. The Mann-Whitney test, also called the rank sum test, is a nonparametric test that compares two unpaired groups. Since we predicted that the results for sera from streptococcal diseases would most likely be higher than normal, we chose the one-tailed P value.
RESULTS
MAb 10B6 recognizes M5 protein peptides C2A and C3.
Anti-M protein MAb 10B6 recognizes the rheumatic fever-associated class I M protein epitope described by Bessen and colleagues (4). Furthermore, Jones and colleagues (23) identified a 15-amino-acid peptide within the conserved region of the M6 protein that contained the MAb 10B6 epitope. For identification of the epitope within the M5 protein recognized by MAb 10B6, it was reacted with 23 overlapping synthetic peptides of the type 5 streptococcal M protein by ELISA. The synthesized M5 peptides represent the amino-terminal and A repeat (NT), B repeat (B), and C repeat (C) regions of the M5 molecule (Table 1). Table 1 shows the reaction of anti-M protein MAb 10B6 with streptococcal M5 peptides in the ELISA. The M5 protein peptides were used in the ELISA to determine the M protein epitopes recognized by a MAb to the class I M protein epitope (10B6, 20 μg/ml). The M5 peptides recognized by MAb 10B6 were peptides C2A and C3 (Table 1). At least four repetitive assays performed in duplicate have verified that these two peptides react with MAb 10B6 by ELISA. A control antibody did not react with these peptides. This highly repeatable result identified the M5 peptides containing the class I epitope. The strong recognition of these two nonadjacent C repeat region peptides can best be explained by the identity in their amino acid sequences: peptides C2A and C3 both contain the sequence KGLRRDLDASREAK (Table 1). The N-terminal half of this peptide shows identity with the 10B6 epitope described by Jones et al. in the M6 protein (23). Because some M protein epitopes have been shown to contain regions of sequence homology with myosin, the M5 sequence KGLRRDLDASREAK was analyzed and found, along with human cardiac myosin, to possess the sequence RRDL, residues 1178 to 1181 (32) (Fig. 1). This segment of cardiac myosin (Fig. 1) is found in the heavy meromyosin region of the myosin β heavy-chain rod and is highly conserved (identical) in human cardiac and skeletal myosins. The identical sequence (RRDL) between the M protein and myosin is highlighted in Fig. 1. However, when myosin peptide AEFQKMRRDLEEATLQHEA was synthesized and reacted with MAb 10B6 by ELISA and Western blotting, no reaction was observed (data not shown). Furthermore, not all M5 peptides containing the RRDL sequence, such as peptide C1A, reacted with MAb 10B6.
FIG. 1.
Alignment of streptococcal M5 peptides C2A and C3 with the β heavy chain of human cardiac myosin (21). Identical residues are indicated with two dots, while a conservative substitution is indicated by a single dot as determined by the FASTA alignment program (Intelligenetics, Mountain View, Calif.). The sequence identified as homologous between C2A, C3, and myosin is highly conserved (identical) between skeletal and cardiac myosin. The identical residues are located in the heavy meromyosin fragment of skeletal and cardiac myosin.
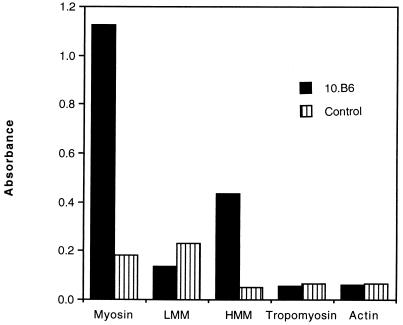
We then tested MAb 10B6 by the ELISA and the immunoblot assay to determine if it could recognize intact myosin. MAb 10B6 reacted with rabbit skeletal (Fig. 2) and human cardiac (Fig. 3) myosin but did not react with actin or tropomyosin (Fig. 2). It was also found to react with the heavy meromyosin fragment of the myosin molecule in the ELISA but did not react with the light meromyosin fragment (Fig. 2). Normal mouse sera diluted 1:100 were used as a negative control in these experiments (Fig. 2 and 3). Although not shown, an IgG MAb isotype control tested at 10, 20, and 50 μg/ml did not react with myosin in the blot.
FIG. 2.
Reaction of MAb 10B6 with myosin. MAb 10B6 (20 μg/ml) and normal mouse sera (pool from three normal BALB/c mice) (normal mouse sera, 1:100) were reacted with myosin, the light meromyosin fragment (LMM), the heavy meromyosin fragment (HMM), tropomyosin, and actin by ELISA. Results with human cardiac and rabbit skeletal myosin were similar.
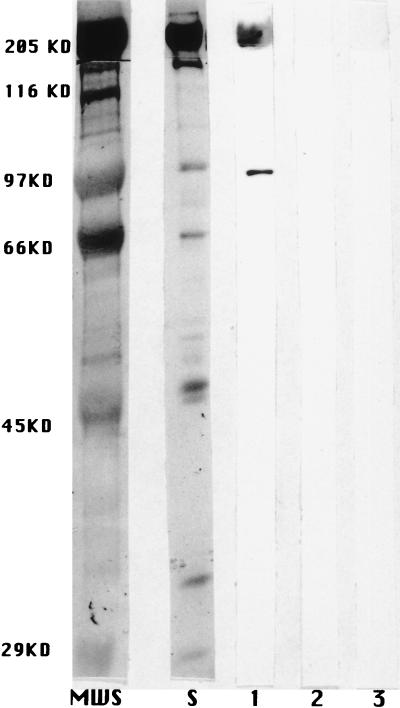
FIG. 3.
Reaction of MAb 10B6 with human cardiac myosin by Western immunoblot analysis. MAb 10B6 (20 μg/ml) (lane 1) and a control antibody were reacted with purified myosin by a Western immunoblot analysis. Similar results were obtained with rabbit skeletal (data not shown) and human cardiac myosin. Lanes 2 and 3, no reactivity of myosin with normal mouse serum (1:100) and an isotype control, respectively; lane S, amido black protein stain of the 200-kDa band of myosin heavy chain. MWS, molecular size standards.
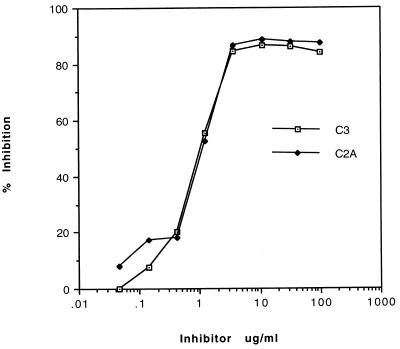
To demonstrate that the antimyosin activity of MAb 10B6 could be adsorbed with sequences found in M protein, a competitive inhibition ELISA was performed in which MAb 10B6 (20 μg/ml) was incubated with various concentrations of either peptide C2A (residues 254 to 271) or C3 (293 to 308) before it was reacted with myosin by ELISA. Both M5 peptides, C2A and C3, inhibited the binding of MAb 10B6 in a dose-dependent fashion. One microgram of either peptide per ml resulted in 50% inhibition of MAb 10B6 binding to rabbit skeletal myosin (data not shown) or human cardiac myosin (Fig. 4). M5 peptide C1C2 (residues 241 to 258) showed no inhibition of MAb 10B6 at any concentration tested (1 to 500 μg/ml; data not shown). Collectively, our results confirm previous studies regarding the location of the 10B6 epitope (23) and indicate that the M5 peptides C2A and C3 (292 to 307) contain the class I M protein epitope, which is immunologically cross-reactive with myosin.
FIG. 4.
Inhibition of the binding of MAb 10B6 to myosin with M5 peptides. MAb 10B6 (20 μg/ml) was preincubated with 100 to 0.05 μg of M5 peptide C3 or C2A per ml before being reacted with skeletal myosin by ELISA. Percentage of inhibition was calculated based on the reaction of MAb 10B6 without inhibitor and diluted in the buffer used in the inhibition. Results were identical with those for purified rabbit skeletal and human cardiac myosin. At concentrations of 1 to 500 μg/ml, M5 peptide C1C2 showed no inhibition of MAb 10B6.
Comparison of anti-peptide C3 reactivity of sera from UNC, ARF, and AGN.
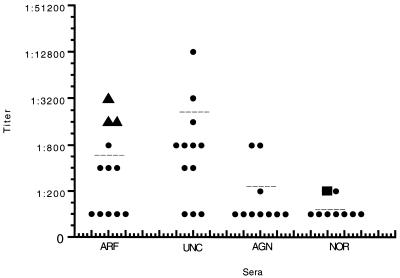
In order to determine if there were quantitative differences in IgG responses to peptide C3 in UNC, ARF, AGN, and NOR sera, titrations of the sera were performed against peptide C3 in the ELISA. Figure 5 shows the titers of individual ARF, UNC, AGN, and NOR sera reacted with peptide C3. There was no significant difference in titers observed for the ARF group versus the UNC group. NOR serum IgG titers to peptide C3 were <100, whereas ARF and UNC titers ranged between <100 to 3,200 and 12,800, respectively. The three carditis patients (triangles in Fig. 5) had the highest IgG titers against peptide C3 in the ARF group; however, several of the UNC group also had high titers without clinical evidence of ARF. With two exceptions, the AGN group had a low or normal IgG response to peptide C3. Statistical comparison of the ARF, UNC, and AGN groups with the normal group demonstrated that the ARF and UNC groups were significantly different from the NOR group, while the AGN group was similar to the NOR group. P values, means, and standard deviations are reported in the legend for Fig. 5, and a scattergram of the individual data and group means is shown in Fig. 5.
FIG. 5.
Scattergram showing the titers of individual ARF, UNC, AGN, and normal sera tested against peptide C3 by ELISA. Titers of each individual serum are shown in the scattergram. Sera were diluted 1:100, and twofold dilutions were performed in PBS. Peptide C3 was coated onto Immunlon 4 microtiter plates at 50 ml/well of a 10-μg/ml solution. The dilutions were added to washed wells, and the ELISA was performed as described previously. OD was read at 405 nm, and an endpoint of 0.3 was used to calculate the titer. Triangles represent carditis patient sera, and a square shows the titer of a normal serum pool from five normal individuals. Individual sera are represented by single dots. Mean titers (± standard deviations) are designated by dashed lines and were calculated for the ARF group (741 ± 950), the UNC group (1,825 ± 3,562), the AGN group (250 ± 292) and the NOR group (112 ± 35). A comparison of the ARF, UNC, and AGN groups with the NOR group yielded the following P values: for ARF versus NOR, P = 0.03; for UNC versus NOR, P = 0.004; and for AGN versus NOR, P = 0.336. ARF and UNC groups were significantly different from the NOR and AGN groups. In addition, the P value for ARF versus AGN was 0.09.
To determine if the ability to react with M5 peptide C3 (residues 293 to 308) was a quality possessed by other human sera containing antimyosin antibodies, serum from nine patients with nonrheumatic myocarditis were pooled and compared with those of the ARF group for reactivity with myosin and peptide C3 by ELISA. Both groups were found to have elevated levels of anti-human cardiac myosin antibodies (optical density [OD] = 1.1), with no apparent difference between their reactivities in the ELISA. However, a comparison of the two groups of sera for reactivity to M5 peptide C3 (residues 293 to 308) indicated that the reactivity of the ARF group was higher (OD = 0.9) than that of the nonrheumatic group (OD = 0.3). The reactivity of the nonrheumatic carditis group with peptide C3 was similar to that seen with normal serum. Although a single high-titered serum could have skewed the results, the data suggest that anti-C3 reactivity is not associated with all sera demonstrating antimyosin antibody, such as myocarditis patient sera. The anti-C3 reactivity was present only in serum from patients exposed to streptococci.
Anti-C3 antibodies.
We then determined if immunization with M5 peptide C3 could induce the production of antimyosin antibodies in Lewis rats, which were chosen because they have been shown to be susceptible to myosin-induced myocarditis and streptococcus-induced arthritis. Groups of three rats were immunized and boosted with M5 peptide C3 (293 to 308) as well as the other 22 M5 peptides. Antisera (1:100 dilution) from each of the three C3-immunized rats demonstrated a strong reaction with M5 peptide C3 and skeletal or cardiac myosin (OD = 1.65) by the ELISA but not with tropomyosin (OD = 0.2) or actin (OD = 0.2). Sera from rats (group of three) immunized with Freund’s complete adjuvant alone and the preimmune sera (group of three) showed no reactivity by the ELISA with any of the antigens tested (OD < 0.2). Rats immunized with the other 22 M5 peptides (groups of three) showed no reaction (OD < 0.2) with myosin by the ELISA. All sera were tested individually in each group by the ELISA. These results confirmed that epitopes are common between the M5 peptide C3 (293 to 308) and myosin. Thus, the two molecules have similar epitopes.
We used ARF sera and peptide C3 to determine if human antibodies against the class I epitope in peptide C3 cross-react with myosin. This test was performed with affinity-purified antibodies to C3 from the sera of three patients with ARF. Affinity-purified anti-C3 antibodies reacted with the homologous peptide, C3, and with myosin but not with actin or tropomyosin by ELISA (Fig. 6). A pool of 5 normal human sera was diluted to the same immunoglobulin concentration as the purified anti-C3 but showed no reaction with peptide C3 (293 to 308) or myosin in the ELISA. Under identical conditions, sera from a patient with streptococcal pharyngitis and a high titer of anti-C3 were placed onto the C3 affinity column. The antibody eluate from the column produced lower reactivity, but the results demonstrated cross-reactivity of the anti-C3 antibody from UNC with myosin as well as peptide C3. These data were similar to those shown for the ARF sera in Fig. 6. However, compared to UNC sera, 6 times more anti-C3 IgG and 29 times more anti-C3 IgM were affinity purified from ARF sera (data not shown). As a control, ARF sera were passed over a column which contained M5 peptide C1C2 (241 to 258) attached to Sepharose beads. The eluate from this column showed no reactivity by ELISA with peptide C3 (293 to 308) or myosin. These observations suggest that during a streptococcal infection, an epitope associated with streptococcal M5 peptide C3 may contribute to the antimyosin response observed in UNC and ARF patients. The data show that human anti-C3 antibodies against the class I epitope react with myosin and that the levels of anti-C3 antibody purified from peptide C3 affinity columns were quantitatively much higher in ARF than in UNC infection.
FIG. 6.
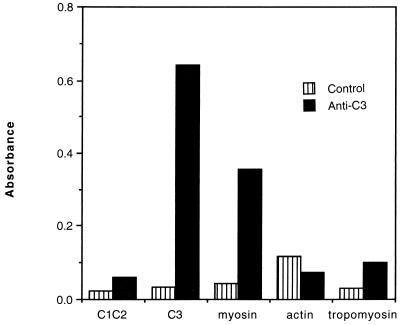
Reaction of affinity-purified anti-C3 antibodies from ARF sera with streptococcal M5 peptide C3 and myosin. Antibodies to M5 peptide C3 were affinity purified from the sera of a patient with rheumatic carditis. The purified antibodies were diluted 1:10 with buffer and reacted with M5 peptides C1C2 and C3 and with skeletal myosin, actin, and tropomyosin by ELISA. Normal human sera (five samples) were pooled and tested as the control. Normal and affinity-purified Igs were reacted at the same Ig concentrations. A second ARF carditis serum was affinity purified and found to react similarly with myosin and peptide C3 as shown. These data show that human anti-C3 antibodies react with myosin following streptococcal infection.
DISCUSSION
The data in this study support the hypothesis that the class I epitope of streptococcal M protein is immunologically similar to myosin. First, anti-M protein MAb 10B6, which recognizes the class I epitope of M proteins, reacted with cardiac and skeletal myosins. Synthetic peptides spanning the A, B, and C repeat regions of M5 protein were reacted with MAb 10B6, and the two M protein peptides recognized by MAb 10B6, peptides C2A and C3, contained the consensus amino acid sequence KGLRRDLDASREAK. Within the common sequence, four amino acids (RRDL) were identical to a site in human cardiac and skeletal myosins (Fig. 1). The myosin sequence is highly conserved among cardiac and skeletal myosins and is located in the heavy meromyosin fragment. The cross-reactivity is most likely due to its homology with a region in the heavy meromyosin fragment. The reaction of MAb 10B6 with the heavy meromyosin fragment (Fig. 2) supports the hypothesis that the myosin cross-reactive epitope recognized by MAb 10B6 is in the heavy meromyosin fragment. It is not clear why M5 peptide C1A (215 to 232) containing the sequence KGLRRDLD did not react strongly with MAb 10B6 unless the ELISA reaction time was very extended (hours). The failure of MAb 10B6 to react strongly with peptide C1A may be due to the overall amino acid composition or conformation of peptide C1A, which could result in a difference in binding capacity or presentation on the microtiter plate. In support of a conformational determinant, we found that when myosin peptide AEFQKMRRDLEEATLQHEA, containing the RRDL identity with the M5 sequence, was synthesized and reacted with MAb 10B6, no reaction was observed, suggesting that this region of myosin may not be involved in the reaction of MAb 10B6 or that the conformation formed by the coiled-coil may be important in the reactivity. The residues omitted from peptide C1A were DASREAK, which may be important in the overall structure of peptides C2A and C3, making them more reactive with MAb 10B6. In attempts to locate a smaller reactive sequence, smaller peptide heptamers of peptide C3 were reacted with MAb 10B6, but this has not resulted in the positive identification of an epitope smaller than KGLRRDLDASREAK. However, five of the six N-terminal amino acids in the M5 sequence KGLRRDLDASREAK are identical to the C-terminal end of the M6-10B6 epitope reported by Jones et al. (23), suggesting that the sequence GLRRD plays an important part in the epitope.
A second part of the evidence is that immunization of rabbits with C repeat region peptides of M6 protein resulted in the production of antimyosin antibody against denatured forms of myosin (43), and in this study immunization of Lewis rats with peptide C3 resulted in antimyosin antibody production. In addition, immunization of BALB/c mice with the M5 C repeat peptides also induced an antimyosin response to several of the C repeat peptides. Histopathological examination of the hearts from C repeat peptide-immunized animals showed no evidence of cellular infiltration or myocardial or valvular damage (11). Collectively, these data suggest that the C repeat region peptides can induce antimyosin antibody but that they do not produce heart lesions in animals. In regard to T-cell responses against the C repeat region, a recent study suggests that peptide C3 is a myosin cross-reactive T-cell epitope in BALB/c mice (11). Human T-cell clones responsive to carboxy-terminal sequences of M protein also proliferate to sequences of human cardiac myosin (38). The data show that sequences within the C repeat and carboxy-terminal region of M5 protein induce antimyosin antibody and are involved in T-cell responses against myosin.
A third line of evidence, suggesting that the class I epitope of M protein cross-reacts immunologically with myosin, is the demonstration that affinity-purified anti-C3 antibody from human ARF and UNC sera reacts with myosin. The affinity purification demonstrated more anti-C3 antibody in ARF, which is not surprising due to the hyperresponsiveness generally observed in ARF. In our study, we investigated ARF, UNC, AGN, and nonrheumatic carditis sera for reactivity with our C repeat region peptides or peptide C3 which contains the class I epitope. Titration of ARF and UNC serum groups against peptide C3 containing the class I epitope showed no significant differences in antibody titers to peptide C3. The C repeat peptides reacted with ARF and UNC sera but to a much lesser degree with AGN, NOR, or nonrheumatic myocarditis sera. In studies in which the ARF sera were shown to have higher reactivity than the UNC sera (37), it is possible that penicillin therapy for the UNC group could have decreased their exposure to streptococci, resulting in a lowered immune response to streptococci and to peptide C3 in particular. Preliminary data for sera from ARF and UNC patients collected during a streptococcal outbreak at the Great Lakes Naval Station prior to the general use of penicillin support the hypothesis that treatment of the UNC patients in our study with penicillin could have decreased exposure to streptococcal infection, thereby reducing the immune response observed against the class I epitope (23a). Prior to the use of penicillin, ARF was more prevalent. The fact that the antimyosin antibody response to the class I epitope is similar in ARF and UNC patients yet contributes to the disease in the susceptible host could be due to a host susceptibility factor. Antimyosin antibodies were shown to deposit in the hearts of only genetically susceptible mice (31).
Penicillin therapy cannot explain the significant differences between the ARF and AGN groups. Differences between ARF and AGN sera might be explained by the fact that since the AGN strains generally do not have the class I epitope, sera from AGN patients would not be expected to react at levels equivalent to those of ARF sera against the C3 peptide. The observation that certain serotypes of streptococci are associated with outbreaks ARF or AGN (4–6) has led to the proposal that unique epitopes or molecules are associated with the development of ARF. It has been reported that patients with ARF elicit a serologic response to a class I group A streptococcal infection (5). Furthermore, it is not known if the high responders to C3 in the UNC group may be at risk for developing ARF from future streptococcal infections.
While there is a close association between the class I epitope- and the rheumatic fever-producing strains, not all strains containing this epitope have been associated with ARF. For example, a reactive 10B6 epitope is also found in M protein isolated from group G streptococci even though this streptococcal group has never been associated with ARF (9, 22). An explanation why some serotypes of group A streptococci are recognized by MAb 10B6 and others are not is that the class II M molecules differ from class I molecules in three amino acid positions within the conserved C repeat (4).
Most important is the question of whether the class I epitope and C repeat region are involved in ARF and, if so, what role they might play in disease. It is important to resolve this question, since a group A streptococcal C repeat vaccine shows potential for providing broad-based protective immunity against streptococcal diseases (3, 7, 20). As shown in Fig. 5, antibodies against the C3 peptide are markers of a class I streptococcal infection and may be involved in the production of the disease. If the C repeat region or the class I epitope is a direct contributor to disease in a susceptible host, it will be important to distinguish protective from disease-producing epitopes.
ACKNOWLEDGMENTS
We thank Janet Heuser and Carol Crossley for expert technical assistance and Kenneth Jackson and the W. K. Warren Molecular Biology Resource Facility at the University of Oklahoma Health Sciences Center for synthesis and purification of the M5 peptides. We thank Kevin Jones for MAb 10B6, Barry Gray, Noel Rose, Penelope Shackelford, and Eyal Taylor for providing serum samples, and Patrick Umeda for his combined amino acid sequence data comparisons of known myosins. We also express appreciation to Kevin Jones and Debra Bessen for the critical review of our manuscript.
This work was supported by grant HL 35280 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Adderson E E, Shikhman A R, Ward K E, Cunningham M W. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetyl-glucosamine/anti-myosin antibody V region genes. J Immunol. 1998;161:2020–2031. [PubMed] [Google Scholar]
- 2.Anderson H C, Kunkel H G, McCarty M. Quantitative antistreptokinase studies in patients infected with group A hemolytic streptococci: a comparison with serum antistreptolysin and gamma globulin levels with special reference to the occurrence of rheumatic fever. J Clin Invest. 1948;27:425–434. doi: 10.1172/JCI101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessen D, Fischetti V A. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol. 1990;145:1251–1256. [PubMed] [Google Scholar]
- 4.Bessen D, Jones K F, Fischetti V A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989;169:269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen D E, Veasy L G, Hill H R, Augustine N H, Fischetti V A. Serologic evidence for a class I group A streptococcal infection among rheumatic fever patients. J Infect Dis. 1995;172:1608–1611. doi: 10.1093/infdis/172.6.1608. [DOI] [PubMed] [Google Scholar]
- 6.Bisno A L. The concept of rheumatogenic and non-rheumatogenic group A streptococci. In: Read S E, Zabriskie J B, editors. Streptococcal diseases and the immune response. New York, N.Y: Academic Press, Inc.; 1980. pp. 789–803. [Google Scholar]
- 7.Bronze M S, Courtney H S, Dale J B. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J Immunol. 1992;148:888–893. [PubMed] [Google Scholar]
- 8.Carpino L A, Han G Y. The 9-fluorenylmethoxy-carbonyl amino protecting group. J Org Chem. 1972;37:3404–3409. [Google Scholar]
- 9.Collins C M, Kimura A, Bisno A L. Group G streptococcal M protein exhibits structural features analogous to those of class I M proteins of group A streptococci. Infect Immun. 1992;60:3689–3696. doi: 10.1128/iai.60.9.3689-3696.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham M W, Antone S M, Gulizia J M, McManus B M, Fischetti V A, Gauntt C J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham M W, Antone S M, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65:3913–3923. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham M W, McCormack J M, Fenderson P G, Ho M K, Beachey E H, Dale J B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. 1989;143:2677–2683. [PubMed] [Google Scholar]
- 13.Cunningham M W, McCormack J M, Talaber L R, Harley J B, Ayoub E M, Muneer R S, Chun L T, Reddy D V. Human monoclonal antibodies reactive with antigens of the group A streptococcus and human heart. J Immunol. 1988;141:2760–2766. [PubMed] [Google Scholar]
- 14.Cunningham M W, Swerlick R A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986;164:998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale J B, Beachey E H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1985;162:583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale J B, Beachey E H. Protective antigenic determinant of streptococcal M protein shared with sarcolemmal membrane protein of human heart. J Exp Med. 1982;156:1165–1176. doi: 10.1084/jem.156.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale J B, Beachey E H. Sequence of myosin-crossreactive epitopes of streptococcal M protein. J Exp Med. 1986;164:1785–1790. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell A, Antone S M, Gauntt C J, Crossley C A, Clark W A, Cunningham M W. Autoimmune determinants of rheumatic carditis: localization of epitopes in human cardiac myosin. Eur Heart J. 1991;12:158–162. doi: 10.1093/eurheartj/12.suppl_d.158. [DOI] [PubMed] [Google Scholar]
- 19.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischetti V A, Hodges W M, Hruby D E. Protection against streptococcal pharyngeal colonization with a vaccinia:M protein recombinant. Science. 1989;244:1487–1490. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- 21.Jaenicke T, Diederich K W, Haas W, Scheich J, Lichter P, Pfordt M, Bach A, Vosberg H P. Complete sequence of human β myosin. Genomics. 1990;8:194–207. doi: 10.1016/0888-7543(90)90272-v. [DOI] [PubMed] [Google Scholar]
- 22.Jones K F, Fischetti V A. Biological and immunochemical identity of M protein on group G streptococci with M protein on group A streptococci. Infect Immun. 1987;55:502–506. doi: 10.1128/iai.55.3.502-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones K F, Khan S A, Erickson B W, Hollingshead S K, Scott J R, Fischetti V A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J Exp Med. 1986;164:1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Jones, K. F., et al. Unpublished data.
- 24.Kaplan M H. Immunologic relation of streptococcal and tissue antigens. I. Properties of an antigen in certain strains of group A streptococci exhibiting an immunologic cross reaction with human heart tissue. J Immunol. 1963;90:595–606. [PubMed] [Google Scholar]
- 25.Kaplan M H. Infection and immunology in the rheumatic diseases. D. C. Dumonde (ed.). Oxford, England: Blackwell Scientific Publications; 1976. [Google Scholar]
- 26.Kaplan M H, Bolande R, Rakita L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever. Association with cardiac failure. N Engl J Med. 1964;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan M H, Dallenbach F D. Immunologic studies of heart tissue. II. Occurrence of bound gamma-globulin in auricular appendages from rheumatic hearts. Relationship to certain histopathologic features of rheumatic heart disease. J Exp Med. 1961;113:1–16. doi: 10.1084/jem.113.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan M H, Svec K H. Immunologic relation of streptococcal and tissue antigens. III. Presence in human sera of streptococcal antibody cross reactive with heart tissue. Association with streptococcal infection, rheumatic fever, and glomerulonephritis. J Exp Med. 1964;119:651–666. doi: 10.1084/jem.119.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krisher K, Cunningham M W. Myosin: a link between streptococci and heart. Science. 1985;227:413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- 30.Lannagan R, Zaki S. Localization of globulin in the endocardium in rheumatic heart disease by the ferritin-labelled antibody technique. Nature. 1968;217:173–174. [Google Scholar]
- 31.Liao L, Sindhwani R, Rojkind M, Factor S, Leinwand L, Diamond B. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med. 1995;187:1123–1131. doi: 10.1084/jem.181.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahoney W C, Hermodson M H. Purification and characterization of synthetic peptides by HPLC chromatography. J Biol Chem. 1980;225:11199–11203. [PubMed] [Google Scholar]
- 33.Manjula B N, Acharya A S, Mische S M, Fairwell T, Fischetti V A. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem. 1984;259:3686–3693. [PubMed] [Google Scholar]
- 34.Manjula B N, Fischetti V A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem Biophys Res Commun. 1986;140:684–690. doi: 10.1016/0006-291x(86)90786-2. [DOI] [PubMed] [Google Scholar]
- 35.Manjula B N, Trus B L, Fischetti V A. Presence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological properties. Proc Natl Acad Sci USA. 1985;82:1064–1068. doi: 10.1073/pnas.82.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller L C, Gray E D, Beachey E H, Kehoe M A. Antigenic variation among group A streptococcal M proteins: nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988;263:5668–5673. [PubMed] [Google Scholar]
- 37.Mori K, Kamakawaji N, Sasazuki T. Persistent elevation of immunoglobulin G titer against the C region of recombinant group A streptococcal M protein in patients with rheumatic fever. Pediatr Res. 1996;55:502–506. doi: 10.1203/00006450-199602000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Pruksakorn S, Currie B, Brandt E, Hunsakunachai P C, S, Manmontri A, Robinson J H, Kehoe M A, Galbraith A, Good M F. Identification of T cell autoepitopes that cross-react with the C-terminal segment of the M protein of group A streptococci. Int Immunol. 1994;6:1235–1244. doi: 10.1093/intimm/6.8.1235. [DOI] [PubMed] [Google Scholar]
- 39.Shikhman A R, Greenspan N S, Cunningham M W. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-d-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. J Immunol. 1994;153:5593–5606. [PubMed] [Google Scholar]
- 40.Stollerman G H. Rheumatic and heritable connective tissue diseases of the cardiovascular system. In: Braunmald E, editor. Heart disease: a textbook of cardiovascular medicine. Vol. 11. Philadelphia, Pa: W. B. Saunders; 1988. pp. 1706–1734. [Google Scholar]
- 41.Stollerman G H. Rheumatic fever and streptococcal infection. In: Hurst J W, Mason D T, editors. Clinical cardiology monographs. Vol. 1. New York, N.Y: Grune and Stratton; 1975. p. 122. [Google Scholar]
- 42.Tobacman L S, Adelstein R S. Enzymatic comparisons between light chain isozymes of human cardiac myosin subfragment-1. J Biol Chem. 1984;259:11226–11230. [PubMed] [Google Scholar]
- 43.Vashishtha A, Fischetti V A. Surface-exposed conserved region of the streptococcal M protein induces antibodies cross-reactive with denatured forms of myosin. J Immunol. 1993;150:4693–4701. [PubMed] [Google Scholar]
- 44.Widdowson J P, Maxted W R, Notley C M, Pinney A M. The antibody responses in man to infection with different serotypes of group A streptococci. J Med Microbiol. 1974;7:483–496. doi: 10.1099/00222615-7-4-483. [DOI] [PubMed] [Google Scholar]
- 45.Widdowson J P, Maxted W R, Pinney A M. An M-associated protein antigen (MAP) of group A streptococci. J Hyg. 1976;69:553–564. doi: 10.1017/s0022172400021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabriskie J B, Hsu K C, Seegal B C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970;7:147–159. [PMC free article] [PubMed] [Google Scholar]