Abstract
Understanding the prognostic significance of acute kidney injury (AKI) stage 1B [serum creatinine (sCr) ≥ 1.5 mg/dL] compared with stage 1A (sCr < 1.5 mg/dL) in a US population is important as it can impact initial management decisions for AKI in hospitalized cirrhosis patients. Therefore, we aimed to define outcomes associated with stage 1B in a nationwide US cohort of hospitalized cirrhosis patients with AKI. Hospitalized cirrhosis patients with AKI in the Cerner-Health-Facts database from January 2009 to September 2017 (n = 6250) were assessed for AKI stage 1 (≥ 1.5–2-fold increase in sCr from baseline) and were followed for 90 days for outcomes. The primary outcome was 90-day mortality; secondary outcomes were in-hospital AKI progression and AKI recovery. Competing-risk multivariable analysis was performed to determine the independent association between stage 1B, 90-day mortality (liver transplant as a competing risk), and AKI recovery (death/liver transplant as a competing risk). Multivariable logistic regression analysis was performed to determine the independent association between stage 1B and AKI progression. In all, 4654 patients with stage 1 were analyzed: 1A (44.3%) and 1B (55.7%). Stage 1B patients had a significantly higher cumulative incidence of 90-day mortality compared with stage 1A patients, 27.2% versus 19.7% (p < 0.001). In multivariable competing-risk analysis, patients with stage 1B (vs. 1A) had a higher risk for mortality at 90 days [sHR 1.52 (95% CI 1.20–1.92), p = 0.001] and decreased probability for AKI recovery [sHR 0.76 (95% CI 0.69–0.83), p < 0.001]. Furthermore, in multivariable logistic regression analysis, AKI stage 1B (vs. 1A) was independently associated with AKI progression, OR 1.42 (95% CI 1.14–1.72) (p < 0.001). AKI stage 1B patients have a significantly higher risk for 90-day mortality, AKI progression, and reduced probability of AKI recovery compared with AKI stage 1A patients. These results could guide initial management decisions for AKI in hospitalized patients with cirrhosis.
INTRODUCTION
Acute kidney injury (AKI) is a frequent complication in hospitalized patients with cirrhosis, occurring in 20%–53%.[1–3] In this setting, AKI is significantly associated with high short-term mortality, which is directly linked to its severity.[1–5] AKI severity is classified into 3 distinct stages that are characterized by the degree of serum creatinine (sCr) increase relative to baseline sCr.[4,5] Stage 1 is an increase of sCr ≥ 1.5–2 times from baseline; stage 2 is an increase of sCr ≥ 2–3 times from baseline; and stage 3 is an increase in sCr ≥ 3 times from baseline or sCr ≥ 4.0 mg/dL with an acute increase of ≥ 0.3 mg/dL or the initiation of hemodialysis (HD).
Once AKI is recognized, the International Club of Ascites (ICA) guidelines recommend i.v. volume expansion and identifying and treating precipitating factors.[5] In the case of AKI stage 2 or higher, volume expansion with i.v. albumin for 2 consecutive days is recommended, and in nonresponders to albumin volume expansion, administration of terlipressin in patients with hepatorenal syndrome-AKI (HRS-AKI) is recommended. In contrast, the European Study of Liver Disease guidelines recommend volume expansion with albumin for AKI stage 1 with sCr ≥ 1.5 mg/dL (known as AKI stage 1B) and initiation of terlipressin in albumin nonresponders with HRS-AKI.[6] This recommendation stems from several European studies showing worse outcomes in patients with AKI stage 1 and sCr ≥ 1.5 mg/dL compared with patients with AKI stage 1 and sCr < 1.5 mg/dL (known as stage 1A).[1,7,8] Although the prognostic significance of AKI stage 1B in a US population is yet to be defined, these recommendations have been endorsed by the American Gastroenterological Association practice update on the evaluation and management of AKI in cirrhosis.[9] Thus, there remains a significant gap in knowledge on the prognostic significance of AKI stage 1B in a US-based population, which is diverse with higher comorbid conditions, such as chronic kidney disease (CKD), compared with a European population.[3,10] Understanding the clinical course and prognosis of AKI stage 1B in a US population is crucial as it can drastically impact AKI management, specifically regarding initial management decisions for AKI (eg, use of i.v. albumin) and the initiation of terlipressin in patients with stage 1B meeting HRS-AKI criteria. Therefore, we aimed to define the clinical characteristics and outcomes associated with AKI stage 1B in a nationwide US cohort of hospitalized patients with cirrhosis and AKI.
METHODS
Consecutive patients with cirrhosis hospitalized between January 1, 2009 and September 1, 2017 were identified in the de-identified Health Insurance Portability and Accountability Act–compliant Cerner Health Facts Database (Cerner Corporation, Kansas City, MI). Hospital characteristics, vital sign data, laboratory data, pharmaceutical data, and procedural codes (through International Classification of Diseases (ICD) 9/10th revision diagnosis codes and current procedural terminology codes) are among the information contributed to the database. We used previously validated ICD-9/10 codes (primary or secondary, summarized in Supplemental Table S1, http://links.lww.com/LVT/A474)[3,11,12] to identify cirrhosis and its etiology, liver-related complications, comorbidities, infections, history of liver/kidney transplantation, and HD status. This study was approved by the Indiana University Institutional Review Board and is in accordance with both Declarations of Helsinki and Istanbul. Informed consent was waived as this was a retrospective study.
Study population
Details on the study cohort have been described here.[13] In brief, patients with cirrhosis over the age of 18 with AKI were included (see Definitions: AKI, AKI progression, and AKI recovery). For patients with multiple qualifying AKI-related hospitalizations during the study period, we only considered the initial hospitalization. We excluded patients admitted for surgical reasons, who had inadequate data to discern AKI, with prior liver/kidney transplant, and who died/were discharged to hospice within 7 days of hospitalization. For the latter, a total of 66 patients had AKI stage 1 (n = 31 stage 1A and n = 35 stage 1B), and the median time to death was 5 days.
Outcomes
Patients were followed from the time of AKI to assess for outcomes. The primary outcome was mortality at 90 days from the time of AKI. Secondary outcomes were in-hospital progression of AKI and complete AKI recovery from the time of AKI.
Definitions: AKI, AKI progression, and AKI recovery
AKI was defined by Kidney Disease Improving Global Outcomes,[4] which has been endorsed by the ICA[5] as either a rise in sCr of ≥ 0.3 mg/dL from baseline within 48 hours or a percentage increase sCr ≥ 50% from baseline, which is known or presumed to have occurred within the prior 7 days. AKI stages 1A and 1B were defined as meeting Kidney Disease Improving Global Outcomes stage 1 criteria [increase in sCr ≥ 0.3 mg/dL within 48 hours or an increase in sCr ≥ 1.5-fold to 2-fold from baseline[4]] and sCr < 1.5 mg/dL and > 1.5 mg/dL,[1,7,8] respectively. AKI stages 2 and 3 were also based on the Kidney Disease Improving Global Outcomes staging system,[4] which has been endorsed by the ICA.[5] Baseline sCr was defined per the ICA,[5] which was the availability of sCr within the previous 3 months of AKI onset. In patients without a baseline sCr within the previous 3 months, the last sCr value within 1 year of AKI onset was used. If an sCr was not available within 1 year of AKI onset, the first sCr value at the time of hospitalization was considered as a baseline as recommended by the ICA.[5] Recurrent AKI was defined as AKI occurring at least 48 hours after AKI recovery from the index AKI.[14]
We defined AKI progression as the increase from AKI stage 1 A/B to stage 2/3 or the initiation of HD[5] during the hospitalization. Complete AKI recovery was defined as the return of sCr to a value within 0.3 mg/dL of baseline during the hospitalization. Partial AKI recovery was defined by a decrease in the AKI stage with sCr ≥ 0.3 mg/dL above the baseline value. Both complete and partial recoveries are analogous to “complete” and “partial” responses to therapy defined by the ICA.[5]
Hospitalization details
See the Supplemental Material, http://links.lww.com/LVT/A474.
Statistical analysis
Patient characteristics were compared between AKI stages 1A and 1B at the time of AKI diagnosis. Categorical variables were compared using chi-squared tests and presented as percentages. Continuous variables were compared using the Wilcoxon rank sum tests and presented as median with interquartile range (IQR).
Primary outcome analysis
Mortality between AKI stage 1A and stage 1B was compared using Fine and Gray competing risks regression, with the creation of a cumulative incidence function. Liver transplantation (LT) during the follow-up period was considered a competing risk, and patients lost to follow-up were censored at the time of the last clinical encounter. Differences between cumulative incidence functions were compared using Gray’s test. Sensitivity analysis comparing the cumulative incidence of mortality was performed between (1) patients with stage 1A and stage 1B who did not have AKI progression, (2) stage 1A patients who progressed to stage 1B and stage 1B nonprogressors, and (3) stage 1A and stage 1B patients who were not admitted to the intensive care unit [(ICU); it includes patients who received vasopressors and mechanical ventilation].
Univariate competing risk regression analyses were performed to identify factors associated with the primary outcome. Variables that were significant in univariate analysis (p < 0.10) for the primary outcome were then entered into a multivariable competing risk analysis to determine the independent association between stage AKI stage 1B and the primary outcome. Variance inflation factors were used to remove variables with high collinearity in the competing risk multivariable analysis. To explore the relationship between sex and AKI stage 1B versus stage 1A, an interaction term between sex and AKI stage 1B versus stage 1A was included in the competing risk multivariable analysis. Sensitivity analysis excluding patients with recurrent AKI during the follow-up period was performed. The relationship between preexisting CKD and AKI stage 1B versus stage 1A was also explored through an interaction term in the competing risk multivariable analysis. In addition, competing risk univariate and multivariable analysis was performed in the following subgroups: patients with and without preexisting CKD. Sub-HR (sHRs) and their corresponding 95% CI were reported.
Secondary outcome analysis
Univariate logistic regression analysis was performed to identify variables associated with AKI progression. Significant variables (p < 0.10) were then entered into a multivariable model to determine the independent association between AKI stage 1B and AKI progression. The final list of covariates was also determined by removing variables that caused high collinearity, as assessed by variance inflation factors. OR and their corresponding 95% CI were reported. The association between AKI stage 1B and complete AKI recovery was assessed using Fine and Gray competing risks regression. Death or LT during hospitalization was considered a competing risk. Multivariable competing risk analyses were performed to assess the association between AKI stage 1B and complete AKI recovery. The final covariates chosen for multivariable modeling were those that were significant in univariate analysis (p < 0.1). SHRs and their 95% CI were reported. In addition, the relationship between preexisting CKD and AKI stage 1B versus stage 1A was for the secondary outcomes that were explored through an interaction term in the multivariable competing risk analysis. Subgroup analysis for both secondary outcomes was also performed in patients with and without preexisting CKD.
A two-sided nominal p-value < 0.05 was considered statistically significant. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
In all, 6250 patients met inclusion criteria, of which 4654 patients with AKI stage 1 at the time of diagnosis [1A: n = 2064 (44.3%); 1B: n = 2590 (55.7%)] were analyzed. The median age (IQR) was 61 (53,70) years, and the majority were White (68.5%) and male (62.7%) and admitted to an urban teaching hospital (62.6%). The most common etiologies of cirrhosis were NASH (41.4%), followed by alcohol (24.3%) and hepatitis C (17.6%). The median baseline sCr was 1.10 (0.79, 1.83) mg/dL; 37.2% had preexisting CKD, and 63.3% had ascites. The median (IQR) days between baseline sCr and sCr at the time of AKI diagnosis were 19 (4, 119) days. The median Model for End-Stage Liver Disease-Sodium (MELD-Na) score at the time of AKI was 22 (16, 27), and the median (IQR) length of hospitalization was 9 (5, 16) days.
Comparisons of patient and clinical characteristics between AKI stages 1A and 1B
Demographic and clinical characteristics of patients with stages 1A and 1B are compared in Table 1. Patients with stage 1B were older [64 (56, 73) vs. 58 (50, 67) y, p < 0.001], more likely to be male [67.3% vs. 54.1%, p < 0.001), and have NASH [47.3% vs. 34.0%, p < 0.001] and ascites [66.3% vs. 59.5%, p < 0.001] compared with patients with stage 1A (Table 1). Furthermore, there was a higher proportion of Black patients with stage 1B compared with stage 1A patients (19.2% vs. 10.6%). Patients with stage 1B had a higher prevalence of preexisting CKD compared with stage 1A patients, 59.9% vs. 8.7% (p < 0.001), and had significantly higher median baseline sCr, 1.66 (1.20, 1.97) vs. 0.74 (0.60, 0.90) mg/dL (p < 0.001), respectively. Moreover, patients with stage 1B had significantly higher median sCr at time of AKI diagnosis compared with stage 1A patients, 2.20 (1.72, 3.34) vs. 1.10 (0.94, 1.30) mg/dL (p < 0.001), respectively, and accordingly had significantly higher MELD-Na scores [25 (20,29) vs. 18 (11,24) in 1A, p < 0.001] at the time of AKI diagnosis. Furthermore, patients with stage 1B had higher rates of nonspontaneous bacterial peritonitis infections compared with stage 1A patients, 30.8% vs. 26.6% (p < 0.001), respectively.
TABLE 1.
Comparisons of demographic, clinical, and laboratory data between patients with AKI stages 1A and 1B
| Stage 1A N = 2064 | Stage 1B N = 2590 | p | |
|---|---|---|---|
| Age, median (IQR) | 58.0 (50.0, 67.0) | 64.0 (56.0, 73.0) | < 0.001 |
| Race, n (%) | |||
| White | 1509 (73.1) | 1683 (65.0) | < 0.001 |
| Black | 219 (10.6) | 496 (19.2) | — |
| Other | 336 (16.3) | 411 (15.8) | — |
| Sex, n (%) male | 1,117 (54.1) | 1,743 (67.3) | < 0.001 |
| Hospital Type, n (%) | |||
| Rural | 418 (20.3) | 620 (24.0) | 0.01 |
| Urban, nonteaching | 322 (15.6) | 379 (14.6) | — |
| Urban, teaching | 1324 (64.1) | 1591 (61.4) | — |
| Etiology of cirrhosis, n (%) | |||
| Hepatitis C | 369 (17.9) | 450 (17.4) | 0.68 |
| Alcohol | 629 (30.5) | 503 (19.5) | < 0.001 |
| NASH | 702 (34.0) | 1,226 (47.3) | < 0.001 |
| Other | 107 (5.1) | 139 (5.3) | 0.83 |
| Unknown etiology | 257 (12.5) | 272 (10.5) | 0.04 |
| HCC, n (%) | 58 (2.8) | 81 (3.1) | 0.59 |
| Ascites, n (%) | 1,229 (59.5) | 1,719 (66.3) | < 0.001 |
| HE, n (%) | 524 (25.4) | 660 (25.5) | 0.97 |
| Esophageal variceal hemorrhage, n (%) | 120 (5.8) | 75 (2.9) | < 0.001 |
| Charlson Comorbidity Index (excl. liver) | 2 (1,4) | 3 (2, 6) | < 0.001 |
| Body mass index, median (IQR) kg/m2 | 27.1 (23.1, 32.3) | 28.0 (24.1, 33.8) | < 0.001 |
| Diabetes, n (%) | 1,026 (49.7) | 1,362 (53.6) | 0.06 |
| Hypertension, n (%) | 1,047 (50.7) | 1,803 (69.6) | < 0.001 |
| Chronic kidney disease, n (%) | 180 (8.7) | 1,551 (59.9) | < 0.001 |
| Baseline creatinine, median (IQR) mg/dL | 0.74 (0.60, 0.90) | 1.66 (1.20, 1.97) | < 0.001 |
| MAP at the time of AKI diagnosis, median (IQR) | 82.3 (73.0, 93.3) | 80.0 (72.0, 92.0) | 0.001 |
| Laboratory at the time of AKI, median (IQR) | |||
| White blood cell count, 109 | 9.0 (5.9, 13.2) | 8.4 (5.9, 12.3) | 0.06 |
| Sodium, mmol/L | 134.0 (128.0, 139.0) | 135.0 (128.0, 139.0) | 0.25 |
| Creatinine, mg/dL | 1.10 (0.94, 1.30) | 2.20 (1.72, 3.34) | < 0.001 |
| Albumin, g/dL | 2.7 (2.2, 3.2) | 2.7 (2.2, 3.3) | 0.21 |
| Total bilirubin, mg/dL | 2.0 (1.0, 4.8) | 1.4 (0.7, 3.1) | < 0.001 |
| International normalized ratio | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.8) | 0.83 |
| MELD-Na at time of AKI, median (IQR) | 18 (11, 24) | 25 (20, 29) | < 0.001 |
| SBP infection, n (%) | 70 (3.4) | 104 (4.0) | 0.30 |
| Non-SBP infection, n (%) | 548 (26.6) | 797 (30.8) | 0.002 |
| Hospital-acquired AKI, n (%) | 1,221(59.1) | 1,938 (74.8) | < 0.001 |
Abbreviations: AKI, acute kidney injury; IQR, interquartile range; MAP, mean arterial pressure; MELD-Na, Model for End-Stage Liver Disease Sodium; SBP, spontaneous bacterial peritonitis.
Comparisons of AKI clinical course between AKI stages 1A and 1B
Comparisons of AKI clinical course between patients with stages 1A and 1B can be found in Table 2. Compared with stage 1A patients, patients with stage 1B had significantly higher rates of AKI progression (p < 0.001), peak AKI stage 3 (p < 0.001), and HD use (p < 0.001). Furthermore, patients with stage 1B had significantly lower rates of in-hospital complete AKI recovery compared with patients with stage 1A, 68.9% vs. 86.9% (p < 0.001), respectively. Albumin use was infrequent in both stages, but stage 1B patients had higher rates of use (19.0% vs. 25.3%, p < 0.001). Of those that had AKI progression (n = 1,494), 33% received albumin (34.7% Stage 1A vs. 32.6% Stage 1B, p = 0.49). The majority of patients received crystalloids, with patients with stage 1A having higher rates of use compared with stage 1B patients (60.9% vs. 57.3%, p = 0.01). In addition, patients with stage 1B were more likely to receive combinational therapy with midodrine, octreotide, and vasopressors compared with patients with stage 1A (Table 2). Although intensive care unit admission/transfer and mechanical ventilation use were similar between both stages, patients with stage 1B had significantly higher rates of in-hospital death compared with patients with stage 1A, 14.0% vs. 11.6% (p = 0.02), respectively.
TABLE 2.
Comparisons of AKI clinical course between patients with AKI stages 1A and 1B
| Stage 1A N = 2,064 | Stage 1B N = 2,590 | p | |
|---|---|---|---|
| Peak creatinine, median (IQR) mg/dL | 1.24 (1.00, 1.49) | 2.76 (1.94, 4.70) | < 0.001 |
| Delta creatinine (peak minus baseline), median (IQR) mg/dL | 0.43 (0.30, 0.61) | 0.80 (0.50, 1.45) | < 0.001 |
| Progression of AKI, n (%) | 453 (21.9) | 1,041 (40.2) | < 0.001 |
| Peak AKI stage, n (%) | |||
| 2 | 257 (12.4) | 191 (7.4) | < 0.001 |
| 3 | 196 (9.5) | 850 (32.8) | — |
| AKI recovery, n (%) | |||
| None | 265 (10.2) | 693 (26.8) | < 0.001 |
| Partial | 6 (2.9) | 114 (4.3) | — |
| Complete | 1,793 (86.9) | 1,783 (68.9) | — |
| Albumin usea, n (%) | 399 (19.3) | 655 (25.3) | < 0.001 |
| Crystalloid useb, n (%) | 1,258 (60.9) | 1,485 (57.3) | 0.01 |
| Midodrine and octreotide combination use, n (%) | 50 (2.4) | 117 (4.5) | < 0.001 |
| Hemodialysis use, n (%) | 4 (< 0.01) | 233 (9.0) | < 0.001 |
| ICU admission/transfer, n (%) | 500 (24.2) | 601 (23.2) | 0.44 |
| ACLF, n (%) | 201 (9.7) | 359 (13.9) | < 0.001 |
| ICU interventions, n (%) | |||
| Vasopressors | 255 (12.4) | 454 (17.5) | < 0.001 |
| Mechanical ventilation | 269 (13.0) | 327 (12.6) | 0.71 |
| In-hospital recurrent AKI, n (%) | 368 (17.8) | 555 (21.4) | 0.003 |
| In-hospital death, n (%) | 240 (11.6) | 363 (14.0) | 0.02 |
| Hospital length of stay, median (IQR) days | 9 (5, 15) | 10 (6, 16) | < 0.001 |
25% albumin.
median (IQR) volume given in both groups was 2500 (1000–4500) mL.
Abbreviations: ACLF, acute-on-chronic-liver failure; AKI, acute kidney injury; ICU, intensive care unit; IQR, interquartile range.
Comparisons of outcomes between AKI stages 1A and 1B
Primary outcome
Comparisons of cumulative incidence for mortality between AKI stages 1A and 1B can be found in Figure 1. The cumulative incidence of mortality was significantly higher in stage 1B patients compared with stage 1A patients: 30-day 17.4% (95% CI 15.9–19.0) vs. 14.4% (95% CI 12.8–16.1) and 90-day 27.2% (95% CI 25.3–29.3) vs. 19.7% (95% CI 17.8–21.6) (Gray’s test p < 0.001). In sensitivity analysis, the cumulative incidence of mortality was significantly higher in nonprogressors with stage 1B compared to stage 1A nonprogressors: 30-day 12.1% (95% CI 10.4–13.9) vs. 7.0% (95% CI 5.7–8.5) and 90-day 20.9% (95% CI 18.6–23.2) vs. 11.1% (95% CI 9.4–13.0) (Gray’s test p < 0.001). Similarly, the cumulative incidence of mortality was significantly higher in non-ICU patients with stage 1B compared with non-ICU stage 1A patients: 30-day 11.5% (95% CI 9.9–13.3) vs. 8.9% (95% CI 7.3–10.6) and 90-day 20.0% (95% CI 17.8–22.3) vs. 13.0% (95% CI 11.0–15.1) (Gray’s test p < 0.001). There was no significant difference in the cumulative incidence of mortality between patients with stage 1A who progressed to stage 1B and stage 1B nonprogressors (Gray’s test p = 0.07).
FIGURE 1.
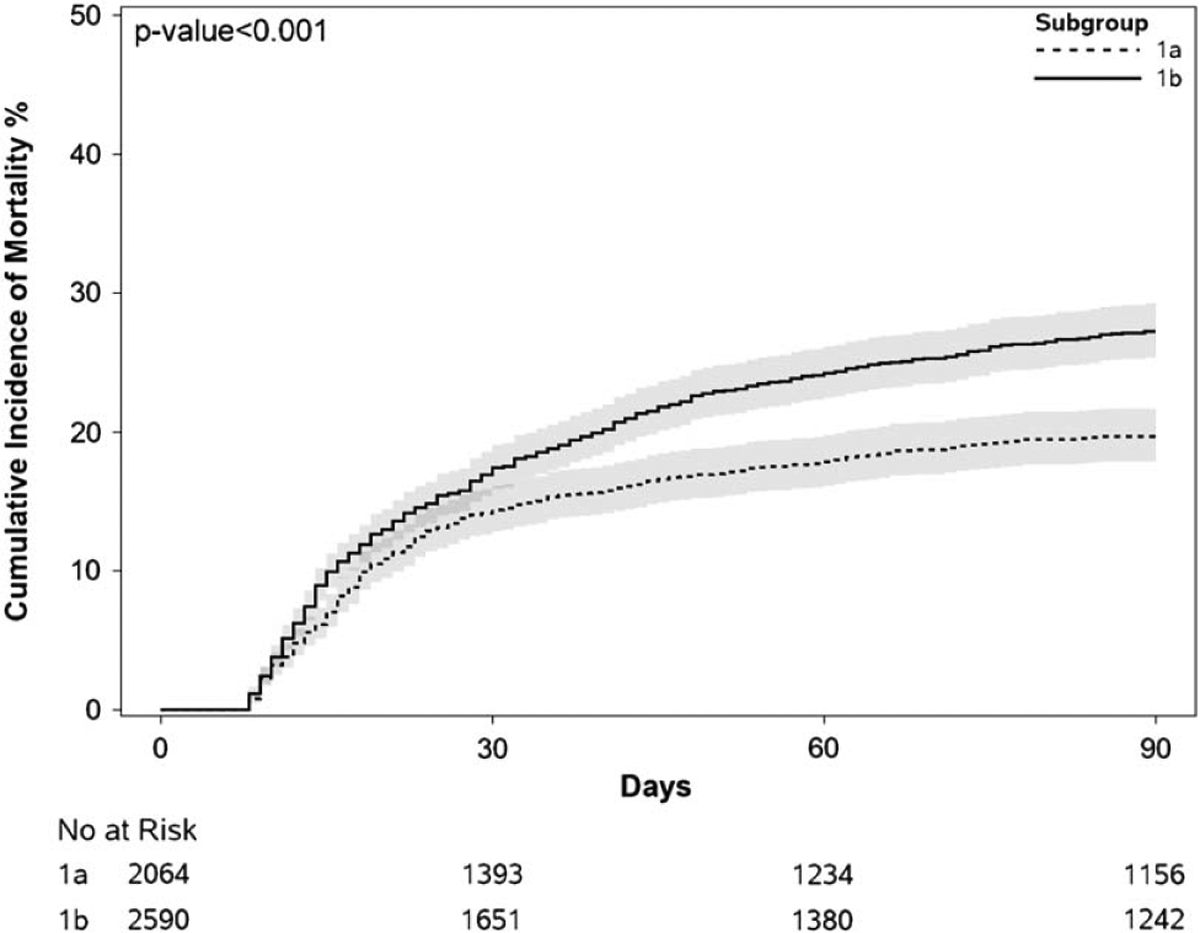
Comparison of cumulative incidence of 90-day mortality between acute kidney injury stages 1A and 1B.
In univariate competing risk analysis, stage 1B was associated with an increased risk of death at 90 days [sHR 1.43 (95% CI 1.23–1.62), p < 0.001]. Additional factors associated with mortality are shown in Supplemental Table S2, http://links.lww.com/LVT/A474. In multivariable competing risk analysis (Table 3), stage 1B was independently associated with an increased risk for mortality at 90 days [sHR 1.52 (95% CI 1.29–1.92), p = 0.001]. No significant interaction was found between sex and stage 1B vs. stage 1A (p = 0.90), but a significant interaction was found between preexisting CKD and stage 1B vs. stage 1A (p < 0.001). When adjusting for recurrent AKI during the 90-day follow-up period (stage 1A n = 678 and stage 1B n = 1009 with recurrent AKI), the results remained unchanged, sHR 1.48 (95% CI 1.17–1.86) (p = 0.001). Sensitivity analysis excluding patients with recurrent AKI during the follow-up period showed similar results [sHR 1.56 (95% CI 1.10–2.23), p = 0.01]. Furthermore, results were similar when the model was adjusted for baseline sCr in lieu of preexisting CKD [sHR 1.47 (95% CI 1.16–1.86), p = 0.002)].
TABLE 3.
Multivariable model for 90-day mortality
| Variable | sHR (95% CI) | p |
|---|---|---|
| Stage 1B (vs. stage 1A) | 1.52 (1.20–1.92) | 0.001 |
| Age (per 1-year increase) | 1.02 (1.02–1.03) | < 0.001 |
| Ascites | 1.42 (1.12–1.79) | 0.003 |
| HE | 1.74 (1.41–2.15) | < 0.001 |
| Hepatitis C cirrhosis | 1.17 (0.86–1.60) | 0.33 |
| NASH cirrhosis | 1.27 (0.99–1.63) | 0.06 |
| Diabetes | 1.27 (1.03–1.58) | 0.03 |
| Hypertension | 0.82 (0.66–1.03) | 0.08 |
| Preexisting chronic kidney disease | 1.06 (0.83–1.36) | 0.63 |
| MAP at the time of AKI (per 1 mm Hg increase) | 1.00 (0.99–1.00) | 0.32 |
| Albumin at the time of AKI (per 1 U increase) | 0.66 (0.57–0.78) | < 0.001 |
| Total bilirubin at the time of AKI (per 1 U increase) | 1.05 (1.03–1.06) | < 0.001 |
| International normalized ratio (per 1 U increase) | 1.06 (1.01–1.11) | 0.02 |
| White blood cell count (per 1 U increase) | 1.02 (1.00–1.03) | 0.01 |
| Any infection | 1.06 (0.86–1.34) | 0.59 |
| ICU transfer during the index hospitalization | 1.25 (0.98–1.58) | 0.07 |
| Mechanical ventilation during the index hospitalization | 1.99 (1.52–2.60) | < 0.001 |
| Vasopressor use during the index hospitalization | 1.25 (0.83–1.35) | 0.07 |
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; MAP, mean arterial pressure.
Subgroup analysis
Univariate competing risk analysis for patients with and without preexisting CKD can be found in Supplemental Table S3, http://links.lww.com/LVT/A474. After adjusting for significant factors associated with mortality, patients with stage 1B had a significantly higher risk for death at 90 days in patients without preexisting CKD [sHR 1.56 (95% CI 1.19–2.04), p = 0.001]. However, stage 1B was not associated with mortality in patients with preexisting CKD [sHR 1.13 (95% CI 0.75–1.71), p = 0.56].
Secondary outcomes
Univariate analysis for factors associated with AKI progression and complete AKI recovery is shown in Supplemental Table S4, http://links.lww.com/LVT/A474. In multivariable analysis (Table 4), stage 1B was independently associated with AKI progression [OR 1.42 (95% CI 1.14–1.76), p = 0.001] and a decreased probability for complete AKI recovery [sHR 0.76 (95% CI 0.69–0.83), p < 0.001]. No significant interactions were found between preexisting CKD and stage 1B versus stage 1A for AKI progression (p = 0.87) and complete AKI recovery (p = 0.91). Subgroup univariable analysis in patients with and without preexisting CKD can be found in Supplemental Tables S5, http://links.lww.com/LVT/A474 and S6, http://links.lww.com/LVT/A474, respectively. In multivariable analysis, stage 1B continued to be independently associated with AKI progression in patients with and without preexisting CKD, OR 1.56 (95% CI 1.06–2.29) (p = 0.03) and OR 1.33 (95% CI 1.03–1.71) (p = 0.03), respectively. Similarly, stage 1B continued to be associated with a decreased probability for complete AKI recovery in patients with and without preexisting CKD, sHR 0.72 (95% CI 0.56–0.92) (p = 0.01), and sHR 0.81 (95% CI 0.74–0.88) (p < 0.001), respectively.
TABLE 4.
Multivariable model for AKI progression and complete AKI recovery
| AKI Progression | ||
|---|---|---|
| Variable | OR (95% CI) | p |
| Stage 1B (vs. stage 1A) | 1.42 (1.14–1.76) | 0.002 |
| Age (per 1-year increase) | 0.99 (0.98–1.00) | 0.001 |
| Type of hospital (urban vs. rural) | 0.67 (0.54–0.82) | < 0.001 |
| Ascites | 1.27 (1.04, 1.55) | 0.02 |
| Variceal hemorrhage | 0.54 (0.32–0.81) | 0.01 |
| HE | 1.24 (1.01–1.52) | 0.04 |
| Hepatitis C cirrhosis | 1.12 (0.87–1.42) | 0.38 |
| Alcohol-associated cirrhosis | 0.80 (0.63–1.02) | 0.07 |
| Diabetes | 1.07 (0.89–1.30) | 0.47 |
| Hypertension | 0.93 (0.76, 1.15) | 0.52 |
| Preexisting chronic kidney disease | 3.39 (2.68–4.28) | < 0.001 |
| Albumin at the time of AKI (per 1 U | 0.76 (0.67–0.87) | < 0.001 |
| increase) | ||
| Total bilirubin at the time of AKI (per | 1.04 (1.03–1.06) | < 0.001 |
| 1 U increase) | ||
| International normalized ratio (per 1 | 1.04 (0.96–1.13) | 0.31 |
| U increase) | ||
| White blood cell count (per1 U increase) | 1.00 (0.99–1.01) | 0.57 |
| Any infection | 1.43 (1.17–1.76) | 0.001 |
| ICU transfer during the index | 1.19 (0.97–1.47) | 0.10 |
| hospitalization | ||
| Mechanical ventilation during the | 1.99 (1.52–2.59) | < 0.001 |
| index hospitalization | ||
| Vasopressor use during the index | 1.81 (1.42–2.30) | < 0.001 |
| hospitalization | ||
| Complete AKI recovery | ||
| Variable | sHR (95% CI) | p |
| Stage 1B (vs. stage 1A) | 0.76 (0.69–0.83) | < 0.001 |
| Age (per 1-year increase) | 1.00 (1.00–1.00) | 0.79 |
| Sex (male vs. female) | 0.98 (0.90–1.07) | 0.66 |
| Race (non-White vs. White) | 0.96 (0.88–1.05) | 0.37 |
| Type of hospital (urban vs. rural) | 1.06 (0.97–1.15) | 0.23 |
| Ascites | 0.87 (0.80–0.94) | 0.001 |
| Variceal hemorrhage | 1.20 (1.03–1.40) | 0.03 |
| Alcohol-associated cirrhosis | 1.12 (1.01–1.24) | 0.04 |
| NASH cirrhosis | 0.95 (0.89–1.04) | 0.23 |
| Diabetes | 0.96 (0.89–1.04) | 0.34 |
| Hypertension | 1.05 (0.96–1.14) | 0.32 |
| Preexisting chronic kidney disease | 0.60 (0.50–0.63) | < 0.001 |
| MAP at the time of AKI (per 1 mm Hg | 1.00 (0.99–1.00) | 0.001 |
| increase) | ||
| Albumin at the time of AKI (per 1 U | 1.16 (1.10–1.23) | < 0.001 |
| increase) | ||
| Total bilirubin at time of AKI (per 1 U | 0.98 (0.97–0.99) | < 0.001 |
| increase) | ||
| White blood cell count (per 1 U increase) | 1.01 (1.00–1.01) | 0.003 |
| Any Infection | 0.94 (0.84–1.04) | 0.15 |
| Vasopressor use during the index | 0.94 (0.84–1.04) | 0.23 |
| hospitalization | ||
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; MAP, mean arterial pressure.
DISCUSSION
In this large nationwide US cohort of hospitalized patients with cirrhosis and AKI, we sought to define the clinical characteristics and prognostic significance of AKI stage 1B. We found AKI stage 1B to be prevalent (41% had stage 1B at the time of AKI diagnosis among all patients with AKI) and occurred frequently in patients who were older, with NASH, preexisting CKD, and ascites. We also found Black patients to have a higher proportion of stage 1B, which is likely attributed to the higher burden of CKD in Black patients.[15] Importantly, we found patients with stage 1B to have a significantly higher risk for 90-day mortality (sHR 1.52), AKI progression (OR 1.42), and lower probability of complete AKI recovery (sHR 0.72) compared with patients with AKI stage 1A. However, it is important to mention that stage 1A should not be regarded as benign as it carried an 11.6% in-hospital mortality rate and progressed to 21.9% in our study. Also, 1 in 10 patients with stage 1A developed acute on chronic liver failure, which is known to negatively impact prognosis. Nevertheless, our findings underscore and validate[1,7,8] the prognostic significance of stage 1B in a US-based population.
The frequency of preexisting CKD was significantly higher in patients with stage 1B compared with patients with stage 1A, 59.9% vs. 9.9%, respectively. Hence, it is possible that the driving force behind stage 1B outcomes could be the influence of preexisting CKD.[16,17] Given this possibility, we performed a subgroup multivariable analysis for our primary and secondary outcomes. We found that stage 1B was not associated with mortality in patients with preexisting CKD but was associated with mortality in patients without preexisting CKD. These findings are further supported by the significant interaction found between preexisting CKD and stage 1B versus stage 1A in our multivariable competing risk analysis for mortality. Hence, the prognostic impact for stage 1B appears to be in patients without preexisting CKD. The reasons for these differences are unclear and possibly related to patient selection, heterogeneity of CKD severity, unmeasured factors associated with the severity of cirrhosis (ie, frailty), and follow-up care after discharge. On the other hand, we did not find significant interactions between stage 1B versus stage 1A and preexisting CKD for our secondary outcomes, and stage 1B was associated with AKI progression and decreased probability of complete AKI recovery independent of preexisting CKD.
Various guidelines use stage 1A versus stage 1B distinction or the presence of key comorbidities when triaging initial management decisions.[5,6,9] In patients with stage 1 AKI and ascites, the ICA guidelines recommend withdrawal or reduction of certain medications (eg, diuretics, vasodilators, and nonsteroidal anti-inflammatory drugs), prompt evaluation and treatment of bacterial infections, and plasma volume expansion with either crystalloids or albumin.[5] The European Study of Liver Disease guidelines[6] and, recently, the American Gastroenterological Association guidance statement for the evaluation and management of AKI in cirrhosis[9] suggest a similar approach but for patients with stage 1B to receive albumin. Our findings support these recommendations, particularly in patients with ascites and infection, known factors that are associated with AKI progression,[5,18] which were also confirmed in the current study. Therefore, the decision to administer albumin versus crystalloids for the initial management of AKI stage 1A or stage 1B could be determined by the presence or absence of ascites.
Our study had several limitations. First, due to the nature of the dataset, we lacked additional granular details on several of the variables, such as details required to discern AKI phenotypes and cause of death, although it could be inferred that most patients were likely to have hypovolemic or prerenal AKI due to the high rates of complete in-hospital AKI recovery and the remainder having a combination of either acute tubular necrosis or HRS-AKI (4% received midodrine and octreotide). Indeed, lower AKI progression and mortality rates observed in patients with stage 1A would suggest a higher proportion of hypovolemic AKI compared with stage 1B patients. Moreover, we are unable to stratify the severity and phenotype of preexisting CKD (eg, structural or functional such as HRS-CKD), which may impact outcomes differentially or account for sarcopenia, which could lead to the overestimation of kidney function[19] and affect the prevalence of preexisting CKD. A further multicenter prospective study with CKD severity stratification and incorporation of structural and functional kidney biomarkers would be needed to understand the impact of CKD and its phenotypes on AKI outcomes. Second, due to the retrospective nature of the study, residual confounding exists. Also, although we used strict criteria to define baseline sCr, the possibility of AKI misdiagnosis exists, given the frequent fluctuations in sCr that may occur in patients with cirrhosis. Third, the underutilization of i.v. albumin could have affected our AKI progression rates. However, compared with Huelin et al,[1] the AKI recovery rates for stage 1A in our study were similar (90% vs. 86.9%) and higher in stage 1B, 50% versus 68.9%. Nevertheless, the underutilization of albumin use provides a key area for quality improvement in the management of AKI in patients with cirrhosis.[20] Lastly, despite using validated ICD-9/10 codes that carry high sensitivity for the diagnosis of cirrhosis, the possibility for cirrhosis misclassification may exist.
Despite the limitations of our study, there are also several strengths. Our sample size was large and diverse and included urban and rural hospitals across the United States, which allowed for meaningful comparisons between patients with AKI stages 1A and 1B. These comparisons provided a better understanding of the natural history of these stages, which could translate to modifications in clinical management, such as earlier administration of albumin and terlipressin in stage 1B nonresponders meeting HRS-AKI criteria. In addition, we used strict definitions for AKI and its progression/recovery, which allowed for accurate estimates of the rates of AKI progression and recovery for stages 1A and 1B. Knowledge of these estimates can help inform the design of interventional studies focused on improving AKI recovery and survival in this population.
In conclusion, AKI stage 1B is prevalent and occurs frequently in patients who are older, with NASH, preexisting CKD, and ascites. Compared with stage 1A patients, stage 1B patients have higher rates of mortality, AKI progression, and lower rates of complete AKI recovery. Therefore, these data support the American Gastroenterological Association and European Study of Liver Disease guideline recommendations for the initial management of AKI stage 1 in patients with cirrhosis. Further international prospective studies evaluating the natural history of AKI are needed to validate the prognostic significance of stage 1B. Ultimately, randomized clinical trials are needed to evaluate if early aggressive therapy with albumin in stage 1B improves AKI-associated outcomes.
Supplementary Material
FUNDING INFORMATION
Andrew S. Allegretti and Giuseppe Cullaro are funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health; Andrew S. Allegretti—K23 DK128567 and Giuseppe Cullaro—K23 DK131278. Giuseppe Cullaro is also funded by the American Association for Study of Liver Disease Clinical, Translational, and Outcomes Research Award.
Abbreviations:
- ACLF
acute-on-chronic-liver failure
- AKI
acute kidney injury
- CKD
chronic kidney disease
- HD
hemodialysis
- HRS-AKI
hepatorenal syndrome acute kidney injury
- ICA
International Club of Ascites
- ICD
International Classification of Diseases
- ICU
intensive care unit
- IQR
interquartile range
- LT
liver transplantation
- MAP
mean arterial pressure
- MELD-Na
Model for End-Stage Liver Disease Sodium
- SBP
spontaneous bacterial peritonitis
- sCr
serum creatinine
- sHR
sub-HR
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.ltxjournal.com.
CONFLICTS OF INTEREST
Giuseppe Cullaro consults for Ocelot Bio and Retro. He owns stock in Eli Lilly and Novo Nordisk. Eric S. Orman advises BioVie. Salvatore Piano consults for Plasma Protein Therapeutics and Resolution Therapeutics. He advises Mallinckrodt. He is on the speakers’ bureau for Grifols. Andrew S. Allegretti consults for Mallinckrodt and Ocelot Bio. The remaining authors have no conflicts to report.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available at Cerner Health Facts. Restrictions apply to the availability of this data, which were used under license for this study. Data are available from Dr. Ananth Grama and Mr. Mobasshir Naved with the permission of Cerner Health Facts. Data in Health Facts were extracted directly from the electronic medical records of hospitals in which Cerner has a data use agreement. Encounters may include pharmacy, clinical and microbiology laboratory, admission, and billing information from affiliated patient care locations. All admissions, medication orders and dispensing, laboratory orders, and specimens are date and time-stamped, providing a temporal relationship between treatment patterns and clinical information. Cerner Corporation has established Health Insurance Portability and Accountability Act-compliant operating policies to establish deidentification for Health Facts. No data were reproduced from other sources.
REFERENCES
- 1.Huelin P, Piano S, Sola E, Stanco M, Sole C, Moreira R, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol. 2017;15:438–445 e435. [DOI] [PubMed] [Google Scholar]
- 2.Wong F, O’Leary JG, Reddy KR, Garcia-Tsao G, Fallon MB, Biggins SW, et al. Acute kidney injury in cirrhosis: Baseline serum creatinine predicts patient outcomes. Am J Gastroenterol. 2017;112:1103–10. [DOI] [PubMed] [Google Scholar]
- 3.Desai AP, Knapp SM, Orman ES, Ghabril MS, Nephew LD, Anderson M, et al. Changing epidemiology and outcomes of acute kidney injury in hospitalized patients with cirrhosis - a US population-based study. J Hepatol. 2020;73:1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013; 61:649–72. [DOI] [PubMed] [Google Scholar]
- 5.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62: 968–74. [DOI] [PubMed] [Google Scholar]
- 6.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- 7.Fagundes C, Barreto R, Guevara M, Garcia E, Sola E, Rodriguez E, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–81. [DOI] [PubMed] [Google Scholar]
- 8.Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013; 59:482–9. [DOI] [PubMed] [Google Scholar]
- 9.Flamm SL, Wong F, Ahn J, Kamath PS. AGA Clinical Practice update on the evaluation and management of acute kidney injury in patients with cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2022;20:2707–16. [DOI] [PubMed] [Google Scholar]
- 10.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–81. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt ML, Barritt AS, Orman ES, Hayashi PH. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology. 2015;148: 967–977 e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive predictive value of International Classification of Diseases, 10th revision, codes for cirrhosis and its related complications. Clin Gastroenterol Hepatol. 2018;16:1677–8. [DOI] [PubMed] [Google Scholar]
- 13.Patidar KR, Naved MA, Grama A, Adibuzzaman M, Aziz Ali A, Slaven JE, et al. Acute kidney disease is common and associated with poor outcomes in patients with cirrhosis and acute kidney injury. J Hepatol. 2022;77:108–15. [DOI] [PubMed] [Google Scholar]
- 14.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–57. [DOI] [PubMed] [Google Scholar]
- 15.Laster M, Shen JI, Norris KC. Kidney disease among African Americans: A population perspective. Am J Kidney Dis. 2018;72:S3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullaro G, Verna EC, McCulloch CE, Lai JC. Improving the Model for End-Stage Liver Disease with sodium by incorporating kidney dysfunction types. Hepatology. 2022;76:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullaro G, Verna EC, Lai JC. Association between renal function pattern and mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17:2364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong F, Reddy KR, Tandon P, O’Leary JG, Garcia-Tsao G, Vargas HE, et al. Progression of Stage 2 and 3 acute kidney injury in patients with decompensated cirrhosis and ascites. Clin Gastroenterol Hepatol. 2021;19:1661–69 e1662. [DOI] [PubMed] [Google Scholar]
- 19.Yoo JJ, Kim SG, Kim YS, Lee B, Lee MH, Jeong SW, et al. Estimation of renal function in patients with liver cirrhosis: Impact of muscle mass and sex. J Hepatol. 2019;70:847–54. [DOI] [PubMed] [Google Scholar]
- 20.Patidar KR, Adibuzzaman M, Naved MA, Rodriquez D, Slaven JE, Grama A, et al. Practice patterns and outcomes associated with intravenous albumin in patients with cirrhosis and acute kidney injury. Liver Int. 2022;42:187–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available at Cerner Health Facts. Restrictions apply to the availability of this data, which were used under license for this study. Data are available from Dr. Ananth Grama and Mr. Mobasshir Naved with the permission of Cerner Health Facts. Data in Health Facts were extracted directly from the electronic medical records of hospitals in which Cerner has a data use agreement. Encounters may include pharmacy, clinical and microbiology laboratory, admission, and billing information from affiliated patient care locations. All admissions, medication orders and dispensing, laboratory orders, and specimens are date and time-stamped, providing a temporal relationship between treatment patterns and clinical information. Cerner Corporation has established Health Insurance Portability and Accountability Act-compliant operating policies to establish deidentification for Health Facts. No data were reproduced from other sources.


