Abstract
Objective
Maternal colonization with Group B Streptococcus (GBS) is a significant risk factor for serious neonatal morbidity. There are limited data on how the cervicovaginal (CV) microbiota and host immune factor β-defensin-2 might influence GBS colonization in pregnant individuals. This study sought to determine if the CV microbiota is associated with GBS colonization in pregnant individuals, and if β-defensin-2 modifies this relationship.
Study Design
This was a secondary analysis of a prospective cohort study of pregnant individuals with singleton pregnancies who had CV microbiota specimens analyzed at 16 to 20, 20 to 24, and 24 to 28 weeks’ gestation, along with a third trimester GBS rectovaginal (RV) culture (n= 492). Microbiota data were analyzed with 16S rRNA gene sequencing and classified into community state types (CSTs). Log-binomial multivariable regression was used to model associations between CST and GBS RV status and to calculate risk ratios. β-defensin-2, an immune factor known to modulate the relationship between CST and pregnancy outcomes, was examined as an effect modifier. Results Of 492 individuals, 34.3% were GBS RV +. Compared with individuals with CST I at 16 to 20 weeks, individuals with CST IV-A and CST II had a significantly elevated relative risk of subsequent GBS RV+ status. When stratified by high and low β-defensin2 levels, β-defensin-2 was found to be an effect modifier of the association between CST IV-A and GBS RV+ status. In individuals with low β-defensin-2 levels, CST VI-A was associated with GBS RV+ status, but among individuals with high β-defensin-2 levels, there was no such association (interaction p-value = 0.03).
Conclusion
Pregnant individuals with CV microbiota characterized by CST IV-A and CST II had significantly elevated risk of GBS RV colonization in the third trimester compared with those with CST I, and β-defensin-2 was an effect modifier of the association between CST IV-A and GBS RV+ status. Future research should investigate if manipulation of the CV microbiota can prevent GBS colonization, thereby reducing intrapartum antibiotic prophylaxis and the risks of neonatal GBS infection.
Keywords: cervicovaginal microbiota, dysbiosis, GBS, β-defensin-2, microbiome, pregnancy
Group B Streptococcus (GBS), or Streptococcus agalactiae, is a gram-positive β-hemolytic bacterium that colonizes the gastrointestinal and vaginal tracts of up to one-third of pregnant individuals.1 While colonization is usually benign for the pregnant individual, GBS rectovaginal (RV) colonization in pregnancy is associated with substantial risks to the neonate, including GBS bacteremia, sepsis, and death. As such, the Centers for Disease Control and Prevention (CDC) recommends routinely screening all pregnant individuals for GBS RV colonization prior to delivery, and approximately 31% of individuals in the United States receive antibiotics in labor for prophylaxis against neonatal GBS disease.1 Despite this, GBS remains the leading cause of early-onset neonatal sepsis in the United States.1 Moreover, studies have shown that up to 40% of individuals who are GBS RV+ when tested in prenatal care are ultimately GBS RV− on delivery, leading to significant overtreatment during labor, which poses risks of increasing antibiotic resistance.2 Accordingly, there is significant need for therapeutics to prevent GBS RV colonization at delivery, rather than relying on imprecise screening and prophylaxis algorithms.
Recent work has begun to elucidate the role of the cervicovaginal (CV) microbiota in reproductive health outcomes, raising the question of whether the CV microbiota could potentially be manipulated to reduce or prevent GBS RV colonization during pregnancy.3–5 While existing data have rigorously characterized the CV microbiota in both pregnant and nonpregnant individuals,3,6–10only a few studies have begun to examine the relationship between the CV microbiota and GBS RV colonization in pregnancy.7–9 A few studies have demonstrated an inverse association between Lactobacillus species and GBS colonization in the third trimester,9,11 whereas others have not confirmed this.7,8 Furthermore, recent works have demonstrated that antimicrobial peptide β-defensin-2 is associated with nonoptimal CV microbiota as well as bacterial vaginosis and sexually transmitted infections, but the impact of β-defensin-2 on GBS colonization in pregnancy has not yet been examined.3,5,12,13 Therefore, we sought to determine whether the CV microbiota was associated with GBS RV colonization in pregnancy. We examined the relationship between GBS RV colonization and various characteristics of the CV microbiome at multiple time points throughout pregnancy. Furthermore, we sought to examine whether β-defensin-2 also modulated the relationship between CV microbiota and GBS RV colonization, with the hypothesis that low β-defensin-2 would be associated with nonoptimal CV microbiota and GBS colonization.
Materials and Methods
Study Design
This was a secondary analysis of a prospective cohort study entitled “Motherhood & Microbiome” or “M&M” (NCT02030106: R01 NR014784–01).3 The methods from this original study, including Institutional Review Board approval from the University of Pennsylvania, have been previously published.3 Two thousand individuals were approached and consented for enrollment during a prenatal care visit between 16 and 20 weeks (visit 1, V1) of gestation at the Hospital of the University of Pennsylvania between December 2013 and February 2017. A CV specimen was collected at the time of enrollment as well as at two additional prenatal visits between 20 and 24 weeks(visit2,V2)and24and28weeks(visit3,V3)of gestation. Individuals were ineligible for enrollment in the study if they met any of the following exclusion criteria: (1) major fetal anomaly; (2) HIV positive status; (3) history of organ transplant; or (4) chronic steroid use (greater than 20mg/d for more than 30 days at the time of first study visit).
Among the 1,943 individuals who completed at least one study visit, 581 were previously selected for microbiota analysis as part of the primary study to evaluate associations of the microbiota and spontaneous preterm birth.3 For the current analysis, of these 581, we excluded individuals who were missing GBS RV culture data, resulting in a final sample size of 492 individuals with both microbiota and GBS RV culture data available for analysis (Fig. 1).
Fig. 1.
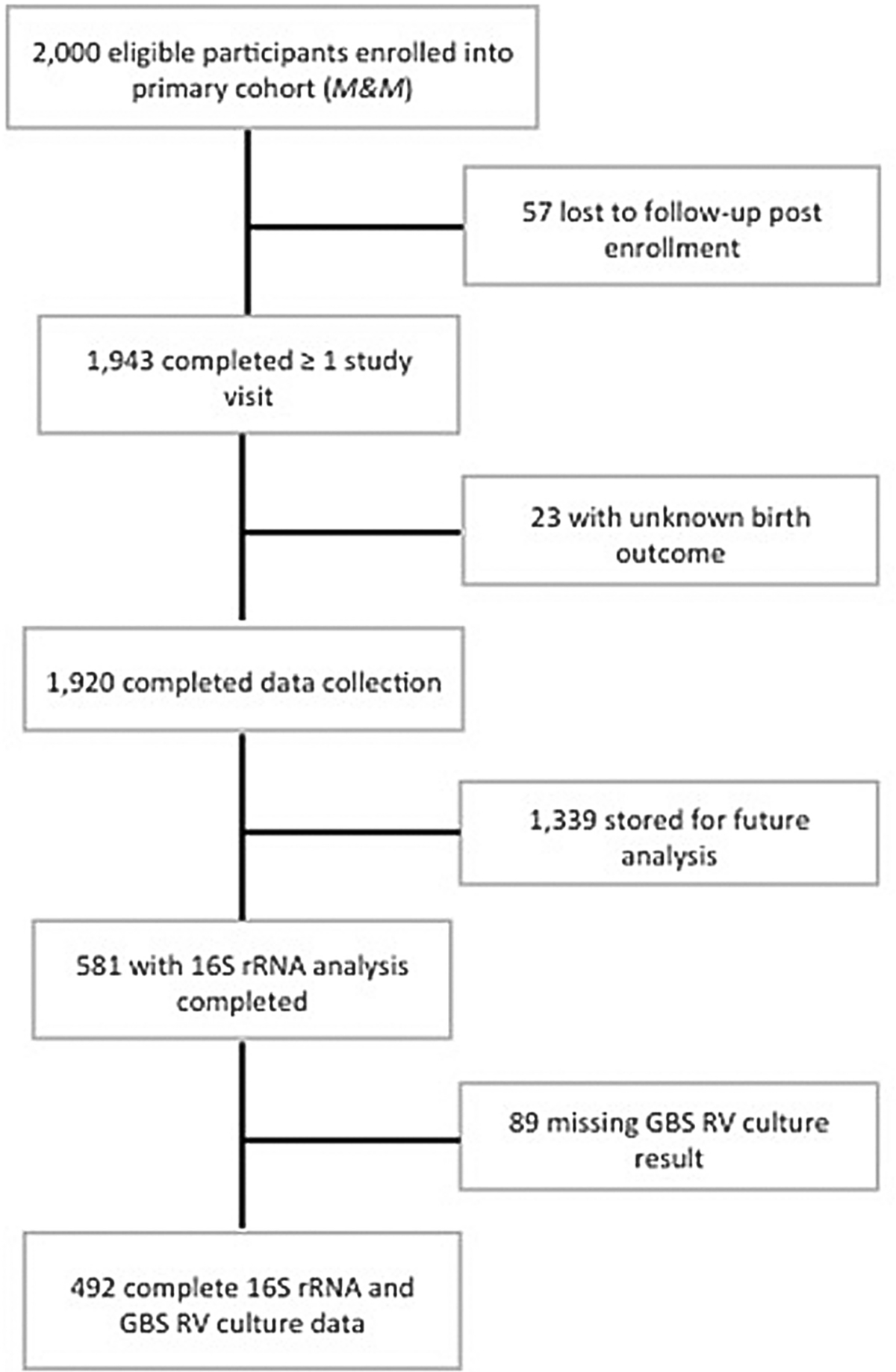
Study enrollment flow chart. GBS, Group B Streptococcus; RV, rectovaginal.
16S rRNA Gene Analyses
Microbial analyses were done as previously reported.3 Briefly, the methods involve DNA extraction, 16S rRNA gene PCR amplification and sequencing (with amplification of the V3–V4 regions of the 16S rRNA gene).14 Bacterial load in each sample was calculated using the methods of Liu et al using a Pan Bacterial qPCR targeting the V3–V4 region of the 16S rRNA gene.15 Total copies of 16S rRNA genes were generated and used to estimate the bacterial load of each bacterial species by multiplying the species relative abundance in each sample by the total 16S rRNA gene copies in that sample. Quality control for these methods has previously been reported.16–18 Taxonomic assignments of each 16S rRNA V3–V4 amplicon sequence was performed using PECAN, a novel and rapid model-based Markov Chain classifier groups each sequence to the species level without the need for OTU clustering (www.ravel-lab.org.pecan) and validated using a phylogenetic-based method as previously reported.19–21 Applying PECAN to this dataset resulted in the identification of a total of 533 species level taxa.
Using previously established methods, the vaginal microbial communities were classified into community state types (CSTs) based on the predominant bacteria in the sample.10 Consistent with prior reports, six CSTs were characterized.3,10 CSTI is defined by predominance of Lactobacillus crispatus, CST II by Lactobacillus gasseri, CST III by Lactobacillus iners, and CST V by Lactobacillus jensenii. CST IV is non-Lactobacillus dominated, characterized instead by facultative and strict anaerobes.3 CST IV-A is distinguished from CST IV-B by a higher abundance of bacterial vaginosis-associated bacteria.3
Group B Streptococcus Colonization Determination
As part of routine prenatal care, individuals had a GBS RV culture collected during the third trimester to screen for GBS colonization in accordance with CDC guidelines.1 Maternal demographics, obstetric history, GBS RV culture result, and pregnancy outcomes were abstracted from electronic medical records by research staff. As standard of care at our hospital, all GBS RV cultures were analyzed by the hospital microbiology laboratory in accordance with standard GBS culture protocol.22 An RV swab was collected by clinical providers during prenatal care according to the CDC guidelines and inoculated into a Carrot Broth tube. The Carrot Broth was examined after 18 to 24 hours of incubation, and any mauve or darker pink colonies were considered positive. All tubes were then subcultured to GBS detect agar and incubated for 12 to 24 hours at 35 to 37°C in 5 to 10% CO2. The plates were examined after 18 to 24 hours of incubation to determine presence or absence of GBS. GBS positive cultures were not further investigated for serotypes. Individuals were considered GBS positive (GBS RV + ) if they had an RV culture collected during routine prenatal care that was positive for GBS. Individuals were considered GBS negative (GBS RV − ) if they had a GBS RV culture that was negative for GBS. The median gestational age at which GBS RV swabs were collected was 37.0 weeks with an interquartile range of 36.0 to 38.1 weeks.
β-defensin-2 Analysis
We have previously demonstrated that the immune factor β-defensin-2 is present in the CV space and can modulate the association between the CV microbiota and adverse pregnancy outcomes.3 Therefore, we sought to examine whether β-defensin-2modulatedtherelationshipbetweenCVmicrobiota and GBS RV colonization. β-defensin-2 was analyzed from swabs obtained at 16 to 20 weeks of gestation (V1) using with ELISA as previously described.3 As done in prior work, β-defensin-2 values were dichotomized at the median <17,098 pg/mL.13 High β-defensin-2 was defined as ≥17,098 pg/mL.
Statistical Analysis
Bivariate comparisons of maternal characteristics were compared between GBS RV+ and GBS RV− groups. All maternal characteristics included in Table 1 were assessed as potential confounders and included in the final model if inclusion modified the association of interest by ≥10%. The primary analysis used participants with samples obtained at 16 to 20 weeks of gestation (V1) to analyze associations of CSTs with subsequent GBS RV status. Secondarily, we analyzed associations CSTs at V2 and V3 with GBS RV status. Log-binomial regression was used to model the association between CST and GBS RV status and modified Poisson regression was used if the log-binomial model did not converge. We used a test of the ratio of relative risks to determine if β-defensin-2 modified the association of CST with GBS RV status. Due to the known association of race with CV microbiota and of race with GBS RV status, we also performed analyses restricted to Black individuals who were the majority of the cohort.3 Finally, we performed a sensitivity analysis restricted to individuals without antibiotic exposure in the 4 weeks prior to sample collection. A nominal p-value <0.05 was considered statistically significant and no adjustment for multiple comparisons was made. All statistical analyses were performed using Stata 15.0.
Table 1.
Maternal characteristics by Group B Streptococcus rectovaginal culture status (n = 492)
| Maternal characteristics | All (n = 492) | GBS RV− (n = 323) | GBS RV+ (n = 169) | p-Value |
|---|---|---|---|---|
| Race | ||||
| White | 107 (21.7) | 73 (22.6) | 34 (20.1) | 0.78 |
| Black | 364 (74.0) | 237 (73.4) | 127 (75.1) | |
| Other | 21 (4.3) | 13 (4.0) | 8 (4.7) | |
| Agea | 28.6 [6.3] | 28.1 [5.8] | 28.3 [6.2] | 0.78 |
| Gestational age at delivery | ||||
| ≥37 wk | 386 (78.5) | 261 (80.8) | 125 (74.0) | 0.08 |
| < 37 wk | 106 (21.5) | 62 (19.2) | 44 (26.0) | |
| BMI at V1 (kg/m2)b | 29.3 [7.2] | 28.9 (7.1) | 29.9 (7.5) | 0.15 |
| Single marital status | 348 (70.7) | 228 (70.6) | 120 (71.0) | 0.92 |
| Publicly insured | 260 (52.8) | 163 (50.5) | 97 (57.4) | 0.14 |
| Multiparous | 283 (57.5) | 175 (54.2) | 108 (63.9) | 0.04 |
| Gestational diabetes | 24 (4.9) | 13 (4.0) | 11 (6.5) | 0.22 |
| Pregestational diabetes | 12 (2.4) | 5 (1.5) | 7 (4.1) | 0.08 |
| Chronic hypertension | 30 (6.1) | 18 (5.6) | 12 (7.1) | 0.50 |
| Gestational hypertension | 76 (15.4) | 50 (15.5) | 26 (15.4) | 0.77 |
| GC/CT infection in pregnancy | 27 (5.5) | 13 (4.0) | 14 (8.3) | 0.05 |
| Term PROMc | 34 (8.9) | 22 (8.5) | 12 (9.6) | 0.25 |
| Clinical chorioamnionitisd | 35 (7.1) | 24 (7.5) | 11 (6.5) | 0.55 |
| Low β-defensin-2 (βD) at V1e | 236 (48.1) | 160 (49.7) | 76 (45.0) | 0.32 |
Abbreviations: BMI, body mass index; GC/CT, Neisseria gonorrhoeae or Chlamydia trachomatis; PROM, premature rupture of membranes; RV, rectovaginal.
Note: All data are presented as n (col%), unless otherwise noted.
Mean [standard deviation].
Missing BMI: n = 4.
n = 92 missing term PROM.
n = 2 missing clinical chorioamnionitis data.
n = 1 missing βD; low βD defined as less than the median as measured in a nested case-control sample of 637 individuals in the parent cohort of 1,943 individuals: <17,098 pg/mL.
Results
Out of the 492 individuals included in this analysis, 169 were GBS RV+ and 323 were GBS RV−, resulting in a prevalence of GBS colonization of 34.3%. This prevalence of GBS colonization is consistent with what is expected in our primarily Black individual population.1 Maternal demographic and clinical characteristics were compared between those who were GBS RV+ and GBS RV− (Table 1). The majority of the study population was Black. The average age was 28 years and the majority had term deliveries. There were no significant differences in body mass index, marital status, or insurance type between groups. Individuals who were GBS RV+ were more likely to be multiparous (63.9 vs. 54.2%, p = 0.04). There were no differences between groups in rates of hypertensive disease, diabetes, premature rupture of membranes, or chorioamnionitis. The groups were also similar in rate of low β-defensin-2 levels at V1, with 45.0% of GBS RV+ and 49.7% of GBS RV− individuals having low β-defensin-2.
Association between Community State Types and Group B Streptococcus Status
The association between CV microbiota at each visit and GBS RV status was examined (Table 2). Individuals with CST IV-A at V1 had a significantly higher risk of subsequent GBS RV+ status compared with individuals with CST I. This association remained significant after adjustment for race, parity, and pregestational diabetes (aRR: 1.56, 95% confidence interval [CI: 1.02–2.37]). As association of similar direction and magnitude was observed at V2 and V3 but did not reach statistical significance (Supplementary Table S1, available in the online version). When restricted to Black individuals, the association between CST IV-A at V1 and GBS RV+ status remained similar in direction and magnitude, but without statistical significance after adjustment for parity and pregestational diabetes (aRR: 1.36, 95% CI [0.86–2.14]).
Table 2.
Association between cervicovaginal microbiota community state type at 16 to 20 weeks’ gestation and Group B Streptococcus status on rectovaginal culture
| CST | GBS RV+ n (%) | RR (95% CI) | aRR (95% CI)a |
|---|---|---|---|
| V1: 16–20 wk (N = 492) | 169 (34.3) | ||
| I (n = 136) | 35 (25.7) | Ref. | Ref. |
| II (n = 32) | 18 (56.3) | 2.19 (1.44–3.32) | 2.08 (1.35–3.22) |
| III (n = 96) | 35 (36.5) | 1.42 (0.96–2.09) | 1.35 (0.90–2.02) |
| IV-A (n = 69) | 29 (42.0) | 1.63 (1.10–2.43) | 1.56 (1.02–2.37) |
| IV-B (n = 115) | 39 (33.9) | 1.32 (0.90–1.93) | 1.26 (0.84–1.88) |
| V (n = 44) | 13 (29.6) | 1.15 (0.67–1.97) | 1.13 (0.66–1.95) |
Abbreviations: CI, confidence interval; CST, community state type; GBS, Group B Streptococcus; Ref., reference; RV, rectovaginal.
Adjusted for Black race, parity, and pregestational diabetes.
There was also a significant association between CST II and GBS RV+ status at both V1 (aRR: 2.08, 95% CI [1.35–3.22]) and V2 (aRR: 1.83, 95% CI [1.18–2.84]), after adjustment for race, parity, and pregestational diabetes. The association at V3 did not reach statistical significance but showed a similar relationship (aRR: 1.80, 95% CI [0.92–3.51]). Among Black individuals, this relationship retained statistical significance only at V1, after adjustment for parity and pregestational diabetes (aRR: 2.07, 95% CI [1.18–3.62]).
In a sensitivity analysis restricted to the 430 individuals without antibiotic use within 4 weeks prior to CV specimen collection, the significant associations between CST IV-A and CST II at V1 and GBS RV+ status persisted (aRR: 1.69 (95% CI: 1.06–2.71); 1.88 (95% CI: 1.13–3.12), respectively; adjusted for race, parity, and pregestational diabetes).
β-defensin-2 and Group B Streptococcus Status
We next stratified by low and high β-defensin-2 levels to investigate whether the local immune response impacted the association between CST and GBS colonization. We found evidence of effect modification between β defensin-2 and CST on the risk of GBS RV+ status. Among individuals with low β-defensin-2, there was a significant association between CST IV-A and GBS RV+ status after adjusting for parity, maternal age, race, and BMI (aRR: 2.68, 95% CI [1.24–5.79]). However, when restricted to individuals with high β-defensin-2, there was no association between CST IV-A and GBS RV+ status (aRR: 0.98, 95% CI [0.46–2.08]; Fig. 2). The interaction term was significant in unadjusted (p = 0.03) but not adjusted models (p = 0.07). While there was also a significant association between CST II and GBS RV+ status among individuals with low β-defensin-2 in the adjusted analysis (aRR: 2.68, 95% CI [1.21–5.92]; Fig. 2), we did not find that β-defensin-2 levels modified the association between CST II and GBS status in the unadjusted analyses (p = 0.49) or adjusted analyses (p = 0.38).
Fig. 2.
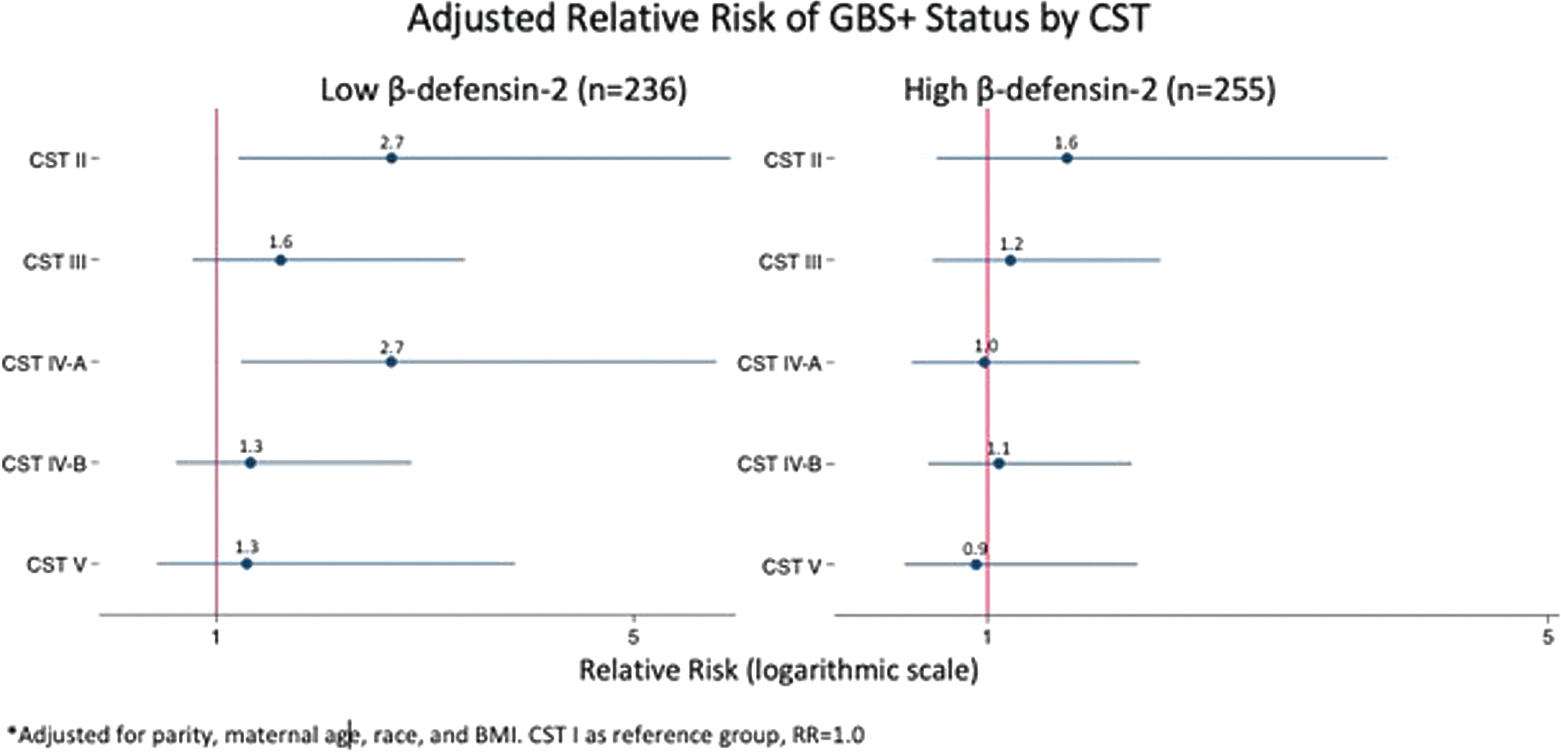
Forest plot showing the association between CST and GBS RV +, stratified by βD (n = 491)*. *n = 1 individual in cohort missing βD value. βD, β-defensin; CST, community state type; GBS, Group B Streptococcus; RV, rectovaginal.
When this same analysis was conducted restricted to Black individuals, a similar relationship was observed (Supplementary Table S2, available in the online version). Black individuals with low β-defensin-2 levels had higher risk of GBS RV+ status when they had concurrent CST IV-A compared with CST I after adjusting for parity, maternal age, and BMI (aRR: 3.07, 95% CI [1.18–7.96]). In contrast, among individuals with high β-defensin-2 levels, there was no association (aRR: 0.79, 95% CI [0.36–1.73]; interaction p = 0.03). Similarly, Black individuals with low β-defensin-2 levels and CST II compared with CST I had higher risk of GBS RV+ status (aRR: 4.62, 95% CI [1.30–16.4]), but individuals with high β-defensin-2 levels did not (aRR: 1.17, 95% CI [0.38–3.58]), but this interaction was only significant in unadjusted models (p = 0.04) but not adjusted models (p = 0.11).
Discussion
Principal Findings
This study demonstrates that CST IV-A, which is characterized by a paucity of Lactobacillus species and a predominance of anaerobic microbes and has previously been shown to be associatedwithadversepregnancyoutcomes,3,4,23isassociated with a significantly increased risk of GBS RV colonization. CST II is also associated with increased risk of GBS RV colonization, although the smaller number of individuals in this subgroup make interpretation of this finding more difficult. Furthermore, we found that β-defensin-2, an antimicrobial peptide known to be part of a protective host immune response in the CV space, is an effect modifier in the relationship between CST and GBS RV status.3 Our data suggest that nonoptimal CV microbiota and low levels of a protective immune factor contribute to increased risk of GBS colonization. These findings may present opportunities for novel therapeutic targets for prevention of GBS colonization in pregnancy.
Results in the Setting of What Is Known
Prior studies have demonstrated inconsistent relationships betweenCVmicrobiotaandGBScolonizationinpregnancy.7–11 One large study of pregnant individuals in Guatemala did not find any significant differences in the CV microbiota when comparing GBS RV+ and GBS RV− individuals; however, 16S rRNA sequencing was completed at >35 weeks’ gestation, and the prevalence of GBS colonization in this population was less than half of that of our population.9 Our findings are consistent with two other studies of pregnant individuals, both of which demonstrated an association between lower abundance of Lactobacilli among GBS colonized individuals. Furthermore, our findings are consistent with previous in vitro work, which has shown that Lactobacillus species block GBS adherence to vaginal epithelial cells and reduce GBS colonization.24–26 These findings may provide a biological mechanisms as to why we observed an increased rate of GBS colonization in individuals who had non-lactobacillus dominated CST IV-A. Consistent with our results, Rosen et al, in a study of nonpregnant individuals, found a significantly higher prevalence of GBS colonization in CST IV-A compared with CST IV-B.27 Whether specific bacterial taxa that differentiate CST IV-A and CST IV-B might encourage or prevent GBS colonization requires further investigation.
Our finding of a significant relationship between CST II and GBS colonization has not been previously documented in the literature. CST II is characterized by a predominance of L. gasseri. While there has been limited prior work assessing the influence of L. gasseri specifically on GBS colonization, Starc et al found a lower risk of vaginal colonization with GBS when lactobacilli were the dominant part of the vaginal microbiota among pregnant people in the third trimester, but L. gasseri had the least antagonistic effect on GBS colonization. Accordingly, we hypothesize that it is possible that colonization with L. gasseri represents a nonoptimal microbiota in our population.
Regarding our findings of the role of β-defensin-2 as a modifier of the relationship between CV microbiota and GBS colonization, this represents an advancement of existing knowledge. Several recent studies have demonstrated an association between low levels of β-defensin-2 and numerous obstetric and gynecologic pathologies, including preterm birth, bacterial vaginosis, lichen sclerosis, sexually transmitted infections.3,12,28,29 In regards to GBS specifically, prior in vitro work by Castro-Leyva et al demonstrated that when incubated with S. agalactiae, choriodecidual cells increased production of β-defensin-2.30 Boldenow et al showed increased β-defensin-2 production by GBS-exposed amnion cells and also found that in bacterial culture, β-defensin-2 decreased GBS viability in a concentration-dependent manner.31 Extrapolating from the in vitro data suggesting that β-defensin-2 may represents a host immune response to combat GBS, we theorize that individuals with low levels of β-defensin-2 in the second trimester have a suboptimal host immune response leading to a microbial–immune milieu that is more permissive to GBS colonization.
Clinical Implications
This study offers a significant contribution to understanding the relationship between the CV microbiota and GBS colonization. This study is the first to report that pregnant individuals with high-risk CV microbiota and low levels of β-defensin-2 in the second trimester may be at higher risk of GBS RV colonization in the third trimester. While further mechanistic studies are needed to bridge the knowledge gap from the associations shown here to useful clinical application, our findings are compelling as they raise the possibility that manipulation of the CV microbiota early in pregnancy may offer a mechanism to decrease the risk of GBS colonization at delivery. Such an intervention, if successful, would decrease the need for intrapartum antibiotic prophylaxis against GBS, thereby reducing the associated risks of allergic reactions and alteration of the maternal and neonatal microbiota. Moreover, the ability to prevent GBS colonization would greatly reduce the neonatal morbidity and mortality associated with intrapartum vertical transmission of GBS. As recent work by our group has shown, antenatal GBS screening protocols are imprecise, and the opportunity to prevent GBS colonization and thereby reduce the need for intrapartum antibiotic prophylaxis would have great clinical impact.2
Research Implications
Our findings have numerous implications for future work. Larger studies are needed to be able to correlate CV microbiota and GBS colonization findings with clinical outcomes of neonatal GBS infection. Furthermore, differences in the CV microbial and immune profiles in pregnancy require further study to understand how they impact each other and important clinical outcomes, as well as how they may change throughout gestation. Recent work from members of our group demonstrated an association between perceived stress, low levels of β-defensin-2, and preterm birth.5,13 Further investigation is warranted to evaluate factors that impact levels of β-defensin-2, how β-defensin-2 may or may not change throughout gestation, and how these could be modulated to improve health outcomes.
Strengths and Limitations
The strengths of this study include examination of both microbiota and the immune factor β-defensin-2 in association with GBS colonization. Additionally, our sample size is relatively large for work of this kind and represents a majority Black population, a group at particularly high risk for GBS colonization and adverse pregnancy outcomes.32 Limitations include that this is a secondary analysis of a larger cohort study, and we had relatively small numbers of individuals within certain CSTs, potentially limiting power. Further, we did not examine the specific serotypes of GBS in our samples. There are numerous known serotypes of GBS, and it is possible that colonization with different serotypes can occur.
Conclusion
Our data suggest that the CV microbiota and local immune response in the CV space in the second trimester are associated with an increased risk of GBS colonization in the third trimester. Microbial communities at various biological sites can be modified without the use of antibiotics. As such, future research should investigate if GBS colonization can be prevented by modifying the CV microbiota, thus limiting, if not preventing, the need for intrapartum antibiotic prophylaxis while also reducing the risks of neonatal infection.
Supplementary Material
Key Points.
The relationship between the CV microbiota and GBS RV colonization is unknown.
A Lactobacillus-deficient, anaerobic rich vaginal community, CST IV-A, is associated with increased risk of GBS RV colonization.
β-defensin-2 is an effect modifier of the association between CST IV-A and GBS RV+ status.
Funding
National Institutes of Health R01NR014784 (PI Elovitz); Department of Pediatrics, Children’s Hospital of Philadelphia (Burris); Individuals Reproductive Health Research Grant 5 K12 HD 1265–22. T32-HD007440 (McCoy). Funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Conflict of Interest None declared.
References
- 1.Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Prevention of perinatal Group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59(RR-10):1–36 [PubMed] [Google Scholar]
- 2.McCoy JA, Elovitz MA, Alby K, Koelper NC, Nissim I, Levine LD. Association of obesity with maternal and cord blood penicillin levels in women with Group B Streptococcus colonization. Obstet Gynecol 2020;136(04):756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elovitz MA, Gajer P, Riis V, et al. Cervicovaginal microbiota and local immune response modulate the riskof spontaneous preterm delivery. Nat Commun 2019;10(01):1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerson KD, McCarthy C, Elovitz MA, Ravel J, Sammel MD, Burris HH. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am J Obstet Gynecol 2020;222(05):491.e1–491.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerson KD, McCarthy C, Ravel J, Elovitz MA, Burris HH. Effect of a nonoptimal cervicovaginal microbiota and psychosocial stress on recurrent spontaneous preterm birth. Am J Perinatol 2021;38 (05):407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Hassan SS, Gajer P, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014;2(01):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altoparlak U, Kadanali A, Kadanali S. Genital flora in pregnancy and its association with Group B streptococcal colonization. Int J Gynaecol Obstet 2004;87(03):245–246 [DOI] [PubMed] [Google Scholar]
- 8.Brzychczy-Włoch M, Pabian W, Majewska E, et al. Dynamics of colonization with Group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol 2014;37(03):307–319(In eng) [PubMed] [Google Scholar]
- 9.Rick AM, Aguilar A, Cortes R, et al. Group B streptococci colonization in pregnant Guatemalan women: prevalence, risk factors, and vaginal microbiome. Open Forum Infect Dis 2017;4(01): ofx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108(suppl 1, suppl 1):4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starc M, Lučovnik M, Eržen Vrlič P, Jeverica S. Protective effect of Lactobacillus crispatus against vaginal colonization with Group B streptococci in the third trimester of pregnancy. Pathogens 2022; 11(09):980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda-Nicolau NM, Silva MC, Bento GFC, et al. Cervicovaginal levels of human beta defensins during bacterial vaginosis. PLoS One 2021;16(12):e0260753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burris HH, Riis VM, Schmidt I, Gerson KD, Brown A, Elovitz MA. Maternal stress, low cervicovaginal β-defensin, and spontaneous preterm birth. Am J Obstet Gynecol MFM 2020;2(02):100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadrosh DW, Ma B, Gajer P, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014;2(01):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CM, Aziz M, Kachur S, et al. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol 2012;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011;27 (21):2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinformatics. 2011;Chapter 10:Unit 10.7. (In Eng). Doi: 10.1002/0471250953.bi1007s36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R. E. UCHIME2: improved chimera prediction for amplicon sequencing. bioRxiv 2016
- 19.Cole JR, Wang Q, Fish JA, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 2014;42(Database issue):D633–D642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liland KH, Vinje H, Snipen L. microclass: an R-package for 16S taxonomy classification. BMC Bioinformatics 2017;18(01):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hug LA, Baker BJ, Anantharaman K, et al. A new view of the tree of life. Nat Microbiol 2016;1:16048. [DOI] [PubMed] [Google Scholar]
- 22.Filkins L, Hauser JR, Robinson-Dunn B, Tibbetts R, Boyanton BL, Revell P. American Society for Microbiology provides 2020 guidelines for detection and identification of Group B Streptococcus. J Clin Microbiol 2020;59(01):e01230–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015;112(35):11060–11065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Gregorio PR, Juárez Tomás MS, Nader-Macías ME. Immuno-modulation of Lactobacillus reuteri CRL1324 on Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Am J Reprod Immunol 2016;75(01):23–35 [DOI] [PubMed] [Google Scholar]
- 25.De Gregorio PR, Juárez Tomás MS, Leccese Terraf MC, Nader-Macías ME. Preventive effect of Lactobacillus reuteri CRL1324 on Group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol 2015;118(04):1034–1047 [DOI] [PubMed] [Google Scholar]
- 26.Ortiz L, Ruiz F, Pascual L, Barberis L. Effect of two probiotic strains of Lactobacillus on in vitro adherence of Listeria monocytogenes, Streptococcus agalactiae, and Staphylococcus aureus to vaginal epithelial cells. Curr Microbiol 2014;68(06):679–684 [DOI] [PubMed] [Google Scholar]
- 27.Rosen GH, Randis TM, Desai PV, et al. Group B Streptococcus and the vaginal microbiota. J Infect Dis 2017;216(06):744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fichorova RN, Morrison CS, Chen PL, et al. Aberrant cervical innate immunity predicts onset of dysbiosis and sexually transmitted infections in women of reproductive age. PLoS One 2020;15(01): e0224359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunner A, Medvecz M, Makra N, et al. Human beta defensin levels and vaginal microbiome composition in post-menopausal women diagnosed with lichen sclerosus. Sci Rep 2021;11(01):15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro-Leyva V, Zaga-Clavellina V, Espejel-Nuñez A, et al. Decidu-alization mediated by steroid hormones modulates the innate immunity in response to Group B streptococcal infection in vitro. Gynecol Obstet Invest 2017;82(06):592–600 [DOI] [PubMed] [Google Scholar]
- 31.Boldenow E, Hogan KA, Chames MC, Aronoff DM, Xi C, Loch-Caruso R. Role of cytokine signaling in Group B Streptococcus-stimulated expression of human beta defensin-2 in human extraplacental membranes. Am J Reprod Immunol 2015;73(03): 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevention of Group B Streptococcal Early-Onset Disease in Newborns. ACOG Committee opinion, number 782. Obstet Gynecol 2019;134(01):19–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


