Abstract
Infection with the parasitic helminth Brugia malayi can result in development of a severe asthmatic response termed tropical pulmonary eosinophilia. This disease, thought to result from a host inflammatory response to blood parasites which become trapped in the lung microvasculature, is characterized by a profound eosinophilic infiltration into the lungs. Recruitment of eosinophils also correlates with the development of airway hyperresponsiveness (AHR) to cholinergic agonists and severe asthmatic symptoms. Our studies examined the role of interleukin-5 (IL-5) in helminth-induced pulmonary eosinophilia and AHR. C57BL/6 mice immunized with killed B. malayi microfilariae and challenged intravenously with live microfilariae exhibit many of the characteristics of human disease, including peripheral and pulmonary eosinophilia. Cells recovered by bronchoalveolar lavage of sensitized mice consisted of 3.8% eosinophils on day 1 postchallenge and 84% on day 10. Extracellular major basic protein was present on the surface of airway epithelial cells as early as day 1 and continued to be evident after 8 days, indicating sustained activation and degranulation of eosinophils in the lung. These histologic changes correlated with the development of AHR to carbachol. In contrast to immunocompetent mice, immunization and challenge with B. malayi in IL-5−/− mice did not induce peripheral or pulmonary eosinophilia, and these mice failed to show AHR in response to cholinergic agonists. Taken together, these data indicate that IL-5 and eosinophils are required for the induction of AHR by filarial helminths.
An estimated 130 million people are infected with Wuchereria bancrofti and Brugia malayi, the parasitic helminths that cause lymphatic filariasis (26). Much of the pathology associated with the disease, including elephantiasis, is attributed to the adult worms in the lymphatics. First-stage larvae (microfilariae) circulate in the blood and generally do not cause pathological sequelae; however, in certain populations of individuals, the presence of microfilariae in the lungs is associated with severe asthmatic symptoms and airway hyperresponsiveness (AHR) (3, 6). This condition, termed tropical pulmonary eosinophilia (TPE), is thought to be caused by microfilariae trapped in the pulmonary vasculature and can be distinguished from allergic asthma by the effectiveness of anthelminthics in relieving clinical symptoms (27).
Previous studies from this laboratory demonstrated that immunization with B. malayi microfilariae selectively induces a Th2-associated response with production of interleukin-5 (IL-5) and eosinophilia (28–30). In the present study, we demonstrate that intravenous (i.v.) injection of microfilariae into sensitized mice stimulates several features similar to those of TPE patients, including the development of profound pulmonary eosinophilia and evidence of eosinophil degranulation. Importantly, the respiratory smooth muscle of isolated tracheas from these animals is hyperresponsive to the cholinergic agonist carbachol, indicating pulmonary dysfunction. Furthermore, as IL-5 is an important regulator of eosinophil growth, differentiation, and activation (4, 7, 20, 21), we used mice in which the IL-5 gene has been disrupted to demonstrate that eosinophils are essential for the development of filaria-induced AHR.
MATERIALS AND METHODS
Parasites.
Microfilariae were obtained from male jirds (Meriones unguiculatis) infected with B. malayi (NIH contract 73262). Microfilariae were harvested by peritoneal lavage with Dulbecco’s modified Eagle’s medium (BioWhittaker, Walkersville, Md.), washed twice in Hanks balanced salt solution, and counted in a Sedgewick-Rafter counting chamber. Parasites were used live for i.v. challenge or stored at −70°C for subcutaneous (s.c.) immunization.
Immunization.
Female C57BL/6 mice (4 to 6 weeks old) were obtained from Taconic Farms (Germantown, N.Y.). IL-5 gene knockout (IL-5−/−) mice on a C57BL/6 background were generated by Manfred Kopf (Basel Institute of Immunology) and kindly provided by Edward Pearce (Cornell University). All mice used in these studies were housed in microisolators until sacrificed. Mice received three weekly s.c. immunizations at the base of the tail with 100,000 killed (frozen) microfilariae in 0.2 ml of saline, followed 10 days later by i.v. injection with 200,000 live microfilariae.
BAL and differential leukocyte counts.
Bronchoalveolar lavage (BAL) was performed by intratracheal instillation of 0.5 ml of phosphate-buffered saline (Sigma, St. Louis, Mo.). Total leukocyte counts in BAL fluids were determined with a hemocytometer.
For differential counts, blood smears and cytocentrifuge preparations from BAL fluids were stained with modified Wright-Giemsa stain (Diff-Quik; Dade Diagnostics, Aguada, P.R.), and 400 cells were counted from two slides for each animal.
Histopathology and immunohistochemistry.
Lungs were fixed in 10% formalin and embedded in paraffin, and 5-μm sections were prepared for histology. Sections were stained with hematoxylin and eosin for the assessment of overall inflammatory response.
To detect eosinophils and major basic protein (MBP), paraffin sections were incubated with rabbit antisera to murine MBP at 1:1,000 dilution in 1% fetal calf serum in 0.05 M Tris-buffered saline at room temperature in a humidified chamber for 2 h (anti-MBP serum was prepared by Kirsten Larsen as described elsewhere [21] and kindly provided by Gerald Gleich, Mayo Clinic, Rochester, Minn.). Biotinylated goat anti-rabbit immunoglobulin (DAKO, Carpenteria, Calif.), diluted 1:200 in 1% fetal calf serum in 0.05 M Tris-buffered saline, was added for 30 min followed by a similar incubation with prediluted alkaline phosphatase-conjugated streptavidin (BioGenex, San Ramon, Calif.). Positive reactivity was visualized with substrate (VectorRed; Sigma) containing 12 mg of levamisole (Sigma) and counterstaining with modified Harris’ hematoxylin (Richard-Allen, Kalamazoo, Mich.).
Isometric measurement of tracheal smooth muscle response to cholinergic agonists.
Tracheal reactivity was determined as described by Garssen et al. (13). Animals were sacrificed by intraperitoneal injection of 1.5 mg of pentobarbital sodium (Nembutal; Abbott Laboratories, North Chicago, Ill.). Tracheas were dissected and kept in modified Krebs-bicarbonate solution (118.1 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.0 mM NaH2PO4, 0.5 mM MgCl2, 2.5 mM CaCl2, 11.1 mM dextrose [pH 7.4]) which was continuously gassed with a mixture containing 95% O2 and 5% CO2. Adventitia and fat tissue were removed from each trachea, and a 3.0-mm cylindrical section was cut from the midportion of the tissue. Tracheal cylinders were suspended between a metal rod and a force displacement transducer (World Precision Instruments, Inc., Sarasota, Fla.) connected to an amplifier. Tissues were equilibrated in an organ bath (Crown Glass Co. Inc., Somerville, N.Y.) filled with 20 ml of Krebs-bicarbonate solution and aerated for 40 to 45 min before stimulation. The temperature was maintained at 37°C by a constant-temperature circulating unit. Starting preload was 1 g followed by 0.1-g adjustments after each stimulation until the reproducible maximal response was obtained. Concentration response curves to carbachol were performed, and isometric force (grams) generated by smooth muscle was monitored and recorded on a rectilinear four-channel chart recorder (Gould, Cleveland, Ohio).
Statistics.
Data are presented as mean ± standard error (SE). Smooth muscle contractile responses were averaged and analyzed by nonlinear regression, using PRISM (Graph Pad Software). Maximal force of smooth muscle contraction (Emax) and half-maximal dose of cholinergic agonist were calculated and compared by an unpaired t test.
RESULTS
Histopathology of filaria-induced pulmonary inflammation in C57BL/6 and IL-5−/− mice.
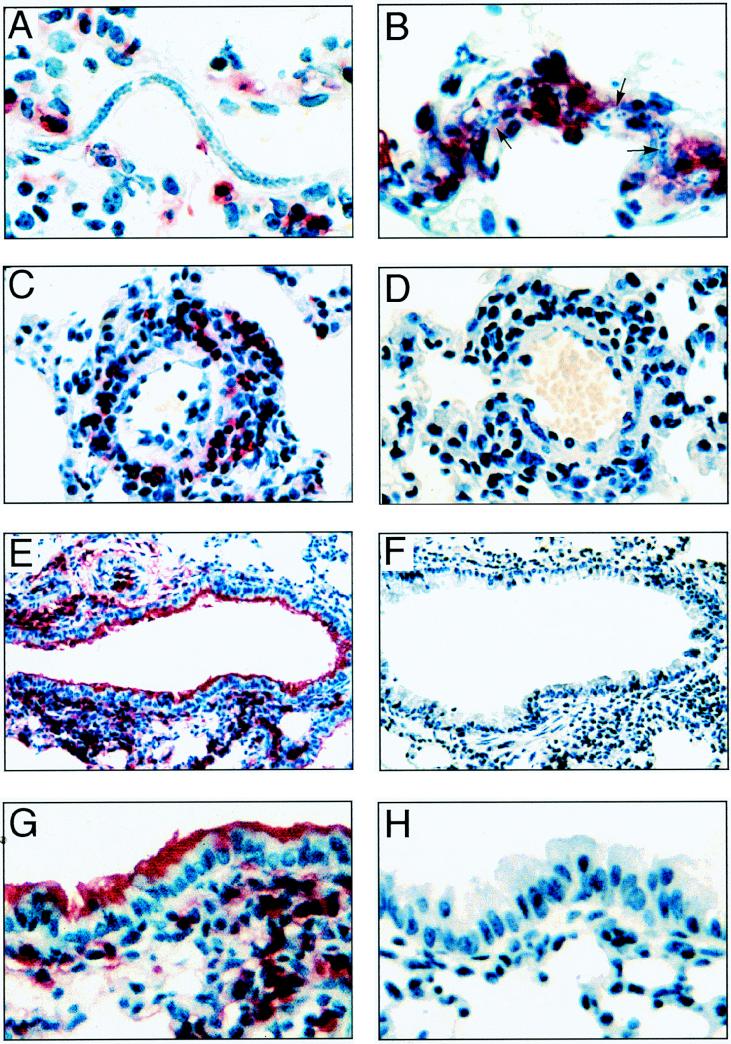
The initiator of the inflammatory response in the lung is thought to be dead and degenerating worms in the lung microvasculature (27). Lung sections were immunostained with antisera to eosinophil MBP. In both mouse strains, microfilariae were detected in peripheral blood at day 1 (82.5 ± 22.6 versus 77.5 ± 15.6 parasites per 80 μl of blood; P > 0.05). Microfilariae could be found in the peripheral blood as late as 10 days postchallenge, although the numbers detected were not significantly different between the two strains of mice at any time. Microfilariae were also observed in lung vessels on day 1 after i.v. challenge. Inflammatory cells were rarely detected on the worm surface in IL-5−/− mice. However, in C57BL/6 mice, the number of inflammatory cells on the worm surface was highly variable; some microfilariae had few associated inflammatory cells (Fig. 1A), whereas others were coated with inflammatory cells, notably eosinophils (Fig. 1B).
FIG. 1.
Eosinophils and MBP in lungs after i.v. inoculation with B. malayi larvae. C57BL/6 and IL-5−/− mice were immunized s.c. with 100,000 killed B. malayi larvae (microfilariae) and injected i.v. with live microfilariae. On day 1 or day 8 after challenge, mice were sacrificed, and lungs were fixed in formalin. All sections were immunostained with antisera to eosinophil MBP and visualized with VectorRed. (A) Microfilariae in lung vasculature of a C57BL/6 mouse day 1 after i.v. challenge; (B) microfilariae surrounded by inflammatory cells, including eosinophils on day 1 (arrowheads indicate visible areas of the worm); (C) perivascular inflammation in a C57BL/6 mouse on day 8 after challenge (note the presence of numerous eosinophils and mononuclear cells); (D) blood vessel from an IL-5−/− mouse sacrificed on day 8; (E) terminal bronchiole from a C57BL/6 mouse on day 8 postchallenge, with deposition of MBP on bronchial epithelial cells; (F) terminal bronchiole from an IL-5−/− mouse on day 8 after i.v. challenge; (G) bronchiolar epithelium from a C57BL/6 mouse on day 8 postchallenge; (H) bronchiolar epithelium from an IL-5−/− mouse. Photomicrographs are representative of five mice per time point in three repeat experiments. Original magnifications: A and B, ×600; C and D, ×400; E and F, ×200; G and H, ×400.
Perivascular infiltrates were evident in the lungs of mice of both strains on day 1 after i.v. challenge but were more pronounced on day 8. Vasculitis was a defining pathological feature in both C57BL/6 and IL-5−/− mice; however, immunostaining with antisera to eosinophil MBP demonstrated that eosinophils were a prominent component of the perivascular infiltrate of C57BL/6 mice (Fig. 1C) but were not detected in IL-5−/− mice (Fig. 1D). Consistent with this observation, eosinophils were also prominent in the peribronchial area (Fig. 1E), whereas the cellular infiltrate in IL-5−/− mice consisted mostly of macrophages and lymphocytes (Fig. 1F), with few eosinophils.
In addition to recruitment of intact eosinophils into the lung parenchyma of immunocompetent mice, there was also significant extracellular MBP detected in the lungs. Figure 1E also shows extensive deposition of MBP in the airways, which at higher magnification (Fig. 1G) was found to coat the apical surface of the respiratory epithelial cells. Although these figures are from day 8 after challenge, MBP was detected in the airways as early as day 1 postchallenge (not shown). In contrast to C57BL/6 mice, no MBP was detected in the airways of IL-5−/− mice at any time (Fig. 1F and H).
Together, these data indicate that the presence of filariae in the lungs of immunocompetent animals stimulates eosinophil recruitment and degranulation, whereas in the absence of IL-5, filariae induce a mononuclear cell infiltrate.
Effect of IL-5 deficiency on cells recovered from BAL fluid.
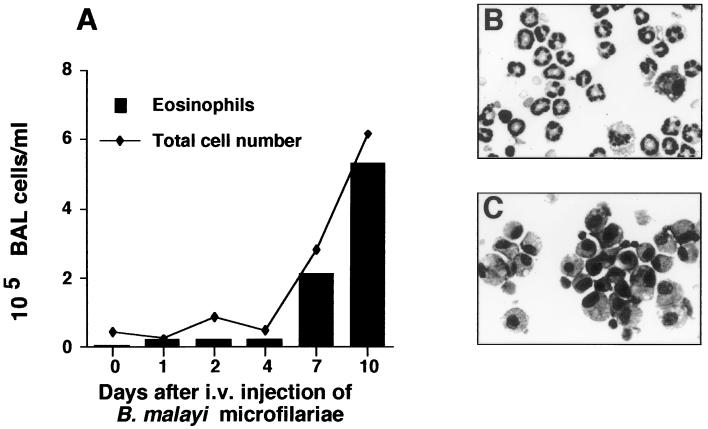
One of the hallmarks of TPE is the presence of eosinophils in BAL fluid (27, 31). To determine the extent of inflammatory cell recruitment to the lung, lavages were performed at various time points after parasite challenge (Fig. 2A). In C57BL/6 mice, the percentage of eosinophils in the BAL fluid increased from 1.2 × 103/ml (3.8%) on day 1 postchallenge to 2.2 × 104/ml (40%) on day 4. By day 10, the total number of cells in the BAL fluid had increased more than 13-fold, with 5.3 × 105/ml (84%) of these cells being eosinophils (Fig. 2B). The BAL fluid of IL-5−/− mice sacrificed day 8 postchallenge contained 105 (± 1.5 × 104) cells/ml, which was not significantly different from the value for C57BL/6 mice. However, in contrast to C57BL/6 mice, the cellular infiltrate was primarily mononuclear (97.2%), composed of lymphocytes (58.2%) and macrophages (39%) (Fig. 2C).
FIG. 2.
Kinetics of inflammatory response in the airway. C57BL/6 mice were immunized three times with 100,000 killed microfilariae and challenged i.v. with live microfilariae. (A) At the time points indicated, BAL fluid was cytocentrifuged and cells were examined after staining with modified Wright-Giemsa stain. The data are the means of five animals/time point and are representative of two repeat experiments. (B) Cytospin preparation of BAL fluid harvested day 10 postchallenge from a C57BL/6 mouse and stained with modified Wright-Giemsa stain (magnification, ×600). (C) Cytospin of BAL fluid from an IL-5−/− mouse (magnification, ×600).
Role of IL-5 in filaria-induced AHR.
To determine if vasculitis, eosinophil recruitment to the lung, and degranulation were associated with physiologic changes in lung function, we evaluated AHR in sensitized C57BL/6 and IL-5−/− mice. Measurement of contractile responses of airway smooth muscle to cholinergic agonists is a standard method to examine AHR (2, 13, 35).
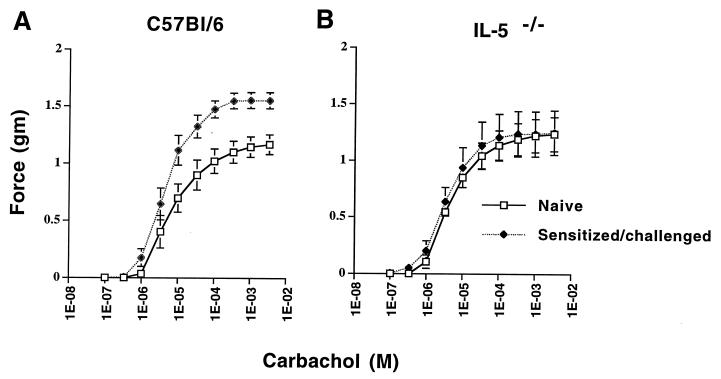
For C57BL/6 mice, the contractile response of respiratory smooth muscle of isolated tracheas to cumulative doses of the cholinergic agonist carbachol was measured on day 8 postchallenge, when pulmonary eosinophilia and MBP deposition were prominent. Tracheas isolated from naive C57BL/6 mice responded to carbachol in a dose-dependent manner (Fig. 3A). The contractile force generated by tracheas from sensitized-challenged mice was greater at all doses of carbachol tested, indicated by the leftward shift of the dose response curve. The maximal contractile force, Emax, of tracheas isolated day 8 postchallenge was significantly higher than for naive mice (1.56 ± 0.07 g versus 1.15 ± 0.08 g; P = 0.002) (Fig. 3A). These data demonstrate that i.v. injection of microfilariae in sensitized mice induces AHR. The concentration of carbachol at which the contractile response is half-maximal was not significantly different from that for naive animals (P = 0.28). Intravenous injection of microfilariae into unsensitized mice did not induce increased airway responsiveness to cholinergic agonists (not shown).
FIG. 3.
Contractile responses of tracheal smooth muscle after i.v. injection of B. malayi microfilariae. C57BL/6 and IL-5−/− mice were immunized and injected i.v. with microfilariae as described in Materials and Methods. On day 8 postchallenge, tracheas were dissected and tracheal cylinders were submerged in an organ bath and exposed to cumulative doses of carbachol. (A) “Naive” data points represent contractile responses of unimmunized, unchallenged C57BL/6 mice. The data points represent force generated in grams (mean ± SE of eight animals per group). Data were analyzed by nonlinear regression using PRISM. Statistical significance was determined by an unpaired t test. Maximal contractile force in tracheas from sensitized animals (1.56 ± 0.07 g) increased significantly compared to naive controls (1.15 ± 0.08 g; P = 0.002). Enhanced tracheal reactivity was also observed at day 1 postchallenge. (B) Tracheal smooth muscle responses of naive IL-5−/− and sensitized/challenged IL-5−/− mice (mean ± SE of eight animals per group). Maximal contractile force in tracheas from sensitized animals (1.25 ± 0.16 g) did not differ significantly from the value for naive controls (1.23 ± 0.22 g; P = 0.96).
Naive IL-5−/− mice had a dose-response curve similar to that for naive C57BL/6 mice (Fig. 3B). However, in contrast to sensitized, immunocompetent mice, tracheas from sensitized IL-5−/− mice did not exhibit hyperresponsiveness to carbachol (Emax, 1.25 ± 0.16 g versus 1.23 ± 0.22 g; P = 0.96) (Fig. 3). Therefore, these data indicate a requirement for IL-5 and eosinophils in filaria-induced AHR.
DISCUSSION
In this study, we used IL-5 gene knockout mice to investigate the role of eosinophils in the inflammatory response and subsequent pathophysiology induced by i.v. injection of B. malayi into sensitized mice. A previous study found that BALB/c mice develop pulmonary eosinophilia and elevated serum immunoglobulin E after immunization and i.v. challenge with B. malayi antigens (8). The data presented here extend these observations by demonstrating that microfilariae injected i.v. into sensitized mice also induce vasculitis, eosinophil degranulation, and release of MBP and stimulate AHR. While IL-5−/− mice also develop vasculitis in response to parasite challenge, the cellular infiltrate is primarily mononuclear. Importantly, IL-5−/− mice do not develop AHR, providing evidence for an essential role for eosinophils in filaria-induced pulmonary dysfunction.
Several murine models of asthma have demonstrated that AHR develops after aerosol challenge with environmental allergens (5, 10, 14, 35). Filaria-induced AHR differs from these models in that (i) it relies on a hematogenous route of challenge; (ii) vasculitis is a prominent pathological feature in the lungs; and (iii) AHR is observed at 8 days postchallenge, indicating a sustained inflammatory response. Despite these differences, our data are in agreement with studies by Foster et al. in demonstrating a role for eosinophils and IL-5 in AHR (10, 18).
Our findings indicate that development of AHR requires both a sensitization phase and a localized inflammatory response. IL-5, which is essential for eosinophil production, is induced after prolonged exposure to parasite antigens (chronic exposure in humans [24, 25, 34] or repeated immunization in mice [4, 10, 33]). Dead and degenerating parasites in the lungs then stimulate a localized inflammatory response that leads to recruitment of eosinophils to the site. Lung biopsies from patients with TPE show microfilariae surrounded by eosinophilic material (6, 19, 39). The stimulus for migration of eosinophils into the airways is unclear but may involve production of IL-1 or tumor necrosis factor alpha from vascular endothelial cells at the site of parasite death which then stimulate respiratory epithelial cells to release eosinophil chemoattractants such as eotaxin (16, 23, 32).
Temporal analysis of eosinophil recruitment into the airways demonstrated that eosinophils continue to accumulate over time, with eosinophils in BAL fluid increasing to greater than 80% of the total inflammatory infiltrate on day 10 after challenge. Our data indicate that eosinophils not only are recruited to the lungs but also degranulate, releasing MBP in the airways. The observed eosinophil degranulation is consistent with the highly activated state of eosinophils recovered by BAL from patients with TPE (31). Our working hypothesis is that eosinophil degranulation and release of MBP are responsible for the pathophysiology observed in TPE. Consistent with this notion, MBP deposition on bronchial epithelial cells correlated with enhanced tracheal contractile responses. This was evident both at day 1 and day 8, indicative of a sustained response.
Although MBP is one of several cationic granule proteins with known cytotoxic activity, including eosinophil cationic protein, eosinophil-derived neurotoxin, and eosinophil peroxidase (1, 15), numerous studies have demonstrated a relationship between MBP and allergic airway disease. For example, MBP concentration has been correlated with severity of bronchial hyperresponsiveness in patients with allergic asthma (12, 37, 38). In addition, intratracheal instillation of MBP induced AHR in rats and monkeys (17, 36), and antibody neutralization of MBP suppressed allergen-induced AHR in guinea pigs (9, 22). Furthermore, in vitro studies have shown that MBP has a dose-dependent cytotoxic effect on tracheal epithelial cells (11). Future studies will determine the role of MBP in relation to other granule proteins in development of filaria-induced AHR and will examine the molecular mechanisms involved in the development of AHR.
In summary, this study demonstrates that i.v. injection of B. malayi microfilariae into sensitized, immunocompetent mice induces immunological and physiological changes which have similarities to those described for TPE (27, 31), notably pulmonary eosinophilia and the development of AHR. Furthermore, the presence of MBP in airways provides a possible causal link between eosinophil granule proteins and AHR. Importantly, the absence of AHR in IL-5−/− mice demonstrates that eosinophils are responsible for the pathophysiology associated with this disease.
ACKNOWLEDGMENTS
We gratefully acknowledge receipt of IL-5−/− mice from Manfred Kopf and Edward Pearce and receipt of anti-MBP sera from Kirsten Larsen and Gerald Gleich. We also appreciate the technical assistance of Bernadette Erokwu and Eugenia Diaconu, and we thank James Kazura, Christopher King, Frederick Heinzel, and Richard Silver for critical review of the manuscript. We also thank Carol Farver and Rosana Cohen for helpful discussions.
This work was supported by Burroughs Wellcome New Investigator Award 0720 (E.P.) and National Institutes of Health grant HL50527 (M.A.H.).
REFERENCES
- 1.Ackerman S J. Characterization and function of eosinophil granule proteins. In: Mkino S, Fukuda T, editors. Eosinophils. Biological and clinical aspects. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 33–74. [Google Scholar]
- 2.Armour C L, Diment L M, Black J L. Relationship between smooth muscle volume and contractile responses in airway tissue. Isometric versus isotonic measurement. J Pharmacol Exp Ther. 1988;245:687–691. [PubMed] [Google Scholar]
- 3.Chhabra S, Gaur S. Airway hyperreactivity in tropical pulmonary eosinophilia. Chest. 1988;93:1105–1106. doi: 10.1378/chest.93.5.1105. [DOI] [PubMed] [Google Scholar]
- 4.Coffman R L, Seymour B W, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 5.Corry D B, Folkesson H G, Warnock M L, Erle D J, Matthay M A, Wiener-Kronish J P, Locksley R M. Interleukin 4, but not interleukin 5 or eosinophils is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danaraj T J, Pacheco G, Shanmugaratnam K, Beaver P C. The etiology and pathology of eosinophilic lung (tropical eosinophilia) Am J Trop Med Hyg. 1966;15:183–189. doi: 10.4269/ajtmh.1966.15.183. [DOI] [PubMed] [Google Scholar]
- 7.Dent L A, Strath M, Mellor A L, Sanderson C J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egwang T G, Kazura J W. The BALB/c mouse as a model for immunological studies of microfilariae-induced pulmonary eosinophilia. Am J Trop Med Hyg. 1990;43:61–66. doi: 10.4269/ajtmh.1990.43.61. [DOI] [PubMed] [Google Scholar]
- 9.Evans C M, Fryer A D, Jacoby D B, Gleich G J, Costello R W. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Investig. 1997;100:2254–2262. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster P. Interleukin 5 deficiency abolishes eosinophilia, airway hyperreactivity and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frigas E, Loegering D, Gleich G. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Investig. 1980;42:35–43. [PubMed] [Google Scholar]
- 12.Frigas E, Loegering D, Solley G, Farrow G, Gleich G. Elevated levels of eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc. 1981;56:345–353. [PubMed] [Google Scholar]
- 13.Garssen J, Van L H, Van D V H, Nijkamp F. An isometric method to study respiratory smooth muscle responses in mice. J Pharmacol Methods. 1990;24:209–217. doi: 10.1016/0160-5402(90)90031-f. [DOI] [PubMed] [Google Scholar]
- 14.Gavett S, O’Hearn D, Li X, Huang S, Finkelman F, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleich G, Adolphson C, Leiferman K. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalo J A, Lloyd C M, Kremer L, Finger E, Martinez-A C, Siegelman M H, Cybulsky M, Gutierrez-Ramos J C. Eosinophil recruitment to the lung in a murine model of allergic inflammation: the role of T cells, chemokines and adhesion receptors. J Exp Med. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gundel R, Letts L, Gleich G. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Investig. 1991;87:1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan S P, Koskinen A, Foster P S. Interleukin-5 and eosinophils induce airway damage and bronchial hyperreactivity during allergic airway inflammation in BALB/c mice. Immunol Cell Biol. 1997;75:284–288. doi: 10.1038/icb.1997.43. [DOI] [PubMed] [Google Scholar]
- 19.Joshi V V, Udwadia F E, Gadgil R K. Etiology of tropical eosinophilia. A study of lung biopsies and review of published reports. Am J Trop Med Hyg. 1969;18:231–240. [PubMed] [Google Scholar]
- 20.Kopf M, Brombacher F, Hodgkin P, Ramsay A, Milbourne E, Dai W, Ovington K, Behm C, Kohler G, Young I, Matthaei K. IL-5 deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:1–20. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee J J, McGarry M P, Farmer S C, Denzler K L, Larson K A, Carrigan P E, Brenneise I E, Horton M A, Haczku A, Gelfand E W, Leikauf G D, Lee N A. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefort J, Nahori M-A, Ruffie C, Vargaftig B B, Pretolani M. In vivo neutralization of eosinophil derived major basic protein inhibits antigen induced bronchial hyperreactivity in sensitized guinea pigs. J Clin Investig. 1996;97:1117–1121. doi: 10.1172/JCI118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilly C M, Nakamura H, Kesselman H, Nagler-Anderson C, Assano K, Garcia-Zepeda E A, Rothenberg M E, Drazen J M, Luster A D. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Investig. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limaye A P, Abrams J S, Silver J E, Awadzi K, Francis H F, Ottesen E A, Nutman T B. Interleukin-5 and the posttreatment eosinophilia in patients with onchocerciasis. J Clin Investig. 1991;88:1418–1421. doi: 10.1172/JCI115449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limaye A P, Abrams J S, Silver J E, Ottesen E A, Nutman T B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990;172:399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael E, Bundy D A P. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13:472–476. doi: 10.1016/s0169-4758(97)01151-4. [DOI] [PubMed] [Google Scholar]
- 27.Ottesen E A, Nutman T B. Tropical pulmonary eosinophilia. Annu Rev Med. 1992;43:417–424. doi: 10.1146/annurev.me.43.020192.002221. [DOI] [PubMed] [Google Scholar]
- 28.Pearlman E, Hazlett F J, Boom W H, Kazura J W. Induction of murine T-helper-cell responses to the filarial nematode Brugia malayi. Infect Immun. 1993;61:1105–1112. doi: 10.1128/iai.61.3.1105-1112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearlman E, Kazura J W, Hazlett F J, Boom W H. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 30.Pearlman E, Kroeze W K, Hazlett F J, Chen S S, Mawhorter S D, Boom W H, Kazura J W. Brugia malayi: acquired resistance to microfilariae in BALB/c mice correlates with local Th2 responses. Exp Parasitol. 1993;76:200–208. doi: 10.1006/expr.1993.1023. [DOI] [PubMed] [Google Scholar]
- 31.Pinkston P, Vijayan V K, Nutman T B, Rom W N, O’Donnell K M, Cornelius M J, Kumaraswami V, Ferrans V J, Takemura T, Yenokida G, Thiruvengadam K V, Tripathy S P, Ottessen E A, Crystal R G. Acute tropical pulmonary eosinophilia. Characterization of the lower respiratory tract inflammation and its response to therapy. J Clin Investig. 1987;80:216–225. doi: 10.1172/JCI113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothenberg M E, MacLean J A, Pearlman E, Leder P. Targeted disruption of the chemokine eotaxin partially reduces peripheral blood and antigen induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sher A, Coffman R L, Hieny S, Scott P, Cheever A W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steel C, Nutman T B. Regulation of IL-5 in onchocerciasis. A critical role for IL-2. J Immunol. 1993;150:5511–5518. [PubMed] [Google Scholar]
- 35.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger W J. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996;157:4173–4180. [PubMed] [Google Scholar]
- 36.Uchida D, Ackerman S, Coyle A, Larsen G, Weller P, Freed J, Irvin C. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am Rev Respir Dis. 1993;147:982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- 37.Wardlaw A, Dunnette S, Gleich G, Collins J, Kay A. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 38.Wassom D, Loegering D, Solley G, Moore S, Schooley R, Fauci A, Gleich G. Elevated serum levels of the eosinophil granule major basic protein in patients with eosinophilia. J Clin Investig. 1981;67:651–661. doi: 10.1172/JCI110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb G K G, Job C K, Gault E W. Tropical eosinophilia. Demonstration of microfilariae in lung, liver and lymph nodes. Lancet. 1960;i:835–842. doi: 10.1016/s0140-6736(60)90730-3. [DOI] [PubMed] [Google Scholar]