Abstract
The sex-determining region Y (SRY)-related high-mobility group (HMG) box (SOX) family, composed of 20 transcription factors, is a conserved family with a highly homologous HMG domain. Due to their crucial role in determining cell fate, the dysregulation of SOX family members is closely associated with tumorigenesis, including tumor invasion, metastasis, proliferation, apoptosis, epithelial-mesenchymal transition, stemness and drug resistance. Despite considerable research to investigate the mechanisms and functions of the SOX family, confusion remains regarding aspects such as the role of the SOX family in tumor immune microenvironment (TIME) and contradictory impacts the SOX family exerts on tumors. This review summarizes the physiological function of the SOX family and their multiple roles in tumors, with a focus on the relationship between the SOX family and TIME, aiming to propose their potential role in cancer and promising methods for treatment.
Subject terms: Cancer genomics, Cancer immunotherapy
Facts
Acetylation and SUMOylation promotes the translocation of SOX TFs from nucleus to cytoplasm.
SOX is overexpressed in multiple tumors and promotes tumor occurrence and development by promoting EMT, stemness, metastasis, and proliferation.
The SOX family promotes the formation of an inhibitory tumor immune microenvironment by regulating the differentiation activation of immune cells and the release of chemokines.
Open questions
Does the subcellular localization of SOX family differ in the occurrence and development of tumors? Is inhibiting or promoting this displacement effective for tumor treatment?
What is the reason for the contradictory role that the SOX family may play in the same downstream signaling pathway in different or even the same tumors?
The vast majority of research has focused on the three members of SOX2, SOX4, and SOX9. Do other members, particularly those in the same subgroup also play important roles in tumors?
Besides serving as a tumor specific antigen, can the regulatory effect of SOX in immune microenvironment be utilized to develop a wider range of effective immunotherapy drugs?
Introduction
Transcription factors (TFs) regulate gene expression by binding to upstream sequences or distal gene elements of the transcription start site. This regulation of gene expression is crucial in the processes of cell proliferation and differentiation [1]. Of all protooncogenes, approximately 19% are transcription factors [2]. Their mutations and aberrant activation exert a significant impact on tumor development and provide potential targets for therapies.
The SOX family was first discovered in mice in 1990, and since then its function has been gradually uncovered [3]. The SOX family plays an essential role in the development of many tissues and organs. Additionally, its involvement in sex determination, maintenance of stem cell function, and differentiation of immune cells has been gradually revealed [4]. Dysfunction of the SOX family leads to the manifestation of various diseases, the most serious of which is cancer.
In this review, we first discuss the structure and function of SOX TFs. Next, we reveal their multiple roles in tumor invasion and metastasis, proliferation and apoptosis, stemness and epithelial-mesenchymal transition (EMT). Finally, we highlight the involvement of the SOX family in the tumor immune microenvironment (TIME) and propose potential therapeutic targets and strategies to address drug resistance.
General overview of SOX TFs
Structures of SOX TFs
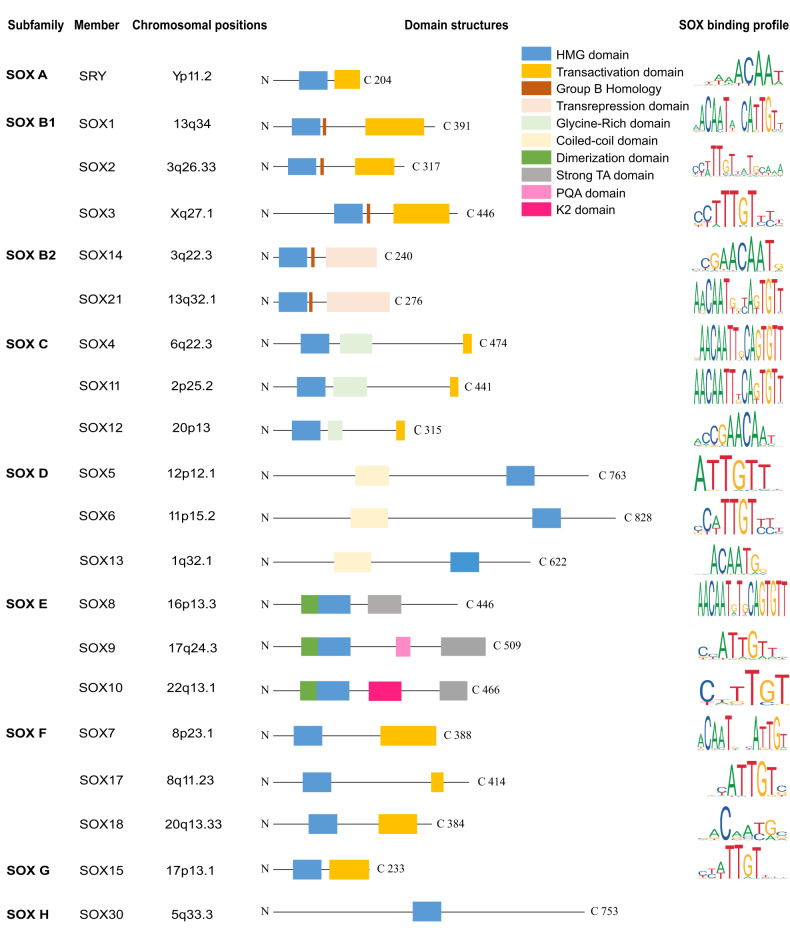
The sex-determining region Y (SRY)-related high-mobility group (HMG) box (SOX) family was first identified due to the homology between its HMG domain and the testicular determining factor SRY [3]. SOX TFs are a conserved family with highly homologous HMG domain, which mediate DNA binding and regulate gene expression [5]. The core domain of the SOX TFs, HMG, contains 79 amino acids residues with a hexameric core sequence WWCAAW (W = A/T) [4]. The sequence RPMNAFMVW is conserved in all SOX TFs except SRY [5]. To date, 20 members of the SOX TFs have been identified in mammals. According to the similarity of their HMG domain, SOX TFs are classified into 8 different subgroups: subgroup A (SRY), subgroup B1 (SOX1, SOX2, SOX3), subgroup B2 (SOX14, SOX21), subgroup C (SOX4, SOX11, SOX12), subgroup D (SOX5, SOX6, SOX13), subgroup E (SOX8, SOX9, SOX10), subgroup F (SOX7, SOX17, SOX18), SOX G (SOX15), SOX H (SOX30) (Fig. 1). Among the subgroups, members share similar functions based on their similar structure. For example, SOX4 and SOX11 in subgroup C possess similar glycine-rich areas in the middle of their domain and exhibit synergistic effects on the neurogenesis [6] and inducing the formation of cartilage growth plate [7].
Fig. 1. Domain structure and binding profile of human SOX transcription factors.
The SOX family comprises nine groups, each exhibiting a highly conserved HMG domain spanning 79 amino acids. Members within the same subgroup exhibit similar structure and functionality. The diagram illustrates several domains, including the high mobility group (HMG) domain (blue box), homology domain of SOXB (red box), activation domain (orange box), inhibitory domain (pink box), dimerization domain (dark green box), glycine-rich domain (light green), strong TA domain (gray box). The chromosomal positions and domain structures of SOX members have been confirmed. The predicted SOX binding profiles were obtained from the JASPAR public database.
Physiological function of SOX TFs
SOX TFs are crucial for numerous aspects of organizational development, including the cardiovascular, skeletal, pulmonary and nervous systems. Besides, they also participate in sex differentiation and stem cell homeostasis maintenance.
Blood cell
The SOX family maintains the stemness of hematopoietic stem cells and regulates their differentiation and maturation. SOX4 facilitates T lymphocyte differentiation in the thymus [8], while SOX6 supports the survival and maturation of erythroid cells [9]. SOX7 regulates the mesodermal bloodline and promotes the formation of hematopoietic progenitor cells and endothelial progenitor cells [10]. SOX17 primes hemogenic potential in endothelial cells (ECs), thereby regulating hematopoietic development from human embryonic stem cells (hESCs)/ induced pluripotent stem cells (iPSCs) [11].
Neuron
The SOX family promotes the development of the central nervous system and peripheral nerves, as well as facilitates the regeneration and repair of damaged nerves. SOX2 promotes the relocalization of N-cadherin to Schwann cells, promoting axonal regrowth [12]. Members in SOX B1 maintain stem cells in CNS, while SOX10 promotes terminal oligodendrocyte differentiation [13]. SOX9 is essential in the formation and maintenance of multipoint neural stem cells [14]. SOX10 is a transcriptional regulator of Schwann cell differentiation and maturation, promoting peripheral nervous system development [15]. The premature termination of SOX10 leads to absence of neural crest in Hirschsprung disease [16].
Cardiovascular
By regulating the proliferation and differentiation of myocardial cells and vascular endothelial cells, the SOX family promotes cardiovascular development. SOX4-/-embryos stuck into the endothelial ridges without further development into the semilunar valves and the outlet portion of the muscular ventricular septum [17]. SOX6 participates in the regulation of L-type Ca2+channels, regulating cardiac myocyte development [18]. SOX7, as the first direct endothelial-specific regulator of Vegfc transcription, can strictly control the number and spatial distribution of lymphatic endothelial cells (LECs) by locally suppressing VEGFC levels [19]. SOX9 is essential for the formation of cardiac valves and septa, while the loss of SOX9 fails to form the endocardial cushions [20]. SOX17 participates in cardiovascular development, helping to form the cardiac muscle [21]. Besides, SOX17 promotes coronary vessels development via activating Nestin’s enhancer [22]. SOX18 and SOX7 has a redundant role in the establishment of proper arteriovenous identity in zebrafish [23].
Sex determination
The SOX family plays a crucial role in the development of male reproductive organs and gender determination. SRY, the only Y-linked gene required to give rise to male development, is able to induce sex reversal in XX transgeneic adult mice [24]. Similarly, SOX9 fosters the development of double potential gonads into testes, which is integral to testicular development [25]. The mutations of SOX9 leads to autosomal sex reversal and campomelic dysplasia [26]. Besides, SOX8 and SOX9 have redundancy in Sertoli cell development, cord formation and testis differentiation. Clarkson et al. believed that such redundancy, which is typical feature of SOX TFs biology, represents a mere evolutionary relic in mammals [27].
Lung development
The SOX family regulates alveolar and bronchial differentiation, promotes the formation of pulmonary vasculature, and contributes to pulmonary tissue development. They are closely associated with pulmonary arterial hypertension. SOX2, SOX9, and SOX17 exhibit a synergistic effect on the occurrence and maintenance of lung tissue. SOX2 is involved in the development of the bronchial branches, primarily expressed in the epithelium of the embryonic proximal airways, while hardly expressed in the adult lungs [28]. SOX2/SOX9 allows the manipulation of branching morphogenesis and the later formation of conducting and respiratory airways [29]. SOX9 promotes the proliferation of lung progenitor cells and inhibits premature initiation of airway and alveolar differentiation [30, 31]. By controlling the balance of proliferation and differentiation, and regulating extracellular matrix (ECM), SOX9 facilitates appropriate morphogenesis of branching [32]. Utilizing a rare population of SOX9 basal cells (BCs) located at airway epithelium rugae, Ma et al. regenerate adult human lung [33]. SOX17 promotes the differentiation of precursor cells into alveolar epithelial cells and bronchial epithelial cells, and regulates the expression of their specific genes [34]. Besides, SOX17 promotes the differentiation of endothelial cells and vascular formation, the variation of its enhancers is one of the important causes for the formation of pulmonary arterial hypertension [35].
Cartilage
Through regulating ECM formation and cellular metabolism, the SOX family participates in cartilage formation and regeneration [36]. As an essential regulator of skeletal progenitor cells, SOX9 plays an important role in cartilage formation, cartilage regeneration, and endochondral osteogenesis [36, 37]. Besides, SOX5, SOX6, and SOX9 are often referred to as SOX Trio, which together regulate the development of cartilage. Even with normal expression of SOX9, knocking out SOX5 and SOX6 simultaneously can lead to mouse death in the uterus due to chondrodysplasia [38]. Through regulating ECM formation and cellular metabolism, the SOX family plays a vital role in the development and regeneration of cartilage [36].
In summary, SOX plays an important regulatory role in different stages of organ growth and development. This requires precise spatiotemporal regulation. Precise regulation of SOX gene expression occurs at multiple levels, including transcriptional and translational regulation, as well as post-translational modification (PTM) [39]. These regulatory systems collectively maintain the normal physiological functions of the SOX family within the human body and, when anomalies occur, can lead to numerous diseases, cancer included (Table 1).
Table 1.
The physical function of SOX family and their role in cancer.
| Subfamily | Member | Physiological function | Role in cancer | Reference |
|---|---|---|---|---|
| SOX A | SRY | Sex determination | Promotes the migration and invasion of HCC cells | [24, 185, 186] |
| SOX B1 | SOX1 | Neural stem cell differentiation | Inhibits the invasion of HCC, lung cancer, nasopharyngeal carcinoma | [132, 187] |
| SOX2 |
Maintains embryonic stem cell properties Axonal regrowth Occurrence and maintenance of lung tissue. |
Inhibits invasion of ESCC, GC Promotes metastasis of bladder cancer, GBM, CRC, lung cancer |
[12, 67, 94, 103–105, 137, 138, 188, 189] | |
| SOX3 | Indispensable for gonadal function | Promotes migration and metastasis of GC and osteosarcoma | [190] | |
| SOX B2 | SOX14 | Promotes neurogenesis | Participates in the proliferation and invasion of cervical cancer | [191] |
| SOX21 | Neural stem cell differentiation | Induces apoptosis of GBM and glioma | [127, 192] | |
| SOX C | SOX4 |
Lymphocyte differentiation Cartilage growth plate formation, neurogenesis, cardiovascular development |
Promotes proliferation and metastasis of ESCC, TNBC, CRC, OSCC, HCC | [6, 7, 17, 54, 58, 68, 95, 99] |
| SOX11 |
Neurogenesis Formation of cartilage growth plate |
Inhibits the proliferation of hematopoietic malignancies and Ewing sarcoma Induces EMT in uterine carcinosarcoma |
[6, 7, 59, 86, 129, 193] | |
| SOX12 | Lymphocyte differentiation | Increases proliferation and metastasis of HCC and GC | [91, 96, 143] | |
| SOX D | SOX5 |
Interference of oligodendrocyte specification Activation of neural crest specification Cartilage formation |
Induces apoptosis of glioma Promotes metastasis of NSCLC, prostate cancer, CRC |
[38, 81, 127, 194] |
| SOX6 |
Survival and maturation of erythroid cells Development of cardiac myocyte and cartilage |
Inhibits proliferation and metastasis of glioma, pancreatic cancer, HCC Promotes EMT in NPC |
[9, 18, 38, 56, 126, 127] | |
| SOX13 | Lymphocyte differentiation |
Promotes metastasis of HCC, CRC Promotes proliferation of BC, thyroid cancer |
[85, 98, 141] | |
| SOX E | SOX8 | Involved in testis differentiation, osteogenesis, | Promotes proliferation of proliferation of gestational trophoblastic neoplasia and TSCC | [24, 195, 196] |
| SOX9 |
Development of testicular and lung tissue, maintains the homeostasis of stem cells in the tissue Promotes delamination of the trunk neural crest |
Tumor suppressor in melanoma Promotes metastasis of HCC, CRC, prostate cancer Promotes proliferation of HCC, CRC, GC |
[25, 70, 71, 89, 90, 108–112, 163, 193, 197] | |
| SOX10 | Peripheral nervous development | Promotes EMT in HCC, biomarker for breast cancer | [84, 171] | |
| SOX F | SOX7 |
Hematopoietic development, regulates lymphatic endothelial cells |
Inhibits proliferation of lung cancer, CRC and AML, Treg infiltration in glioma, recruitment of TAMs | [19, 123, 124, 128, 150, 154, 198] |
| SOX17 | Development of lung tissue and cardiovascular | Promotes angiogenesis in ovarian cancer, recruit Tregs in glioma | [21, 100, 193] | |
| SOX18 | Development of the endothelial cells lining the blood and lymphatic vessels heart and skeletal muscle | Induces metastasis of GC cells | [23, 93, 199] | |
| SOX G | SOX15 | Population of myogenic progenitor cells and regeneration | Inhibits proliferation of prostate cancer and glioma | [125, 130] |
| SOX H | SOX30 | Promotes or maintain meiosis and gender differentiation of testicular germ cell | Inhibits invasion and metastasis of lung cancer and prostate cancer | [64, 200] |
AML acute myeloid leukemia, BC breast cancer, CRC colorectal cancer, EMT epithelial-mesenchymal transition, ESCC esophageal squamous cell carcinoma, GBM glioblastoma, GC gastric cancer, HCC hepatic cell carcinoma, NSCLC non-small cell lung cancer, NPC nasopharyngeal carcinoma, OSCC oral squamous cell carcinoma, SOX sex determining region Y (SRY)- HMG box, TAM tumor-associated macrophages, TNBC triple-negative breast cancer, TREG regulatory T cell, TSCC tongue squamous cell carcinoma.
Regulation of SOX TFs
Post-translational modifications of SOX TFs
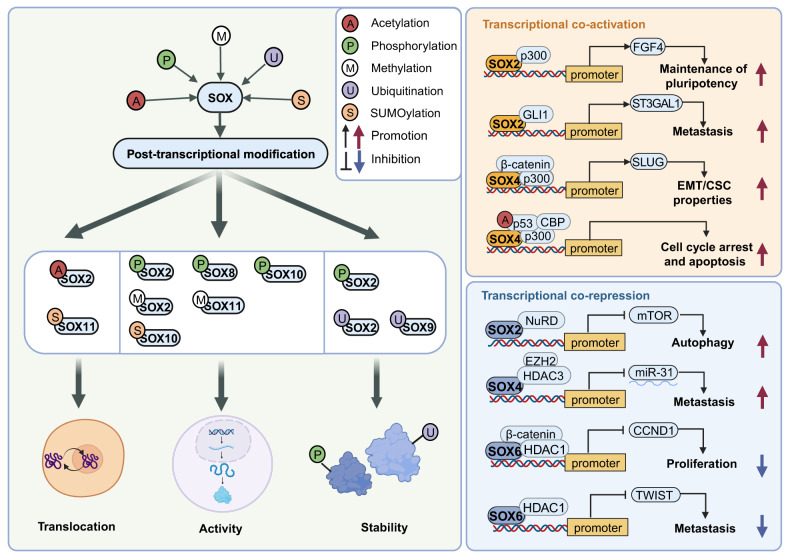
PTMs serve as crucial regulatory mechanisms for modulating the expression of SOX TFs and orchestrating various oncogenic processes by controlling their localization, activity, and interactions with partner molecules. Elucidating intricate mechanisms of PTMs in the SOX family is of immense importance in advancing novel therapeutic targets. PTM refers to covalent modifications of proteins after RNA translation, mainly involving the addition of chemical groups to one or multiple residues of the target protein or small molecules to form the protein [40]. As for SOX TFs, most studies regarding PTMs concentrate on SOX2, SOX4 and SOX9. Here, the phosphorylation, SUMOylation, methylation, acetylation and ubiquitination of SOX TFs are presented (Fig. 2).
Fig. 2. Post-transcriptional modifications and cofactors of SOX TFs.
Post-transcriptional modification of SOX TFs mainly includes acetylation [48], phosphorylation [41, 42, 120], methylation [40, 59], ubiquitination [45, 46] and SUMOylation [43, 44], which infects the translocation, activity and stability of SOX TFs. Cofactors interact with SOX TFs and exert co-activation [50–53] or co-repression [54–57] effects on the physiological and pathological functions of SOX TFs.
Phosphorylation, the most prevalent form of PTM, is critical for regulating protein activity and function [40]. PKCι phosphorylates Thr118 of SOX2, which upregulates hedgehog acyltransferase (HHAT) and maintains the stem-like and tumorigenic properties of lung squamous cell carcinoma (LSCC) [41]. Moreover, in melanoma, the phosphorylation of SOX10 by ERK at T240 and T244 residues inhibits SOX10 SUMOylation, dampening its transcriptional activity [42]. SUMOylation mainly involves the reversible binding of Small Ubiquitin-like Modifier (SUMO) to the target protein, which usually reduces DNA binding activity [40]. In nasopharyngeal carcinoma (NPC), phosphorylation of SOX2 by ERK permits its subsequent SUMOylation at K245, leading to the autophagic degradation of SOX2 [43]. SUMOylation of SOX11 suppresses its nuclear localization and binding ability. This mechanism is correlated with the proper expression of SOX4, which is critical for retinal ganglion cell development [44]. Ubiquitination often affects protein stability and promotes its degradation [40]. Setd7 mediates the methylation of SOX2 at K119, resulting in the ubiquitination and subsequent degradation of SOX2 [45]. Moreover, DDRGK domain-containing protein 1 (DDRGK1) inhibits ubiquitination and the subsequent degradation of SOX9 during chondrogenesis [46]. Methylation and acetylation are another two important forms of PTM. Methylation mainly impacts pioneer factor activity, while acetylation of SOX TFs promotes nuclear localization [40]. In embryonic stem cells (ESCs), methylation of SOX2 by CARM1 at Arg113 promotes SOX2 self-association and enhances SOX2 transactivation [47]. Regarding acetylation, p300 and CEP promote the acetylation of SOX2, while the mSin3A/ histone deacetylases (HDAC) complex promotes its deacetylation. The interplay between these proteins acts as a switch, rapidly regulating gene expression and facilitating nuclear export [48].
In addition to these chemical modifications, transcription factors can achieve co-activation or co-repression through protein-protein interactions. P300 and its homolog, CBP, are typical co-activators that serve as bridging molecules connecting promoters and proximal enhancer regions [49]. SOX2 interacts with p300 and cooperatively activates FGF4, maintaining pluripotency in embryonal carcinoma [50]. In uterine carcinosarcoma, the complex comprising SOX4, β-catenin, and p300 promotes β-catenin-mediated transcription of SLUG, contributing to EMT/CSC properties [51]. DNA damage response (DDR) acts as a tumorigenesis barrier. In DDR-associated cancer, Pan et al. discovered that SOX4 enhances p53 acetylation via interacting with p300/CBP and facilitating p300/CBP/p53 complex formation, which further promotes cell cycle arrest and apoptosis [52]. Besides p300, in melanoma, SOX2 forms a complex with GLI1 and cooperatively transactivates ST3GAL1, which promotes melanoma metastasis via upregulating AXL [53]. Similarly, HDAC and NuRD are the main co-repressors of SOX TFs. HDAC is a typical co-repressor factor that often exerts repressive functions through increasing the chromatin compaction of their target genes [40]. In esophageal squamous cell carcinoma (ESCC), SOX4 cooperates with EZH2 and HDAC3 to enhance tumor cell progression and metastasis through epigenetic silencing of miR-31 by H3K27me [54]. Through interacting with β-catenin and HDAC1, SOX6 suppresses the activity of the cyclin D1 (CCND1) promoter, while downregulation of SOX6 induces the proliferation of pancreatic β-cells [55]. In pancreatic cancer, SOX6 downregulates TWIST1 by recruiting HDAC1 to the TWIST1 promoter, which inhibits pancreatic cancer metastasis [56]. SOX2 recruits NuRD and cooperatively represses mTOR, inducing autophagy and subsequent reprogramming to pluripotency [57]. These intricate and complex interactions collectively contribute to the transcriptional regulation of the SOX family in both physiological and tumorigenic contexts.
Epigenetic regulation of SOX TFs
Apart from PTM, SOX proteins are also regulated at epigenetic levels, which mainly involves methylation and non-coding RNAs (ncRNAs). DNA methylation typically leads to gene silencing and repression, which primarily occurs in SOX with a suppressor role in cancer. METTL14 raises YTHDF2 to m6A-modified sites of SOX4 mRNA and promotes SOX4 degradation [58]. The methylation of SOX11 promoter leads to SOX11 silencing in hematopoietic malignancies [59]. In lung cancers, loss of SOX30 frequently occurs due to its methylation [60]. The methylation of SOX17 promoter contributes to its epigenetic silencing in papillary thyroid carcinoma [61].
NcRNAs mainly include miRNAs and lncRNAs. LncRNAs can competitively bind to the target mRNA of miRNAs, or act as a molecular sponge to relieve the inhibition of miRNAs on target mRNA and promote the expression of target genes [62]. MiR-183-5p downregulates MUC15, inducing SOX2 expression via the c-MET/PI3K/AKT axis [63], while miR-653-5p upregulates SOX30 in prostate cancer [64]. LncRNA PCAT1 upregulates SOX2 in non-small cell lung cancer (NSCLC) [65]. In most cases, they collectively regulate the expression of SOX TFs. In cholangiocarcinoma cells, YY1 upregulates lncRNA DLEU1, which competitively binds to miR-149-5p, elevating Yes-associated protein 1 (YAP1) expression. YAP1 upregulates SOX2 via binding to TEAD on the promoter region of SOX2, ultimately promoting the proliferation of cholangiocarcinoma cells [66]. Besides, lncRNA PTV1 upregulates SOX2 expression by competitively bind to miR-136 [67]. LncRNA SNHG47 upregulates SOX4 by sponging miR-338-3p, promoting the proliferation of ESCC cells [68]. Additionally, SOX5 expression is upregulated by lncRNA SOX2OT via miR-194-5p, ultimately promoting colorectal cancer (CRC) invasion [69]. Moreover, SOX9 upregulates lncRNA FARSA-AS1 expression by binding to its promoter, while FARSA-AS1 upregulates SOX9 by impeding miR-18b-5p, forming a feedback loop to promote CRC metastasis [70]. Similarly, miR-424-5p sponged by LncRNA HCG18, miR-361-3p sponged by lncRNA PVT1 and miR-296-5p sponged by lncRNA PRR34-AS1 promote SOX9 expression in cholangiocarcinoma [71], NSCLC [72] and HCC [73] respectively. Altogether, methylation and ncRNAs epigenetically silence tumor-suppressor role of SOX proteins in cancers, while enhancing the expression of oncogenic SOX proteins, thereby facilitating tumor initiation and progression.
SOX TFs in cancer
Cancer is characterized by eight core hallmarks, including the ability to sustain proliferative signaling, resist growth suppressors and cell death, enable replicative immortality, access vasculature, activate invasion and metastasis, reprogram cellular metabolism, and avoid immune destruction [74]. The SOX family influences these cancer characteristics through various mechanisms, encompassing promoting cancer invasion and metastasis, influencing proliferation and apoptosis, aiding in stemness, facilitating EMT, and modulating tumor immunity. Therefore, the disturbance of the SOX family runs through the occurrence and development of many tumors (Fig. 3).
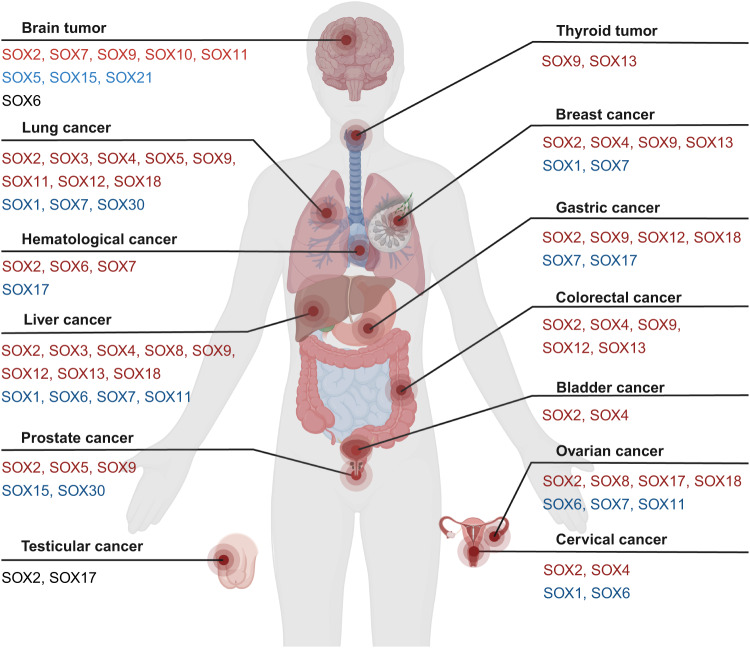
Fig. 3. Involvement of human SOX TFs in cancer.
The participation of SOX TFs in various cancers has been disclosed, including brain tumor [103, 105, 107, 113, 118, 125, 127, 131, 154, 155, 176, 177, 183, 184, 188, 194], thyroid cancer [98], lung cancer [41, 65, 73, 104, 135, 136, 151, 164, 193], breast cancer [121, 145, 146, 153, 158, 171, 181], hematologic cancer [123, 178], liver cancer [82, 84, 87, 89, 93, 95, 108, 111, 115, 132, 152], gastric cancer [91, 92, 112, 137, 138, 160, 190], colorectal cancer [58, 69, 70, 77, 85, 96, 110, 124, 149, 189], ovarian cancer [100, 120, 169, 173], bladder cancer [201], prostate cancer [64, 81, 90, 116, 117, 130, 163], testicular cancer [110, 195] and cervical cancer [114, 134]. The red fonts stand for upregulation, blue indicates downregulation and black for either upregulation or downregulation.
SOX family acts as an oncogene
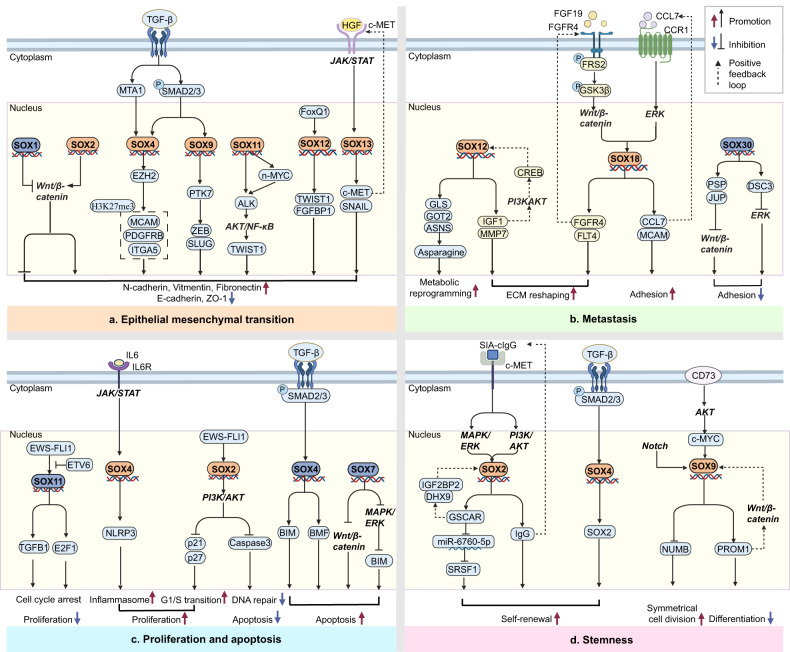
Numerous members of the SOX family significantly contribute to the initiation and progression of tumors. They primarily promote EMT, invasion and metastasis, proliferation and apoptosis, and stemness of tumors. In some cases, they also relate to treatment insensitivity and poor prognosis (Fig. 4).
Fig. 4. SOX TFs acts as the hub of signaling pathways in the tumorigenesis.
SOX TFs in critical positions of the signaling pathway that mediates EMT [76, 82, 85, 87, 132, 78–80, 86], invasion and metastasis [91–93, 96, 135], proliferation and apoptosis [59, 97, 99, 123, 124, 128], stemness of cancer [104, 105, 107, 108, 110, 111].
EMT
EMT refers to the process by which epithelial cells acquire interstitial features under certain conditions [75]. Hypoxia and cytokines secreted by stromal cells significantly induce EMT and promote tumor invasion for cells at the margins of many solid tumors [40]. Transcription factors are crucial in controlling multiple cellular events like proliferation, survival, differentiation, adhesion, and migration during the EMT [75]. So far, several transcription factors, including SNAIL, TWIST1, ZEB, and SLUG, are extensively involved in the EMT process. SOX TFs serve as the hub of these signaling pathways and mediate the EMT of tumors.
Wnt/β-catenin signaling pathway mediates EMT of tumors. Through activating the Wnt/β-catenin pathway, SOX2 induces EMT in breast cancer and prostate cancer [76]. Recent studies have demonstrated that DVL3 upregulates SOX2 expression by activating the Wnt/β-catenin/c-MYC pathway, thus promoting and maintaining EMT in CRC [77]. Besides, SOX family is a crucial mediator of the TGF-β-induced EMT in cancer. SOX4 is upregulated by TGF-β-induced-MTA1 and contributes to EMT via inducing expression of EZH2, which mediates H3K27me3 epigenetic imprint of Mcam, Pdgfrb, Itga5 [78, 79]. Aside from being a direct TGF-β target, SOX4 also performs as an effector of TGF-β signaling via interacting with SMAD3 in a phosphorylation-independent manner [80]. The upregulation of SOX5 by the TGF-β/SMAD3 signaling pathway activates TWIST1, ultimately contributing to EMT in prostate cancer [81]. The upregulation of SOX9 via the TGF-β/SMAD2/3 pathway induces the expression of PTK7, which subsequently upregulates ZEB1 and SLUG, leading to EMT in hepatic cell carcinoma (HCC) [82]. Moreover, SOX TFs induces EMT through directly stimulating the expression of SNAIL or TWIST. SOX3 induces EMT in osteosarcoma cells by transcriptionally activating SNAIL1 [83]. In HCC, ELMO1-induced upregulation of SOX10 activates the PI3K/AKT signaling pathway. Subsequently, this pathway enhances the expression of SNAIL, which ultimately induces EMT [84]. HGF upregulates SOX13 through the JAK/STAT pathway. Consequently, the upregulation of SOX13 promotes the EMT of CRC by transactivating SNAIL and c-MET [85]. SOX11 activates ALK directly or via upregulation of n-MYC, which activates the downstream transduction cascades containing AKT, NF-κB and TWIST1, leading to EMT in uterine carcinosarcoma [86]. FoxQ1 upregulates SOX12 expression, which transactivates the expression of TWIST1 and FGFBP1, promoting EMT in HCC [87].
Invasion and metastasis
Tumor invasion and metastasis are the major causes of cancer progression and treatment failure. Therefore, studying and understanding the mechanisms behind tumor invasion and metastasis are crucial for cancer treatment and prognosis. Through remodeling the metabolism, extracellular matrix and tumor microenvironment, SOX TFs contribute to the invasion and metastasis of tumors.
ECM is a non-cellular component of the extracellular matrix in tissues, containing various proteins, polysaccharides, and other molecules, providing structural support and biological signal transduction for cells [88]. By regulating ECM composition and structure, SOX promotes tumor metastasis. SOX9 promotes the secretion of activin B from HCC cells via upregulating INHBB. Activin B promotes the activation of surrounding hepatic stellate cells, which promotes the deposition of extracellular matrix, contributing to the metastasis of HCC [89]. Zbtb7a, a member of POK transcription factors, interacts with SOX9 to antagonize its transcriptional activity on key target genes. In advanced prostate cancer, the genetic loss of Zbtb7a activates SOX9-targeted genes such as H19 and MIA, ultimately reducing the expression of integrin to promote cancer metastasis [90]. In GC, SOX12 promotes tumor extracellular matrix (ECM) reshaping by upregulating matrix metalloproteinase (MMP) 7 and IGF1. In turn, IGF1 induces SOX12 expression through IGF1/CREB/SOX12 pathway, forming a feedback loop to promote GC metastasis [91]. The CCL7-CCR1 axis upregulates SOX18 via the ERK/ELK1 pathway. In turn, SOX18 induces the expression of MCAM and CCL7, forming a feedback loop to promote GC adhesion and metastasis [92]. In addition, Chen et al. discovered that in HCC, FGFR4 and its ligand FGF19 transactivate the SOX18 promoter directly by activating the p-FRS2/p-GSK3β/Wnt/β-catenin pathway. In turn, SOX18 directly increases the expression of FGFR4 and FTL4, forming a feedback loop to promote the ECM reshaping and metastasis of HCC [93].
Besides ECM, SOX TFs also mediate tumor metastasis via remodeling metabolism and microenvironment. SOX2 upregulates IL6 by inducing the expression of FOSL2. IL6 activates JAK/STAT signaling, sustaining inflammation to promote tumor metastasis [94]. SOX4 upregulates CXCL12 which promotes neovascularization of microenvironment via activating CXCL12/CXCR4/CXCR7 axis, ultimately catalyzing HCC metastasis [95]. Moreover, SOX12 transactivates GLS, GOT2 and ASNS, which promotes metabolic reprogramming by inducing asparagine synthesis, ultimately promoting CRC metastasis [96].
Proliferation and apoptosis
Evading growth suppressors and resisting cell death are two core hallmarks of cancer [74]. Through promoting G1/S transition, angiogenesis and activating inflammasome, SOX TFs promotes cancer proliferation. In Ewing sarcoma, SOX2 upregulated by EWS-FLI1 activates PI3K/AKT signaling. The activated AKT then promotes G1/S transition via inhibiting p21 and p27 proteins. Simultaneously, the activated AKT promotes poly (ADP-ribose) polymerase (PARP)-induced DNA repair through impeding caspase3, which suppresses cell apoptosis. Altogether, SOX2 promotes Ewing sarcoma growth [97]. SOX13 upregulates TRIM11, which inhibits the degradation of YAP, ultimately promoting the G1/S transition of anaplastic thyroid cancer cells [98]. In OSCC, SOX4 is upregulated by IL-6 through the JAK/STAT pathway. SOX4 induces the expression of NLRP3, promoting OSCC proliferation via activating inflammasome [99]. Moreover, The interaction between PAX8 and SOX17 promotes vascular endothelial growth factor (VEGFR) expression by downregulating SERPINE1, ultimately leading to angiogenesis in ovarian cancer (OC) [100].
Stemness
Cancer stem cells (CSCs) refer to a group of stem cells with abnormal differentiation and uncontrollable division [101]. Several signaling pathways, including Wnt/β-catenin, NF-κB, Notch, Hedgehog, PI3K and JAK/STAT, are vital in maintaining CSCs [101]. By acting as the upstream or downstream in these signaling pathways, the SOX family contributes to stemness of cancer cells via regulating the self-renewal ability, division and differentiation.
SOX2 is considered one of the four key transcription factors that mark the pluripotency along with KLF4, OCT3/4, KLF5 and MYC [102]. Hypoxia-inducible factor (HIF) induces the expression of both SOX2 and KLF4 in glioblastoma (GBM), which in turn upregulate CD133/15, ultimately promoting stemness expression [103]. In lung cancer, SIA-cIgG binds to c-Met, upregulating SOX2/OCT4 through the PI3K/AKT/mTOR and RAS/RAF/MAPK/ERK signaling pathways. In turn, SOX2 induces the expression of IgG and promotes its translocation to the cytoplasm, forming a feedback loop. Altogether, SOX2 promotes the self-renewal and cancer cell stemness [104]. Moreover, SOX2 upregulates the expression of lncRNA GSCAR, which competes with miR-6760-5p to upregulate SRSF1 expression, thereby maintaining glioma stem cell (GSC) self-renewal ability. Additionally, GSCAR enhances the interaction between IGF2BP2 and DHX9, promoting SOX2 stabilization and forming a positive feedback loop [105].
Induced by cisplatin, CLU triggers mitochondrial fission by activating DNM1L through AKT-mediated phosphorylation. CLU promotes the mitophagic degradation of MSX2 and induces the expression of SOX2, thereby enhancing stemness in oral cancer [106]. Han et al. discovered that SOX4 upregulated by TGF-β-SMAD2/3 signaling increases the self-renewal ability of glioma stem cells via inducing SOX2 expression, indicating a crucial role of SOX2 and SOX4 in stemness maintenance [107]. SOX9 is a critical regulator of cell stemness. Liu et al. discovered that SOX9 is upregulated via Notch signaling, which enhances cancer stem cells’ self-renewal and symmetrical cell division (SCD) of liver CSCs via downregulating Numb expression [108]. Song et al. reported that YAP1 induces SOX9 expression via interacting with TEAD, a conserved binding site in the SOX9 promoter. This upregulation of SOX9 increases SCD and maintains stemness [109]. In CRC, SOX9 directly activates PROM1 via a Wnt/β-catenin-responsive intronic enhancer. Additionally, PROM1 also upregulates SOX9 via stabilizing β-catenin. This interaction creates a positive feedback loop between SOX9 and PROM1, impeding differentiation and enhancing the stem cell properties of CRC [110]. CD73 upregulates SOX9 through the AKT/c-MYC signaling pathway and impedes its ubiquitination and subsequent degradation via inhibiting GSK3β, thus promoting dedifferentiation and maintaining the stemness of HCC [111].Recently, Chen et al. established a hereditary GC model expressing both SOX2 and SOX9, with distinctive cell populations and metastasis in the liver and peritoneum. They discovered that these metastatic cells originated from SOX9+ progenitor cells in the stomach, and SOX9 plays an indispensable role in the asymmetric division of GC stem cells [112].
Therapy resistance
The development of drug resistance in cancer treatment remains a significant obstacle to successful clinical outcomes. Therefore, exploring drug resistance mechanisms is paramount to enhancing therapeutic effect and patient prognosis.
The disturbance of ATP-binding cassette subfamily G member (ABCG) is implicated in SOX-induced drug resistance. In CD133-positive glioma stem cells (GSCs), the miR-145/OCT4/SOX2 axis confers radio-chemo resistance, while inhibition of this axis increases the sensitivity to temozolomide (TMZ) and radiation, following a decreased expression of ABCG2, MDR1 and Bcl2 [113]. SOX4 increases the expression of ABCG2 in cervical cancer and subsequently promotes resistance to cisplatin [114]. SOX9 upregulates ABCG2 via activating the AKT pathway, which confers sorafenib resistance of HCC [115].
Besides ABCG, multiple mechanisms like aberrant signaling pathways, including NF-κB, Wnt/β-catenin, AKT and mTOR, also participate in SOX-induced drug resistance. In prostate cancer, SOX2 dysregulates the cell cycle via upregulating WEE1 and CDK1, resulting in the insensitivity of prostate cancer to NHRSI [116]. Gao et al. discovered that in NSCLC, SOX2 suppresses radioimmune responses via activating the cGAS/STING signaling pathway [65]. The expression of SOX2 is inhibited by TP53 through the upregulation of miR-34, whereas RB1 represses SOX2 expression via directly binding to the E2F binding sites of the SOX2 promoter. The deficiency of TP53 and RB1 in prostate cancer promotes the expression of SOX2, contributing to antiandrogen resistance. Consequently, this leads to the transdifferentiation of treatment-sensitive adenocarcinoma cells into derivatives that resemble neuroendocrine cell states [117]. Furthermore, SOX4 promotes TMZ resistance in GBM by interacting with EZH2 and coactivating METTL3, resulting in transcriptional plasticity of tumor cells [118]. TEAD inhibitor induces SOX4 expression via upregulation of VGLL3, promoting cancer cell survival and resistance to TEAD inhibitors via activating the PI3K/AKT signaling pathway [119]. In OC, SOX8 phosphorylated by Aurora-A promotes glucose metabolism and inhibits cell senescence via upregulating FoxK1, ultimately promoting resistance to cisplatin [120]. By upregulating HDAC5, c-MYC promotes the deacetylation of SOX9, which subsequently leads to its nuclear translocation, contributing to tamoxifen resistance in breast cancer [121].
SOX family acts as a suppressor
Despite most research focusing on the oncogenic characteristics of SOX TFs, they also exhibit anticancer effects in specific types of tumors. SOX TFs act as double-edged swords, exerting divergent effects in different tumor types via distinct signaling pathways. A comprehensive understanding of the multifaceted roles of SOX TFs in tumors is of great significance for elucidating the mechanisms of tumor development and informing therapeutic approaches.
Proliferation and apoptosis
Uncontrolled cell proliferation and apoptosis are key features in tumor development. Certain members of the SOX protein family can impede cell proliferation and promote apoptosis, thus inhibiting tumor growth and improving patient prognosis. TGF-β signaling is also a dual mediator of tumor proliferation and metastasis. During TGF-β/SMAD-induced EMT, the upregulation of SOX4 by TGF-β activates the pro-apoptotic proteins BIM and BMF, ultimately leading to apoptosis in pancreatic cancer. Notably, this shift of SOX4’s function from pro-tumor to pro-apoptosis is triggered by the suppression of KLF5, which is downregulated by TGF-β-induced SNAIL [122]. Overexpression of SOX11 inhibits proliferation in hematopoietic malignancies, with the activation of the TGF-β signaling pathway and reduction of E2F1, while the methylation of SOX11 promoter leads to SOX11 silencing and the proliferation of tumors [59]. Besides TGF-β, Wnt/β-catenin is a typical pro-tumor signaling. Through antagonizing the Wnt/β-catenin signaling pathway, SOX7 inhibits the proliferation of acute myeloid leukemia (AML) and CRC [123, 124]. Additionally, through downregulating Wnt/β-catenin signaling, SOX15 inhibits cell proliferation of glioma cells [125]. P53 is a crucial tumor suppressor gene that regulates cell growth, DNA repair, and apoptosis. In HCC, SOX6 inhibits NPM1 via repressing c-MYC, which subsequently stabilizes and activates p53 via promoting the formation of the p14ARF/HDM2/p53 complex, ultimately inhibiting HCC proliferation [126]. In primary human GBM cells, SOX5, SOX6, and SOX21 induce apoptosis by upregulating CDK inhibitors and downregulating p53 protein turnover [127]. In addition to these classical signaling pathways and interacting proteins, there are also other mechanisms involved in cancer inhibition ability of SOX. In lung cancer, SOX7 induces apoptosis through the MAPK/ERK/BIM pathway [128]. In Ewing sarcoma, ETV6 competes with EWS-FLI1 to bind SOX11. Inactivation of ETV6 enhances the interaction between EWS-FLI1 and SOX11, leading to increased expression of SOX11. This, in turn, causes cell cycle arrest and inhibits proliferation [129]. In prostate cancer, SOX15 functions as a tumor suppressor by upregulating AOC1, which reduces the proliferation and migration of prostate cancer through the activation of reactive oxygen species and ferroptosis [130]. Moreover, the overexpression of SOX21 forms a complex with SOX2, altering the balance and composition of SOX2 and SOX21, consequently inducing apoptosis in glioma cells [131].
Invasion and metastasis
Invasion and metastasis contribute to the poor prognosis and are responsible for the fatal outcome in cancer patients. Some members of the SOX family can inhibit cancer invasion and metastasis, elucidating the complex dual effects of SOX TFs in cancer. Specifically, through inhibiting the Wnt/β-catenin signaling pathway, SOX1 impedes EMT and subsequent metastasis in HCC [132], nasopharyngeal carcinoma [133], and cervical cancers [134]. Moreover, in lung adenocarcinoma, SOX30 downregulates Wnt/β-catenin and ERK signaling by promoting the transcriptional activation of DSP, JUP and DSC3, suppressing cancer adhesion and metastasis [135]. Similarly, SOX30 suppresses metastasis of lung cancer [136] and prostate cancer [136] via inhibiting Wnt/β-catenin signaling. Besides Wnt/β-catenin signaling, phosphatase and tensin homolog (PTEN) is another mediator of SOX’s suppressing role. Furthermore, SOX2 upregulates PTEN, which in turn dephosphorylates AKT, thereby suppressing invasion in GC [137]. The lncRNA BC002811 disrupts the interaction between SOX2 and PTEN, consequently suppressing PTEN transcription. The downregulation of PTEN ultimately promotes GC metastasis via upregulating MMP2 and MMP9 [138].
SOX TFs in tumor immune microenvironment
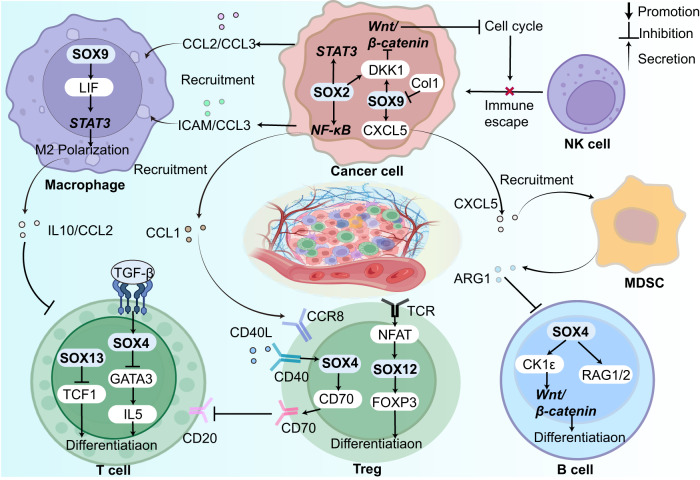
The immune system monitors the internal environment, identifying and eliminating abnormal cells primarily through T cells, B cells, and natural killer (NK) cells. However, a suppressive TIME consisting of tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) facilitates immune evasion and tumor cell survival. These cells interact via complex communication networks using secreted cytokines, chemokines, growth factors, and ECM proteins, contributing to a stable immune microenvironment [139]. SOX TFs regulate the differentiation, recruitment, and activation of these immune cells, thus maintaining the homeostasis of the immune microenvironment. Dysregulation of immune reactions, either excessive or weak, can lead to the development of diseases such as autoimmune diseases and cancer. In the context of cancer, the SOX family typically establishes a suppressive TIME by recruiting suppressive immune cells, enhancing the secretion of immune inhibitory molecules and suppressive cytokines, which confers resistance to immunotherapy in many patients (Fig. 5).
Fig. 5. SOX TFs as multifaceted players in immune microenvironment.
Extensive involvement of SOX factors in various kinds of immune cells, including TAMs [140, 145], Tregs [143, 153, 156], CD8 + T cell [140, 141, 160], B cell [144], NK cells [165] and MDSCs [167], renders them as crucial regulators and promising targets for immunotherapy.
SOX TFs inside immune cells
SOX TFs participate in the differentiation and maturation of multiple immune cells in both physiological and pathological contexts. Inside TAMs, Fan et al. demonstrated that SOX9-regulated leukemia inhibitory factor (LIF) mediates macrophage repolarization to an M2 state via activating STAT signaling. This repolarization process induces the secretion of immunosuppressive factors, including IL10 and CCL2, by TAMs, ultimately leading to immune suppression of T cells. In airway inflammation, SOX4 is upregulated by TGF-β and inhibits TH2 differentiation by downregulating GATA3-induced IL5 [140]. Additionally, SOX13 acts as a switch of αβ and γδ T cell differentiation via antagonizing TCF1 [141]. CD39 is expressed on some surfaces of human thymus-derived Tregs, which regulates the immune suppressive capacity of Tregs. The overexpression of CD39 in Tregs maintains its status via autocrine/paracrine TGF-β signaling, which induces CD39 expression via upregulating SOX4 [142]. Moreover, in adoptive transfer colitis, SOX12 is upregulated by T cell receptor (TCR)-mediated NFAT activation in CD4 + T cells. SOX12 induces Foxp3 expression via directly binding to its promoter, subsequently inducing the differentiation of Tregs in the periphery [143]. However, the relationship between SOX12 and Tregs in cancer remains unknown. Physiologically, SOX4 induces the differentiation of early B cells by inhibiting Wnt/β-catenin through upregulating CK1ε. Simultaneously, SOX4 also upregulates RAG1/2, thereby allowing for VDJ gene rearrangements at the Igh locus [144].
SOX TFs in the interaction of cancer cells and immune cells
SOX TFs mediate interactions between cancer cells and TAMs/TANs
M2 TAMs and tumor-associated neutrophils (TANs) secrete many cytokines that contribute to a suppressive TIME for tumor survival. In breast cancer, the overexpression of SOX2 upregulates CCL3 and ICAM1 via activating NF-κB. Simultaneously, SOX2 induces CCL2 and CCL3 via activating STAT3 signaling pathway. These changes ultimately result in the recruitment of TAMs in the TME [145]. The secretion of CXCL1 by TAMs promotes the upregulation of SOX4 by activating NF-κB signaling pathway, ultimately promoting breast cancer metastasis [146]. In GSCs, the co-expression of SOX2 and OCT upregulates BRD4, which subsequently promotes the secretion of cytokines and chemokines, such as SPP1, CXCL5, and IL8, ultimately leading to the recruitment and polarization of M2 TAMs [147]. SOX2 is upregulated in head and neck squamous cell carcinoma (HNSCC) due to the enhancing effect of TAMs on the availability of HA, which binds to CD44 receptors on the surface of cancer cells. Through activating PI3K/4EBP1 axis, CD44 upregulates SOX2, thereby promoting the CSC properties of the cancer cells [148]. In CRC, the expression of SOX4 can be induced by TGF-β, which is secreted by TAMs under hypoxia stimulation. As a result of this upregulation, SOX4 increased the expression of TMEM2 and concomitantly inhibited endoplasmic reticulum stress (ERS) in CRC cells [149]. PDGF-BB, which is secreted by tumor cells, facilitates the expression of IL-33 through the upregulation of SOX7. This molecular cascade subsequently promotes cancer metastasis by recruiting TAMs [150]. TANs recruitment is driven by the upregulation of CXCL5 mediated by SOX2 and the loss of NKX2-1 [151].
SOX TFs mediate interactions between cancer cells and Tregs
Tregs are a subset of CD4 + CD25 + T cells that exert a significant impact on maintaining the body’s immune homeostasis. However, in the tumor microenvironment, the suppressive effect of Tregs inhibits the anti-tumor response of T cells and weakens the body’s immune response against tumors, thereby promoting tumor growth [152]. SOX2 reduces the binding of H3K27Me on the promoter region of CCL1, inducing CCL1 expression. Simultaneously, SOX2 upregulates CCL1 via activating NF-κB signaling pathway, ultimately recruiting Tregs to the TME, which maintains the CSCs of breast cancer [153]. In high-grade glioma, deletion of SOX7 reduced CCL17, CCL22, and CCL5, which subsequently decreased Treg cells, while deletion of SOX17 increased CCL17 concentration and recruitment of Treg cells [154]. Xiao et al. analyzed data from TCGA and CCGA and identified an association between SOX10 overexpression in glioma and DNA replication, mismatch pair and regulation of negative regulatory T-cell differentiation [155]. Balsas et al. found that SOX11 expression in mantle cell lymphoma (MCL) is correlated with CD70 overexpression, increased Tregs infiltration and T-cell activation, contributing to the invasion of MCL [156].
SOX TFs mediate interactions between cancer cells and responsive T/B cells
Responsive T cells and responsive B cells are the primary effector cells in the human body that carry out immune clearance, kill pathogenic cells, and maintain the microenvironment. CD8 + T cell is the main cytotoxic T cell in responsive T cells, which can eliminate tumor cells through the production of cytokines, activation of memory T cells, or direct cytotoxic effects. Responsive B cells mainly produce specific antibodies to help eliminate tumor cells or inhibit their growth. In HNSCC, Tan et al. found that SOX2 inhibits the infiltration of CD8 + T cells via inhibiting STING-type I interferon (IFN) signaling and facilitating autophagy-dependent degradation of STING, which contributes to the immune escape of tumors [157]. In triple-negative breast cancer, integrin αvβ6 on the surface of epithelial cancer cells activates TGF-β to induce SOX4 expression, promoting the immune escape via suppressing cytotoxic CD8 T cells [158]. Chimeric antigen receptor (CAR) T cell therapy remains ineffective in solid tumor environment for the exhaustion in TME. Good et al. found that this dysfunction of CAR T cells, including the transition of conventional CD8 + T-to-NK-like T cells, can be prevented by the downmodulation of ID3 and SOX4 efficiency [159]. SOX9 continuously suppresses CD8 + T cells in GAC patients through the upregulation of LIF or the recruitment of TAMs mentioned above, which is independent of its suppression of NK cells in PC patients [160]. As for B cells, few SOX family members have been found to be associated with B cells in TIME. In MCL, SOX11 blocks the B-cell differentiation program and activates the B-cell receptor (BCR) signaling pathway, leading to MCL’s oncogenesis and aggression [156].
SOX TFs mediate interactions between cancer cells and NK cells
NK cells are lymphocytes lacking a specific antigen receptor that can eliminate tumor and virus-infected cells without prior sensitization [161]. Through analyzing the TIMER 2.0 database, Qin et al. concluded that NK cells were negatively correlated with most SOX family genes, namely SOX4, SOX8, SOX11, SOX17 and SOX18 [162]. SOX9 activates NANOG, which represses ICAM1 expression by inhibiting histone acetyltransferase p300 recruitment to ICAM1 promoter. This leads to immune evasion of pancreatic cancer from NK cells and promotes pancreatic cancer recurrence [163]. Moreover, SOX2 and SOX9 have been found to confer the latency CSCs the ability to evade NK cell killing via inducing MHC1 markers of self, promoting metastatic relapse [164]. Malladi et al. discovered that Latency Competent Cancer (LCC) in lung and breast cancer, which expresses high levels of SOX2 and SOX9, undergoes autocrine Wnt/β-catenin inhibition through DKK1. This inhibition results in a slow-cycling state in LCC cells, leading to their escape from recognition by NK cells and eventually causing an enrichment of LCC [165].
SOX TFs mediate interactions between cancer cells and MDSC
Myeloid-derived suppressor cell (MDSC) is a group of immature immune cells derived from hematopoietic stem cell (HSCs) in the bone marrow, which inhibits immune responses to promote tumor growth and escape [166]. In pancreatic cancer, Chen et al. found that the upregulation of SOX9 occurs due to the deletion of Col1, leading to the elevated expression and secretion of CXCL5. Consequently, MDSCs are recruited to the tumor site, where they mediate the suppression of T and B cell function through the secretion of arginase, ultimately leading to the progression of pancreatic cancer [167].
Association between the expression of SOX TFs and immune checkpoints in cancers
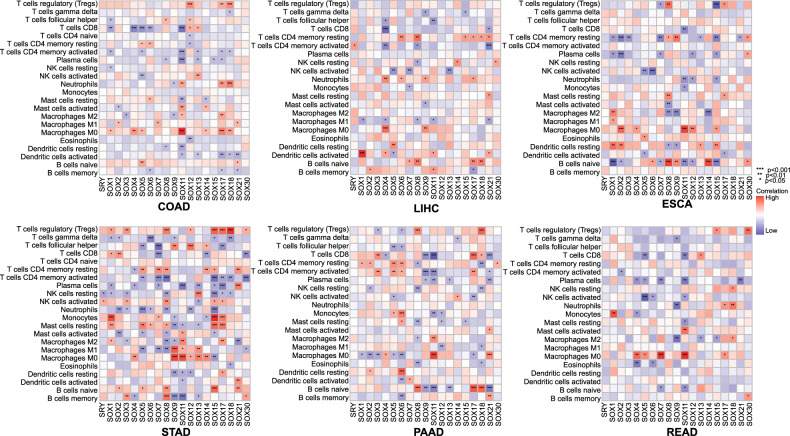
The unresponsive TIME contributes to the ineffectiveness of many anticancer drugs. By manipulating immune checkpoints, tumor cells can regulate the activity of immune cells and facilitate immune evasion. The therapeutic effects of several anticancer drugs are greatly enhanced by combining them with immune checkpoint inhibitors (ICIs). An instance is the front-line treatment for liver cancer, where Lenvatinib and ICIs are combined [168]. More research is required to explore in-depth the underlying mechanisms and improve the therapy efficacy. Using TCGA data, we analyze the correlation between the SOX family and the infiltration of 22 immune cells in a panel of six common digestive tumors (Fig. 6). These heatmaps illustrate a clear correlation between the SOX family and the vast majority of immune cells in these tumors. In most studied tumors in the digestive system, SOX18 exhibits a strong correlation with Tregs, while SOX11 is highly correlated with TAMs and CD4 + /CD8 + T cells. These potential associations may inform future studies of tumor immunity.
Fig. 6. The association heatmap of SOX transcription factors expression in immune cells in digestive cancers.
The association heatmap between SOX transcription factor expression and immune cells in digestive system malignancies. Red indicates positive correlation and blue indicates negative correlation. The color is darker, the correlation is stronger. The data was obtained from the TCGA public database. STAD stomach adenocarcinoma, LIHC Liver hepatocellular carcinoma, ESCA esophageal carcinoma, PAAD pancreatic adenocarcinoma, READ rectum adenocarcinoma, COAD colon adenocarcinoma.
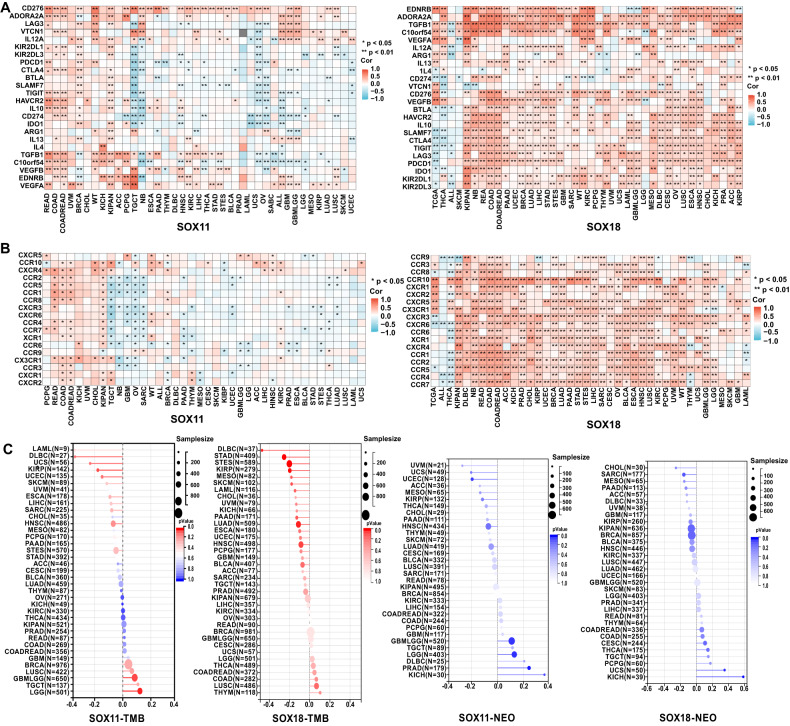
Based on the findings above, we infer that SOX11 and SOX18 have an intimate connection with tumor immunity. Subsequently, we examined the co-expression of these two genes in immune checkpoints associated with cancer (Fig. 7A) and analyzed their correlation with chemokine and receptor (Fig. 7B), tumor mutation burden (TMB), and Neoantigen (NEO) (Fig. 7C). As shown in the figures, SOX18 exhibits a strong positive correlation with several immune checkpoints, including TNFRSF4, CX3CL1, ENTPD1, and SELP, as well as chemokines present in tumors such as EDNRB, ADORA2A, TGFB1, CCL2, CXCL12. Moreover, SOX11 and SOX18 are closely associated with tumor TMB and NEO, which is of significant importance in predicting immune efficacy and patient prognosis, providing new treatment strategies for personalized immunotherapy. These findings offer a new perspective for a deeper understanding of tumor immunology and hold promise for further opportunities in the research and treatment of tumor-associated antigens.
Fig. 7. Pan-cancer-expression, immune checkpoints association, TMB and NEO of SOX TFs in tumors.
Correlation between the expression of SOX TFs and that of immune-related genes in cancers. The pan-cancer dataset (TCGA TARGET GTEx, PANCAN, N = 19131, G = 60499) is downloaded from the UCSC database (https://xenabrowser.net/). After filtering out all normal samples and samples with zero expression levels, we performed log2(x + 1) transformation on each expression value and calculated the correlation between SOX family and the above indicators. We extracted the expression data of (A) immune checkpoint pathway genes (Inhibitory [24]); (B) Chemokine and their receptor genes (Chemokine [41], Receptor [18]) in each sample. Besides, the Simple Nucleotide Variation dataset is acquired from GDC (https://portal.gdc.cancer.gov/). The (C) tumor mutational burden (TMB) for each cancer is calculated using the R package maftools. Neoantigen data is obtained from previous studies [202]. Red indicates positive correlation and blue indicates negative correlation. The darker the color, the stronger the correlation. ACC adrenocortical carcinoma, BLCA bladder urothelial carcinoma, BRCA breast invasive carcinoma, CESC cervical squamous cell carcinoma and endocervical adenocarcinoma, CHOL cholangiocarcinoma, COAD colon adenocarcinoma, COADREAD colon adenocarcinoma/rectum adenocarcinoma, DLBC lymphoid neoplasm diffuse large B-cell lymphoma, ESCA esophageal carcinoma, FPPP FFPE Pilot Phase II, GBM glioblastoma multiforme, GBMLGG glioma, HNSC head and neck squamous cell carcinoma, KICH kidney chromophobe, KIPAN pan-kidney cohort (KICH + KIRC + KIRP), KIRC kidney renal clear cell carcinoma, KIRP kidney renal papillary cell carcinoma, LAML acute myeloid leukemia, LGG brain lower grade glioma, LIHC liver hepatocellular carcinoma, LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, MESO mesothelioma, OV ovarian serous cystadenocarcinoma, PAAD pancreatic adenocarcinoma, PCPG pheochromocytoma and paraganglioma, PRAD prostate adenocarcinoma, READ rectum adenocarcinoma, SARC sarcoma, STAD stomach adenocarcinoma, SKCM skin cutaneous melanoma, STES stomach and esophageal carcinoma, TGCT testicular germ cell tumors, THCA thyroid carcinoma, THYM thymoma, UCEC uterine corpus endometrial carcinoma, UCS uterine carcinosarcoma, UVM uveal melanoma, OS osteosarcoma, ALL acute lymphoblastic leukemia, NB neuroblastoma, WT high-risk Wilms tumor.
In summary, the SOX family significantly contributes to the tumor immune microenvironment by interacting with various components that jointly mediate immune evasion in tumors and serve as a potential prognosis biomarker in tumor immunotherapy.
SOX and tumor treatment
Despite years of research and drug development, many patients are still deprived of suitable cancer treatment options. The roles and mechanisms of SOX TFs in tumors have garnered increasing attention, stimulating research into therapeutic strategies targeting SOX proteins. As transcription factors, the SOX family has long been considered undruggable due to the absence of binding sites for small molecule drugs that target these proteins [169]. Thus, the primary methods currently involve developing drugs that interfere with upstream and downstream of SOX TFs, designing peptides derived from SOX and stimulating immune responses (Table 2).
Table 2.
SOX-targeted therapeutics.
| Compounds | Target | Biomarker | Cancer | Mechanism | Reference |
|---|---|---|---|---|---|
| BAY-11-7082 | CXCL1 | SOX4 | Breast cancer | Inhibits the EMT process via blocking NF-κB pathway | [146] |
| Rapamycin | mTOR | SOX2, SOX9 | GBM | Inhibits the properties of GSCs | [177] |
| Gemcitabine | EGFR | SOX9 | PaC | Inhibits the metastasis of PaC via inhibiting EGFR/FOXA2/SOX9 axis | [179] |
| Luoteolin | PI3K/AKT | SOX2 | ESCC | Downregulates SOX2 through inhibition of the PI3K/AKT signaling pathway | [174] |
| Thiolutin | PSMD14 | SOX2 | HNSCC | Promotes chemosensitivity via inhibiting E2F1/AKT/SOX2 axis | [175] |
| P42 | SOX2 | SOX2 | ESCC | Interacts with SOX2 via a constrained peptide expression cassette | [180] |
| ZF-ATFs | SOX2 | SOX2 | / | Binds to the promoter of SOX2 with a transcriptional repressor SKD domain. | [181] |
| Peptide | S9pep | / | Colon cancer | Mimics SOX9 tumor-suppressive properties and inhibit colon cancer cell growth | [182] |
| S6pep | / | GBM | Induces SOX6 peptide-specific cytotoxic T lymphocytes, lyse GSCs derived from GBM. | [183] | |
| S11pep | / | Glioma | Generates SOX11-specific CD8 + T cells | [184] |
EGFR epidermal growth factor receptor, ESCC esophageal squamous cell carcinoma, GBM glioblastoma, GSC glioma stem cell, HNSCC head and neck squamous cell carcinoma, PaC pancreatic cancer, SOX sex determining region Y (SRY)- HMG box.
SOX TFs as biomarkers
Since SOX TFs have a tight connection with the development, drug resistance and prognosis of tumors, SOX TFs have been extensively studied as potential prognostic biomarkers. Although there are established biomarkers for stem cells in HCC, Ruzinova et al. conducted a comparison of the prognostic value of positive SOX9 immunohistochemistry with other markers, such as EpCAM and K19 in a large cohort of North American patients (n = 216). The findings indicate that SOX9 outperforms K19 and EpCAM as a stem cell marker in predicting the prognosis for HCC [170]. SOX10 is an immunochemical biomarker with moderate sensitivity and high specificity for the basal-like intrinsic subtype of breast cancer, which is independent of hormone receptor status [171]. SOX11, cooperated with CD23 and CD200, is considered the best-characterized biomarker in the leukemic variant MCL [172]. Moreover, with high sensitivity and specificity, SOX17 is overexpressed in most ovarian and endometrial cancers, making it a proper diagnostic biomarker [173].
Targeting SOX TFs indirectly
SOX TFs are involved in various processes in tumorigenesis, such as EMT, stemness, proliferation and apoptosis, invasion and metastasis, as mentioned before. Targeting the upstream and downstream signaling pathways of SOX can inhibit these processes and, in turn, improve patient prognosis. For instance, Luoteolin, a kind of natural flavonoid, attenuates cancer cell stemness in paclitaxel-resistant esophageal cancer cells, which is achieved by downregulating SOX2 through inhibition of the PI3K/AKT signaling pathway [174]. In HNSCC, PSMD14 promotes chemoresistance via enforcing the E2F1/AKT/SOX2 axis. Therefore, Thiolutin, a PSMD14 inhibitor, robustly enhances chemosensitivity and improves patient prognosis [175]. Polydatin, a monocrystalline compound obtained from Polygonum cuspidatum, impedes GBM invasion via inhibiting multiple components of the epidermal growth factor receptor (EGFR)-AKT/ERK1/2/STAT3-SOX2/SNAIL signaling pathway [176]. Besides, cyclopamine, an inhibitor of the SHH pathway, combined with the mTOR inhibitor rapamycin, reduces the expression of SOX2 and SOX9 in GBM cell lines and subsequently inhibits the properties of GSCs [177]. In addition, using BAY-11-7082, a NF-κB inhibitor, hampers the CXCL-induced NF-κB/SOX4 interaction, which inhibits the metastasis of breast cancer [146]. Inhibition of the protein lysine methyltransferase G9A suppresses the self-renewal of chronic myeloid leukemia (CML) by upregulating SOX6 [178]. Moreover, gemcitabine, an EGFR inhibitor, reduces the metastasis of pancreatic cancer via inhibiting the EGFR/FOXA2/SOX9 axis [179].
Targeting SOX TFs directly
Several methods are available to interfere with SOX TFs directly. Liu et al. designed a P42 peptide that can interact with SOX2 and inhibit its function without affecting its levels. The results showed that this peptide effectively inhibits tumor growth and metastasis without impacting normal esophageal epithelium [180]. Additionally, SOX2 is silenced by designing sequence-specific artificial transcription factors (ZF-ATFs) based on the ZF domain. The ZF-ATFs bind to the SOX2 promoter using a transcriptional SKD domain that represses their transcription [181]. With a minimum off-target effect and the easiness of delivery, ZF-ATFs would become a promising method for tumor treatment.
Peptides derived from SOX TFs
Due to the suppressive role of SOX families in certain tumors, peptides that replicate their tumor-suppressing function are developed. Blache et al. synthesized a short SOX9 peptide located at the hinge between the HMG DNA-binding domain and the SOX E central conserved domain. This peptide mimics the tumor-suppressive properties of SOX9 and has been shown to inhibit the Wnt/β-catenin signaling pathway, impeding CRC growth [182]. Moreover, SOX TFs are promising tumor-associated antigens that only overexpressed in tumor cells. Peptides derived from SOX TFs are developed as antigen peptide epitopes to stimulate T cell response. Ueda et al. synthesized a SOX6 peptide that induces specific cytotoxic T lymphocytes capable of lysing GSCs derived from GBM [183]. Recently, a peptide derived from the amino acid sequence of SOX11 has been developed to treat GBM. This FMACSPVAL peptide demonstrates the highest efficiency in generating SOX11-specific CD8+ T cells, making it a promising epitope for T cell-based immunotherapy in glioma [184].
Conclusion and prospects
Over the last three decades, the role of SOX transcription factors in various tumors has gradually been uncovered, including their involvement in metastasis, invasion, proliferation, apoptosis, EMT, stemness, and drug resistance. The Wnt/β-catenin, Notch, TGF-β, TWIST1, JAK/STAT, and NF-κB pathways are the main pathways that intimately interact with SOX TFs. However, most research has focused on a few members of the SOX family, such as SOX2, SOX4, and SOX9, while the characters of other SOX TFs in tumors require further research. Additionally, the research regarding SOX family and TIME is just getting started. Although we have identified some SOX family members closely associated with immunity, further experiments are needed to validate their roles and more detailed mechanisms in TIME. Moreover, despite TFs being undruggable, many drugs have been developed targeting these factors. However, relevant therapeutic drugs that have been put into clinical practice remain limited. Therefore, extensive research on SOX’s roles in tumors, particularly in TIME, has excellent prospects for advancing novel therapies.
Author contributions
LX, BZ and XC conceptualized this study. JJ, DH and MS drafted the manuscript. SL, YW, YW and XX revised the manuscript. ZZ, JZ and XL performed the bioinformatics analysis of SOX family. SW, SX and ZW helped perform the analysis with constructive discussions. LX and WH obtained funding. All authors read and approved the final manuscript.
Funding
Research was supported by grants from the National Natural Science Foundation of China No. 82372917 (WH), No. U23A20451 (LX), No. 82273310 (LX), No. 82173313 (WH), the Natural Science Foundation of Hubei Province 2022CFA016 (LX), and the Basic Research Support Program of Huazhong University of Science and Technology 2023BR038 (LX).
Data availability
The datasets generated and/or analysed during the current study are available in the UCSC database (https://xenabrowser.net/) and GDC database (https://portal.gdc.cancer.gov/).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Junqing Jiang, Yufei Wang, Mengyu Sun.
Contributor Information
Wenjie Huang, Email: huangwenjie@tjh.tjmu.edu.cn.
Limin Xia, Email: xialimin@tjh.tjmu.edu.cn.
References
- 1.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–77. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 2.Darnell JE. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–9. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 3.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–50. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 4.Xu YR, Yang WX. SOX-mediated molecular crosstalk during the progression of tumorigenesis. Semin Cell Dev Biol. 2017;63:23–34. doi: 10.1016/j.semcdb.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–55. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 6.Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006;20:3475–86. doi: 10.1101/gad.403406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato K, Bhattaram P, Penzo-Méndez A, Gadi A, Lefebvre V. SOXC transcription factors induce cartilage growth plate formation in mouse embryos by promoting noncanonical WNT signaling. J Bone Min Res. 2015;30:1560–71. doi: 10.1002/jbmr.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van De Wetering M, Oosterwegel M, Van Norren K, Clevers H. Sox-4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J. 1993;12:3847–54. doi: 10.1002/j.1460-2075.1993.tb06063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumitriu B, Patrick MR, Petschek JP, Cherukuri S, Klingmuller U, Fox PL, et al. Sox6 cell-autonomously stimulates erythroid cell survival, proliferation, and terminal maturation and is thereby an important enhancer of definitive erythropoiesis during mouse development. Blood. 2006;108:1198–207. doi: 10.1182/blood-2006-02-004184. [DOI] [PubMed] [Google Scholar]
- 10.Gandillet A, Serrano AG, Pearson S, Lie-A-Ling M, Lacaud G, Kouskoff V. Sox7-sustained expression alters the balance between proliferation and differentiation of hematopoietic progenitors at the onset of blood specification. Blood. 2009;114:4813–22. doi: 10.1182/blood-2009-06-226290. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima-Takagi Y, Osawa M, Oshima M, Takagi H, Miyagi S, Endoh M, et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121:447–58. doi: 10.1182/blood-2012-05-431403. [DOI] [PubMed] [Google Scholar]
- 12.Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–55. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28:583–8. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gomez Gaviro MV, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13:1181–9. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 15.Weider M, Wegner M. SoxE factors: transcriptional regulators of neural differentiation and nervous system development. Semin Cell Dev Biol. 2017;63:35–42. doi: 10.1016/j.semcdb.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998;18:60–4. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- 17.Schilham MW, Oosterwegel MA, Moerer P, Ya J, de Boer PAJ, van de Wetering M, et al. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature. 1996;380:711–4. doi: 10.1038/380711a0. [DOI] [PubMed] [Google Scholar]
- 18.Cohen-Barak O, Yi Z, Hagiwara N, Monzen K, Komuro I, Brilliant MH. Sox6 regulation of cardiac myocyte development. Nucleic Acids Res. 2003;31:5941–8. doi: 10.1093/nar/gkg807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang IKN, Graus MS, Kirschnick N, Davidson T, Luu W, Harwood R, et al. The blood vasculature instructs lymphatic patterning in a SOX7-dependent manner. EMBO J. 2023;42:e109032. doi: 10.15252/embj.2021109032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, et al. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci USA. 2004;101:6502–7. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Asakura M, Inoue H, Nakamura T, Sano M, Niu Z, et al. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:3859–64. doi: 10.1073/pnas.0609100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Hernández S, Gómez MJ, Sánchez-Cabo F, Méndez-Ferrer S, Muñoz-Cánoves P, Isern J. Sox17 controls emergence and remodeling of nestin-expressing coronary vessels. Circulation Res. 2020;127:e252–70. doi: 10.1161/CIRCRESAHA.120.317121. [DOI] [PubMed] [Google Scholar]
- 23.Cermenati S, Moleri S, Cimbro S, Corti P, Del Giacco L, Amodeo R, et al. Sox18 and Sox7 play redundant roles in vascular development. Blood. 2008;111:2657–66. doi: 10.1182/blood-2007-07-100412. [DOI] [PubMed] [Google Scholar]
- 24.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–21. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 25.Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, et al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun. 2018;9:5319. doi: 10.1038/s41467-018-07784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–20. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson MJ, Harley VR. Sex with two SOX on: SRY and SOX9 in testis development. Trends Endocrinol Metab. 2002;13:106–11. doi: 10.1016/s1043-2760(01)00541-0. [DOI] [PubMed] [Google Scholar]
- 28.Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife. 2017;6:e26575. doi: 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonçalves AN, Correia-Pinto J, Nogueira-Silva C. ROBO2 signaling in lung development regulates SOX2/SOX9 balance, branching morphogenesis and is dysregulated in nitrofen-induced congenital diaphragmatic hernia. Respir Res. 2020;21:302. doi: 10.1186/s12931-020-01568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Llora Batlle O, van den Ameele J, Thomas JC, He P, Lim K, et al. SOX9 maintains human foetal lung tip progenitor state by enhancing WNT and RTK signalling. EMBO J. 2022;41:e111338. doi: 10.15252/embj.2022111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, et al. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci USA. 2013;110:18042–51. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MAH, Kopp JL, et al. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci USA. 2013;110:E4456–4464. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Q, Ma Y, Dai X, Ren T, Fu Y, Liu W, et al. Regeneration of functional alveoli by adult human SOX9+ airway basal cell transplantation. Protein Cell. 2018;9:267–82. doi: 10.1007/s13238-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange AW, Keiser AR, Wells JM, Zorn AM, Whitsett JA. Sox17 promotes cell cycle progression and inhibits TGF-beta/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS One. 2009;4:e5711. doi: 10.1371/journal.pone.0005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, et al. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med. 2019;7:227–38. doi: 10.1016/S2213-2600(18)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitsillides AA, Beier F. Keep your Sox on, chondrocytes! Nat Rev Rheumatol. 2021;17:383–4. doi: 10.1038/s41584-021-00628-9. [DOI] [PubMed] [Google Scholar]
- 37.van Gastel N, Stegen S, Eelen G, Schoors S, Carlier A, Daniëls VW, et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020;579:111–7. doi: 10.1038/s41586-020-2050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, et al. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–90. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 39.Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Dev (Camb, Engl) 2013;140:4129–44. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- 40.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25:139–51. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han S, Ren Y, He W, Liu H, Zhi Z, Zhu X, et al. ERK-mediated phosphorylation regulates SOX10 sumoylation and targets expression in mutant BRAF melanoma. Nat Commun. 2018;9:28. doi: 10.1038/s41467-017-02354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji J, Yu Y, Li ZL, Chen MY, Deng R, Huang X, et al. XIAP limits autophagic degradation of Sox2 and is a therapeutic target in nasopharyngeal carcinoma stem cells. Theranostics. 2018;8:1494–510. doi: 10.7150/thno.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang KC, Hertz J, Zhang X, Jin XL, Shaw P, Derosa BA, et al. Novel regulatory mechanisms for the SoxC transcriptional network required for visual pathway development. J Neurosci. 2017;37:4967–81. doi: 10.1523/JNEUROSCI.3430-13.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang L, Zhang L, Wei W, Jin X, Wang P, Tong Y, et al. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol Cell. 2014;55:537–51. doi: 10.1016/j.molcel.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Egunsola AT, Bae Y, Jiang MM, Liu DS, Chen-Evenson Y, Bertin T, et al. Loss of DDRGK1 modulates SOX9 ubiquitination in spondyloepimetaphyseal dysplasia. J Clin Invest. 2017;127:1475–84. doi: 10.1172/JCI90193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao HY, Zhang YJ, Dai H, Zhang Y, Shen YF. CARM1 mediates modulation of Sox2. PLoS One. 2011;6:e27026. doi: 10.1371/journal.pone.0027026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baltus GA, Kowalski MP, Zhai H, Tutter AV, Quinn D, Wall D, et al. Acetylation of sox2 induces its nuclear export in embryonic stem cells. Stem Cells. 2009;27:2175–84. doi: 10.1002/stem.168. [DOI] [PubMed] [Google Scholar]
- 49.Lundblad JR, Kwok RP, Laurance ME, Harter ML, Goodman RH. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–8. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 50.Nowling T, Bernadt C, Johnson L, Desler M, Rizzino A. The co-activator p300 associates physically with and can mediate the action of the distal enhancer of the FGF-4 gene. J Biol Chem. 2003;278:13696–705. doi: 10.1074/jbc.M207567200. [DOI] [PubMed] [Google Scholar]
- 51.Inoue H, Takahashi H, Hashimura M, Eshima K, Akiya M, Matsumoto T, et al. Cooperation of Sox4 with β-catenin/p300 complex in transcriptional regulation of the Slug gene during divergent sarcomatous differentiation in uterine carcinosarcoma. BMC Cancer. 2016;16:53. doi: 10.1186/s12885-016-2090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou T, et al. Induction of SOX4 by DNA damage is critical for p53 stabilization and function. Proc Natl Acad Sci USA. 2009;106:3788–93. doi: 10.1073/pnas.0810147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pietrobono S, Anichini G, Sala C, Manetti F, Almada LL, Pepe S, et al. ST3GAL1 is a target of the SOX2-GLI1 transcriptional complex and promotes melanoma metastasis through AXL. Nat Commun. 2020;11:5865. doi: 10.1038/s41467-020-19575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koumangoye RB, Andl T, Taubenslag KJ, Zilberman ST, Taylor CJ, Loomans HA, et al. SOX4 interacts with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal cancer cells. Mol Cancer. 2015;14:24. doi: 10.1186/s12943-014-0284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T, et al. SOX6 suppresses cyclin D1 promoter activity by interacting with beta-catenin and histone deacetylase 1, and its down-regulation induces pancreatic beta-cell proliferation. J Biol Chem. 2007;282:19052–61. doi: 10.1074/jbc.M700460200. [DOI] [PubMed] [Google Scholar]
- 56.Jiang W, Yuan Q, Jiang Y, Huang L, Chen C, Hu G, et al. Identification of Sox6 as a regulator of pancreatic cancer development. J Cell Mol Med. 2018;22:1864–72. doi: 10.1111/jcmm.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S, Xia P, Ye B, Huang G, Liu J, Fan Z. Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell. 2013;13:617–25. doi: 10.1016/j.stem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19:106. doi: 10.1186/s12943-020-01220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gustavsson E, Sernbo S, Andersson E, Brennan DJ, Dictor M, Jerkeman M, et al. SOX11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Mol Cancer. 2010;9:187. doi: 10.1186/1476-4598-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han F, Liu W, Jiang X, Shi X, Yin L, Ao L, et al. SOX30, a novel epigenetic silenced tumor suppressor, promotes tumor cell apoptosis by transcriptional activating p53 in lung cancer. Oncogene. 2015;34:4391–402. doi: 10.1038/onc.2014.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li JY, Han C, Zheng LL, Guo MZ. Epigenetic regulation of Wnt signaling pathway gene SRY-related HMG-box 17 in papillary thyroid carcinoma. Chin Med J. 2012;125:3526. [PubMed] [Google Scholar]
- 62.Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–51. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han T, Zheng H, Zhang J, Yang P, Li H, Cheng Z, et al. Downregulation of MUC15 by miR-183-5p.1 promotes liver tumor-initiating cells properties and tumorigenesis via regulating c-MET/PI3K/AKT/SOX2 axis. Cell Death Dis. 2022;13:200. doi: 10.1038/s41419-022-04652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Q, Sun Z, Yang F, Mao T, Gao Y, Wang H. SOX30, a target gene of miR-653-5p, represses the proliferation and invasion of prostate cancer cells through inhibition of Wnt/β-catenin signaling. Cell Mol Biol Lett. 2019;24:71. doi: 10.1186/s11658-019-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao Y, Zhang N, Zeng Z, Wu Q, Jiang X, Li S, et al. LncRNA PCAT1 activates SOX2 and suppresses radioimmune responses via regulating cGAS/STING signalling in non-small cell lung cancer. Clin Transl Med. 2022;12:e792. doi: 10.1002/ctm2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Jiang X, Xu Y, Kang P, Huang P, Meng N, et al. YY1-induced DLEU1/miR-149-5p promotes malignant biological behavior of cholangiocarcinoma through upregulating YAP1/TEAD2/SOX2. Int J Biol Sci. 2022;18:4301–15. doi: 10.7150/ijbs.66224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Q, Kong F, Cong R, Ma J, Wang C, Ma X. PVT1/miR-136/Sox2/UPF1 axis regulates the malignant phenotypes of endometrial cancer stem cells. Cell Death Dis. 2023;14:177. doi: 10.1038/s41419-023-05651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W, Wang L, Li X, Zhao C, Shi L, Zhao H, et al. LncRNA SNHG17 regulates cell proliferation and invasion by targeting miR-338-3p/SOX4 axis in esophageal squamous cell carcinoma. Cell Death Dis. 2021;12:806. doi: 10.1038/s41419-021-04093-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng Y, Xu Y, Gao Y, Chen Y, Wang X, Chen Z. A novel lncRNA SOX2OT promotes the malignancy of human colorectal cancer by interacting with miR-194-5p/SOX5 axis. Cell Death Dis. 2021;12:499. doi: 10.1038/s41419-021-03756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou T, Wu L, Ma N, Tang F, Yu Z, Jiang Z, et al. SOX9-activated FARSA-AS1 predetermines cell growth, stemness, and metastasis in colorectal cancer through upregulating FARSA and SOX9. Cell Death Dis. 2020;11:1071. doi: 10.1038/s41419-020-03273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ni Q, Zhang H, Shi X, Li X. Exosomal lncRNA HCG18 contributes to cholangiocarcinoma growth and metastasis through mediating miR-424-5p/SOX9 axis through PI3K/AKT pathway. Cancer Gene Ther. 2023;30:582–95. doi: 10.1038/s41417-022-00500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin M, Meng Y, Luo C, He S, Qin F, Yin Y, et al. lncRNA PRR34-AS1 promotes HCC development via modulating Wnt/β-catenin pathway by absorbing miR-296-5p and upregulating E2F2 and SOX12. Mol Ther Nucleic Acids. 2021;25:37–52. doi: 10.1016/j.omtn.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qi G, Li L. Long non-coding RNA PVT1 contributes to cell growth and metastasis in non-small-cell lung cancer by regulating miR-361-3p/SOX9 axis and activating Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2020;126:110100. doi: 10.1016/j.biopha.2020.110100. [DOI] [PubMed] [Google Scholar]
- 74.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 75.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30:764–76. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013;336:379–89. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Yang Z, Liu W, Zhu W, Yin L, Han Z, et al. Disheveled3 enhanced EMT and cancer stem-like cells properties via Wnt/β-catenin/c-Myc/SOX2 pathway in colorectal cancer. J Transl Med. 2023;21:302. doi: 10.1186/s12967-023-04120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–83. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Li L, Liu J, Xue H, Li C, Liu Q, Zhou Y, et al. A TGF-β-MTA1-SOX4-EZH2 signaling axis drives epithelial–mesenchymal transition in tumor metastasis. Oncogene. 2020;39:2125–39. doi: 10.1038/s41388-019-1132-8. [DOI] [PubMed] [Google Scholar]
- 80.Vervoort SJ, Lourenço AR, Tufegdzic Vidakovic A, Mocholi E, Sandoval JL, Rueda OM, et al. SOX4 can redirect TGF-β-mediated SMAD3-transcriptional output in a context-dependent manner to promote tumorigenesis. Nucleic Acids Res. 2018;46:9578–90. doi: 10.1093/nar/gky755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu J, Tian J, Zhu S, Sun L, Yu J, Tian H, et al. Sox5 contributes to prostate cancer metastasis and is a master regulator of TGF-β-induced epithelial mesenchymal transition through controlling Twist1 expression. Br J cancer. 2018;118:88–97. doi: 10.1038/bjc.2017.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong TLM, Wong TL, Zhou L, Man K, Purcell J, Lee TK, et al. Protein Tyrosine Kinase 7 (PTK7) Promotes Metastasis in Hepatocellular Carcinoma via SOX9 Regulation and TGF-β Signaling. Cell Mol Gastroenter. 2023;15:13–37. doi: 10.1016/j.jcmgh.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu M, Chen D, Shen C, Shen J, Zhao H, He Y. Sex-determining region Y-box protein 3 induces epithelial-mesenchymal transition in osteosarcoma cells via transcriptional activation of Snail1. J Exp Clin Cancer Res. 2017;36:46. doi: 10.1186/s13046-017-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng H, Zhang Y, Zhou Z, Guo Y, Huang X, Westover KD, et al. Intergrated analysis of ELMO1, serves as a link between tumour mutation burden and epithelial-mesenchymal transition in hepatocellular carcinoma. Ebiomedicine. 2019;46:105–18. doi: 10.1016/j.ebiom.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du F, Li X, Feng W, Qiao C, Chen J, Jiang M, et al. SOX13 promotes colorectal cancer metastasis by transactivating SNAI2 and c-MET. Oncogene. 2020;39:3522–40. doi: 10.1038/s41388-020-1233-4. [DOI] [PubMed] [Google Scholar]
- 86.Inoue H, Hashimura M, Akiya M, Chiba R, Saegusa M. Functional role of ALK-related signal cascades on modulation of epithelial-mesenchymal transition and apoptosis in uterine carcinosarcoma. Mol Cancer. 2017;16:37. doi: 10.1186/s12943-017-0609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang W, Chen Z, Shang X, Tian D, Wang D, Wu K, et al. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. 2015;61:1920–33. doi: 10.1002/hep.27756. [DOI] [PubMed] [Google Scholar]
- 88.Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. 2021;6:153. doi: 10.1038/s41392-021-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y, Qian B, Sun X, Kang Z, Huang Z, Ding Z, et al. Sox9/INHBB axis-mediated crosstalk between the hepatoma and hepatic stellate cells promotes the metastasis of hepatocellular carcinoma. Cancer Lett. 2021;499:243–54. doi: 10.1016/j.canlet.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 90.Wang G, Lunardi A, Zhang J, Chen Z, Ala U, Webster KA, et al. Zbtb7a suppresses prostate cancer through repression of a Sox9-dependent pathway for cellular senescence bypass and tumor invasion. Nat Genet. 2013;45:739–46. doi: 10.1038/ng.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Du F, Feng W, Chen S, Wu S, Cao T, Yuan T, et al. Sex determining region Y-box 12 (SOX12) promotes gastric cancer metastasis by upregulating MMP7 and IGF1. Cancer Lett. 2019;452:103–18. doi: 10.1016/j.canlet.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 92.Chen J, Dang Y, Feng W, Qiao C, Liu D, Zhang T, et al. SOX18 promotes gastric cancer metastasis through transactivating MCAM and CCL7. Oncogene. 2020;39:5536–52. doi: 10.1038/s41388-020-1378-1. [DOI] [PubMed] [Google Scholar]
- 93.Chen J, Du F, Dang Y, Li X, Qian M, Feng W, et al. Fibroblast growth factor 19-mediated up-regulation of SYR-related high-mobility group box 18 promotes hepatocellular carcinoma metastasis by transactivating fibroblast growth factor receptor 4 and Fms-related tyrosine kinase 4. Hepatology. 2020;71:1712–31. doi: 10.1002/hep.30951. [DOI] [PubMed] [Google Scholar]
- 94.Njouendou AJ, Szarvas T, Tiofack AAZ, Kenfack RN, Tonouo PD, Ananga SN, et al. SOX2 dosage sustains tumor-promoting inflammation to drive disease aggressiveness by modulating the FOSL2/IL6 axis. Mol Cancer. 2023;22:52. doi: 10.1186/s12943-023-01734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsai CN, Yu SC, Lee CW, Pang JS, Wu CH, Lin SE, et al. SOX4 activates CXCL12 in hepatocellular carcinoma cells to modulate endothelial cell migration and angiogenesis in vivo. Oncogene. 2020;39:4695–710. doi: 10.1038/s41388-020-1319-z. [DOI] [PubMed] [Google Scholar]
- 96.Du F, Chen J, Liu H, Cai Y, Cao T, Han W, et al. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019;10:239. doi: 10.1038/s41419-019-1481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren C, Ren T, Yang K, Wang S, Bao X, Zhang F, et al. Inhibition of SOX2 induces cell apoptosis and G1/S arrest in Ewing’s sarcoma through the PI3K/Akt pathway. J Exp Clin Cancer Res. 2016;35:44. doi: 10.1186/s13046-016-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang J, Tian Z, Liao X, Wu G. SOX13/TRIM11/YAP axis promotes the proliferation, migration and chemoresistance of anaplastic thyroid cancer. Int J Biol Sci. 2021;17:417–29. doi: 10.7150/ijbs.54194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao L, Li X, Cao P, Fei W, Zhou H, Tang N, et al. Interleukin-6 mediated inflammasome activation promotes oral squamous cell carcinoma progression via JAK2/STAT3/Sox4/NLRP3 signaling pathway. J Exp Clin Cancer Res. 2022;41:166. doi: 10.1186/s13046-022-02376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaves-Moreira, Mitchell MA D, Arruza C, Rawat P, Sidoli S, Nameki R, et al. The transcription factor PAX8 promotes angiogenesis in ovarian cancer through interaction with SOX17. Sci Signal. 2022;15:eabm2496. doi: 10.1126/scisignal.abm2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–34. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17:183–93. doi: 10.1038/nrm.2016.8. [DOI] [PubMed] [Google Scholar]
- 103.Wang P, Zhao L, Gong S, Xiong S, Wang J, Zou D, et al. HIF1α/HIF2α-Sox2/Klf4 promotes the malignant progression of glioblastoma via the EGFR-PI3K/AKT signalling pathway with positive feedback under hypoxia. Cell Death Dis. 2021;12:312. doi: 10.1038/s41419-021-03598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang X, Zhang S, Tang J, Tian T, Pan Y, Wu L, et al. A Self-Propagating c-Met-SOX2 Axis Drives Cancer-Derived IgG Signaling That Promotes Lung Cancer Cell Stemness. Cancer Res. 2023;83:1866–82. doi: 10.1158/0008-5472.CAN-22-2733. [DOI] [PubMed] [Google Scholar]
- 105.Jiang X, Zhang Y, Yuan Y, Jin Z, Zhai H, Liu B, et al. LncRNA GSCAR promotes glioma stem cell maintenance via stabilizing SOX2 expression. Int J Biol Sci. 2023;19:1681–97. doi: 10.7150/ijbs.80873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Praharaj PP, Patra S, Mishra SR, Mukhopadhyay S, Klionsky DJ, Patil S, et al. CLU (clusterin) promotes mitophagic degradation of MSX2 through an AKT-DNM1L/Drp1 axis to maintain SOX2-mediated stemness in oral cancer stem cells. Autophagy. 2023;19:1–21. [DOI] [PMC free article] [PubMed]
- 107.Han W, Hu P, Wu F, Wang S, Hu Y, Li S, et al. FHL3 links cell growth and self-renewal by modulating SOX4 in glioma. Cell Death Differ. 2019;26:796–811. doi: 10.1038/s41418-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C, Liu L, Chen X, Cheng J, Zhang H, Shen J, et al. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology. 2016;64:117–29. doi: 10.1002/hep.28509. [DOI] [PubMed] [Google Scholar]
- 109.Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74:4170–82. doi: 10.1158/0008-5472.CAN-13-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liang X, Duronio GN, Yang Y, Bala P, Hebbar P, Spisak S, et al. An enhancer-driven stem cell-like program mediated by SOX9 blocks intestinal differentiation in colorectal cancer. Gastroenterology. 2022;162:209–22. doi: 10.1053/j.gastro.2021.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma XL, Hu B, Tang WG, Xie SH, Ren N, Guo L, et al. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13:11. doi: 10.1186/s13045-020-0845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Q, Weng K, Lin M, Jiang M, Fang Y, Chung SSW, et al. SOX9 modulates the transformation of gastric stem cells through biased symmetric cell division. Gastroenterology. 2023;164:1119–1136.e12. doi: 10.1053/j.gastro.2023.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang YP, Chien Y, Chiou GY, Cherng JY, Wang ML, Lo WL, et al. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 2012;33:1462–76. doi: 10.1016/j.biomaterials.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 114.Sun R, Jiang B, Qi H, Zhang X, Yang J, Duan J, et al. SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis. 2015;6:e1990. doi: 10.1038/cddis.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang M, Wang Z, Zhi X, Ding W, Xiong J, Tao T, et al. SOX9 enhances sorafenib resistance through upregulating ABCG2 expression in hepatocellular carcinoma. Biomed Pharmacother. 2020;129:110315. doi: 10.1016/j.biopha.2020.110315. [DOI] [PubMed] [Google Scholar]
- 116.Williams A, Gutgesell L, de Wet L, Selman P, Dey A, Avineni M, et al. SOX2 expression in prostate cancer drives resistance to nuclear hormone receptor signaling inhibition through the WEE1/CDK1 signaling axis. Cancer Lett. 2023;565:216209. doi: 10.1016/j.canlet.2023.216209. [DOI] [PubMed] [Google Scholar]
- 117.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–8. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li F, Chen S, Yu J, Gao Z, Sun Z, Yi Y, et al. Interplay of m6 A and histone modifications contributes to temozolomide resistance in glioblastoma. Clin Transl Med. 2021;11:e553. doi: 10.1002/ctm2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun Y, Hu L, Tao Z, Jarugumilli GK, Erb H, Singh A, et al. Pharmacological blockade of TEAD-YAP reveals its therapeutic limitation in cancer cells. Nat Commun. 2022;13:6744. doi: 10.1038/s41467-022-34559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun H, Wang H, Wang X, Aoki Y, Wang X, Yang Y, et al. Aurora-A/SOX8/FOXK1 signaling axis promotes chemoresistance via suppression of cell senescence and induction of glucose metabolism in ovarian cancer organoids and cells. Theranostics. 2020;10:6928–45. doi: 10.7150/thno.43811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xue Y, Lian W, Zhi J, Yang W, Li Q, Guo X, et al. HDAC5-mediated deacetylation and nuclear localisation of SOX9 is critical for tamoxifen resistance in breast cancer. Brit J Cancer. 2019;121:1039–49. doi: 10.1038/s41416-019-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-β tumor suppression through a lethal EMT. Cell. 2016;164:1015–30. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Man CH, Fung TK, Wan H, Cher CY, Fan A, Ng N, et al. Suppression of SOX7 by DNA methylation and its tumor suppressor function in acute myeloid leukemia. Blood. 2015;125:3928–36. doi: 10.1182/blood-2014-06-580993. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Huang S, Dong W, Li L, Feng Y, Pan L, et al. SOX7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 2009;277:29–37. doi: 10.1016/j.canlet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 125.Zhang D, Guo S, Wang H, Hu Y. SOX15 exerts antitumor function in glioma by inhibiting cell proliferation and invasion via downregulation of Wnt/β-catenin signaling. Life Sci. 2020;255:117792. doi: 10.1016/j.lfs.2020.117792. [DOI] [PubMed] [Google Scholar]
- 126.Wang J, Ding S, Duan Z, Xie Q, Zhang T, Zhang X, et al. Role of p14ARF-HDM2-p53 axis in SOX6-mediated tumor suppression. Oncogene. 2016;35:1692–702. doi: 10.1038/onc.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kurtsdotter I, Topcic D, Karlén A, Singla B, Hagey DW, Bergsland M, et al. SOX5/6/21 prevent oncogene-driven transformation of brain stem cells. Cancer Res. 2017;77:4985–97. doi: 10.1158/0008-5472.CAN-17-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun QY, Ding LW, Johnson K, Zhou S, Tyner JW, Yang H, et al. SOX7 regulates MAPK/ERK-BIM mediated apoptosis in cancer cells. Oncogene. 2019;38:6196–210. doi: 10.1038/s41388-019-0865-8. [DOI] [PubMed] [Google Scholar]
- 129.Gao Y, He XY, Wu XS, Huang YH, Toneyan S, Ha T, et al. ETV6 dependency in Ewing sarcoma by antagonism of EWS-FLI1-mediated enhancer activation. Nat Cell Biol. 2023;25:298–308. doi: 10.1038/s41556-022-01060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ding Y, Feng Y, Huang Z, Zhang Y, Li X, Liu R, et al. SOX15 transcriptionally increases the function of AOC1 to modulate ferroptosis and progression in prostate cancer. Cell Death Dis. 2022;13:673. doi: 10.1038/s41419-022-05108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caglayan D, Lundin E, Kastemar M, Westermark B, Ferletta M. Sox21 inhibits glioma progression in vivo by forming complexes with Sox2 and stimulating aberrant differentiation. Int J Cancer. 2013;133:1345–56. doi: 10.1002/ijc.28147. [DOI] [PubMed] [Google Scholar]
- 132.Tsao CM, Yan MD, Shih YL, Yu PN, Kuo CC, Lin WC, et al. SOX1 functions as a tumor suppressor by antagonizing the WNT/β-catenin signaling pathway in hepatocellular carcinoma. Hepatol (Baltim, Md) 2012;56:2277–87. doi: 10.1002/hep.25933. [DOI] [PubMed] [Google Scholar]
- 133.Guan Z, Zhang J, Wang J, Wang H, Zheng F, Peng J, et al. SOX1 down-regulates β-catenin and reverses malignant phenotype in nasopharyngeal carcinoma. Mol Cancer. 2014;13:257. doi: 10.1186/1476-4598-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yw L, Cm T, Pn Y, Yl S, Ch L, Md Y. SOX1 suppresses cell growth and invasion in cervical cancer. Gynecologic Oncol. 2013;131:174–81. doi: 10.1016/j.ygyno.2013.07.111. [DOI] [PubMed] [Google Scholar]
- 135.Hao X, Han F, Ma B, Zhang N, Chen H, Jiang X, et al. SOX30 is a key regulator of desmosomal gene suppressing tumor growth and metastasis in lung adenocarcinoma. J Exp Clin Cancer Res. 2018;37:111. doi: 10.1186/s13046-018-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han F, Liu WB, Shi XY, Yang JT, Zhang X, Li ZM, et al. SOX30 inhibits tumor metastasis through attenuating wnt-signaling via transcriptional and posttranslational regulation of β-catenin in lung cancer. Ebiomedicine. 2018;31:253–66. doi: 10.1016/j.ebiom.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang S, Tie J, Wang R, Hu F, Gao L, Wang W, et al. SOX2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating PTEN. Cancer Lett. 2015;358:210–9. doi: 10.1016/j.canlet.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 138.Lin X, Li G, Yan X, Fu W, Ruan J, Ding H, et al. Long non-coding RNA BC002811 promotes gastric cancer metastasis by regulating SOX2 binding to the PTEN promoter. Int J Biol Sci. 2023;19:967–80. doi: 10.7150/ijbs.76407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pansy K, Uhl B, Krstic J, Szmyra M, Fechter K, Santiso A, et al. Immune regulatory processes of the tumor microenvironment under malignant conditions. Int J Mol Sci. 2021;22:13311. doi: 10.3390/ijms222413311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-β and suppresses T(H)2 differentiation. Nat Immunol. 2012;13:778–86. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–3. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 142.Gerner MC, Ziegler LS, Schmidt RLJ, Krenn M, Zimprich F, Uyanik-Ünal K, et al. The TGF-b/SOX4 axis and ROS-driven autophagy co-mediate CD39 expression in regulatory T-cells. FASEB J. 2020;34:8367–84. doi: 10.1096/fj.201902664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanaka S, Suto A, Iwamoto T, Kageyama T, Tamachi T, Takatori H, et al. Sox12 promotes T reg differentiation in the periphery during colitis. J Exp Med. 2018;215:2509–19. doi: 10.1084/jem.20172082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mallampati S, Sun B, Lu Y, Ma H, Gong Y, Wang D, et al. Integrated genetic approaches identify the molecular mechanisms of Sox4 in early B-cell development: intricate roles for RAG1/2 and CK1ε. Blood. 2014;123:4064–76. doi: 10.1182/blood-2013-12-543801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mou W, Xu Y, Ye Y, Chen S, Li X, Gong K, et al. Expression of Sox2 in breast cancer cells promotes the recruitment of M2 macrophages to tumor microenvironment. Cancer Lett. 2015;358:115–23. doi: 10.1016/j.canlet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 146.Wang N, Liu W, Zheng Y, Wang S, Yang B, Li M, et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018;9:880. doi: 10.1038/s41419-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma T, Hu C, Lal B, Zhou W, Ma Y, Ying M, et al. Reprogramming transcription factors Oct4 and Sox2 induce a BRD-dependent immunosuppressive transcriptome in GBM-propagating cells. Cancer Res. 2021;81:2457–69. doi: 10.1158/0008-5472.CAN-20-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gomez KE, Wu F, Keysar SB, Morton JJ, Miller B, Chimed TS, et al. Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Cancer Res. 2020;80:4185–98. doi: 10.1158/0008-5472.CAN-20-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kang Y, Lv R, Feng Z, Zhu J. Tumor-associated macrophages improve hypoxia-induced endoplasmic reticulum stress response in colorectal cancer cells by regulating TGF-β1/SOX4. Cell Signal. 2022;99:110430. doi: 10.1016/j.cellsig.2022.110430. [DOI] [PubMed] [Google Scholar]
- 150.Yang Y, Andersson P, Hosaka K, Zhang Y, Cao R, Iwamoto H, et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat Commun. 2016;7:11385. doi: 10.1038/ncomms11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mollaoglu G, Jones A, Wait SJ, Mukhopadhyay A, Jeong S, Arya R, et al. The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity. 2018;49:764–779.e9. doi: 10.1016/j.immuni.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75:1271–83. doi: 10.1016/j.jhep.2021.07.032. [DOI] [PubMed] [Google Scholar]
- 153.Xu Y, Dong X, Qi P, Ye Y, Shen W, Leng L, et al. Sox2 communicates with Tregs through CCL1 to promote the stemness property of breast cancer cells. Stem Cells. 2017;35:2351–65. doi: 10.1002/stem.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim IK, Kim K, Lee E, Oh DS, Park CS, Park S, et al. Sox7 promotes high-grade glioma by increasing VEGFR2-mediated vascular abnormality. J Exp Med. 2018;215:963–83. doi: 10.1084/jem.20170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xiao G, Wang K, Wang Z, Dai Z, Liang X, Ye W, et al. Machine learning-based identification of SOX10 as an immune regulator of macrophage in gliomas. Front Immunol. 2022;13:1007461. doi: 10.3389/fimmu.2022.1007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balsas P, Veloza L, Clot G, Sureda-Gómez M, Rodríguez ML, Masaoutis C, et al. SOX11, CD70, and Treg cells configure the tumor-immune microenvironment of aggressive mantle cell lymphoma. Blood. 2021;138:2202–15. doi: 10.1182/blood.2020010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tan YS, Sansanaphongpricha K, Xie Y, Donnelly CR, Luo X, Heath BR, et al. Mitigating SOX2-potentiated immune escape of head and neck squamous cell carcinoma with a STING-inducing nanosatellite vaccine. Clin Cancer Res. 2018;24:4242–55. doi: 10.1158/1078-0432.CCR-17-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bagati A, Kumar S, Jiang P, Pyrdol J, Zou AE, Godicelj A, et al. Integrin αvβ6-TGFβ-SOX4 pathway drives immune evasion in triple-negative breast cancer. Cancer cell. 2021;39:54–67.e9. doi: 10.1016/j.ccell.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Good CR, Aznar MA, Kuramitsu S, Samareh P, Agarwal S, Donahue G, et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell. 2021;184:6081–6100.e26. doi: 10.1016/j.cell.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fan Y, Li Y, Yao X, Jin J, Scott A, Liu B, et al. Epithelial SOX9 drives progression and metastases of gastric adenocarcinoma by promoting immunosuppressive tumour microenvironment. Gut. 2023;72:624–37. doi: 10.1136/gutjnl-2021-326581. [DOI] [PubMed] [Google Scholar]
- 161.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin S, Liu G, Jin H, Chen X, He J, Xiao J, et al. The dysregulation of SOX family correlates with DNA methylation and immune microenvironment characteristics to predict prognosis in hepatocellular carcinoma. Dis Markers. 2022;2022:2676114. doi: 10.1155/2022/2676114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu Y, Xu M, Li X, Weng X, Su Z, Zhang M, et al. SOX9 and HMGB3 co-operatively transactivate NANOG and promote prostate cancer progression. Prostate. 2023;83:440–53. doi: 10.1002/pros.24476. [DOI] [PubMed] [Google Scholar]
- 164.Laughney AM, Hu J, Campbell NR, Bakhoum SF, Setty M, Lavallée VP, et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med. 2020;26:259–69. doi: 10.1038/s41591-019-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362. doi: 10.1038/s41392-021-00670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen Y, Kim J, Yang S, Wang H, Wu CJ, Sugimoto H, et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39:548–565.e6. doi: 10.1016/j.ccell.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fulgenzi CAM, Scheiner B, Korolewicz J, Stikas CV, Gennari A, Vincenzi B, et al. Efficacy and safety of frontline systemic therapy for advanced HCC: A network meta-analysis of landmark phase III trials. JHEP Rep. 2023;5:100702. doi: 10.1016/j.jhepr.2023.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ashrafizadeh M, Taeb S, Hushmandi K, Orouei S, Shahinozzaman M, Zabolian A, et al. Cancer and SOX proteins: new insight into their role in ovarian cancer progression/inhibition. Pharm Res. 2020;161:105159. doi: 10.1016/j.phrs.2020.105159. [DOI] [PubMed] [Google Scholar]
- 170.Ruzinova MB, Ma C, Brunt EM, Goss CW, Vachharajani N, Chapman WC, et al. SOX9 expression is superior to other stem cell markers K19 and EpCAM in predicting prognosis in hepatocellular carcinoma. Am J Surg Pathol. 2023;47:1–11. doi: 10.1097/PAS.0000000000001990. [DOI] [PubMed] [Google Scholar]
- 171.Klaric KA, Riaz N, Asleh K, Wang XQ, Atalla T, Strickland S, et al. SRY-box transcription factor 10 is a highly specific biomarker of basal-like breast cancer. Histopathology. 2022;80:589–97. doi: 10.1111/his.14592. [DOI] [PubMed] [Google Scholar]
- 172.Ek S, Dictor M, Jerkeman M, Jirström K, Borrebaeck CAK. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 2008;111:800–5. doi: 10.1182/blood-2007-06-093401. [DOI] [PubMed] [Google Scholar]
- 173.Shaker N, Chen W, Sinclair W, Parwani AV, Li Z. Identifying SOX17 as a sensitive and specific marker for ovarian and endometrial carcinomas. Mod Pathol. 2023;36:100038. doi: 10.1016/j.modpat.2022.100038. [DOI] [PubMed] [Google Scholar]
- 174.Zhao J, Li L, Wang Z, Li L, He M, Han S, et al. Luteolin attenuates cancer cell stemness in PTX-resistant oesophageal cancer cells through mediating SOX2 protein stability. Pharmacol Res. 2021;174:105939. doi: 10.1016/j.phrs.2021.105939. [DOI] [PubMed] [Google Scholar]
- 175.Jing C, Duan Y, Zhou M, Yue K, Zhuo S, Li X, et al. Blockade of deubiquitinating enzyme PSMD14 overcomes chemoresistance in head and neck squamous cell carcinoma by antagonizing E2F1/Akt/SOX2-mediated stemness. Theranostics. 2021;11:2655–69. doi: 10.7150/thno.48375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen Y, Niu J, Li L, Li Z, Jiang J, Zhu M, et al. Polydatin executes anticancer effects against glioblastoma multiforme by inhibiting the EGFR-AKT/ERK1/2/STAT3-SOX2/Snail signaling pathway. Life Sci. 2020;258:118158. doi: 10.1016/j.lfs.2020.118158. [DOI] [PubMed] [Google Scholar]
- 177.Garros-Regulez L, Aldaz P, Arrizabalaga O, Moncho-Amor V, Carrasco-Garcia E, Manterola L, et al. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin Ther Targets. 2016;20:393–405. doi: 10.1517/14728222.2016.1151002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou M, Zhang X, Liu C, Nie D, Li S, Lai P, et al. Targeting protein lysine methyltransferase G9A impairs self-renewal of chronic myelogenous leukemia stem cells via upregulation of SOX6. Oncogene. 2021;40:3564–77. doi: 10.1038/s41388-021-01799-1. [DOI] [PubMed] [Google Scholar]
- 179.Kaushik G, Seshacharyulu P, Rauth S, Nallasamy P, Rachagani S, Nimmakayala RK, et al. Selective inhibition of stemness through EGFR/FOXA2/SOX9 axis reduces pancreatic cancer metastasis. Oncogene. 2021;40:848–62. doi: 10.1038/s41388-020-01564-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu K, Xie F, Zhao T, Zhang R, Gao A, Chen Y, et al. Targeting SOX2 protein with peptide aptamers for therapeutic gains against esophageal squamous cell carcinoma. Mol Ther. 2020;28:901–13. doi: 10.1016/j.ymthe.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stolzenburg S, Beltran AS, Swift-Scanlan T, Rivenbark AG, Rashwan R, Blancafort P. Stable oncogenic silencing in vivo by programmable and targeted de novo DNA methylation in breast cancer. Oncogene. 2015;34:5427–35. doi: 10.1038/onc.2014.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Blache P, Canterel-Thouennon L, Busson M, Verdié P, Subra G, Ychou M, et al. A short SOX9 peptide mimics SOX9 tumor suppressor activity and is sufficient to inhibit colon cancer cell growth. Mol Cancer Ther. 2019;18:1386–95. doi: 10.1158/1535-7163.MCT-18-1149. [DOI] [PubMed] [Google Scholar]
- 183.Ueda R, Ohkusu-Tsukada K, Fusaki N, Soeda A, Kawase T, Kawakami Y, et al. Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. Int J Cancer. 2010;126:919–29. doi: 10.1002/ijc.24851. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y, Keib A, Neuber B, Wang L, Riemer AB, Bonsack M, et al. Definition and characterization of SOX11-derived T cell epitopes towards immunotherapy of glioma. Int J Mol Sci. 2023;24:1943. doi: 10.3390/ijms24031943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Peng H, Ivanov AV, Oh HJ, Lau YFC, Rauscher FJ. Epigenetic gene silencing by the SRY protein is mediated by a KRAB-O protein that recruits the KAP1 co-repressor machinery. J Biol Chem. 2009;284:35670–80. doi: 10.1074/jbc.M109.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Murakami S, Ninomiya W, Sakamoto E, Shibata T, Akiyama H, Tashiro F. SRY and OCT4 are required for the acquisition of cancer stem cell-like properties and are potential differentiation therapy targets. Stem Cells. 2015;33:2652–63. doi: 10.1002/stem.2059. [DOI] [PubMed] [Google Scholar]
- 187.Ahmad A, Strohbuecker S, Tufarelli C, Sottile V. Expression of a SOX1 overlapping transcript in neural differentiation and cancer models. Cell Mol Life Sci. 2017;74:4245–58. doi: 10.1007/s00018-017-2580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang XL, Jiao BH, Wu JL, Yang JK, Hu YH, Cui K. Mechanism of RIP2 enhancing stemness of glioma cells induces temozolomide resistance. CNS Neurosci Ther. 2022;28:2319–30. doi: 10.1111/cns.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fu Y, Bai C, Wang S, Chen D, Zhang P, Wei H, et al. AKT1 phosphorylates RBM17 to promote Sox2 transcription by modulating alternative splicing of FOXM1 to enhance cancer stem cell properties in colorectal cancer cells. FASEB J. 2023;37:e22707. doi: 10.1096/fj.202201255R. [DOI] [PubMed] [Google Scholar]
- 190.Shen J, Zhai J, Wu X, Xie G, Shen L. Serum proteome profiling reveals SOX3 as a candidate prognostic marker for gastric cancer. J Cell Mol Med. 2020;24:6750–61. doi: 10.1111/jcmm.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Delogu A, Sellers K, Zagoraiou L, Bocianowska-Zbrog A, Mandal S, Guimera J, et al. Subcortical visual shell nuclei targeted by ipRGCs develop from a Sox14+-GABAergic progenitor and require Sox14 to regulate daily activity rhythms. Neuron. 2012;75:648–62. doi: 10.1016/j.neuron.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 192.Wang H, Ma ZW, Ho FM, Sethi G, Tang FR. Dual Effects of miR-181b-2-3p/SOX21 interaction on microglia and neural stem cells after gamma irradiation. Cells-basel. 2023;12:649. doi: 10.3390/cells12040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhu Y, Li Y, Jun Wei JW, Liu X. The role of Sox genes in lung morphogenesis and cancer. Int J Mol Sci. 2012;13:15767–83. doi: 10.3390/ijms131215767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baroti T, Zimmermann Y, Schillinger A, Liu L, Lommes P, Wegner M, et al. Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain. Glia. 2016;64:122–38. doi: 10.1002/glia.22919. [DOI] [PubMed] [Google Scholar]
- 195.Rjiba K, Mougou-Zerelli S, Hamida IH, Saad G, Khadija B, Jelloul A, et al. Additional evidence for the role of chromosomal imbalances and SOX8, ZNRF3 and HHAT gene variants in early human testis development. Reprod Biol Endocrinol. 2023;21:2. doi: 10.1186/s12958-022-01045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie S-L, Fan S, Zhang S-Y, Chen W-X, Li Q-X, Pan G-K, et al. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/β-catenin pathway. Int J cancer. 2018;142:1252–65. doi: 10.1002/ijc.31134. [DOI] [PubMed] [Google Scholar]
- 197.Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lilly AJ, Costa G, Largeot A, Fadlullah MZH, Lie-A-Ling M, Lacaud G, et al. Interplay between SOX7 and RUNX1 regulates hemogenic endothelial fate in the yolk sac. Development. 2016;143:4341–51. doi: 10.1242/dev.140970. [DOI] [PubMed] [Google Scholar]
- 199.Hosking BM, Wyeth JR, Pennisi DJ, Wang SC, Koopman P, Muscat GE. Cloning and functional analysis of the Sry-related HMG box gene, Sox18. Gene. 2001;262:239–47. doi: 10.1016/s0378-1119(00)00525-4. [DOI] [PubMed] [Google Scholar]
- 200.Han F, Jiang X, Li ZM, Zhuang X, Zhang X, Ouyang WM, et al. Epigenetic inactivation of SOX30 is associated with male infertility and offers a therapy target for non-obstructive azoospermia. Mol Ther Nucleic Acids. 2020;19:72–83. doi: 10.1016/j.omtn.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xie Q, Hua X, Huang C, Liao X, Tian Z, Xu J, et al. SOX2 promotes invasion in human bladder cancers through MMP2 upregulation and FOXO1 downregulation. Int J Mol Sci. 2022;23:12532. doi: 10.3390/ijms232012532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2018;48:812–830.e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the UCSC database (https://xenabrowser.net/) and GDC database (https://portal.gdc.cancer.gov/).