Abstract
Pneumocystis carinii is an important pulmonary pathogen responsible for morbidity and mortality in patients with AIDS. The acute-phase response (APR), the primary mechanism used by the body to restore homeostasis following infection, is characterized by increased levels of circulating fibrinogen (FBG). Although the liver is the primary site of increased FBG synthesis during the APR, we unexpectedly discovered that FBG is synthesized and secreted by lung alveolar epithelial cells in vitro during an inflammatory stimulus. Therefore, we sought to determine whether lung epithelial cells produce FBG in vivo using animal models of P. carinii pneumonia (PCP). Inflammation was noted by an influx of macrophages to P. carinii-infected alveoli. Northern hybridization revealed that γ-FBG mRNA increased two- to fivefold in P. carinii-infected lung tissue, while RNA in situ hybridization demonstrated increased levels of γ-FBG mRNA in the lung epithelium. Immunoelectron microscopy detected lung epithelial cell-specific production of FBG, suggesting induction of a localized inflammatory response resembling the APR. A systemic APR was confirmed by a two- to fivefold upregulation of the levels of hepatic γ-FBG mRNA in animals with PCP, resulting in a corresponding increase in levels of FBG in plasma. Furthermore, immunoelectron microscopy revealed the presence of FBG at the junction of cell membranes of trophic forms of P. carinii organisms aggregated along the alveolar epithelium. These results implicate FBG in the pathogenesis of PCP in a manner similar to that of the adhesive glycoproteins fibronectin and vitronectin, which are known to participate in intra-alveolar aggregation of organisms and adherence of P. carinii to the lung epithelium.
Pneumocystis carinii pneumonia (PCP) has been demonstrated in a variety of immunosuppressed animal models with manifestations similar to those observed in afflicted humans (22, 27, 39, 49). Attachment of the organisms to alveolar type I epithelium is an essential step in establishing P. carinii infection (27, 30, 31, 41, 42). Ultrastructural studies indicate that P. carinii trophic forms attach to the surface of type I alveolar epithelial cells early in infection with limited interaction of organisms with type II cells as PCP progresses (27, 31, 32). The consequences of P. carinii attachment to the alveolar epithelium are only beginning to be understood. In the early stages of P. carinii infection, mononuclear cell infiltration represents the most prominent feature of the host inflammatory response, while little polymorphonuclear leukocyte or lymphocyte infiltration is observed (27, 29). Osmiophilic material, suggestive of changes in surfactant homeostasis, is observed in areas of marked necrosis (31) and during late stages of infection (32). Levels of surfactant protein-D (SP-D) increase in the lower respiratory tract during PCP (37), and increased amounts of SP-A are recovered in bronchoalveolar lavage fluids (40). Analyses of bronchoalveolar lavage fluids from patients with PCP, both with and without AIDS, revealed an accumulation of extracellular phospholipid surfactant-like material suggestive of an associated proteinosis, which was not observed in immunocompromised patients without PCP (52).
P. carinii infection is complicated by pulmonary fibrin deposition and the development of interstitial fibrosis (54). In a study of lung biopsies from patients having PCP, marked interstitial fibrosis was observed which was characterized by the absence of intra-alveolar exudate and the atypical clustering of cysts within the connective tissue rather than in the alveolar spaces (54). Focal hyaline membranes, indicative of fibrin deposition are found in PCP (54). Presently it is believed that alveolar fibrin results from leakage of plasma fibrinogen (FBG) followed by its conversion to fibrin in the final stages of coagulation. Fibrin deposition influences inflammatory cell traffic and fibroblast proliferation (24). Furthermore, FBG, fibrin, and fibrin degradation products (FDPs), resulting from plasmin-mediated fibrinolysis, interfere with or inactivate surfactant function (24, 34, 45). Fibrosis in patients with PCP is characterized by fibrous thickening and fibroblastic proliferation within the alveolar septum (54). Patients with fibrosis on the initial biopsy tend to have abnormal pulmonary function tests after recovery from PCP. Thus, P. carinii infection clearly results in a disruption of pulmonary function and homeostasis.
FBG is the major blood clotting factor involved in the maintenance of hemostasis. During a systemic inflammatory response, FBG synthesis by hepatocytes is upregulated 2- to 10-fold such that levels of FBG in plasma increase from an average of 3 mg/ml to 6 to 30 mg/ml (10, 53). Increased levels of FBG in plasma and subsequent fibrin formation serve to restore homeostasis by providing a provisional matrix to support the cell interactions of wound healing (7). This upregulation of FBG gene expression during inflammation is one of a myriad of responses associated with the disruption of homeostasis due to infection, tissue injury, or neoplasia, collectively termed the acute-phase response or reaction (APR) (10, 53). Recently, we showed that FBG is synthesized and secreted by lung alveolar epithelial cells in vitro (46). Importantly, FBG synthesis by pneumocytes occurs in response to interleukin-6 (IL-6) and dexamethasone (DEX). Our more recent observations indicate that FBG, not fibrin, produced in vitro by lung epithelial cells in response to IL-6–DEX treatment, is secreted basolaterally (14) and becomes incorporated into the extracellular matrix (15). Thus, it is likely that locally synthesized FBG functions in the maintenance or restoration of the epithelial barrier during lung injury. We propose that increased synthesis and secretion of lung epithelial cell-derived FBG is one of the physiological events occurring during PCP. In this report, we demonstrate that expression of the γ-FBG gene is elevated in lung epithelial cells during PCP. Because subsequent fibrin deposition can occur during lung injury, these studies suggest that lung cell-type specific expression of FBG contributes to the balance between appropriate wound healing and the development of tissue fibrosis and scarring.
MATERIALS AND METHODS
Animal models and sample preparation.
Ferrets were obtained from Marshall Farms (Northrose, N.Y.). PCP was induced in ferrets fed a normal diet by adding DEX (2 mg/liter) and tetracycline (500 mg/liter) to the drinking water (49). Immunosuppression caused by the steroid rendered the animals susceptible to natural infection by P. carinii. Control animals were treated with DEX and tetracycline or tetracycline only, as stated above, plus Bactrim (20 ml/liter) in the drinking water to prevent P. carinii infection. C.B-17 scid/scid mice, 8 to 10 weeks old, were obtained from the Trudeau Animal Breeding Facility (22). These SCID mice spontaneously develop detectable P. carinii infection at about 4 weeks of age. All forms of P. carinii were visualized by light microscopy after various staining procedures; the cyst form of P. carinii was visualized in lung sections after Gomori’s methenamine silver (GMS) staining (19). To assess P. carinii-infected lungs for fungal contamination and to confirm the presence of P. carinii cysts and trophozoites, cytospin preparations of P. carinii-infected lung homogenates deposited by cytocentrifugation onto slides were stained with modified Diff-Quik (Difco, Detroit, Mich.) (22).
Lungs and livers were harvested after sacrificing the animals with an intraperitoneal injection of a lethal dose of Beuthanasia-D (390-mg/ml pentobarbital–50-mg/ml phenytoin; 1 ml per animal). At times, ferrets were exsanguinated by intracardiac puncture after the animals reached a plane of anesthesia but before death as determined by toe pinch reflex. Blood was anticoagulated with heparin for preparation of plasma. To retain airway macrophages (3), infected or control tissues used for histology and in situ hybridizations were sliced into 2- to 3-mm sections, fixed by immersion in formalin, and then embedded in paraffin blocks.
RNA isolation and Northern analysis.
Molecular biology reagents were obtained from Life Technologies (Gaithersburg, Md.), chemical reagents were obtained from Sigma (St. Louis, Mo.), radionucleotides were purchased from DuPont New England Nuclear (Boston, Mass.), and the Zetaprobe nylon membrane was from Bio-Rad (Centreville, N.Y.). Total RNA and poly(A)+ mRNA were isolated as previously described (20), and Northern blot hybridization was carried out with 32P-labeled ferret lung-specific γ-FBG cDNA, pFLGγ3 (47), or the rat liver-specific γ-FBG cDNA, pRγBP18 (17).
In situ RNA:RNA hybridization.
In situ hybridization was performed essentially as previously described (9, 16, 17, 19, 47, 51). The pFLGγ3 cDNA was cloned from ferret lung mRNA as previously described (47); the sequence is deposited with GenBank under accession no. U28494. The sense and antisense pFLGγ3, γ-actin, and P. carinii surface glycoprotein A (gpA) riboprobes were labeled to a specific activity of 2.93 × 107 cpm/μg by using [3H]CTP and [3H]UTP. The riboprobe specific activity was calculated from the specific activity of the input ribonucleotides as previously described (1). Thus, probes generated from the same batches of radionucleotides will have the same specific activity, while the total mass synthesized is dependent on the efficiency of in vitro transcription, which varies from probe to probe. The antisense orientation of the riboprobe detects the positive expression of the mRNA, whereas the sense orientation of the riboprobe is used to detect background levels of silver grains in the absence of specific mRNA expression. The slides probed in situ were exposed to NTB-2 emulsion (Kodak, Rochester, N.Y.) for 8 weeks at 4°C. After photographic development, slides were counterstained with Mayer’s hematoxylin and eosin (H&E) to visualize tissue and cell morphology by brightfield microscopy; silver grains were visualized under darkfield microscopy. Some slides upon which the in situ hybridization procedure was carried out were counterstained with GMS as previously described (19).
SDS-PAGE and Western blot analysis of human and ferret plasma FBG.
Human FBG was purchased from KabiVitrum (Franklin, Ohio), and mouse and rat FBG were from Sigma. Ferret FBG was quantitatively precipitated from plasma (13) or purified as previously described (27). Commercially obtained FBGs were purified further to remove contaminating plasminogen and fibronectin (FN) (46). For Western blotting, 20 μg of protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted to nitrocellulose. Immunoblotting was performed with monoclonal antibody (MAb) H9B7 generated against the γ chain of human FBG (18) and shown to cross-react with ferret FBG (47). Secondary antibodies and substrate color development were performed as previously described (18).
IEM.
Polyclonal antiserum was raised in rabbits against purified mouse FBG, and the immunoglobulin G (IgG) fraction was affinity purified (28, 46). P. carinii-infected SCID mice were sacrificed, and the lungs were inflation fixed in situ with 4% paraformaldehyde–0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, as previously described (57). Fixed lungs were removed and processed into embedding resin, and immunoelectron microscopy (IEM) was performed as previously described (14).
RESULTS
Establishment of P. carinii infection and inflammatory cell response.
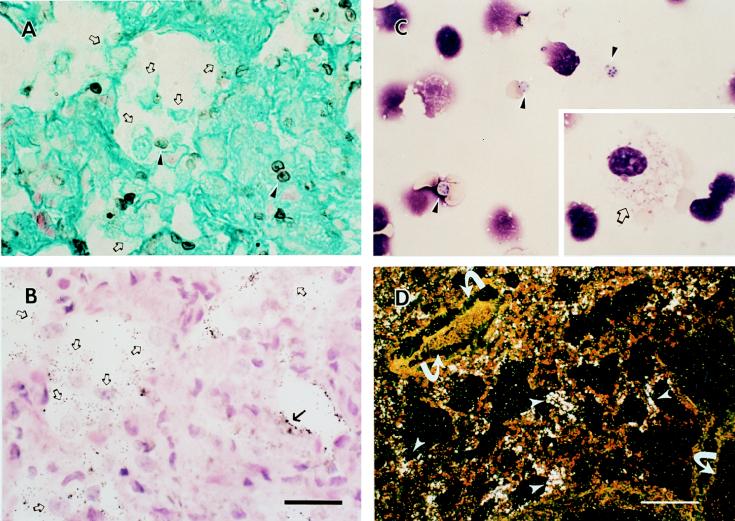
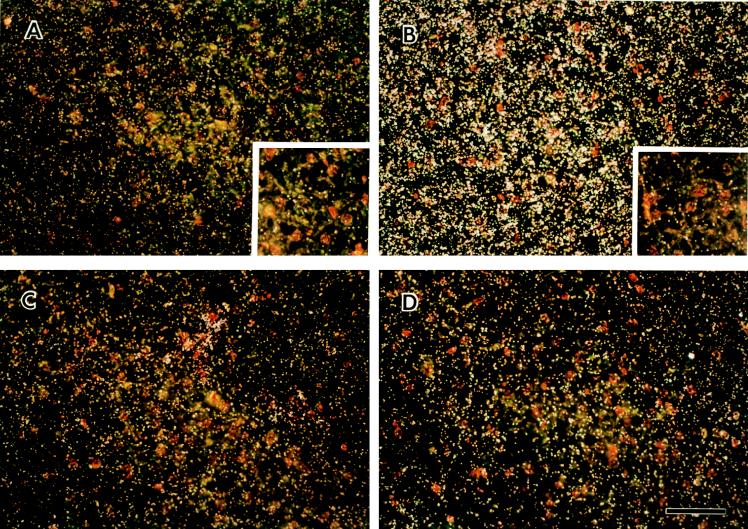
P. carinii infection was documented after 4 to 6 weeks of DEX treatment by staining of tissue sections with GMS (Fig. 1A) and H&E (Fig. 1B), cytocentrifugation and Diff Quik staining of organisms from infected lung homogenates (Fig. 1C), and in situ hybridization of ferret lung tissue sections for expression of P. carinii-specific surface glycoprotein A (gpA) mRNA (Fig. 1B and D). Positive detection of mRNA is denoted under darkfield microscopy as abundant, white silver grains localized to organisms. Note that not all alveoli were uniformly infected. Panels A and B of Fig. 1 were also hybridized with the gpA antisense riboprobe. Under brightfield illumination, the silver grains denoting a positive signal for gpA mRNA in P. carinii appear as black dots. In addition, P. carinii-infected ferrets demonstrated the characteristic inflammatory cell response of alveolar monocyte/macrophage accumulation with little detection of polymorphonuclear leukocytes (Fig. 1A to C). The course of P. carinii infection was similar in SCID mice (not shown).
FIG. 1.
Pathology of P. carinii-infected lung tissue. Alveolar macrophages (open arrows) were detected in P. carinii-infected ferret lung tissue by GMS (A) and H&E (B) staining (cysts, arrowheads; trophs, arrows). Diff Quik staining demonstrates both trophic (inset, open arrow) and cyst (arrowheads) nuclei in a cytospin preparation of infected ferret lung homogenate (C). Note that the cyst forms of P. carinii shown in this field (arrowheads) contain between four and eight nuclei representative of intracystic bodies that correspond to the individual nuclei of the trophic forms released upon excystation during a stage in the P. carinii life cycle. The bar in panel B represents 20 μm for panels A to C. Trophic nuclei within a macrophage cytoplasm were occasionally detected (C, inset). (D) A low-power darkfield exposure of P. carinii-infected lung probed with the gpA antisense riboprobe specific for P. carinii gpA mRNA Bar, 70 μm. Arrows indicate blood vessels.
PCP induces an acute-phase inflammatory reactant in the lung.
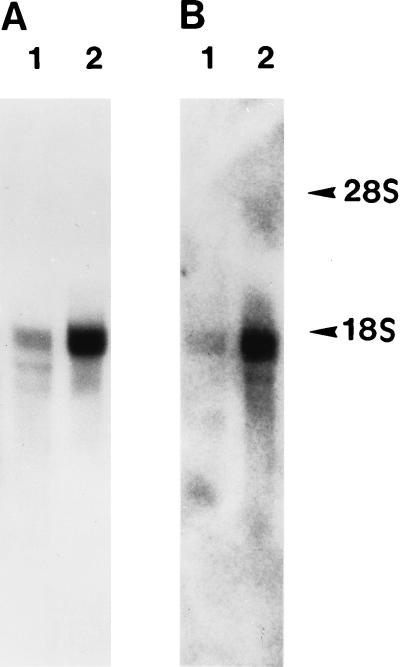
Because cultured lung epithelial cells synthesize and secrete FBG, and hepatic FBG gene expression is upregulated during an inflammatory response, we determined whether P. carinii infection induced FBG gene expression in the lung. The results of Northern hybridization indicated that the expression of γ-FBG mRNA was elevated four- to fivefold in the lungs of P. carinii-infected animals compared to the level in normal animals (Fig. 2B), indicative of a localized inflammatory response. Equal loading of host mRNA was indicated by similar staining of the 28S rRNA by acridine orange-stained gels run in parallel (not shown). Figure 2 shows autoradiographs of liver and lung tissues exposed at room temperature for 15 min and at −70°C for 6 h, respectively, indicating that, while the overall relative abundance of γ-FBG mRNA per microgram of RNA is much lower in the lung compared to the liver, the relative induction of γ-FBG mRNA was four- to fivefold higher in both the lung and liver of a P. carinii-infected animal (Fig. 2A and B, lanes 2) than in those of a control. To determine the cell-type specificity of γ-FBG mRNA expression in the infected lung, RNA:RNA in situ hybridization was performed. In both uninfected and infected lungs, γ-FBG mRNA expression was observed in the epithelium. The levels of γ-FBG mRNA were increased in airway epithelial cells of infected lung tissue (Fig. 3).
FIG. 2.
Northern blot analysis of normal and PCP ferret liver and lung mRNAs. Poly(A)+ mRNA (5 μg) from normal (lanes 1) and P. carinii-infected (lanes 2) ferret liver (A) and lung (B) probed with pFLGγ3. The positions of the host rRNAs are indicated.
FIG. 3.
In situ hybridization of lung tissues from normal and P. carinii-infected ferrets. Tissue sections shown in panels A to D were probed with pFLGγ3 antisense probe; insets were probed with pFLGγ3 sense probe. The arrows indicate bronchial epithelial cells in P. carinii-infected (C) and normal (A) ferret lung tissues. H&E stains of the same fields are also shown (B and D, respectively). Positive detection of mRNA is denoted under darkfield microscopy as abundant, white silver grains localized to the cytoplasm of cells (panels A and C).
Alveolar epithelium produces FBG protein during PCP.
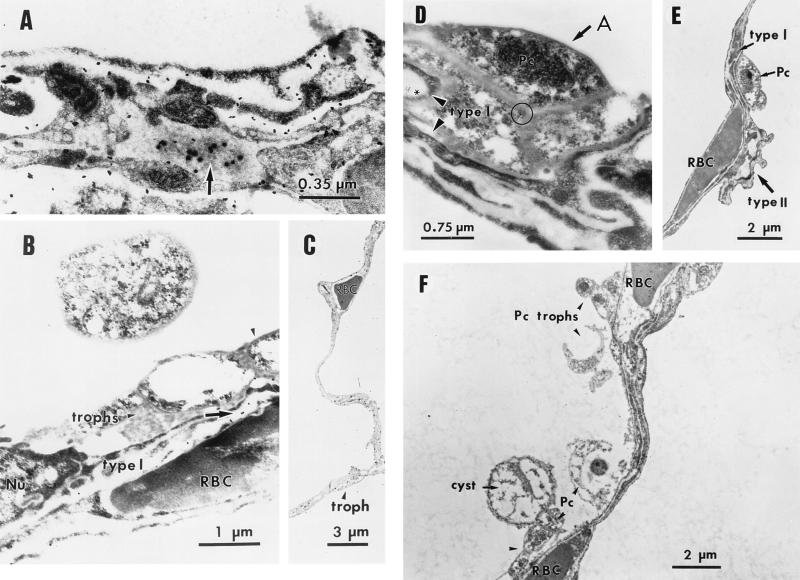
To determine whether lung epithelial cells synthesize FBG protein in vivo, IEM of P. carinii-infected lung tissue was performed. The results showed the intracellular presence of FBG in secretory granules of epithelium lining the fused basement membrane of the septa between adjacent alveoli (Fig. 4A). P. carinii organisms were observed attached to alveolar epithelial cells where intracellular FBG protein was detected (Fig. 4B). While the complete life cycle of P. carinii has not been fully elucidated due to the lack of a continuous in vitro culture system, several developmental stages of the organism have been identified by light and electron microscopy (23, 27, 31, 32, 59). The two major developmental forms of the organism, the amporhous trophic form (also known as troph, trophozoite, or intracystic body) and the rigid cyst form differ in the composition of their cell walls (59). During the putative life cycle, the trophozoite matures into a precyst which develops up to 8 intracystic bodies. Upon excystation, these “daughter” trophs are released to renew the cycle. In this study, the characteristic trophic and cyst forms of P. carinii were observed by both light (Fig. 1) and transmission electron microscopy (Fig. 4). Furthermore, intra-alveolar FBG was detected at the junction of cell membranes of trophic forms of P. carinii aggregated at the apical face of the alveolar epithelium (Fig. 4D), suggesting that FBG may participate in aggregation and adherence of the organisms to type I epithelium.
FIG. 4.
IEM localization of FBG in the lung epithelium. The intracellular presence of FBG in P. carinii-infected lung tissue was determined by IEM by using 100 μg of anti-FBG IgG/ml detected with Protein-A-gold (diameter, 20 nm) conjugate (panels A and B, arrows; panel D, asterisk). FBG was detected at the junction of cell membranes of trophic forms of P. carinii organisms (panel D, circle) aggregated (panel D, arrow) along the alveolar epithelium (panel D, arrowheads). Purified rabbit IgG (100 μg/ml) was used as the negative control in the primary antibody step (F). The morphologically rigid cyst and pleomorphic trophic forms of P. carinii organisms attached to type I epithelium and aggregated in the alveolar spaces are shown in panels B to F. Lower magnifications show the integrity of septal walls between alveoli (C and F). Alveolar capillaries are indicated by the presence of the erythrocytes (RBC). The appropriate magnification for each field is indicated. A, alveolar lumen; Nu, nucleus; Pc, P. carinii.
PCP induces a systemic APR.
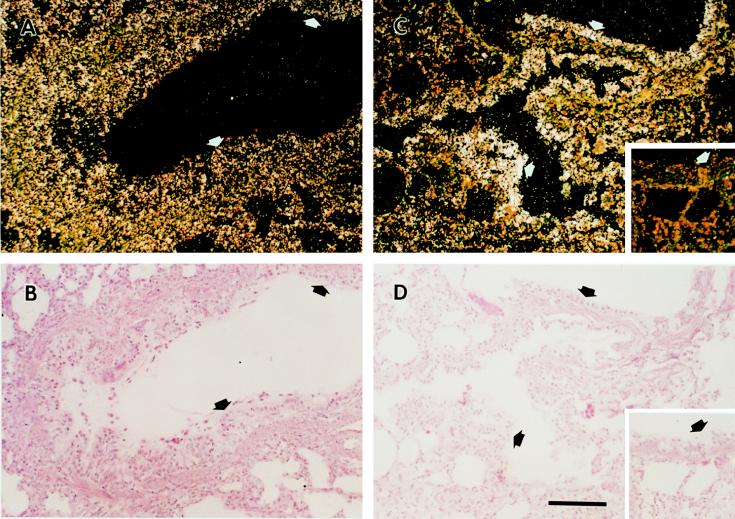
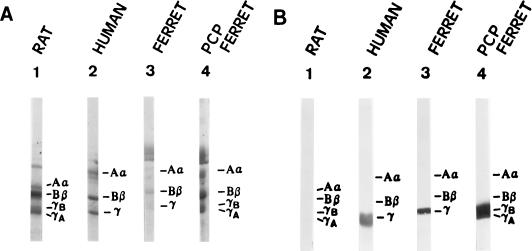
Northern hybridization revealed that P. carinii infection induced upregulation of the γ-FBG gene as determined by increases in the steady-state levels of liver γ-FBG mRNA (Fig. 2A). RNA in situ hybridization studies demonstrated that the levels of γ-FBG mRNA were significantly upregulated in all hepatocytes during PCP, while the levels of γ-actin remained the same (Fig. 5). This observation is consistent with a systemic APR to the infection. The induction of systemic inflammation in the ferret model of PCP was further supported by increased levels of FBG in plasma (Fig. 6A), also detected by Western blot identification of the ferret FBG γ-chain with the cross-reacting MAb, H9B7 (Fig. 6B).
FIG. 5.
In situ hybridization of liver tissue from normal and P. carinii-infected ferrets. Normal (A and C) and P. carinii-infected (B and D) livers were probed with pFLGγ3+ (panels A and B) or γ-actin antisense probes (panels C and D); insets were probed with pFLGγ3− sense probe.
FIG. 6.
SDS-PAGE and Western blot of plasma FBG. Ferret plasma FBG was quantitatively precipitated from 8 μl of plasma and resolved by SDS–7.5% PAGE. (A) Coomassie blue stain; (B) immunoblot with cross-reacting anti-human γ chain MAb, H9B7.
The role of P. carinii infection in the absence of DEX immunosuppression.
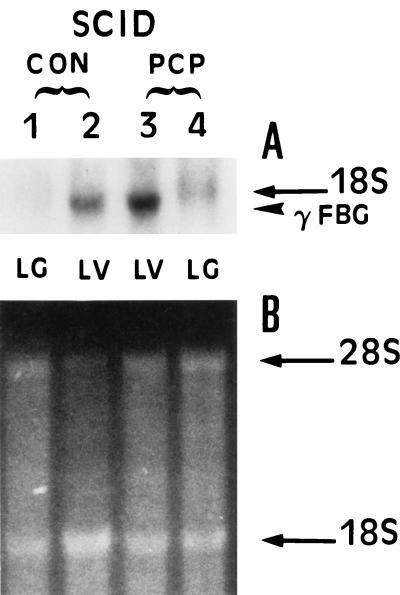
To determine whether P. carinii infection in the absence of DEX immunosuppression resulted in increased lung expression of γ-FBG, the SCID mouse model of PCP was utilized. The levels of γ-FBG mRNA were increased twofold in P. carinii-infected SCID mouse liver consistent with the level of induction of FBG gene expression in a variety of in vivo and in vitro models of inflammation. While the basal level of γ-FBG mRNA was undetectable in control lung total RNA, γ-FBG mRNA was detectable in P. carinii-infected lung total RNA, indicating an increase in steady-state levels of γ-FBG mRNA during P. carinii infection (Fig. 7). These results indicate that P. carinii infection alone in the absence of DEX immunosuppression is sufficient to induce the mediator(s) of γ-FBG expression locally in the lung and systemically in the liver.
FIG. 7.
Northern blot analysis of γ-FBG mRNA in P. carinii-infected SCID mouse liver and lung. (A) Total RNA (20 μg) from uninfected (CON, lanes 1 and 2) and P. carinii-infected (PCP, lanes 3 and 4) SCID mouse liver (LV, lanes 2 and 3) and lung (LG, lanes 1 and 4) was probed with γ-FBG cDNA pRγBP18. (B) Acridine orange-stained gel of duplicate set of denatured RNA.
The role of DEX immunosuppression in the absence of P. carinii infection.
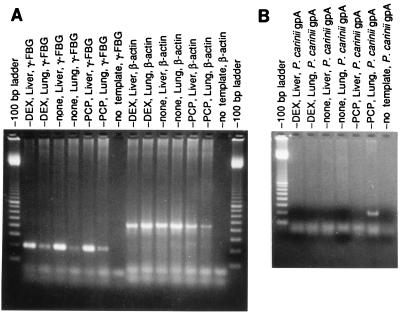
To determine whether DEX treatment alone, in the absence of P. carinii infection, resulted in elevated γ-FBG gene expression, ferrets were treated with tetracycline, Bactrim, and DEX to achieve immunosuppression but prevent PCP; control animals were treated with tetracycline and Bactrim but not with DEX. RNA was prepared from the lungs and livers of matched pairs of animals 3 and 4 weeks after initiation of DEX immunosuppression. Microscopic examination of GMS-stained histological sections cut from the Bactrim-treated immunosuppressed and control ferret lungs indicated that Bactrim was successful in preventing P. carinii infection (not shown). Total RNAs (20 μg) from 4-week DEX-immunosuppressed tissues and normal tissues were probed with the ferret specific γ-FBG cDNA, pFLGγ3. Equivalent amounts of γ-FBG mRNA were found in DEX-treated compared to normal ferret liver, indicating that prolonged exposure of the animal to DEX immunosuppression did not result in induction of γ-FBG gene expression (not shown). To demonstrate basal amounts of γ-FBG mRNA in lung tissues of the control and DEX-treated ferrets, reverse transcriptase (RT) PCR amplification of total RNA (10μg) from P. carinii-infected liver and lung tissues was carried out. The results demonstrated that the lung tissue mRNA was intact and that basal amounts of steady-state γ-FBG mRNA were present in normal lung tissue (Fig. 8A), as shown above by Northern (Fig. 2) and in situ hybridizations (Fig. 3). The primer pairs used for amplification of the RNA:DNA heteroduplex resulted in a 200-bp product for γ-FBG and a 450-bp product for β-actin (Fig. 8A). To confirm the absence of P. carinii organisms at the microscopic level, RT-PCR was performed using primer pairs specific for ferret P. carinii gpA. The results indicated that none of the Bactrim-treated animals were susceptible to breakthrough infection with P. carinii, as the characteristic 220-bp product for ferret-derived P. carinii gpA was not obtained (Fig. 8B). Taken together, these results confirm that the observed increase in γ-FBG gene expression in both liver and lung tissues occurs only during P. carinii infection and is not a result of prolonged DEX immunosuppression.
FIG. 8.
RT-PCR of RNA isolated from ferrets treated in the presence or absence of DEX and Bactrim. Bactrim-treated DEX-immunosuppressed (DEX), control (none), and P. carinii-infected (PCP) ferret lungs and livers were amplified with γ-FBG and β-actin primer pairs (A). Total RNAs from the livers and lungs of P. carinii-infected ferrets were amplified with a gpA-specific primer pair as positive controls for the RT-PCR (B). In both panels, no template served as the negative control reaction. The molecular size markers are in 100-bp increments.
DISCUSSION
Our data show clearly that P. carinii infection results in local and systemic inflammatory responses as measured by increased levels of γ-FBG mRNA in lung and liver tissues, respectively. Prolonged immunosuppression of animals with DEX, in the absence of P. carinii infection, did not result in an increase in γ-FBG mRNA expression in either the lung or liver. In light of these results, the induction of FBG mRNAs in the ferret is not likely the result of nonspecific upregulation secondary to DEX administration but is a specific consequence of PCP. Finally, we showed the in vivo production of FBG by the lung alveolar epithelium by IEM and have previously demonstrated that IL-6 and DEX, known positive regulators in the induction of hepatic FBG gene expression (10, 53), induce the synthesis and secretion of intact FBG from a human lung epithelial cell line (46). Furthermore, elevated expression of γFBG mRNA in the liver and lung during PCP suggested that, in addition to a systemic (liver) inflammatory reaction, a tissue-specific (i.e., lung) inflammatory reaction akin to the APR was induced. Haptoglobin, another APR protein whose expression was considered liver specific, is also expressed in the lung epithelium during inflammation (58).
Until recently, little was known about the induction of an APR during PCP. Syrjala et al. (50) monitored the levels of C-reactive protein (CRP) in human immunodeficiency virus-infected and immunocompromised patients with or without PCP; CRP is upregulated several hundredfold in a systemic APR (10, 53). The levels of CRP were elevated over 200-fold in patients with a fatal outcome of PCP, while human immunodeficiency virus infection itself did not significantly increase CRP levels (50). The erythrocyte sedimentation rate was increased in PCP patients as well, indicative of elevated levels of FBG in plasma in response to a systemic inflammation (10, 53). A review of case histories examining AIDS patients with PCP revealed that 7 of 37 cases had hypoalbuminemia (8); albumin is downregulated during the APR (10, 53). The peripheral blood monocyte/macrophage accessory cell population represents the body’s major source of IL-6, the cytokine which plays a central role in regulating the coordinated cytokine response to injury or infection during the host response to acute or chronic inflammation (53); lung cells express elevated levels of IL-6 during PCP (2, 57). Alveolar macrophages were the predominant inflammatory cell type observed during PCP in ferrets, as was also shown in other animal models of PCP (29, 57). Macrophages contribute to the control of P. carinii infection by the production of the proinflammatory cytokines IL-1β, IL-6 and tumor necrosis factor alpha (TNF-α). During PCP, IL-1 and TNF-α are present only in the lungs (5, 6, 21), whereas IL-6 is found in both the lungs and circulation (4, 57). These findings suggest that TNF-α and IL-1 function principally at the site of infection, whereas IL-6 acts both at the site of infection and systemically in the upregulation of FBG gene expression in the lung and liver, respectively, as shown in this study. Together, these observations indicate that P. carinii infection induces a systemic inflammatory response. Moreover, production of FBG by the lung epithelium is indicative of a local inflammatory response during infection, providing the first demonstration of the extrahepatic expression of FBG during an acute or chronic inflammatory response in vivo.
A growing body of literature has emerged elucidating interrelationships among inflammatory cells, cytokines, and FBG and its related products, fibrin and FDPs, that is applicable to understanding the progression of PCP. During P. carinii infection, elevated levels of TNF-α may induce pulmonary expression of intercellular adhesion molecule 1 (ICAM-1, CD54) (60), which is involved in cell-cell interactions (48). It is known that the γ-chain of FBG can enhance leukocyte binding to cells through a bridging mechanism involving either two ICAM-1s on opposing cells or through CD11b-CD18 (CR3, Mac-1) and ICAM-1 (26, 56). Additionally, the presence of FDPs may adversely affect functional CD4+ T lymphocytes (24, 43), which are essential for host immunity to P. carinii infection (39). Furthermore, FDPs induce monocytes to express both procoagulant (tissue factor) and antifibrinolytic activities (tissue plasminogen activator inhibitor 1) (44). These studies demonstrate that lung-specific injury induces a systemic inflammatory response and suggest that excessive fibrin(ogen) in lung tissue poses a serious threat to adequate resolution of lung infection and injury.
The mechanism of P. carinii attachment and how the lung epithelium responds to this attachment has been investigated extensively. P. carinii surface gpA (12) mediates attachment of the organism to the lung epithelium (30, 41, 42). Several host proteins bind to P. carinii gpA, including SP-A (61), SP-D (37), mannose binding protein (11, 38), and the extracellular matrix components FN (41, 42) and vitronectin (VN) (30, 55). Aside from its known functions in fibrin formation and platelet aggregation, the γ chain has been shown to mediate the adhesion of microorganisms to FBG and fibrin (2, 25). Adhesion of microorganisms to surfaces is the critical early event in colonization. Adherence of host proteins to the surface of microorganisms may allow them to escape immune surveillance by disguising themselves as “self” (25). FN (41, 42) and VN (30, 55) also affect attachment of P. carinii to the lung epithelium by functioning as bridging molecules. Adherence of P. carinii to host molecules takes advantage of the well-described RGD-adhesive domains and heparin binding domains of FN or VN (41, 55). Levels of FN, VN (33), and now FBG have been shown to increase in the lung during PCP. FBG also contains RGD and heparin binding domains (35, 36) and functions as a bridging molecule in known cell-cell interactions (26, 56). Thus, it is likely that FBG synthesized by the lung epithelium may promote adhesion of P. carinii and/or adhesion of inflammatory cells to sites of infection. In support of this hypothesis, we observed FBG immunogold staining that appeared to bridge between P. carinii organisms adhered to the alveolar epithelium. We postulate that the FBG RGD and heparin binding domains participate in such intra-alveolar clumping of the organisms.
Tissue response to injury involves the activation of repair mechanisms that promote removal of debris and tissue remodeling to restore normal architecture and function. Extensive or prolonged lung injury is likely to result in excessive tissue destruction and development of fibrosis (24). Synthesis of FBG in lung may serve to restore homeostasis by aiding in wound repair or extracellular matrix remodeling after injury, similar to the function of circulating hepatic FBG produced in response to acute or chronic inflammation. Indeed, neutralization of IL-6 during PCP results in more severe inflammation and delayed clearance of the organism from the lung (4). However, elevated levels of FBG at the sites of inflammation, particularly associated with lung injury, may result in pathogenesis caused by overactive repair mechanisms. The association of high levels of FBG and fibrin deposition in lung disease becomes of greater concern when considering the possibility that local synthesis of FBG could contribute to rearranged lung architecture. Moreover, formation of fibrin and subsequent fibrinolysis in lung tissue at sites of inflammation may further enhance production of proinflammatory cytokines and thereby lead to pathophysiologic events. An imbalance of procoagulant and fibrinolytic activities of resident and infiltrating mononuclear cells may also lead to abnormalities in surfactant function (34, 45). When surfactant production is reduced or surfactant is damaged, alveoli collapse, causing hypoxia and the conditions that favor edema formation, resulting in a potentially fatal outcome in patients suffering from PCP or other severe lung diseases. Therefore, synthesis of FBG upon induction by proinflammatory mediators in the pulmonary epithelium is a significant new finding that may have dramatic implications for improving the management of patients with lung diseases or infections.
ACKNOWLEDGMENTS
We thank Barbara J. Earnest, Karl Van Der Meid, and Jean Brennan for their expert technical assistance with the animal models of PCP; Sarah O. Lawrence for assistance with immunoelectron microscopy; and Gayle Guadiz and C. G. Haidaris for their critical reading of the manuscript.
This work was supported by PHS grants HL50615, AI23302, AI07362, HL30616, AI28354, and HL59833 from the National Institutes of Health, Bethesda, Md., and a grant from the Strong Children’s Research Center of the University of Rochester.
REFERENCES
- 1.Angerer L M, Cox K H, Angerer R C. Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol. 1987;152:649–661. doi: 10.1016/0076-6879(87)52071-7. [DOI] [PubMed] [Google Scholar]
- 2.Bolyard M G, Lord S T. Expression of altered γ chain molecules in E. coli to study calcium binding and staphylococcal clumping. In: Matsuda M, Iwanaga S, Takada A, Henschen A, editors. Fibrinogen 4: current and basic clinical aspects. Amsterdam, The Netherlands: Elsevier; 1990. pp. 21–24. [Google Scholar]
- 3.Brain J D, Gehr P, Kavet R I. Airway macrophages. The importance of the fixation method. Am Rev Respir Dis. 1984;129:823–826. doi: 10.1164/arrd.1984.129.5.823. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Havell E A, Gigliotti F, Harmsen A G. Interleukin-6 production in a murine model of Pneumocystis carinii pneumonia: relation to resistance and inflammatory response. Infect Immun. 1993;61:97–102. doi: 10.1128/iai.61.1.97-102.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Havell E A, Harmsen A G. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect Immun. 1992;60:1279–1284. doi: 10.1128/iai.60.4.1279-1284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Havell E A, Moldawer L L, McIntyre K W, Chizzonite R A, Harmsen A G. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J Exp Med. 1992;176:713–718. doi: 10.1084/jem.176.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark R A F, editor. Wound repair. 2nd ed. New York, N.Y: Plenum Press; 1996. [Google Scholar]
- 8.Cohen O J, Stoeckle M Y. Extrapulmonary Pneumocystis carinii infections in the acquired immunodeficiency syndrome. Arch Intern Med. 1991;151:1205–1214. [PubMed] [Google Scholar]
- 9.Courtney M A, Stoler M H, Marder V J, Haidaris P J. Developmental expression of mRNAs encoding platelet proteins in rat megakaryocytes. Blood. 1991;77:560–568. [PubMed] [Google Scholar]
- 10.Dowton S B, Colten H R. Acute phase reactants in inflammation and infection. Semin Hematol. 1988;25:84–90. [PubMed] [Google Scholar]
- 11.Ezekowitz R A, Williams D J, Koziel H, Armstrong M Y, Warner A, Richards F F, Rose R M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991;351:155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 12.Gigliotti F. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1992;165:329–336. doi: 10.1093/infdis/165.2.329. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin J F. An evaluation of techniques for the separation and estimation of plasma fibrinogen. Clin Chem. 1965;11:63–73. [PubMed] [Google Scholar]
- 14.Guadiz G, Sporn L A, Goss R A, Lawrence S O, Marder V J, Simpson-Haidaris P J. Polarized secretion of fibrinogen by lung epithelial cells. Am J Respir Cell Mol Biol. 1997;17:60–69. doi: 10.1165/ajrcmb.17.1.2730. [DOI] [PubMed] [Google Scholar]
- 15.Guadiz G, Sporn L A, Simpson-Haidaris P J. Thrombin cleavage-independent deposition of fibrinogen in extracellular matrices. Blood. 1997;90:2644–2653. [PubMed] [Google Scholar]
- 16.Haidaris P J, Courtney M A. Liver-specific RNA processing of the ubiquitously transcribed rat fibrinogen γ-chain gene. Blood. 1992;79:1218–1224. [PubMed] [Google Scholar]
- 17.Haidaris P J, Courtney M A. Tissue-specific and ubiquitous expression of fibrinogen γ-chain mRNA. Blood Coagul Fibrinolysis. 1990;1:433–437. doi: 10.1097/00001721-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Haidaris P J, Peerschke E I, Marder V J, Francis C W. The C-terminal sequences of the γ 57.5 chain of human fibrinogen constitute a plasmin sensitive epitope that is exposed in crosslinked fibrin. Blood. 1989;74:2437–2444. [PubMed] [Google Scholar]
- 19.Haidaris P J, Wright T W, Gigliotti F, Fallon M A, Whitbeck A A, Haidaris C G. In situ hybridization analysis of developmental stages of Pneumocystis carinii that are transcriptionally active for a major surface glycoprotein gene. Mol Microbiol. 1993;7:647–656. doi: 10.1111/j.1365-2958.1993.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 20.Haidaris P J, Wright T W, Gigliotti F, Haidaris C G. Expression and characterization of a cDNA clone encoding an immunodominant surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1992;166:1113–1123. doi: 10.1093/infdis/166.5.1113. [DOI] [PubMed] [Google Scholar]
- 21.Harmsen A G, Chen W. Resolution of Pneumocystis carinii pneumonia in CD4+ lymphocyte-depleted mice given aerosols of heat-treated Escherichia coli. J Exp Med. 1992;176:881–886. doi: 10.1084/jem.176.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmsen A G, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasleton P S, Curry A, Rankin E M. Pneumocystis carinii pneumonia: a light microscopical and ultrastructural study. J Clin Pathol. 1981;34:1138–1146. doi: 10.1136/jcp.34.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idell S, Koenig K B, Fair D S, Martin T R, McLarty J, Maunder R J. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;261:L240–L248. doi: 10.1152/ajplung.1991.261.4.L240. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy M J, Calderone R A, Cutler J E, Kanabe T, Riesselman M H, Robert R, Senet J M, Annaix V, Bouali A, Mahaza C, et al. Molecular basis of Candida albicans adhesion. J Med Vet Mycol. 1992;30:95–122. [PubMed] [Google Scholar]
- 26.Languino L R, Plescia J, Duperray A, Brian A A, Plow E F, Geltosky J E, Altieri D C. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 27.Lanken P N, Minda M, Pietra G G, Fishman A P. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol. 1980;99:561–588. [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence S O, Wright T W, Francis C W, Fay P J, Haidaris P J. Purification and functional characterization of homodimeric γ B-γ B fibrinogen from rat plasma. Blood. 1993;82:2406–2413. [PubMed] [Google Scholar]
- 29.Limper A H, Hoyte J S, Standing J E. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Investig. 1997;99:2110–2117. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limper A H, Standing J E, Hoffman O A, Castro M, Neese L W. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect Immun. 1993;61:4302–4309. doi: 10.1128/iai.61.10.4302-4309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long E G, Smith J S, Meier J L. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Investig. 1986;54:609–615. [PubMed] [Google Scholar]
- 32.Millard P R, Wakefield A E, Hopkin J M. A sequential ultrastructural study of rat lungs infected with Pneumocystis carinii to investigate the appearances of the organism, its relationships and its effects on pneumocytes. Int J Exp Pathol. 1990;71:895–904. [PMC free article] [PubMed] [Google Scholar]
- 33.Neese L W, Standing J E, Olson E J, Castro M, Limper A H. Vitronectin, fibronectin, and gp120 antibody enhance macrophage release of TNF-α in response to Pneumocystis carinii. J Immunol. 1994;152:4549–4556. [PubMed] [Google Scholar]
- 34.O’Brodovich H M, Weitz J I, Possmayer F. Effect of fibrinogen degradation products and lung ground substance on surfactant function. Biol Neonate. 1990;57:325–333. doi: 10.1159/000243209. [DOI] [PubMed] [Google Scholar]
- 35.Odrljin T M, Francis C W, Sporn L A, Bunce L A, Marder V J, Simpson-Haidaris P J. Heparin-binding domain of fibrin mediates its binding to endothelial cells. Arterioscler Thromb Vasc Biol. 1996;16:1544–1551. doi: 10.1161/01.atv.16.12.1544. [DOI] [PubMed] [Google Scholar]
- 36.Odrljin T M, Shainoff J R, Lawrence S O, Simpson-Haidaris P J. Thrombin cleavage enhances exposure of a heparin binding domain in the N-terminus of the fibrin β chain. Blood. 1996;88:2050–2061. [PubMed] [Google Scholar]
- 37.O’Riordan D M, Standing J E, Kwon K Y, Chang D, Crouch E C, Limper A H. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J Clin Investig. 1995;95:2699–2710. doi: 10.1172/JCI117972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Riordan D M, Standing J E, Limper A H. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect Immun. 1995;63:779–784. doi: 10.1128/iai.63.3.779-784.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palluault F, Dei-Cas E, Slomianny C, Soulez B, Camus D. Golgi complex and lysomsomes in rabbit derived Pneumocystis carinii. Biol Cell. 1990;70:73–82. doi: 10.1016/0248-4900(90)90362-7. [DOI] [PubMed] [Google Scholar]
- 40.Phelps D S, Rose R M. Increased recovery of surfactant protein A in AIDS-related pneumonia. Am Rev Respir Dis. 1991;143:1072–1075. doi: 10.1164/ajrccm/143.5_Pt_1.1072. [DOI] [PubMed] [Google Scholar]
- 41.Pottratz S T, Paulsrud J, Smith J S, Martin W J D. Pneumocystis carinii attachment to cultured lung cells by pneumocystis gp 120, a fibronectin binding protein. J Clin Investig. 1991;88:403–407. doi: 10.1172/JCI115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pottratz S T, Weir A L. Attachment of Pneumocystis carinii to primary cultures of rat alveolar epithelial cells. Exp Cell Res. 1995;221:357–362. doi: 10.1006/excr.1995.1385. [DOI] [PubMed] [Google Scholar]
- 43.Robson S C, Saunders R, Purves L R, de Jager C, Corrigall A, Kirsch R E. Fibrin and fibrinogen degradation products with an intact D-domain C-terminal γ chain inhibit an early step in accessory cell-dependent lymphocyte mitogenesis. Blood. 1993;81:3006–3014. [PubMed] [Google Scholar]
- 44.Robson S C, Shephard E G, Kirsch R E. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 β, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haemotol. 1994;86:322–326. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 45.Seeger W, Stohr G, Wolf H R, Neuhof H. Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J App Physiol. 1985;58:326–338. doi: 10.1152/jappl.1985.58.2.326. [DOI] [PubMed] [Google Scholar]
- 46.Simpson-Haidaris P J. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood. 1997;89:873–882. [PubMed] [Google Scholar]
- 47.Simpson-Haidaris P J, Wright T W, Earnest B J, Hui Z, Neroni L A, Courtney M A. Cloning and characterization of a lung-specific cDNA corresponding to the γ chain of hepatic fibrinogen. Gene. 1995;167:273–278. doi: 10.1016/0378-1119(95)00679-6. [DOI] [PubMed] [Google Scholar]
- 48.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 49.Stokes D C, Gigliotti F, Rehg J E, Snellgrove R L, Hughes W T. Experimental Pneumocystis carinii pneumonia in the ferret. Br J Exp Pathol. 1987;68:267–276. [PMC free article] [PubMed] [Google Scholar]
- 50.Syrjala H, Lahdevirta J, Ruutu P, Jokipii L, Jokipii A M, Ruutu T. Acute phase response in Pneumocystis carinii pneumonia. Scand J Infect Dis. 1990;22:713–716. doi: 10.3109/00365549009027125. [DOI] [PubMed] [Google Scholar]
- 51.Thertulien R, Simpson-Haidaris P J, Haidaris C G. Intracellular localization of a Trypanosoma cruzi kDNA minicircle transcript using RNA: RNA in situ hybridization. J Eukaryot Microbiol. 1994;41:402–407. doi: 10.1111/j.1550-7408.1994.tb06097.x. [DOI] [PubMed] [Google Scholar]
- 52.Tran Van Nhieu J, Vojtek A M, Bernaudin J F, Escudier E, Fleury-Feith J. Pulmonary alveolar proteinosis associated with Pneumocystis carinii. Ultrastructural identification in bronchoalveolar lavage in AIDS and immunocompromised non-AIDS patients. Chest. 1990;98:801–805. doi: 10.1378/chest.98.4.801. [DOI] [PubMed] [Google Scholar]
- 53.Weber J. Interleukin-6: multifunctional cytokine. Biol Ther Cancer Updates. 1993;3:1–9. [Google Scholar]
- 54.Weber W R, Askin F B, Dehner L P. Lung biopsy in Pneumocystis carinii pneumonia: a histopathologic study of typical and atypical features. Am J Clin Pathol. 1977;67:11–19. doi: 10.1093/ajcp/67.1.11. [DOI] [PubMed] [Google Scholar]
- 55.Wisniowski P, Martin W J., 2nd Interaction of vitronectin with Pneumocystis carinii: evidence for binding via the heparin binding domain. J Lab Clin Med. 1995;125:38–45. [PubMed] [Google Scholar]
- 56.Wright S D, Weitz J I, Huang A J, Levin S M, Silverstein S C, Loike J D. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci USA. 1988;85:7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright T W, Johnston C J, Harmsen A G, Finkelstein J N. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Am J Respir Cell Mol Biol. 1997;17:491–500. doi: 10.1165/ajrcmb.17.4.2851. [DOI] [PubMed] [Google Scholar]
- 58.Yang F, Friedrichs W E, Navarijo-Ashbaugh A L, deGraffenried L A, Bowman B H, Coalson J J. Cell type-specific and inflammatory-induced expression of haptoglobin gene in lung. Lab Investig. 1995;73:433–440. [PubMed] [Google Scholar]
- 59.Yoshikawa H, Morioka H, Yoshida Y. Ultrastructural detection of carbohydrates in the pellicle of Pneumocystis carinii. Parasitol Res. 1988;74:537–543. doi: 10.1007/BF00531631. [DOI] [PubMed] [Google Scholar]
- 60.Yu M L, Limper A H. Pneumocystis carinii induces ICAM-1 expression in lung epithelial cells through a TNF-α-mediated mechanism. Am J Physiol. 1997;273:L1103–L1111. doi: 10.1152/ajplung.1997.273.6.L1103. [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman P E, Voelker D R, McCormack F X, Paulsrud J R, Martin W J., II 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J Clin Investig. 1992;89:143–149. doi: 10.1172/JCI115554. [DOI] [PMC free article] [PubMed] [Google Scholar]