Abstract
Transgenic (Tg) mice whose epidermal keratinocytes constitutively overexpress either B7-1 (CD80) or B7-2 (CD86) exhibited exaggerated cutaneous delayed type hypersensitivity (DTH) to haptens compared to non-Tg mice. To determine whether enhanced DTH in these Tg mice is seen in response to cutaneous fungal infections, a primary infection with Candida albicans was established by inoculating this organism on the occluded skin of Tg and non-Tg mice. These infections resolved 7 days after removal of occlusive dressing in all three groups of mice, without evidence of exaggerated inflammation in either the Tg or non-Tg mice. Only B7-2 Tg mice developed enhanced Th1-lymphocyte-mediated immune responses to C. albicans antigens after resolving this infection: enhanced footpad swelling in response to intradermal C. albicans antigens, enhanced production of mRNA encoding Th1 lymphokines in draining lymph nodes, and increased gamma interferon secreted into culture supernatants by lymph node T lymphocytes stimulated with Candida antigens in vitro. Lastly, Western blotting of sera from mice that had resolved this fungal infection indicated that only B7-2 Tg mice recognized a wide range of Candida-associated antigens. These data suggest that these two costimulatory molecules, when expressed by keratinocytes, do not deliver identical signals to C. albicans antigen-reactive Th1 lymphocytes. The enhanced immune response in B7-2 Tg mice to a cutaneous C. albicans infection demonstrates the importance of antigen presentation and costimulation in immune reactivity to fungi. Furthermore, B7-2 Tg mice may be useful in identification of protective Candida antigens.
Recurrent and chronic cutaneous infections with Candida albicans are common and medically important in humans in a variety of clinical settings (27, 41). The skin, mucous membranes, and the gastrointestinal tract are frequent sites of colonization and infection by this fungus. Host defense mechanisms that result in resistance to superficial or invasive infections with C. albicans involve, in part, CD4+ T-lymphocyte-mediated immunity (1, 3, 5, 18).
In some experimental mouse model systems, the outcomes of such candidal infections or the responses to vaccination with C. albicans antigens are dependent on the quality of the T-lymphocyte-mediated immune responses to C. albicans antigens. CD4+ T-helper lymphocytes can be divided into subsets based on their lymphokine profiles. Th1 clones produce interleukin-2 (IL-2) and gamma interferon and support delayed type hypersensitivity (DTH) (8, 34), and Th2 clones produce IL-4 and IL-5 and drive antigen-specific immunoglobulin synthesis (both immunoglobulin G1 [IgG1] and IgE) (8, 34). Th1-dominant, Candida-specific responses are associated with protective immunity (6, 7, 42–45), and Th2-dominant, Candida-specific responses are associated with disease promotion because of a lack of protective immunity (6, 7, 42–45). This paradigm of Th1-mediated protection and Th2-mediated disease promotion has been established by using responses to immunization followed by an invasive C. albicans infection whereby fungemia is established by intravenous injection of viable organisms (44). Similarly, mucosal infections with C. albicans indicate that this route of infection also induces Th1-mediated immunity in resistant mice (5, 13).
Percutaneous immunization of experimental mice with C. albicans antigens also results in cell-mediated immunity (11). Skin challenges of experimental animals that have preexisting immunity to C. albicans antigens will result in an anamnestic response (footpad swelling in mice) (6, 42–44). These data indicate that the skin immune system is an effective route for processing C. albicans antigens in a manner that results in local and/or systemic immunity. Mouse models of primary cutaneous C. albicans infections have been established (48–50), but the effects of a primary cutaneous C. albicans infection in the priming of cell-mediated immunity are poorly understood. The skin immune system that can respond to superficial C. albicans infections includes epidermal Langerhans cells, keratinocytes, and skin-associated lymphoid tissue (51). However, a direct role for gamma interferon in protection against murine C. albicans infections that originate either from a mucosal site or by direct inoculation into the bloodstream is controversial (6, 40).
Cutaneous cell-mediated immune responses can be manipulated in transgenic (Tg) mice. When either of the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) is overexpressed by keratinocytes in the skin by using a keratin promoter to target these second-signal molecules to epidermis in Tg mice, an amplification of cutaneous DTH to topical haptens is seen (2, 14, 36). The stable expression of B7-1 or B7-2 on the cell surfaces of epidermal keratinocytes induces it to act as a professional antigen presenting cell (APC) for CD4+ T-lymphocyte activation (2, 14, 15, 36), thus amplifying antigen presentation in the skin and resulting in exaggerated cutaneous DTH. This is in contrast to keratinocytes from non-Tg mice (16), which normally act as nonprofessional APCs for T-lymphocyte activation because they cannot deliver costimulatory signals to Th lymphocytes during antigen presentation. In physiologic situations, keratinocytes may downregulate cutaneous immunity because they induce Th1-lymphocyte clonal anergy (16). Thus, epicutaneous hapten challenge in B7-1 and B7-2 Tg mice results in exaggerated and persistent cutaneous DTH. In these Tg mice, the duration of ear swelling is longer than 6 weeks after a single hapten challenge and is accompanied by persistent Th1 lymphokine transcripts (2, 14, 36). The kinetics of DTH in B7-1 and B7-2 Tg mice are therefore profoundly altered compared to those in normal mice, in which ear swelling and Th1 lymphokine transcripts resolve within 72 h of hapten challenge.
To determine whether this exaggerated cutaneous DTH in B7-1 and B7-2 Tg mice would alter host-fungus interactions during a primary cutaneous infection with C. albicans, we utilized Tg mice in a superficial skin infection model and compared them to normal control mice. Herein we report that the overexpression of costimulatory molecules B7-1 and B7-2 by epidermal keratinocytes differentially affects the host immune responses to a primary cutaneous C. albicans infection. B7-1 Tg and non-Tg mice responded similarly to C. albicans infections, with weak Th1-mediated immune responses. In contrast, there was a significant augmentation of Th1-mediated immunity to C. albicans antigens in B7-2 Tg mice. These data suggest that these two costimulatory molecules, when expressed by keratinocytes, do not deliver identical signals to Candida antigen-reactive Th1 lymphocytes. The data also suggest that the intraepithelial microenvironment plays a critical role in modulating epidermal antigen presentation of C. albicans antigens.
MATERIALS AND METHODS
Tg mice.
The lines of heterozygous B7-1 and B7-2 Tg mice [(B6 × DBA/2)F1 background] described herein were created by standard methodologies previously described (36). The keratinocyte-specific K14 promoter targeted the overexpression of B7-1 or B7-2 to basal keratinocytes (36). Tg mice were identified by Southern blotting, and the transgene copy number was estimated (14, 36). Non-Tg control mice were age-matched littermates of either the B7-1 or B7-2 Tg mice. For the experimental C. albicans infections, we selected lines of B7-1 and B7-2 Tg mice that expressed similarly high levels of cell surface costimulatory molecules. The animals were housed in a pathogen-free environment at the vivarium of the University of Rochester School of Medicine and Dentistry, Rochester, N.Y., and provided with food and water ad libitum. All experimental animals were between the ages of 6 and 8 weeks when used in these studies. All experiments were reviewed and approved by the University Committee on Animal Resources.
Stool cultures for C. albicans were taken from representative non-Tg, B7-1 Tg, and B7-2 Tg mice to determine whether there was gastrointestinal colonization of these animals. Stool cultures were consistently negative for C. albicans, indicating that none of the mice in these animal colonies carried this fungus. None of 10 sentinel mice from our colony had detectable fungus in their stool specimens. They were thus considered immunologically naive to C. albicans antigens.
Histologic specimens.
Skin biopsy specimens were fixed in 10% formaldehyde and processed for paraffin embedding by standard methods. Ten-micrometer-thick sections were cut onto glass slides and stained with hematoxylin and eosin to study the characteristics of the tissue inflammatory reaction to the fungal infection or Gomori’s methenamine silver (GMS) stain to detect C. albicans in tissue samples (12).
Immunofluorescence detection of B7 transgene expression.
To identify Tg mice, skin biopsy specimens were obtained from distal tail, embedded in OCT medium (Miles Laboratories, Elkhart, Ind.), and snap frozen, and 5-μm-thick cryostat sections were cut onto glass slides (36). They were fixed for 5 min at 4°C in acetone and washed. The specimens were then stained with uncoupled CTLA4/Ig (10 μg/ml) (a generous gift from Peter S. Linsley, Seattle, Wash.) or with control Ig. Staining was detected with fluorescein coupled F(ab′)2 fragments of goat anti-human Ig. The specimens were examined with a Jena Lumar epifluorescence microscope (Zeiss, Oberkochen, Federal Republic of Germany), and photomicrographs were obtained with the same microscope.
Analysis of B7 transgene expression in mouse epidermis by CSLC.
The level of expression of B7-1 and B7-2 transgene expression in the epidermis of the Tg mice was analyzed by fluorescence-based confocal scanning laser cytometry (CSLC) with a Meridian Ultima Adherent Cell Analysis System (ACAS) and its accompanying Data Analysis System (DASY) Master Program , v. 3.32 (Meridian Instruments, Inc., Okemos, Mich.). CSLC with the ACAS and DASY provided imaging capacity on stationary samples comparable to conventional confocal scanning laser microscopy but with the added quantitative analysis of fluorescence intensity provided by flow cytometry. This obviated the need for enzymatic dissociation of tissue prior to analysis and the consequent loss of antigen due to proteolytic degradation during the dissociation process. Samples were excited with a 488-nm argon laser line, and emissions below 575 nm were detected with a photomultiplier tube. All samples were examined with a 40× objective and scanned with an identical set of parameters so that results from different tissue sections could be compared. In all cases, the laser beam (approximately 0.3 μm in thickness) scanned the sample in a Raster pattern at intervals of 1.0 μm. Fluorescence was detected from the entire thickness of the epidermis of a 5-μm tail section over an area of approximately 2,500 mm2.
From the data points collected, digitized images of scanned sections were generated that corresponded to a scale of pixel values ranging from 0 to 4,095 relative fluorescence units (RFU). From the digitized images, statistical analysis was performed with programs in the DASY software package. These included (i) the number of fluorescent pixels in the scanned area above the background threshold level; (ii) the average fluorescence in RFU, which was obtained by dividing the total integrated value of the foreground RFU by the number of data points collected; and (iii) the average background threshold level. In the images derived from the B7-1 and B7-2 Tg mice, pixels with relative fluorescence values of 400 or less were subtracted as background from the original scan prior to the statistical analysis. Since the average fluorescence of the tissue sections derived from the non-Tg mice was well below 400 RFU, no pixels were subtracted prior to statistical analysis.
C. albicans.
Strain 613p of C. albicans (22, 23, 33, 52) was used for all cutaneous infections, footpad intradermal injections for DTH, and extraction of cell wall antigens for T-lymphocyte proliferation assays. For the primary cutaneous infections, viable blastoconidia of C. albicans 613p cells were grown overnight in yeast extract-peptone-dextrose medium (YEPD) broth at 37°C. The concentration of blastoconidia at the time they were collected was approximately 4 × 108/ml. The blastoconidia were washed twice in sterile phosphate buffered saline (PBS) and utilized for primary cutaneous fungal infections (see below).
Preparation of C. albicans-derived antigens.
To provide soluble C. albicans antigen for T-lymphocyte proliferation assays, a cell wall extract was prepared from blastoconidia of strain 613p as described previously (31, 39). Antigens for Western blotting were also derived from blastoconidia of strain 613p. Cells were grown to stationary phase by overnight culture in YEPD and washed twice in PBS. The cell pellets from 20 ml of culture (approximately 4 × 108 cells/ml) were suspended in 2 ml of concentrated sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 3% SDS and 15% β-mercaptoethanol. The cell suspension was incubated in a boiling water bath for 15 min, the insoluble material was pelleted by centrifugation, and the supernatant containing solubilized antigens was removed and stored at −20°C until use. The extracted antigens were separated by preparative SDS-PAGE and electro-transferred to nitrocellulose for Western blotting (19).
Primary cutaneous fungal infections.
Groups of experimental animals (either non-Tg, B7-1 Tg, or B7-2 Tg mice) were prepared for primary cutaneous fungal infections as follows. A 2- by 2-cm area of abdominal skin was shaved until all fur was removed. The exposed skin was then gently abraded with fine-grade, sterile sandpaper (#3 grade; 3M Company, St. Paul, Minn.) until the skin was glistening but not bleeding. Next, 107 C. albicans blastoconidia were pipetted onto the surface of the skin in a 20-μl volume of sterile PBS. The area was occluded with a 0.5- by 0.5-cm piece of sterile gauze that was moistened with sterile PBS. This layer was further occluded with a 1- by 1-cm piece of plastic sheeting (Reynolds Wrap, R.J. Reynolds Company, Richmond, Va.). The final layer of the dressing was an elasticized adhesive gauze (Elastacon dressing; 3M Company), which was kept wrapped circumferentially around the experimental animals’ torsos during the 5 days of the experimental occlusion. This dressing was examined daily and reinforced as needed. The normal activities of the experimental animals were not affected by the presence of these dressings. This occlusive dressing remained on the skin for 5 days and was then removed to expose the infected skin to the air. In some cases, the animals were allowed to recover from infection and the lesion healed spontaneously. In other cases, animals were sacrificed by asphyxia in a CO2 atmosphere, and skin biopsy specimens were obtained at predetermined times for quantitative fungal cultures or histology.
Monitoring of C. albicans in experimental skin infections.
After establishing a skin infection with C. albicans or PBS (sham control), occlusive dressings were removed, the mice were sacrificed, and skin biopsy specimens were taken from the infection site at selected times. The skin biopsy specimens were weighed on a Mettler triple beam balance to the nearest 0.01 g and were then transferred to a sterile microfuge tube. To each tube, 400 μl of tissue culture grade 0.25% trypsin-EDTA solution (Gibco/BRL, Gaithersburg, Md.) was added. The tubes were incubated at 37°C for 30 min with intermittent vortexing. Pilot studies indicated that this enzymatic solution did not affect the viability or the colony-forming ability of C. albicans (which had 100% plating efficiency after exposure to 0.25% trypsin-EDTA). Serial dilutions of the dissociated tissue slurry were plated on YEPD agar plates, the plates were incubated for 48 h at 37°C, and colony forming units (CFU) were counted. Data on the total CFU were divided by the tissue weight (in grams), resulting in values for CFU/gram of tissue. The CFU/gram of tissue values for each of the mice in an experimental group were pooled together and expressed as a mean ± standard error of the mean (SEM). A semiquantitative analysis of the presence of C. albicans in mice allowed to recover from infection by the removal of the occlusive dressing was performed by swabbing the previously infected area with a cotton swab dipped in sterile PBS and rolling the swab onto YEPD plates. A relative evaluation of organism numbers was performed by assessing the C. albicans CFU obtained in this manner.
Measurement of DTH.
DTH responses in mice were determined by measurement of footpad swelling following subcutaneous injection of C. albicans organisms. This was accomplished by the injection with a micrometer syringe of 107 heat-killed C. albicans (HKCA) organisms in 25 μl of sterile PBS (right footpad) or sterile PBS alone (control, left footpad) into the footpads of naive or immunized mice or into mice that had resolved primary cutaneous skin infections. Footpad thickness was measured with thickness gauge calipers (Swiss Precision Instruments) initially to establish a baseline (before the injection) and then at intervals 24 to 96 h after the HKCA or PBS injection. The mean net increase in footpad thickness for each group was determined after the subtraction of pre- and postinjection values. In each experiment, the animals were randomized, coded, injected by one individual, and measured by a second individual to whom the code was unknown. At the time of footpad testing, animals were housed in a random manner as well, so that a group representing one experimental condition was not housed in the same cage. The changes in footpad thickness from all the mice in an experimental group were pooled together and expressed as a mean value ± SEM.
In vitro T-cell proliferation assay.
To assess local T-cell proliferative responses to C. albicans antigens, we used a modified draining lymph node assay (11). Experimental animals (either non-Tg, B7-1 Tg, or B7-2 Tg mice, n = 5 mice per group) were challenged with 107 C. albicans organisms (as described above) or sham infected with PBS. The occlusive dressing was removed, and the infection was allowed to resolve. One week after the infection resolved, experimental mice (either C. albicans infected or sham infected) were challenged with a single injection of 107 HKCA organisms in a 25 μl volume into the dermis of their footpads. One week after this injection, single-cell suspensions of lymph node cells were prepared from the peripheral lymph nodes from non-Tg, B7-1 Tg, and B7-2 Tg mice (repeated in three separate experiments). T-lymphocyte culture was performed in 96-well round-bottom plates in a humidified 5% CO2 atmosphere for 120 h. T lymphocytes were cultured with medium alone, medium containing 10 U of recombinant human IL-2 (rIL-2; Genzyme Corp., Boston, Mass.)/ml, or C. albicans cell wall extract (10 μg/ml final concentration in culture medium). During the last 18 h of culture, 1 μCi of [3H]thymidine (TdR) was added to the culture well. Cells were harvested and [3H]TdR incorporation into DNA was measured by liquid scintillation counting. T-lymphocyte medium consisted of RPMI (MA Biofluids, Walkersville, Md.) with 10% fetal calf serum, antibiotic solution, glutamine (GIBCO Laboratories, Grand Island, N.Y.), and indomethacin (1 μg/ml) (Sigma Chemical Co., St. Louis, Mo.). Samples were assayed in triplicate, and the data are presented as means ± standard deviations.
Lymphokine ELISA.
To measure the Th1-derived lymphokine, gamma interferon, and the Th2-derived lymphokine, IL-4, commercially available specific enzyme-linked immunosorbent assays (ELISAs) were utilized (R & D Systems, Minneapolis, Minn.). Cell-free culture supernatants were collected from lymph node T lymphocytes that were stimulated with medium alone or medium containing 10 μg of C. albicans cell wall extract/ml. The supernatants were collected after 72 h of culture and stored at −20°C until their use in an ELISA. All samples were assayed in duplicate. Gamma interferon or IL-4 content in supernatants was estimated by comparing the optical densities of the unknowns to those of the standards.
RNA extraction.
Lymph nodes or abdominal skin was harvested from sham-infected or C. albicans-infected mice. For skin samples, the subcutaneous fat was removed by microdissection. The remaining tissue was snap frozen in liquid nitrogen and pulverized with a mortar and pestle. Total RNA was then extracted with phenol-chloroform (14) and quantitated by spectrophotometry.
RNase protection assay.
Eight micrograms of total RNA isolated from the skin or lymph nodes was used in the RiboQuant Multiprobe RNase Protection Assay System employing the mCK-3 template set (Pharmingen, San Diego, Calif.) (17, 37, 46). The protected RNA duplexes were resolved on a 40-cm, 6%, 19:1 acrylamide sequencing gel. The dried gel was then used in several rounds of autoradiography such that exposure was within the linear recording range of the film (X-Omat; Eastman Kodak, Rochester, N.Y.) for the cytokines of interest. Optical density readings of the resultant bands were obtained with a Molecular Dynamics Personal Densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). Specific cytokine transcripts were determined by comparing the relative migration of unprotected, undigested 32P-labelled cytokine probes to that of RNase-digested, protected 32P-labelled cytokine probes derived from a mouse mRNA standard set (EL-4 RNA). Hybridized, protected probes demonstrated specific, predictable alterations in their relative migration on the acrylamide gel (17, 37, 46).
Western blotting.
Immunodetection of C. albicans antigens with sera from individual mice that had recovered from infection was performed by a methodology described previously (19). Sera from infected mice were obtained 21 days after resolution of the infection. Sera from mice vaccinated with HKCA in complete Freund’s adjuvant (CFA) were collected after confirmation that the animals had developed DTH as measured by footpad swelling following antigen challenge. All sera were used at a dilution of 1:100 in the Western blotting.
Statistical analysis.
Data were analyzed for statistical significance with the two-tailed unpaired Student’s t test (Statview student software program; Abacus Concepts, Berkeley, Calif.). Probability values of <0.05 were considered statistically significant.
RESULTS
Expression of B7-1 and B7-2 costimulatory molecules in keratinocytes of Tg mice.
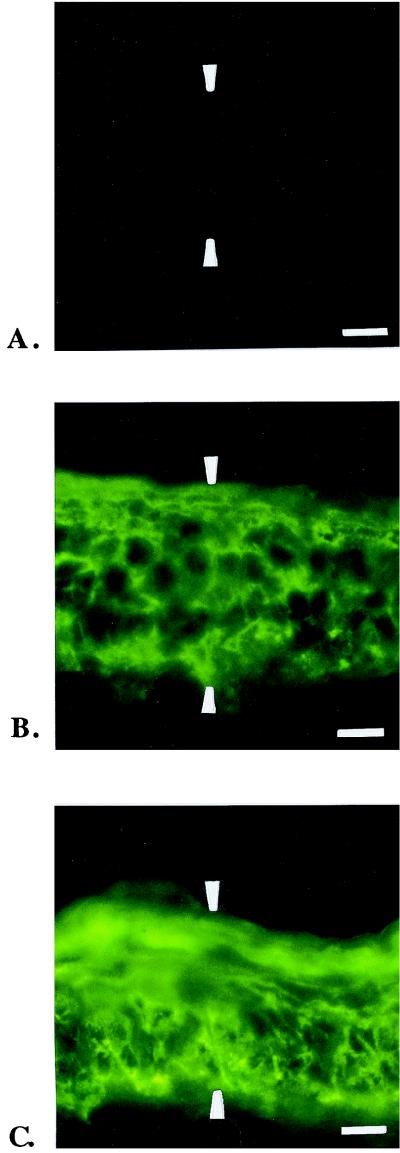
Immunofluorescence analysis of mouse skin confirmed high levels of cell surface expression of either B7-1 or B7-2 in the Tg mice and a lack of expression of these costimulatory molecules in non-Tg mice (Fig. 1A, B, and C). To provide quantitative evidence that the relative level of expression of each costimulatory molecule was equivalent in the keratinocytes from representative animals of the respective Tg mouse strains, tail sections from B7-1, B7-2, and non-Tg mice were stained with CTLA4/Ig and detected with fluorescein-coupled goat anti-human Ig. These stained sections were then analyzed by CSLC, and the relative level of fluorescence in each section was quantitated. A statistical analysis of the scanned tissue sections from two representative animals of each mouse strain is shown in Table 1. The relative levels of B7-1 and B7-2 expression were nearly equivalent in the epidermis of the respective Tg mice, with the expression of B7-1 slightly higher, on average, than B7-2 expression. The epidermis of the non-Tg mouse strain did not express detectable levels of B7.
FIG. 1.
Keratinocytes in the epidermis of Tg mice express equally high levels of B7-1 and B7-2 costimulatory molecules. Fluorescence photomicrography of tail skin from non-Tg mice (A), B7-2 Tg mice (B), and B7-1 Tg mice (C) stained with CTLA-4/Ig fusion protein to detect B7-1 or B7-2 expression on the cell surfaces of epidermial keratinocytes. Tissue outlined by arrowheads represents epidermis. Scale bars, 20 μm. Staining of the epidermis with control Ig was negative in all three groups of mice (data not shown). For the C. albicans skin infection model, lines of B7-1 Tg and B7-2 Tg mice that had similar high levels of cell surface expression of these costimulatory molecules by keratinocytes were selected.
TABLE 1.
Analysis of B7 transgene expression in epidermis of mice
| Samplea | No. of fluorescent pixels | Avg fluorescence (RFU)b | Avg background (RFU) |
|---|---|---|---|
| B7-2 Tg | 6,676 | 1,104 | 122 |
| B7-2 Tg | 7,878 | 880 | 174 |
| B7-1 Tg | 10,741 | 1,248 | 207 |
| B7-1 Tg | 10,046 | 1,284 | 146 |
| Non-Tg | 2,822 | 121 | 70 |
| Non-Tg | 2,051 | 106 | 74 |
Strain of mouse from which tissue was derived. Tissue sections from two animals from each strain were examined by indirect immunofluorescence and CSLC.
Relative fluorescence units.
Normal resolution of primary cutaneous C. albicans infections in B7-1 and B7-2 Tg mice.
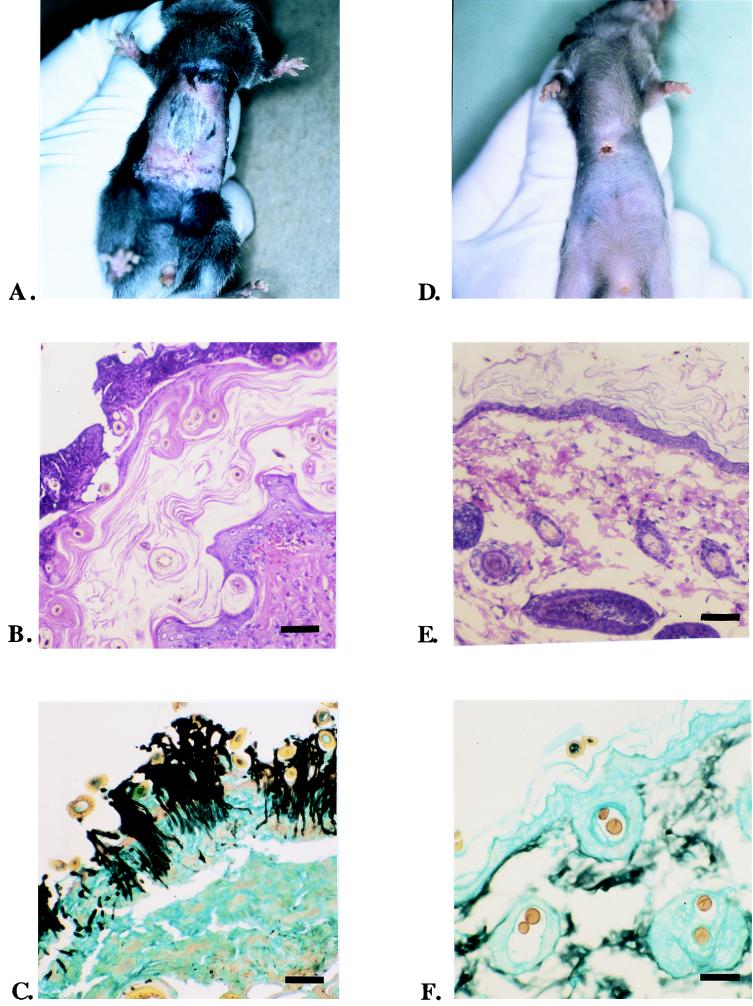
To determine whether B7-1 and B7-2 Tg mice resolved primary C. albicans infections normally, we established an infection of abdominal skin in groups of five animals of the Tg mice and non-Tg control mice. Histologic study of skin biopsy specimens from the sites of primary infection was done with hematoxylin and eosin- and GMS-stained sections to assess qualitative aspects of the skin infection and the host inflammatory response. As indicated in Fig. 2A, there was a vigorous primary cutaneous C. albicans infection of the skin of all animals (B7-1 Tg, B7-2 Tg, and non-Tg mice) on day 0, when the occlusive dressings were removed from the skins of the experimentally infected animals. As demonstrated by GMS staining of the biopsy specimens taken from the skin infection sites (Fig. 2C), there were multiple infectious foci in the thickened stratum corneum of infected animals, characterized by the presence of numerous pseudohyphae and blastoconidia. Although this infection remained localized to the stratum corneum of the epidermis, rarely invaded the viable epidermis, and never invaded the dermis, there was a vigorous host response, as indicated in hematoxylin and eosin-stained skin biopsy specimens (Fig. 2B and G). The inflammatory infiltrate induced by a primary cutaneous C. albicans infection consisted of a mixture of monocytes, lymphocytes, and neutrophils, with exocytosis of some of these inflammatory cells into the epidermis occurring at the site of the infection. There were no qualitative differences in the nature of the inflammatory infiltrate induced by the superficial fungal infection among B7-1 Tg, B7-2 Tg, and non-Tg mice. Histologic studies of skin biopsy specimens from sham-infected mice demonstrated neither the presence of yeast forms in skin nor an inflammatory skin reaction (data not shown).
FIG. 2.
Clinical, histologic, and microbiologic features of primary cutaneous C. albicans skin infections in B7-1 Tg, B7-2 Tg, and non-Tg mice. Primary skin infections with C. albicans were established as described in Materials and Methods. (A) Appearance of skin lesion induced by this fungal infection after 5 days of occlusion of 107 blastoconidia of C. albicans on a non-Tg mouse. This is the appearance immediately after removing the occlusive dressing. (B) Hematoxylin and eosin stain of skin biopsy specimen taken from the infection site, revealing a vigorous inflammatory reaction consisting of a mixture of monocytes, lymphocytes, and neutrophils. (C) GMS stain of skin biopsy specimen taken from the infection site on the same day, indicating the presence of pseudohyphae and blastoconidia. (D) Healing of skin lesion 7 days after removing the occlusive skin dressing. (E) Hematoxylin and eosin stain of skin biopsy specimen taken the same day. (F) GMS stain of skin biopsy site of healed skin on the same day. Pseudohyphae and blastoconidia are no longer present. Inflammatory infiltrate has completely resolved. (G) Higher magnification of panel B, revealing the nature of inflammatory infiltrate in the papillary dermis. The infiltrate was composed of lymphocytes, macrophages, and granulocytes. Scale bars, 150 μm (B and E); 20 μm (C, F, and G). The skin lesions healed, and the histologic abnormalities resolved, similarly in all three groups of mice (data not shown).
Our observations of the healing of the skin lesions induced by the experimental C. albicans skin infections in B7-1 Tg, B7-2 Tg, and non-Tg control mice indicated that the skin lesions induced by the infection healed by approximately 7 days after exposure to air (Fig. 2D). Histologic study of GMS-stained skin biopsy specimens (Fig. 2F) confirmed that the organism was absent from the epidermis at this time and that the host inflammatory response to this fungal infection subsided (Fig. 2E). As assessed by observations of the healing of skin lesions and histologic study of skin biopsy specimens, there were no differences in the rates of resolution of the cutaneous infections among the B7-1 Tg, B7-2 Tg, and non-Tg mice.
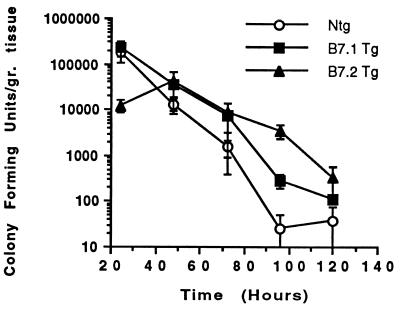
We confirmed the resolution of the primary skin infection by a microbiologic method for the presence of CFU derived by swabbing the skin infection sites and plating the samples. This study showed that primary cutaneous C. albicans skin infections resolved over 5 to 7 days in the B7-1 Tg, B7-2 Tg, and non-Tg control mice (Fig. 3). Although the B7-2 Tg mice had significantly lower CFU at the 24-h time point compared to the B7-1 Tg and non-Tg mice, all three groups of animals resolved the infections after 5 to 7 days. Cultures of the skin of sham-infected mice (Tg or non-Tg mice) indicated that there were no C. albicans organisms on the skin surfaces of these control animals (data not shown). These microbiologic data are consistent with our observations of the skin lesions as well as our histologic studies of skin biopsy specimens derived from the infection sites.
FIG. 3.
Primary cutaneous C. albicans infections resolve normally in B7-1 Tg, B7-2 Tg, and non-Tg mice. Assessment of the rate of resolution in groups of experimental B7-1 Tg, B7-2 Tg, and non-Tg (Ntg) controls (n = 5 mice per group for each time point). C. albicans CFU per gram of tissue were assessed daily over 5 days after removing the occlusive dressings that initiated the infections. All three groups of animals resolved their skin infections at a similar rate. Data depicted are the means ± SEM in semi-log scale.
Cutaneous C. albicans infections induce enhanced DTH in B7-2 Tg mice.
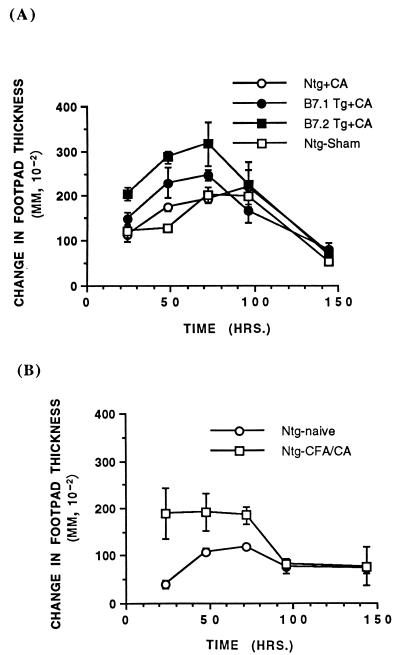
To determine whether these primary cutaneous C. albicans infections induced DTH to this fungus, we challenged groups of B7-1 Tg, B7-2 Tg, and non-Tg mice with a single intradermal footpad injection of 107 HKCA organisms 1 week after the animals had resolved a primary cutaneous infection. As depicted in Fig. 4A, B7-2 Tg mice developed the most vigorous DTH in response to HKCA. Their response was significantly greater than that of the non-Tg infected mice and the sham-infected mice (P < 0.05, two-tailed, unpaired Student’s t test). It is noteworthy that the non-Tg mice that had resolved the cutaneous C. albicans infection demonstrated weak footpad DTH to HKCA that was not significantly different from that of the sham-infected mice. Although the B7-1 Tg mice demonstrated greater DTH compared to the non-Tg C. albicans-infected or sham-infected control mice, this difference was not statistically significant. These data imply that B7-2 expression by keratinocytes augmented an immune response to a primary cutaneous C. albicans infection.
FIG. 4.
B7-2 Tg mice that had resolved a primary skin infection exhibit enhanced footpad swelling in response to intradermal challenge with C. albicans antigens. (A) Seven days after resolving a primary cutaneous infection with C. albicans (or sham infections), groups of B7-1 Tg, B7-2 Tg, and non-Tg (Ntg) mice (n = 3 mice per group) were challenged with an intradermal injection of 107 HKCA organisms (CA) in sterile PBS (right footpad) or PBS alone (left footpad). (B) Non-Tg mice were immunized with 107 HKCA organisms emulsified in CFA and boosted with similar numbers of C. albicans organisms 1 week later; naive mice served as a negative control. After another week, these animals were challenged with an intradermal injection of 107 HKCA organisms in sterile PBS (right footpad) or PBS alone (left footpad). Change in footpad thickness was followed over time. Data depicted represent the means ± SEM of changes in footpad thickness over time.
Since the immunization of mice with whole pathogens emulsified in an adjuvant such as CFA produces an immune response, we immunized non-Tg mice with a single subcutaneous injection of 107 HKCA organisms emulsified in CFA. This group of animals was studied to determine whether direct immunization with HKCA would result in more vigorous DTH than would a primary cutaneous infection. These immunized mice developed significant DTH on rechallenge (intradermal challenge into the footpad) with HKCA compared to naive mice (Fig. 4B). However, the resolution of the primary fungal infection in B7-2 Tg mice resulted in a greater DTH than did HKCA-CFA immunization of naive mice.
Cutaneous C. albicans infections resulted in enhanced Th1 lymphokine transcripts in situ in lymph nodes from B7-2 Tg mice.
To determine the mechanism of the enhanced DTH to C. albicans antigens in the B7-2 Tg mice, we studied lymphokine transcript profiles (RNase protection assay) in the draining lymph nodes (inguinal chain) in C. albicans-infected B7-1 Tg, B7-2 Tg, and non-Tg mice as well as sham-infected non-Tg (negative control) or noninfected HKCA-CFA-immunized mice (positive control) (Fig. 5). Transcripts for IL-2 and gamma interferon (Th1 lymphokines) and for IL-12 p40 (an APC-derived cytokine that enhances gamma interferon production) were detected in RNA extracted from lymph nodes from all groups of these experimental mice. Lymph node cells from B7-2 Tg mice responded to the C. albicans infection most vigorously, resulting in the highest Th1 lymphokine transcript response when compared to the other experimental groups, including the HKCA-CFA-immunized mice. Transcripts for IL-4 or IL-10 (Th2 lymphokines) were not detected in mRNA from any of these groups of animals. However, IL-4 and IL-10 transcripts were detectable in mRNA from EL-4 mouse thymoma, which was a positive control for these cytokines in the RNase protection assay.
FIG. 5.
B7-2 Tg mice exhibit enhanced production of Th1-cytokine transcripts in regional lymph nodes after primary cutaneous fungal infections. Primary infections with C. albicans (or sham infections) were established on the skin of groups of B7-1 Tg, B7-2 Tg, and non-Tg (Ntg) mice (n = 3 mice per group). One week after removing the dressing, regional lymph nodes were harvested, RNA was extracted, and cytokine transcripts were studied by RNase protection assay. Control groups include sham-infected non-Tg and CFA-immunized mice, but not infected mice. Data depicted represent the ratio of the optical density of the cytokine gene to that of the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) for each of the groups of experimental mice. RNA extracted from lymph nodes of B7-2 Tg mice had consistently higher Th1 lymphokine profiles than that from B7-1 Tg or non-Tg mice.
T lymphocytes from B7-2 Tg mice respond to soluble C. albicans antigens in vitro with enhanced production of gamma interferon.
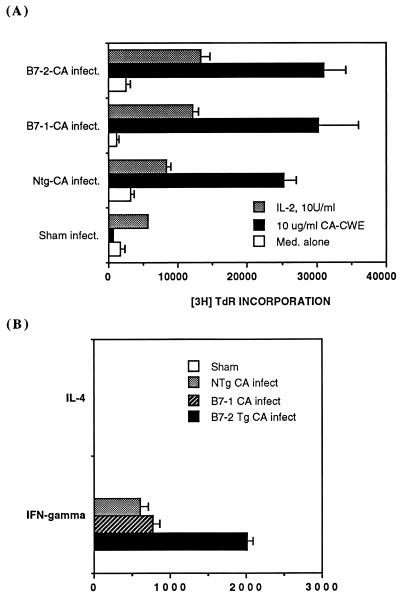
To further confirm the significance of the Th1 lymphokine transcript profile observed in RNase protection studies from draining lymph nodes from C. albicans-infected mice, T-helper lymphocyte proliferation and lymphokine production in response to C. albicans antigens in an in vitro assay (Fig. 6A and B) were studied. One week after the resolution of a primary cutaneous C. albicans infection or sham infection, B7-1 Tg, B7-2 Tg, and non-Tg mice were challenged with a single intradermal injection of 107 HKCA organisms into the footpad. One week later, draining lymph nodes (popliteal) were dissected, and lymphocyte cell suspensions were isolated and stimulated with a water-soluble cell wall extract of C. albicans. As depicted in Fig. 6A, lymph node T lymphocytes from animals that had resolved this fungal infection proliferated vigorously in response to a C. albicans cell wall extract. In contrast, lymph node T lymphocytes from sham-infected animals did not respond to this cell wall extract. IL-4 and gamma interferon contents of the cell-free supernatants of T lymphocytes stimulated with medium alone (not shown) or a water soluble C. albicans cell wall extract (Fig. 6B) were studied by a specific ELISA. Lymph node T lymphocytes from animals that had resolved primary cutaneous infections with C. albicans all produced gamma interferon but not IL-4 in response to the C. albicans antigens. T lymphocytes from B7-2 Tg mice produced significantly more gamma interferon compared to C. albicans-infected B7-1 Tg and non-Tg mice and sham-infected control mice. Thus, the direct assay of in vitro T-lymphocyte responses to C. albicans antigens confirmed the data of the footpad DTH assay and the RNase protection assay, which both indicated that B7-2 Tg mice responded to a primary cutaneous infection with a more vigorous Th1-mediated immune response than B7-1 Tg mice or non-Tg control mice.
FIG. 6.
Lymph node lymphocytes from B7-2 Tg mice exhibit enhanced production of gamma interferon when rechallenged with C. albicans antigens in vitro. Primary infections with C. albicans (or sham infections) were established on the skin of groups of B7-1 Tg, B7-2 Tg, and non-Tg mice (n = 3 mice per group). One week after removing the dressing, 107 HKCA were injected into their footpads, and after another 7 days, their draining lymph nodes were harvested and studied in vitro. (A) Proliferative response ([3H]TdR incorporation) of T lymphocytes from draining lymph node to medium alone, C. albicans cell wall extract (CA-CWE), or 10 U of rIL-2/ml. (B) Cytokines (IL-4 or gamma interferon) secreted into supernatants by T lymphocytes stimulated by C. albicans cell wall extracts. Data represent means ± standard deviations of triplicate specimens derived from one representative experimental animal from each group. This pattern of results was reproducible in all experimental animals studied (n = 3).
Primary cutaneous infections in B7-2 Tg mice induce vigorous antibody responses against numerous C. albicans antigens.
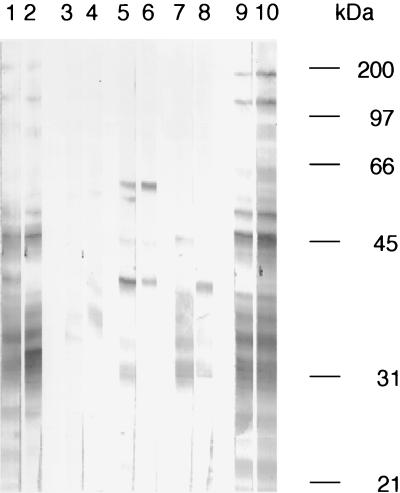
To determine whether primary cutaneous C. albicans infections affected T-lymphocyte-derived help for a specific antibody response against C. albicans antigens, we tested the sera of mice that had resolved a primary skin infection for Candida-specific antibodies by Western blotting (Fig. 7). Candida-specific antibody responses of naive and parenterally immunized non-Tg mice, as well as non-Tg, B7-1, and B7-2 Tg mice that had resolved primary C. albicans or sham infections were examined. Neither preinfection sera from infected mice (data not shown) nor postinfection sera from sham-infected animals (Fig. 7, lanes 3 and 4) demonstrated any reactivity by Western blotting to C. albicans antigens extracted from blastoconidia. Sera from either non-Tg mice (Fig. 7, lanes 5 and 6) or B7-1 Tg mice (Fig. 7, lanes 7 and 8) reacted weakly with C. albicans antigens, whereas sera from parenterally immunized non-Tg mice reacted more vigorously (Fig. 7, lanes 1 and 2). In striking contrast to the reactivity of the sera from the infected non-Tg and B7-1 Tg mice, sera from B7-2 Tg mice that had resolved cutaneous infections reacted very strongly with numerous polypeptides over a broad range of antigens derived from C. albicans (Fig. 7, lanes 9 and 10).
FIG. 7.
Primary cutaneous C. albicans infection induces vigorous antibody responses in B7-2 Tg mice. Primary infections with C. albicans (or sham infections) were established on the skin of groups of B7-1 Tg, B7-2 Tg, and non-Tg mice. Sera were obtained at 21 days after resolving the infection. Sera were also obtained from mice immunized parenterally with HKCA blastoconidia, after confirmation that the animals had developed DTH as measured by footpad swelling following antigen challenge. Sera were used to probe a Western blot of C. albicans blastoconidial antigens separated on an SDS–12% PAGE/gel. Sera from two different animals in each experimental group are shown. All sera were used at a dilution of 1:100 in the Western blot. Lanes: 1 and 2, non-Tg mice immunized with HKCA emulsified in CFA; 3 and 4, non-Tg mice, sham infected; 5 and 6, non-Tg mice infected with C. albicans; 7 and 8, B7-1 Tg mice infected with C. albicans; 9 and 10, B7-2 Tg mice infected with C. albicans.
DISCUSSION
This study utilized Tg mice in which keratinocytes expressed either CD80 (B7-1) or CD86 (B7-2) and compared their immune responses to primary cutaneous C. albicans infections to those of non-Tg mice. These lines of Tg mice were selected because of their exaggerated and persistent cutaneous DTH to epicutaneous haptens (2, 14, 36). DTH to epicutaneous haptens is a result of hapten-specific, CD4+ T-lymphocytes that usually have a Th1 lymphokine profile (9, 10, 47). Thus, these lines of Tg mice provide an opportunity to determine whether exaggerated DTH to haptens was also seen in response to an infectious organism such as C. albicans, in which CD4+ T lymphocytes with a Th1 lymphokine profile mediate protective host immunity (6, 7, 42–45).
Although the experimental data indicated that there was an enhanced immune response after a primary cutaneous C. albicans infection in Tg mice, there were differences noted in the characteristics of the host response to this fungus compared to the response to haptens. First, primary or secondary applications of haptens to B7-1 or B7-2 Tg mouse skin results in exaggerated inflammation, which was apparent upon measurement of ear swelling and examination of histology of skin biopsy specimens (2, 14, 36, 53). There was no evidence of exaggerated inflammation in B7-1 Tg or B7-2 Tg mouse skin during the resolution of the primary cutaneous C. albicans infection (Fig. 2). Primary or secondary applications of haptens to Tg mouse skin resulted in chronic inflammation that lasted weeks instead of 48 to 72 h as in non-Tg mice, which was apparent upon measurement of ear swelling and examination of histology of skin biopsy specimens (2, 14, 36). There was no evidence of persistent inflammation after the resolution of the fungal infection in B7-1 Tg or B7-2 Tg mice, since the inflammatory infiltrates resolved with the healing of the skin infection (Fig. 2). These data indicate that the type of inflammatory response is dependent on the specific insult (hapten versus infectious agent) to Tg mouse skin that elicits DTH. One possible explanation for persistence of DTH to haptens but not C. albicans infections is that there is evidence that some haptens, which form chemical conjugates to skin proteins, may persist for long periods of time (54). This may provoke a chronic T-lymphocyte inflammatory response in Tg mice. In contrast, microbial antigens such as those associated with C. albicans may be cleared from the skin more rapidly (in 5 to 7 days), as suggested by the results of the fungal cultures from the skin of infected mice (Fig. 3).
There was another important difference between the hapten model and the C. albicans infection model in these Tg mice. After hapten applications, B7-1 Tg mice had the most vigorous inflammatory responses and cytokine transcript upregulation in response to primary and secondary applications of haptens or irritants, greater than those of B7-2 Tg mice and non-Tg controls (2, 14, 36). In the C. albicans skin infection model, B7-2 expression in the epidermis of Tg mice amplified Th1-lymphocyte-mediated Candida-specific responses more than B7-1 in the epidermis of Tg mice. B7-1 expression in the epidermis of Tg mice did not amplify Th1-lymphocyte-mediated responses, since these Tg mice responded as weakly to C. albicans antigens as did non-Tg control mice. These data suggest that the expression of costimulatory molecules in the epidermis has differential importance for DTH to haptens versus microbial agents such as C. albicans, with B7-1 costimulation being more important for hapten-induced cutaneous DTH, and B7-2 costimulation being more important for C. albicans-induced cutaneous DTH.
B7-1 (CD80) and B7-2 (CD86) are members of the immunoglobulin gene superfamily, and share limited sequence homology in their extracellular domain (30). During antigen presentation by professional antigen presenting cells, such as activated macrophages, dendritic cells or B-lymphocytes, either B7-1 or B7-2 provides costimulatory signals by binding to their counter-receptors CD28 and CTLA-4 (CD152) on the cell surfaces of CD4+ Th lymphocytes (26, 30). This APC-derived second signal is necessary for Th-lymphocyte activation, since antigen presentation by ‘nonprofessional’ APCs (such as epidermal keratinocytes) that do not express these costimulatory molecules will result in Th-lymphocyte clonal anergy, a type of T-lymphocyte tolerance (38).
These two costimulatory molecules have overlapping but distinct properties. B7-2 is expressed constitutively on dendritic antigen-presenting cells, and is rapidly upregulated after activation (hours), whereas B7-1 has a slower kinetics of upregulation (days) (21, 24, 25). Based on these properties, it has been postulated that B7-2 may be more important in the initiation of primary immune responses (32).
The role of B7-1 and B7-2 in favoring the development of Th-1 versus Th2-lymphocyte-mediated immunity has been controversial. One in vivo mouse model suggested that B7-1-mediated costimulation favored the development of Th1-lymphocyte responses, and that B7-2 favored Th2-lymphocyte responses (28), but other studies contradicted this finding (29, 38). The current model for the role of B7-1 and B7-2 costimulation in Th1- versus Th2-lymphocyte development evokes a strength of signal model for T-lymphocyte activation, in which T-cell receptor and costimulatory molecules both play a role in Th1- and Th2-lymphocyte development. Many variables contribute to this strength of signal, including the nature of the antigen (e.g., self, transplantation, or microbial antigens), the antigen-presenting cells involved, the site (tissue) of the antigen presentation, the duration of the immune response, and the effects of genes regulating the immune response (i.e., those of DBA/2 mice, which tend to demonstrate stronger Th2 responses, versus those of C57BL/6 mice, which tend to demonstrate stronger Th1 responses). This suggests that the individual roles of B7-1 and B7-2 may differ in various in vivo situations.
Previous studies of DBA/2 mice suggested that these animals were susceptible to fungal infections because these mice respond to C. albicans infection with a Th-2-lymphocyte-predominant immune response (6, 42–44). Our model system utilized (C57BL/6 × DBA/2)F1 mice as the hosts for primary cutaneous C. albicans infections. The finding that all three strains of these F1 mice (non-Tg, B7-1 Tg, and B7-2 Tg) produced exclusively Th1-lymphocyte-mediated immune responses to C. albicans antigens suggests that the C57BL/6 haplotype, with its tendency for Th1-lymphocyte responses to infections (35), is dominant over the tendency of the DBA/2 haplotype to respond to C. albicans infections with Th2-lymphocyte-dominant responses (6, 42–44).
There is little information regarding the role of APC-derived B7-1 and B7-2 costimulation in the development of Candida antigen specific CD4+ Th1-lymphocytes. Our model system consistently indicated that the constitutive expression of B7-2 by keratinocytes in the epidermis had a significant adjuvant effect for Th1-lymphocyte-mediated immune response against Candida antigens. B7-2 Tg mice displayed enhanced DTH footpad swelling in response to Candida antigens (Fig. 4) and enhanced production of transcripts encoding the Th1 cytokines IL-2, gamma interferon, and IL-12 p40 (Fig. 5). CD4+ Th-lymphocytes secreted more gamma interferon after stimulation with Candida antigens, and Th-lymphocytes provided vigorous help for antibody synthesis against Candida antigens (Fig. 7). Epidermal B7-1 expression was ineffective in this regard, since these Tg mice did not respond differently than non-Tg mice, even though the levels of expression of B7-1 and B7-2 were equivalent in the respective Tg mouse strains (Fig. 1 and Table 1). Since antibody-mediated immunity may have a protective role in some invasive fungal infections (4, 20), the enhanced antibody production by B7-2 Tg mice against Candida antigens may be important in protecting these mice in secondary invasive challenges.
These data suggest that the intraepithelial antigen-presenting microenvironment plays an important role in determining the strength of Th1-mediated immune responses after a primary skin infection with C. albicans. The data indicate that local skin infections in the appropriate microenvironment can result in systemic immunity at sites distal to the local infection. Further studies of secondary, invasive C. albicans infections in these B7-2 Tg mice will determine whether this enhanced Th1-lymphocyte immune response will provide enhanced protection. If primary cutaneous fungal infections in the presence of B7-2 transgene expression result in enhanced protective immunity, it should be considered as a strategy for genetic immunization to C. albicans antigens, by using genes encoding C. albicans antigens along with genes encoding B7-2.
ACKNOWLEDGMENTS
We thank Barbara Ferbel, Julia Gish, and Lani Sherrill for their expert technical assistance.
This research was supported by institutional postdoctoral training grants 532 AR07472 (R. Burns) and 1R29AR40933-01 (A. Gaspari). The Tg mice were produced at the University of Rochester Tg mouse facility (supported by Cancer Center core grant CA 11198-5).
REFERENCES
- 1.Ashman R B. Murine candidiasis: cell-mediated immune response correlates directly with susceptibility and resistance to infection. Immunol Cell Biol. 1990;60:15–20. doi: 10.1038/icb.1990.2. [DOI] [PubMed] [Google Scholar]
- 2.Burns, R., A. Nasir, B. Ferbel, D. Ramirez, R. Barth, and A. A. Gaspari. The T-cell costimulatory molecules B7-1 (CD80) and B7-2 (CD86) when expressed on keratinocytes deliver different signals during contact hypersensitivity responses. J. Invest. Dermatol., in press.
- 3.Cantorna M T, Balish E. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect Immun. 1991;59:2447–2455. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A. Antibody immunity and invasive infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenci E, Romani L, Vecchiarelli A, Puccetti P, Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989;57:3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cenci E, Romani L, Vecchiarelli A, Puccetti P, Bistoni F. T-cell subsets and IFN-gamma production in resistance to systemic candidosis in immunized mice. J Immunol. 1990;144:4333–4339. [PubMed] [Google Scholar]
- 7.Cenci E, Menacci A, Del Sero G, Bistoni F, Romani L. Induction of protective Th1 responses to Candida albicans by antifungal therapy alone or in combination with an interleukin-4 antagonist. J Infect Dis. 1997;176:217–226. doi: 10.1086/514027. [DOI] [PubMed] [Google Scholar]
- 8.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dearman R J, Moussavi A, Kemeny D M, Kimber I. Contribution of CD4+ and CD8+ T lymphocyte subsets to the cytokine secretion patterns induced in mice during sensitization to contact and respiratory chemical allergens. Immunology. 1996;89:502–510. doi: 10.1046/j.1365-2567.1996.d01-778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamantstein T, Eckert R, Volk H D, Kupier-Weglinski J W. Reversal by interferon-gamma of inhibition of delayed-type hypersensitivity induction by anti-CD4 or anti-interleukin-2 receptor (CD25) monoclonal antibodies. Evidence for the physiological role of the CD4+ Th1+ subset in mice. Eur J Immunol. 1988;18:2101–2103. doi: 10.1002/eji.1830181237. [DOI] [PubMed] [Google Scholar]
- 11.Domer J E, Moser S A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978;20:88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elenitsas R, Van Belle P, Elder D. Laboratory methods. In: Elder D, Elenitsas R, Jaworsky C, Johnson B Jr, editors. Lever’s histopathology of skin. 8th ed. Philadelphia, Pa: Lippincott-Raven; 1994. pp. 51–60. [Google Scholar]
- 13.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaspari A A, Burns R P, Jr, Kondo S, Nasir A, Kurup A, Mlodynia D, Sauder D, Barth R K. Characterization of the altered cutaneous reactivity in transgenic mice whose keratinocytes overexpress B7-1. Clin Immunol Immunopathol. 1998;86:259–270. doi: 10.1006/clin.1997.4491. [DOI] [PubMed] [Google Scholar]
- 15.Gaspari A A, Ferbel B, Chen Z, Razvi F, Polakowska R. Accessory and alloantigen presenting cell functions of A431 keratinocytes that stably express the B7 antigen. Cell Immunol. 1993;149:291–302. doi: 10.1006/cimm.1993.1156. [DOI] [PubMed] [Google Scholar]
- 16.Gaspari A A, Jenkins M K, Katz S I. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific TH1 clones. J Immunol. 1988;141:2216–2220. [PubMed] [Google Scholar]
- 17.Gilman M. Ribonuclease protection assay. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Stuhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley and Sons, Inc.; 1993. pp. 4.7.1–4.7.8. [Google Scholar]
- 18.Greenfield R A. Host defense system interactions with Candida. J Med Vet Mycol. 1992;30:89–104. [PubMed] [Google Scholar]
- 19.Haidaris P J, Wright T W, Gigliotti F, Haidaris C G. Expression and characterization of a cDNA clone encoding an immunodominant surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1992;166:1113–1123. doi: 10.1093/infdis/166.5.1113. [DOI] [PubMed] [Google Scholar]
- 20.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatchcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman M, Haidaris C G. Analysis of Candida albicans adhesion to salivary mucin. Infect Immun. 1993;61:1940–1949. doi: 10.1128/iai.61.5.1940-1949.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman M P, Haidaris C G. Identification and characterization of a Candida albicans binding proteoglycan secreted from rat submandibular salivary glands. Infect Immun. 1994;62:828–836. doi: 10.1128/iai.62.3.828-836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inaba K, Inaba M, Witmer-Pack M, Hatchcock K, Hodes R, Steinman R M. Expression of B7 costimulator molecules on mouse dendritic cells. Adv Exp Med Biol. 1995;378:65–70. doi: 10.1007/978-1-4615-1971-3_13. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Witmer-Pack M, Hatchcock K, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 27.Kirkpatrick C H. Chronic mucocutaneous candidiasis. Eur J Microbiol Infect Dis. 1989;8:448–456. doi: 10.1007/BF01964059. [DOI] [PubMed] [Google Scholar]
- 28.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 29.Lenschow D J, Ho S C, Sattar H, Rhee L, Gray G, Babavi N, Herold K C, Bluestone J A. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd K O. Isolation, characterization and partial structure of peptido-galactomannans from the yeast form of Cladosporium werneckii. Biochemistry. 1970;9:3446–3453. doi: 10.1021/bi00819a025. [DOI] [PubMed] [Google Scholar]
- 32.Lu P, Wang Y L, Linsley P S. Regulation of self-tolerance by CD80/CD86 interactions. Curr Opin Immunol. 1997;9:858–862. doi: 10.1016/s0952-7915(97)80190-2. [DOI] [PubMed] [Google Scholar]
- 33.Meitner S W, Bowen W H, Haidaris C G. Oral and esophageal Candida albicans infection in the hyposalivatory rat. Infect Immun. 1990;58:2228–2236. doi: 10.1128/iai.58.7.2228-2236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosmann T R, Cherwinski H, Bond M W, Gredlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;144:1472–1477. [PubMed] [Google Scholar]
- 35.Nabors G S, Nolan T, Croop W, Li J, Farrell J P. The influence of the site of parasite inoculation on the development of Th1 and Th2 type immune responses in (BALB/c × C57BL/6)F1 mice infected with Leishmania major. Parasite Immunol. 1995;17:569–579. doi: 10.1111/j.1365-3024.1995.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 36.Nasir A, Ferbel B, Salminen W, Barth R K, Gaspari A A. Exaggerated and persistent cutaneous-delayed type hypersensitivity in transgenic mice whose epidermal keratinocytes constitutively express B7-1 antigen. J Clin Invest. 1994;94:892–898. doi: 10.1172/JCI117411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naylor M S, Relf M, Balkwill F R. Northern analysis, ribonuclease protection and in situ analysis of cytokine messenger RNA. In: Balkwill F R, editor. Cytokines. A practical approach. Oxford, England: Oxford University Press; 1995. pp. 35–56. [Google Scholar]
- 38.Nicholson L B, Kuchroo V K. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837–842. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 39.Peat S, Whelan W J, Edwards T E. Polysaccharides of baker’s yeast. IV. Mannan. J Chem Soc. 1961;1:29–34. [Google Scholar]
- 40.Qian Q, Cutler J E. Gamma interferon is not essential in host defense against disseminated candidiasis in mice. Infect Immun. 1997;65:1748–1753. doi: 10.1128/iai.65.5.1748-1753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rippon J W. Candidiasis and the pathogenic yeasts. In: Rippon J W, editor. Medical mycology. Vol. 3. Philadelphia, Pa: W. B. Saunders; 1988. p. 532. [Google Scholar]
- 42.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 45.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 46.Sambrook J, Romball C G, Hobbs M V, Ernst D N, Shultz L, Weigle W O. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saulnier M, Huang S, Aguet M, Ryffel B. Role of interferon-gamma in contact hypersensitivity assessed in interferon-gamma receptor-deficient mice. Toxicology. 1995;102:301–312. doi: 10.1016/0300-483x(95)03101-k. [DOI] [PubMed] [Google Scholar]
- 48.Sohnle P G, Hahn B L. Effect of immunosuppression on epidermal defenses in a murine model of cutaneous candidiasis. J Lab Clin Med. 1989;113:700–707. [PubMed] [Google Scholar]
- 49.Sohnle P G, Hahn B L. Epidermal proliferation and the neutrophilic infiltrates of experimental cutaneous candidiasis in mice. Arch Dermatol Res. 1989;281:279–283. doi: 10.1007/BF00431063. [DOI] [PubMed] [Google Scholar]
- 50.Sohnle P G, Hahn B L. The fate of individual organisms during clearance of experimental cutaneous Candida albicans infections in mice. Acta Dermato-Venereol. 1992;72:241–244. [PubMed] [Google Scholar]
- 51.Strelein J. Lymphocyte traffic, T-cell malignancies and the skin. J Investig Dermatol. 1978;71:167–171. doi: 10.1111/1523-1747.ep12547071. [DOI] [PubMed] [Google Scholar]
- 52.Vasilas A, Molina L, Hoffman M, Haidaris C G. The influence of morphological variation on Candida albicans adhesion to denture acrylic in vitro. Arch Oral Biol. 1992;37:613–622. doi: 10.1016/0003-9969(92)90123-p. [DOI] [PubMed] [Google Scholar]
- 53.Williams I R, Ort R J, Kupper T S. Keratinocyte expression of B7-1 in transgenic mice amplifies the primary immune response to cutaneous antigens. Proc Natl Acad Sci USA. 1994;91:12780–12784. doi: 10.1073/pnas.91.26.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willis I, Kligman A M. The mechanism of the persistent light reactor. J Investig Dermatol. 1968;51:385–394. [PubMed] [Google Scholar]