Abstract
As a key molecular scaffold for various flavonoids, naringenin is a value-added chemical with broad pharmaceutical applicability. For efficient production of naringenin from acetate, it is crucial to precisely regulate the carbon flux of the oxaloacetate-phosphoenolpyruvate (OAA-PEP) regulatory node through appropriate pckA expression control, as excessive overexpression of pckA can cause extensive loss of OAA and metabolic imbalance. However, considering the critical impact of pckA on naringenin biosynthesis, the conventional strategy of transcriptional regulation of gene expression is limited in its ability to cover the large and balanced solution space. To overcome this hurdle, in this study, pckA expression was fine-tuned at both the transcriptional and translational levels in a combinatorial expression library for the precise exploration of optimal naringenin production from acetate. Additionally, we identified the effects of regulating pckA expression by validating the correlation between phosphoenolpyruvate kinase (PCK) activity and naringenin production. As a result, the flux-optimized strain exhibited a 49.8-fold increase compared with the unoptimized strain, producing 122.12 mg/L of naringenin. Collectively, this study demonstrated the significance of transcriptional and translational flux rebalancing at the key regulatory node, proposing a pivotal metabolic engineering strategy for the biosynthesis of various flavonoids derived from naringenin using acetate.
One-sentence summary
In this study, transcriptional and translational regulation of pckA expression at the crucial regulatory node was conducted to optimize naringenin biosynthesis using acetate in E. coli.
Keywords: Flavonoids, Acetate, Phosphoenolpyruvate carboxykinase, Transcriptional and translational regulation, Metabolic engineering
Graphical Abstract
Graphical Abstract.

Introduction
Naringenin, a valuable chemical compound known for its diverse pharmaceutical applications and significance as a fundamental scaffold for the biosynthesis of various flavonoids, has been broadly utilized, showing increasing market demands (Fowler & Koffas, 2009; Karim et al., 2018; Ng et al., 2019; Salehi et al., 2019; Wang et al., 2019). Owing to limitations such as low yield, high requirements for solvents, and high cost from traditional extraction methods of naringenin from natural plants (Lin et al., 2014; Xu et al., 2020; Zhou et al., 2020), the biosynthesis of naringenin through microbial fermentation via heterologous production has been studied as a breakthrough with advances in metabolic engineering and synthetic biology. Furthermore, to accomplish economically feasible microbial production of naringenin, the utilization of acetate as a highly promising feedstock was examined (Kim et al., 2022) due to its potential as an affordable and abundant availability from inexpensive sources such as lignocellulosic biomass and carbon dioxide (S. J. Lim et al., 2008; Nam et al., 2016; H. G. Lim et al., 2018; Roy et al., 2021; Harahap & Ahring, 2023).
For the bioconversion of acetate to naringenin, improving acetyl-CoA availability is necessary for a sufficient supply of malonyl-CoA for the naringenin biosynthetic pathway (Zha et al., 2009; Veronica et al., 2015; Milke & Marienhagen, 2020). Indeed, the previous study has identified key regulatory nodes for naringenin biosynthesis from acetate, indicating enhanced naringenin production with increased acetyl-CoA availability through the flux redistribution between cell growth and naringenin production (Kim et al., 2022). Particularly, the upregulation of pckA gene encoding phosphoenolpyruvate carboxykinase (PCK) at the oxaloacetate (OAA) node acted as a critical linkage that reroutes tricarboxylic acid (TCA) cycle intermediates via anaplerosis of the glyoxylate cycle to the naringenin biosynthetic pathway (Oh et al., 2002; Waegeman et al., 2011).
To maximize naringenin production from acetate, it is required to optimize the carbon flux at the OAA node through appropriate expression levels of pckA. From this perspective, gene expression control for efficient microbial conversion has been conducted in previous studies by altering transcription efficiency or translation efficiency (Xu et al., 2013; Biggs et al., 2014; Noh et al., 2020; Sohn et al., 2020; Chen et al., 2022). However, these strategies for the optimization of gene expression have mainly regulated the single-level stage, which could lead to a suboptimal point with limited coverage of large and balanced solution space (J. A. Jones, Toparlak, et al., 2015; Hwang et al., 2021). Specifically, at the OAA node, excessive overexpression of pckA can cause the extensive loss of OAA, resulting in a metabolic imbalance between cell growth and naringenin production. As acetyl-CoA is crucial for both cell growth and naringenin biosynthesis, the overexpression of pckA could lead to a shortage of OAA, essential for the TCA cycle, resulting in inferior cell growth and decreased total naringenin production (K. L. Jones et al., 2000; Pitera et al., 2007). Conversely, low expression of pckA may not effectively reroute OAA to acetyl-CoA, thereby maintaining an insufficient supply of acetyl-CoA for naringenin production in cells. Thus, the expression of pckA must be precisely regulated to achieve efficient biosynthesis of naringenin, given its considerable influence on flux distribution. In this context, at the branch point between cell growth and production, such as the OAA node, optimal flux rebalancing achieved through fine-tuning of gene expression, beyond conventional promoter-level regulation, would be an effective strategy for enhanced naringenin production from acetate.
In this study, we conducted fine-tuning of pckA gene expression in both transcriptional and translational levels to explore a large and balanced solution space at the OAA node (Fig. 1). To precisely tune pckA expression, we constructed systematic mutant libraries using promoters with varying strengths and 5′-untranslated region (5′-UTR) variants that could generate different expression levels. Specifically, we rationally designed 5′-UTRs with predicted translation efficiencies using the UTR Library Designer to establish a more balanced expression range beyond transcriptional variation. With the constructed variants of pckA expression, we examined the capacity of these pckA expression variants for naringenin production and the enzymatic activity of PCK, investigating the optimal expression of pckA at the OAA node under flux rebalancing at the transcriptional and translational levels. Consequently, the flux-optimized strain demonstrated a significant increase in naringenin production capacity compared with the unoptimized strain, achieving competitive results to those obtained from conventional substrates (Supplementary information, Table S1). Collectively, our study highlights the importance of transcriptional and translational optimization of gene expression at the key regulatory node to maximize product formation, presenting an effective metabolic engineering strategy for the biosynthesis of naringenin and its various flavonoid derivatives using acetate.
Fig. 1.
Schematic diagram of systematic engineering of pckA at both transcriptional and translational levels. PEP, phosphoenolpyruvate; 5′-UTR, 5′-untranslated region; TCA cycle, tricarboxylic acid cycle. TX and TL are the abbreviations of transcription and translation, respectively.
Material and Methods
Reagents and Primers
Plasmid and genomic DNA were purified using GeneAll® Plasmid SV kit and GeneAll® Exgene™ Cell SV kit (GeneAll Biotechnology, Seoul, Korea), respectively. Q5® High-Fidelity DNA Polymerase, restriction endonucleases, and T4 DNA ligase were purchased from New England Biolabs (Ipswich, MA, USA). T4 Polynucleotide Kinase and EmeraldAmp® PCR Master Mix were purchased from Takara Bio Inc. (Shiga, Japan). Oligonucleotides were synthesized by Cosmogenetech (Seoul, Korea). Other reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless specified.
Construction of Bacterial Strains and Plasmids
Bacterial strains and plasmids used in this study are listed in Supplementary information, Table S4. Escherichia coli Mach1-T1® was used to proliferate all plasmids for cloning experiments and naringenin production was performed in BL21 Star™ (DE3) and its derivatives. For the naringenin production, transcriptionally optimized pFlavoopt, containing 4-coumaroyl-CoA ligase (4CL) from Arabidopsis thaliana, chalcone synthase (CHS) from Petunia hybrida, and chalcone isomerase (CHI) from Citrus maxima, was obtained from a previous study (J. A. Jones et al., 2016), and other genes were derived from the genomic DNA of E. coli BL21 Star™ (DE3). The genetic information on constitutive promoters (Anderson promoter series) and terminator (BBa_B1006) was acquired from the Registry of Standard Biological Parts (http://parts.igem.org). 5′-UTRs with specific predicted expression levels were designed using the UTR Library Designer (Seo et al., 2014).
Restriction enzyme digestion and ligation were used for the construction of plasmids pACYCA and pACYCAP115. Specifically, acs and pckA genes were amplified from the genomic DNA of E. coli BL21 Star™ (DE3) via two sequential PCRs to introduce the constitutive promoter and synthetic 5′-UTR. For the insertion of acs, the vector fragment was obtained from pACYCduet-1, as a template, using Vector_acs_speI_F/Vector_acs_notI_R. The purified PCR fragments of acs and its vector were digested using SpeI and NotI and assembled, resulting in pACYCA. Similarly, the vector fragment was amplified from pACYCA as a template, using Vector_pckA_kpnI_F/Vector_pckA_notI_R, for the insertion of pckA. The resulting fragment and the pckA PCR fragment were digested using KpnI and NotI, and assembled to construct pACYCAP115. Plasmids with all variants of pckA (pACYCAP106, pACYCAP109, pACYCAP113, pACYCAP115U2, pACYCAP115U3, pACYCAP115U4, pACYCAP115U5, pACYCAP109U2, pACYCAP109U3, pACYCAP109U4, and pACYCAP109U5) were constructed through blunt-end cloning using the forward primers with corresponding constitutive promoter sequence (J23106_pckA_blunt_F, J23109_pckA_blunt_F, J23113_pckA_blunt_F, and J23115_U2_pckA_blunt_F, J23115_U3_pckA_blunt_F, J23115_U4_pckA_blunt_F, J23115_U5_pckA_blunt_F, J23109_U2_pckA_blunt_F, J23109_U3_pckA_blunt_F, J23109_U4_pckA_blunt_F, J23109_U5_pckA_blunt_F, respectively) and the reverse primer (pckA_blunt_R). Deletion of the chromosomal iclR gene was conducted by the Lambda-Red recombination method using the plasmids pKD46 and pCP20 (Datsenko & Wanner, 2000).
Culture Conditions for Naringenin Production
Escherichia coli strains were cultivated in Andrew's Magic Medium (AMM) containing 100 mL of 10 × MOPS mixture (J. A. Jones, Vernacchio, et al., 2015), 5.0 g/L K2HPO4, 3.5 g/L KH2PO4, 3.5 g/L (NH4)2HPO4, 2 g/L casamino acid, 0.1 mL of 5 g/L thiamine-HCl, and 0.1 mL of 1 M CaCl2 supplemented with 10 g/L NaOH-neutralized acetate (pH 7.0) as a carbon source. Antibiotics (100 mg/L ampicillin and 34 mg/L chloramphenicol) were added to the medium for plasmid maintenance.
For naringenin production, single colonies of each strain were inoculated in 15-mL test tubes containing 3 mL fresh AMM and incubated overnight at 37°C with continuous shaking (200 rpm). Thereafter, saturated broths were inoculated in 300-mL Erlenmeyer flasks containing 25 mL fresh medium, at an OD600 of 0.1, and incubated to reach an OD600 of 1.0. Refreshed culture broths were re-inoculated into 25 mL fresh medium with OD600 of 0.1 and incubated at a culture temperature of 37 °C, with agitation at 200 rpm. When culture broths reached an OD600 of 1.0 200 mg/L p-coumaric acid and 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) for the induction of 4cl, chs, and chi genes were added to the broth, lowering the temperature to 30°C after induction. All experiments were performed in biological triplicates. OD600 of the broths was recorded using a UV-1700 spectrophotometer (Shimadzu Co., Kyoto, Japan) and the pH was adjusted to 6.8–7.1, with a 5 M HCl solution, using an Orion™ 8103BN ROSS™ pH meter (Thermo Fisher Scientific, Waltham, MA, USA). Culture samples were periodically collected and stored at −80°C for further analysis.
Enzyme Activity Assay
The promoter strength variants were cultivated until OD600 value of 2.0, then the cell pellets were harvested by centrifugation at 15 814×g for 10 min at 4°C. Cell lysates were prepared through the addition of 0.1 mM phosphate buffer solution (pH 8.0) to the cell pellets and disruption by the sonication (Q125 Sonicator, Qsonica, CT, USA). Enzyme activity was assayed according to the previous study with minor modifications (Hou et al., 1995). The reaction mixture containing 4 mM ATP, 4 mM MgCl2, 4 mM OAA, and 0.1 mM phosphate buffer solution was added to cell lysates up to 180 µL, then enzyme reactions were performed for 60 min at 30°C. The consumption of OAA was measured using an Aminex HPX-87H column (Bio-Rad Laboratories, Richmond, CA, USA) and Shodex RI-101 detector (Shodex, Klokkerfaldet, Denmark), using 5 mM H2SO4 as the mobile phase at a flow rate of 0.6 mL/min at 14°C (Hou et al., 1995).
Metabolites Analysis
Ultimate 3000 high-performance liquid chromatography system (Dionex, Sunnyvale, CA, USA) was used to analyze the metabolites contained in culture broths. For the detection of naringenin production and p-coumaric acid consumption, the culture broth and an equal amount of absolute ethanol were mixed and centrifuged for 10 min at 13 000 rpm. Thereafter, the supernatant was analyzed with an Acclaim 120 C18 column (Dionex) and a UV–vis diode array detector. Acetonitrile and water, each containing 0.1% formic acid, were used as the mobile phase, at a flow rate of 1 mL/min, using the following multi-gradient flow program: 10–40% acetonitrile for 0–10 min and 40–60% acetonitrile for 10–15 min with absorbance detection at 280 nm. Acetate consumption was identified with Aminex HPX-87H column (Bio-Rad Laboratories, Richmond, CA, USA) and Shodex RI-101 detector (Shodex, Klokkerfaldet, Denmark), using 5 mM H2SO4 as the mobile phase at a flow rate of 0.6 mL/min at 14°C.
Results and Discussion
Systematic Regulation of pckA Expression for Improved Production of Naringenin from Acetate
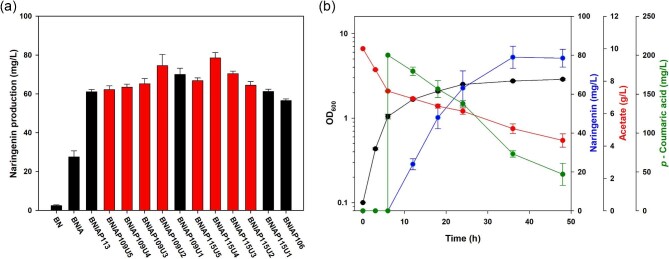
In our previous study, naringenin production was improved through overexpression of acs encoding acetyl-CoA synthase and pckA genes to enhance acetyl-CoA supply, indicating that insufficient acetyl-CoA availability was a bottleneck for further naringenin biosynthesis (Kim et al., 2022). Building on this finding, we utilized BNIAP variants of the previous study as base strains, with activation of the glyoxylate shunt pathway and acetate assimilation through the acs overexpression. In particular, based on the previous examination of pckA expression variance under different promoters and the corresponding naringenin production (Kim et al., 2022), in this study, we thoroughly investigated the solution space of PCK activity between the synthetic promoter BBa_J23115 and BBa_J23109 for further optimization of naringenin production (Fig. 2a). To this end, mutant libraries of gene expression were generated using rationally designed 5′-UTR variants for optimum flux at the OAA regulatory node at both transcriptional and translational levels. Specifically, we extensively constructed five variants of pckA expression with different 5′-UTR sequences, utilizing computationally predicted translation efficiency for the promoters through the UTR Library Designer (Seo et al., 2014): BNIAP115U1, BNIAP115U2, BNIAP115U3, BNIAP115U4, and BNIAP115U5 for the promoter BBa_J23115; BNIAP109U1, BNIAP109U2, BNIAP109U3, NIAP109U4, and BNIAP109U5 for the promoter BBa_J23109 (from high to low translation efficiency, in order).
Fig. 2.
(a) Comparison of the constructed variants with transcriptional and translational regulation of pckA expression in naringenin production and (b) the fermentation profile of the BNIAP115U4 strain. pckA expression variants with synthetic 5′-untranslated region (UTR) sequence designed from UTR Library Designer are indicated with red bars. Flask cultures were performed for 48 h in biological triplicates. Error bars indicate the standard deviations of biological triplicates.
The naringenin production capacity of all pckA variants was evaluated using acetate as a carbon source (Fig. 2a). Among them, the BNIAP115U4 with the transcriptional and translational regulation exhibited the highest naringenin production of 78.47 mg/L and specific production of 91.50 mg/g dry cell weight, which is a 32.1-fold and 61.41-fold increase over those of the base strain BN, which harbors the recombinant pathway of naringenin biosynthesis (Fig. 2a,b, and Supplementary information, Table S2). Additionally, the naringenin yield on p-coumaric acid in the BNIAP115U4 strain (0.590 g naringenin/g p-coumaric acid) was 17.4-fold higher than that of the BN strain (0.034 g naringenin/g p-coumaric acid), indicating a significant enhancement in the conversion of p-coumaric acid to naringenin. Overall, we demonstrated that the transcriptional and translational regulation of pckA expression beyond the promoter-level stage was important for optimizing naringenin production from acetate, leading to the optimum flux redistribution at the key branch node.
Validation of the Effects of Transcriptional and Translational Modulation of pckA Expression on Naringenin Production
Upregulating pckA to facilitate carbon flux from OAA to PEP would be an effective strategy for maximizing naringenin production. However, pckA overexpression could cause extensive OAA loss, leading to a critical metabolic imbalance and decreased naringenin production. Therefore, to explore the optimal point of flux redistribution at the OAA node, we generated pckA mutants with variations at the transcriptional and translational levels in gene expression and evaluated their naringenin production. To thoroughly validate the effects of precise and balanced expression control on naringenin biosynthesis, the PCK activities of the mutants were examined. Specifically, we verified the correlation between PCK activity and naringenin production among the pckA mutants whose expression was complexly regulated at both transcriptional and translational levels.
To examine the pckA expression levels of the constructed expression library, we conducted PCK activity assays on the variants in the mutant library designed with different promoter strengths and computed translation efficiencies of the 5′-UTRs (Fig. 3). The results indicated that the balanced expression range was successfully generated within the specific exploration range, and naringenin production capacity was enhanced with increased PCK activity. However, as we speculated that an extensive loss of OAA could have detrimental effects on the cells, the specific growth rate decreased when pckA was overexpressed, resulting in decreased specific naringenin productivity and naringenin production titers (Fig. 3, Supplementary information, Fig. S2). Furthermore, the difference in PCK activity arising from translational regulation beyond the transcriptional level led to differences in naringenin production capacity, indicating that a detailed investigation into the appropriate pckA expression was essential for flux optimization. Despite limitations making it challenging to consider the broad global effects caused by pckA upregulation, the verifications of the enhanced flux of OAA to acetyl-CoA and the subsequent increased production of naringenin were successfully achieved.
Fig. 3.
Effects of fine-tuning of pckA at the transcriptional and translational levels for the production of naringenin. The bar plot illustrates normalized phosphoenolpyruvate carboxykinase (PCK) enzyme activities for various mutants with expression levels modulated by distinct promoters and engineered 5′-UTRs. The pckA mutants with regulated expression are ordered in the x-axis as an activity-increasing row. The naringenin production capacity of each variant is marked as a scatter plot. Error bars indicate the standard deviations of biological triplicates.
Collectively, systematic engineering of the proper pckA expression at both transcriptional and translational levels was an effective strategy for maximizing naringenin biosynthesis. By assessing the impact of pckA expression regulation, we demonstrated that precise regulation at the branch node of cell growth and production was highly important for flux rebalancing, leading to efficient naringenin production from acetate.
Optimization of Cultivation Conditions for Enhanced Naringenin Production
For further improvement of naringenin production, we conducted the optimization of culture conditions for the BNIAP115U4 strain by regulating the expression of pckA at multiple levels. In this study, 4CL, CHS, and CHI, the key heterologous enzymes, were expressed under T7 promoters using IPTG as an inducer for naringenin biosynthesis. Although the culture conditions of the BNIAP109 strain, which was engineered at the promoter strength level, were examined in a previous study, the optimum culture conditions need to be specified experimentally for a particular system again, as changes in pckA gene expression level would result in differences in resource utilization (Kim et al., 2022). Since the optimum induction point and induction level were associated with the allocation of cellular resources for protein biosynthesis (Andrianantoandro et al., 2006; Gomes et al., 2020; J. A. Jones, Collins, et al., 2015), the optimum culture conditions may vary depending on the host cells and carbon substrates for naringenin production (J. A. Jones et al., 2016; J. A. Jones & Koffas, 2016). Therefore, to achieve maximized naringenin biosynthesis, we investigated the optimal IPTG induction condition for the heterologous key enzymes in the BNIAP115U4 strain to balance the distribution of cellular components between cell growth and naringenin production.
To achieve this, we evaluated the naringenin production capacity of the BNIAP115U4 strain according to different induction points and IPTG concentrations. First, in the range of OD600 0.6 to 3.0 as induction points, the BNIAP115U4 strain exhibited the highest naringenin titer at the induction point of OD600 1.0 (Fig. 4a), indicating the importance of the proper transition point from cell growth to production. Additionally, the BNIAP115U4 strain showed the highest naringenin production with the addition of 0.05 mM IPTG in the concentration range of 0.001–1.0, emphasizing the appropriate heterologous expression of 4CL, CHS, and CHI for the optimized production of naringenin from acetate. Notably, compared with BNIAP109U1 with only transcriptional optimization of pckA expression under the optimized culture condition, BNIAP115U4 with pckA expression regulated at both the transcriptional and translational levels had a 32% increase in naringenin production (Supplementary information, Fig. S1). Consequently, we achieved the highest naringenin titer of 122.12 mg/L under the optimized culture conditions at the induction point of OD600 1.0 with the addition of 0.05 mM IPTG (Fig. 4b), which is competitive with the results from previous studies on conventional substrates such as glucose or glycerol (Supplementary information, Table S1).
Fig. 4.
Culture condition optimization of the BNIAP115U4 strain. (a) Evaluation of naringenin production capacity under different conditions of IPTG concentrations and induction times. (b) Fermentation profile of the BNIAP115U4 strain under optimized culture conditions, such as induction time at OD600 of 1.0 with the addition of 0.05 mM IPTG. Flask cultures were performed for 48 h in biological triplicates. Error bars indicate the standard deviations of biological triplicates. BNIAP115U4 strain refers to E. coli BL21 Star™(DE3) with heterologous expression of essential enzymes for naringenin production, acs overexpression, iclR knockout, and pckA upregulation under constitutive promoter BBa_J23115 and synUTRpck4; IPTG, isopropyl β-d-thiogalactopyranoside.
Conclusion
In this study, our goal was to optimize naringenin biosynthesis from acetate by systematically engineering the expression of pckA. The efficient microbial production of naringenin from acetate requires enhancing acetyl-CoA availability for a sufficient supply of malonyl-CoA, a precursor of naringenin biosynthesis. From this perspective, upregulating pckA, which converts OAA to PEP, to reroute TCA cycle intermediates to the naringenin production pathway can increase acetyl-CoA availability. However, a detailed exploration of the appropriate pckA expression level is essential to prevent metabolic imbalance caused by extensive OAA loss, contributing to the optimization of naringenin biosynthesis.
To this end, in the study, we fine-tuned the transcriptional and translational levels of pckA expression. Using computational design through the UTR Library Designer based on synthetic promoters, we constructed a balanced mutant library of pckA expression regulated at both transcriptional and translational levels. The resulting strain, BNIAP115U4, exhibited the highest naringenin production capacity of 122.12 mg/L under the optimized condition, a 49.8-fold increase in production compared with the unoptimized strain. In addition, we validated the impact of optimization of pckA expression on naringenin biosynthesis by evaluating the correlation between PCK activity and naringenin production. While achieving precise control of pckA expression in the strain with the overexpression of the acs gene to enhance acetyl-CoA availability, we anticipated that overexpressing genes encoding acetate transporter or kinase-phosphate acetyltransferase could serve as effective engineering targets for further enhancing naringenin production. Collectively, this study demonstrated the importance of systematic engineering with transcriptional and translational control of gene expression at the key regulatory node involved in the flux redistribution between cell growth and naringenin production, laying the groundwork for the production of various flavonoids using acetate.
Supplementary Material
Contributor Information
Dong H Kim, Department of Chemical Engineering, Pohang University of Science and Technology, 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Korea.
Hyun G Hwang, Institute of Environmental and Energy Technology, Pohang University of Science and Technology, 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Korea.
Dae-yeol Ye, Department of Chemical Engineering, Pohang University of Science and Technology, 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Korea.
Gyoo Y Jung, Department of Chemical Engineering, Pohang University of Science and Technology, 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Korea; School of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology, 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Korea.
Author contributions
D.H.K., H.G.H., and G.Y.J. designed the project. D.H.K. and H.G.H. performed experiments. D.H.K., H.G.H., and G.Y.J. analyzed the data and D.H.K., H.G.H., D.Y.Y., and G.Y.J. wrote the manuscript. G.Y.J. supervised the project. All authors approved the final version of the manuscript.
Funding
This research was supported by grants from the Korea Institute of Marine Science & Technology Promotion funded by the Ministry of Oceans and Fisheries (20220258).
Conflict of interest
The authors declare no conflict of interest.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- Andrianantoandro E., Basu S., Karig D. K., Weiss R. (2006). Synthetic biology: New engineering rules for an emerging discipline. Molecular Systems Biology, 2(1), 2006–2028. 10.1038/msb4100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs B. W., De Paepe B., Santos C. N. S., De Mey M., Kumaran Ajikumar P. (2014). Multivariate modular metabolic engineering for pathway and strain optimization. Current Opinion in Biotechnology, 29(1), 156–162. 10.1016/J.COPBIO.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Chen R., Liu Y., Zhong W., Hao X., Mu T., Yang M., Xing J. (2022). Ribosome-binding sequences (RBS) engineering of key genes in Escherichia coli for high production of fatty alcohols. Biotechnology and Bioprocess Engineering, 27(4), 615–623. 10.1007/s12257-021-0354-0 [DOI] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences, 97(12), 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler Z. L., Koffas M. A. G. (2009). Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Applied Microbiology and Biotechnology, 83(5), 799–808. 10.1007/s00253-009-2039-z [DOI] [PubMed] [Google Scholar]
- Gomes L., Monteiro G., Mergulhão F. (2020). The impact of IPTG induction on plasmid stability and heterologous protein expression by Escherichia coli biofilms. International Journal of Molecular Sciences, 21(2), 576. 10.3390/ijms21020576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harahap B. M., Ahring B. K. (2023). Acetate production from syngas produced from lignocellulosic biomass materials along with gaseous fermentation of the syngas: A review. Microorganisms, 11(4), 995. 10.3390/microorganisms11040995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Chao Y., Liao J. (1995). A mutant phosphoenolpyruvate carboxykinase in Escherichia coli conferring oxaloacetate decarboxylase activity. Journal of Bacteriology, 177(6), 1620–1623. 10.1128/jb.177.6.1620-1623.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H. G., Noh M. H., Koffas M. A. G., Jang S., Jung G. Y. (2021). Multi-level rebalancing of the naringenin pathway using riboswitch-guided high-throughput screening. Metabolic Engineering, 67, 417–427. 10.1016/J.YMBEN.2021.08.003 [DOI] [PubMed] [Google Scholar]
- Jones J. A., Collins S. M., Vernacchio V. R., Lachance D. M., Koffas M. A. G. (2015). Optimization of naringenin and p-coumaric acid hydroxylation using the native E. coli hydroxylase complex, HpaBC. Biotechnology Progress, 32(1), 21–25. 10.1002/btpr.2185 [DOI] [PubMed] [Google Scholar]
- Jones J. A., Koffas M. A. G. (2016). Optimizing metabolic pathways for the improved production of natural products. Methods in Enzymology, 575, 179–193. 10.1016/BS.MIE.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Jones J. A., Toparlak T. D., Koffas M. A. G. (2015). Metabolic pathway balancing and its role in the production of biofuels and chemicals. Current Opinion in Biotechnology, 33, 52–59. 10.1016/J.COPBIO.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Jones J. A., Vernacchio V. R., Lachance D. M., Lebovich M., Fu L., Shirke A. N., Schultz V. L., Cress B., Linhardt R. J., Koffas M. A. G. (2015). ePathOptimize: A combinatorial approach for transcriptional balancing of metabolic pathways. Scientific Reports, 5(1), 11301. 10.1038/srep11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. A., Vernacchio V. R., Sinkoe A. L., Collins S. M., Ibrahim M. H. A., Lachance D. M., Hahn J., Koffas M. A. G. (2016). Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metabolic Engineering, 35, 55–63. 10.1016/J.YMBEN.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Jones K. L., Kim S.-W., Keasling J. D. (2000). Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metabolic Engineering, 2(4), 328–338. 10.1006/mben.2000.0161 [DOI] [PubMed] [Google Scholar]
- Karim N., Jia Z., Zheng X., Cui S., Chen W. (2018). A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends in Food Science & Technology, 79, 35–54. 10.1016/j.tifs.2018.06.012 [DOI] [Google Scholar]
- Kim D. H., Hwang H. G., Jung G. Y. (2022). Optimum flux rerouting for efficient production of naringenin from acetate in engineered Escherichia coli. Biotechnology for Biofuels and Bioproducts, 15(1), 90. 10.1186/s13068-022-02188-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. G., Lee J. H., Noh M. H., Jung G. Y. (2018). Rediscovering acetate metabolism: Its potential sources and utilization for biobased transformation into value-added chemicals. Journal of Agricultural and Food Chemistry, 66(16), 3998–4006. 10.1021/acs.jafc.8b00458 [DOI] [PubMed] [Google Scholar]
- Lim S. J., Kim B. J., Jeong C. M., Choi J., Ahn Y. H., Chang H. N. (2008). Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresource Technology, 99(16), 7866–7874. 10.1016/j.biortech.2007.06.028 [DOI] [PubMed] [Google Scholar]
- Lin Y., Jain R., Yan Y. (2014). Microbial production of antioxidant food ingredients via metabolic engineering. Current Opinion in Biotechnology, 26, 71–78. 10.1016/j.copbio.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Milke L., Marienhagen J. (2020). Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis. Applied Microbiology and Biotechnology, 104(14), 6057–6065. 10.1007/s00253-020-10643-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam C. W., Jung K. A., Park J. M. (2016). Biological carbon monoxide conversion to acetate production by mixed culture. Bioresource Technology, 211, 478–485. 10.1016/j.biortech.2016.03.100 [DOI] [PubMed] [Google Scholar]
- Ng K. R., Lyu X., Mark R., Chen W. N. (2019). Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chemistry, 270, 123–129. 10.1016/j.foodchem.2018.07.077 [DOI] [PubMed] [Google Scholar]
- Noh M. H., Cha S., Kim M., Jung G. Y. (2020). Recent advances in microbial cell growth regulation strategies for metabolic engineering. Biotechnology and Bioprocess Engineering, 25(6), 810–828. 10.1007/s12257-019-0511-x [DOI] [Google Scholar]
- Oh M. K., Rohlin L., Kao K. C., Liao J. C. (2002). Global expression profiling of acetate-grown Escherichia coli. Journal of Biological Chemistry, 277(15), 13175–13183. 10.1074/JBC.M110809200 [DOI] [PubMed] [Google Scholar]
- Pitera D. J., Paddon C. J., Newman J. D., Keasling J. D. (2007). Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metabolic Engineering, 9(2), 193–207. 10.1016/J.YMBEN.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Roy M., Yadav R., Chiranjeevi P., Patil S. A. (2021). Direct utilization of industrial carbon dioxide with low impurities for acetate production via microbial electrosynthesis. Bioresource Technology, 320, 124289. 10.1016/j.biortech.2020.124289 [DOI] [PubMed] [Google Scholar]
- Salehi B., Fokou P. V. T., Sharifi-Rad M., Zucca P., Pezzani R., Martins N., Sharifi-Rad J. (2019). The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals, 12(1), 11. 10.3390/ph12010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S. W., Yang J. S., Cho H. S., Yang J., Kim S. C., Park J. M., Kim S., Jung G. Y. (2014). Predictive combinatorial design of mRNA translation initiation regions for systematic optimization of gene expression levels. Scientific Reports, 4(1), 4515. 10.1038/SREP04515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn Y. J., Kim H. T., Jo S. Y., Song H. M., Baritugo K.-A., Pyo J., Choi J., Joo J. C., Park S. J. (2020). Recent advances in systems metabolic engineering strategies for the production of biopolymers. Biotechnology and Bioprocess Engineering, 25(6), 848–861. 10.1007/s12257-019-0508-5 [DOI] [Google Scholar]
- Veronica van S.-W. P., Jan M., Pettinari M. J. (2015). Metabolic engineering of Escherichia coli for the synthesis of the plant polyphenol pinosylvin. Applied and Environmental Microbiology, 81(3), 840–849. 10.1128/AEM.02966-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waegeman H., Beauprez J., Moens H., Maertens J., De Mey M., Foulquié-Moreno M. R., Heijnen J. J., Charlier D., Soetaert W. (2011). Effect of iclR and arcA knockouts on biomass formation and metabolic fluxes in Escherichia coli K12 and its implications on understanding the metabolism of Escherichia coli BL21 (DE3). BMC Microbiology, 11(1), 70. 10.1186/1471-2180-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Cress B. F., Yang Z., Hordines J. C., Zhao S., Jung G. Y., Wang Z., Koffas A. G., M. (2019). Design and characterization of biosensors for the screening of modular assembled naringenin biosynthetic library in Saccharomyces cerevisiae. ACS Synthetic Biology, 8(9), 2121–2130. 10.1021/acssynbio.9b00212 [DOI] [PubMed] [Google Scholar]
- Xu P., Gu Q., Wang W., Wong L., Bower A. G. W., Collins C. H., Koffas M. A. G. (2013). Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nature Communications, 4(1), 1409. 10.1038/ncomms2425 [DOI] [PubMed] [Google Scholar]
- Xu P., Marsafari M., Zha J., Koffas M. (2020). Microbial coculture for flavonoid synthesis. Trends in Biotechnology, 38(7), 686–688. 10.1016/J.TIBTECH.2020.01.008 [DOI] [PubMed] [Google Scholar]
- Zha W., Rubin-Pitel S. B., Shao Z., Zhao H. (2009). Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metabolic Engineering, 11(3), 192–198. 10.1016/J.YMBEN.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Zhou S., Hao T., Zhou J. (2020). Fermentation and metabolic pathway optimization to de novo synthesize (2S)-naringenin in Escherichia coli. Journal of Microbiology and Biotechnology, 30(10), 1574–1582. 10.4014/jmb.2008.08005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.