Abstract
The Drosophila Smad-interacting co-factor, Schnurri (Shn) confers transcriptional repression in response to Decapentaplegic (Dpp) signaling. Shn zinc fingers 6-8 mediate this Smad interaction but are lacking in vertebrate Shn homologs. In contrast, the vertebrate-conserved zinc finger 1,2 and 4,5 pairs have been reported to engage in Smad-mediated transcriptional activation in fly and vertebrate systems, and to contribute to Dpp-dependent tissue repair in the fly retina. We report that mutation of zinc coordination residues within vertebrate-conserved Shn zinc finger pairs 1,2 and 4,5 results in ectopic venation that is sensitive to Dpp signaling.
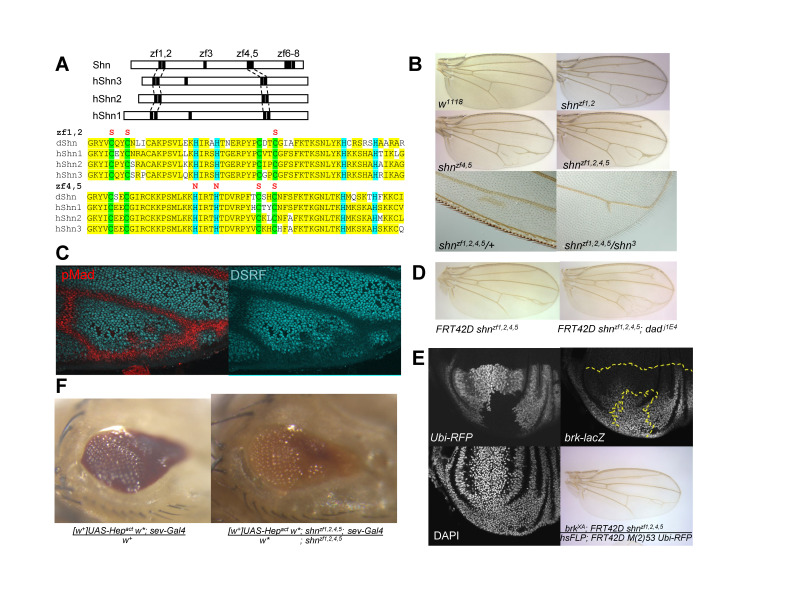
Figure 1. Mutations that disrupt Schnurri zinc finger pairs 1,2 and 4,5 in Drosophila result in ectopic wing venation.
(A) Homologous zinc finger pairs in Drosophila Shn and human Shn family members. Shown below are the cys>ser and his>asn amino acid substitutions introduced into Shn zinc finger pairs 1,2 and 4,5 together with alignment illustrating the extensive homology with corresponding human Shn1, Shn2 and Shn3 zinc fingers (highlighted yellow). (B) Wings from wildtype, shn zf1,2 , shn zf4,5 and shn zf1,2,4,5 females, a short patch of ectopic vein adjacent to L2 in a shn zf1,2,4,5 /+ female, and L4 branching in a shn zf1,2,4,5 /shn 3 female. (C) shn zf4,5 pupal wing stained to detect pMad (red; marks pre-vein) and DSRF (cyan; marks intervein tissue). The observed reciprocal patterns of ectopic pMad and reduced DSRF territory in pupal wings resemble the patterns of ectopic venation observed in adult wings. (D) Comparison of ectopic venation in FRT42D shn zf1,2,4,5 and FRT42D shn zf1,2,4,5 ; dad j1E4 (note that severity of the shn zf1,2,4,5 phenotype was somewhat reduced in a FRT42 background) . (E) Third instar wing disc expression of brk XA , marked by β-galactosidase, is not reduced in shn zf1,2,4,5 clones. Large FRT42D M(2)53 + shn zf1,2,4,5 clones are marked by absence of Ubi-RFP (clone boundaries marked by yellow dashed lines in brk-lacZ panel). Clones were induced in y 1 w brk XA /FM7 i ; P{w + act-GFP} ; FRT42D shn zf1,2,4,5 / y w hsFLP; FRT42D P{w + piM}45F M(2)53 P{Ubi-RFP, w + }60E larvae . A resulting adult wing exhibits clonal ectopic venation. (F) shn zf1,2,4,5 fails to increase ommatidial loss caused by sevenless-Gal4 driven expression of an activated form of Hemipterous. Left panel: P{w+UAS-Hep.Act} 1 , w*/+; P{ry+GAL4-Hsp70.sev} 332.5 , M{UAS-nlsTimer-NA} ZH-86Fb /+ . Right panel: P{w+UAS-Hep.Act} 1 , w*/ w*; shn zf1,2,4,5 ; P{ry+GAL4-Hsp70.sev} 332.5 , M{UAS-nlsTimer-NA} ZH-86Fb /+ . Eye color difference is due to a [ w+ ] element that fails to fully rescue w- . Both crosses were done at 19 o C and were semi-lethal with a fraction of the sev-Gal4 >UAS-Hep act progeny dying as pupae, some partially eclosed.
Description
In response to Dpp (a Drosophila member of the BMP-TGFβ family), Smad proteins Mothers Against Dpp (Mad) and Medea (Med) form a complex that activates some target genes while repressing others (Hamaratoglu et al., 2014) . Repression occurs by binding of Mad-Med trimers to silencer elements (SE) that allows recruitment of Shn, a large zinc finger protein that interacts with additional co-repressors (Müller et al., 2003; Pyrowolakis et al., 2004; Gao et al., 2005; Cai and Laughon, 2009). Shn binds the Mad-Med-SE complex via zinc fingers 6-8 without apparent involvement of zinc fingers 1-5 ( Fig. 1A ). Vertebrate Schnurri proteins ( e.g. , human Shn1, Shn2 and Shn3) contain zinc finger pairs homologous to Shn zf 1,2 and zf 4,5 but lack counterparts to the more C-terminal zf 6-8 (Arora et al., 1995; Grieder et al., 1995; Staehling-Hampton et al., 1995; Blitz and Cho, 2009; Fig. 1A ). Unlike Shn zinc fingers 6-8, zinc finger pairs 1,2 and 4,5 have intrinsic DNA-binding specificity that resembles that of Rel proteins (Fan and Maniatis, 1990; Baldwin et al. 1990, van’t Veer et al., 1992; Dai et al., 2000). Fly Shn was found to synergize with Mad and Med in activating expression via such κB-like sites in a Ubx B-lux reporter (Dai et al., 2000) , evidence of functionally direct Shn-DNA contact. Experiments in Xenopus and mammalian systems found Shn proteins contributing to BMP-induced transcriptional activation by interaction with DNA-bound Smad complexes (Jin et al., 2006; Yao et al., 2006; Javier et al., 2012) . Shn has also been identified as contributing to mitigation of JNK-induced tissue damage to the fly retina, a property localizing to the region of Shn spanning zinc fingers 3-5 (Kelsey et al., 2012) . This repair/recovery of photoreceptor cells was found to be dependent on Dpp signaling through Mad (Kramer et al., 2021) .
To investigate the function of Shn zinc finger pairs 1,2 and 4,5, CRISPR-Cas9 gene editing was used to disrupt zinc coordination ( Fig. 1A ). For a variety of zinc finger proteins, it has been found that even a single Cys to Ser mutation disrupts zinc coordination and function, including DNA binding (Redemann et al., 1988; Severne et al., 1988; Witte et al., 1988; Webster et al. 1991 ). For zinc fingers 1 and 2, serine substitutions were introduced in place of three zinc-coordinating cysteines (two in zf1, one in zf2). Separately, asparagine substitutions were introduced in place of the two zinc-coordinating histidines in zf4, along with serine substitutions for the two zinc-coordinating cysteines in zf5. A resulting zf4,5 mutant line was then targeted to add the three zf1,2 substitutions, resulting in lines with a total of 7 substitutions predicted to disrupt zinc coordination by both zinc finger pairs. For all constructs, multiple correctly targeted fly lines were confirmed by DNA sequencing.
The resulting shn zf1, 2 , shn zf4,5 and shn zf1, 2,4,5 lines were homozygous viable with completely penetrant ectopic venation extending from or neighboring L2 and L4 ( Fig. 1B ) and phenotypic severity of s hn zf1, 2,4,5 > shn zf4,5 > shn zf1, 2 . With low penetrance, heterozygotes displayed small ectopic patches of vein tissue limited to the intervein territories adjacent to L2 and L4, while complementation tests against the amorphic allele, shn 3 (aka shn TD5 ), resulted in mild ectopic venation with incomplete penetrance. Together, these results suggest a dosage-dependent gain-of-function in Shn activity that is competed by wild-type in heterozygotes. The mutations have no obvious effect on viability (e.g., shn zf1,2,4,5 homozgyotes recovered as a third or more of progeny from shn zf1,2,4,5 /CyO parents) with no discerned effect on developmental timing, life span or fertility.
The characterized role of Dpp signaling in L2 and L4 formation and positioning (de Celis, 1997; reviewed by Blair 2007) , suggests that the zf1,2 and zf4,5 mutations result in an elevated response to Dpp signaling in the context of vein pattern. Consistent with this, anti-pMad staining of shn zf4,5 pupal discs revealed expanded pre-vein invasion at the expense of dSRF-positive intervein territory ( Fig. 1C ). If pMad acts directly to repress dSRF in the context of pupal vein patterning, presumably as a pMad-Med-Shn complex, the observed ectopic venation would indicate elevated transcriptional silencing resulting from disruption of Shn zinc finger pairs 1,2 and 4,5. This inference suggests that zf1,2 and 4,5 normally restrain or compete with the ability of Shn to engage in pMad-Med-Shn transcriptional silencing.
In comparison to the embryonic lethal phenotypes of classic shn alleles, the homozygous viable ectopic venation phenotypes suggest a function for zinc finger pairs 1,2 and 4,5 both distinct and separable from the carboxy-terminal zinc fingers 6-8 that have been found to be essential and sufficient for Dpp-dependent gene silencing in patterning during embryonic and larval development (Muller et al., 2003; Pyrowolakis et al., 2004). Ectopic shn zf1,2,4,5 venation was enhanced by a hypomorphic dad allele ( Fig. 1D ) that increases the activity of Mad-Med complexes in response to Dpp signaling (Tsuneizumi et al., 1997) , a result consistent with Dpp signaling activity contributing to this apparent gain-of-function shn zf1, 2,4,5 phenotype.
The normal viability and fertility of shn zf1,2,4,5 flies suggests that these mutations have little or no effect on the ability of Shn to repress critical targets in other developmental contexts (Marty et al., 2000; Chen D, McKearin D. 2003; Pyrowolakis et al., 2004; Vuilleumier et al., 2010; Crocker and Erives, 2013; Beira et al., 2014) . Consistent with this, FRT42 shn zf1,2,4,5 clones in 3 rd instar wing imaginal discs showed no evident decrease in expression of brk-lacZ ( Fig. 1E ). Localized clonal ectopic venation was observed in resulting adult flies, consistent with pupal stage vein patterning being sensitive to disruption of zinc finger pairs 1,2 and 4,5.
The results raise questions regarding the role of the highly conserved Shn zinc finger pairs 1,2 and 4,5 in Dpp signaling and possibly other interactions yet to be discovered. First, how do zinc finger pairs 1,2 and 4,5 serve to prevent ectopic vein formation? Do they dampen or compete with the ability of Shn zinc fingers 6-8 to interact with silencer-bound Mad-Med complexes, perhaps by alternative Smad interactions and/or sequestering of Shn at other genomic locations? Perhaps relevant to this question, the zinc finger pairs 1,2 and 4,5 each have DNA binding affinities that overlap those of Rel proteins ( e.g. , NFkB) and have been found to contribute to transcription activation in vertebrate contexts. Second, the high phylogenetic conservation in vertebrates lacking homology to zinc fingers 6-8 suggests that Drosophila zinc finger pairs 1,2 and 4,5 may also function in ways that are distinct from zf6-8 mediated silencing, or one that impacts Dpp signaling conditionally, perhaps in response to stress (Clark et al. 2011) . The identification of shn as a loss-of-function enhancer of JNK-induced photoreceptor loss suppressible by a segment of Shn spanning zinc fingers 3-5 is suggestive of such a scenario (Kelsey et al. 2012) , although we failed to find an obvious effect of shn zf1,2,4,5 on the severity of such JNK-induced photoreceptor loss ( Fig. 1F ). Such a noncanonical role may align with studies of vertebrate Shn paralogs that mediate or intersect with BMP-regulated gene expression. Further investigation of how Shn zinc finger pairs 1,2 and 4,5 delimit vein patterning may provide mechanistic clues applicable to the diverse roles of mammalian Shn paralogs (Jones, et al. 2010; Staton et al. 2012; Takao et al. 2013; Choi et al. 2015; Srivastava et al. 2016) .
Methods
CRISPR guide RNA target sites were chosen using the online tool CRISPR Optimal Target Finder, targetfinder.flycrispr.neuro.brown.edu (Gratz et al., 2014) . Guide RNAs for targeting zf1,2 and zf4,5 were engineered in pU6-BbsI-chiRNA by inverse PCR with Phusion polymerase (NEB) using oligos listed below. Homology repair template plasmids containing the desired point mutations were designed as previously described (Gratz et al. 2015a, 2015b; https://flycrispr.org/scarless-gene-editing/ ). First, targeted shn segments spanning the zf1,2 and zf4,5 regions (~2 kb for each zf pair) were PCR amplified from genomic DNA of the to-be-injected w - ; vas-Cas9 stock. Next, each region was divided into “left arm” and “right arm” segments that overlap a TTAA cleavage site for piggyBac transposase that allows precise excision of an interrupting dsRed marker after correct targeting (see Gibson assembly primers listed below). Point mutations in zf1, zf4 and zf5 were then introduced using NEB Q5 Base-Changer site-directed mutagenesis (primers listed below). Each “arm” was then transferred into pScarlessHD-DsRed-w + by Gibson assembly (NEB Builder HiFi Assembly kit with Phusion DNA polymerase) positioning the left and right arms on opposite sides of dsRed (primers listed below) . The single zf2 cys>ser change was introduced by a primer during Gibson assembly.
For the initial set of zf1,2 and zf4,5 mutations, individual adults recovered from injected w - ; vas-Cas9 embryos were crossed to w - ; Sco/CyO , screening for w - ; dsRed + progeny indicative of successful shn zf1,2/zf4,5 targeting. Correct, error-free targeting was verified by genomic DNA sequencing extending across shn-dsRed junctions. Piggybac transposase was then used to precisely excise dsRed by crossing the balanced lines to w - ; Gla/CyO; pBacT . Resulting dsRed - lines were sequenced across 2 kb spanning the targeted sites to verify precise dsRed excision and lack of spurious alterations in sequence. This yielded five correctly excised zf1,2 lines from eighteen dsRed + lines, and three correctly excised zf4,5 lines from ten dsRed + lines. Allelism was verified by crosses among the resulting lines, scoring for the ectopic vein phenotype. A single w - ; shn zf 4,5 ; vas-Cas9 line was then used to generate seven w - ; shn zf1,2,4,5 dsRed + /CyO lines (injections for this set by Best Gene). From these, seven w - ; shn zf1,2,4,5 lines were generated by dsRed excision, with sequencing verification and allelism testing.
For mosaic analysis, the brk XA lacZ reporter was used to detect brk expression in FRT42D M(2)53 + shn 1,2,4,5 / FRT42D M(2)53 + shn 1,2,4,5 clones marked by loss of Ubi-RFP . Immunostaining and confocal imagining of imaginal discs was performed as previously described (Blair 2000; Schleede and Blair, 2015) using the following antibodies: mouse anti-β-galactosidase (Developmental Studies Hybridoma Bank, University of Iowa), Alexa Fluor 488 conjugated goat anti-mouse IgG (Jackson ImmunoResearch); antibody reagents used to detect pMad and DSRF were as described previously (Schleede and Blair, 2015) . Sanger DNA sequencing reagents and processing were provided by the UW Biotechnology Center.
Reagents
Drosophila stocks
w - ; vas-Cas9 (O’Connor-Giles Lab)
w - ; Gla/CyO; pBacT (O’Connor-Giles Lab)
w - ; shn zf1,2 (this study)
w - ; shn zf 4,5 ; vas-Cas9 (this study)
w - ; shn zf1,2, 4,5 (this study)
w; FRT42D mago 3 shn 3 /CyO (Bloomington 52289, donor: Nicholas Baker)
y 1 w 1118 ; PlacW-dad j1E4 /TM3, Sb 1 (Bloomington 10305, source: Yuh Nung Jan)
y 1 w 111 8 hsFLP ; FRT42D shn zf1,2,4,5 ; PlacW-dad j1E4 /TM3-TM6 (this study)
y 1 w brk XA /FM7 i ,P{w + act-GFP} ; Sco/CyO (Bloomington 58792, source: Gerard Campbell)
y 1 w brk XA /FM7 i ,P{w + act-GFP} ; FRT42D shn zf1,2,4,5 (this study)
w ; FRT42D P{w + piM}45F M(2)53/CyO (Bloomington 5698, donor: Gerald M. Rubin)
y w hsFLP; FRT42D P{w + piM}45F M(2)53 P{Ubi-RFP, w + }60E/SM5a-TM6Tb (this study)
P{w + UAS-Hep.Act} 1 , w*/FM6, w* (Bloomington 9305, donor: Marek Mlodzik)
P{ry + GAL4-Hsp70.sev} 332.5 , M{UAS-nlsTimer-NA} ZH-86Fb (Bloomington 78057; donor: Peter Lidsky)
P{w + UAS-Hep.Act} 1 , w*/FM6, w*; shn zf1,2,4,5 (this study)
w*; shn zf1,2,4,5 ; P{ry + GAL4-Hsp70.sev} 332.5 , M{UAS-nlsTimer-NA} ZH-86Fb (this study)
Primers for engineering gRNAs in pU6-BbsI-chiRNA (bold = target sequence)
zf1,2gRNA-F GTACTGACAAACGTATCGTC GTTTTAGAGCTAGAAATAGCAAG
zf4,5gRNA-F G TGTGCTTCTTGAGCATCGA GTTTTAGAGCTAGAAATAGCAAG
Primers for Q5 Base Changer site-directed mutagenesis (bold = targeted mutation; underlined = silent change disrupting gRNA seed region)
zf1 cys1>ser_R TGA G A G ACGTATCGTCCGGACTTC
zf1 cys2>ser_F GTACT C TAACTTGATCTGTGCCAAG
zf4 his1>asn_R ATGT T CTT T TTGAGCATCGACGGCTTC
zf4 his2>asn_F TCGCACT A ACACGGACGTGAGGCCAT
zf5 cys1>ser_R GCTG G ATGTGAATGGCCTCACGTC
zf5 cys2>ser_F CATT C CAACTTCAGGTGAGTCATTG
Primers for Gibson assembly of shn zf1,2 and zf4,5 genomic segments into pScarlessHD-DsRed-w + (upper case = shn sequence; bold = targeted mutation)
Primers for zf1,2 segment
Left arm set:
ScarlessDsRedLA_rev tcggccccgaagacacta
1-2LA_fwd tatagtgtcttcggggccgaGTGCCGCTGCCGACTGTT 38mer
1-2LA_rev (zf2 cys>ser) atatgattatctttctagggTTAAACGCAATGCCG G ACG 39mer
ScarlessDsRedLA_fwd ccctagaaagataatcatattgtg
Right arm set:
ScarlessDsRedRA_rev ccctagaaagatagtctgcg
1-2RA_fwd cgcagactatctttctagggTTAAGACGAAGAGTAATTTGTACAAAC
1-2RA_rev cgtatatggtcttcttttccGGCACGCACCAACTGTAA
ScarlessDsRedRA_fwd ggaaaagaagaccatatacg
Primers for zf4,5 segment
Left arm set:
ScarlessDsRedLA_rev tcggccccgaagacacta
4-5LA_fwd tatagtgtcttcggggccgaAACAGCAAGGAGGCACCG
4-5LA_rev atatgattatctttctagggTTAAAACTGTGGAATGGAACAAAG
ScarlessDsRedLA_fwd ccctagaaagataatcatattgtg
Right arm set:
ScarlessDsRedRA_rev ccctagaaagatagtctgcg
4-5RA_fwd cgcagactatctttctagggTTAAGACCAAGGGAAATCTG
4-5RA_rev cgtatatggtcttcttttccACGCCGCTGTGTATAATG
ScarlessDsRedRA_fwd ggaaaagaagaccatatacg
Acknowledgments
Acknowledgments
The authors thank Kate O’Connor-Giles and Scott Gratz (Brown University) for contributing fly stocks, plasmids and embryo injection, Seth Blair (UW Madison) for fly stocks and confocal microscopy, and the Bloomington Drosophila Stock Center for fly stocks.
Funding Statement
This work was supported by UW Laboratory of Genetics Funding for Undergraduate Research (A.L.).
References
- Arora K, Dai H, Kazuko SG, Jamal J, O'Connor MB, Letsou A, Warrior R. The Drosophila schnurri gene acts in the Dpp/TGF beta signaling pathway and encodes a transcription factor homologous to the human MBP family. Cell. 1995 Jun 2;81(5):781–790. doi: 10.1016/0092-8674(95)90539-1. [DOI] [PubMed] [Google Scholar]
- Baldwin AS Jr, LeClair KP, Singh H, Sharp PA. A large protein containing zinc finger domains binds to related sequence elements in the enhancers of the class I major histocompatibility complex and kappa immunoglobulin genes. Mol Cell Biol. 1990 Apr 1;10(4):1406–1414. doi: 10.1128/mcb.10.4.1406-1414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beira JV, Springhorn A, Gunther S, Hufnagel L, Pyrowolakis G, Vincent JP. The Dpp/TGFβ-dependent corepressor Schnurri protects epithelial cells from JNK-induced apoptosis in drosophila embryos. Dev Cell. 2014 Oct 9;31(2):240–247. doi: 10.1016/j.devcel.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Blair SS. 2000. Chapter 10: Imaginal Discs. Drosophila Protocols, Cold Spring Harbor Press. Sullivan W, Ashburner M, Hawley RS, editors.
- Blitz IL, Cho KW. Finding partners: how BMPs select their targets. Dev Dyn. 2009 Jun 1;238(6):1321–1331. doi: 10.1002/dvdy.21984. [DOI] [PubMed] [Google Scholar]
- Cai Y, Laughon A. The Drosophila Smad cofactor Schnurri engages in redundant and synergistic interactions with multiple corepressors. Biochim Biophys Acta. 2009 Mar 1;1789(3):232–245. doi: 10.1016/j.bbagrm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003 Oct 14;13(20):1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Choi JK, Zhu A, Jenkins BG, Hattori S, Kil KE, Takagi T, Ishii S, Miyakawa T, Brownell AL. Combined behavioral studies and in vivo imaging of inflammatory response and expression of mGlu5 receptors in schnurri-2 knockout mice. Neurosci Lett. 2015 Oct 23;609:159–164. doi: 10.1016/j.neulet.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Clark RI, Woodcock KJ, Geissmann F, Trouillet C, Dionne MS. Multiple TGF-β superfamily signals modulate the adult Drosophila immune response. Curr Biol. 2011 Sep 29;21(19):1672–1677. doi: 10.1016/j.cub.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J, Erives A. A Schnurri/Mad/Medea complex attenuates the dorsal-twist gradient readout at vnd. Dev Biol. 2013 Mar 13;378(1):64–72. doi: 10.1016/j.ydbio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Dai H, Hogan C, Gopalakrishnan B, Torres-Vazquez J, Nguyen M, Park S, Raftery LA, Warrior R, Arora K. The zinc finger protein schnurri acts as a Smad partner in mediating the transcriptional response to decapentaplegic. Dev Biol. 2000 Nov 15;227(2):373–387. doi: 10.1006/dbio.2000.9901. [DOI] [PubMed] [Google Scholar]
- de Celis JF. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 1997 Mar 1;124(5):1007–1018. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- Fan CM, Maniatis T. A DNA-binding protein containing two widely separated zinc finger motifs that recognize the same DNA sequence. Genes Dev. 1990 Jan 1;4(1):29–42. doi: 10.1101/gad.4.1.29. [DOI] [PubMed] [Google Scholar]
- Gao S, Steffen J, Laughon A. Dpp-responsive silencers are bound by a trimeric Mad-Medea complex. J Biol Chem. 2005 Aug 17;280(43):36158–36164. doi: 10.1074/jbc.M506882200. [DOI] [PubMed] [Google Scholar]
- Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O'Connor-Giles KM. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 2014 Jan 29;196(4):961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Harrison MM, Wildonger J, O'Connor-Giles KM. Precise Genome Editing of Drosophila with CRISPR RNA-Guided Cas9. Methods Mol Biol. 2015;1311:335–348. doi: 10.1007/978-1-4939-2687-9_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Rubinstein CD, Harrison MM, Wildonger J, O'Connor-Giles KM. CRISPR-Cas9 Genome Editing in Drosophila. Curr Protoc Mol Biol. 2015 Jul 1;111:31.2.1–31.2.20. doi: 10.1002/0471142727.mb3102s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder NC, Nellen D, Burke R, Basler K, Affolter M. Schnurri is required for Drosophila Dpp signaling and encodes a zinc finger protein similar to the mammalian transcription factor PRDII-BF1. Cell. 1995 Jun 2;81(5):791–800. doi: 10.1016/0092-8674(95)90540-5. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Affolter M, Pyrowolakis G. Dpp/BMP signaling in flies: from molecules to biology. Semin Cell Dev Biol. 2014 May 9;32:128–136. doi: 10.1016/j.semcdb.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Jones DC, Schweitzer MN, Wein M, Sigrist K, Takagi T, Ishii S, Glimcher LH. Uncoupling of growth plate maturation and bone formation in mice lacking both Schnurri-2 and Schnurri-3. Proc Natl Acad Sci U S A. 2010 Apr 19;107(18):8254–8258. doi: 10.1073/pnas.1003727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier AL, Doan LT, Luong M, Reyes de Mochel NS, Sun A, Monuki ES, Cho KW. Bmp indicator mice reveal dynamic regulation of transcriptional response. PLoS One. 2012 Sep 11;7(9):e42566–e42566. doi: 10.1371/journal.pone.0042566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006 Apr 1;10(4):461–471. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kelsey EM, Luo X, Brückner K, Jasper H. Schnurri regulates hemocyte function to promote tissue recovery after DNA damage. J Cell Sci. 2012 Jan 24;125(Pt 6):1393–1400. doi: 10.1242/jcs.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J, Neves J, Koniikusic M, Jasper H, Lamba DA. Dpp/TGFβ-superfamily play a dual conserved role in mediating the damage response in the retina. PLoS One. 2021 Oct 26;16(10):e0258872–e0258872. doi: 10.1371/journal.pone.0258872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Lints R, Foehr ML, Tokarz R, Yu L, Emmons SW, Liu J, Savage-Dunn C. The Caenorhabditis elegans schnurri homolog sma-9 mediates stage- and cell type-specific responses to DBL-1 BMP-related signaling. Development. 2003 Nov 19;130(26):6453–6464. doi: 10.1242/dev.00863. [DOI] [PubMed] [Google Scholar]
- Marty T, Müller B, Basler K, Affolter M. Schnurri mediates Dpp-dependent repression of brinker transcription. Nat Cell Biol. 2000 Oct 1;2(10):745–749. doi: 10.1038/35036383. [DOI] [PubMed] [Google Scholar]
- Müller B, Hartmann B, Pyrowolakis G, Affolter M, Basler K. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell. 2003 Apr 18;113(2):221–233. doi: 10.1016/s0092-8674(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Pyrowolakis G, Hartmann B, Müller B, Basler K, Affolter M. A simple molecular complex mediates widespread BMP-induced repression during Drosophila development. Dev Cell. 2004 Aug 1;7(2):229–240. doi: 10.1016/j.devcel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Redemann N, Gaul U, Jäckle H. 1988. Disruption of a putative Cys-zinc interaction eliminates the biological activity of the Krüppel finger protein. Nature 332(6159): 90-2. [DOI] [PubMed]
- Rustgi AK, Van 't Veer LJ, Bernards R. 1990. Two genes encode factors with NF-kappa B- and H2TF1-like DNA-binding properties. Proc Natl Acad Sci U S A 87(22): 8707-10. [DOI] [PMC free article] [PubMed]
- Schleede J, Blair SS. The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of Drosophila melanogaster. PLoS Genet. 2015 Oct 6;11(10):e1005576–e1005576. doi: 10.1371/journal.pgen.1005576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severne Y, Wieland S, Schaffner W, Rusconi S. Metal binding 'finger' structures in the glucocorticoid receptor defined by site-directed mutagenesis. EMBO J. 1988 Aug 1;7(8):2503–2508. doi: 10.1002/j.1460-2075.1988.tb03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Engels H, Schanze I, Cremer K, Wieland T, Menzel M, Schubach M, Biskup S, Kreiß M, Endele S, Strom TM, Wieczorek D, Zenker M, Gupta S, Cohen J, Zink AM, Naidu S. Loss-of-function variants in HIVEP2 are a cause of intellectual disability. Eur J Hum Genet. 2015 Jul 8;24(4):556–561. doi: 10.1038/ejhg.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K, Laughon AS, Hoffmann FM. A Drosophila protein related to the human zinc finger transcription factor PRDII/MBPI/HIV-EP1 is required for dpp signaling. Development. 1995 Oct 1;121(10):3393–3403. doi: 10.1242/dev.121.10.3393. [DOI] [PubMed] [Google Scholar]
- Staton TL, Lazarevic V, Jones DC, Lanser AJ, Takagi T, Ishii S, Glimcher LH. Dampening of death pathways by schnurri-2 is essential for T-cell development. Nature. 2011 Apr 7;472(7341):105–109. doi: 10.1038/nature09848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vazquez J, Park S, Warrior R, Arora K. The transcription factor Schnurri plays a dual role in mediating Dpp signaling during embryogenesis. Development. 2001 May 1;128(9):1657–1670. doi: 10.1242/dev.128.9.1657. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997 Oct 9;389(6651):627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, Pyrowolakis G. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol. 2010 May 9;12(6):611–617. doi: 10.1038/ncb2064. [DOI] [PubMed] [Google Scholar]
- Webster LC, Zhang K, Chance B, Ayene I, Culp JS, Huang WJ, Wu FY, Ricciardi RP. Conversion of the E1A Cys4 zinc finger to a nonfunctional His2,Cys2 zinc finger by a single point mutation. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9989–9993. doi: 10.1073/pnas.88.22.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MM, Dickson RC. Cysteine residues in the zinc finger and amino acids adjacent to the finger are necessary for DNA binding by the LAC9 regulatory protein of Kluyveromyces lactis. Mol Cell Biol. 1988 Sep 1;8(9):3726–3733. doi: 10.1128/mcb.8.9.3726-3733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LC, Blitz IL, Peiffer DA, Phin S, Wang Y, Ogata S, Cho KW, Arora K, Warrior R. Schnurri transcription factors from Drosophila and vertebrates can mediate Bmp signaling through a phylogenetically conserved mechanism. Development. 2006 Oct 1;133(20):4025–4034. doi: 10.1242/dev.02561. [DOI] [PubMed] [Google Scholar]