Key Points
Question
In patients undergoing tongue base mucosectomy (TBM) for head and neck squamous cell carcinoma of unknown primary, is step serial sectioning (SSS) histopathological processing associated with better outcomes than conventional techniques in identifying primary cancers in diagnostic oropharyngeal tissue samples?
Findings
In this multicenter cohort study of 58 prospectively identified patients with suspected head and neck squamous cell carcinoma of unknown primary, SSS was associated with added considerable workload with minimal benefit vs conventional histology techniques. The overall identification rate for TBM following SSS was 50.0%, which varied with the timing of the tonsillectomy with respect to the TBM.
Meaning
The results of this cohort study suggest that a review of conventional histology by a second pathologist may be more beneficial than SSS in identifying unknown primary head and neck cancers.
Abstract
Importance
Patients with suspected head and neck squamous cell carcinoma of unknown primary (HNSCCUP) may undergo tonsillectomy and tongue base mucosectomy (TBM) to help identify clinicoradiologically occult primary disease. It is hypothesized that when these diagnostic specimens are analyzed, conventional histopathological (CH) techniques risk missing small primary tumors that may be hidden in the tissue blocks.
Objective
To establish the outcomes of a step serial sectioning (SSS) histopathological technique vs CH when analyzing diagnostic tissue specimens from TBM and tonsillectomy performed for HNSCCUP.
Design, Setting, and Participants
The MOSES prospective multicenter noninterventional cohort study was conducted over a 25-month period from November 2019 at secondary and tertiary care ear, nose, and throat departments in the United Kingdom and included adults with clinicoradiologically occult HNSCCUP who were undergoing TBM.
Intervention
Conventional histopathological techniques performed on TBM and tonsillectomy specimens at participating centers, followed by SSS performed at the central laboratory.
Main Outcome
Identification of cancer on central histopathological review of TBM and tonsillectomy specimens.
Results
Tissue from 58 eligible patients was analyzed (median [range] age, 58 [47-82] years; 10 women [17%]), with 20 480 sections cut in the laboratory and 4096 sections directly examined by a pathologist (median [range], 64 [28-135] per patient). The overall identification rate for TBM following SSS according to study protocol was 50.0% (95% CI, 37.5%-62.5%) and by subgroups was 42.9% (95% CI, 21.4%-67.4%) when performed following a negative bilateral tonsillectomy, 46.7% (95% CI, 24.8%-69.9%) at the same time as bilateral tonsillectomy, and 57.1% (95% CI, 36.5%-75.5%) following historic tonsillectomy. Conventional histopathological techniques at central review identified 2 undiagnosed primary tumors and revised the diagnosis of 2 other cases (1 nonmalignant and another down staged). Step serial sectioning identified a single additional tumor: an ipsilateral synchronous tongue base tumor for which a contralateral tumor had been identified on CH. Multifocal disease was seen in 5 (8.6%); all were human papillomavirus–related and in the tongue base.
Conclusions and Relevance
In this multicenter cohort study of patients undergoing TBM for HNSCCUP, SSS was associated with added considerable histopathological workload with minimal additional diagnostic benefit. A second opinion for conventional histological techniques may be more beneficial. Synchronous primary disease should be considered when planning diagnostic oropharyngeal surgery for these patients.
This cohort study examines the outcomes of step serial sectioning vs conventional histopathology when analyzing diagnostic tissue specimens from tongue base mucosectomy and tonsillectomy performed for head and neck squamous cell carcinoma of unknown primary.
Introduction
Head and neck squamous cell carcinoma of unknown primary (HNSCCUP) presents a diagnostic challenge. Many patients present with neck lumps that are suspicious of metastatic cancer, but identification of the primary site is not always straightforward.1,2 Some primary sites are obvious on clinical examination, although this may require flexible nasendoscopy to adequately visualize the upper aerodigestive tract mucosa. Others require cross-sectional and/or functional imaging to help localize probable primary disease.3 In either case, the goal is histological diagnosis, as identifying the primary site allows tissue-specific oncological therapy and the best chance of improved oncological and functional outcomes.4 However, in a minority of patients, the primary site remains clinically and radiologically occult.1 In these patients, speculative diagnostic surgery to putative primary sites is commonly advocated in a final attempt to identify a subclinical cancer before definitive treatment is undertaken.5
During recent years, there has been an increasing recognition of the role of human papillomavirus (HPV) infection in head and neck cancers,1,2,6,7 and this association has also been observed in unknown primary disease.3 In parallel, tongue base mucosectomy (TBM) has been increasingly adopted in clinicoradiologically occult primary disease,8 in which the oropharyngeal lymphoepithelial tissue is likely to be the origin of most HPV-related cases.4,5,9,10 However, several fundamental issues remain with the rationale for performing this speculative diagnostic surgery. First and most fundamentally, identification of a primary site has not been definitively associated with improved oncological outcomes. Second, the biological and clinical behavior of these tumors has not been shown to be similar to clinically or radiologically evident oropharyngeal tumors. Third, the occurrence of multifocal disease means that incomplete oropharyngeal sampling may be falsely reassuring even after a single primary has been identified.6,7,11,12 Regardless, despite extensive diagnostic workup, including speculative diagnostic surgery, some patients will go on to initiate treatment with a diagnosis of HNSCCUP.8,13 Accepting that current practice may include palatine tonsillectomy and TBM, it may then be asked if there is any more that could be done with the resultant specimens to improve identification rates.
Once diagnostic tissue samples have been obtained from putative primary sites, they undergo histopathological processing, including fixation into tissue blocks, commonly measuring around 3 mm in depth. Conventional histology (CH) techniques obtain a representative section from each block that is subjected to microscopic analysis by a qualified pathologist. Theoretically, small clinicoradiologically occult primary cancers could reside within this tissue block and be overlooked using these standard techniques. A histopathological processing technique called step serial sectioning (SSS) has been used to examine tissue specimens with greater fidelity by sampling the entire tissue block at regular intervals, reducing the chance of missing small tumors that may otherwise be overlooked. The use of this technique has previously been reported in head and neck cancer,14,15,16,17,18 but to our knowledge, it has not been applied to diagnostic specimens in this challenging subset of patients with unknown primary disease. This study aimed to establish the outcomes of conventional techniques and SSS histopathological processing in analyzing diagnostic oropharyngeal tissue samples in a prospectively identified multicenter cohort undergoing TBM for HNSCCUP.
Methods
Ethical Considerations and Regulatory Approvals
The study protocol was reviewed and approved by the study sponsor (The Royal Marsden NHS Foundation Trust), London-Riverside Research Ethics Committee, and the Health Research Authority. Written informed consent was provided by all patients.
Study Design and Setting
A prospective multicenter noninterventional cohort study was conducted in UK secondary care head and neck departments. The primary end point was diagnosis of cancer in oropharyngeal tissue specimens on histopathological processing.
Participant Eligibility Criteria and Identification
Patients 16 years and older with cytologically or pathologically confirmed cervical metastatic SCC who were undergoing TBM for identification of primary site were included. Patients with a history of previous head and neck cancer or history of receiving radiation to the head and neck region or undergoing targeted biopsies were excluded. Patients were identified at the multidisciplinary team meetings of participating centers at any time in their diagnostic pathway before undergoing TBM.
Standard-of-Care Practice
Inclusion in the study was not associated with indications for surgery or the technical aspects of the surgery itself. The choice of method of TBM remained with the participating center. Following resection of the lingual tonsil tissue via TBM, routine histopathological processing was conducted locally according to their usual practices and procedures, with anatomically orientated specimens fixed in 10% neutral buffered formalin and cut into 2-mm to 3-mm parallel pieces and then processed to paraffin wax. Four-μm sections were cut and stained with a hematoxylin and eosin stain and then examined by a pathologist for evidence of small-cell carcinoma. This conventional histopathological assessment was confirmed as the standard operating procedure at the participating sites and contributed to the multidisciplinary team–recommended management plan.
SSS Histopathology
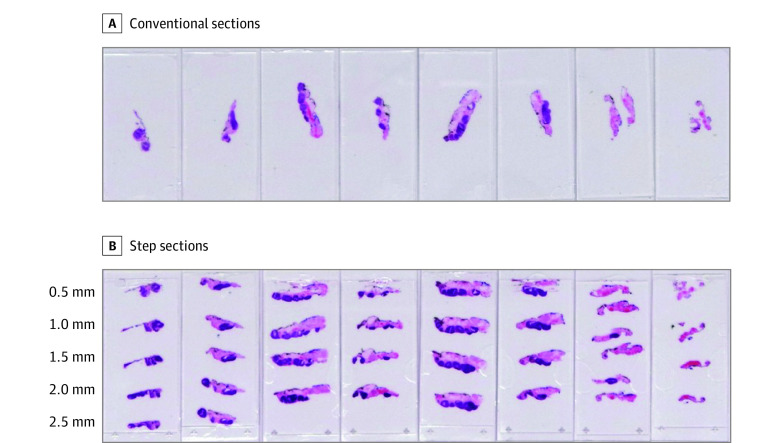
The lingual tonsil obtained via TBM, along with any palatine tonsillar tissue removed as part of the patients’ diagnostic work up to identify a primary tumor, were subsequently transferred to the study sponsor for SSS. Five 4-μm serial sections were cut every 500 μm through the formalin-fixed paraffin-embedded blocks until the tissue was depleted. At each step, section 3 was stained with hematoxylin and eosin. If a small-cell carcinoma was identified, then serial sections 2 and 4 were submitted for HPV testing (p16 immunohistochemistry and high-risk HPV DNA in situ hybridization). Serial sections 1 and 5 were retained for repeated tests, as required. Interval sections between the steps were discarded. Figure 1 demonstrates the additional diagnostic advantage afforded by SSS.
Figure 1. Comparison of the Fidelity of Step Serial Sectioning With Conventional Histology for Palatine Tonsillar Specimens in the MOSES Study.
A, Conventional sections from 8 blocks stained with hematoxylin and eosin. B, Step sections taken at 500-μm intervals through the block generates a further 33 sections for examination.
Data Collection
Data were recorded on patient demographic characteristics, medical and surgical history, relevant investigations, perioperative outcomes, CH results, the initial planned definitive treatment, and oncological status up to 12 months. Patients also completed questionnaires on pain and swallowing function using the MD Anderson Dysphagia Index. The oncological and functional outcomes were not the focus of this article.
The first phase of the study opened to recruitment in November 2019. A second phase of the study will extend the recruitment from 60 to 100 patients, with reporting of oncological and functional follow-up to 5 years, although these additional participants will not have SSS performed on their oropharyngeal tissues.
Statistical Analysis
No a priori sample size calculation was conducted, as recruitment was limited by funding to specimens of 60 patients. Descriptive statistics are presented with 95% CIs using the Wilson score when appropriate. Subgroups were defined by the timing of tonsillectomy in association with TBM, as this was associated with the probable pick-up rates. Loss of follow-up was not applicable, as the end point was contemporaneous. Missing or ambiguous data were clarified with the participating sites, and persistently missing data points were excluded from relevant analyses.
Results
Centers and Patients
Between November 2019 and December 2021, 60 patients from 19 centers across the United Kingdom were recruited and underwent TBM (median [range], 3 [1-9] cases per center). Two patients were subsequently withdrawn before their oropharyngeal tissue underwent SSS (due to reclassification as salivary gland cancer and a patient withdrawal), but recruitment was paused to allow interim analysis after 25 months, giving 58 complete cases. Following the interim analysis, the study management team agreed not to recruit further patients for SSS as part of the ongoing study. The median age for all included patients was 58 (range, 47-82) years, and 10 (17.2%) were female.
TBM Technique and Timing
Techniques for TBM were transoral robotic surgery in 44 patients (75.9%), transoral electrocautery in 11 patients (19.0%), and transoral laser microsurgery in 3 patients (5.2%). Table 1 gives a further breakdown by subgroup.
Table 1. Clinicopathological Features for All Patients and for the Most Common Diagnostic Surgical Scenarios.
| Variable/classification | No. (%) | |||
|---|---|---|---|---|
| All patients (M = 58) | Bilateral tonsillectomy and bilateral TBM (n = 15) | Bilateral tonsillectomy then bilateral TBM (n = 14) | Bilateral TBM (historic tonsillectomy) (n = 21) | |
| Age, y | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| Median (range) | 58 (47-82) | 53 (47-70) | 57 (51-78) | 61 (52-82) |
| Mean (SD) | 59.7 (8) | 55.8 (6.5) | 59.5 (8) | 63.3 (8.4) |
| Sex | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| Female | 10 (17.2) | 1 (6.7) | 1 (7.1) | 5 (23.8) |
| Male | 48 (82.8) | 14 (93.3) | 13 (92.9) | 16 (76.2) |
| Smoking history | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| Never smoker | 22 (37.9) | 7 (46.7) | 5 (35.7) | 7 (33.3) |
| Previous smoker | 28 (48.3) | 7 (46.7) | 7 (50) | 10 (47.6) |
| Current smoker | 8 (13.8) | 1 (6.7) | 2 (14.3) | 4 (19) |
| Alcohol use history | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| Never | 5 (8.6) | 1 (6.7) | 3 (21.4) | 1 (4.8) |
| Occasional | 25 (43.1) | 9 (60) | 7 (50) | 6 (28.6) |
| Moderate | 20 (34.5) | 2 (13.3) | 4 (28.6) | 10 (47.6) |
| Heavy | 8 (13.8) | 3 (20) | 0 | 4 (19) |
| p16 Statusa | ||||
| Data available | 56 (96.6) | 15 (100) | 14 (100) | 19 (90.5) |
| Negative | 9 (16.1) | 1 (6.7) | 3 (21.4) | 4 (21.1) |
| Positive | 47 (83.9) | 14 (93.3) | 11 (78.6) | 15 (78.9) |
| HPV-ISH statusa | ||||
| Data available | 19 (32.8) | 8 (53.3) | 2 (14.3) | 6 (28.6) |
| Negative | 2 (10.5) | 0 | 0 | 2 (33.3) |
| Positive | 17 (89.5) | 8 (100) | 2 (100) | 4 (66.7) |
| EBER-ISH statusa | ||||
| Data available | 12 (20.7) | 3 (20) | 5 (35.7) | 4 (19) |
| Negative | 10 (83.3) | 1 (33.3) | 5 (100) | 4 (100) |
| Positive | 2 (16.7) | 2 (66.7) | 0 | 0 |
| CT neck | ||||
| Data available | 31 (53.4) | 5 (33.3) | 10 (71.4) | 12 (57.1) |
| Yes | 31 (53.4) | 5 (33.3) | 10 (71.4) | 12 (57.1) |
| No | 0 | 0 | 0 | 0 |
| MRI neck | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| Yes | 51 (87.9) | 15 (100) | 11 (78.6) | 18 (85.7) |
| No | 7 (12.1) | 0 | 3 (21.4) | 3 (14.3) |
| PET-CT | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| Yes | 57 (98.3) | 15 (100) | 14 (100) | 20 (95.2) |
| No | 1 (1.7) | 0 | 0 | 1 (4.8) |
| Clinical nodal status (TNM8)b | ||||
| Data available | 58 (98.3) | 14 (93.3) | 14 (100) | 21 (100) |
| cN1 | 49 (84.5) | 13 (86.7) | 12 (85.7) | 18 (85.7) |
| cN2 (HPV-positive) | 1 (1.7) | 1 (6.7) | 0 | 0 |
| cN2a | 2 (3.4) | 0 | 1 (7.1) | 0 |
| cN2b | 5 (8.6) | 1 (6.7) | 1 (7.1) | 3 (14.3) |
| cN3b | 1 (1.7) | 0 | 0 | 0 |
| TBM method | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| TORS | 44 (75.9) | 10 (66.7) | 10 (71.4) | 16 (76.2) |
| TLM | 3 (5.2) | 2 (13.3) | 1 (7.1) | 0 |
| TOEC | 11 (19) | 3 (20) | 3 (21.4) | 5 (23.8) |
| Pathological tumor status (TNM8) | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| pT0 | 22 (37.9) | 3 (20.0) | 8 (57.1) | 9 (42.9) |
| pT1 | 33 (56.9) | 9 (60.0) | 6 (42.9) | 12 (57.1) |
| pT2 | 3 (5.2) | 3 (20.0) | 0 | 0 |
| Maximum tumor sizec | ||||
| Data available | 33 (97.1) | 10 (90.9) | 6 (100) | 13 (100) |
| Median (range), mm | 8 (2-26) | 7.25 (2-26) | 10.75 (4-18) | 8 (2-17) |
| Tumor margind | ||||
| Data available | 33 (97.1) | 11 (100) | 6 (100) | 12 (92.3) |
| Positive (<1 mm) | 18 (54.5) | 7 (63.6) | 2 (33.3) | 7 (58.3) |
| Close (1-3 mm) | 9 (27.3) | 2 (18.2) | 2 (33.3) | 5 (41.7) |
| Clear (>3 mm) | 6 (18.2) | 2 (18.2) | 2 (33.3) | 0 |
| Step serial sectioning result | ||||
| Total sections | 4096 | 1300 | 1175 | 1188 |
| Median (range), sections | 64 (28-135) | 90 (35-135) | 92 (39-128) | 52 (31-107) |
| Mean sections | 70.6 | 86.7 | 83.9 | 56.6 |
| Additional primary | 1 (1.7) | 1 (6.7) | 0 | 0 |
| No additional primary | 57 (98.3) | 14 (93.3) | 14 (100) | 21 (100) |
| Final pathology | ||||
| Data available | 58 (100) | 15 (100) | 14 (100) | 21 (100) |
| No foci | 22 (37.9) | 3 (20) | 8 (57.1) | 9 (42.9) |
| Single foci | 31 (53.4) | 9 (60.0) | 5 (35.7) | 11 (52.4) |
| Multiple foci | 5 (8.6) | 3 (20) | 1 (7.1) | 1 (4.8) |
Abbreviations: CT, computed tomography; EBER-ISH, Epstein-Barr virus-encoded RNA in situ hybridization; HPV-ISH, high-risk human papillomavirus in situ hybridization; MRI, magnetic resonance imaging; PET-CT, 18F-fluorodeoxyglucose–positron emission tomography–computed tomography; TBM, tongue base mucosectomy; TLM, transoral laser microsurgery; TOEC, transoral electrocautery; TORS, transoral robotic surgery.
Final histopathological status.
Taking account of final HPV status by p16 immunohistochemistry.
Size given for largest focus of primary site disease in cases of multifocal disease.
Closest of any margins in cases of multifocal disease.
Tongue base mucosectomy was most commonly performed following an historic tonsillectomy (21 [36.2%]). It was performed as a staged procedure after negative palatine tonsillectomy for 15 patients (25.9%) and concurrently with tonsillectomy in 14 (24.1%). Other combinations of timings of TBM surgery and completeness of palatine and lingual lymphoepithelial resection accounted for 8 patients (13.8%; Table 2).
Table 2. Locations of Primary Tumors for All Diagnostic Surgical Scenarios.
| Diagnostic surgical scenario | No. (%) | Location of primary disease, No. (%) | Rates, % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No foci | Ipsilateral tonsil | Ipsilateral tongue base, >1 cm from midline | Tongue base within 1 cm of midline | Contralateral tongue base, >1 cm from midline | Bilateral synchronous tongue base | Tongue base cancer | Any OP cancer | ||
| Bilateral tonsillectomy and bilateral TBM | 15 (25.9) | 3 (20) | 5 (33.3) | 4 (27.7) | 0 | 0 | 3 (20) | 20.0 (7.0-45.2) | 80.0 (54.8-93.0) |
| Bilateral tonsillectomy then bilateral TBM | 14 (24.1) | 8 (57.1) | 0 | 5 (35.7) | 0 | 0 | 1 (7.1) | 7.1 (1.3-31.5) | 42.9 (21.4-67.4) |
| Bilateral TBM (historic tonsillectomy) | 21 (36.2) | 9 (42.9) | 0 | 3 (14.3) | 8 (38.1) | 0 | 1 (4.8) | 42.9 (24.5-63.5) | 57.1 (36.5-75.5) |
| Ipsilateral tonsillectomy and bilateral TBM | 5 (8.6) | 1 (20.0) | 2 (40.0) | 0 | 1 (20.0) | 1 (20.0) | 0 | 40.0 (11.8-76.9) | 80.0 (37.6-96.4) |
| Ipsilateral tonsillectomy and ipsilateral TBM | 1 (1.7) | 0 | 0 | 0 | 1 (100) | 0 | 0 | 100 (20.7-100) | 100 (20.7-100) |
| Ipsilateral tonsillectomy, then contralateral tonsillectomy and TBM | 2 (3.4) | 1 (50.0) | 0 | 1 (50.0) | 0 | 0 | 0 | 0 (0-65.8) | 50.0 (9.5-90.5) |
| Overall | 58 (100) | 22 (37.9) | 7 (12.1) | 13 (22.4) | 10 (17.2) | 1 (1.7) | 5 (8.6) | 27.6 (17.8-40.2) | 62.1 (49.2-73.4) |
Abbreviations: OP, oropharyngeal; TBM, tongue base mucosectomy.
Conventional Histology
At central review with CH techniques (including original slides), 2 cases were upgraded and 2 were downgraded. The upgraded cases had new ipsilateral tongue base primaries identified that had not been diagnosed by the participating site. The downgraded cases included a case in which the diagnosis of squamous cell carcinoma was changed to inflammatory ulceration and necrotizing sialometaplasia and another case in which the tumor had been diagnosed as a single large tumor but was shown to be 2 separate, smaller synchronous tumors. There was a single case identified as of carcinoma in situ (in the contralateral base of tongue lymphoepithelial tissue) that was reclassified as pT1 for final histology, consistent with the latest World Health Organization classification of head and neck tumors.19
SSS Histopathology
A total of 20 480 sections were cut in the laboratory, with 4096 sections directly examined by the pathologist (median [range], 64 [28-135] per patient; Table 1). Step serial sectioning identified a single additional synchronous tumor: an HPV-positive ipsilateral tongue base tumor in a patient who had had a contralateral tongue base primary identified on CH (Figure 2). The additional tumor was contained entirely within the tissue block (dimensions on the section 5.8 mm × 3.0 mm and estimated to measure 2.0 mm within the block). Review of the original sections from the participating site confirmed the tumor was not evident.
Figure 2. Right Tongue Base Human Papillomavirus–Associated Squamous Cell Carcinoma Discovered by Serial Step Sections in the MOSES Study.
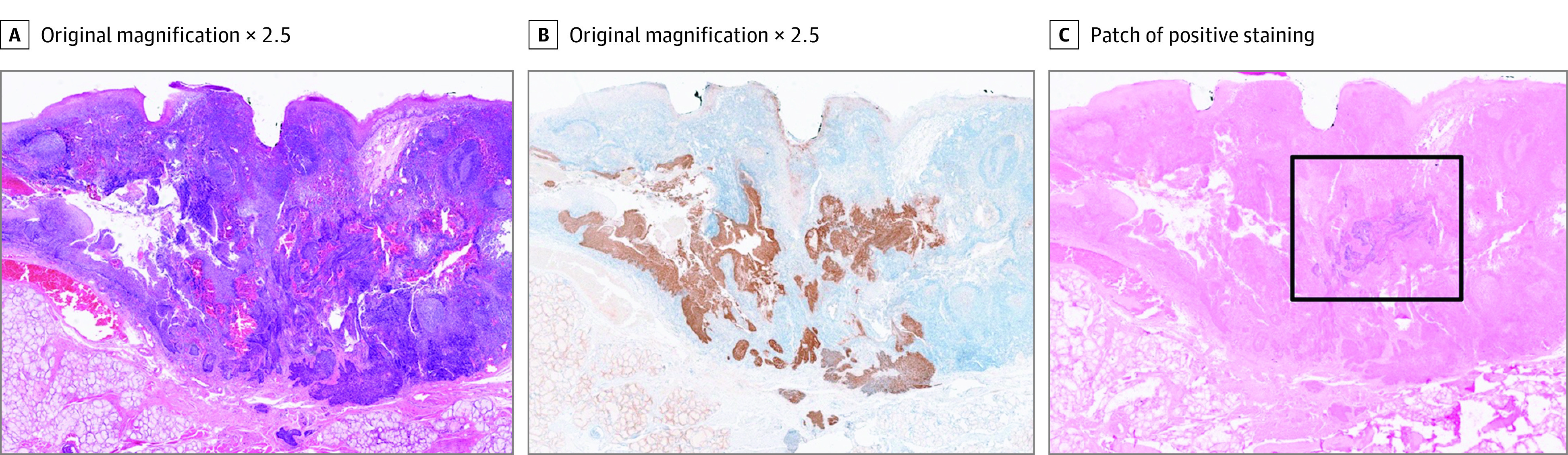
Hematoxylin and eosin (A), p16 immunohistochemistry (B), and high-risk human papillomavirus DNA in situ hybridization (C). The black box highlights patch of positive staining (original magnification ×2.5).
Overall, 5 TBM patients (8.6%; 95% CI, 3.7%-18.6%) in this cohort had multifocal disease, all found bilaterally in the tongue base. Notably, 3 (20%; 95% CI, 7.0%-45.2%) of those having tonsillectomy and TBM in a single theater episode had synchronous disease. All primary cancers identified on central laboratory testing were HPV-related small-cell carcinomas.
TBM Identification Rates
The overall identification rate for TBM following SSS according to study protocol was 50.0% (95% CI, 37.5%-62.5%). By subgroup, the rates were when performed following a negative bilateral tonsillectomy (42.9%; 95% CI, 21.4%-67.4%), at the same time as bilateral tonsillectomy (46.7%; 95% CI, 24.8%-69.9%), and in the context of an historic tonsillectomy (57.1%; 95% CI, 36.5%-75.5%).
Discussion
Summary of Findings
This multicenter cohort study prospectively analyzed 58 patients with clinically and radiologically occult primary disease who were undergoing TBM for investigation of HPV-positive and HPV-negative HNSCCUP. A TBM was performed using various transoral surgical techniques, reflecting contemporary UK practice. A TBM was most commonly performed following an historic tonsillectomy (36%), as a staged procedure after negative palatine tonsillectomy (26%), and then concurrently with tonsillectomy (24%).
All resected oropharyngeal tissues underwent CH at their local center followed by SSS, according to the study protocol, with a median of 320 sections being cut and 64 sections analyzed per patient. Only a single additional 5.8-mm ipsilateral tongue base tumor was identified using SSS, in addition to a contralateral tongue base tumor in the same patient. Review by a second pathologist led to the identification of 2 additional cancers and downgrading of 2 cancers (1 felt to be inflammatory change and 1 found to be 2 separate lesions, not a single entity). Multifocal disease was seen in 5 (8.6%), all HPV-related and all in the tongue base. The overall TBM identification rate was 50.0%, although this varied, as expected, in association with the timing of removal of the palatine tonsils.
TBM Identification Rates
To our knowledge, there are no published randomized clinical trials investigating the benefits of tongue base mucosectomy; thus observational data alone must be relied on. The MOSES study hypothesized that CH techniques may miss very small oropharyngeal tumors and sought to establish the true incidence of clinicoradiologically occult tumors in this population. Recently, Al-Lami et al20 published their systematic review of histopathological detection of a primary tumor in HNSCCUP, which included more than 700 patients.20 The focus was on comparing the effectiveness of different transoral surgical techniques. The timing of palatine tonsillectomy was not considered. Whether conducted concurrently, as a staged procedure or not at all, this will have a substantial association with the apparent incidence of primary disease in the lingual tonsil. In the present study, with a prospectively recruited cohort, this can be accounted for and has shown that the TBM identification rates vary, as expected, with the highest rate seen in the absence of any palatine tonsil tissue due to historic tonsillectomy (57.1%).
Farooq et al21 conducted a very similar systematic review with searches performed only 2 years previously. They identified that 17 studies had reported TBM results associated with their timing with palatine tonsillectomy, although unfortunately they did not use this to stratify their pooled analysis. Inclusion criteria differed slightly between these 2 systematic reviews, and the present study but overall tongue base primary pick-up rates were shown to be fairly similar at 45%, 53% and 50%, respectively.
Incomplete Diagnostic Oropharyngeal Surgery
Several patients underwent some level of TBM to be included in the MOSES study but did not have full clearance of the putative oropharyngeal lymphoepithelial tissue (bilateral tonsillectomy and bilateral TBM). Interpreting incidence rates within the oropharynx should consider the extent of surgery, not least due to the presence of multifocal disease. Farooq et al21 reported only 4 of 432 cases (0.9%) with multifocal disease (1 bilateral tongue base, 3 bilateral palatine tonsils). The MOSES cohort saw a markedly higher rate of 8.6%, all as synchronous tumors within the tongue base, which was more in keeping with other studies around 5% to 10%.11,12
Contralateral disease was also more common in the MOSES cohort than previously reported, although this was entirely confined to the tongue base and was predominantly seen with multifocal disease (ie, a rate of 8.6%). No contralateral palatine tonsil tumors were identified either as single entities or as part of multifocal disease. Farooq et al21 identified a contralateral tonsil primary rate of only 0.9% (4 of 432; 3 in patients with bilateral tonsil primaries). Further, the HNSCCUP National Cohort Study delivered by INTEGRATE in the UK corroborated these findings with higher rates of contralateral tongue base primaries than contralateral tonsil, despite significantly more palatine tonsillectomy surgery being performed.1
Regardless of low pick-up rates, full clearance of the oropharyngeal lymphoepithelial tissue would be associated with increased primary site identification rates. However, this must be weighed against the inherent morbidity associated with the procedure: most notably, pain and bleeding risk in the short term and pharyngeal stenosis in the longer term.9,22,23 The psychological effect of persistent unknown primary disease is also not to be overlooked. Patients have reported frustration from not knowing the original site of disease, anxiety from not being able to have focused treatment, and even denial of the cancer diagnosis when unable to relate to its origin.24,25,26
The rates of pharyngeal stenosis are not well established, although the extension of the MOSES trial will potentially be able to report on this from a prospectively recruited cohort in due course. What has not been satisfactorily demonstrated is a longer-term functional or oncological benefit from either identifying or not identifying these clinically and radiologically occult primary sites. Subsequent treatment has been shown to vary considerably for patients with HNSCCUP,13 with no randomized clinical trials to reference. Many of these patients will receive radiation therapy to at least some part of the upper aerodigestive tract mucosa and so diagnostic surgery, which may well leave residual disease (54.5% had a margin of <1 mm in this study) and could be seen as superfluous. The 8th edition of the American Joint Committee on Cancer cancer staging manual considers p16-positive and negative cervical metastases without an identified primary tumor separately, as Tx and T0 disease, respectively.27 However, the required diagnostic workup to reach this categorization is not stipulated and can vary considerably between patients and centers.1,12 Due to its high prevalence in oropharyngeal disease, HPV-associated HNSCCUP is presumed to have originated in either the palatine or lingual tonsil.7,12,28 With the high rates of clinico-radiologically occult tongue base primaries seen, should TBM take precedence as a minimum requirement for diagnostic work-up over and above a contralateral tonsillectomy? The data presented here would suggest so.
Particularly in the case of p16-negative disease, oropharyngeal sampling alone, through palatine tonsillectomy and TBM, will not allow histological analysis of all tissues that may give rise to cancers associated with metastatic cervical SCC. Random biopsy specimens of skin or elsewhere in the upper aerodigestive tract that appear clinically and radiologically normal are unlikely to be followed by identification of a primary site. It is hoped that a now funded extension of the MOSES study may allow for further insight into these patients through genetic sequencing. However, in several cases, especially where primary site emergence is not seen, regression of the primary tumor before initial presentation remains a plausible explanation for this occult disease.
Limitations
The MOSES cohort study reports the largest prospectively identified cohort of HNSCCUP undergoing TBM but is not without its limitations. First, stratification of patients by timing of palatine tonsillectomy delivered relatively small subgroups for analysis. However, SSS was the focus of this initial phase of the MOSES study, and it is felt this question was answered adequately. Second, various surgical techniques were used to obtain the tissue (transoral robotic surgery/transoral laser microsurgery/transoral electrocautery), and surgery did not mandate a standard operating procedure across centers. While homogenization for quality assurance may seem desirable, the study intentionally set out to report on the contemporary clinical practice in a pragmatic national setting to ensure that results were as generalizable as possible to day-to-day care.
Conclusions
In a prospectively identified multicenter cohort study of patients undergoing TBM for HNSCCUP, comprehensive SSS was associated with added considerable histopathological workload with minimal additional diagnostic benefit. In practice, a second opinion using CH techniques for HNSCCUP diagnostic specimens may be more beneficial than upfront SSS for all cases. Multifocal oropharyngeal disease was commonly seen in tongue base specimens associated with HPV disease. The role of oropharyngeal diagnostic surgery, and in particular the benefit of contralateral palatine tonsillectomy, is yet to be established.
Data sharing statement
References
- 1.INTEGRATE (The UK ENT Trainee Research Network) . National audit of the Management of Head and Neck Squamous Cell Carcinoma of Unknown Primary. Accessed February 10, 2022. entintegrate.co.uk/hnsccup
- 2.National Collaborating Centre for Cancer . Diagnosis and Management of Metastatic Malignant Disease of Unknown Primary Origin. National Collaborating Centre for Cancer; 2010. [PubMed] [Google Scholar]
- 3.Junn JC, Soderlund KA, Glastonbury CM. Imaging of head and neck cancer with CT, MRI, and US. Semin Nucl Med. 2021;51(1):3-12. doi: 10.1053/j.semnuclmed.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 4.Maghami E, Ismaila N, Alvarez A, et al. Diagnosis and management of squamous cell carcinoma of unknown primary in the head and neck: ASCO guideline. J Clin Oncol. 2020;38(22):2570-2596. doi: 10.1200/JCO.20.00275 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . Head and neck cancer guidelines (version 1.2022) [internet]. Accessed January 6, 2022. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 6.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386-396. doi: 10.1016/j.mayocp.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Lechner M, Liu J, Masterson L, Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022;19(5):306-327. doi: 10.1038/s41571-022-00603-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen MHH, Channir HI, von Buchwald C. Human papillomavirus and squamous cell carcinoma of unknown primary in the head and neck region: a comprehensive review on clinical implications. Viruses. 2021;13(7):1297. doi: 10.3390/v13071297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter SC, Ofo E, Meikle D, et al. Trans-oral robotic assisted tongue base mucosectomy for investigation of cancer of unknown primary in the head and neck region. The UK experience. Clin Otolaryngol. 2017;42(6):1247-1251. doi: 10.1111/coa.12860 [DOI] [PubMed] [Google Scholar]
- 10.de Almeida JR. Role of transoral robotic surgery in the work-up of the unknown primary. Otolaryngol Clin North Am. 2020;53(6):965-980. doi: 10.1016/j.otc.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 11.Jain KS, Sikora AG, Baxi SS, Morris LGT. Synchronous cancers in patients with head and neck cancer: risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer. 2013;119(10):1832-1837. doi: 10.1002/cncr.27988 [DOI] [PubMed] [Google Scholar]
- 12.Motz K, Qualliotine JR, Rettig E, Richmon JD, Eisele DW, Fakhry C. Changes in unknown primary squamous cell carcinoma of the head and neck at initial presentation in the era of human papillomavirus. JAMA Otolaryngol Head Neck Surg. 2016;142(3):223-228. doi: 10.1001/jamaoto.2015.3228 [DOI] [PubMed] [Google Scholar]
- 13.Axelsson L, Holmberg E, Nyman J, et al. Swedish national multicenter study on head and neck cancer of unknown primary: prognostic factors and impact of treatment on survival. Int Arch Otorhinolaryngol. 2021;25(3):e433-e442. doi: 10.1055/s-0040-1712106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilling C, Stoeckli SJ, Haerle SK, et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer. 2015;51(18):2777-2784. doi: 10.1016/j.ejca.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 15.Nieuwenhuis ER, Kolenaar B, van Bemmel AJM, et al. A complete magnetic sentinel lymph node biopsy procedure in oral cancer patients: a pilot study. Oral Oncol. 2021;121:105464. doi: 10.1016/j.oraloncology.2021.105464 [DOI] [PubMed] [Google Scholar]
- 16.de Bree R, de Keizer B, Civantos FJ, et al. What is the role of sentinel lymph node biopsy in the management of oral cancer in 2020? Eur Arch Otorhinolaryngol. 2021;278(9):3181-3191. doi: 10.1007/s00405-020-06538-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panda S, Thakar A, Kakkar A, et al. Is the retropharyngeal lymph node the first echelon node for carcinoma tonsil? prospective evaluation and literature review. Eur Arch Otorhinolaryngol. 2021;278(10):3995-4004. doi: 10.1007/s00405-020-06585-5 [DOI] [PubMed] [Google Scholar]
- 18.Siddiq S, Cartlidge D, Stephen S, et al. Robotic lateral oropharyngectomy following diagnostic tonsillectomy is oncologically safe in patients with high risk human papillomavirus related squamous cell cancer. Eur Arch Otorhinolaryngol. 2018;275(7):1853-1860. doi: 10.1007/s00405-018-4968-6 [DOI] [PubMed] [Google Scholar]
- 19.Westra W, Fujii S, Bishop J, et al. Oropharyngeal tumours (base of tongue, tonsils adenoids), squamous cell carcinoma, HPV associated. Accessed October 8, 2023. https://tumourclassification.iarc.who.int/chapters/52
- 20.Al-Lami A, Gao C, Saddiq M, et al. Reducing the unknowns: a systematic review & meta-analysis of the effectiveness of trans-oral surgical techniques in identifying head and neck primary cancer in carcinoma unknown primary. Oral Oncol. 2022;126:105748. doi: 10.1016/j.oraloncology.2022.105748 [DOI] [PubMed] [Google Scholar]
- 21.Farooq S, Khandavilli S, Dretzke J, et al. Transoral tongue base mucosectomy for the identification of the primary site in the work-up of cancers of unknown origin: Systematic review and meta-analysis. Oral Oncol. 2019;91:97-106. doi: 10.1016/j.oraloncology.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 22.Owen S, Puvanendran M, Meikle D, et al. Baseline swallowing measures predict recovery at 6 weeks after transoral robotic surgery for head and neck cancer. Clin Otolaryngol. 2017;42(2):366-372. doi: 10.1111/coa.12731 [DOI] [PubMed] [Google Scholar]
- 23.Muderris T, Sevil E, Bercin S, Gul F, Kiris M. Oropharyngeal stenosis after transoral robotic lingual tonsillectomy. J Craniofac Surg. 2015;26(3):853-855. doi: 10.1097/SCS.0000000000001584 [DOI] [PubMed] [Google Scholar]
- 24.Boyland L, Davis C. Patients’ experiences of carcinoma of unknown primary site: dealing with uncertainty. Palliat Med. 2008;22(2):177-183. doi: 10.1177/0269216307085341 [DOI] [PubMed] [Google Scholar]
- 25.Richardson A, Wagland R, Foster R, et al. Uncertainty and anxiety in the cancer of unknown primary patient journey: a multiperspective qualitative study. BMJ Support Palliat Care. 2015;5(4):366-372. doi: 10.1136/bmjspcare-2013-000482 [DOI] [PubMed] [Google Scholar]
- 26.Ishida K, Ando S, Komatsu H, Kinoshita S, Mori Y, Akechi T. Psychological burden on patients with cancer of unknown primary: from onset of symptoms to initial treatment. Jpn J Clin Oncol. 2016;46(7):652-660. doi: 10.1093/jjco/hyw048 [DOI] [PubMed] [Google Scholar]
- 27.American Joint Committee on Cancer . AJCC cancer staging manual. Accessed March 28, 2017. https://www.springer.com/us/book/9783319406176
- 28.Schroeder L, Boscolo-Rizzo P, Dal Cin E, et al. Human papillomavirus as prognostic marker with rising prevalence in neck squamous cell carcinoma of unknown primary: a retrospective multicentre study. Eur J Cancer. 2017;74:73-81. doi: 10.1016/j.ejca.2016.12.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data sharing statement