Abstract
Background:
Gankyrin is an ankyrin-repeat protein that promotes cell proliferation, tumor development and cancer progression when overexpressed.
Aim:
To design and synthesize a novel series of gankyrin-binding small molecules predicated on a 2,5-pyrimidine scaffold.
Materials & methods:
The synthesized compounds were evaluated for their antiproliferative activity, ability to bind gankyrin and effects on cell cycle progression and the proteasomal degradation pathway.
Results:
Compounds 188 and 193 demonstrated the most potent antiproliferative activity against MCF7 and A549 cells, respectively. Both compounds also demonstrated the ability to effectively bind gankyrin, disrupt proteasomal degradation and inhibit cell cycle progression.
Conclusion:
The 2,5-pyrimidine scaffold exhibits a novel and promising strategy for binding gankyrin and inhibiting cancer cell proliferation.
Keywords: breast cancer, gankyrin, lung cancer, protein–protein interactions, tumor suppressor proteins
Gankyrin is a seven ankyrin repeat (AR)-containing protein that facilitates the proteolysis of numerous substrates and modified tumor suppressor proteins (TSPs) [1–3]. As an essential component of the 19S regulatory particle of the 26S proteasome, gankyrin is an upstream regulator of essential proteins identified in both oncogenic and inflammatory pathways [2,4–8]. Gankyrin was first observed in 2000 to be overexpressed in patients with early-stage liver cancer and has since been reported in various other cancer types [9–18]. Recent insights into the oncogenic role of gankyrin in breast and lung cancer development supports its promising therapeutic potential through targeted inhibition.
Gankyrin's ability to regulate cancer proliferation is largely attributed to its interaction with the S6 ATPase as a component of the 26S proteasome [1] to enhance the proteolysis of TSPs such as p53 and Rb [3,19–21]. Overactive TSP degradation results in uncontrolled cell growth and cancer proliferation [22–24]. Studies have also shown that small-molecule binders of gankyrin can decrease cancer cell proliferation by increasing levels of Rb and p53 [22,25–29].
In 2021, the current author's group discovered a novel gankyrin binding scaffold (LAS789, compound 1) from a high-throughput virtual screen. Despite a significant increase in gankyrin binding ability (∼16-fold over cjoc42) [27], only modest improvements in antiproliferative activity were observed. The current study was designed to establish the first structure–activity relationships (SARs) for the LAS789 scaffold in terms of both targeting gankyrin and inhibiting the proliferation of gankyrin-overexpressing cancer cells.
The initial discovery of LAS789 as a potent gankyrin binder suggested a similar mode of action to the small molecule–protein interaction seen with cjoc42 in molecular modeling studies (Figure 1A) [25,27]. Such analysis predicted that ring B of LAS789 (compound 1) interacts with Trp46 of gankyrin through a parallel-displaced π–π interaction, while ring A likely interacts with Trp74 of gankyrin through a T-shaped π–π interaction. Interestingly, ring C does not appear to engage in any interactions with gankyrin and may be insignificant for binding (Figure 1B). Therefore, the aim of this study was to develop an extensive SAR for rings A and B and determine the significance of ring C for gankyrin binding and antiproliferative activity against gankyrin-overexpressing cancer cells.
Figure 1. . Proposed binding of cjoc42 and compound 1 (LAS789) to gankyrin.
(A) cjoc42 docked into its proposed gankyrin binding site (Protein Data Base [PDB]: 1QYM) [30]. (B) Compound 1 (LAS789) docked into its proposed binding site (PDB: 1QYM) [30]. (C) Chemical structures of cjoc42 and compound 1 (LAS789).
Materials & methods
Chemistry
Full synthetic protocols and spectral characterization are provided in the Supplementary Information.
Cell culture
A549 cells (ATCC CCL-185) were grown in Roswell Park Memorial Institute (RPMI) 1640 media (VWR #45000-396) supplemented with 10% fetal bovine serum (FBS), 1% sodium pyruvate and 1% penicillin/streptomycin. MCF7 cells (ATCC HTB-22) were grown in Dulbecco's modified Eagle medium (DMEM; VWR #45000-304) supplemented with 10% FBS and 1% penicillin/streptomycin. HEK-293 cells (ATCC CRL-1573) were grown in Dulbecco's modified Eagle medium (DMEM; VWR #45000-304) supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were grown and maintained at 37°C in a CO2 incubator.
Antiproliferative assay
The antiproliferative activity of compounds 130–194 against A549 (2.5 × 104 cells/well), MCF7 (2.5 × 104 cells/well) and HEK-293 (2.5 × 104 cells/well) cells was determined as previously described [26].
Protein expression & purification of recombinant gankyrin
Recombinant gankyrin was overexpressed and purified as previously described [26].
Circular dichroism & thermal denaturation
Evaluation of the global secondary structure content and thermal stability of gankyrin in the presence of compounds 188 and 193 using spectropolarimetry were completed as previously described [26].
Cellular thermal shift assay
Compounds 188 and 193 were evaluated for their ability to bind gankyrin in A549 and MCF7 as previously described [26,31].
Western blot analysis
Compounds 188 and 193 were evaluated for their ability to disrupt the proteasomal degradation pathway in A549 and MCF7 cells by western blot analysis as previously described [26].
Cell cycle analysis
Compounds 188 and 193 were evaluated for their ability to inhibit cell cycle progression in A549 and MCF7 cells as previously described [26].
Statistical analysis
GraphPad Prism Version 9.0.1 (CA, USA) was used for data analysis. A two-way analysis of variance was used to determine statistical significance. A p-value < 0.05 was considered statistically significant.
Results & discussion
To experimentally verify the molecular docking experiments (Figure 1), compound 1 was evaluated against both A549 (non-small cell lung cancer) and MCF7 (breast cancer) cell lines for its antiproliferative activity (Table 1). Both non-multidrug resistant cell lines were chosen due to reported gankyrin overexpression, wild-type p53 status and their sensitivity to gankyrin levels [32–36]. Compound 1 exhibited modest antiproliferative activity against both A549 and MCF7 cells, with IC50 values of 45.6 and 35.3 μM, respectively [27]. Additionally, compound 1 demonstrated a significant improvement in gankyrin binding compared with cjoc42, as determined through a protein thermal shift assay (Supplementary Table 1) [27]. Efforts to optimize the compound 1 scaffold were undertaken to explore the significance of ring C and whether the inclusion of a carboxylic acid or ester group was most important to improving antiproliferative activity. This resulted in the synthesis of compounds 130 and 194 (Figure 2).
Table 1. . Antiproliferative evaluation of cjoc42 and compounds 1, 130 and 194 against A549 and MCF7 cells†,‡,§,¶.
| Compound | Structure | A549 IC50 (μM) | MCF7 IC50 (μM) | Ref. |
|---|---|---|---|---|
| Cjoc42 |

|
>50 | >50 | |
| 1 |

|
45.6 (±9.7) | 35.3 (±3.2) | [27] |
| 130 |

|
>50 | 17.2 (±0.9) | |
| 194 |

|
>50 | >50 |
A549 and MCF7 cells were incubated for 24 h prior to drug addition.
A549 and MCF7 cells were incubated for 72 h at 37°C in 5% CO2 with the respective drug.
Cell proliferation was determined using an MTT assay.
All experiments were performed in replicates of 6–8.
Figure 2. . Synthesis of compounds 130–194.

Reagents and conditions: (A) substituted phenol, PPh3, DIAD, THF, reflux, (B) TFA, CH2Cl2, RT, (C) 2-chloro-5-pyrimidine carboxylic acid, K2CO3, DMF, 120°C, (D) methyl ester glycine, TEA, then NaBH4, MeOH, RT, (E) 55–81: oxalyl chloride, DMF, DIPEA, CH2Cl2, (F) LiOH (aq.), MeOH, RT.
DIAD: Diisopropyl azodicarboxylate; DIPEA: N,N-Diisopropylethylamine; DMF: N,N-Dimethylformamide; MeOH: Methanol; RT: Room temperature; TEA: Triethylamine; TFA: Trifluoroacetic acid; THF: Tetrahydrofuran.
Compounds 130 and 194 were then evaluated for their antiproliferative activity (Table 1) against A549 and MCF7 cells. Compound 130, which lacked the phenethyl group of compound 1, enhanced antiproliferative activity against MCF7 cells, however, it did not improve antiproliferative activity against A549 cells (IC50 >50 μM). Interestingly, compound 194 exhibited a decrease in antiproliferative activity against MCF7 cells compared with compound 130, suggesting the decreased lipophilicity caused by the addition of the carboxylic acid group decreased cell penetration. Based on these results, it was determined that the phenethyl group of compound 1 was not essential to its antiproliferative activity. Therefore, derivatives of compound 130 were designed with the goal of optimizing the functional group substitutions on rings A and B to further enhance its antiproliferative activity against A549 and MCF7 cell lines.
Synthesis
Compounds 131–193 were then synthesized (Figure 2) with the goal of replacing the 4-chloro group of each phenyl ring with different functional groups at various positions. The synthesis of derivatives 131–193 began with a Mitsunobu reaction between a series of substituted phenols and N-Boc-L-prolinol (2), followed by acid-mediated removal of the Boc-protecting group to obtain intermediates 29–54. Intermediates 29–54 then underwent a nucleophilic aromatic substitution with 2-chloropyrimidine-5-carboxylic acid to obtain key intermediates 55–81. In parallel, a series of benzaldehydes (82–105) each underwent reductive amination with glycine methyl ester to obtain intermediates 106–129. Carboxylic acid intermediates 55–81 were then converted to the corresponding acid chloride with oxalyl chloride followed by a nucleophilic substitution with amine intermediates 106–129 to ultimately obtain final compounds 130–193. Additionally, the hydrolysis of compound 130 afforded carboxylic acid 194.
Antiproliferative evaluation
Compounds 130–155 were then evaluated for their antiproliferative activity against gankyrin-overexpressing A549 and MCF7 cell lines (Table 2). Compounds 131–139 contain various substitutions at the para position of ring A. Electron-withdrawing groups at the para position, such as 4-CN (133) and 4-NO2 (134), did not exhibit any significant improvement in antiproliferative activity against A549 or MCF7 cells compared with 130, while 4-CF3 (135) and 4-CO2CH3 (136) significantly improved activity against only A549 cells. Large halogen substitutions, such as 4-Br (131) and 4-I (132), significantly enhanced antiproliferative activity against A549 cells while activity against MCF7 cells decreased. Additionally, small electron-donating groups, such as 4-CH3 (137) and 4-OCH3 (139), exhibited significant improvements in antiproliferative activity against A549 cells but with a loss of activity against MCF7 cells. Larger electron-withdrawing groups, such as 4-Ph (138), exhibited a significant improvement in activity against A549 cells but a slight improvement against MCF7 cells. At the meta position, halogen substitutions (140, 141 and 142) resulted in a moderate to significant increase in antiproliferative activity against A549 cells, while only a Br substitution (142) caused a slight improvement in activity against MCF7 cells. Additionally, electron-withdrawing groups, such as 3-CN (143) and 3-CO2CH3 (146), resulted in moderate and significant improvements in antiproliferative activity, respectively, against only A549 cells. A significant improvement in antiproliferative activity against A549 cells and a moderate improvement against MCF7 cells were also demonstrated by 3-CF3 (145). Smaller electron-donating groups, such as 3-CH3 (147) and 3-OCH3 (149), resulted in significant improvements in antiproliferative activity against A549 cells along with a loss of activity against MCF7 cells. Additionally, substitutions at the ortho position (150–156) all caused moderate to significant improvements against A549 cells, except for 2-NO2 (153), while they all caused a loss in antiproliferative activity against MCF7 cells compared with compound 130.
Table 2. . Antiproliferative evaluation of compounds 130–178 against A549 and MCF7 cells†,‡,§,¶.

| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | A549 IC50 (μM) | MCF7 IC50 (μM) |
| 130 | 4-Cl | 4-Cl | >50 | 17.2 (±0.9) |
| 131 | 4-Br | 4-Cl | 10.9 (±0.7) | 21.3 (±1.6) |
| 132 | 4-I | 4-Cl | 8.7 (±0.6) | 41.1 (±3.1) |
| 133 | 4-CN | 4-Cl | 41.1 (±3.8) | 18.8 (±1.6) |
| 134 | 4-NO2 | 4-Cl | >50 | 16.7 (±1.3) |
| 135 | 4-CF3 | 4-Cl | 19.5 (±0.8) | 14.7 (±0.7) |
| 136 | 4-CO2CH3 | 4-Cl | 26.3 (±1.1) | 13.7 (±1.2) |
| 137 | 4-CH3 | 4-Cl | 14.6 (±0.9) | 23.0 (±1.8) |
| 138 | 4-Ph | 4-Cl | 17.2 (±1.5) | 9.8 (±0.7) |
| 139 | 4-OCH3 | 4-Cl | 22.5 (±2.1) | 23.1 (±2.0) |
| 140 | 3-F | 4-Cl | 24.6 (±2.2) | 24.3 (±2.1) |
| 141 | 3-Cl | 4-Cl | 27.0 (±2.4) | 18.2 (±1.6) |
| 142 | 3-Br | 4-Cl | 13.1 (±1.2) | 15.8 (±1.2) |
| 143 | 3-CN | 4-Cl | 27.8 (±1.6) | 42.4 (±3.3) |
| 144 | 3-NO2 | 4-Cl | >50 | 19.3 (±1.5) |
| 145 | 3-CF3 | 4-Cl | 12.9 (±0.9) | 9.8 (±0.8) |
| 146 | 3-CO2CH3 | 4-Cl | 23.9 (±1.9) | 32.0 (±2.2) |
| 147 | 3-CH3 | 4-Cl | 16.4 (±1.1) | 26.0 (±2.1) |
| 148 | 3-Ph | 4-Cl | >50 | >50 |
| 149 | 3-OCH3 | 4-Cl | 7.6 (±0.7) | 21.0 (±1.2) |
| 150 | 2-Cl | 4-Cl | 20.2 (±1.5) | 23.2 (±1.9) |
| 151 | 2-I | 4-Cl | 36.3 (±3.3) | 26.2 (±1.9) |
| 152 | 2-CN | 4-Cl | 23.1 (±1.7) | 40.1 (±3.3) |
| 153 | 2-NO2 | 4-Cl | >50 | >50 |
| 154 | 2-CF3 | 4-Cl | 38.5 (±2.8) | 43.3 (±4.1) |
| 155 | 2-CH3 | 4-Cl | 28.0 (±2.6) | 19.1 (±1.8) |
| 156 | 4-Cl | 4-F | 21.2 (±1.6) | 8.2 (±0.7) |
| 157 | 4-Cl | 4-Br | 46.7 (±3.7) | 18.5 (±1.5) |
| 158 | 4-Cl | 4-I | 21.1 (±2.1) | 14.1 (±1.2) |
| 159 | 4-Cl | 4-CF3 | 22.2 (±2.1) | 12.4 (±1.3) |
| 160 | 4-Cl | 4-CH3 | 21.0 (±2.0) | 11.7 (±1.1) |
| 161 | 4-Cl | 4-Ph | >50 | 19.8 (±1.4) |
| 162 | 4-Cl | 4-OCH3 | 20.4 (±0.9) | 8.0 (±0.6) |
| 163 | 4-Cl | 3-F | 21.2 (±1.8) | 13.5 (±1.1) |
| 164 | 4-Cl | 3-Cl | 22.8 (±1.8) | 14.7 (±1.2) |
| 165 | 4-Cl | 3-Br | >50 | 18.5 (±1.7) |
| 166 | 4-Cl | 3-I | 40.5 (±3.4) | 21.6 (±1.7) |
| 167 | 4-Cl | 3-CN | >50 | 19.4 (±1.5) |
| 168 | 4-Cl | 3-CF3 | 20.2 (±1.9) | 10.5 (±1.0) |
| 169 | 4-Cl | 3-CH3 | 20.4 (±1.0) | 14.1 (±1.1) |
| 170 | 4-Cl | 3-Ph | >50 | >50 |
| 171 | 4-Cl | 3-OCH3 | 21.1 (±1.9) | 18.8 (±1.4) |
| 172 | 4-Cl | 2-F | >50 | 17.6 (±1.4) |
| 173 | 4-Cl | 2-Br | 25.0 (±2.0) | 6.9 (±0.5) |
| 174 | 4-Cl | 2-I | >50 | 32.9 (±2.4) |
| 175 | 4-Cl | 2-CF3 | 22.6 (±1.7) | >50 |
| 176 | 4-Cl | 2-CH3 | 19.2 (±1.9) | 7.4 (±0.6) |
| 177 | 4-Cl | 2-Ph | 23.3 (±1.8) | 18.3 (±1.3) |
| 178 | 4-Cl | 2-OCH3 | 34.3 (±2.6) | 5.8 (±0.7) |
A549 and MCF7 cells were incubated for 24 h prior to drug addition.
A549 and MCF7 cells were incubated for 72 h at 37°C in 5% CO2 with the respective drug.
Cell proliferation was determined using an MTT assay.
All experiments were performed in replicates of 6–8.
Compounds 156–178, which involved various substitutions on ring B, were also evaluated for their antiproliferative activity against A549 and MCF7 cells (Table 2). Electron-withdrawing groups at the para position (156–159) demonstrated significant improvements in antiproliferative activity against A549 cells except for 157 (4-Br). Interestingly, compounds 156–159 resulted in slight to no improvement in activity against MCF7 cells, with 4-F (156) resulting in the most substantial improvement in antiproliferative activity. Small electron-donating groups, such as 4-CH3 (160) and 4-OCH3 (162), exhibited significant enhancements in activity against A549 cells along with a slight improvement in activity against MCF7 cells. Larger groups, such as 4-Ph (161), caused a loss of activity against both cell lines. Electron-withdrawing groups at the meta position, such as 3-F (163) and 3-CF3 (168), exhibited only slight improvements in activity against MCF7 cells and significant improvements in activity against A549 cells. At the ortho position, electron-withdrawing groups, such as 2-Br (173), demonstrated a moderate increase in antiproliferative activity against MCF7 cells and a significant improvement in activity against A549 cells. Furthermore, small electron-donating groups, such as 2-CH3 (176) and 2-OCH3 (178), exhibited moderate to significant improvement in antiproliferative activity against A549 cells and moderate improvements against MCF7 cells compared with 130.
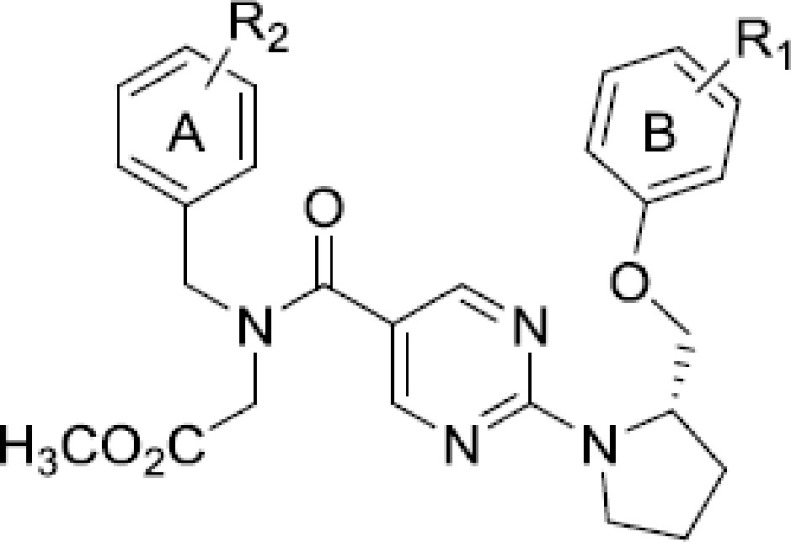
Based on the previously described findings, the most potent substitutions from both phenyl rings (ring A and ring B) were combined to further optimize this scaffold's antiproliferative activity. Therefore, compounds 179–193 were synthesized to combine the optimal R1 and R2 substitutions (Figure 2). Compounds 185, 186 and 188 (IC50 = 3.1, 4.7 and 1.0 μM, respectively) all exhibited potent antiproliferative activity against MCF7 cells (Table 3). Compounds 192 (IC50 = 6.4 μM) and 193 (IC50 = 5.7 μM) demonstrated potent antiproliferative activity against A549 cells. Furthermore, the antiproliferative activity of compounds 185, 186, 188, 189, 190, 191 and 193 was determined against the noncancerous HEK293 cell line, where each compound exhibited an IC50 >50 μM. This suggests these compounds are selective for gankyrin-overexpressing cancer cell lines and are nontoxic to noncancerous cells.
Table 3. . Antiproliferative evaluation of compounds 179–193 against A549 and MCF7 cells†,‡,§,¶.
| |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | A549 IC50 (μM) | MCF7 IC50 (μM) | HEK293 IC50 (μM) |
| 179 | 3-OCH3 | 2-Br | 32.9 (±2.9) | 6.1 (±0.6) | NT |
| 180 | 3-OCH3 | 2-CH3 | 17.6 (±1.7) | 14.0 (±1.3) | NT |
| 181 | 3-OCH3 | 4-F | 17.4 (±1.9) | 10.9 (±0.9) | NT |
| 182 | 3-OCH3 | 4-OCH3 | 16.6 (±2.0) | 8.3 (±0.7) | NT |
| 183 | 3-OCH3 | 2-OCH3 | 17.8 (±1.4) | 12.0 (±1.0) | NT |
| 184 | 4-Ph | 2-OCH3 | 38.0 (±2.6) | 9.8 (±1.0) | NT |
| 185 | 4-Ph | 2-Br | 39.2 (±3.6) | 3.1 (±0.3) | >50 |
| 186 | 4-Ph | 2-CH3 | 9.9 (±0.9) | 4.7 (±0.4) | >50 |
| 187 | 4-Ph | 4-F | 35.0 (±2.7) | 25.5 (±1.6) | NT |
| 188 | 4-Ph | 4-OCH3 | 10.0 (±0.8) | 1.0 (±0.1) | >50 |
| 189 | 3-CF3 | 2-Br | 24.0 (±1.9) | 5.9 (±0.5) | >50 |
| 190 | 3-CF3 | 2-CH3 | 9.6 (±0.9) | >50 | >50 |
| 191 | 3-CF3 | 4-F | 8.1 (±0.8) | 6.9 (±1.1) | >50 |
| 192 | 3-CF3 | 4-OCH3 | 6.4 (±0.5) | 5.9 (±0.8) | NT |
| 193 | 3-CF3 | 2-OCH3 | 5.7 (±0.5) | 8.4 (±0.9) | >50 |
A549 and MCF7 cells were incubated for 24 h prior to drug addition.
A549 and MCF7 cells were incubated for 72 h at 37°C in 5% CO2 with the respective drug.
Cell proliferation was determined using an MTT assay.
All experiments were performed in replicates of 6–8.
NT: Not tested.
Compound effects on gankyrin conformation
In addition to their potent antiproliferative activity, compounds 185, 186, 188 and 193 also demonstrated the ability to effectively bind gankyrin, with compounds 188 and 193 proving the most potent binders (Supplementary Table 1). To decipher the impact of compounds 188 and 193 on the structure of gankyrin, the thermal stability and secondary structure content were evaluated through circular dichroism (CD) and thermal denaturation (Tm) as previously described (Figure 3) [26].
Figure 3. . Circular dichroism spectra and thermal denaturation of gankyrin in presence of compounds 188 and 193.
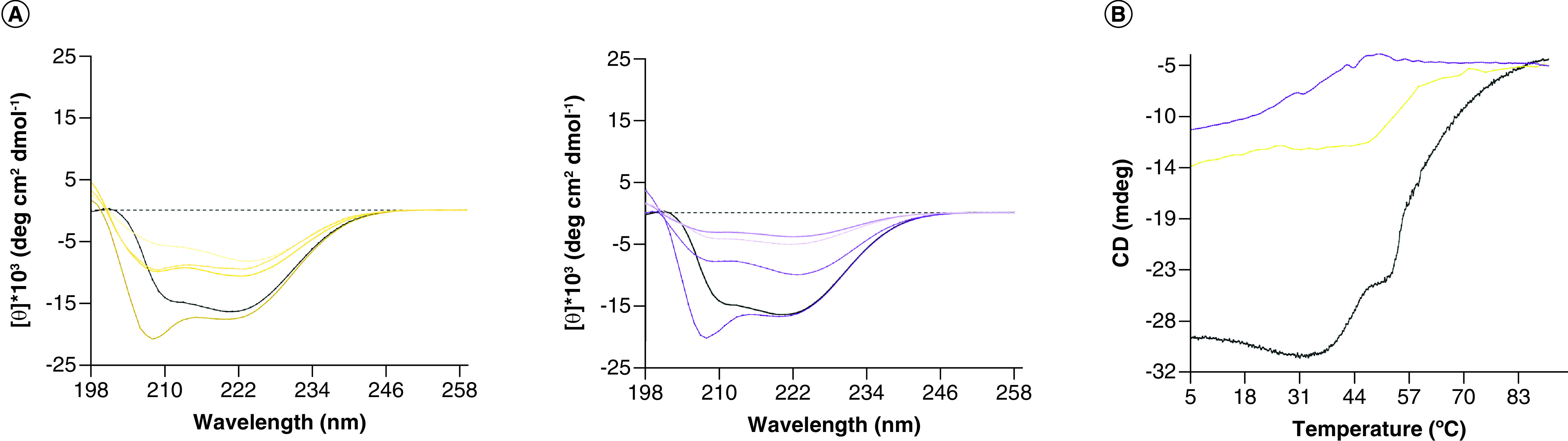
(A) CD spectral analysis of gankyrin alone (black) and in presence of increasing concentrations of compounds 188 (yellow) and 193 (purple). Individual samples of gankyrin were saturated with each compound from 100 nM to 1.5 μM, represented in a dark-to-light color gradient on each graph. CD spectra of gankyrin alone demonstrates expected α-helical minima at 208 and 222 nm, whereas gankyrin in presence of each compound showed a decrease in signal intensity. Indicative of a decrease in global secondary structure content of gankyrin when titrated with either compound. (B) Tm of gankyrin alone (black) and in presence of highest saturation of compounds 188 (yellow) and 193 (purple). All Tm studies were monitored at 217 nm. Both compounds induce partial unfolding with improved gankyrin stability observed from previously reported compounds [26]. Evident through restoration of a one-state transition at 41°C (188) and 53°C (193) rather than complete unfolding as previously described.
CD: Circular dichroism; Tm: Thermal denaturation.
As shown, the overall structural integrity of gankyrin is relatively protected with the modifications incorporated into compounds 188 and 193, with a recovery in the thermal stability and folding expected with AR-containing proteins. Specifically, AR domains have a two-state transition that can be evaluated through biophysical techniques such as CD and Tm. Furthermore, this phenomenon is understood as a major driving force in the stability of the AR domain due to a highly conserved and reoccurring N-terminal motif [37] that also supports the increased number of binding partners for one AR-containing protein [38,39]. Previously reported cjoc42 derivatives severely impaired the stability of gankyrin and were predicted to inhibit nonspecific protein–protein interactions [26]. These data suggests that compounds 188 and 193 affect gankyrin's global fold and stability that may be maintained through distal AR repeats.
The ability of compounds 188 and 193 to effectively bind gankyrin in both MCF7 and A549 cells, respectively, was determined by cellular thermal shift assay (CETSA; Supplementary Figure 1). Compound 188 decreased the thermal stability of gankyrin in MCF7 cells at its respective antiproliferative IC50 concentration (1 μM; Supplementary Figure 1A & B). Similarly, compound 193 exhibited a decrease in the thermal stability of gankyrin in A549 cells at its antiproliferative IC50 concentration (5 μM; Supplementary Figure 1C & D). From the CETSA results, it was concluded that both compounds 188 and 193 bind gankyrin while also promoting the unfolding and destabilization of its 3D structure.
Western blot analysis
Western blot analyses of both compounds 188 and 193 were performed to determine if their antiproliferative activity was due to disruption of the proteasomal degradation pathway (Figure 4). Compound 188 treatment of MCF7 cells caused an increase in levels of p53 and phospho–p53 (Ser6), whereas gankyrin levels remained relatively unchanged (Figure 4A). Similarly, when A549 cells were treated with compound 193, increased levels of p53 and phospho–p53 (Ser6) were also observed while gankyrin levels decreased (Figure 4B). This is in good agreement with previously reported data for cjoc42 and its derivatives [22,26,28,29]. Interestingly, treatment with both compounds resulted in an unexpected decrease in Rb levels. This observation suggests these compounds primarily disrupt the gankyrin–p53 and/or gankyrin–proteasome interaction and likely do not target the gankyrin–Rb or gankyrin–CDK4 interactions that regulate Rb hyperphosphorylation and proteolysis [3,21]. These cumulative findings support the idea that compounds 188 and 193 inhibit gankyrin activity and disrupt the proteasomal degradation pathway in MCF7 and A549 cells.
Figure 4. . Western blot analysis of tumor suppressor protein levels from MCF7 and A549 lysate upon treatment with compounds (A) 188 and (B) 193, respectively.

Cell cycle analysis
Since gankyrin plays an important role in cell cycle regulation through mediating p53 expression, the effects of compounds 188 and 193 on cell cycle progression were investigated (Figure 5). Treatment of MCF7 cells with compound 188 caused a significant dose-dependent increase in the number of cells in the G0/G1 phase while also significantly decreasing the cell population in the S and G2/M phases (Figure 5E). Similarly, treatment of A549 cells with compound 193 caused a significant increase in the number of cells in the G0/G1 phase while also significantly decreasing the cell population in the G2/M phases (Figure 5J). These findings are in good agreement with the previously discussed western blot analyses, where treatment with 188 and 193 caused an increase in p53 levels (Figure 4). These findings were also as expected due to the ability of p53 and phospho–p53 (Ser6) to inhibit the G1/S transition of the cell cycle [40,41]. This further supports the findings that compounds 188 and 193 inhibit gankyrin and disrupt the proteasomal degradation pathway causing cell cycle arrest and decreased cancer cell proliferation as previously observed for other gankyrin-binding small molecules [26].
Figure 5. . Cell cycle analysis of compounds 188 and 193 in MCF7 and A549 cells, respectively.

(A) Cell cycle analysis by flow cytometry of MCF7 cells treated with DMSO. (B) Cell cycle analysis by flow cytometry of MCF7 cells treated with 0.25 μM of compound 188. (C) Cell cycle analysis by flow cytometry of MCF7 cells treated with 0.5 μM of compound 188. (D) Cell cycle analysis by flow cytometry of MCF7 cells treated with 1 μM of compound 188. (E) Histogram data of cell cycle analysis of MCF7 cells treated with DMSO and different concentrations of compound 188. (F) Cell cycle analysis by flow cytometry of A549 cells treated with DMSO. (G) Cell cycle analysis by flow cytometry of A549 cells treated with 1.25 μM of compound 193. (H) Cell cycle analysis by flow cytometry of A549 cells treated with 2.5 μM of compound 193. (I) Cell cycle analysis by flow cytometry of A549 cells treated with 5 μM of compound 193. (J) Histogram data of cell cycle analysis of A549 cells treated with DMSO and different concentrations of compound 193. Data represents the mean ± standard deviation (n = 3).
*0.01 < p < 0.05; **0.001 < p < 0.01; ***0.0001 < p < 0.001; ****0.00001 < p < 0.0001.
DMSO: Dimethyl sulfoxide.
Conclusion & future perspective
The previously identified compound 1 (LAS789) exhibited a significant improvement over cjoc42 in gankyrin binding and antiproliferative activity against A549 and MCF7 cells. To further improve the antiproliferative activity of compound 1, the presence of the phenethyl group, ring A substitutions and ring B substitutions were investigated. Compounds 188 and 193 exhibited potent antiproliferative activity against gankyrin-overexpressing A549 and MCF7 cells while also demonstrating a lack of antiproliferative activity against noncancerous HEK293 cells. Protein thermal shift data also demonstrated that these two compounds retained gankyrin-binding ability compared with compound 1. CETSA, CD and Tm analyses all demonstrated that compounds 188 and 193 bind and destabilize the 3D structure of gankyrin. Western blot analysis established that compounds 188 and 193 effectively disrupt the proteasomal degradation pathway while also reducing cell cycle progression. The cumulative results show that compounds 188 and 193 represent significant advancements in gankyrin-binding small molecules as the novel compound 1 scaffold continues to evolve.
The development of small-molecule gankyrin binders continues to advance as an interesting and promising anticancer strategy. This work presents a novel scaffold for targeting gankyrin resulting in two compounds with a significant ability to inhibit the proliferation of A549 and MCF7 cells. Going forward, these compounds will help determine the role of gankyrin in lung cancer and breast cancer and set the stage for the further development of this novel 2,5-pyrimidine scaffold for targeting gankyrin to inhibit cancer cell growth.
Summary points.
A novel gankyrin-binding scaffold was modified to optimize gankyrin binding and antiproliferative activity.
Ring C of compound 1 was nonessential for gankyrin binding and antiproliferative activity.
Several 2,5-pyrimidine derivatives of compound 1 were synthesized and biologically evaluated.
Substitutions to both phenyl rings (R1 and R2) improved antiproliferative activity against MCF7 and A549 cancer cells and maintained gankyrin binding.
Combining the most potent R1 and R2 substitutions provided compounds 188 and 193 with potent antiproliferative activity.
Investigations of the mechanisms of action of 188 and 193 demonstrated their ability to disrupt the proteasomal degradation pathway and inhibit cell cycle progression.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge and thank the College of Pharmacy and Health Sciences, the Department of Pharmaceutical Sciences and the Office of Grants and Sponsored Research at St. John's University, and the Gustaf H. Carlson School of Chemistry & Biochemistry at Clark University for providing the facilities and financial support for this research.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/suppl/10.4155/fmc-2023-0124
Financial disclosure
This work was supported in part by NIH grants SC2GM139672 (AM) and R15GM126432 (DES). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Nakamura Y, Nakano K, Umehara T et al. Structure of the oncoprotein gankyrin in complex with S6 ATPase of the 26S proteasome. Structure 15(2), 179–189 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Cheng L. Gankyrin as a potential therapeutic target for cancer. Invest. New Drugs 35(5), 655–661 (2017). [DOI] [PubMed] [Google Scholar]; •• Review addressing gankyrin's role in cancer and the importance of targeting gankyrin.
- 3.Li J, Tsai M-D. Novel insights into the INK4-CDK4/6-Rb pathway: counter action of gankyrin against INK4 proteins regulates the CDK4-mediated phosphorylation of Rb. Biochemistry 41(12), 3977–3983 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Jiang B, Zhang Y. Gankyrin regulates cell signaling network. Tumour Biol. 37(5), 5675–5682 (2016). [DOI] [PubMed] [Google Scholar]; •• Review addressing gankyrin's interaction with and regulation of tumor suppressor proteins p53 and Rb.
- 5.Camacho-Moll ME, Macdonald J, Looijenga LHJ et al. The oncogene gankyrin is expressed in testicular cancer and contributes to cisplatin sensitivity in embryonal carcinoma cells. BMC Cancer 19(1), 1124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Fu J, Xu A et al. Gankyrin drives malignant transformation of chronic liver damage-mediated fibrosis via the Rac1/JNK pathway. Cell Death Dis. 6(5), e1751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han J, Wang F, Lan Y et al. KIFC1 regulated by miR-532-3p promotes epithelial-to-mesenchymal transition and metastasis of hepatocellular carcinoma via gankyrin/AKT signaling. Oncogene 38(3), 406–420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Sakurai T, Higashitsuji H, Kashida H et al. The oncoprotein gankyrin promotes the development of colitis-associated cancer through activation of STAT3. Oncotarget 8(15), 24762–24776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashitsuji H, Itoh K, Nagao T et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat. Med. 6(1), 96–99 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Li J, Guo Y. Gankyrin oncoprotein: structure, function, and involvement in cancer. Curr. Chem. Biol. 4(1), 13–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L, Wu B, Zhang L et al. Gankyrin promotes osteosarcoma tumorigenesis by forming a positive feedback loop with YAP. Cell. Signal. 65, 109460 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Li X, Zhang Y, Xiong C et al. Overexpression of a new gene P28GANK confers multidrug resistance of gastric cancer cells. Cancer Invest. 27(2), 129–139 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Bai Z, Tai Y, Li W et al. Gankyrin activates IL-8 to promote hepatic metastasis of colorectal cancer. Cancer Res. 73(14), 4548–4558 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Meng Y, He L, Guo X et al. Gankyrin promotes the proliferation of human pancreatic cancer. Cancer Lett. 297(1), 9–17 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Zhen C, Chen L, Zhao Q et al. Gankyrin promotes breast cancer cell metastasis by regulating Rac1 activity. Oncogene 32(29), 3452–3460 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Luo X, Chen L, Dai J et al. Gankyrin gene deletion followed by proteomic analysis: insight into the roles of gankyrin in tumorigenesis and metastasis. BMC Med. Genomics 5, 36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Zhang J, Zhen C, Yang B, Feng L. Gankyrin as a potential target for tumor therapy: evidence and perspectives. Am. J. Transl. Res. 10(7), 1949–1960 (2018). [PMC free article] [PubMed] [Google Scholar]
- 18.Jahangiri R, Mosaffa F, EmamiRazavi A, Gharib M, Jamialahmadi K. Increased expression of gankyrin and stemness factor Oct-4 are associated with unfavorable clinical outcomes and poor benefit of tamoxifen in breast carcinoma patients. Pathol. Oncol. Res. 26(3), 1921–1934 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Higashitsuji H, Higashitsuji H, Itoh K et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 8(1), 75–87 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Qiu W, Wu J, Walsh EM et al. Retinoblastoma protein modulates gankyrin-MDM2 in regulation of p53 stability and chemosensitivity in cancer cells. Oncogene 27(29), 4034–4043 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Higashitsuji H, Liu Y, Mayer RJ, Fujita J. The oncoprotein gankyrin negatively regulates both p53 and RB by enhancing proteasomal degradation. Cell Cycle 4(10), 1335–1337 (2005). [DOI] [PubMed] [Google Scholar]
- 22.D'Souza AM, Jiang Y, Cast A et al. Gankyrin promotes tumor-suppressor protein degradation to drive hepatocyte proliferation. Cell Mol. Gastroenterol. Hepatol. 6(3), 239–255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates in vivo utility of a gankyrin-binding small molecule.
- 23.Zheng T, Hong X, Wang J et al. Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology 59(3), 935–946 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Mahajan A, Guo Y, Yuan C, Weghorst CM, Tsai M-D, Li J. Dissection of protein-protein interaction and CDK4 inhibition in the oncogenic versus tumor suppressing functions of gankyrin and P16. J. Mol. Biol. 373(4), 990–1005 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chattopadhyay A, O'Connor CJ, Zhang F et al. Discovery of a small-molecule binder of the oncoprotein gankyrin that modulates gankyrin activity in the cell. Sci. Rep. 6, 23732 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discovery of the first small-molecule binder of gankyrin.
- 26.Kanabar D, Goyal M, Kane EI et al. Small-molecule gankyrin inhibition as a therapeutic strategy for breast and lung cancer. J. Med. Chem. 65(13), 8975–8997 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanabar D, Kabir A, Chavan T, Kong J, Yoganathan S, Muth A. Identification of novel gankyrin binding scaffolds by high throughput virtual screening. Bioorg. Med. Chem. Lett. 43, 128043 (2021). [DOI] [PubMed] [Google Scholar]
- 28.D'Souza AM, Gnanamony M, Thomas M et al. Second generation small molecule inhibitors of gankyrin for the treatment of pediatric liver cancer. Cancers (Basel) 14(13), 3068 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Souza AM, Cast A, Kumbaji M et al. Small molecule Cjoc42 improves chemo-sensitivity and increases levels of tumor suppressor proteins in hepatoblastoma cells and in mice by inhibiting oncogene gankyrin. Front. Pharmacol. 12, 580722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manjasetty BA, Quedenau C, Sievert V et al. X-ray structure of human gankyrin, the product of a gene linked to hepatocellular carcinoma. Proteins 55(1), 214–217 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Jafari R, Almqvist H, Axelsson H et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 9(9), 2100–2122 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Taheri T, Jamialahmadi K, Khadijeh F. Unexpected lower expression of oncoprotein gankyrin in drug resistant ABCG2 overexpressing breast cancer cell lines. Asian Pac. J. Cancer Prev. 18(12), 3413–3418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YH, Kim J-H, Choi YW et al. Gankyrin is frequently overexpressed in breast cancer and is associated with ErbB2 expression. Exp. Mol. Pathol. 94(2), 360–365 (2013). [DOI] [PubMed] [Google Scholar]; • Examines gankyrin's role in MCF7 cells and breast cancer.
- 34.Gao L, Xie H, Dong L et al. Gankyrin is essential for hypoxia enhanced metastatic potential in breast cancer cells. Mol. Med. Rep. 9(3), 1032–1036 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Yu T, Liu Y, Xue J et al. Gankyrin modulated non-small cell lung cancer progression via glycolysis metabolism in a YAP1-dependent manner. Cell Death Discov. 8(1), 312 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W-P, Sun Y, Lu Q et al. Gankyrin promotes epithelial-mesenchymal transition and metastasis in NSCLC through forming a closed circle with IL-6/STAT3 and TGF-β/SMAD3 signaling pathway. Oncotarget 8(4), 5909–5923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Examines gankyrin's role in A549 cells and non-small cell lung cancer.
- 37.Mosavi LK, Cammett TJ, Desrosiers DC, Peng Z-Y. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 13(6), 1435–1448 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane EI, Spratt DE. Structural insights into ankyrin repeat-containing proteins and their influence in ubiquitylation. Int. J. Mol. Sci. 22(2), 609 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripp KW, Barrick D. The tolerance of a modular protein to duplication and deletion of internal repeats. J. Mol. Biol. 344(1), 169–178 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene 25(38), 5220–5227 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Gordon EM, Ravicz JR, Liu S, Chawla SP, Hall FL. Cell cycle checkpoint control: the cyclin G1/Mdm2/p53 axis emerges as a strategic target for broad-spectrum cancer gene therapy–a review of molecular mechanisms for oncologists. Mol. Clin. Oncol. 9(2), 115–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



