Abstract
Aims
We performed quality control of lipid-lowering therapy (LLT) in patients with acute coronary syndrome (ACS), with a view to proposing corrective actions.
Methods and results
Using a Define Measure Analysis Improve Control (DMAIC) approach applied to data from the ACS EuroPath IV survey, we measured attainment of two quality indicators (QIs) related to lipid-lowering treatment: (i) prescription of high-intensity statins (or equipotent treatment) before discharge, and (ii) proportion with LDL-cholesterol <55 mg/dL (1.4 mmol/L) during follow-up. A total of 530 European cardiologists responded and provided data for up to 5 patients from their centre, for acute and follow-up phases. Corrective measures are proposed to increase the rate of attainment of both QIs. Attainment of the first QI was measured in 929 acute-phase patients, 99% had LLT prescribed at discharge and 75% of patients fulfilled the first QI. Attainment of the second QI was assessed in 1721 patients with follow-up. The second QI was reached in 31% of patients. The DMAIC approach yielded 10 potential changes in prescription, 3 for the first and 7 for the second QI. The overall strategy is ‘Fire to Target’, i.e. early intensification of the LLT using statins, ezetimibe, bempedoic acid, and proprotein convertase subtilisin/kexin type-9 inhibitors, and is presented as an algorithm for routine application.
Conclusion
Quality control for LLT, based on the ACS EuroPath IV survey, detected 10 potential changes in prescription that could enhance attainment of 2 QIs. Whether the Fire to Target strategy will be adopted and effective needs to be assessed in further steps of the EuroPath Quality programme.
Keywords: Acute coronary syndrome, Lipid-lowering therapy, Statin, Ezetimibe, Proprotein convertase subtilisin/kexin type-9 inhibitors, Bempedoic acid
Graphical Abstract
Graphical Abstract.
Introduction
There is a growing interest in the quality of health services, which involves providing effective, safe, people-centred care that is timely, equitable, integrated, and efficient. This heightened awareness of the quality of care explains why the European Society of Cardiology (ESC) guidelines are currently systematically complemented with the publication of a set of quality indicators (QIs). These QIs are tools specifically developed for the assessment of quality of care, linked to the guidelines and intended to be used for quality control, a key step in quality assurance. Although assessments of QIs from registries have been published, for example, in the management of acute myocardial infarction (AMI),1–4 the remaining actions needed to comply with quality control are not usually implemented.5 Strategies of quality control routinely applied in industry might also be suitable for implementation in the healthcare sector. Examples include ‘Six Sigma’, ‘Lean’, or the combination, ‘Lean Six Sigma’, which aim to reduce variability in both quality and the timing of care.6 In an attempt to improve the quality of lipid management in patients admitted for AMI, we undertook quality control using the ‘Define Measure Analysis Improve Control’ (DMAIC) strategy. The quality control focused on two objectives, identified as QIs by current ESC guidelines, namely prescription of statins at high intensity,7 and the proportion of patients achieving LDL-cholesterol (LDL-c) goals8 within 12 months after acute coronary syndrome (ACS).
Methods
The quality control process followed four of the five steps of the DMAIC approach, namely:
The ‘determine’ step: Despite high-intensity statins and ezetimibe being widely available, recent observational studies have shown that among patients with atherosclerotic cardiovascular disease, only 20% reach the therapeutic goal.9,10 One of the leading reported reasons for this failure to reach therapeutic objectives is suboptimal intensity of lipid-lowering therapy (LLT), both at the initial prescription and during follow-up.10
-
The ‘measure’ step: Data from ACS EuroPath IV survey were used for the assessment and measurement of the two QIs investigated, and defined as follows:
The first QI was assessed at discharge and defined as ‘prescription of high-intensity statins before discharge or a combination of a statin (any intensity) plus ezetimibe, or a prescription of proprotein convertase subtilisin/kexin type-9 inhibitor (PCSK9i); alone or combined with statins’. Only patients at the acute phase were considered. This QI is derived from the QI defined by ESC-ACVC, namely ‘discharge with high-intensity statins (defined as atorvastatin ≥40 mg or rosuvastatin ≥20 mg) unless contra indicated’.7 It takes account of patients with a documented history of intolerance in whom the combination of low/moderate-intensity statin and ezetimibe or PCSK9i can be used and provides a similar reduction in LDL-c to that achieved with high-intensity statin monotherapy.
The second QI was defined as ‘the rate of patients who had an LDL-c level <55 mg/dL (1.4 mmol/L) during follow-up’ and considered only patients during the follow-up phase. This QI is derived from the ESC QIs for cardiovascular disease prevention, namely ‘LDL-c level at or below that recommended for their estimated cardiovascular risk’, considered as both an LDL-c <55 mg/dL (1.4 mmol/L) and a >50% reduction in baseline LDL-c.10
The ‘analyse’ step: Based on the responses recorded in the ACS EuroPath IV survey, the reasons for LLT prescription, in terms of type of drug, intensity, and timing of prescription were analysed. The ACS EuroPath project was designed to assess clinical practice in terms of LLT in post-ACS patients, with a view to improving the quality of lipid management and prevention, both during the acute phase, and during the first year of follow-up.11 The ACS EuroPath IV survey was conducted in 2022 and involved 530 physicians [137 (26%) interventional cardiologists and 393 (74%) general cardiologists] and collected data for 2650 patients with ACS, 929 (35%) at the acute phase and 1721 (65%) at the follow-up phase. The results of the ACS Europath IV survey have been published elsewhere.15 The project included online questionnaires using a patient record form, in which each respondent provided data for the last five patients of their centre, for the acute and follow-up phases of ACS. The surveys were designed to capture important clinical parameters to identify the overall level of quality and potential weak points. The survey design is illustrated in Supplementary material online, Figure S1. The questions of the survey covered five major sections, namely: (i) patient’s risk profile and therapeutic lipid goals; (ii) content, use of, and expectations for lipid-lowering management protocols at the acute phase (from admission to discharge); (iii) content, use of, and expectations for lipid-lowering management protocols for the post-discharge phase; (iv) rules and recommendations during follow-up; and (v) patient record data collection. The survey included specific questions about the details of LLT prescription, notably the type and intensity of LLT prescribed (statin intensity, ezetimibe, use of PCSK9i); the time of prescription; LDL-c levels at baseline and during follow-up (up to 12 months). In addition, the time frame to achieve the two QIs was also measured with an objective of a 4- to 6-week interval between the initiation (or changes) of LLT and the assessment of tolerance and efficacy.
The ‘improve’ step: Proposals for change were made, considering the results of recent randomized and observational studies, with the aim of improving the rate and timing of attainment of the two QIs. Proposals for change were retained providing that the changes were in agreement with the ESC guidelines, applicable at reasonable cost and effort, with minimal disruption of routine practice, and demonstrated to be safe and effective.
Results
Measure: attainment of quality indicators
Overall, attainment of the LDL-c objective was one of the main priorities, after smoking cessation and diabetes control, when managing post-ACS patients. Regarding the LDL-c objectives, 68% of cardiologists expected to reduce LDL-c by >50%, and 42% aimed to achieve an LDL-c level <55 mg/dL (1.4 mmol/L). The timeframe to reach these LDL-c objectives was within 3 months in 54%, and within 6 months in 91% (see Supplementary material online, Figure S2).
The first QI was measured in 929 acute-phase patients included in the survey. Lipid-lowering therapy was prescribed during the early phase of hospitalization in 99% of cases and comprised high-intensity statins in 75% (monotherapy in 44%, in combination with ezetimibe in 29% and with PCSK9i in 2%). As a result, the proportion of patients fulfilling the first QI was 75%, while the QI was not satisfied in 25%. Figure 1 displays the type of LLT during hospitalization and at discharge among patients who satisfied QI1 and those who did not.
Figure 1.
Type of lipid-lowering therapy during hospitalization and at discharge among patients who met the first quality indicator, and those who did not. QI, quality indicator; HIS, high-intensity statins; mono, monotherapy; PCSK9i, proprotein convertase subtilisin/kexin type-9 inhibitors; Mod, moderate; LLT, lipid-lowering therapy.
Attainment of the second QI was assessed in 1721 patients from the follow-up phase of the survey. For patients in the acute phase, the first follow-up visit was planned before discharge in 80% of patients, and 62% were referred to cardiac rehabilitation. Among patients who were followed up, the first follow-up visit took place on average at 16 weeks, and before 6 weeks in 31% of cases, with LDL-c assessment performed in 76%. The mean LDL-c level was 85 ± 45 vs. 125 ± 53 mg/dL at admission (2.1 ± 1.1 vs. 3.1 ± 1.3 mmol/L). The second QI was reached in 18% of patients in whom LDL-c was tested at the first follow-up visit. A second visit was performed in 53%; mean LDL-c was 71 ± 37 mg/dL (1.78 ± 0.9 mmol/L) and the QI was satisfied in 34% of patients receiving a second follow-up and in whom LDL-c was measured. A third visit was performed in 20% of patients [mean LDL-c 67 ± 33 mg/dL (1.7 ± 0.8 mmol/L)] and a fourth in 5% [mean LDL-c 73 ± 38 mg/dL (1.8 ± 1.0 mmol/L)].
The main changes in LLT observed over successive visits were an increase in LLT intensity: not in the intensity of statin therapy, but rather by introducing combination therapy with ezetimibe (up to 36%) and/or PCSK9i (up to 20%). As a result, overall, the second QI was achieved in 31% of the patients who were followed up and had LDL-c measured at least once during follow-up. The rate of use of high-intensity statins at discharge was higher in patients who had LDL-c <55 mg/dL (1.4 mmol/L) at the first follow-up visit (overall 88%; 49% monotherapy) compared with those who had LDL-c ≥55 mg/dL (1.4 mmol/L) at the first follow-up (overall 77%; 43% monotherapy; Figure 2).
Figure 2.
Lipid-lowering treatment received at discharge and changes in lipid-lowering treatment at the first follow-up visits in patients with LDL-cholesterol <55 mg/dL (<1.4 mmol/L) at the first follow-up (lower bars), compared with those with LDL-cholesterol ≥ 55 mg/dL (≥1.4 mmol/L) at the first follow-up (upper bars). Mono, monotherapy; combi, combination; PCSK9i, proprotein convertase subtilisin/kexin type-9 inhibitors; Mod, moderate; LLT, lipid-lowering therapy; FU, follow-up.
Analysis and proposals for improvement of prescription strategies
Based on the analysis of the results of the survey, 10 proposals for change were identified, with a view to improving the attainment of the 2 QIs (Figure 3).
Figure 3.
Overview of the 10 proposals for quality improvement derived from the quality control: 2 measures to improve the attainment of QI1 and 8 measures to improve the attainment of QI2.
Initial statin prescription should be at high intensity, regardless of baseline LDL-c: The reasons why 13% of patients were treated with low/moderate-intensity statin monotherapy cannot be accurately determined from the results of the survey. However, misinterpretation of the LDL-c goals and an overestimation of the risk of intolerance could have played a role. Interestingly, a substantial proportion of physicians may limit statin intensity, aiming for an LDL-c goal above the threshold recommended by guidelines. Indeed, 26% set the LDL-c goal at <55 mg/dL (1.4 mmol/L), while 50% considered the LDL-c goal to be between 55 and 70 mg/dL (1.4 and 1.8 mmol/L), underlining a relevant gap in guidelines awareness. Regarding patients with a history of intolerance of high-intensity statins, re-challenge with a different high-intensity statin is possible, or use of low/moderate intensity statin monotherapy combined with ezetimibe would provide a decrease in LDL-c comparable with that of high-intensity statins.13 When statin plus ezetimibe are not sufficient, we propose a two-pill-three-drug step strategy using the highest tolerated dose of a potent statin (atorvastatin or rosuvastatin) plus the fixed-dose combination of bempedoic acid with ezetimibe. In patients with complete statin intolerance, bempedoic acid in combination with ezetimibe lowers LDL-c by 35–45%.14
High-intensity statins should be initiated at admission: In the survey, LLT was introduced at admission in 26%, and within the first day in 63%. The ESC guidelines recommend initiating high-intensity statins ‘as early as possible’, but without an explicit timeline for the first prescription. This should be interpreted to mean ‘at admission’, given the IIa B recommendation for initiation of high-intensity statins before planned percutaneous coronary intervention, which is highly likely to occur early in patients admitted for ACS. Delaying the initiation of LLT until after lipid testing is not necessary, because neither the non-fasting state nor 1 day of treatment with statins have a substantial impact on the LDL-c level.
-
Statins in combination with ezetimibe before discharge: The first-line combination of statins and ezetimibe is not explicitly recommended by the ESC guidelines, but rather, as a second step after failure to lower LDL-c to the therapeutic goal with lifestyle measures and statins alone. Nevertheless, a strategy comprising first-line treatment with the combination of high-intensity statins + ezetimibe (‘Strike Early and Strike Strong’) is now proposed in a clinical consensus statement from Association for Acute CardioVascular Care in collaboration with the European Association of Preventive Cardiology and the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy.15 Recently, evidence from randomized trials has shown that bempedoic acid, either alone or in combination with ezetimibe, is capable of achieving further LDL-c reductions16–19 and improved clinical outcomes. There is converging evidence to support the prescription of combination treatment before discharge, as follows:
According to the biological effects of statins and ezetimibe, in order to reach the recommended LDL-c goals, the combination is required, in order to decrease LDL-c sufficiently whenever baseline LDL-c is >110 mg/dL. In the ACS EuroPath IV survey, the mean LDL-c level was 129 ± 54 mg/dL (3.66 ± 1.4 mmol/L). Accordingly, high-intensity stain monotherapy would be needed in the majority of patients. Despite the fact that 85% of discharge LLT prescriptions included high-intensity statins (or an equipotent treatment), the mean LDL-c at the first visit was 85 ± 45 mg/dL (1.9 ± 1.26 mmol/L), and only 18% had LDL-c <55 mg/dL (1.4 mmol/L). A similarly low rate of attainment of LDL-c goals was observed among very high-risk patients in a large contemporary registry9 where the combination of a statin plus ezetimibe was used in fewer than 10% of patients.
The results of the survey show wide variations across countries in the use of statins and ezetimibe at discharge. In countries where the rate of use was high (>40% in Italy, Spain, and France), more patients had LDL-c <55 mg/dL (1.4 mmol/L) at the first visit compared with countries with the lowest rate of use (<35% in Germany, UK, and The Netherlands; rate of combination of 14, 21, 37 vs. 6, 8, 4%, respectively; Supplementary material online, Figure S3). In an observational study where >90% of patients received high-intensity statins and 65% received ezetimibe before discharge post ACS, the rate of patients at LDL-c goals was found to be as high as 50%, without the need for prescription of a PCSK9i.20
Adding ezetimibe to high-intensity statins before discharge when baseline LDL-c >100 mg/dL [70 mg/dL (1.8 mmol/L)] for patients already under LLT has been proposed by the European Atherosclerosis Society.21,22 Although this strategy is somewhat complex, it is applicable in routine practice, and makes it possible to get half of the patients to LDL-c goals.20 The systematic prescription of the combination of statins plus ezetimibe as first-line therapy23 is easier to apply in practice, with better biological and clinical efficacy, and without safety concerns.24
Combination with bempedoic acid and/or PCSK9i during hospitalization: In a large, nationwide, observational study of patients with ACS, a greater decrease in LDL-c at 6–8 weeks was associated with better long-term outcomes.25 This concept pleads in favour of the routine addition of other lipid-lowering drugs, such as ezetimibe but also bempedoic acid or PCSK9i on top of high-intensity statins. On average, a combination of statin and ezetimibe yields a 65% reduction in LDL-c at 4 weeks.13 Another option for oral treatment is bempedoic acid in addition to a statin and ezetimibe. Bempedoic acid is especially effective in patients with little or no background statin treatment. The reduction in LDL-c with bempedoic acid monotherapy is around 25% in statin-naive patients, and around 18% on top of statins. Bempedoic acid in combination with ezetimibe, ideally as a fixed-dose tablet, lowers LDL-c by around 40% in statin-naive patients and by around 35% on top of statins. Such a combination of bempedoic acid and maximally tolerated statins, before discharge or during follow-up is likely to increase the rate of attainment of QI2, and its clinical benefit has been demonstrated.14 Finally, the combination with PCSK9i is proposed by the ESC guidelines as the final step in the optimization cascade for patients whose LDL-c levels are not at goal, despite already taking the maximal tolerated statin dose plus ezetimibe. However, starting such a combination earlier, notably during hospitalization for ACS, can also be considered in patients already taking the maximal tolerated statin dose and ezetimibe. From the survey, 4% of patients received a PCSK9i during the acute stay, even though 53% of the physicians agreed with the use of PCSK9i before discharge for patients who have experienced a second vascular event within the last 2 years, and 50% of physicians agreed for patients admitted with maximally tolerated statins and ezetimibe. Of note, local regulatory rules may hinder the adoption of this strategy in several European countries.
Screening for familial hypercholesterolaemia (FH): a lipid profile was obtained during the acute stay in 90%, and LDL-c was measured in 96% of those tested, HDL-c in 92% and triglycerides in 84%. Screening for FH, based on clinical history and LDL-c level, was performed in 13% (FH was already known in a further 5%), and a high probability of FH was identified in 7%, based on clinical assessment (67%), risk score (28%), and genetic confirmation (10%). Although this rate of FH is comparable with that reported in other observational studies,26 adding a specific alert for FH screening, when admission LDL-c is >190 mg/dL without LLT, or >130 mg/dL under LLT is a simple method that could improve screening. This approach is recommended by the ESC guidelines13 and has already been proposed in LLT management protocols for patients with ACS.21,22 A diagnosis of FH could also have an impact on the discharge prescription because, for these patients, 78% of physicians agreed that a combination of a statin and ezetimibe should be systematically prescribed before discharge, and 62% agreed to the systematic use of a PCSK9i.
Schedule the first follow-up visit at 4–6 weeks: The timing of the first post-discharge visit is not explicit in the ESC guidelines, nor is the type of healthcare provider (HCP) to be preferred (general practitioner or cardiologist), but 4–6 weeks after the introduction of LLT, or any change in LLT, has been suggested as a suitable timeframe.13 The utility of the 4- to 6-week visit is three-fold, namely to check for tolerance, adherence, and efficacy. In the survey, 80% of patients had an appointment for their first follow-up visit when they were discharged, but less than half of those visits were scheduled within 6 weeks. In other words, many patients are followed up later than recommended. In the end, the average time to the first follow-up was 16 weeks: for 17%, the first follow-up was before the fourth week, for 14% between 4 and 6 weeks, for 11% between 6 and 8 weeks, and >8 weeks for the remaining 58% of patients.
Transmission of information at discharge: In the discharge document, specific information regarding lipid management should be given. In the survey, for example, the LDL-c goal for the patient is expected to be included in the discharge document by 67% of HCPs, the timing of the first lipid assessment by 67%, and strategies for LLT intensification by 56%. In practice, the LDL-c goal for the patient is included in the discharge document for 84% of patients, timing of the first lipid assessment for 51%, and strategies for LLT intensification by 42%, and including an algorithm for the LLT management for 28%. Improving the hospital discharge letters would palliate the gaps in communication between specialists and primary care providers, both in terms of the letter’s content and the timeframe of delivery. To date, only an expert consensus27 has focused on the form and content of information to be communicated at discharge post ACS. The discharge document should include key elements such as the patient’s risk level, the recommended LDL-c goal, the discharge LLT, the timing of the first visit, and information about the need for rehabilitation. Moreover, if intolerance, non-adherence, or a need for LLT intensification is likely to arise for the specific patient, the strategies to address these issues, as described below, should be mentioned in the discharge document. It should also be acknowledged that the discharge letter is an important source of information for the patient. If carefully drafted, it can promote the consolidation of the knowledge acquired during the hospital admission thus empowering the patient and leading to improved treatment adherence.
Management of statin-associated muscular symptoms (SAMS): Statin-associated muscular symptoms are the main cause of LLT intolerance and the main reason for the de-escalation of LLT intensity, cessation of treatment, or non-adherence. Statin-associated muscular symptoms are reported in 9% in randomized studies, but in up to 17% of patients in observational studies.28 The discrepancy in these rates between different types of study is likely explained by the ‘nocebo’ effect, highlighted by an ‘N-of-one’ study29 where patients ‘intolerant to statins’ report similar SAMS when treated with statins as when they are treated with a placebo. Irrespective of the underlying mechanism, the management of patients with SAMS has been clearly described.30 The ‘de-challenge–re-challenge’ sequence is helpful for both the diagnosis of SAMS and to determine the ‘maximally tolerated statin intensity’. Bempedoic acid offers an interesting alternative to statins.16,17,31,32 After any decrease in statin intensity or after a switch from statin to bempedoic acid, a lack of efficacy is likely, and this can be compensated for by adding ezetimibe,33 and/or a PCSK9i.32,34
Optimization of LLT for efficacy during the follow-up: According to the recommended step-by-step intensification process, the use of PCSK9i is considered when the LDL-c goals are not reached, despite a combination of statins and ezetimibe. Data from the survey show that all patients had at least one follow-up visit, 53% had at least two, 15% had three, and 4% had four follow-up visits in the 12 months after discharge. Among the 82% patients who were not at LDL-c objectives at the first visit, LLT was modified in 61%, notably by an increase in the dose of statins (18%), addition of another LLT (29%) or both (7%). After the first visit, among patients who had further visits and were still not at LDL-c objectives (66%), LLT was changed in 38%, notably by an increase in the statin dose (12%), addition of another LLT (17%) or both (3%). Among patients with a third visit, 63% were still not at the LDL-c objective, and changes to the LLT were made in 24%. There was also a decrease in LLT intensity to optimize tolerance or compliance, or to avoid a ‘too low LDL-c’ (see Supplementary material online, Figure S4). As a result, a combination of statins and ezetimibe (39% at discharge, 41% at the first and second visits) or with a PCSK9i (9% at discharge, 22% at the first visit, and 29% at the second visit) increased; and beyond the first follow-up visit, changes in LLT were limited. The routine prescription of combination therapy with a statin plus ezetimibe before discharge would reduce the number of follow-up visits, and the next LLT optimization steps would then be the addition bempedoic acid and/or a PCSK9i if needed.
Simplification of LLT and patient information for long-term adherence: Both intensity of and adherence to LLT decrease over time,35 resulting in suboptimal exposure to LLT over the years. In the survey, two-thirds of the patients received information about lipid management during the acute stay, and 59% of physicians expect the use of telemedicine to increase as a tool for LLT adherence and monitoring. An expert consensus on statin adherence concluded that simplification of the treatment, by using fixed combinations, as well as adequate information to the patients, and management of side effects could improve adherence.36 Better LDL-c control and adherence have been shown with moderate intensity statins plus ezetimibe compared with high-intensity statin monotherapy.33 Among patients treated with a PCSK9i in ‘real life practice’ in Europe, discontinuation of PCSK9i was infrequent, and most patients were at LDL-c goals.23,37 Nevertheless, reducing the number of PCSK9i injections by using monthly or even a twice-yearly treatment formulation could improve adherence. Once treatment is optimized and even when it is well tolerated, a common cause of non-adherence is the fear of side-effects related to ‘too low’ levels of LDL-c, despite a large body of data showing the safety and better clinical prevention achieved when LDL-c is <30 mg/dL (0.9 mmol/L).38
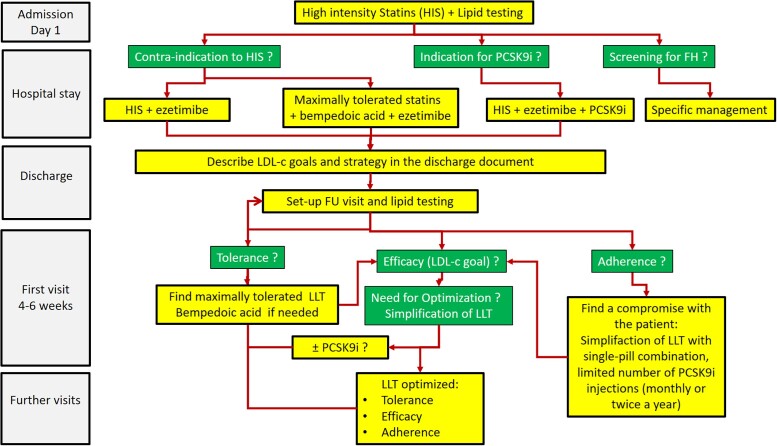
For the routine application of these changes in lipid-lowering treatment for patients admitted for ACS, a Fire to Target treatment algorithm is illustrated in Figure 4.
Figure 4.
Proposed lipid management algorithm (‘Fire to Target’) for routine application of the proposed changes.
Discussion
Quality control focusing on two QIs for LLT management confirms the suboptimal quality of LDL-c control, particularly regarding the objective of LDL-c levels at follow-up. The analysis of potential causes using the ACS EuroPath IV survey data made it possible to propose 10 potential solutions, in line with ESC guidelines. To complete the DMAIC approach, a further assessment of the same QIs should be performed again after implementation of the proposed corrective measures.
Performing quality control of LLT after ACS is timely, because recent observational studies consistently report a failure to reach the 2019 LDL-c objectives in the majority of post-ACS patients, despite the availability of effective drugs to lower LDL-c. EuroPath is a quality programme,39 with a first survey of lipid management post ACS in 2018 (EuroPath I) followed by analysis and proposal for changes.40,41 The ACS EuroPath IV evaluation of lipid management was performed in 2022, after the changes to the recommended LDL-c objectives.13 The ACS EuroPath IV survey was specifically designed to capture the different phases of the prescription of LLT between the acute phase and 12 months follow-up in the context of ‘post 2019’ guidelines (see Supplementary material online, Figure S1).12 To the best of our knowledge, this is the first quality control initiative to improve the attainment of QIs defined by the ESC for LLT post ACS with a DMAIC approach. To ensure that the quality control process is pursued, future iterations of the ACS EuroPath survey should focus on measuring the change in the QIs, after introduction of changes in practice based on the implementation of the measures proposed here.
In ACS EuroPath IV, the overall rate of prescription of high-intensity statins at discharge post ACS was 75% (and 86% of the patients had a discharge LLT capable of decreasing LDL-c by 50% or more), which is higher than reported in previous studies42,43 and also higher than the rates observed in the EuroPath I survey (66%), performed in 2018 with a comparable design (Figure 1).12 The improvement in discharge prescriptions may reflect the changes introduced in the ESC/EAS recommendations for LDL-c goals for very high-risk patients.13 There is greater room for improvement regarding the second QI, since it was reached in only 31% of patients who were followed up and had their LDL-c measured. Among the 10 proposals for change, it is likely feasible to apply the majority of them in practice, at reasonable cost and effort, without a complex strategy, and probably even with a simplification of routine practice.
The systematic use of the combination of high-intensity statins and ezetimibe before discharge is an important improvement, not only because of the early decrease in LDL-c, ease of application, and limited cost, but also because it is likely to have a clinical impact by enabling earlier optimization with PCSK9i, when indicated. Given the observed timing of the first and subsequent follow-up visits, the decision to add PCSK9i could be brought forward by 6–12 months. The clinical impact of the earlier use of PCSK9i was not only demonstrated in the ODYSSEY-OUTCOMES study44 but also in the FOURIER study (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk), where the clinical benefit of the additional PSCK9i was more pronounced among patients included <12 months after an ACS, when compared with those with remote (>12 months) ACS.45 In addition, delayed introduction of PCSK9i in eligible patients has a clinical cost as shown in the FOURIER-Open Label Extension, where patients from the active group (i.e. who had a 2.2-year head start on the introduction of PCSK9i) had a 20% relative reduction in cardiovascular death, myocardial infarction or stroke, and a 23% relative reduction in cardiovascular death.36 However, the optimization of LLT with PCSK9i suffers from one important limitation, namely the local conditions for reimbursement of PCSK9i in each country. Wide variations exist across European countries regarding the availability and/or reimbursement of PCSK9i. To date, PCSK9i are not reimbursed in European countries for patients with LDL-c <70 mg/dL under oral LLT, even if the LDL-c remains >55 mg/dL and even if the ESC/EAS recommended goals are not reached. Thus, accountability for the failure to satisfy the second QI needs to be carefully examined46 when it depends on the local possibility to prescribe a PCSK9i.
Limitations
First, the quality control is based on the analysis of online survey data and not on available patient records. Second, information was provided by physicians, who selected the patients who were included, and therefore, data are subject to selection bias and may not be representative of the overall ACS population. Furthermore, the survey was only performed in countries of the European Union, which limits extrapolation of the algorithm. Lastly, the regulatory limitations of each healthcare system may preclude initiating treatments such as PCSK9i from admission Day 1.
Conclusions
Quality control using a DMAIC approach, based on data from ACS EuroPath IV and focused on 2 QIs for LDL-c, led to 10 proposals for changes to be made during the acute phase and up to 12 months after discharge, with the aim of improving lipid management. The areas targeted for change include the systematic use of high-intensity statins, prescription at admission, combination with ezetimibe before discharge, screening for FH during hospitalization, scheduling the first follow-up visit within 4–6 weeks, early and effective transmission of lipid guidance in the discharge letter, management of intolerance to detect the maximally tolerated LLT, management of lack of efficacy with optimization using PCSK9i, and fostering of patient adherence by treatment simplification and patient information.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care.
Supplementary Material
Acknowledgements
Medical writing was performed by an independent medical writer, under responsibility of the author’s group. The work of A.L.C. is supported in part by Ministero della Salute Ricerca Corrente.
Contributor Information
François Schiele, Department of Cardiology, University Hospital Jean Minjoz, Boulevard Fleming, Besançon 25000, France; EA3920, University of Franche-Comte, 19 rue Ambroise Pare, 25000 Besançon, France.
Alberico L Catapano, Department of Pharmacological and Biomolecular Sciences, University of Milan, Via Balzaretti 9, 20133 Milan, Italy; IRCCS MultiMedica, Via Milanese 300, 20099 Sesto S. Giovanni, Milan, Italy.
Raffaele De Caterina, Chair of Cardiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy; Cardiovascular Division, Pisa University Hospital, Via Paradisa 2, 56124 Pisa, Italy.
Ulrich Laufs, Klinik und Poliklinik für Kardiologie, Universitätsklinikum Leipzig, Liebigstrasse 20, 04103 Leipzig, Germany.
J Wouter Jukema, Department of Cardiology, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; The Netherlands Heart Institute, Moreelsepark 1, 3511 EP Utrecht, The Netherlands.
Azfar Zaman, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Trust and Newcastle University, NE7 7DN Newcastle-upon-Tyne, UK.
Alessandro Sionis, Department of Cardiology, Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Universitat Autònoma de Barcelona, Barcelona 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid 28029, Spain.
Funding
The ACS EuroPath IV project and survey are funded by Sanofi. Sanofi supported the medical writing but made no contribution to the content of the manuscript.
Data availability
Qualified researchers may request access to patient level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
References
- 1. Schiele F, Gale CP, Simon T, Fox KAA, Bueno H, Lettino M, et al. The 2020 ESC-ACVC quality indicators for the management of acute myocardial infarction applied to the FAST-MI registries. Eur Heart J Acute Cardiovasc Care 2021;10:207–215. [DOI] [PubMed] [Google Scholar]
- 2. Zusman O, Bebb O, Hall M, Dondo TB, Timmis A, Schiele F, et al. International comparison of acute myocardial infarction care and outcomes using quality indicators. Heart 2019;105:820–825. [DOI] [PubMed] [Google Scholar]
- 3. Aktaa S, Yadegarfar ME, Wu J, Rashid M, de Belder M, Deanfield J, et al. Quality of acute myocardial infarction care in England and Wales during the COVID-19 pandemic: linked nationwide cohort study. BMJ Qual Saf 2022;31:116–122. [DOI] [PubMed] [Google Scholar]
- 4. Bebb O, Hall M, Fox KAA, Dondo TB, Timmis A, Bueno H, et al. Performance of hospitals according to the ESC ACCA quality indicators and 30-day mortality for acute myocardial infarction: national cohort study using the United Kingdom Myocardial Ischaemia National Audit Project (MINAP) register. Eur Heart J 2017;38:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiele F. After the success of ‘quality indicators’ season 1, it is time for the sequel: ‘quality assurance’. Eur Heart J Acute Cardiovasc Care 2022;11:806–807. [DOI] [PubMed] [Google Scholar]
- 6. Rathi R, Vakharia A, Shadab M. Lean six sigma in the healthcare sector: a systematic literature review. Mater Today Proc 2022;50:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiele F, Aktaa S, Rossello X, Ahrens I, Claeys MaJ, Collet JP, et al. 2020 update of the quality indicators for acute myocardial infarction: a position paper of the Association for Acute Cardiovascular Care: the study group for quality indicators from the ACVC and the NSTE-ACS guideline group. Eur Heart J Acute Cardiovasc Care 2021;10:224–233. [DOI] [PubMed] [Google Scholar]
- 8. Aktaa S, Gencer B, Arbelo E, Davos CH, Désormais I, Hollander M, et al. European Society of Cardiology quality indicators for cardiovascular disease prevention: developed by the working group for cardiovascular disease prevention quality indicators in collaboration with the European Association for Preventive Cardiology of the European Society of Cardiology. Eur J Prev Cardiol 2022;29:1060–1071. [DOI] [PubMed] [Google Scholar]
- 9. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279–1289. [DOI] [PubMed] [Google Scholar]
- 10. De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Ž, Rydén L, et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019;285:135–146. [DOI] [PubMed] [Google Scholar]
- 11. Landmesser U, Pirillo A, Farnier M, Jukema JW, Laufs U, Mach F, et al. Lipid-lowering therapy and low-density lipoprotein cholesterol goal achievement in patients with acute coronary syndromes: the ACS patient pathway project. Atheroscler Suppl 2020;42:e49–e58. [DOI] [PubMed] [Google Scholar]
- 12. Laufs U, Catapano AL, De Caterina R, Schiele F, Sionis A, Zaman A, et al. The effect of the 2019 ESC/EAS dyslipidaemia guidelines on low-density lipoprotein cholesterol goal achievement in patients with acute coronary syndromes: the ACS EuroPath IV project. Vascul Pharmacol 2023;148:107141. [DOI] [PubMed] [Google Scholar]
- 13. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 14. Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason De, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 2023;388:1353–1364. [DOI] [PubMed] [Google Scholar]
- 15. Krychtiuk KA, Ahrens I, Drexel H, Halvorsen S, Hassager C, Huber K, et al. Acute LDL-C reduction post ACS: strike early and strike strong: from evidence to clinical practice. A clinical consensus statement of the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Association of Preventive Cardiology (EAPC) and the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur Heart J Acute Cardiovasc Care 2022;11:939–949. [DOI] [PubMed] [Google Scholar]
- 16. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019;8:e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 18. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 19. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buonvino C, Chopard R, Guillon B, Puymirat E, Farnier M, Ferrières J, et al. An innovative lipid-lowering approach to enhance attainment of low-density lipoprotein cholesterol goals. Eur Heart J Acute Cardiovasc Care 2020;9:879–887. [DOI] [PubMed] [Google Scholar]
- 21. Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: a statement from a European Atherosclerosis Society Task Force. Atherosclerosis 2021;325:99–109. [DOI] [PubMed] [Google Scholar]
- 22. Schiele F, Farnier M, Krempf M, Bruckert E, Ferrières J, Angoulvant D, et al. A consensus statement on lipid management after acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2018;7:532–543. [DOI] [PubMed] [Google Scholar]
- 23. Ray KK, Reeskamp LF, Laufs U, Banach M, Mach F, Tokgözoğlu LS, et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J 2022;43:830–833. [DOI] [PubMed] [Google Scholar]
- 24. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 25. Schubert J, Lindahl B, Melhus H, Renlund H, Leosdottir M, Yari A, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J 2021;42:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danchin N, Farnier M, Zeller M, Puymirat E, Cottin Y, Belle L, et al. Long-term outcomes after acute myocardial infarction in patients with familial hypercholesterolemia: the French registry of acute ST-elevation and non-ST-elevation myocardial infarction program. J Clin Lipidol 2020;14:352–360 e6. [DOI] [PubMed] [Google Scholar]
- 27. Schiele F, Lemesle G, Angoulvant D, Krempf M, Kownator S, Cheggour S, et al. Proposal for a standardized discharge letter after hospital stay for acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2020;9:788–801. [DOI] [PubMed] [Google Scholar]
- 28. Bytyci I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J 2022;43:3213–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joy TR, Monjed A, Zou GY, Hegele RA, McDonald CG, Mahon JL. N-of-1 (single-patient) trials for statin-related myalgia. Ann Intern Med 2014;160:301–310. [DOI] [PubMed] [Google Scholar]
- 30. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicholls S, Lincoff AM, Bays HE, Cho L, Grobbee DE, Kastelein JJ, et al. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J 2021;235:104–112. [DOI] [PubMed] [Google Scholar]
- 32. Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580–1590. [DOI] [PubMed] [Google Scholar]
- 33. Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet 2022;400:380–390. [DOI] [PubMed] [Google Scholar]
- 34. Moriarty PM, Parhofer KG, Babirak SP, Cornier M-A, Duell PB, Hohenstein B, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J 2016;37:3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khunti K, Danese MD, Kutikova L, Catterick D, Sorio-Vilela F, Gleeson M, et al. Association of a combined measure of adherence and treatment intensity with cardiovascular outcomes in patients with atherosclerosis or other cardiovascular risk factors treated with statins and/or ezetimibe. JAMA Netw Open 2018;1:e185554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drexel H, Coats AJS, Spoletini I, Bilato C, Mollace V, Perrone Filardi P, et al. An expert opinion paper on statin adherence and implementation of new lipid-lowering medications by the ESC Working Group on Cardiovascular Pharmacotherapy: barriers to be overcome. Eur Heart J Cardiovasc Pharmacother 2020;6:115–121. [DOI] [PubMed] [Google Scholar]
- 37. O'Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech A, Kuder JF, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 2022;146:1109–1119. [DOI] [PubMed] [Google Scholar]
- 38. Karagiannis AD, Mehta A, Dhindsa DS, Virani SS, Orringer CE, Blumenthal RS, et al. How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol. Eur Heart J. 2021;42:2154–2169 [DOI] [PubMed] [Google Scholar]
- 39. Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf 2014;23:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alings M, Descamps O, Guillon B, Leosdottir M, Maggioni AP, Recasens L, et al. Implementation of clinical practices and pathways optimizing ACS patients lipid management: focus on eight European initiatives. Atheroscler Suppl 2020;42:e59–e64. [DOI] [PubMed] [Google Scholar]
- 41. Sionis A, Catapano AL, De Ferrari GM, Dudek D, Jukema JW, Landmesser U, et al. Improving lipid management in patients with acute coronary syndrome: the ACS Lipid EuroPath tool. Atheroscler Suppl 2020;42:e65–e71. [DOI] [PubMed] [Google Scholar]
- 42. Gitt AK, Lautsch D, Ferrieres J, De Ferrari GM, Vyas A, Baxter CA, et al. Contemporary data on treatment practices for low-density lipoprotein cholesterol in 3867 patients who had suffered an acute coronary syndrome across the world. Data Brief 2018;16:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leonardi S, Montalto C, Carrara G, Casella G, Grosseto De, Galazzi M, et al. Clinical governance of patients with acute coronary syndromes. Eur Heart J Acute Cardiovasc Care 2022;11:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 45. Gencer B, Mach F, Murphy SA, De Ferrari GM, Huber K, Lewis BS, et al. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER trial. JAMA Cardiol 2020;5:952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chassin MR, Loeb JM, Schmaltz SP, Wachter RM. Accountability measures–using measurement to promote quality improvement. N Engl J Med 2010;363:683–688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.