Key Points
Question
Among patients with large-vessel occlusion acute ischemic stroke, does adjunctive intravenous methylprednisolone added to endovascular thrombectomy improve clinical outcomes?
Findings
In this randomized clinical trial that included 1680 patients, intravenous methylprednisolone, compared with placebo, as adjunct to endovascular thrombectomy resulted in no significant difference in disability severity between groups as measured by the overall distribution of the modified Rankin Scale score at 90 days (adjusted generalized odds ratio for a lower level of disability, 1.10).
Meaning
Adjunctive methylprednisolone therapy added to endovascular treatment for acute ischemic stroke did not significantly improve the degree of overall disability.
Abstract
Importance
It is uncertain whether intravenous methylprednisolone improves outcomes for patients with acute ischemic stroke due to large-vessel occlusion (LVO) undergoing endovascular thrombectomy.
Objective
To assess the efficacy and adverse events of adjunctive intravenous low-dose methylprednisolone to endovascular thrombectomy for acute ischemic stroke secondary to LVO.
Design, Setting, and Participants
This investigator-initiated, randomized, double-blind, placebo-controlled trial was implemented at 82 hospitals in China, enrolling 1680 patients with stroke and proximal intracranial LVO presenting within 24 hours of time last known to be well. Recruitment took place between February 9, 2022, and June 30, 2023, with a final follow-up on September 30, 2023.
Interventions
Eligible patients were randomly assigned to intravenous methylprednisolone (n = 839) at 2 mg/kg/d or placebo (n = 841) for 3 days adjunctive to endovascular thrombectomy.
Main Outcomes and Measures
The primary efficacy outcome was disability level at 90 days as measured by the overall distribution of the modified Rankin Scale scores (range, 0 [no symptoms] to 6 [death]). The primary safety outcomes included mortality at 90 days and the incidence of symptomatic intracranial hemorrhage within 48 hours.
Results
Among 1680 patients randomized (median age, 69 years; 727 female [43.3%]), 1673 (99.6%) completed the trial. The median 90-day modified Rankin Scale score was 3 (IQR, 1-5) in the methylprednisolone group vs 3 (IQR, 1-6) in the placebo group (adjusted generalized odds ratio for a lower level of disability, 1.10 [95% CI, 0.96-1.25]; P = .17). In the methylprednisolone group, there was a lower mortality rate (23.2% vs 28.5%; adjusted risk ratio, 0.84 [95% CI, 0.71-0.98]; P = .03) and a lower rate of symptomatic intracranial hemorrhage (8.6% vs 11.7%; adjusted risk ratio, 0.74 [95% CI, 0.55-0.99]; P = .04) compared with placebo.
Conclusions and Relevance
Among patients with acute ischemic stroke due to LVO undergoing endovascular thrombectomy, adjunctive methylprednisolone added to endovascular thrombectomy did not significantly improve the degree of overall disability.
Trial Registration
ChiCTR.org.cn Identifier: ChiCTR2100051729
This clinical trial assesses the efficacy and adverse events of adjunctive intravenous low-dose methylprednisolone to endovascular thrombectomy for patients with acute ischemic stroke secondary to large-vessel occlusion.
Introduction
Adjunctive treatment is needed to complement acute stroke reperfusion therapies, which remain unsatisfactory. Over the past decades, preclinical models have suggested potential benefits of corticosteroids in stroke treatment, including the reduction of infarct area, regulation of cerebral blood flow, improvement of the no-reflow phenomenon (which refers to the lack of tissue perfusion despite recanalization of an occluded artery), stabilization of the blood-brain barrier, prevention of angiogenic edema, inhibition of oxygen free radical–induced lipid peroxidation, and modulation of the immune response,1,2,3,4,5,6,7,8,9,10,11 yet none have been validated by clinical trials. Most of the prior trials investigating corticosteroids were conducted before the advent of mechanical thrombectomy as a viable treatment option.7,12,13,14 Consequently, corticosteroids were likely previously examined under the more challenging conditions of permanent, as opposed to transient, brain ischemia.15 The presence or lack of reperfusion has been identified as a critical determinant of corticosteroid effectiveness in animal models.9 In addition, many of the prior trials were composed of patients who did not undergo brain imaging so only had “presumed ischemic stroke” and the sample sizes were limited. In a 2011 Cochrane review of 24 published stroke trials involving corticosteroids, only 8 were deemed acceptable for meta-analysis synthesis, encompassing a total of 466 patients. Moreover, these studies used high doses and long duration of corticosteroids, which may have led to more adverse effects.16
The current study sought to determine the effects of intravenous low-dose methylprednisolone in patients with acute ischemic stroke due to large-vessel occlusion (LVO) in the setting of rapid cerebral reperfusion now attainable by endovascular treatment.
Methods
Trial Design and Oversight
The Methylprednisolone as Adjunctive to Endovascular Treatment for Acute Large Vessel Occlusion (MARVEL) trial was an investigator-initiated, multicenter, randomized, double-blind, placebo-controlled clinical trial. The trial was performed at 82 comprehensive stroke centers in China. It was approved both by the central medical ethics committee and the research board at each site. Signed informed consent was obtained from the patient or their legally authorized representative before enrollment. The study protocol has been published and is available in Supplement 1, and the statistical analysis plan is available in Supplement 2.17
The trial was designed and conducted by a steering committee composed of independent academic investigators and was monitored by an independent data and safety monitoring board. All data analyses and end point adjudication were performed by an independent clinical events committee. The trial was conducted according to the Declaration of Helsinki Harmonization Guidelines.
Participants
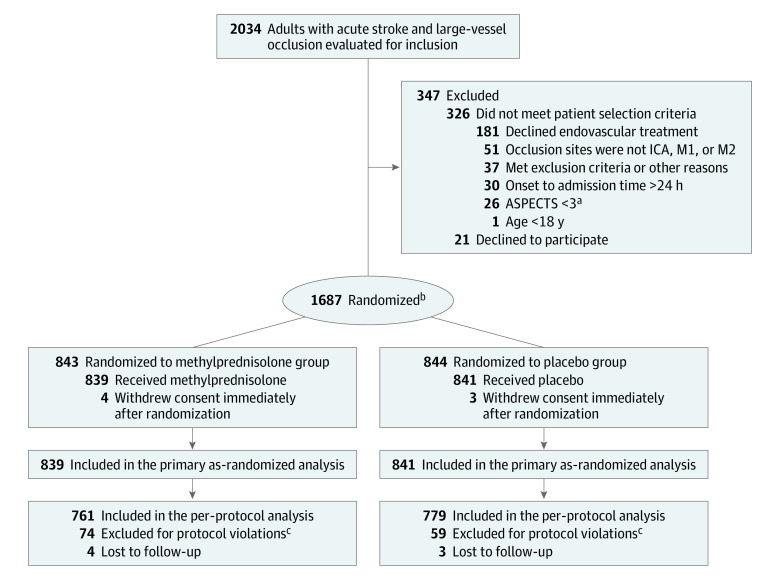
Eligible patients were adults aged 18 years or older with a disabling ischemic stroke at the time of randomization (baseline National Institutes of Health Stroke Scale [NIHSS] score ≥6; range, 0 to 42, with higher scores indicating greater stroke severity) who had been able to complete usual activities in daily life without support before the stroke (modified Rankin Scale [mRS] score <2; the mRS score ranges from 0 [no symptoms] to 6 [death] for the evaluation of neurologic functional disability), and were enrolled up to 24 hours from the time the patient was last known to be well (Figure 1 and Table 1). Computed tomographic (CT) angiography, magnetic resonance angiography, or digital subtraction angiography were performed to identify patients with an occlusion of the intracranial internal carotid artery or the first or second segment of the middle cerebral artery. The trial included patients with small-to-large ischemic core (defined as an Alberta Stroke Program Early CT Score18 [ASPECTS] of 3-10; range, 0–10, with 1 point subtracted for early ischemic change in each defined region on the noncontrast CT scan). Detailed inclusion and exclusion criteria are provided in eMethods 1 in Supplement 3.
Figure 1. Patients in a Study of the Effect of Intravenous Methylprednisolone vs Placebo as Adjunct to Endovascular Thrombectomy.
ASPECTS indicates Alberta Stroke Program Early CT Score; ICA, intracranial carotid artery; M1, the first segment of middle cerebral artery; and M2, the second segment of the middle cerebral artery.
aASPECTS ranges from 0 to 10, with 1 point subtracted for early ischemic change in each defined region on the noncontrast computed tomographic scan.
bRandomization was stratified by participating center.
ceFigure 1 in Supplement 3 provides detailed explanations of protocol violations.
Table 1. Demographic and Clinical Characteristics of the Patients at Baseline.
| Characteristic | No. (%) | |
|---|---|---|
| Methylprednisolone (n = 839) | Placebo (n = 841) | |
| Age, median (IQR), y | 68.0 (59.0-75.0) | 69.0 (59.0-77.0) |
| Sex | ||
| Female | 359 (42.8) | 368 (43.8) |
| Male | 480 (57.2) | 473 (56.2) |
| Medical historya | ||
| Hypertension | 504 (60.1) | 523 (62.2) |
| Atrial fibrillation | 348 (41.5) | 353 (42.0) |
| Hyperlipidemia | 267 (31.8) | 245 (29.1) |
| Diabetes | 170 (20.3) | 152 (18.1) |
| Coronary heart disease | 147 (17.5) | 169 (20.1) |
| Cerebral ischemia | 136 (16.2) | 117 (13.9) |
| Valvular heart disease | 114 (13.6) | 115 (13.7) |
| Intracranial hemorrhage | 12 (1.4) | 10 (1.2) |
| Transient ischemic attack | 6 (0.7) | 4 (0.5) |
| Smoking (current) | 236 (28.1) | 241 (28.7) |
| Prestroke modified Rankin Scale scoreb | ||
| 0 | 803 (95.7) | 817 (97.1) |
| 1 | 28 (3.3) | 14 (1.7) |
| 2 | 6 (0.7) | 8 (1.0) |
| 3 | 1 (0.1) | 1 (0.1) |
| 4 | 1 (0.1) | 1 (0.1) |
| Glucose level at hospital arrival median (IQR), mmol/L | 7.2 (6.2-8.9) [n = 736] | 7.0 (6.0-8.6) [n = 736] |
| Baseline NIHSS score, median (IQR)c | 19.0 (16.0-21.0) | 19.0 (16.0-21.0) |
| Baseline ASPECTS, median (IQR)d | 6.0 (4.0-7.0) | 6.0 (4.0-7.0) |
| TOAST etiologye | n = 838 | |
| Cardioembolic | 422 (50.4) | 393 (46.7) |
| Large artery atherosclerosis | 302 (36.0) | 334 (39.7) |
| Others | 32 (3.8) | 41 (4.9) |
| Unknown | 82 (9.8) | 73 (8.7) |
| Occlusion site on CT or MR angiography | ||
| Intracranial internal carotid artery | 286 (34.1) | 293 (34.8) |
| M1 middle cerebral artery segment | 452 (53.9) | 426 (50.7) |
| M2 middle cerebral artery segment | 99 (11.8) | 122 (14.5) |
| Otherf | 2 (0.2) | 0 |
| Intravenous thrombolysis | 295 (35.2) | 333 (39.6) |
| Time from last known well, median (IQR), h | ||
| To puncture | 5.8 (3.9-10.2) | 6.0 (4.0-9.8) |
| To randomization | 5.9 (4.0-10.2) | 6.1 (4.0-9.8) |
| To recanalization | 7.2 (5.2-11.6) | 7.4 (5.2-11.5) [n = 840] |
| Time from randomization to initial treatment, median (IQR), min | 8.0 (6.0-14.0) | 8.0 (6.0-13.0) [n = 839] |
| Blood pressure at hospital arrival, median (IQR), mm Hg | ||
| Systolic | 142.0 (126.0-160.0) | 145.0 (127.0-160.0) |
| Diastolic | 83.0 (74.0-92.0) | 84.0 (75.0-94.0) |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography; MR, magnetic resonance; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
SI conversion factor: To convert glucose to mg/dL, divide by 0.0555.
Comorbidities based on family or patient report.
Scores on the mRS of functional disability range from 0 (no symptoms) to 6 (death).
Scores on the NIHSS range from 0 to 42, with higher scores indicating more severe neurologic deficit.
The ASPECTS is an imaging measure of the extent of ischemic stroke. Scores range from 0 to 10, with higher scores indicating a smaller infarct core. Listed are values for the core laboratory assessment. Data were missing for 3 patients in the methylprednisolone group and 3 patients in the placebo group.
The TOAST classification system is a widely used method for classifying ischemic stroke and transient ischemic attack (TIA). It divides ischemic stroke and TIA into 5 subtypes based on their likely causes: large artery atherosclerosis, cardioembolism, small-artery occlusion, other determined etiology, and undetermined etiology. Data were missing for 1 patient in the methylprednisolone group.
One patient had a basilar artery occlusion and 1 patient had an anterior cerebral artery occlusion in the methylprednisolone group.
Randomization and Blinding
Patients were randomly assigned in 1:1 ratio to receive methylprednisolone or a placebo infusion. We randomly assigned patients using a web-based mobile phone app or computer. Randomization was stratified according to participating center with a permutation block size of 4. Patients were assigned a random serial number according to the time they were enrolled, and corresponding blinded and numbered medications were provided. Methylprednisolone and placebo were prepared as white lyophilized powder in numbered vials. These vials were visually identical, except for a unique vial number. All trial personnel and patients were unaware of the treatment assignment.
Interventions
Patients received either intravenous methylprednisolone or placebo at a dose of 2 mg/kg/d (up to a maximum dose of 160 mg) for 3 days. The study drug was administered as soon as possible after randomization, ideally before arterial access closure but not to be delayed for more than 2 hours after arterial access closure for endovascular treatment. After qualifying imaging, all patients underwent rapid endovascular thrombectomy (EVT) using available devices. Pretreatment intravenous thrombolysis was allowed. Methylprednisolone and placebo were provided by Chongqing Lummy Pharmaceutical Co Ltd. Sites were expected to adhere to stroke national practice guidelines for concomitant medical therapy.19
Outcomes
The primary efficacy outcome was the distribution of the mRS score (shift analysis) at 90 days after randomization. The score assessment was based on central evaluation by means of video or audio by evaluators who were unaware of the treatment assignment. If video or audio recordings were unavailable, outcomes were determined in-person by local investigators, who were also unaware of the treatment assignment.
The secondary efficacy outcomes included the NIHSS score at 5 to 7 days or at early discharge (range, 0 to 42, with higher scores indicating greater stroke severity), health-related quality of life measured with the European Quality of Life 5-Dimension visual analog scale (EQ-5D-VAS; range, 0 to 100, with higher scores indicating better quality of life, and the minimal clinically important difference for health-related quality of life on a 0-100 scale is 320) at 90 days, and the following prespecified dichotomizations of the mRS score at 90 days: 0 to 4 vs 5 to 6, 0 to 3 vs 4 to 6, 0 to 2 vs 3 to 6, and 0 or 1 (or return to premorbid mRS score) vs 2 to 6.
The primary safety prespecified outcomes were death from any cause within 90 days and symptomatic intracranial hemorrhage, as assessed according to the modified Heidelberg bleeding classification within 48 hours after EVT.21 Other prespecified safety measures included any intracranial hemorrhage within 48 hours after EVT, and adverse events including gastrointestinal bleeding within 7 days after EVT and pneumonia. Other adverse events, including new-onset diabetes, hyperosmolar hyperglycemic state, and insulin use in hospital stay, were also recorded.
Sample Size Calculation
The sample-size calculation was based on previous studies22,23 with an expected cumulative proportion of patients with an mRS score of 0 to 2 (43.0% in the placebo group and 50.0% in the methylprednisolone group, indicating an odds ratio of 1.33). We expected that a 7% absolute risk difference between the 2 groups would be appropriate to power the study because it exceeds the reported minimally clinically important difference for a novel stroke treatment, which ranges from 1.1% to 1.5%.24 We calculated that 794 patients per group would be required to have a power of 80% to show the expected treatment effect with a 2-sided α of .05. Considering an approximate 5% nonadherence or dropout rate, we intended to enroll 1672 patients.
Statistical Analysis
The primary analysis of the primary outcome was based on the complete case of the as-randomized, full analysis set population, which included patients according to their randomization assignment, with a valid assessment of mRS at day 90 (eMethods 2 in Supplement 3). The primary analyses for all outcomes were based on adjusted analyses for 7 prespecified covariates: age, baseline NIHSS score, prestroke mRS score, baseline ASPECTS, use of intravenous thrombolysis, time from onset to randomization, and occlusion location. Per-protocol analyses for the primary and secondary outcomes included patients who received the complete intervention as specified in the protocol. Analyses of adverse events were based on the safety population, which consisted of all randomized participants who received at least 1 dose of the study drug. We also performed sensitivity analyses of the primary outcome including a per-protocol analysis; imputation of missing primary outcome under best-case, worst-case, and best-worst case scenarios, and using multiple imputation; and a random effect model to control for possible center effect (eMethods 3 in Supplement 3).
The treatment effect for the primary outcome was measured by generalized odds ratio (genOR) with 95% CIs.25 The genOR was identified as one of the primary statistical methods for analysis of the primary outcome in the initial statistical analysis plan if the proportional odds assumption was not met. It was designated as the final primary analysis method for analysis of the primary outcome in the final statistical analysis plan. Both unadjusted genOR and adjusted genOR for prespecified covariates using the inverse probability of treatment weighting method were calculated.26 Secondary binary outcomes were analyzed by modified Poisson regression model with robust error estimation, from which unadjusted risk ratios (RRs) and adjusted RRs with their 95% CIs using inverse probability of treatment weighting were derived. The treatment effects for the non-normal continuous outcomes were measured using unadjusted and adjusted win ratios.26 Efficacy outcomes were assessed in the as-randomized, full analysis set population and repeated in the per-protocol set population. Safety outcomes were assessed in the safety population using modified Poisson regression model and survival analysis methods. Testing for modification of the treatment effect on the primary efficacy and safety outcomes were conducted in 10 subgroups: age, sex, NIHSS score, prestroke mRS score, ASPECTS, intravenous thrombolysis, time to randomization, occlusion location, reperfusion at EVT, and treatment during or outside the peak COVID-19 period. Consistent with regulatory and other guidance to reduce the effect of the COVID-19 pandemic on trial results, a prespecified subgroup analysis was performed. The control period included patients with valid 90-day follow-up prior to January 8, 2023, when the Chinese government downgraded the control of COVID-19 from grade A control (the same level of control as with the plague and cholera) to grade B control. Formal interaction tests were not performed because there is no reliable technique for calculating the modification effect when genORs are analyzed.
For the primary and secondary outcomes, a 2-sided P value of less than .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings from analyses of secondary outcomes and subgroups should be interpreted as exploratory. SAS version 9.4 (SAS Institute) and R version 4.3.0 (R Development Core Team; http://www.r-project.org) were used for the statistical analyses.
Results
Characteristics of the Patients
Between February 9, 2022, and June 30, 2023, 1687 patients underwent randomization (839 to the methylprednisolone group and 841 to the placebo group) at 82 centers in China. Seven patients, whose representatives withdrew consent immediately after randomization, could not be included in the as-randomized full analysis set (Figure 1; eFigures 1 and 2 in Supplement 3). Demographic and clinical characteristics were well balanced in the 2 study groups (Table 1; eTable 1 in Supplement 3). The median age of participants was 68 years (IQR, 59-75) in the methylprednisolone group and 69 years (IQR, 59-77) in the placebo group, and 359 of 839 participants (42.8%) were female in the methylprednisolone group and 368 of 841 participants (43.8%) were female in the placebo group. The median baseline NIHSS score was 19.0 (IQR, 16.0-21.0) in both groups; the median ASPECTS was 6.0 in both groups; and the median time from stroke onset to randomization was 5.9 hours (IQR, 4.0-10.2) in the methylprednisolone group and 6.1 hours (IQR, 4.0-9.8) in the placebo group.
Primary Efficacy Outcome
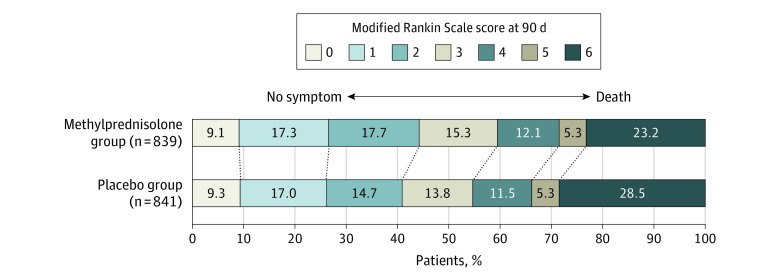
In the primary analysis, the median 90-day mRS score was 3 (IQR, 1-5) in the methylprednisolone group vs 3 (IQR, 1-6) in the placebo group. There was no significant difference in the distribution of 90-day mRS disability outcomes on the global mRS between the methylprednisolone group and the placebo group, yielding an unadjusted genOR of 1.12 (95% CI, 0.98-1.28; P = .09) and an adjusted genOR of 1.10 (95% CI, 0.96-1.25; P = .17) (Table 2 and Figure 2). The sensitivity and per-protocol analyses yielded similar results (eTables 2-4 and eFigure 3 in Supplement 3).
Table 2. Efficacy and Safety Outcomes in a Study of the Effect of Intravenous Methylprednisolone vs Placebo Before Endovascular Thrombectomy.
| Outcome | No./total (%) | Unadjusted value (95% CI) | P value | Adjusted value (95% CI)a | P value | |
|---|---|---|---|---|---|---|
| Methylprednisolone (n = 839) | Placebo (n = 841) | |||||
| Primary efficacy outcome | ||||||
| mRS score at 90 d, win proportion (%)b | 307 623/699 730 (44.0) | 274 681/699 730 (39.3) | GenORc: 1.12 (0.98 to 1.28) | .09 | GenORc: 1.10 (0.96 to 1.25) | .17 |
| mRS score at 90 d, median (IQR) | 3 (1 to 5) | 3 (1 to 6) | ||||
| Secondary efficacy outcomes d | ||||||
| mRS score of 0-4 at 90 d | 597/835 (71.5) | 555/838 (66.2) | RR: 1.08 (1.01 to 1.15) | .02 | RR: 1.07 (1.00 to 1.14) | .04 |
| RD: 0.05 (0.01 to 0.1) | .02 | RD: 0.05 (0.01 to 0.08) | .004 | |||
| mRS score of 0-3 at 90 d | 496/835 (59.4) | 459/838 (54.8) | RR: 1.08 (1 to 1.18) | .056 | RR: 1.07 (0.98 to 1.16) | .11 |
| RD: 0.05 (0 to 0.09) | .056 | RD: 0.04 (0.01 to 0.07) | .02 | |||
| mRS score of 0-2 at 90 d | 368/835 (44.1) | 343/838 (40.9) | RR: 1.08 (0.96 to 1.20) | .19 | RR: 1.07 (0.95 to 1.19) | .26 |
| RD: 0.03 (−0.02 to 0.08) | .19 | RD: 0.03 (−0.01 to 0.06) | .11 | |||
| mRS score of 0-1 or return to premorbid score at 90 d | 223/835 (26.7) | 222/838 (26.5) | RR: 1.01 (0.86 to 1.18) | .92 | RR: 0.99 (0.84 to 1.16) | .89 |
| RD: 0 (−0.04 to 0.04) | .92 | RD: 0 (−0.03 to 0.03) | .85 | |||
| NIHSS score at 5-7 d or earlier if discharge (IQR)e | 11.0 (4.0 to 23.0) | 12.0 (4.0 to 28.0) | WR: 1.08 (0.96 to 1.21) | .20 | WR: 1.07 (0.95 to 1.20) | .25 |
| EQ-5D-VAS score at 90 d (IQR)f | 55.0 (5.0 to 80.0) | 50.0 (0.0 to 80.0) | WR: 1.13 (1.00 to 1.28) | .051 | WR: 1.11 (0.98 to 1.25) | .11 |
| Primary safety outcomes | ||||||
| Mortality | 194/835 (23.2) | 239/838 (28.5) | RR: 0.81 (0.69 to 0.94) | .01 | RR: 0.84 (0.71 to 0.98) | .03 |
| Symptomatic intracranial hemorrhageg | 71/823 (8.6) | 97/830 (11.7) | RR: 0.74 (0.55 to 0.99) | .04 | RR: 0.74 (0.55 to 0.99) | .04 |
| Secondary safety outcomes | ||||||
| Any radiologic intracranial hemorrhage | 314/823 (38.2) | 308/830 (37.1) | RR: 1.03 (0.91 to 1.16) | .66 | RR: 1.03 (0.91 to 1.16) | .71 |
| Pneumonia | 390 (46.5) | 466 (55.4) | RR: 0.84 (0.76 to 0.92) | <.001 | RR: 0.85 (0.77 to 0.93) | <.001 |
| Gastrointestinal bleeding within 7 d after EVT | 33 (3.9) | 50 (6.0) | RR: 0.66 (0.43 to 1.02) | .06 | RR: 0.66 (0.43 to 1.02) | .06 |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; EVT, endovascular thrombectomy; EQ-5D-VAS, European Quality of Life 5-Dimension visual analog score; genOR, generalized odds ratio; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; RD, risk difference; RR, risk ratio; WR, win ratio.
Adjusted values were adjusted for age, baseline NIHSS score, prestroke mRS score, baseline ASPECTS, use of intravenous thrombolysis, time from onset to randomization, and occlusion location using the inverse probability of treatment weighting method.
The mRS of functional disability ranges from 0 (no symptoms) to 6 (death). The win proportion was calculated by the number of wins in the methylprednisolone group over the placebo group in mRS among all possible pairs of mRS taking 1 patient from the methylprednisolone group and 1 patient from the placebo group divided by the total number of pairs. Data were missing for 4 patients in the methylprednisolone group and 3 patients in the placebo group.
The genOR indicated the probability of mRS score was lower than the other group. GenORs, common odds ratios, RRs, and RDs were adjusted for age, baseline NIHSS score, prestroke mRS, baseline ASPECTS, use of intravenous thrombolysis, time from onset to randomization, and occlusion location using the inverse probability treatment weighting method and were not adjusted for multiple comparisons.
The widths of the confidence intervals for the secondary outcomes were not adjusted for multiple comparisons.
Scores on the NIHSS range from 0 to 42, with higher values reflecting more severe neurologic impairment.
EQ-5D-VAS is a continuous scale measure of self-reported quality of life. Scores range from 0 to 100, with 0 indicating the worst possible quality of life and 100, the best possible quality of life. Data were missing for 4 patients in the methylprednisolone group and 3 patients in the placebo group.
Symptomatic intracranial hemorrhage was defined according to the Heidelberg bleeding classification (an increase in the NIHSS score of ≥4 points or an increase in the score for an NIHSS subcategory of ≥2 points with any intracranial hemorrhage on imaging).
Figure 2. Distribution of Global Disability at 90 Days Based on the Modified Rankin Scale Score.
Shown are the distribution of the modified Rankin Scale score among patients in the methylprednisolone and placebo groups. Scores range from 0 to 6, with 0 indicating no symptoms; 1, no clinically significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death. Numbers indicate rounded proportions. Data of 7 patients who withdrew consent were not included. Data of 4 patients in the methylprednisolone group and 3 patients in the placebo group with missing data were not included. The sum was not 100 because of rounding. Treatment with methylprednisolone was associated with an adjusted generalized odds ratio of 1.10 (95% CI, 0.96-1.25; P = .17) for the distribution of the modified Rankin Scale at 90 days.
Secondary Efficacy Outcomes
An mRS score of 0 to 4 at 90 days occurred in 597 of 835 patients (71.5%) in the methylprednisolone group and 555 of 838 patients (66.2%) in the placebo group, and had a significant difference in both unadjusted and adjusted analysis, yielding an unadjusted RR of 1.08 (95% CI, 1.01-1.15; P = .02) and an adjusted RR of 1.07 (95% CI, 1.00-1.14; P = .04) as well as adjusted risk difference (RD) of 0.05 (95% CI, 0.01-0.08; P = .004). There was no significant difference in other prespecified secondary efficacy end points between the 2 groups. There were 496 of 835 patients (59.4%) who achieved an mRS score of 0 to 3 in the methylprednisolone group compared with 459 of 838 patients (54.8%) in the placebo group (adjusted RR, 1.07 [95% CI, 0.98-1.16], P = .11; adjusted RD, 0.04 [95% CI, 0.01-0.07], P = .02). The EQ-5D-VAS score was numerically but not statistically significantly higher in the methylprednisolone group compared with the placebo group (adjusted win ratio, 1.11 [95% CI, 0.98-1.25], P = .11) (Table 2; eTable 2 in Supplement 3).
Adverse Events
As compared with placebo, methylprednisolone was associated with significantly lower 90-day mortality (23.2% vs 28.5%), yielding an unadjusted RR of 0.81 (95% CI, 0.69-0.94; P = .01) and an adjusted RR of 0.84 (95% CI, 0.71-0.98; P = .03) (Table 2; eFigures 4 and 5 in Supplement 3). Methylprednisolone was associated with a significantly lower rate of symptomatic intracranial hemorrhage at 48 hours (8.6% vs 11.7%; unadjusted RR, 0.74 [95% CI, 0.55-0.99], P = .04; adjusted RR, 0.74 [95% CI, 0.55-0.99], P = .04) compared with placebo. Pneumonia occurred in 390 of 839 patients (46.5%) in the methylprednisolone group and 466 of 841 patients (55.4%) in the placebo group (adjusted RR, 0.85 [95% CI, 0.77-0.93]). The proportion with gastrointestinal bleeding within 7 days after EVT was 33 of 839 patients (3.9%) in the methylprednisolone group and 50 of 841 patients (6.0%) in the placebo group (adjusted RR, 0.66 [95% CI, 0.43-1.02]; P = .06). Insulin treatment during inpatient hospital stay was administered to 165 of 839 patients (19.7%) in the methylprednisolone group and 110 of 841 patients (13.1%) in the placebo group. The incidence of new diabetes occurred in 40 of 839 patients (4.8%) in the methylprednisolone group and 24 of 841 patients (2.9%) in the placebo group (Table 2; eTables 5 and 6 in Supplement 3).
Subgroup and Sensitivity Analyses of Primary End Point
Results for prespecified subgroup analyses are displayed in Figure 3. In the period when COVID-19 was under strict control and no patients presumably had COVID-19, the adjusted genOR for a lower level of disability with methylprednisolone vs placebo was 1.25 (95% CI, 1.04-1.50). The genOR was 0.95 (95% CI, 0.79-1.15) for the period when the Chinese government downgraded the control of COVID-19 (Figure 3; eFigures 5 and 6 in Supplement 3).
Figure 3. Heterogeneity of Treatment Effect for Less Disability Among Prespecified Subgroups.
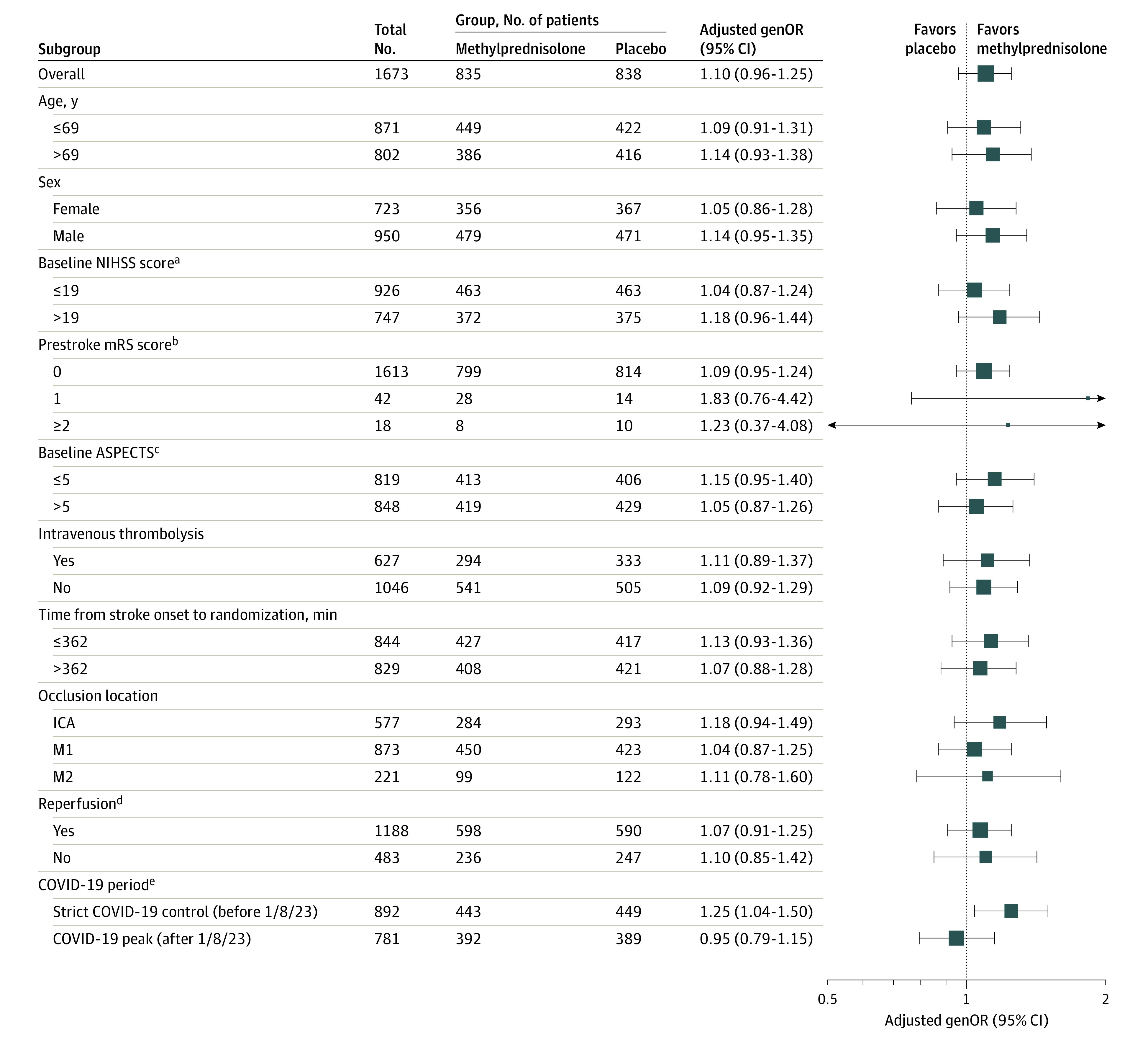
Shown is the subgroup analysis based on the adjusted generalized odds ratio (genOR), indicating the odds that the trial patients assigned to the methylprednisolone group would have better functional recovery at 90 days than patients assigned to receive placebo. The widths of the confidence intervals were not adjusted for multiple comparisons, and the reported confidence intervals should not be used for hypothesis testing. The age, National Institutes of Health Stroke Scale (NIHSS) score, and time from stroke onset to randomization were divided at median of the whole population as prespecified in the statistical analysis plan. The sizes of the boxes in the plot correspond to the number of patients in each subgroup. The arrow indicates that the 95% CI was beyond the scale. The prespecified subgroup analysis by status of hemorrhage was not represented here because they are postrandomization characteristics. ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; ICA, intracranial carotid artery; M1, the first segment of middle cerebral artery; M2, the second segment of the middle cerebral artery; and mRS, modified Rankin Scale.
aScores on the NIHSS range from 0 to 42, with higher scores indicating worse neurologic deficits.
bScores on the mRS of functional disability range from 0 (no symptoms) to 6 (death).
cASPECTS range from 0 to 10, with lower values indicating larger infarction; data were not available for 6 patients.
dData for reperfusion were not available for 2 patients.
eThe strict COVID-19 control period (before January 8, 2023) subgroup in the COVID-19 category included all patients who completed their primary outcome assessment before January 8, 2023, which marks the beginning of the downgrade in COVID-19 management measures. In the strict COVID-19 control period, no patients had COVID-19 infection. The COVID-19 peak period (after January 8, 2023) included patients who completed the primary outcome assessment after January 8, 2023.
Discussion
The results of this trial did not support the primary hypothesis that the administration of methylprednisolone, 2 mg/kg/d, intravenously as adjunct to EVT in patients with acute stroke due to LVO would significantly reduce the overall level of disability at 90 days. However, methylprednisolone treatment was associated with significantly lower rates of symptomatic intracranial hemorrhage within 48 hours and mortality at 90 days.
This trial provides new insights on the effects of corticosteroids in stroke therapy when used as an adjunct to endovascular therapy. Use of conventional or large doses of methylprednisolone are currently recommended to be avoided by the American Heart Association/American Stroke Association guidelines (class III harm) because of a lack of efficacy and the potential to increase the risk of infectious complications.27 In this study, low-dose methylprednisolone was associated with lower mortality, lower rate of symptomatic intracranial hemorrhage, and less pneumonia. This study is therefore one of the first, to our knowledge, to provide evidence regarding the potential role of corticosteroid therapy in the setting of endovascular stroke reperfusion.
There are several potential explanations for the neutral result of the primary outcome with respect to methylprednisolone. First, even though this was one of the largest EVT trials performed to date, the sample size was inadequate to capture a smaller but still clinically relevant treatment effect. This study was modeled under a general expectation of a 7% improvement in the rate of mRS score of 0 to 2 at 90 days, yet there is expert-derived consensus that the minimal clinically important difference for a safe new acute ischemic stroke treatment may be as low as 1.1% to 1.5%.24 Second, because this study included a broad range of patients with LVO and small to moderate ischemic core who underwent EVT, the trial population may have encompassed individuals with minimal blood-brain barrier disruption who potentially do not respond to corticosteroid treatment.
The secondary outcome finding that a higher proportion of patients in the methylprednisolone group were alive and not bedridden (mRS score, 0-4) at 90 days suggests that corticosteroid therapy has the potential effect of increasing the proportion of patients with an outcome of not requiring constant nursing care. The finding may in part reflect the ability of methylprednisolone to lessen blood-brain barrier disruption and vasogenic brain edema,28,29 which is related to fatal brain herniation.28,29 However, corticosteroids have less effect on cytotoxic edema due to cellular energetic failure, so combination therapy with agents targeting cytotoxic edema merits investigation. The findings of reduced symptomatic intracranial hemorrhage support stabilization of the blood-brain barrier as a potential beneficial effect of methylprednisolone. Moreover, the trial’s finding of the effect of corticosteroids on decreased incidence of pneumonia and lower incidence of circulation failure is also worth exploring. A similar effect of low-dose corticosteroids has been observed in the prevention of hospital-acquired pneumonia in patients with traumatic brain injury and patients with severe community-acquired pneumonia.30,31
The strengths of this trial included assessing a drug that is relatively inexpensive and readily available worldwide. Additionally, the high rates of reperfusion achieved with EVT provided a human ischemia-reperfusion model to test corticosteroid agents.
Limitations
This trial had several limitations. First, the COVID-19 pandemic may have had an influence on trial results. In the prespecified COVID-19 subgroup analysis, a favorable shift in the distribution of scores on the mRS toward better outcomes was noted during the period when COVID-19 was under strict control. Second, multiple testing of secondary and safety end points, such as mortality and symptomatic intracranial hemorrhage, were not corrected for. Therefore, for these end points, differences and P values should be interpreted with caution. Third, the rate of intravenous thrombolysis was slightly higher in the placebo group than in the methylprednisolone group, but the results remained robust in the adjusted analysis. Fourth, all patients enrolled in the trial were from China, and further research is needed to understand the generalizability of the results to other populations.
Conclusions
Among patients with acute ischemic stroke due to LVO undergoing EVT, adjunctive methylprednisolone added to EVT did not significantly improve the degree of overall disability.
Trial Protocol
Statistical Analysis Plan
eMethods 1. Detailed Inclusion and Exclusion Criteria
eMethods 2. Definition of Analyzed Population
eMethods 3. Handling of Missing Values and Sensitivity Analyses
eTable 1. Demographic and Clinical Characteristics of Patients at Baseline (Per-Protocol Population)
eTable 2. Detailed Analysis of Primary and Secondary Outcomes (Intention-to-Treat Population)
eTable 3. Primary and Secondary Efficacy Outcomes (Per-Protocol Population)
eTable 4. Primary Efficacy Outcomes—Sensitivity Analysis
eTable 5. Summary of Adverse Events With Special Interest (Safety Population)
eTable 6 Summary of Main Outcome by Participating Centers
eFigure 1. Flowchart
eFigure 2. Distribution of 82 Enrolling Centers in 17 Provinces in China
eFigure 3. Distribution of the Modified Rankin Scale Score at 90 Days (Per-Protocol Population)
eFigure 4. Kaplan-Meier Estimates of Survival Probability (Intention-to-Treat Population)
eFigure 5. Subgroup Analysis of Death (Safety Population)
eFigure 6. Subgroup Analysis of sICH (Safety Population)
Nonauthor Collaborators. MARVEL Investigators
Data Sharing Statement
References
- 1.Laha RK, Dujovny M, Barrionuevo PJ, DeCastro SC, Hellstrom HR, Maroon JC. Protective effects of methyl prednisolone and dimethyl sulfoxide in experimental middle cerebral artery embolectomy. J Neurosurg. 1978;49(4):508-516. doi: 10.3171/jns.1978.49.4.0508 [DOI] [PubMed] [Google Scholar]
- 2.de Courten-Myers GM, Kleinholz M, Wagner KR, Xi G, Myers RE. Efficacious experimental stroke treatment with high-dose methylprednisolone. Stroke. 1994;25(2):487-492. doi: 10.1161/01.STR.25.2.487 [DOI] [PubMed] [Google Scholar]
- 3.Barbosa-Coutinho LM, Hartmann A, Hossmann KA, Rommel T. Effect of dexamethasone on serum protein extravasation in experimental brain infarcts of monkey: an immunohistochemical study. Acta Neuropathol. 1985;65(3-4):255-260. doi: 10.1007/BF00687005 [DOI] [PubMed] [Google Scholar]
- 4.Espinosa A, Meneses G, Chavarría A, et al. Intranasal dexamethasone reduces mortality and brain damage in a mouse experimental ischemic stroke model. Neurotherapeutics. 2020;17(4):1907-1918. doi: 10.1007/s13311-020-00884-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altamentova S, Rumajogee P, Hong J, et al. Methylprednisolone reduces persistent post-ischemic inflammation in a rat hypoxia-ischemia model of perinatal stroke. Transl Stroke Res. 2020;11(5):1117-1136. doi: 10.1007/s12975-020-00792-2 [DOI] [PubMed] [Google Scholar]
- 6.Limbourg FP, Huang Z, Plumier JC, et al. Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J Clin Invest. 2002;110(11):1729-1738. doi: 10.1172/JCI0215481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norris JW. Steroids may have a role in stroke therapy. Stroke. 2004;35(1):228-229. doi: 10.1161/01.STR.0000105930.29558.DB [DOI] [PubMed] [Google Scholar]
- 8.Hall ED. Steroids and neuronal destruction or stabilization. Ciba Found Symp. 1990;153:206-214. [PubMed] [Google Scholar]
- 9.Slivka AP, Murphy EJ. High-dose methylprednisolone treatment in experimental focal cerebral ischemia. Exp Neurol. 2001;167(1):166-172. doi: 10.1006/exnr.2000.7532 [DOI] [PubMed] [Google Scholar]
- 10.Nellis SH, Roberts BH, Kinney EL, Field J, Ummat A, Zelis R. Beneficial effect of dexamethasone on the “no reflow” phenomenon in canine myocardium. Cardiovasc Res. 1980;14(3):137-141. doi: 10.1093/cvr/14.3.137 [DOI] [PubMed] [Google Scholar]
- 11.Mujanovic A, Ng F, Meinel TR, et al. No-reflow phenomenon in stroke patients: A systematic literature review and meta-analysis of clinical data. Int J Stroke. 2024;19(1):58-67. doi: 10.1177/17474930231180434 [DOI] [PubMed] [Google Scholar]
- 12.Kumar N, Jain S, Maheshwari MC. Role of dexamethasone in the outcome from acute stroke. J Assoc Physicians India. 1989;37(5):315-317. [PubMed] [Google Scholar]
- 13.Norris JW, Hachinski VC. High dose steroid treatment in cerebral infarction. BMJ (Clin Res Ed). 1986;292(6512):21-23. doi: 10.1136/bmj.292.6512.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poungvarin N. Steroids have no role in stroke therapy. Stroke. 2004;35(1):229-230. doi: 10.1161/01.STR.0000105931.81723.26 [DOI] [PubMed] [Google Scholar]
- 15.Lyden P, Buchan A, Boltze J, Fisher M; STAIR XI Consortium . Top priorities for cerebroprotective studies: a paradigm shift: report from STAIR XI. Stroke. 2021;52(9):3063-3071. doi: 10.1161/STROKEAHA.121.034947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandercock PA, Soane T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst Rev. 2011;2011(9):CD000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qingwu Y, Changwei G, Chengsong Y. MARVEL: a randomized double-blind, placebo-controlled trial in patients undergoing endovascular therapy: study rationale and design. Stroke Vasc Interv Neurol. Published January 27, 2024. doi: 10.1161/SVIN.123.001090 [DOI] [Google Scholar]
- 18.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group: Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670-1674. doi: 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Han S, Qin H, et al. ; Chinese Stroke Association Stroke Council Guideline Writing Committee . Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of the management of high-risk population. Stroke Vasc Neurol. 2020;5(3):270-278. doi: 10.1136/svn-2020-000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD. 2005;2(1):91-97. doi: 10.1081/COPD-200052090 [DOI] [PubMed] [Google Scholar]
- 21.von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 22.Zi W, Wang H, Yang D, et al. ; ACTUAL Investigators . Clinical effectiveness and safety outcomes of endovascular treatment for acute anterior circulation ischemic stroke in China. Cerebrovasc Dis. 2017;44(5-6):248-258. doi: 10.1159/000478667 [DOI] [PubMed] [Google Scholar]
- 23.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 24.Cranston JS, Kaplan BD, Saver JL. Minimal clinically important difference for safe and simple novel acute ischemic stroke therapies. Stroke. 2017;48(11):2946-2951. doi: 10.1161/STROKEAHA.117.017496 [DOI] [PubMed] [Google Scholar]
- 25.Agresti A. Generalized odds ratios for ordinal data. Biometrics. 1980;36:59-67. doi: 10.2307/2530495 [DOI] [Google Scholar]
- 26.Wang D, Zheng S, Cui Y, He N, Chen T, Huang B. Adjusted win ratio using the inverse probability of treatment weighting. J Biopharm Stat. 2023;1-16. doi: 10.1080/10543406.2023.2275759 [DOI] [PubMed] [Google Scholar]
- 27.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 28.Kozler P, Marešová D, Pokorný J. Effect of methylprednisolone on experimental brain edema in rats: own experience reviewed. Physiol Res. 2021;70(S3):S289-S300. doi: 10.33549/physiolres.934818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leenders KL, Beaney RP, Brooks DJ, Lammertsma AA, Heather JD, McKenzie CG. Dexamethasone treatment of brain tumor patients: effects on regional cerebral blood flow, blood volume, and oxygen utilization. Neurology. 1985;35(11):1610-1616. doi: 10.1212/WNL.35.11.1610 [DOI] [PubMed] [Google Scholar]
- 30.Asehnoune K, Seguin P, Allary J, et al. ; Corti-TC Study Group . Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet Respir Med. 2014;2(9):706-716. doi: 10.1016/S2213-2600(14)70144-4 [DOI] [PubMed] [Google Scholar]
- 31.Dequin PF, Meziani F, Quenot JP, et al. ; CRICS-TriGGERSep Network . Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi: 10.1056/NEJMoa2215145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods 1. Detailed Inclusion and Exclusion Criteria
eMethods 2. Definition of Analyzed Population
eMethods 3. Handling of Missing Values and Sensitivity Analyses
eTable 1. Demographic and Clinical Characteristics of Patients at Baseline (Per-Protocol Population)
eTable 2. Detailed Analysis of Primary and Secondary Outcomes (Intention-to-Treat Population)
eTable 3. Primary and Secondary Efficacy Outcomes (Per-Protocol Population)
eTable 4. Primary Efficacy Outcomes—Sensitivity Analysis
eTable 5. Summary of Adverse Events With Special Interest (Safety Population)
eTable 6 Summary of Main Outcome by Participating Centers
eFigure 1. Flowchart
eFigure 2. Distribution of 82 Enrolling Centers in 17 Provinces in China
eFigure 3. Distribution of the Modified Rankin Scale Score at 90 Days (Per-Protocol Population)
eFigure 4. Kaplan-Meier Estimates of Survival Probability (Intention-to-Treat Population)
eFigure 5. Subgroup Analysis of Death (Safety Population)
eFigure 6. Subgroup Analysis of sICH (Safety Population)
Nonauthor Collaborators. MARVEL Investigators
Data Sharing Statement