Abstract
Chronic liver diseases, e.g., cholestasis, are negatively impacted by inflammation, which further aggravates liver injury. Pharmacotherapy targeting the peroxisome proliferator-activated receptor alpha (PPARα), e.g., fenofibrate, has recently become an off-label therapeutic option for patients with refractory cholestasis. Clinical studies show that fibrates can reduce some pro-inflammatory cytokines in primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), however, its anti-inflammatory mechanisms have not been established. Numerous cytokines are regulated by the transcription factor nuclear receptor kappa B (NF-κB) and PPARα has been shown to interfere with NF-κB signaling. This study investigates the anti-inflammatory mechanism of fenofibrate by inhibiting NF-κB signaling in human macrophages and clinical outcomes in patients with PBC. For adult patients with PBC and an incomplete biochemical response to Ursodiol (13-15 mg/kg/day), the addition of fenofibrate (145 – 160 mg/day) reduced serum levels of TNF-α, IL-17A, IL-1β, IL-6, IL-8, and MCP-1 and increased IL-10. In THP-1 cells, pretreatment with fenofibrate (125 μM) reduced LPS-stimulated peak concentrations of IL-1β (−63%), TNF-α (−88%), and IL-8 (−54%), in a PPARα-dependent manner. Treatment with fenofibrate prior to LPS significantly decreased nuclear NF-κB p50 and p65 subunit binding by 49% and 31%, respectively. Additionally, fenofibrate decreased nuclear NF-κB p50 and p65 protein expression by 66% and 55% and increased cytoplasmic levels by 53% and 54% versus LPS alone, respectively. Lastly, fenofibrate increased IκBα levels by 2.7-fold (p<0.001) vs. LPS. These data demonstrate that fenofibrate reduces pro-inflammatory cytokines section by inhibiting in NF-κB signaling, which likely contribute to its anti-inflammatory effects during chronic liver diseases.
Keywords: fenofibrate, cholestasis, NF-κB, inflammation, cytokines, macrophages
Inflammation contributes to the pathogenesis of several chronic liver diseases, including nonalcoholic fatty liver disease (NAFLD), hepatitis, primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), where it plays a significant role in fibrosis and disease progression [1]. Without proper treatment, these diseases often progress to cirrhosis or hepatocellular carcinoma.
Pro-inflammatory mediators are regulated through multiple pathways, with the nuclear receptor kappa B (NF-κB) transcription factor playing a major role in regulating genes involved in inflammation in chronic liver diseases, including cholestasis and hepatocellular carcinoma [2, 3]. Briefly, NF-κB remains inactive when bound to the inhibitory kappa B (IκB) protein in the cytoplasm until the toll-like receptor 4 (TLR4) is activated by a stimulus, e.g., lipopolysaccharide (LPS). Classically, phosphorylation of IκB results in its dissociation from the NF-κB subunits p50 and p65, which in turn allows for their nuclear translocation to initiate the transcription of pro-inflammatory cytokines [4].
NF-κB regulates the production of many proinflammatory cytokines, making it a major target of interest to reduce inflammation during chronic liver diseases. Among the several pro-inflammatory mediators, interleukin- (IL-) 17 promotes the production of IL-6, IL-1β, and tumor necrosis factor alpha (TNFα) from hepatic macrophages [3]. In particular, IL-8 and IL-17 have been found to be upregulated in PBC, PSC, and NAFLD [5-7] and IL-8 has been identified as a prognostic cytokine in patients with PSC [8]. Additionally, TNFα, IL-8, IL-6, and IL-1β, have been implicated in the progression of fibrosis, all of which are regulated by NF-κB [2, 9, 10].
The peroxisome proliferator-activated receptor alpha (PPARα) inhibits NF-κB signaling by directly binding to the NF-κB subunit p65 and increasing the activity of IκB, through the deactivation and deacetylation of NF-κB [11]. Blocking the NF-κB signaling pathway has been suggested to alleviate PBC [12], whereas activation of the NF-κB pathway promotes the release of proinflammatory cytokines in a mouse model of PBC [13]. Clinically, PPARα agonists, including fibrates, reduce serum biomarkers of liver injury, as well as the toxicity of the bile acid pool, and pro-inflammatory cytokines in PBC and PSC [7, 14-16]. In addition, NF-κB binding sites are located within the IL-8 promoter [17], suggesting that the anti-inflammatory effects of fenofibrate, a PPARα agonist, during cholestasis may be mediated through downregulation of the NF-κB pathway. The current study investigates the inhibitory effects of fenofibrate on proinflammatory cytokines in adult patients with PBC and its inhibitory role on NF-κB signaling and subsequent downregulation of proinflammatory cytokines in a human macrophage model of inflammation.
MATERIAL AND METHODS
Multiplex ELISA
Adult patients with PBC (n=5) who received Ursodiol monotherapy (13-15 mg/kg/day) for at least 6 months and continued to experience an incomplete biochemical response subsequently received combination treatment with Ursodiol and off-label Fenofibrate (145-160 mg/day). Treatments were a part of routine clinical care at the Yale Autoimmune and Cholestatic Liver Diseases Program (New Haven, CT), as previously described [7]. Written consent was provided by patients to have their de-identified blood samples stored at the Yale Liver Center Clinical Registry (New Haven, CT) for research purposes. These de-identified samples were determined to be exempt from the Institutional Review Boards at Yale University (New Haven, CT) and the University of Rhode Island (Kingston, RI). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional review committee. Serum pro-inflammatory cytokine levels were measured using the MILLIPLEX® MAP Human Cytokine/Chemokine Magnetic Bead Panel (Millipore Sigma, Burlington, MA) according to the manufacturer’s protocol.
Materials
Fenofibrate and GW-6471 obtained from Cayman Chemical (Ann Arbor, MI). Lipopolysaccharide (LPS, 055:B5), bovine serum albumin (BSA), 2-mercaptoethanol, fetal bovine serum (FBS), phosphate buffered saline (PBS), and phorbol 12-myristate13-acetate (PMA) obtained from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide was obtained from Fisher Scientific (Pittsburgh, PA). RPMI-1640 medium was purchased from American Type Culture Collection (ATCC, Manassas, VA). M-PER mammalian protein extraction reagent, protease and phosphatase inhibitors, TBS-Tween 20, Streptavidin-HRP then Turbo TMB detection reagent, and chemiluminescent reagents (ThermoFisher Scientific, Rockford, IL). Antibodies against human IKBα (4812), phospho-IKBα (2859), NF-κB p65 (8242), NF-κB p50 (3035), c-Jun (9165S) and phospho-c-Jun (9261S) from Cell Signaling Technologies, Danvers, MA; histone 3, β-actin, peroxidase goat anti-rabbit IgG, p-38, and phospho-p-38 (49900, 1791, 97051, 170099, 195049), nuclear extraction reagent, NF-κB transcription factor binding assays (Abcam, Cambridge, MA). ELISAs were purchased from R&D Systems (Minneapolis, MN).
Cell Culture
THP-1 human monocytes (ATCC, TIB-202™, Manassas, VA) were cultured in RPMI-1640 medium supplemented with 2-mercaptoethanol (0.05 μM) and 10% FBS and maintained in a 37 °C, 5 % CO2 incubator. Cells were differentiated by the addition of PMA (5 ng/mL) to RPMI-1640 medium for 48 hours then replenished with serum-free medium containing drug treatments. THP1-Lucia™ cells (InvivoGen, San Diego, CA) are derived from the human THP-1 monocyte cell line by stable integration of an NF-κB-inducible Luc reporter construct and were cultured per manufacturer’s recommendation, in RPMI-1640 medium containing 2 mM L-glutamine, 25 mM HEPES, 2-mercaptoethanol (0.05 μM), 10% FBS, 100 μg/mL Normocin™ and Zeocin™, and Penicillin-Streptomycin (100 μg/mL). Cells were passaged according to the manufacturer’s protocol.
Enzyme-Linked Immunosorbent Assay (ELISA)
TNFα, IL-1β and IL-8 concentrations in THP-1 cell supernatant were measured by ELISA (R&D systems). Antibody concentrations were determined according to the certificate of analysis for each assay. Capture antibody for each cytokine was applied to plates overnight. Wells were then blocked with 1% BSA at room temperature. Cell supernatant was added to each well and incubated at room temperature. Detection antibodies were added to each well, followed by Streptavidin-HRP then Turbo TMB detection reagent. The optical density was measured at 540 nm.
NF-κB Binding Activity
Nuclear extracts from THP-1 Lucia cells stimulated by LPS ± fenofibrate were used to measure NF-κB subunit p50 and p65 nuclear translocation by ELISA. Nuclear proteins were isolated and exposed to pre-labeled wells containing the specific double stranded DNA sequence containing the NF-κB response element. Primary antibodies for p50 and p65 were applied, followed by a secondary antibody conjugated to horseradish peroxidase to provide a colorimetric readout at 450 nm.
Western Blot Analysis
Total, nuclear, or cytoplasmic protein levels of human IKBα, phospho-IKBα, NF-κB p50, NF-κB p65, p-38, phospho-p-38, c-Jun, and phospho-c-Jun were measured by western blotting. Proteins were separated by 10% polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, incubated with primary antibodies overnight, and followed by goat anti-rabbit conjugated to HRP. Immunodetection was performed using West Pico Plus Chemiluminescent Substrate. Protein bands were quantified by ImageJ, normalized to β-actin (total, cytoplasmic) or Histone 3 (H3, nuclear), and expressed as fold-change over DMSO.
Statistical Analysis
Data are presented as mean ± SEM. Statistical analyses were carried out by one-way or two-way ANOVA, followed by Tukey’s or Dunnett’s multiple comparisons test (GraphPad Prism 9). Paired analysis was performed by Wilcoxon matched-pairs signed rank test (GraphPad Prism 9). Differences with a P-value <0.05 were considered statistically different.
RESULTS
Fenofibrate inhibits pro-inflammatory cytokines in patients with PBC
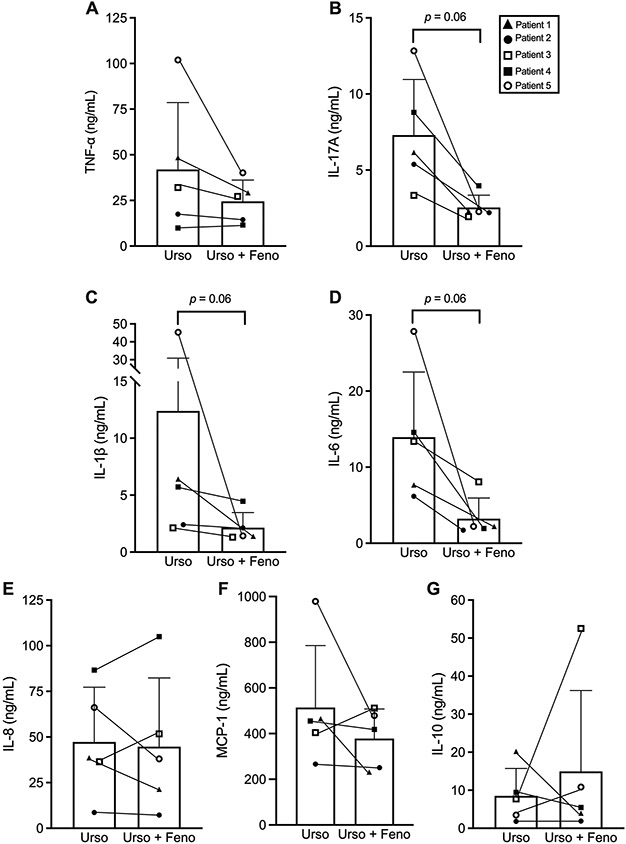
Human serum samples obtained from patients with PBC (n=5) who received Ursodiol monotherapy followed by combination therapy with Ursodiol and fenofibrate were analyzed for pro-inflammatory cytokines and chemokines levels by ELISA. The combination of fenofibrate (145-160 mg/day) plus Ursodiol (13-15 mg/kg/day) reduced serum levels of TNF-α (−42%), IL-17A (−65%, p=0.06), IL-1β (−83%, p=0.06), IL-6 (−77%, p=0.06), IL-8 and MCP-1 (−26%) in comparison to Ursodiol monotherapy (Fig. 1A-F). In addition, adjunct fenofibrate treatment increased the anti-inflammatory cytokine serum IL-10 levels by 75% vs. Ursodiol monotherapy (Fig. 1G). These data support the anti-inflammatory actions of fenofibrate during adult PBC.
Fig. 1.
Paired analysis of serum pro-inflammatory cytokine levels in patients with PBC (n=5) treated with Ursodiol monotherapy (13-15 mg/kg/day) followed by combination therapy with Ursodiol and fenofibrate (145 – 160 mg/day): (A) TNF-α, (B) Il-17A, (C) IL-1β, (D) IL-6, (E) IL-8, (F) MCP-1 and (G) IL-10. Data represent mean ± SD. Statistical analysis was performed using the Wilcoxon matched-pairs signed rank test.
Fenofibrate reduces LPS-induced inflammation in a PPARα-dependent manner
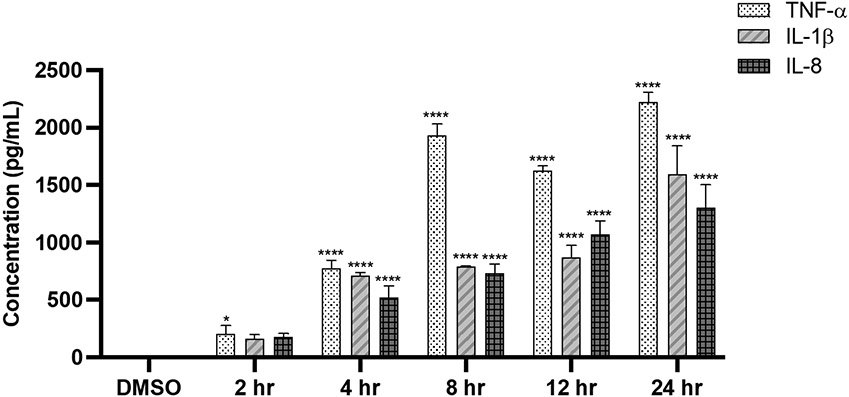
Differentiated THP-1 monocytes are a very commonly used model to study macrophage functions and exposure to LPS is the most potent activator of the macrophage secretory response to activate inflammation in vitro [7, 18]. These properties make THP-1 cells an ideal model to investigate how fenofibrate alters cytokine production. Treatment with fenofibrate (5-250 μM) did not alter THP-1 cell viability compared to DMSO control, Fig. S1. Pro-inflammatory cytokine concentrations of TNFα, IL-1β, and IL-8 were measured in THP-1 cells treated with LPS (10 ng/mL) for 2-24 hours by ELISA. Time-dependent increases in TNFα (p<0.0001), IL-1β (p<0.0001), and IL-8 (p<0.0001) levels were found as early as 4 hours after LPS treatment vs. DMSO, with peak levels reached after 8 and 24 hours of LPS treatment, Figure 2.
Fig. 2.
LPS (10 ng/mL) stimulates pro-inflammatory cytokine secretion in THP-1 cells (2-24 hrs). Data represent mean ± SD, n=3-4. Statistical analysis was performed using ANOVA followed by Dunnett’s multiple comparisons test, *p<0.05, ****p<0.0001 vs. DMSO.
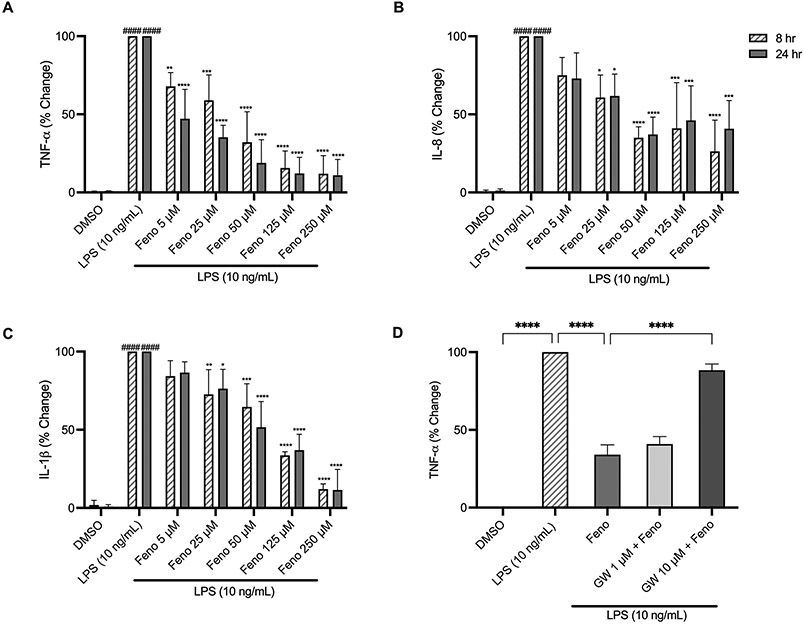
To investigate the anti-inflammatory mechanisms of fenofibrate on cytokine secretion, THP-1 cells were pretreated with fenofibrate (5-250 μM) for 2 hours prior to LPS stimulation. Treatment with LPS (10 ng/mL) significantly upregulated TNF-α, IL-1β, and IL-8 at both 8 and 24 hours (p<0.0001), Figure 3A-C. Alternatively, pretreatment with fenofibrate (50 μM) significantly decreased TNF-α secretion by 68% (p<0.0001) at 8 hours and by 81% (p<0.0001) at 24 hours, respectively, compared to LPS alone. Fig. 3A. Similarly, treatment with fenofibrate (50 μM) significantly reduced IL-8 and IL-1β levels by 65- (p<0.0001) and 35% (p<0.001) at 8 hours and 63- (p<0.0001) and 48% (p<0.0001) at 24 hours, respectively, compared to LPS alone, Fig. 3B-C. The reductions in cytokine levels by fenofibrate (5-125 μM) occurred in a dose-dependent manner. THP-1 cells were further treated with GW-6471, a PPARα antagonist. Pretreatment of THP-1 cells with fenofibrate (50 μM) significantly attenuated the secretion of TNF-α by LPS (10 ng/mL) by 65% (p<0.0001), and GW-6471 (1 and 10 μM) abolished the anti-inflammatory effect of fenofibrate in a dose-dependent manner, Figure 3D. These data demonstrate that pre-treatment with fenofibrate inhibits LPS-induced pro-inflammatory cytokine secretion in a PPARα-dependent manner.
Fig. 3.
Dose-dependent changes of pro-inflammatory cytokine levels. Treatment with LPS (10 ng/mL) for 8- and 24-hr ± 2-hour fenofibrate pretreatment. (A) TNF-α, (B) IL-8, (C) IL-1β, (D) TNF-α production prior to fenofibrate (50 μM) and LPS (10 ng/mL) treatment ± GW6471 (PPAR-α antagonist). Data represent mean ± SD, n=3. Statistical analysis was performed using two-way ANOVA with Dunnett’s multiple comparisons test, *p<0.05, **p<0.01, ***p<0.001 and ****p<0.001 vs. LPS; ####p<0.0001 vs. DMSO (A-C) or one-way ANOVA with Tukey’s multiple comparisons test, ****p<0.0001.
Fenofibrate attenuates NF-κB p65 and p50 binding activity and NF-κB activity
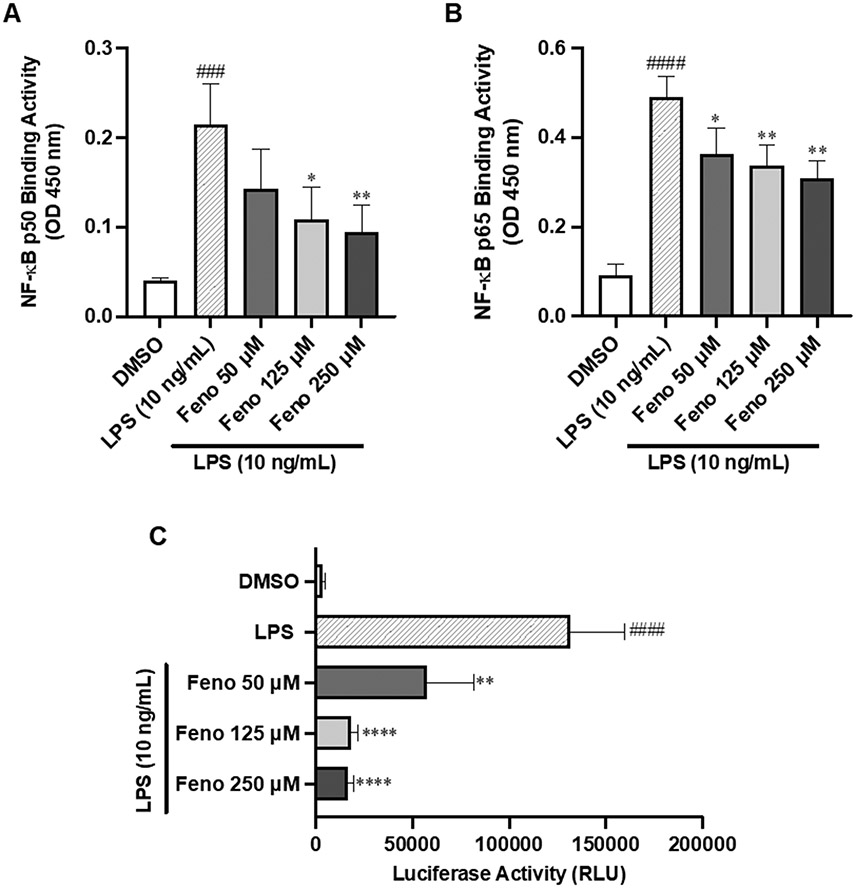
Next, we measured the DNA binding activity of NF-κB p50 and p65 in the nuclear extract of THP-1 cells. Treatment with LPS (10 ng/mL) significantly induced both NF-κB p50 and NF-κB p65 nuclear binding activity by 5.4-fold, compared to DMSO (p<0.001 and p<0.0001, respectively), Figure 4A-B. In contrast, pre-treatment with fenofibrate (125 μM) significantly decreased NF-κB p50 and p65 nuclear binding activity by 49% (p<0.05) and 31% (p<0.01), respectively, compared to LPS treatment alone, Figure 4A-B. These results indicate that fenofibrate interferes with the nuclear translocation of the NF-κB subunits p50 and p65 to inhibit NF-κB p50 and p65 binding activity. Additionally, THP-1-Lucia cells treated with LPS (10 ng/mL) for 24 hours induced NF-κB activity by 36.3-fold (p<0.0001) vs. DMSO, whereas pretreatment with fenofibrate (50–250 μM) significantly attenuated NF-κB activation vs. LPS alone (p<0.01, p<0.0001, respectively), Figure 4C. Together, these data demonstrate that fenofibrate inhibits LPS-mediated NF-κB activation and activity.
Fig. 4.
Effects of fenofibrate on LPS-Induced NF-κB p50 and p65 binding activity. One-hour LPS (10 ng/mL) ± 2-hour fenofibrate pretreatment on nuclear NF-κB (A) p50 and (B) p65 binding activity and (C) NF-κB activity in THP-1 cells treated with LPS (10 ng/mL) for 24 hours ± fenofibrate. Data represent mean ± SD, n=3. Statistical analysis was performed by one-way ANOVA with Dunnett’s multiple comparisons test, *p<0.05, **p<0.01 and ****p<0.0001 vs. LPS; ###p<0.001 and ####p<0.0001 vs. DMSO.
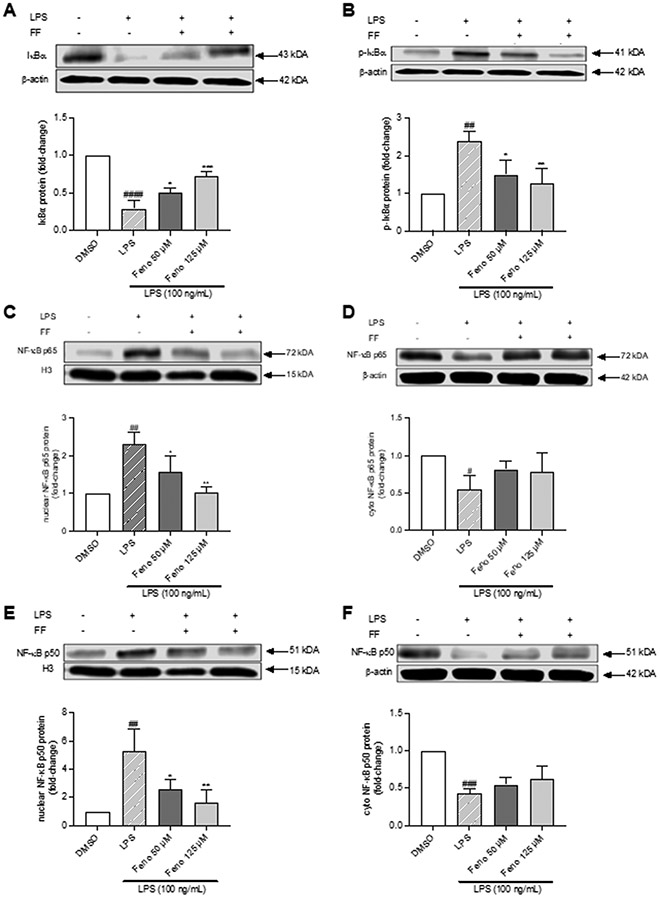
Fenofibrate inhibited IκBα and NF-κB p50 and p65 protein expression
We further investigated the anti-inflammatory effects of fenofibrate on the protein expression of NF-κB targets. Treatment of THP-1 cells with LPS (100 ng/mL) significantly downregulated IκBα protein expression by 70% (p<0.0001) vs. DMSO, which treatment with fenofibrate (125 μM) increased by 2.7-fold (p<0.001), Figure 5A. Treatment with LPS (100 ng/mL) also increased IκBα phosphorylation by 2.4-fold vs. DMSO (p<0.01), which fenofibrate (125 μM) reduced by 48% (p<0.01) vs. LPS, Figure 5B.
Fig. 5.
Effects of treatment with LPS (100 ng/mL) ± 2-hour fenofibrate pretreatment (50, 125 μM) on: (A) IκBα, (B) p-IκBα, (C, E) nuclear NF-κB p50 and p65 and (D, F) cytoplasmic NF-κB p50 and p65 protein levels. Data represent mean ± SD, n=3. Statistical analysis was performed by one-way ANOVA with Dunnett’s multiple comparisons test, *p<0.05, **p<0.01, ***p<0.001 vs. LPS; ##p<0.01, ###p<0.001, and ####p<0.0001 vs. DMSO.
In parallel, stimulation of THP-1 cells with LPS (100 ng/mL) significantly up-regulated nuclear NF-κB p50 and p65 protein expression by 5.3- and 2.3-fold (p<0.01, respectively), and reduced their corresponding cytoplasmic expression by 57% (p<0.001) and 45% (p<0.05), respectively, relative to DMSO, Figure 5C-F. Alternatively, pretreatment with fenofibrate (125 μM) significantly decreased nuclear NF-κB p50 and p65 protein expression by 66 (p<0.01) and 55% ((p<0.01) vs. LPS, respectively), Figure 5C, 5E, which increased cytoplasmic levels of NF-κB p50 and p65 by 53% and 54% versus LPS, Figure 5D, 5F. These findings suggest that fenofibrate sequestered p50/p65 in the cytoplasm thereby reduced NF-κB p50/p65 nuclear translocation, resulting in decreased inflammation. Taken together, these data support the conclusion that PPARα activation by fenofibrate inhibits inflammation via downregulation of the NF-κB signaling pathway.
Lastly, knowing that multiple pathways contribute to inflammation, we investigated whether fenofibrate inhibits the p-38 group of mitogen-activated protein kinases (MAPK) or c-Jun signaling. Interestingly, fenofibrate did not reduce LPS-stimulated p-38 phosphorylation (Fig. S2A), which suggests that fenofibrate may directly modulate NF-KB, independent of MAPK. On the other hand, fenofibrate decreased c-Jun protein phosphorylation by 87% (p<0.01) versus LPS (Fig. S2B), which indicates that the anti-inflammatory effects of fenofibrate may also be mediated through the JNK pathway.
DISCUSSION
Chronic liver diseases, e.g., PBC, PSC, are negatively impacted by inflammation and, as the inflammation persists, it can contribute to fibrosis and the development of hepatocellular carcinoma [1, 19]. Recently, fenofibrate, a PPARα ligand and an FDA-approved therapy to reduce hyperlipidemia, has emerged as a potential adjunct therapy for patients with PBC who experience a subtherapeutic response to UDCA monotherapy [14, 16]. PPARα activation via fenofibrate, along with the activation of PPAR isoforms δ/γ by other agonists, reduces serum cytokine levels in adult patients with PBC and PSC [7], as well reduces hepatic inflammation in rodent models of non-alcoholic steatohepatitis [20]. However, the anti-inflammatory mechanism(s) of fenofibrate during liver diseases have not been fully established. In this study, we hypothesized that fenofibrate reduces inflammation through PPARα-mediated inhibition of NF-κB signaling. Our data demonstrate that fenofibrate blocks NF-κB p50 and p65 binding thereby attenuating NF-κB nuclear translocation and inhibition of subsequent secretion of pro-inflammatory cytokines during adult PBC.
Innate immune cells, e.g., monocytes, have been implicated in the pathogenesis of cholestasis [1] and monocyte-derived macrophages are part of the cellular crosstalk in NAFLD [21]. Upon infection, circulating monocytes circulate to the tissues where they become resident tissue macrophages [22]. THP-1 cells are a human leukemia monocyte cell line that differentiates into a macrophage-like phenotype when treated with PMA and serve as a commonly used in vitro model to study macrophage functions of inflammatory disease including inflammation during cholestasis [23, 24]. Thus, THP-1 macrophages are a suitable model to test the mechanism(s) of fenofibrate on inflammation during PBC.
Fenofibrate-mediated PPARα activation modulates inflammatory pathways and reduces the production of proinflammatory cytokines in both animal studies [7, 25, 26] and in vitro [27]. Our clinical findings herein agree with previous studies showing reduced pro-inflammatory cytokines by fenofibrate treatment [7, 15, 16]. While our patient cohort is small, our findings provide additional anti-inflammatory data on cytokines reduced by fenofibrate. Additionally, our in vitro data are in agreement with others that have demonstrated PPARα activation by Wy-14,643 decreased the production of IL-6 in mouse spleen cells [28] and fenofibrate inhibits NF-κB activation in human endothelial cells [29], while providing additional evidence of fenofibrate’s ability to inhibit NF-κB activity. The current study supports the anti-inflammatory mechanism of fenofibrate in reducing pro-inflammatory cytokines in adult patients with PBC as well as the secretion in the human macrophage cell line THP-1.
The anti-inflammatory actions of PPARα are mediated through the inhibition of several transcription factors including NF-κB [30, 31], activator protein-1 (AP-1) and signal transducers and activators of transcription (STATs) [27]. Many pro-inflammatory cytokines elevated during cholestatic inflammation are regulated by NF-κB [32]. The most common heterodimer form of NF-KB is p50/p65, which remains inactive when sequestered in the cytoplasm due to binding to IκB [33]. Phosphorylation of IκB by IKK and ubiquitination in response to stimuli results in IκB degradation [34]. As a result, the active NF-κB subunits p50 and 65, translocate to the nucleus to enhance target gene expression, e.g., NLRP3 inflammasome and caspase-1, and upregulate pro-inflammatory cytokines. Results of the current study support that fenofibrate significantly decreased NF-κB p50 and p65 nuclear translocation after LPS-induced inflammation in human macrophages. The data presented in this study also show that fenofibrate significantly reduced LPS-induced NF-κB activity in THP-1 Lucia cells. Delerive et al. showed that fibrates induced IκBα protein levels in human aortic smooth muscle cells and reduced NF-κB binding [35]. Indeed, the current study demonstrates that fenofibrate upregulates IκBα, which is otherwise degraded by LPS, and thereby reduced IκBα phosphorylation and subsequent cytokine secretion.
Phosphorylation of the p38 group of the MAPK pathway plays an important role in producing pro-inflammatory cytokines [36] and its downregulation is associated with the downregulation of NF-κB [37]. In addition, phosphorylation of c-Jun induces the AP-1 transcription factor and pro-inflammatory cytokines [38]. Here, we found that LPS-induced inflammation in THP-1 cells upregulated p-38 phosphorylation, however, fenofibrate did not lower p38 protein levels. Alternatively, fenofibrate reduced the LPS-stimulated up-regulation of phospho-c-Jun protein expression, similar with previous reports [39]. Together, the data presented herein demonstrate that fenofibrate reduced pro-inflammatory cytokine production in THP-1 cells through the downregulation of NF-κB signaling and phosphorylation of c-Jun.
CONCLUSION
Therapeutic benefits of PPARα-targeted therapy combined with other pharmacological agents have been shown for the treatment of non-alcoholic steatohepatitis [40] and cholestasis [7, 14-16, 41]. Mechanistically, our current data demonstrate that fenofibrate reduced the LPS-induced pro-inflammatory response by inhibiting the NF-κB signaling pathway in a PPARα-dependent manner. Specifically, LPS significantly induced IκBα phosphorylation and degradation which correspondingly induced NF-κB p50 and p65 subunit nuclear translocation, resulting in the activation of pro-inflammatory cytokines. Furthermore, our clinical data obtained from patients with PBC who received combination therapy with Ursodiol and fenofibrate had reduced proinflammatory cytokines compared to when they received Ursodiol monotherapy, which exemplifies the anti-inflammatory effects of fenofibrate in vivo and supports our in vitro data. Together, these findings support the conclusion that fenofibrate reduces human inflammation by interfering in NF-κB signaling to reduce pro-inflammatory cytokines production in vitro and reduces pro-inflammatory cytokines in patients with PBC. In conclusion, these data indicate that interruption of the NF-κB pathway by fenofibrate could be beneficial for patients with chronic liver diseases.
Supplementary Material
Funding:
Research reported in this publication was in part made possible through the RI-INBRE Centralized Research Core Facility which is supported by the Institutional Development Award Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health (P20-GM103430), and from the Yale Liver Center award NIH P30 DK034989 Clinical-Translational Core.
ABBREVIATIONS
- PBC
Primary biliary cholangitis
- PSC
Primary sclerosing cholangitis
- PPARα
Peroxisome proliferator-activated receptor alpha
- TLR4
Toll-like receptor 4
- LPS
Lipopolysaccharide
- NF-κB
Nuclear factor kappa B
- IκB
Inhibitory kappa B
- IL-
Interleukin-
- BSA
Bovine serum albumin
- FBS
Fetal bovine serum
- PBS
Phosphate buffer saline
- PMA
Phorbol 12-myristate-13-acetate
- ELISA
Enzyme-linked immunosorbent assay
- TNFα
Tumor necrosis factor alpha
- NAFLD
Nonalcoholic fatty liver disease
Footnotes
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- 1.Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets 2011;10(6):509–536 [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Chen D, Jia Y, et al. Methane-Rich Saline Counteracts Cholestasis-Induced Liver Damage via Regulating the TLR4/NF-kappaB/NLRP3 Inflammasome Pathway. Oxid Med Cell Longev 2019;2019:6565283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res 2011;21(1):159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatziieremia S, Gray AI, Ferro VA, et al. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br J Pharmacol 2006;149(2):188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi T, Zhang T, Zhang L, et al. The Distribution and the Fibrotic Role of Elevated Inflammatory Th17 Cells in Patients With Primary Biliary Cirrhosis. Medicine (Baltimore) 2015;94(44):e1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Hwang S, Ahmed YA, et al. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell Mol Immunol 2021;18(1):18–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghonem NS, Auclair AM, Hemme CL, et al. Fenofibrate Improves Liver Function and Reduces the Toxicity of the Bile Acid Pool in Patients With Primary Biliary Cholangitis and Primary Sclerosing Cholangitis Who Are Partial Responders to Ursodiol. Clin Pharmacol Ther 2020;108(6):1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gindin Y, Chung C, Jiang Z, et al. A Fibrosis-Independent Hepatic Transcriptomic Signature Identifies Drivers of Disease Progression in Primary Sclerosing Cholangitis. Hepatology 2021;73(3):1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann HW, Seidler S, Gassler N, et al. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One 2011;6(6):e21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben Ari Z, Avlas O, Pappo O, et al. Reduced hepatic injury in Toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem 2012;29(1-2):41–50 [DOI] [PubMed] [Google Scholar]
- 11.Korbecki J, Bobiński R, Dutka M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflammation Research 2019;68:443–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Xi Y, Tao G, et al. Sirtuin 1 activation alleviates primary biliary cholangitis via the blocking of the NF-kappaB signaling pathway. Int Immunopharmacol 2020;83:106386. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Li MP, Xu B, et al. A study of regulatory effects of TLR4 and NF-kappaB on primary biliary cholangitis. Eur Rev Med Pharmacol Sci 2019;23(9):3951–3959 [DOI] [PubMed] [Google Scholar]
- 14.Lemoinne S, Pares A, Reig A, et al. Primary sclerosing cholangitis response to the combination of fibrates with ursodeoxycholic acid: French-Spanish experience. Clin Res Hepatol Gastroenterol 2018;42(6):521–528 [DOI] [PubMed] [Google Scholar]
- 15.Corpechot C, Chazouilleres O, Rousseau A, et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med 2018;378(23):2171–2181 [DOI] [PubMed] [Google Scholar]
- 16.Levy C, Peter JA, Nelson DR, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther 2011;33(2):235–242 [DOI] [PubMed] [Google Scholar]
- 17.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 1999;19(5):429–438 [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KE, Cutilli J, Piliero LM, et al. Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol 2000;7(6):920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lleo A, de Boer YS, Liberal R, et al. The risk of liver cancer in autoimmune liver diseases. Ther Adv Med Oncol 2019;11:1758835919861914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol 2015;62(3):720–733 [DOI] [PubMed] [Google Scholar]
- 21.Thibaut R, Gage MC, Pineda-Torra I, et al. Liver macrophages and inflammation in physiology and physiopathology of non-alcoholic fatty liver disease. FEBS J 2022;289(11):3024–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharif O, Bolshakov VN, Raines S, et al. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schildberger A, Rossmanith E, Eichhorn T, et al. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm 2013;2013:697972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therien A, Cieslak A, Verreault M, et al. Omega-3 Polyunsaturated Fatty Acid: A Pharmaco-Nutraceutical Approach to Improve the Responsiveness to Ursodeoxycholic Acid. Nutrients 2021;13(8):2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cindoruk M, Kerem M, Karakan T, et al. Peroxisome proliferators-activated alpha agonist treatment ameliorates hepatic damage in rats with obstructive jaundice: an experimental study. BMC Gastroenterol 2007;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill MR, Clarke S, Rodgers K, et al. Effect of peroxisome proliferator-activated receptor alpha activators on tumor necrosis factor expression in mice during endotoxemia. Infect Immun 1999;67(7):3488–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grau R, Diaz-Munoz MD, Cacheiro-Llaguno C, et al. Role of peroxisome proliferator-activated receptor alpha in the control of cyclooxygenase 2 and vascular endothelial growth factor: involvement in tumor growth. PPAR Res 2008:352437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem 1998;273(49):32833–32841 [DOI] [PubMed] [Google Scholar]
- 29.Marx N, Sukhova GK, Collins T, et al. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 1999;99(24):3125–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geier A, Fickert P, Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol 2006;3(10):574–585 [DOI] [PubMed] [Google Scholar]
- 31.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1(6):a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Zhang L, Joo D, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017;2:e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreiro DU, Komives EA. Molecular mechanisms of system control of NF-kappaB signaling by IkappaBalpha. Biochemistry 2010;49(8):1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, Gong J. The role of Kupffer cells in the progression of acute pancreatitis. OA Emergency Medicine 2013;1(1):4 [Google Scholar]
- 35.Delerive P, Gervois P, Fruchart JC, et al. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem 2000;275(47):36703–36707 [DOI] [PubMed] [Google Scholar]
- 36.Dong N, Li X, Xue C, et al. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-kappaB/MAPK signaling pathway. J Cell Physiol 2020;235(7-8):5525–5540 [DOI] [PubMed] [Google Scholar]
- 37.Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol 2007;179(10):7101–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novoszel P, Holcmann M, Stulnig G, et al. Psoriatic skin inflammation is promoted by c-Jun/AP-1-dependent CCL2 and IL-23 expression in dendritic cells. EMBO Mol Med 2021;13(4):e12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano H. Signaling crosstalk between NF-kappaB and JNK. Trends Immunol 2004;25(8):402–405 [DOI] [PubMed] [Google Scholar]
- 40.Rajamoorthi A, Arias N, Basta J, et al. Amelioration of diet-induced steatohepatitis in mice following combined therapy with ASO-Fsp27 and fenofibrate. J Lipid Res 2017;58(11):2127–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallucci GM, Trottier J, Hemme C, et al. Adjunct Fenofibrate Up-regulates Bile Acid Glucuronidation and Improves Treatment Response For Patients With Cholestasis. Hepatol Commun 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.