1. INTRODUCTION
Kaposi's sarcoma (KS) is an endothelial cell cancer. It is more common in patients with human immunodeficiency virus (HIV) infection and is especially prevalent in patients with <200 CD4+ T‐cells. 1
The CD4:CD8 T lymphocytes (TL) ratio is an immunological indicator reflecting the status of patients with HIV infection. A normal CD4:CD8 ratio is ≥1, with CD4+ TL ranging from 500 to 1200/mm3 and CD8+ TL ranging from 150 to 1000/mm3. During infection, HIV generates a decrease in the number of CD4+ TL simultaneous to a consistent activation and expansion of CD8+ TL, which results in a low or inverted serum CD4:CD8 ratio. 2 A low serum CD4:CD8 ratio is associated with depletion and senescence of CD4+ TL, as well as increased activation of CD8+ TL. 3
Recently, Caby et al., found that a serum CD4:CD8 ratio of ≤0.3 is a risk factor for the development of KS, despite combination of antiretroviral treatment (c‐ART) and a CD4+ TL count ≥500 cells/mm3. 4 Similarly, Poizot‐Martin et al., found this risk factor with a serum CD4:CD8 ratio ≤0.5 regardless of the CD4+ TL count. Inverted CD4:CD8 ratio was present in 25% of HIV‐positive patients without KS compared to 72% of patients living with HIV and KS. 5
Certainly, CD8+ TL in HIV infection suffers alterations of their differentiation with a progressive dysfunction undergoing immune exhaustion. Chronic viral infections including HIV and vascular endothelial growth factor (VEGF‐A) production in other diseases, have been implicated in the production of exhausted lymphocytes. 6 , 7
The role of seric and cutaneous CD8+ TL are poorly understood in KS, and no studies have evaluated the role of cutaneous CD8+ TL and CD4:CD8 cutaneous ratio in skin biopsies. The objective of this study is to determine the relationship between the cutaneous CD4:CD8 ratio in skin biopsies and serum CD4:CD8 ratio of KS patients with HIV infection.
2. METHODS
We performed a cross‐sectional, descriptive, and observational study in a single dermatology center. We included cutaneous biopsy samples with a clinical and histopathological diagnosis of KS and HIV infection. Only serum CD4:CD8 TL ratio determinations within 6 months before the diagnosis of KS or 6 months thereafter were included in the study. Patients under 18 years of age and those with nonviable paraffin‐embedded tissue samples were excluded.
Once identified, the slides were processed for immunohistochemistry using the conjugated alkaline phosphatase and peroxidase methods with specific results for CD4+ and CD8+ TL, respectively, and visualized under a light microscope. CD8+ TL were stained brown and CD4+ TL red. Subsequently, three researchers independently counted CD8+ and CD4+ TL per field at ×60 magnification using the Image J program. An average was performed for each case. Finally, we divided the distribution of CD8+ TL in five patterns: perivascular‐peritumoral (defined as an infiltrate around the vascular and spindle cell proliferation of KS), perivascular‐peritumoral and peri‐adnexal, perivascular‐peritumoral and interstitial, and interstitial‐only.
Software R version 4.2.1 was used for statistical analysis. We used the Fisher test to determine the correlation between cutaneous and serum CD4:CD8 ratio with a cut‐off point of ≤0.5 CD4:CD8 ratio.
3. RESULTS
Fifty cutaneous biopsies were included. Among the demographic variables, 49 patients were male, and one patient was female with a mean age of 34 years. On histological examination, 12 cases were classified as patch stage, 22 as plaque stage, and 16 as tumoral stage.
The cutaneous CD4:CD8 ratio was inverted (≤0.5 cells/field) in 44 cases, ranged from 0.5 to 0.8 cells/field in four cases and ≥0.8 cells/field in two cases. On the other hand, the serum ratio was inverted (≤0.5 cells/mm3) in 47 patients, ranged from 0.5 to 0.8 cells/mm3 in two cases, and ≥0.8 cells/mm3 in one case. An inverted cutaneous CD4:CD8 ratio correlated with inversion in serum CD4:CD8 ratio (p = 0.001).
A perivascular‐peritumoral pattern of CD8+ TL distribution was the most frequent (38 cases, 76%), followed by a mixed perivascular‐peritumoral and periadnexal pattern (six cases, 12%), perivascular‐peritumoral and interstitial (four cases, 8%) and only interstitial (two cases, 4%) (Figure 1). Tumoral stage, had a lower cutaneous CD8+ TL count compared with the other two stages (Figure 2), while seric CD8 TL+ count remained elevated.
Figure 1.
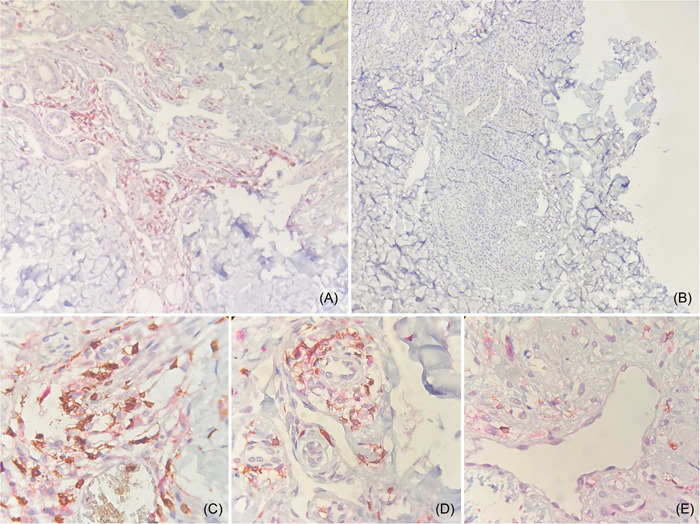
Immunochemistry of Kaposi sarcoma (KS). CD8+ TL cells are illustrated in brown color and CD4+ TL cells in red color. Plaque stage (A) and tumoral stage (B) of KS in a ×20 field. Patch stage of KS in a ×60 field (C). Plaque stage of KS in a ×60 field (D). Tumoral stage of KS in a ×60 field (E). A perivascular CD8+ TL predominance in the endothelial proliferation of KS are observed, however, a lower CD8+ TL count was presented in tumoral stages compared with patch and plaque stages.
Figure 2.
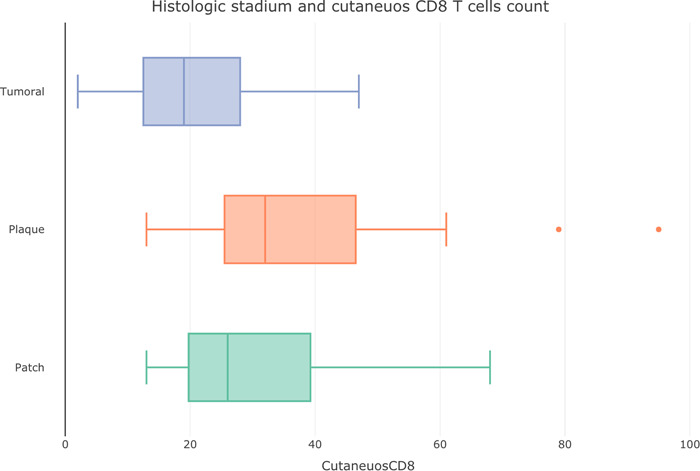
Box plot of histological stadium in Kaposi sarcoma and cutaneous CD8+ T‐cells counts.
Inversion of CD4:CD8 cutaneous ratio ≤0.5 was observed despite viral suppression of HIV (plasma HIV RNA <50 copies/mL) in seven cases. Among these cases, two of them had ≥200 CD4+ TL in blood samples, and other two cases had ≥500 serum CD4+ TL. The other three cases had <200 CD4+ TL.
4. DISCUSSION
In the past, more attention has been paid to CD4+ TL counts in HIV patients, while the CD8+ TL counts were relatively underappreciated. CD4+ TL recovery to ≥500 cells/mm3 is frequently observed in patients under c‐ART, however, CD8+ TL counts are consistently elevated even after long‐term treatment. Elevation of CD8+ TL is associated with increased immune energy and risks of non‐acquired immunodeficiency syndrome‐related clinical events in HIV‐infected patients during c‐ART. 4 , 8 , 9
All patients with virological suppression (plasma HIV RNA <50 copies/mL) had an inversion of cutaneous CD4:CD8 ratio, despite CD4+ TL count ≥200 cells/mm3 in four patients. Therefore, risk of KS persists regardless c‐ART, virological suppression, and CD4+ TL recovery in HIV. In 2017, Caby et al. conducted a cohort to study factors implicated in “virological failure” in 10,012 HIV‐infected patients. Main factors associated with ratio restoration were c‐ART started during primary HIV infection whatever the CD4+ TL count or starting CD4+ TL at least 500 cells/mm3. Higher baseline CD8+ TL counts was negatively associated with ratio restoration at 8 years, only one‐third of individuals achieved CD4:CD8 ratio restoration with sustained virological control. 10
Exhausted CD8+ TL with dysfunction in many subsets are presented in chronic viral infections and tumoral diseases with VEGF‐A production as HIV infection and spindle cells of KS, respectively. The reason is still unknown; however, some authors have raised the possibility that VEGF‐A has a role in the depletion of intratumoral CD8+ TL without affecting circulating CD8+ TL, generating an immunosuppressive tumor microenvironment. 7 , 11 , 12 , 13 On; the other hand, CD8+ TL in HIV infection expresses immune exhaustion markers such as programmed death‐1 and T‐cell immunoglobulin and mucin‐domain containing‐3. Despite c‐ART, CD8+ TL counts remain increased. After treatment initiation, viral load is rapidly controlled and CD4+ TL counts are progressively recovered, however, a persistent elevation of exhausted CD8+ TL with dysfunction in several subsets and a low CD4:CD8 ratio are observed. 6 In our study, the tumoral stage had the least amount of cutaneous CD8+ TL, regardless of elevated blood CD8+ TL count. Our hypothesis is that both VEGF‐A, and chronic viral infection of HIV are implicated in the production of exhausted lymphocytes and could be an important factor to KS histological progression.
The limitations of our study were the retrospective design and the sample size. Our objective in future studies will be to determine if the VEGF‐A is implicated in the pathogenesis of the KS progression.
5. CONCLUSIONS
The role of CD8+ TL is poorly understood in KS. To our knowledge, this is the first study that has evaluated the CD4:CD8 cutaneous ratio in comparison with the serum ratio. A cutaneous CD4:CD8 ratio <0.5 cells/field correlated with a serum CD4:CD8 <0.5 cells/mm3 ratio.
AUTHOR CONTRIBUTIONS
Alejandro García‐Irigoyen: Conceptualization; formal analysis; investigation; methodology; writing—original draft; writing—review and editing. Simón Guzmán‐Bucio: Data curation; investigation; methodology; software. Mercedes Aranda‐Audelo: Investigation; methodology. Zonía R. Moore: Investigation; methodology; writing—original draft. Héctor Trinidad‐Bibiano: Investigation; methodology. Vega‐Memije María Elisa: Conceptualization; investigation; project administration; supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Alejandro García‐Irigoyen affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
García‐Irigoyen A, Guzmán‐Bucio S, Aranda‐Audelo M, Moore ZR, Trinidad‐Bibiano H, Elisa V‐MM. Inversion of CD4:CD8 ratio in 50 cutaneous biopsies of patients with Kaposi sarcoma and human immunodeficiency virus infection: a cross‐sectional, descriptive, and observational study in a single dermatology center. Health Sci Rep. 2024;7:e1855. 10.1002/hsr2.1855
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV‐positive patients. J Int AIDS Soc. 2015;18(1):20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shiels MS, Engels EA. Evolving epidemiology of HIV‐associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caby F, Guiguet M, Weiss L, et al. CD4/CD8 ratio and the risk of Kaposi sarcoma or non‐Hodgkin lymphoma in the context of efficiently treated human immunodeficiency virus (HIV) infection: a collaborative analysis of 20 European Cohort Studies. Clin Infect Dis. 2021;73(1):50‐59. [DOI] [PubMed] [Google Scholar]
- 5. Poizot‐Martin I, Lions C, Cheret A, et al. Kaposi sarcoma in people living with HIV: incidence and associated factors in a French cohort between 2010 and 2015. AIDS. 2020;34(4):569‐577. [DOI] [PubMed] [Google Scholar]
- 6. Perdomo‐Celis F, Taborda NA, Rugeles MT. CD8+ T‐cell response to HIV infection in the era of antiretroviral therapy. Front Immunol. 2019;10:1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voron T, Colussi O, Marcheteau E, et al. VEGF‐A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gras L, May M, Ryder LP, et al. Determinants of restoration of CD4 and CD8 cell counts and their ratio in HIV‐1‐positive individuals with sustained virological suppression on antiretroviral therapy. J Acquir Immune Defic Syndr. 2019;80(3):292‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L‐X, Jiao Y‐M, Zhang C, et al. HIV reservoir decay and CD4 recovery associated with high CD8 counts in immune restored patients on long‐term ART. Front Immunol. 2020;11:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caby F, Guihot A, Lambert‐Niclot S, et al. Determinants of a low CD4/CD8 ratio in HIV‐1‐infected individuals despite long‐term viral suppression. Clin Infect Dis. 2016;62(10):1297‐1303. [DOI] [PubMed] [Google Scholar]
- 11. Baitsch L, Baumgaertner P, Devêvre E, et al. Exhaustion of tumor‐specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol. 2019;19(8):490‐502. [DOI] [PubMed] [Google Scholar]
- 13. Gurzu S, Ciortea D, Munteanu T, Kezdi‐Zaharia I, Jung I. Mesenchymal‐to‐endothelial transition in Kaposi sarcoma: a histogenetic hypothesis based on a case series and literature review. PLoS ONE. 2013;8(8):e71530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


