Abstract
Listeria monocytogenes is an intracellular pathogen that causes severe central nervous system infection in humans and animals. The ability of this bacterium to penetrate nerve cells was investigated by using rat spinal cell cultures. Entry into distinct cell types, i.e., glial cells and neurons, was monitored by a differential immunofluorescence technique with antibodies against cell type-specific markers and the bacterial pathogen. L. monocytogenes was detected predominantly within macrophages constituting the microglia. Astrocytes and oligodendrocytes, the major components of macroglia, were infected to a lesser extent. Surprisingly, Listeria innocua, a noninvasive and nonpathogenic species, also has the capacity to enter into these three types of glial cells. Entry into neurons was a very rare event. In contrast, we found that L. monocytogenes could efficiently invade neurons when these latter cells were cocultivated with Listeria-infected mouse macrophages. In this case, infection of neurons occurs by cell-to-cell spread via an actA-dependent mechanism. These data support the notion that infected phagocytes can be vectors by which L. monocytogenes gains access to privileged niches such as the central nervous system.
Listeria monocytogenes is a ubiquitous, gram-positive, facultative intracellular bacterium responsible for infrequent but often serious opportunistic infections in humans and animals. It has emerged as an important model system for understanding molecular mechanisms of intracellular bacterial pathogenesis (for a review, see reference 27). Host cell infection begins with the internalization of the bacteria by a process similar to phagocytosis. Bacteria escape from phagocytic vacuoles and multiply in the cytoplasm of the host. Rapid movement of bacteria through the cytoplasm of infected cells and the projection of bacteria into pseudopod-like cellular extensions that play a role in cell-to-cell spread of the infection are driven by directed polymerization of host actin monomers. Many of the genes whose products are required for the execution of this infection cycle have already been identified. Probably the most important factor in the escape from phagocytic vacuoles is a pore-forming hemolysin, listeriolysin O, which is encoded by the hly gene. Two distinct phospholipase C proteins (encoded by plcA and plcB) have been shown to have overlapping functions during primary vacuolar lysis and cell-to-cell spread. The actin-based motility process requires a bacterial surface protein, ActA, the actA gene product.
Entry into the host normally occurs in the gut after ingestion of contaminated food. Bacteria pass through the gastrointestinal barrier and spread via the lymph and the blood to distant tissues. In murine experimental infection, bacteria accumulate predominantly in the liver. Depending on the immune response of the host, bacteria either are eliminated or undergo further hematogenous dissemination to the brain and/or the placenta. Among the various clinical manifestations of listeriosis, infections of the central nervous system (CNS) are of critical importance.
Two main forms of nervous system involvement have been described: encephalitis, which is the predominant form in small ruminants (6, 7), and meningitis or meningoencephalitis, which is the most frequent form in humans (21). In naturally occurring animal listerial encephalitis, involvement of the brain stem is thought to be a consequence of retrograde ascension of L. monocytogenes along cranial nerves (1, 6, 7, 16), whereas in cases of meningitis in humans and other species, spread of the microorganism to the CNS is assumed to be hematogenous (3, 21). In most cases, meningitis is associated with a diffuse encephalitis mainly located in the rhombencephalon, where intracerebral abscesses are multiple, necrotic, and coalescent. In experimental murine listeriosis, both types of CNS alteration, i.e., encephalitis and meningoencephalitis, have been observed, depending on the route of infection (22).
While CNS listeriosis poses a significant clinical problem, its pathogenesis is largely unknown. The purpose of the present study was to examine which cell types of spinal cell cultures are permissive to L. monocytogenes. With respect to the suspected intra-axonal migration of L. monocytogenes, it was particularly relevant to examine invasion of neurons by this pathogen.
MATERIALS AND METHODS
Animals.
Cultures of spinal cord neurons and glial cells were prepared from 14-day-old embryonic rats and newborn rats, respectively (Sprague-Dawley; IFFA-Credo, L’Arbresle, France).
Tissue cultures.
Spinal cell cultures were prepared according to a modification of the protocol of Nicola et al. (14).
(i) Glial cells.
After dissection, spinal cords were minced and incubated with 0.05% trypsin-EDTA (GIBCO) diluted in phosphate-buffered saline (PBS) (120 mM, pH 7.4) for 15 min at 37°C. The enzymatic reaction was stopped by addition of horse serum (GIBCO). The tissue was mechanically dissociated with DNase (200 U/ml; Boehringer) and bovine serum albumin (0.4% wt/vol; Sigma) in L15 complete medium as used by Camu et al. (5). Cells were seeded on poly-dl-ornithine-coated 35-mm dishes (15 mg/ml; Sigma) at 3 × 105 cells per ml in L15 complete medium with 10% horse serum (GIBCO) and were incubated at 37°C in a 5% CO2 incubator. The culture medium was renewed after 3 days of seeding. After reaching confluence, the cells were trypsinized (as described above), diluted three times, and seeded on poly-dl-ornithine-coated 12-mm glass coverslips in 4-well plates (Nunc). In all experiments, cells were used after 5 days in vitro.
(ii) Primary cultures of spinal neurons.
Spinal cords were dissociated as described above. Cells were plated at a density of 3 × 105 cells per ml on sterilized glass coverslips (12 mm in diameter) in 4-well plates (Nunc). Coverslips were previously coated with 15 mg of poly-dl-ornithine (Sigma)/ml. Culture supports were successively incubated until plating in medium with 5% inactivated fetal calf serum (GIBCO). Cultures were kept at 37°C in 5% CO2 in the L15 serum-free medium as used by Camu et al. (5) for 5 days.
Cell line.
The mouse macrophage-like cell line J774 (ECACC 85011428) was propagated without antibiotics in RPMI 1640 medium (GIBCO) supplemented with 2 mM l-glutamine and 10% fetal calf serum (decomplemented for 30 min at 56°C).
Bacteria.
L. monocytogenes EGD was originally obtained from P. Berche. L. monocytogenes CIP 71408, 71456, and 71468 and Listeria innocua CLIP 11262T (BUG 499) were obtained from J. Rocourt. L. monocytogenes wild-type strain DP10403S and its isogenic ΔactA mutant strain were kindly provided by D. Portnoy. For each experiment, a log-phase culture of bacteria was prepared by inoculating 1 ml of an overnight culture into 9 ml of brain heart infusion broth. The new culture was incubated for about 3 h at 37°C with agitation until the optical density at 600 nm reached 0.8 to 1. Bacteria were washed twice by centrifugation at 12,000 × g for 2 min and then resuspended and vortex mixed in PBS. Bacteria were diluted in L15 medium supplemented with 7.5% sodium bicarbonate for the infection.
Antibodies.
L. monocytogenes was labeled with a rabbit antiserum against heat-killed L. monocytogenes strain LO28 (named R11). L. innocua was labeled with a rabbit antiserum raised against live L. innocua type strain CLIP 11262T (named R6). The R11 and R6 rabbit polyclonal antibodies were prepared as follows. New Zealand rabbits were inoculated with approximately 109 bacteria from an overnight culture washed twice in PBS. Animals were rechallenged with 109 bacteria 3 and 8 weeks after the first injection. Blood was collected 2 weeks after the last challenge. Astrocytes were labeled with a mouse monoclonal antibody (MAb) directed against pig glial fibrillary acidic protein (GFAP) from Sigma (clone G-A-5). Oligodendrocytes were detected with a mouse MAb directed against the myelin protein Rip (12). Microglial cells were labeled with a mouse MAb raised against the rat CD11b (clone Ox42; Serotec). Neurons were labeled with a mouse MAb directed against the microtubule-associated protein 2A (MAP-2A) that specifically stains perikarya and dendrites (4).
Direct infection of spinal cell cultures by L. monocytogenes.
Infections of glial and neuronal cell cultures by L. monocytogenes were carried out after 5 days in vitro. Approximately 1 × 105 cells per coverslip were infected with 5 × 106 L. monocytogenes cells (multiplicity of infection of about 50). Infected cultures were incubated for 40 min at 37°C in a 5% CO2 atmosphere. The infection process was stopped by rinsing the cultures with prewarmed Leibovitz L15 medium and was followed by the addition of cell culture medium containing 10 μg of gentamicin (Sigma) per ml. This concentration of gentamicin kills extracellular bacteria but leaves intracellular bacteria unaffected. Cells were incubated for 2 or 5 h at 37°C in a 5% CO2 incubator. The incubation time of 2 h was chosen in order to avoid cell-to-cell spread of bacteria and that of 5 h was chosen in order to visualize actively spreading bacteria (28). The cell medium was then removed and the infected cultures were processed for immunocytochemistry.
Indirect infection of spinal cell cultures by L. monocytogenes-infected macrophages.
Mouse J774 macrophages grown in 6-well plates were infected with L. monocytogenes at a multiplicity of infection of about 20 for 1 h. The monolayers were then washed three times with prewarmed PBS and were further incubated in the cell culture medium containing gentamicin (20 μg/ml) for 3.5 h. The monolayers were then washed and trypsinized, and the number of viable cells was determined. After centrifugation, the L. monocytogenes-infected J774 cells were resuspended in L15 complete medium containing gentamicin (20 μg/ml) and plated over the glial or neuronal cells at 5 days in vitro after removal of the cell culture medium. The cell ratio was 1 macrophage per 10 spinal cells. The mixed cultures were incubated for approximately 15 to 19 h to allow the passage of L. monocytogenes from the infected macrophages to the spinal cells by cell-to-cell spread. These infected cultures were then processed for immunofluorescence labeling.
Immunofluorescence labeling of infected cell cultures.
Cells were fixed with 4% paraformaldehyde (wt/vol) in PBS for 20 min at room temperature. After extensive washings in PBS, cells were successively incubated in PBS containing 50 mM ammonium chloride and PBS containing 0.1% gelatin (wt/vol) (PBSg) for 10 min each. Cells were then incubated with primary rabbit polyclonal anti-Listeria antibodies (R11 or R6) (dilution 1/500) for 1 h to label extracellular bacteria. Cells were rinsed with PBSg and incubated with secondary goat anti-rabbit fluorescein (DTAF)-conjugated antibody (Jackson Laboratories, dilution 1/200) for 45 min. Cells were then permeabilized with 0.25% Triton X-100 in PBSg for 15 min and incubated simultaneously with anti-Listeria antibodies (as described above) and MAb directed against a cell-type-specific marker for 1 h. After several washes in PBSg, the cells were incubated simultaneously with secondary goat anti-rabbit CY3-conjugated antibody (Jackson Laboratories, dilution 1/600) to label intracellular bacteria and with secondary goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated fluorescent antibody (Vector Laboratories, dilution 1/200) to label the cells for 45 min. In some experiments, the cell-type-specific labeling was replaced by actin cytoskeleton staining with FITC phalloidin (5 μg/ml). In all experiments, washes and antibody incubation steps were performed at room temperature. The specificity of immunolabeling and the absence of antibody cross-reaction were controlled by omission of the primary antibodies. After immunodetection, cultures were mounted on slides with Vectashield (Vector Laboratories) and observed with a standard fluorescence microscope equipped with appropriate filters.
RESULTS
Morphology and composition of rat spinal cord cultures.
The major cellular components of the spinal cord are neurons and glial cells. These two types of cells were cultivated separately. As previously described, neurons form a dense meshwork of neurites during their in vitro maturation (8). Two functionally different compartments can be distinguished: the cell soma with the dendrites, and the axons. These two neuronal compartments could be identified by immunocytochemistry with a MAb raised against MAP-2A and a MAb raised against rat neurofilament H-subunit (2H-3, hybridoma bank) respectively. In our culture conditions, neurons constituted more than 95% of the total cell population (data not shown).
In the glial cell cultures, three types of cells could be identified by immunocytochemistry: astrocytes, oligodendrocytes, and microglial cells (microglia). Astrocytes were identified by the binding of antibodies against GFAP, the major component of astrocyte intermediate filaments. GFAP-immunoreactive cells were subsequently divided into two groups with distinct morphologies: one composed of polygonal, flat, and non-process-bearing cells and the other composed of stellate-shaped cells characterized by small somata and long branched processes. They are referred to as type 1 (or protoplasmic) and type 2 (or fibrous) astrocytes, respectively. In vivo, oligodendrocytes produce myelin sheets around axons. Therefore, they can be identified by the binding of antibodies against Rip, a basic protein of myelin (12). Oligodendrocytes have a characteristic morphology, i.e., a round somata with numerous highly branched processes arising from it. Microglial cells, which are tissular macrophages, were identified with antibodies against the complement receptor type 3 (CD11b/CD18), a molecule present in most phagocytic cells. Although the composition of glial cell cultures can vary from one preparation to another, they are typically composed of approximately 80 to 90% astrocytes, 1 to 2% oligodendrocytes, and a mix of microglial cells, fibroblasts, and progenitor cells (data not shown).
Entry into glial cells is not a specific property of L. monocytogenes.
In this work, invasion of rat spinal cell cultures by Listeria was studied by differential fluorescence microscopy. This technique allows discrimination between extracellular and intracellular bacteria, as previously described (13). Infected cells were fixed and stained with anti-Listeria antibodies followed by labeling with a fluorescein-conjugated secondary antibody. Permeabilization was then performed, and cells were relabeled with the same primary anti-Listeria antibodies followed by labeling with a rhodamine-conjugated secondary antibody. Since the eucaryotic cell membrane prevents the penetration of antibodies unless it is permeabilized, extracellular bacteria were labeled with both fluorescent dyes, while the intracellular bacteria were only stained with one fluorescent dye.
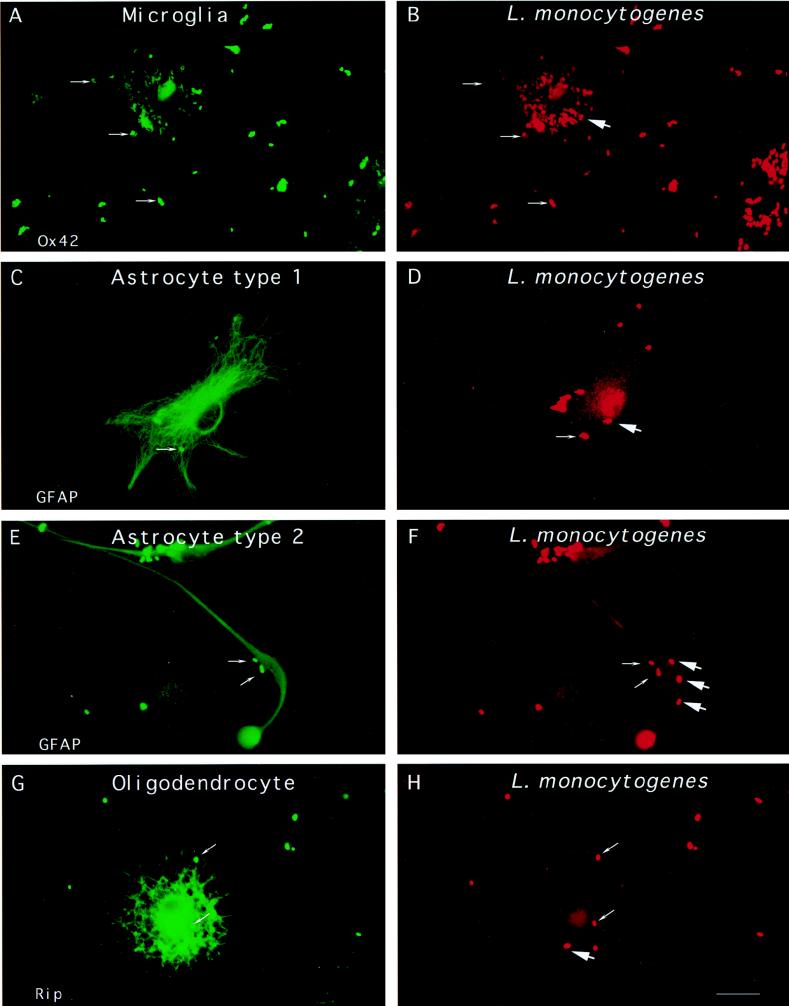
Following infection of glial cells by L. monocytogenes EGD, bacteria were essentially located within macrophages. While some microglial cells looked as if they were completely filled with L. monocytogenes (Fig. 1A and B), the number of bacteria in other labeled cells never exceeded 10 microorganisms per cell. GFAP-positive astrocytes showed the second largest infection rates (Fig. 1C through F) followed by Rip-positive oligodendrocytes (Fig. 1G and H). The average number of intracellular bacteria per astrocyte over 300 cells examined was 0.5 ± 0.2. It was not possible to quantify the entry of L. monocytogenes into oligodendrocytes since the integrity of most of these cells was affected by bacterial infection.
FIG. 1.
Entry of L. monocytogenes into glial cell cultures. Glial cells were incubated with L. monocytogenes for 40 min, unbound bacteria were washed away, and the cells were resuspended in medium containing gentamicin and incubated for 2 h. The cells were fixed and stained with antibodies against cell type-specific markers (Ox42, GFAP, or Rip) and against the bacterial pathogen. The different types of glial cells are shown on the left panels. Differential immunofluorescence labeling of the bacteria was performed in order to discriminate between extracellular and intracellular bacteria, as previously described (see Material and Methods). Extracellular bacteria are indicated by thin arrows and are both green and red whereas intracellular bacteria, indicated by thick arrows, are only red. Two microglial cells are shown in panel A and a bulk of intracellular Listeria cells is shown in panel B. Astrocyte type 1, astrocyte type 2, and oligodendrocyte are shown in panels C, E, and G, respectively, and extracellular L. monocytogenes (thin arrows) and intracellular L. monocytogenes (thick arrows) are shown in panels D, F, and H. Scale bar, 10 μm.
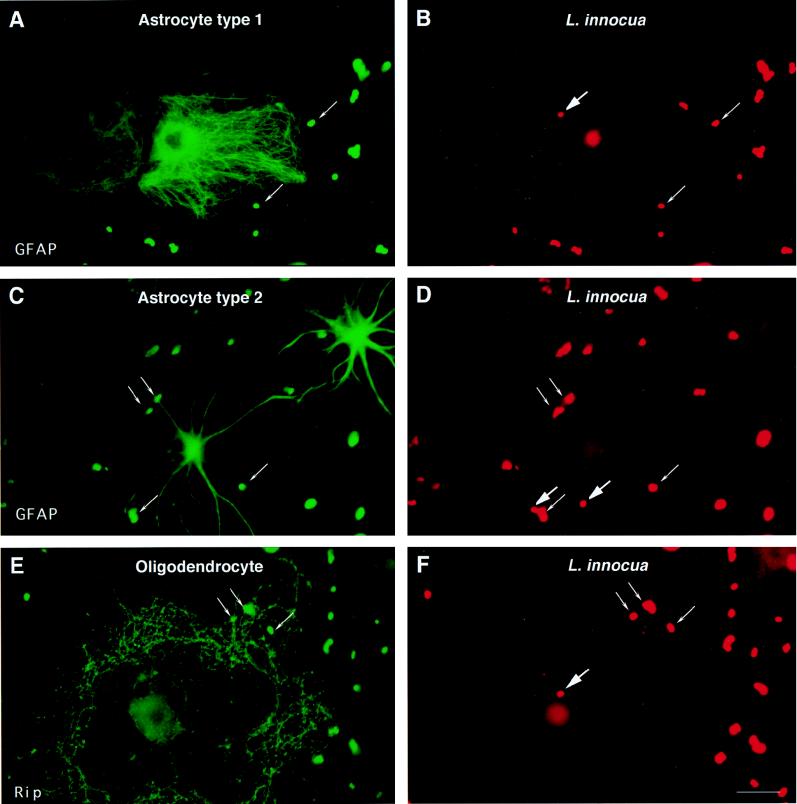
The next series of experiments tested whether entry of L. monocytogenes into glial cells was restricted to pathogenic Listeria species. We analyzed the entry of L. innocua, a nonpathogenic and noninvasive bacterium, into glial cells. L. innocua was found to enter into glial cells as efficiently as L. monocytogenes (Table 1). Examples of intracellular L. innocua in astrocytes and oligodendrocytes are illustrated in Fig. 2A through D and 2E and F, respectively. The average number of intracellular bacteria per astrocyte over 224 cells examined was 0.15 ± 0.1. We noticed that infection with L. innocua had less deleterious effects on glial cells than that with L. monocytogenes, particularly on the integrity of oligodendrocyte processes, a property which is probably due to the absence of listeriolysin O production.
TABLE 1.
Rates of infection of different neuronal cells with L. monocytogenes and L. innocua as determined by differential immunofluorescence labeling
| Cell type | % of cells associated with bacteria
|
No. of intracellular bacteria per cell
|
||
|---|---|---|---|---|
| L. monocytogenes | L. innocua | L. monocytogenes | L. innocua | |
| Microglia | 85 ± 10 (55)a | 80 ± 11 (35) | >20 | >20 |
| Astrocytes | 40 ± 26 (339) | 25 ± 15 (224) | 0.5 ± 0.2 | 0.15 ± 0.1 |
| Oligodendrocytes | NDb | 10 (55) | ND | 0.06 |
| Neurons | 7 ± 3 (500) | ND | 0.002 | ND |
Numbers in parentheses are total numbers of cells observed for each cell type in three independent experiments.
ND, not determined.
FIG. 2.
Entry of L. innocua into glial cell cultures. Details of this infection experiment and immunofluorescence analysis are the same as those described in the legend for Fig. 1 except that L. innocua, a noninvasive and nonpathogenic species of the genus Listeria, was used.
L. monocytogenes can move into glial cells and spread between different types of glial cells.
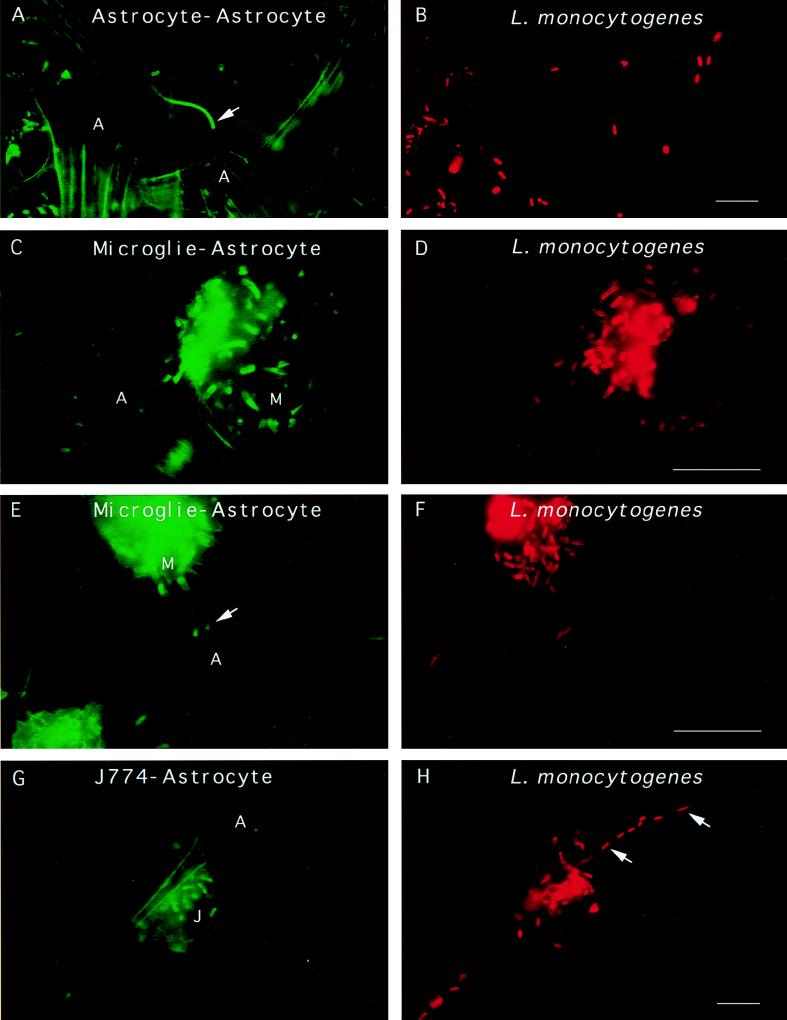
As seen in many other cell types, fluorescence staining for F-actin of infected glial cells showed the presence of typical actin comet tails associated with L. monocytogenes. Actin tails were seen in both astrocytes and microglial cells (Fig. 3A and E). Interestingly, some L. monocytogenes cells were seen extending outward from an infected glial cell to a noninfected cell, suggesting that L. monocytogenes can move between glial cells, from astrocyte to astrocyte (Fig. 3A), and from microglial cell to astrocyte (Fig. 3C and E). Moreover, cocultivation of noninfected glial cells with murine J774 macrophages previously infected with L. monocytogenes showed the passage of L. monocytogenes from macrophages of external origin to astrocytes by cell-to-cell spread (Fig. 3G and H). In this case, infection of glial cells certainly occurs by cell-to-cell spread since cocultivation was performed in a culture medium containing gentamicin, an antibiotic belonging to the family of aminoglycosides that kills extracellular Listeria.
FIG. 3.
L. monocytogenes can move and spread from cell to cell in glial cell cultures. Glial cells were incubated with L. monocytogenes for 40 min, unbound bacteria were washed away, and the cells were resuspended in medium containing gentamicin and incubated for 19 h. The cells were fixed and stained for F-actin with FITC phalloidin. The bacteria were revealed by the technique of differential immunostaining to distinguish between extracellular (green and red) and intracellular (only red) bacteria. Panels A, C, and E show three different fields in which Listeria cells are clearly seen projecting away from astrocytes or microglial cells. Typical Listeria actin tails are indicated by arrows. Panels B, D, and F show the labeling of the bacteria in red. (G and H) J774 macrophages previously infected with L. monocytogenes were cultured with primary glial cell cultures for 19 h in a cell culture medium containing gentamicin to kill extracellular listeriae. The cells were fixed and stained for F-actin, and the presence of intracellular bacteria was determined by differential immunofluorescence. Panel G shows an infected macrophage near an astrocyte. Panel H shows the presence of intracellular L. monocytogenes in an astrocyte (thick arrows). Scale bar, 5 μm.
Direct entry of L. monocytogenes into neurons is a rare event.
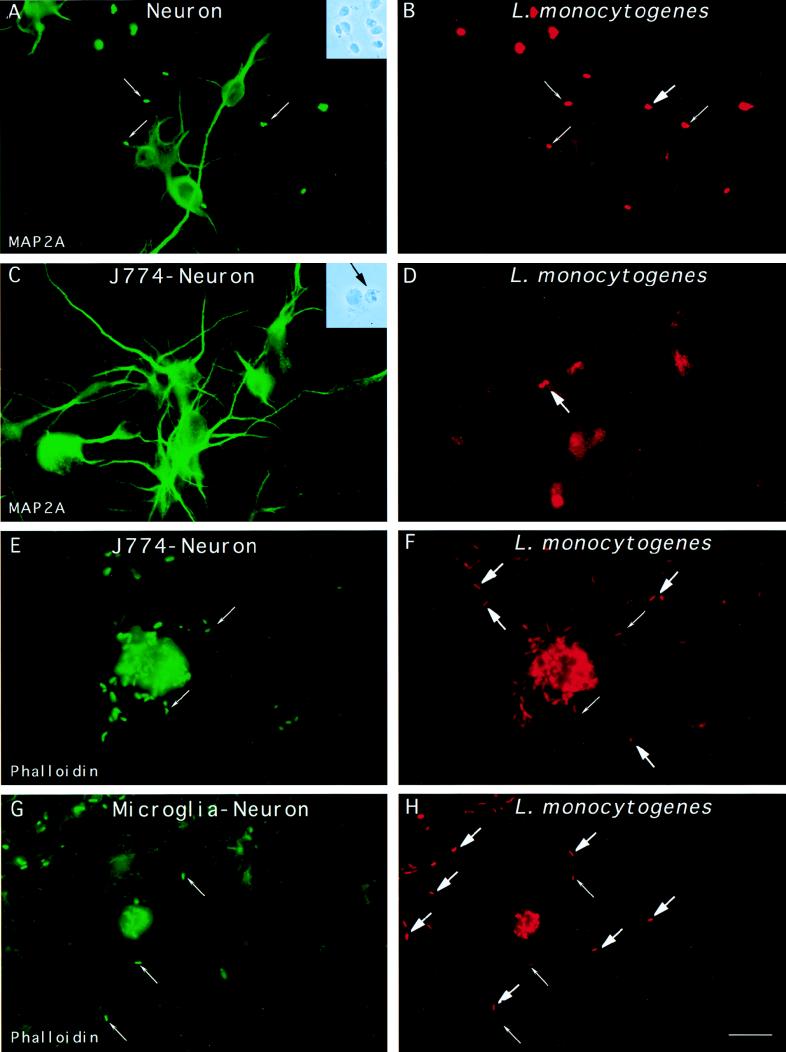
Observation of L. monocytogenes in neurons during natural infection of ruminants (16) as well as in experimental mouse listeriosis (25) led us to test the entry of L. monocytogenes into cultured neurons. Infection of rat spinal cultures of neurons with L. monocytogenes showed that entry into these cells was a very rare event (Table 1). Analysis of approximately 500 neurons presenting associated bacteria by differential immunofluorescence revealed only one intracellular L. monocytogenes cell in the soma of a neuron (Fig. 4A and B). In addition, infection of neurons with L. monocytogenes, in contrast to infection with L. innocua, often had deleterious effects on the integrity of neurites. Similar results were obtained with three clinical strains of L. monocytogenes (CIP 71408, 71456, and 71468) isolated from patients with CNS infections (data not shown).
FIG. 4.
Entry of L. monocytogenes into cultured neurons. (A and B) Neurons were incubated with L. monocytogenes for 40 min, unbound bacteria were washed away, and the cells were resuspended in medium containing gentamicin and incubated for 2 h. The cells were fixed and stained with a MAb raised against MAP-2A (panel A) and against the bacterial pathogen. Extracellular bacteria are indicated by thin arrows and are both green and red, whereas intracellular bacteria indicated by thick arrows are only red. The unique intracellular bacterium that we found in neurons is shown in panel B. Insets: phase-contrast microscopy of primary cultures of neurons infected with L. monocytogenes (A) and of neuron-infected J774 macrophage cocultures (B). The black arrow shows a macrophage adjacent to a neuron. (C through F) J774 macrophages previously infected with L. monocytogenes were cultured with neurons for 19 h in tissue culture medium containing gentamicin. The cells were fixed and stained with a MAb raised against MAP-2A (panel C) or stained for F-actin (panel E), and the presence of intracellular bacteria was examined by differential immunofluorescence. Panel C shows neurons. Panel D shows the presence of two intracellular L. monocytogenes in a neuron. Panel E shows an infected macrophage and the actin network of the neurons. Panel F shows the presence of intracellular L. monocytogenes cells in neurons close to the infected macrophage. (G and H) Cultured neurons were infected with L. monocytogenes for 40 min, washed, and incubated for 15 h in L15 complete medium containing gentamicin. Cells were fixed and stained for F-actin, and the presence of intracellular bacteria was determined by differential immunofluorescence. Panel G shows a microglial cell in the neuronal monolayers. In the neuronal processes located in the vicinity of the infected microglial cell, intracellular bacteria, indicated by thick arrows in panel H, were detected. Scale bar, 10 μm.
L. monocytogenes can efficiently enter neurons by cell-to-cell spread.
The next series of experiments tested whether neurons could be infected by cell-to-cell spread from L. monocytogenes- infected phagocytes. For this purpose, we infected J774 macrophages with L. monocytogenes and then incubated the infected J774 cells with neurons in a cell culture medium containing gentamicin. After 15 h of cocultivation, intracellular Listeria cells were found in neurons distant from J774 cells, and bacteria had infected most neurons by cell-to-cell spread (Fig. 4C, D, E, and F). As expected, an L. monocytogenes actA− strain unable to polymerize cellular actin, when used to infect J774 cells, was unable to spread from cell to cell and could not invade neurons (data not shown). These data indicate that L. monocytogenes, when phagocytosed by J774 macrophages, can invade primary cultured neurons as a consequence of cell-to-cell spread. Interestingly, in one neuronal culture, we found intracellular L. monocytogenes in neurons near infected microglial cells, suggesting that cell-to-cell spread from infected microglial cells to neurons can occur in vivo (Fig. 4G and H).
DISCUSSION
In the present study, the entry of L. monocytogenes into rat spinal cell cultures was examined by using antibodies against cell-type-specific markers and against the bacteria. Our results showed that L. monocytogenes, as well as the closely related noninvasive species L. innocua, can invade glial cells. Microglial cells were filled with both L. monocytogenes and L. innocua. Since serum-free medium was used during the infection process, the possibility of a complement-mediated phagocytosis can be excluded. This finding shows the highly efficient phagocytic activity of microglial cells. Both types of GFAP-positive astrocytes (type 1 or protoplasmic and type 2 or fibrous) were also infected by L. monocytogenes and L. innocua, albeit to a lesser extent, suggesting that invasion genes of L. monocytogenes are not required for entry into glial cells. The fact that L. innocua, a nonpathogenic species, invades astrocytes is not very surprising since astrocytes were shown to display phagocytic activity in situations where phagocytes are not numerous, such as in primary brain cell cultures (15). Invasion of oligodendrocytes was demonstrated but was difficult to evaluate since infection with L. monocytogenes had cytotoxic effects. Clearly, and in contrast to the results with glial cells, entry of L. monocytogenes into cultured neurons was a rare event. Even clinical strains of L. monocytogenes isolated from patients with neuromeningeal listeriosis did not enter into cultured neuronal cells. These results in rat spinal cord cultures are in perfect agreement with those reported in primary mouse fetal brain cultures (20). Indeed, Peters and Hewicker-Trautwein previously showed that L. monocytogenes was primarily associated with microglial cells, while astrocytes, oligodendrocytes, and fibronectin-expressing cells were infected to a lesser extent (20). However, in that study, the authors could not clearly discriminate between adherent bacteria and intracellular bacteria. Peters and Hewicker-Trautwein also found that cultured neurons were not permissive cells to L. monocytogenes invasion. Taken together, these results suggest that L. monocytogenes does not invade cultured neurons. We cannot, however, exclude the possibility that L. monocytogenes infects a very small subset of neurons which are not present in our culture conditions.
Since it has been shown that L. monocytogenes invades human umbilical vein endothelial cells by cell-to-cell spread from mononuclear phagocytes infected by this bacterium (10), we tested whether infected phagocytes were also efficient vectors by which L. monocytogenes could enter neurons. Our results clearly indicate that entry of L. monocytogenes into cultured neurons can occur by cell-to-cell spread from infected phagocytes (J774 macrophages or microglial cells). This Trojan horse mechanism of traveling to and infecting the CNS has also been proposed for visna virus and human immunodeficiency virus (18, 19). In the macrophage-neuron cocultures, actin assembly around intracellular L. monocytogenes was detected in both macrophages and neurons, although actin comet tails were only seen in macrophages. The presence of actin tails is the visualization of intracellular movement. Whether the lack of actin tails in neurons represents a real absence of movement or is an artifact of the culture conditions has not been elucidated. Another intriguing observation was that intracellular L. monocytogenes cells were located in the soma of spinal neurons, a surprising result since intra-axonal movement of L. monocytogenes has been suspected in spontaneous listerial encephalitis of small ruminants (1, 6, 7, 16). It will be of interest to elucidate whether Listeria can move intra-axonally.
Bacterial encephalitis is rare because the brain is well protected from external aggressions by the presence of the blood-brain barrier (BBB). This structure is interposed between the circulatory system and the CNS and is relatively impermeable to ions, amino acids, small peptides, and proteins. In vertebrates, the BBB exists at the level of endothelial cells that make up the brain capillaries (17). These cells form high-resistance tight junctions and exhibit low rates of paracellular leakage and pinocytosis. Close examination of brain capillaries shows that astrocytic endfeet are in close proximity to the endothelial cell plasma membrane and are separated only by the basal lamina (23). Development of in vitro models for the BBB (9, 24) revealed that astrocytes participate in the formation of tight junctions between endothelial cells. How L. monocytogenes crosses the BBB and invades the CNS is unknown. The bacteria may adhere to and directly enter the endothelial cells as described for Nocardia asteroides (2). Alternatively, L. monocytogenes may cross the endothelia by diapedesis or by cell-to-cell spread, being carried by infected blood monocytes. Alternatively and/or simultaneously, the endothelium may induce the secretion of inflammatory mediators and the expression of adhesion molecules that enhance adherence of infected phagocytic cells (11, 26). The results presented in this study suggest that astrocytes may be among the cell targets that carry Listeria to the brain across the BBB.
In certain areas of the brain, however, endothelial cells do not form tight junctions and allow a free exchange of molecules between the blood and adjacent cells. For example, at the level of the choroid plexus, which consists of epithelial cells that produce the cerebrospinal fluid, the barrier is constituted by the tight junctions between epithelial cells which separate the blood from the cerebrospinal fluid. Ultrastructural studies of infected brains in murine experimental listeriosis have shown that L. monocytogenes can be found within the epithelial cells of the choroid plexus, within ependymal cells, and within periventricular neurons (25). Thus, we cannot exclude the possibility that L. monocytogenes, like Streptococcus suis, enters the CNS via the cerebrospinal fluid spaces.
To date, experimental evidence together with the in vivo data does not exclude the possibility that the mechanism used by L. monocytogenes involves a combination of the different pathways mentioned above, which could act cooperatively or separately.
ACKNOWLEDGMENTS
We are indebted to E. Gouin for the production of the rabbit polyclonal antibodies against L. monocytogenes (R11) and L. innocua (R6). We wish to thank B. Zalc for the gift of antibodies against myelin protein Rip and B. Reiderer for providing us with antibodies against MAP-2A.
REFERENCES
- 1.Barlow R M, McGorum B. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Vet Rec. 1985;116:233–236. doi: 10.1136/vr.116.9.233. [DOI] [PubMed] [Google Scholar]
- 2.Beaman B L, Ogata S A. Ultrastructural analysis of attachment to and penetration of capillaries in the murine pons, midbrain, thalamus, and hypothalamus by Nocardia asteroides. Infect Immun. 1993;61:955–965. doi: 10.1128/iai.61.3.955-965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 4.Binder L I, Frankfurter A, Kim H, Caceres A, Payne M R, Rebhun L I. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proc Natl Acad Sci USA. 1984;81:5613–5617. doi: 10.1073/pnas.81.17.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camu W, Bloch-Gallego E, Henderson C E. Purification of spinal motoneurons from chicken and rat embryos by immunospanning. Neuroprotocols. 1993;2:191–199. [Google Scholar]
- 6.Charlton K M. Spontaneous listeric encephalitis in sheep. Electron microscopic studies. Vet Pathol. 1977;14:429–434. doi: 10.1177/030098587701400501. [DOI] [PubMed] [Google Scholar]
- 7.Charlton K M, Garcia M M. Spontaneous listeric encephalitis and neuritis in sheep. Light microscopic studies. Vet Pathol. 1977;14:297–313. doi: 10.1177/030098587701400401. [DOI] [PubMed] [Google Scholar]
- 8.Colin I, Rostaing P, Triller A. Gephyrin accumulates at specific plasmalemma loci during neuronal maturation in vitro. J Comp Neurol. 1996;374:467–479. doi: 10.1002/(SICI)1096-9861(19961021)374:3<467::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Dehouck M P, Méresse S, Delorme P, Fruchart J C, Cecchelli R. An easier, reproducible, and mass production method to study the blood-brain barrier. J Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 10.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frei K, Nadal D, Pfister H-W, Fontana A. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J Exp Med. 1993;178:1255–1261. doi: 10.1084/jem.178.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman B, Hockfield S, Black J, Woodruff K, Waxman S. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, rip. Glia. 1989;2:380–390. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- 13.Heesemann J, Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985;22:168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicola M A, Becker C M, Triller A. Development of glycine receptor alpha subunit in cultivated rat spinal neurons: an immunocytochemical study. Neurosci Lett. 1992;138:173–178. doi: 10.1016/0304-3940(92)90499-w. [DOI] [PubMed] [Google Scholar]
- 15.Noske W, Lentzen H, Lange K, Keller K. Phagocytic activity of glial cells in culture. Exp Cell Res. 1982;142:437–445. doi: 10.1016/0014-4827(82)90385-8. [DOI] [PubMed] [Google Scholar]
- 16.Otter A, Blakemore W F. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiol Hung. 1989;36:125–131. [PubMed] [Google Scholar]
- 17.Pardridge W M. The blood-brain barrier: cellular and molecular biology. New York, N.Y: Raven Press; 1993. [Google Scholar]
- 18.Peluso R, Haase A, Strowring L, Edwards M, Venturs P. A Trojan horse mechanism for the spread of Visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- 19.Perry V H, Lawson L J, Reid D M. Biology of the mononuclear phagocyte system of the central nervous system and HIV infection. J Leukocyte Biol. 1994;56:399–406. doi: 10.1002/jlb.56.3.399. [DOI] [PubMed] [Google Scholar]
- 20.Peters M, Hewicker-Trautwein M. Infection of murine fetal brain cell cultures with Listeria monocytogenes. Vet Microbiol. 1994;41:19–28. doi: 10.1016/0378-1135(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 21.Pollock S S, Pollock T M, Harrison M J G. Infection of the central nervous system by Listeria monocytogenes: a review of 54 adult and juvenile cases. Q J Med. 1984;211:331–340. [PubMed] [Google Scholar]
- 22.Prats N, Briones V, Blanco M M, Altimira J, Ramos J A, Dominguez L, Marco A. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1992;11:744–747. doi: 10.1007/BF01989983. [DOI] [PubMed] [Google Scholar]
- 23.Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- 24.Rubin L L, Hall D E, Porter S, Barbu K, Cannon C, Horner H C, Janatpour M, Liaw C W, Manning K, Morales J, Tanner L I, Tomaselli K J, Bard F. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlüter D, Chahoud S, Lassmann H, Schumann A, Hof H, Deckert-Schlüter M. Intracerebral targets and immunomodulation of murine Listeria monocytogenes meningoencephalitis. J Neuropathol Exp Neurol. 1996;55:14–24. doi: 10.1097/00005072-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Seebach J, Bartholdi D, Frei K, Spanaus K-S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H-W, Fontana A. Experimental Listeria meningoencephalitis. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 27.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A, Mengaud J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 28.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]