Abstract
BACKGROUND
Both targeted decolonization and universal decolonization of patients in intensive care units (ICUs) are candidate strategies to prevent health care–associated infections, particularly those caused by methicillin-resistant Staphylococcus aureus (MRSA).
METHODS
We conducted a pragmatic, cluster-randomized trial. Hospitals were randomly assigned to one of three strategies, with all adult ICUs in a given hospital assigned to the same strategy. Group 1 implemented MRSA screening and isolation; group 2, targeted decolonization (i.e., screening, isolation, and decolonization of MRSA carriers); and group 3, universal decolonization (i.e., no screening, and decolonization of all patients). Proportional-hazards models were used to assess differences in infection reductions across the study groups, with clustering according to hospital.
RESULTS
A total of 43 hospitals (including 74 ICUs and 74,256 patients during the intervention period) underwent randomization. In the intervention period versus the baseline period, modeled hazard ratios for MRSA clinical isolates were 0.92 for screening and isolation (crude rate, 3.2 vs. 3.4 isolates per 1000 days), 0.75 for targeted decolonization (3.2 vs. 4.3 isolates per 1000 days), and 0.63 for universal decolonization (2.1 vs. 3.4 isolates per 1000 days) (P = 0.01 for test of all groups being equal). In the intervention versus baseline periods, hazard ratios for bloodstream infection with any pathogen in the three groups were 0.99 (crude rate, 4.1 vs. 4.2 infections per 1000 days), 0.78 (3.7 vs. 4.8 infections per 1000 days), and 0.56 (3.6 vs. 6.1 infections per 1000 days), respectively (P<0.001 for test of all groups being equal). Universal decolonization resulted in a significantly greater reduction in the rate of all bloodstream infections than either targeted decolonization or screening and isolation. One bloodstream infection was prevented per 99 patients who underwent decolonization. The reductions in rates of MRSA bloodstream infection were similar to those of all bloodstream infections, but the difference was not significant. Adverse events, which occurred in 7 patients, were mild and related to chlorhexidine.
CONCLUSIONS
In routine ICU practice, universal decolonization was more effective than targeted decolonization or screening and isolation in reducing rates of MRSA clinical isolates and bloodstream infection from any pathogen. (Funded by the Agency for Healthcare Research and the Centers for Disease Control and Prevention; REDUCE MRSA ClinicalTrials.gov number, NCT00980980.)
Health care–associated infection is a leading cause of preventable illness and death and often results from colonizing bacteria that overcome body defenses.1–5 Among the pathogens causing health care–associated infection, methicillin-resistant Staphylococcus aureus (MRSA) has been given priority as a target of reduction efforts because of its virulence and disease spectrum, multidrug-resistant profile, and increasing prevalence in health care settings, particularly among patients in the intensive care unit (ICU). Hospitals commonly screen patients in the ICU for nasal carriage of MRSA and use contact precautions with carriers.2–6 Nine states mandate such screening.7
Decolonization has been used to reduce transmission and prevent disease in S. aureus carriers, primarily carriers of methicillin-resistant strains but also carriers of methicillin-sensitive ones.8,9 S. aureus, including both methicillin-resistant and methicillin-susceptible strains, accounts for more health care–associated infections than any other pathogen.4 It is the most common cause of ventilator-associated pneumonia and surgical-site infection and the second most common cause of central-catheter–associated bloodstream infection.4 Decolonization commonly involves a multiday regimen of intranasal mupirocin and chlorhexidine bathing.
There is debate about whether decolonization should be used and, if so, whether to target high-risk pathogens or patient populations that are susceptible to infection from many pathogens.10 In particular, the broad antimicrobial activity of chlorhexidine makes it attractive for preventing health care–associated infection from many pathogens.11–14 Several studies have shown that daily chlorhexidine bathing of all patients in the ICU can reduce MRSA acquisition, the concentration of bacteria on the body surface, and bloodstream infection from all pathogens.11–14 A comparative-effectiveness trial is needed to determine what type of decolonization strategy works best to reduce MRSA and other pathogens in ICUs.15 In addition, it is important to know whether decolonization can be effective in routine ICU care. We conducted a cluster-randomized, pragmatic, comparative-effectiveness trial in adult ICUs to compare targeted and universal decolonization with one another and with MRSA screening and contact precautions alone.
METHODS
STUDY DESIGN
We designed the Randomized Evaluation of Decolonization versus Universal Clearance to Eliminate MRSA (REDUCE MRSA) trial, a three-group, cluster-randomized trial, to compare strategies for preventing MRSA clinical isolates and infections in adult ICUs in Hospital Corporation of America (HCA) hospitals. The trial design has been described previously,15 and the protocols are available with the full text of this article at NEJM.org. The training materials are provided in the Supplementary Appendix, available at NEJM.org. All the authors vouch for the accuracy of the reported data and the fidelity of the study to the protocol. There was a 12-month baseline period from January 1 through December 31, 2009; a phase-in period from January 1 through April 7, 2010; and an 18-month intervention period from April 8, 2010, through September 30, 2011.
The three strategy groups were defined as follows. In group 1 (screening and isolation), bilateral screening of the nares for MRSA was performed on ICU admission, and contact precautions were implemented for patients with a history of MRSA colonization or infection and for those who had any positive MRSA test. This was the previous standard of care in all hospitals. The MRSA screening program for patients in the ICU, who are a group at high risk for infection, began in 2007 at HCA hospitals.16 More than 90% of the patients admitted to the ICU underwent screening, and contact precautions were implemented for carriers of MRSA and other multidrug-resistant pathogens.
In group 2 (targeted decolonization), MRSA screening and contact precautions were similar to those in group 1. Patients known to have MRSA colonization or infection underwent a 5-day decolonization regimen consisting of twice-daily intranasal mupirocin and daily bathing with chlorhexidine-impregnated cloths.
In group 3 (universal decolonization), there was no screening for MRSA on admission to the ICU. Contact precautions were similar to those in group 1. All patients received twice-daily intranasal mupirocin for 5 days, plus daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay.
All adult ICUs in a participating hospital were assigned to the same study group. Contact-precaution policies, which were based on longstanding guidance from the Centers for Disease Control and Prevention (CDC), were identical and unchanged for all hospitals. Precautions were initiated on the basis of current or historical MRSA cultures or other standard indications.6 Results of cultures obtained on admission became available the next day.
STUDY OUTCOMES
The primary outcome was ICU-attributable, MRSA-positive clinical cultures. Screening tests were excluded from all analyses because hospitals implementing universal decolonization discontinued such cultures. Secondary outcomes included ICU-attributable bloodstream infection caused by MRSA and ICU-attributable bloodstream infection caused by any pathogen. Clinical cultures were obtained at the clinician’s discretion.
RECRUITMENT AND ELIGIBILITY CRITERIA
Recruitment occurred among the 160 HCA hospitals. Most were community hospitals with single-occupancy ICU rooms. Eligibility criteria included commitment by the hospital administration to have the hospital undergo randomization for the trial, less than 30% of patients in participating adult ICUs receiving either chlorhexidine bathing or intranasal mupirocin at baseline, stable use of infection-prevention initiatives and products during the baseline period, and agreement to refrain from adopting new initiatives that would conflict with the trial. Throughout the study, corporate-wide campaigns were used to ensure compliance with national practice guidelines.16–18
Each hospital obtained approval from an institutional review board, with more than 90% of the hospitals delegating review to the Harvard Pilgrim Health Care institutional review board. Patient notices about group-specific protocols were posted in each ICU room. The requirement for written informed consent was waived.19
RANDOMIZATION
Randomization was stratified to optimize balance in patient volume and baseline prevalence of MRSA carriage on the basis of clinical cultures and screening tests from July 2008 through June 2009. Hospitals were ranked according to ICU volume and were grouped into sets of six. Within each set, we ordered the hospitals according to the prevalence of MRSA carriage in the ICU. Each group of three consecutive hospitals was randomly assigned, one to each strategy group, with the use of block randomization. Hospitals in states with legislative mandates for MRSA screening in the ICU were similarly and separately randomly assigned to group 1 or 2.
IMPLEMENTATION
On-site activities were implemented by hospital personnel responsible for quality-improvement initiatives, including ICU directors, infection preventionists, and nurse educators. Standard communication channels were used, including group-specific, computer-based training modules and daily electronic documentation by nursing staff for all groups. On-site training in bathing with chlorhexidine-impregnated cloths was provided to hospitals assigned to a decolonization regimen (i.e., group 2 or 3). Nursing directors performed at least three quarterly observations of bathing, including questioning staff about protocol details.
Investigators hosted group-specific coaching teleconferences at least monthly to discuss implementation, compliance, and any new, potentially conflicting initiatives. Compliance assessment involved verification on 1 day per week for each ICU. HCA leadership evaluated trial processes during routine hospital visits. Additional site visits were made at the request of the hospital or if compliance was found to be low.
Intranasal mupirocin ointment 2% (Bactroban, GlaxoSmithKline) and 2% chlorhexidine–impregnated cloths (Sage Products) were used for decolonization. All mupirocin and chlorhexidine-impregnated cloths were purchased at their usual cost by the participating hospitals. In groups 2 and 3, bathing products and products used for wound prophylaxis that were incompatible with chlorhexidine were replaced with compatible products. Adverse events were managed by treating physicians.
DATA COLLECTION AND OUTCOME ASSIGNMENT
Census (i.e., the unit location of each patient for every hospitalization day), microbiologic, pharmacy, supply-chain, nursing-query, and administrative data were obtained from corporate data warehouses, which undergo line-item validation until 99% accuracy is achieved. CDC criteria were used for microbiologic outcomes (first outcome per patient). Pathogens were attributed to an ICU if the collection date occurred during the period from the third day after ICU admission through the second day after ICU discharge. For bloodstream infections to be attributed to skin-commensal organisms, the same organism had to be isolated from two or more blood cultures obtained within 2 calendar days of one another.20
STATISTICAL ANALYSIS
We powered the trial on the basis of the rarest outcome, MRSA bloodstream infection. The study was designed to have 80% power to detect a 40% relative reduction in the rate of MRSA bloodstream infection in group 2, and a 60% relative reduction in the rate in group 3, as compared with group 1. The primary analyses were conducted according to the intention-to-treat principle (as-assigned analyses) and were unadjusted. Proportional-hazards models with shared frailties accounted for clustering within hospitals (see the Supplementary Appendix).21,22 The intervention effect was assessed on the basis of the interaction between group and study period, reflecting the difference in hazard between the baseline and intervention periods among the groups. Data from the phase-in period were excluded from all analyses. When the null hypothesis of equal changes across the groups was rejected, we examined pairwise comparisons.
Sensitivity analyses included multivariable covariate-adjusted models, as-treated models, models that excluded hospitals in states mandating MRSA screening in the ICU, models that accounted for assigned randomization strata, and models that excluded the small numbers of medical-only and surgical-only ICUs. Adjusted models accounted for age, sex, race, insurance type, coexisting conditions (defined with the use of codes from the International Classification of Diseases, 9th Revision), and surgery during the hospital stay. Analyses were performed with the use of SAS software, version 9.3 (SAS Institute).
RESULTS
STUDY PARTICIPANTS
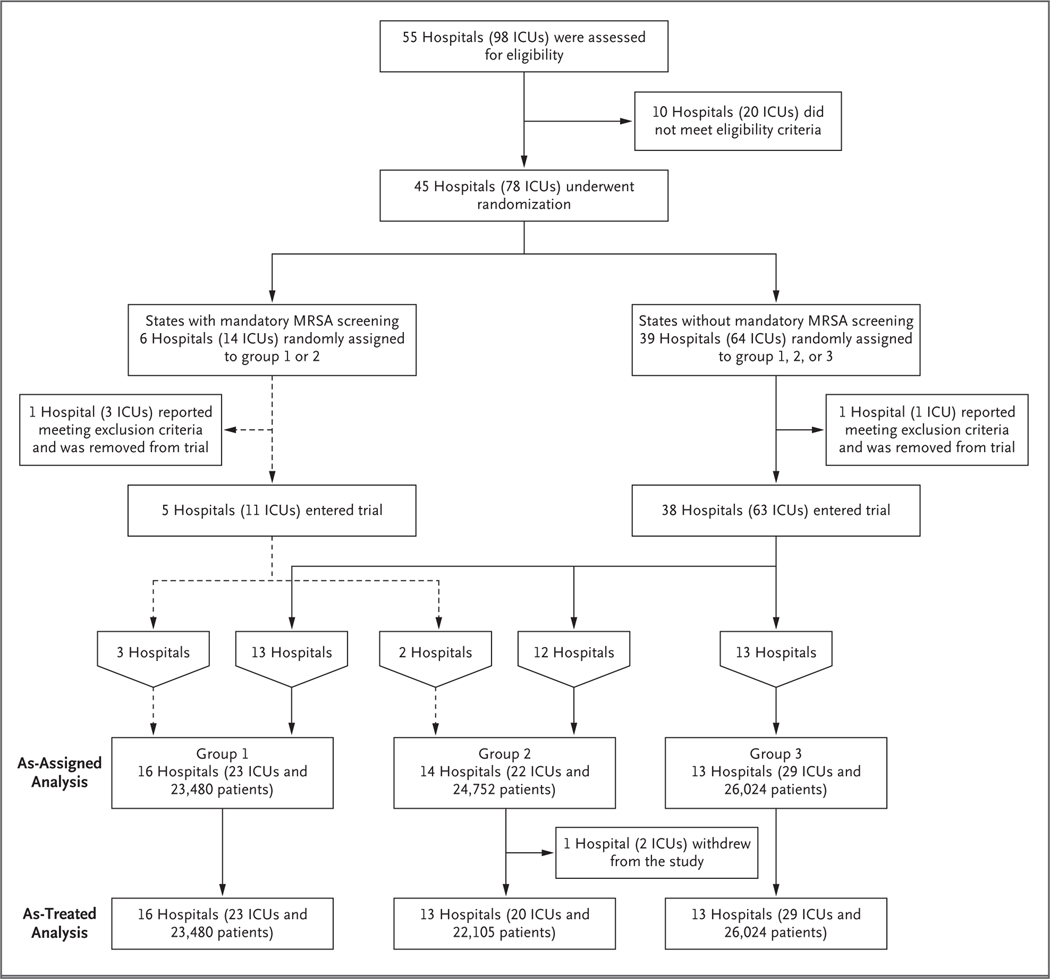
A total of 45 hospitals in 16 states underwent randomization (Fig. 1). A total of 43 (comprising 74 ICUs) implemented the assigned intervention; 2 hospitals that underwent randomization were excluded from all analyses because preexisting exclusion criteria were discovered before the intervention started. One hospital in group 2 (assigned to targeted decolonization) withdrew after the intervention started and was included in the as-assigned analyses but not in the as-treated analyses.
Figure 1. Recruitment, Randomization, and Inclusion in As-Assigned and As-Treated Analyses.
A total of 45 hospitals in 16 states were randomly assigned to a study group, with 43 (comprising 74 ICUs) beginning the assigned intervention; 2 hospitals were excluded from all analyses because preexisting exclusion criteria were discovered before the intervention started. One hospital in group 2 (assigned to targeted decolonization) withdrew after the intervention started and was included in the as-assigned analyses but not the as-treated analyses. The numbers of patients shown in each group are the numbers from the intervention period.
Patient characteristics were similar across groups and between the baseline and intervention periods (Table 1). There was excellent separation of interventions across groups. In group 1, less than 1.0% of patients (range for hospitals in group, 0 to 2.1%) received mupirocin or chlorhexidine. In group 2, a total of 90.8% of MRSA carriers (range for hospitals in group, 56.5 to 100%) received mupirocin and 88.8% (range for hospitals in group, 54.2 to 98.4%) received chlorhexidine. In group 3, a total of 86.1% of patients (range for hospitals in group, 41.0 to 99.1%) received mupirocin and 80.8% (range for hospitals in group, 53.1 to 98.6%) received chlorhexidine.
Table 1.
Characteristics of the Intensive Care Unit (ICU) Population, According to Study Period and Group.*
| Variable | 12-Mo Baseline Period (N = 48,390) | 18-Mo Intervention Period (N = 74,256) | ||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |
| Admission with ICU stay (no.) | 15,816 | 15,218 | 17,356 | 23,480 | 24,752 | 26,024 |
| Attributable ICU patient-days (no.) | 63,135 | 57,418 | 69,668 | 88,222 | 92,978 | 101,603 |
| ICU type (no.)† | ||||||
| Medical | 3 | 5 | 5 | 3 | 5 | 5 |
| Surgical | 1 | 2 | 6 | 1 | 2 | 6 |
| Mixed medical and surgical | 19 | 14 | 18 | 19 | 15 | 17 |
| Hospital stay (days) | ||||||
| Median | 7 | 7 | 8 | 7 | 7 | 7 |
| Interquartile range | 5–12 | 5–12 | 5–12 | 5–12 | 5–12 | 5–12 |
| ICU stay (days) | ||||||
| Median | 3 | 3 | 3 | 3 | 3 | 3 |
| Interquartile range | 2–5 | 2–5 | 2–5 | 1–5 | 2–5 | 2–5 |
| Age (yr) | ||||||
| Median | 65 | 66 | 65 | 65 | 66 | 65 |
| Interquartile range | 52–77 | 53–77 | 51–77 | 52–77 | 53–77 | 52–77 |
| Female sex (%)‡ | 47.2 | 47.2 | 47.9 | 47.6 | 47.2 | 47.5 |
| Nonwhite race (%)§ | 25.9 | 22.1 | 30.8 | 25.9 | 23.5 | 31.7 |
| Coexisting condition (%) | ||||||
| Diabetes | 31.3 | 33.0 | 30.7 | 31.8 | 32.7 | 31.5 |
| Renal failure | 20.0 | 20.4 | 19.0 | 20.3 | 22.2 | 19.7 |
| Cancer | 10.4 | 10.8 | 14.1 | 9.9 | 10.8 | 13.0 |
| Liver failure | 3.4 | 4.4 | 3.9 | 4.0 | 4.1 | 4.2 |
| History of MRSA infection (%)¶ | 10.2 | 11.5 | 10.6 | 9.7 | 11.1 | 3.9 |
| Surgery during hospitalization (%) | 40.5 | 38.6 | 47.5 | 38.7 | 37.7 | 46.2 |
Group 1 implemented methicillin-resistant Staphylococcus aureus (MRSA) screening and isolation; group 2, targeted decolonization (i.e., screening, isolation, and decolonization of MRSA carriers with chlorhexidine and mupirocin); and group 3, universal decolonization (i.e., no screening and all patients underwent decolonization). At baseline, there were no significant between-group differences. For additional details, see the Supplementary Appendix.
Differences in the number of ICUs in the groups between the baseline and intervention periods reflect the fact that one ICU in group 2 opened during the trial and one in group 3 closed.
Data were missing for eight patients.
Race was determined from electronic administrative data at each hospital.
A history of MRSA infection was identified with the use of all available screening and clinical cultures, with the history defined as MRSA carriage documented by the Hospital Corporation of America during the period from 1 year before admission to day 2 of the ICU stay. Data from group 3 during the intervention period are not comparable to data from the other groups because universal decolonization, without screening, was performed for all patients in this group. As the intervention progressed, patients who were readmitted to the ICU were less likely to be identified as MRSA-positive.
Reasons for noncompliance included discharge before scheduled bathing or mupirocin administration, discharge before MRSA-positive results were obtained, moribund state of the patient, length of ICU stay of less than 1 day, and patient’s decision to decline the intervention. MRSA screening occurred in 97.5% of patients (hospital range, 90.6 to 100%) in group 1, in 98.6% (hospital range, 95.6 to 100%) in group 2, and in 0.7% (hospital range, 0 to 4.7%) in group 3. Of the 69 proposed practice changes that occurred at various hospitals during the trial, 36 conflicted with the trial protocol and were not implemented.
OUTCOMES
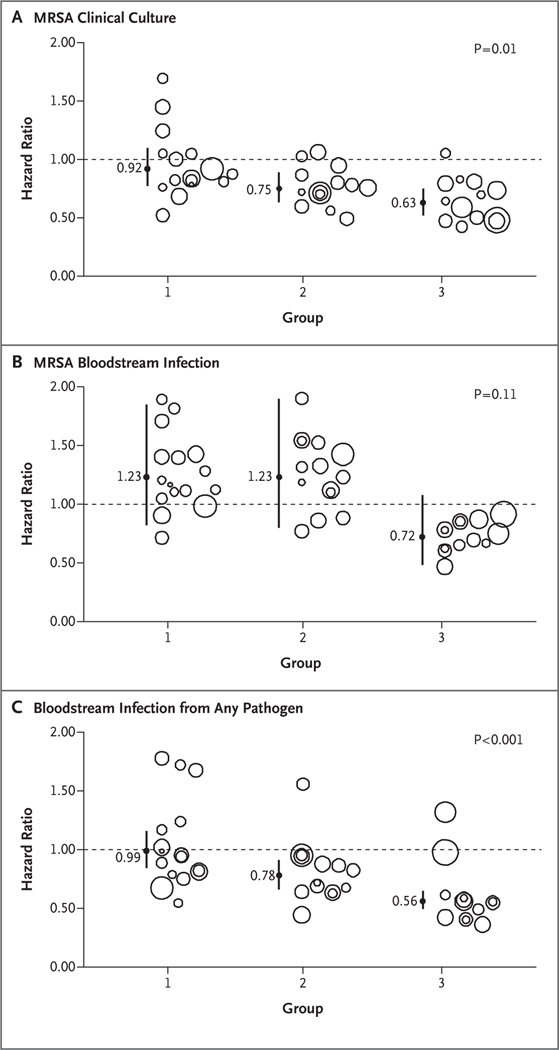
For the primary outcome of ICU-attributable, MRSA-positive clinical cultures in the as-assigned analysis, the relative hazards differed significantly among the groups in a comparison of the intervention period with the baseline period (P = 0.01) (Fig. 2). Pairwise analyses showed that universal decolonization resulted in a significantly greater reduction in the hazard of MRSA-positive clinical cultures than did screening and isolation (hazard ratio in group 3, 0.63; 95% confidence interval [CI], 0.52 to 0.75; hazard ratio in group 1, 0.92; 95% CI, 0.77 to 1.10; P = 0.003 for test of all groups being equal).
Figure 2. Effect of Trial Interventions on Outcomes.
Shown are group-specific hazard ratios and 95% confidence intervals (indicated by vertical lines) for outcomes attributable to the intensive care unit. Results are based on unadjusted proportional-hazards models that accounted for clustering within hospitals. Analyses were based on the as-assigned status of hospitals. Panel A shows hazard ratios for clinical cultures that were positive for methicillin-resistant Staphylococcus aureus (MRSA) infection, Panel B hazard ratios for MRSA bloodstream infection, and Panel C hazard ratios for bloodstream infection from any pathogen. Bubble plots of hazard ratios (predicted random effects or exponentiated frailties) from individual hospitals relative to their group effects are shown. The size of the bubble indicates the relative number of patients contributing data to the trial.
The effects of the strategies on ICU-attributable MRSA bloodstream infection were not significantly different across the study groups (P = 0.11 for test of all groups being equal), although the hazard reduction with universal decolonization was greater than the reductions with the other strategies (hazard ratio, 0.72 [95% CI, 0.48 to 1.08] vs. 1.23 [95% CI, 0.82 to 1.85] for screening and isolation and 1.23 [95% CI, 0.80 to 1.90] for targeted decolonization). For ICU-attributable bloodstream infection from any pathogen, differences among the groups were significant (P<0.001 for test of all groups being equal). In pairwise comparisons, universal decolonization resulted in a significantly greater reduction in the hazard of infection (hazard ratio, 0.56; 95% CI, 0.49 to 0.65) than either screening and isolation (hazard ratio, 0.99; 95% CI, 0.84 to 1.16; P<0.001) or targeted decolonization (hazard ratio, 0.78; 95% CI, 0.66 to 0.91; P = 0.03). We found no significant difference in mortality across the groups, although the trial was inadequately powered to observe even relatively large effects on death.
The effect of targeted decolonization was intermediate between the effects of usual care (i.e., screening and isolation) and universal decolonization for ICU-attributable MRSA cultures and bloodstream infection from any pathogen. Targeted decolonization resulted in significantly lower rates of bloodstream infection from any pathogen than did screening and isolation; other outcomes did not differ significantly between these two groups. Findings in all sensitivity analyses were similar to those in the as-assigned analysis (Table 2).
Table 2.
Hazard Ratios for Primary and Secondary Trial Outcomes.
| Variable | Hazard Ratio (95% CI) | Overall P Value | ||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||
| MRSA | ||||
| Clinical culture | ||||
| As-assigned analysis | ||||
| Unadjusted* | 0.92 (0.77–1.10) | 0.75 (0.63–0.89) | 0.63 (0.52–0.75) | 0.01 |
| Adjusted | 0.92 (0.77–1.10) | 0.74 (0.62–0.88) | 0.64 (0.53–0.77) | 0.02 |
| As-treated analysis, unadjusted | 0.93 (0.78–1.11) | 0.78 (0.65–0.94) | 0.63 (0.52–0.75) | 0.01 |
| Randomization to all three groups, unadjusted analysis† | 0.93 (0.76–1.13) | 0.74 (0.62–0.89) | 0.63 (0.52–0.75) | 0.02 |
| Randomization strata accounted for, unadjusted analysis | 0.93 (0.78–1.11) | 0.75 (0.63–0.89) | 0.63 (0.52–0.75) | 0.01 |
| Mixed medical and surgical ICUs only, unadjusted analysis | 0.93 (0.76–1.12) | 0.71 (0.59–0.86) | 0.57 (0.46–0.71) | 0.004 |
| Bloodstream infection | ||||
| As-assigned analysis | ||||
| Unadjusted | 1.23 (0.82–1.85) | 1.23 (0.80–1.90) | 0.72 (0.48–1.08) | 0.11 |
| Adjusted | 1.20 (0.80–1.81) | 1.19 (0.77–1.84) | 0.74 (0.49–1.12) | 0.18 |
| As-treated analysis, unadjusted | 1.24 (0.82–1.86) | 1.34 (0.84–2.15) | 0.72 (0.48–1.08) | 0.08 |
| Randomization to all three groups, unadjusted analysis† | 1.15 (0.74–1.79) | 1.18 (0.74–1.89) | 0.72 (0.48–1.08) | 0.19 |
| Randomization strata accounted for, unadjusted analysis | 1.24 (0.83–1.86) | 1.22 (0.79–1.88) | 0.73 (0.48–1.09) | 0.12 |
| Mixed medical and surgical ICUs only, unadjusted analysis | 1.15 (0.75–1.77) | 1.20 (0.75–1.93) | 0.72 (0.44–1.20) | 0.28 |
| Bloodstream infection from any pathogen | ||||
| As-assigned analysis | ||||
| Unadjusted‡ | 0.99 (0.84–1.16) | 0.78 (0.66–0.91) | 0.56 (0.49–0.65) | <0.001 |
| Adjusted | 0.98 (0.84–1.15) | 0.77 (0.65–0.90) | 0.55 (0.48–0.64) | <0.001 |
| As-treated analysis, unadjusted | 0.99 (0.84–1.16) | 0.78 (0.66–0.92) | 0.56 (0.49–0.65) | <0.001 |
| Randomization to all three groups, unadjusted analysis† | 0.93 (0.78–1.10) | 0.77 (0.65–0.91) | 0.56 (0.49–0.65) | <0.001 |
| Randomization strata accounted for, unadjusted analysis | 0.99 (0.84–1.16) | 0.78 (0.66–0.91) | 0.56 (0.49–0.65) | <0.001 |
| Mixed medical and surgical ICUs only, unadjusted analysis | 0.96 (0.81–1.13) | 0.80 (0.67–0.96) | 0.59 (0.50–0.69) | <0.001 |
P values in the pairwise analysis were as follows: P = 0.09 for the comparison of group 2 with group 1, P = 0.003 for the comparison of group 3 with group 1, and P = 0.16 for the comparison of group 3 with group 2.
This analysis excluded the five hospitals in states with laws requiring MRSA screening in the ICU.
P values in the pairwise analysis were as follows: P = 0.04 for the comparison of group 2 with group 1, P<0.001 for the comparison of group 3 with group 1, and P = 0.003 for the comparison of group 3 with group 2.
Outcome events and their associated rates are shown in Table 3 and in the Supplementary Appendix. There were no significant between-group differences at baseline (P≥0.30 for all outcomes). The baseline rate of MRSA-positive clinical cultures was higher in group 2 (4.3 per 1000 attributable days) than in the other strategy groups (3.4 per 1000 attributable days in each), but the difference was not significant. At baseline, the rate of bloodstream infections from any pathogen was higher in group 3 (6.1 infections per 1000 attributable days) than in groups 2 and 3 (4.2 and 4.8 infections per 1000 attributable days, respectively), but the difference was not significant (P = 0.87).
Table 3.
Frequency and Rates of Outcomes during the Baseline and Intervention Periods, According to Study Group.*
| Outcome | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | Baseline | Intervention | |
| no. of events (crude rate per 1000 patient-days) | ||||||
| MRSA clinical cultures | 216 (3.4) | 279 (3.2) | 245 (4.3) | 301 (3.2) | 240 (3.4) | 217 (2.1) |
| Bloodstream infection | ||||||
| MRSA | 37 (0.6) | 63 (0.7) | 31 (0.5) | 61 (0.6) | 46 (0.6) | 48 (0.5) |
| Any pathogen† | 265 (4.2) | 360 (4.1) | 273 (4.8) | 341 (3.7) | 412 (6.1) | 356 (3.6) |
| Gram-positive organism | 165 (2.6) | 228 (2.6) | 159 (2.8) | 203 (2.2) | 253 (3.7) | 187 (1.9) |
| Skin commensal organism | 50 (0.8) | 55 (0.6) | 49 (0.9) | 46 (0.5) | 120 (1.8) | 38 (0.4) |
| Noncommensal organism | 115 (1.8) | 173 (2.0) | 110 (1.9) | 157 (1.7) | 133 (2.0) | 149 (1.5) |
| Gram-negative organism | 62 (1.0) | 83 (0.9) | 58 (1.0) | 75 (0.8) | 100 (1.5) | 107 (1.1) |
| Candida species | 38 (0.6) | 49 (0.6) | 56 (1.0) | 63 (0.7) | 59 (0.9) | 62 (0.6) |
Provided rates are crude rates, defined as the number of events per 1000 ICU-attributable patient-days at risk for the event. Patient-days after each event were excluded from the analysis; thus, denominators are different for each cell and are not included.
The distribution of all bloodstream events is based on the first eligible event from any pathogen per patient. For example, a patient with a first ICU-associated bloodstream infection (due to a gram-positive organism) followed by a second ICU-associated bloodstream infection (due to a gram-negative organism) would be counted only in the listing for gram-positive organisms.
By chance, group 3 contained three of the four hospitals that performed bone marrow and solid-organ transplantations. These three hospitals accounted for much of the excess risk in this group, including 72% of the baseline coagulase-negative staphylococcal bloodstream infections (baseline risk of 0.01 events per patient in these three hospitals). The baseline risk per patient in all other hospitals in group 3 (0.004 events) was similar to the baseline risks in all hospitals in groups 1 and 2 (0.003 events in each group). During the intervention period, the risk declined in the three hospitals (0.002) and in all other hospitals implementing universal decolonization (0.0004), as compared with the baseline risks and as compared with the intervention risk for groups 1 and 2 (0.002 in each group). Analyses with adjustment for coexisting conditions such as cancer supported the findings of the as-assigned analyses (Table 2).
ADVERSE EVENTS
There were seven adverse events (two in group 2 and five in group 3) (see the Supplementary Appendix). All involved mild pruritus or rash after chlorhexidine bathing and resolved on discontinuation of the use of chlorhexidine-impregnated cloths.
DISCUSSION
Universal decolonization of patients in the ICU was the most effective strategy, significantly reducing MRSA-positive clinical cultures by 37% and bloodstream infections from any pathogen by 44%. This effect was observed under usual practice conditions in a wide array of hospitals, including community hospitals, that had already implemented national, evidence-based recommendations for preventing health care–associated MRSA infection. A total of 181 patients would need to undergo decolonization to prevent one MRSA-positive clinical culture, and 99 patients would need to undergo decolonization to prevent one bloodstream infection from any pathogen.
Several factors may account for our observation that universal decolonization had a greater preventive effect than the two other strategies. First, chlorhexidine reduces skin colonization by many pathogens, thus protecting patients in the ICU from their own microbiota during a period of heightened vulnerability to infection.11–14 Second, universal decolonization reduces the environmental microbial burden, reducing opportunities for patient-to-patient transmission.14,23 Third, universal decolonization began on the first ICU day, thus avoiding the delay in decolonization pending the results of screening tests.
Another potential benefit of universal decolonization is the elimination of MRSA surveillance tests and the associated reduction in contact precautions, which can interfere with care.24 These findings have implications for legislative mandates requiring MRSA screening in the ICU.25 Nevertheless, there may be occasions when screening is warranted, such as periodic monitoring of resistance. Formal cost-effectiveness analysis is needed to understand whether the observed cessation of screening, reduced contact precautions, and reduced infections offset the product costs and the potential emergence of resistance. It remains to be seen whether universal decolonization can obviate the need for all contact precautions for carriers of MRSA or other multidrug-resistant organisms.
The benefits attributable to universal decolonization are notable for several reasons. First, the large reductions in infections that we observed were achieved over and above the substantial reductions in bloodstream infections due to MRSA and other pathogens that have occurred at HCA hospitals and other hospitals nationally within the past decade.3,26,27 Our study included a direct comparison with high-compliance active surveillance and accompanying contact precautions, which have been associated with decreased rates of MRSA transmission and MRSA bloodstream infection.9,16,25,27,28 Hospitals that have not fully implemented a strategy of screening and isolation may derive additional benefit from this intervention. Second, universal decolonization was implemented as part of routine practice with the use of the usual infrastructure of the hospital for practice change, without the need for on-site study personnel. These results are thus likely to be generally achievable as part of regular practice. Third, the intervention was effective in community hospitals, which make up the majority of U.S. hospitals.
The reduction in bloodstream infections from any pathogen occurred in the context of the relatively higher baseline rates of infection for all pathogen types (gram-positive, gram-negative, and fungal) in group 3, as compared with the other groups. One explanation for these high rates is that this group included three of the four hospitals providing bone marrow and solid-organ transplantations. Such differences across groups are largely accounted for by comparing the outcome rate in each hospital with that hospital’s baseline rate, providing reassurance that the benefit is attributable to decolonization rather than to baseline variation in case mix or clinical practices across groups. In addition, group 3 did not have higher baseline rates of MRSA-positive clinical cultures than the other groups did, so regression to the mean would not explain the beneficial effect on that outcome.
It is unknown whether a threshold level of compliance with universal decolonization is required to achieve the observed benefit or whether a compliance rate higher than the rate in our study (85%) would yield further improvement. Although hospital staff members were aware of the assigned strategy, which could have resulted in unmeasured behavior that affected trial outcomes,29 it is unclear what unmeasured behavior could effect a 44% improvement.
This trial provides no information on the attributable benefit of mupirocin, either alone or in combination with chlorhexidine. On the basis of microbiologic activity, any reduction in non–S. aureus bloodstream infections should be attributed to chlorhexidine. However, for S. aureus, the most common cause of health care–associated infection,4 clearance of the nasal reservoir in combination with body decolonization may be superior to either method alone.30
Widespread use of chlorhexidine and mupirocin could possibly engender resistance.9,31,32 Mupirocin resistance has been reported in some studies of MRSA decolonization,9,30 but not all such studies.8,32–35 MRSA resistance to chlorhexidine lacks a standard definition, but recent reports suggest that resistant strains are rare in the United States.36,37 A gene encoding a multidrug efflux pump that is active against chlorhexidine has been reported in MRSA,38 but its clinical significance is not understood. Reduced susceptibility to chlorhexidine has also been reported in gram-negative bacteria.39 It will therefore be important for surveillance programs to monitor mupirocin and chlorhexidine resistance.3,8
This trial was designed as a pragmatic, comparative-effectiveness trial implemented primarily through usual hospital processes.15,19 We chose this design to obtain results that could be generalized to the broadest set of hospitals, to use processes potentially adoptable by many hospitals, and to conduct a study of sufficient size — all ICUs in dozens of hospitals — with the available resources. Randomization of entire hospitals allowed us to recruit a broad array of hospitals, including community hospitals with no prior experience in clinical research. Finally, the efficient design meant that the total cost of the trial, including the decolonizing product and contributed personnel effort, was less than $3 million, or approximately $40 per patient.
Opportunities to integrate comparative-effectiveness research into routine clinical settings with the use of methods such as those used in the current study will increase as more hospitals adopt electronic health data systems and as multicenter care-improvement collaboratives develop. This trial also highlights the importance of performing rigorous evaluation of quality-improvement initiatives and controlling the introduction of new processes and products. Harnessing such initiatives to identify best practices is an important tenet of the advocacy by the Institute of Medicine for a learning health system.40
In conclusion, we found that universal decolonization prevented infection, obviated the need for surveillance testing, and reduced contact isolation. If this practice is widely implemented, vigilance for emerging resistance will be required.
Supplementary Material
Acknowledgments
Supported by a contract with the AHRQ Healthcare-Associated Infections Program (HHSA290201000008I) and by a grant from the CDC Prevention Epicenters Program (1U01 CI000344, to Dr. Platt).
Dr. Septimus reports receiving consulting fees from 3M and lecture fees from Sage Products; Dr. Hayden, conducting research involving a contributed product from Sage Products; Dr. Weinstein, serving as an unpaid consultant for Sage Products; and Dr. Fraser, owning stock in Express Scripts. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views of the Agency for Healthcare Research and Quality (AHRQ), the Department of Health and Human Services, or the Centers for Disease Control and Prevention (CDC).
Investigators for the Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program and the Agency for Healthcare Research and Quality (AHRQ) Developing Evidence to Inform Decisions about Effectiveness (DECIDE) Network and Healthcare-Associated Infections Program are listed in the Supplementary Appendix, available at NEJM.org.
REFERENCES
- 1.Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis WR, Jarvis AA, Chinn RY. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities, 2010. Am J Infect Control 2012;40:194–200. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007;298:1763–71. [DOI] [PubMed] [Google Scholar]
- 4.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013;34:1–14. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One 2011;6(9):e24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for Isolation Precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007;35:Suppl 2:S65–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MRSA laws. Washington, DC: Association for Professionals in Infection Control and Epidemiology; (http://www.apic.org/Resource_/TinyMceFileManager/Advocacy-PDFs/MRSA_map.gif). [Google Scholar]
- 8.Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol 2007;28:1155–61. [DOI] [PubMed] [Google Scholar]
- 9.Robicsek A, Beaumont JL, Thomson RB, Govindarajan G, Peterson LR. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: impact on infection risk. Infect Control Hosp Epidemiol 2009;30:623–32. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis 2010;14:Suppl 4:S3–S5. [DOI] [PubMed] [Google Scholar]
- 11.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013;368:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9. [DOI] [PubMed] [Google Scholar]
- 13.Popovich KJ, Hota B, Hayes B, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol 2009;30:959–63. [DOI] [PubMed] [Google Scholar]
- 14.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and health-care-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65. [DOI] [PubMed] [Google Scholar]
- 15.Platt R, Takvorian SU, Septimus E, et al. Cluster randomized trials in comparative effectiveness research: randomizing hospitals to test methods for prevention of healthcare-associated infections. Med Care 2010;48:Suppl:S52–S57. [DOI] [PubMed] [Google Scholar]
- 16.Perlin JB, Hickok JD, Septimus EJ, Moody JA, Englebright JD, Bracken RM. A bundled approach to reduce methicillin-resistant Staphylococcus aureus infections in a system of community hospitals. J Healthc Qual 2013;35:57–69. [DOI] [PubMed] [Google Scholar]
- 17.Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol 2008;29:Suppl 1:S12–S21. [DOI] [PubMed] [Google Scholar]
- 18.Healthcare Infection Control Practices Advisory Committee (HICPAC) guidelines. Atlanta: Centers for Disease Control and Prevention; (http://www.cdc.gov/hicpac/). [Google Scholar]
- 19.Solomon MZ, Bonham AC. Ethical oversight of learning health care systems. Hastings Cent Rep 2013;43:Suppl:S1-S44. [DOI] [PubMed] [Google Scholar]
- 20.National Healthcare Safety Network. Patient safety component manual. Atlanta: Centers for Disease Control and Prevention; (http://www.cdc.gov/nhsn/settings.html). [Google Scholar]
- 21.Hayes RH, Moulton LH. Cluster randomized trials. New York: CRC Press, 2009:207. [Google Scholar]
- 22.Ripatti S, Palmgren J. Estimation of multivariate frailty models using penalized partial likelihood. Biometrics 2000; 56:1016–22. [DOI] [PubMed] [Google Scholar]
- 23.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166: 306–12. [DOI] [PubMed] [Google Scholar]
- 24.Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control 2009;37:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber SG, Huang SS, Oriola S, et al. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: position statement from the Joint SHEA and APIC Task Force. Infect Control Hosp Epidemiol 2007;28:249–60. [DOI] [PubMed] [Google Scholar]
- 26.Vital signs: central line–associated blood stream infections — United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011;60:243–8. [PubMed] [Google Scholar]
- 27.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30. [DOI] [PubMed] [Google Scholar]
- 28.Moody J, Septimus E, Hickok J et al. Infection prevention practices in adult intensive care units in a large community hospital system after implementing strategies to reduce healthcare-associated methicillin-resistant Staphylococcus aureus infections. Am J Infect Control 2013;41:126–30. [DOI] [PubMed] [Google Scholar]
- 29.Nijssen S, Bonten MJ, Weinstein RA. Are active microbiological surveillance and subsequent isolation needed to prevent the spread of methicillin-resistant Staphylococcus aureus? Clin Infect Dis 2005; 40:405–9. [DOI] [PubMed] [Google Scholar]
- 30.Harbarth S, Dharan S, Liassine N, Herrault P, Auckenthaler R, Pittet D. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1999;43:1412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother 2003;51: 613–7. [DOI] [PubMed] [Google Scholar]
- 32.Simor AE, Stuart TL, Louie L, et al. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus strains in Canadian hospitals. Antimicrob Agents Chemother 2007;51:3880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother 2007;51:3591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones JC, Rogers TJ, Brookmeyer P, et al. Mupirocin resistance in patients colonized with methicillin-resistant Staphylococcus aureus in a surgical intensive care unit. Clin Infect Dis 2007;45:541–7. [DOI] [PubMed] [Google Scholar]
- 35.Harbarth S, Liassine N, Dharan S, Herrault P, Auckenthaler R, Pittet D. Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2000;31:1380–5. [DOI] [PubMed] [Google Scholar]
- 36.Fritz SA, Hogan PG, Camins BC, et al. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother 2013;57:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeil JC, Hulten KG, Kaplan SL, Mahoney DH, Mason EO. Staphylococcus aureus infections in pediatric oncology patients: high rates of antimicrobial resistance, antiseptic tolerance and complications. Pediatr Infect Dis J 2013;32:124–8. [DOI] [PubMed] [Google Scholar]
- 38.Batra R, Cooper VS, Whiteley C, Patel AK, Wyncoll D, Edgeworth JD. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis 2010;50:210–7. [DOI] [PubMed] [Google Scholar]
- 39.Stickler DJ. Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J Appl Microbiol 2002; 92:Suppl:163S–S70S. [PubMed] [Google Scholar]
- 40.The learning health system series. Washington, DC: National Academies Press; (http://www.nap.edu/catalog.php?record_id=13301). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.