Abstract

The ever-increasing landscape of heterogeneous catalysis, pure and applied, utilizes many different catalysts. Academic insights along with many industrial adaptations paved the way for the growth. In designing a catalyst, it is desirable to have a priori knowledge of what structure needs to be targeted to help in achieving the goal. When focusing on catalysis, one needs to cope with a vast corpus of knowledge and information. The overwhelming desire to exploit catalysis toward commercial ends is irresistible. In today’s world, one of the requirements of developing a new catalyst is to address the environmental concerns. The well-established heterogeneous catalysts have microporous structures (<25 Å), which find use in many industrial processes. The metal–organic framework (MOF) compounds, being pursued vigorously during the last two decades, have similar microporosity with well-defined pores and channels. The MOFs possess large surface area and assemble to delicate structural and compositional variations either during the preparation or through postsynthetic modifications (PSMs). The MOFs, in fact, offer excellent scope as simple Lewis acidic, Brönsted acidic, Lewis basic, and more importantly bifunctional (acidic as well as basic) agents for carrying out catalysis. The many advances that happened over the years in biology helped in the design of many good biocatalysts. The tools and techniques (advanced preparative approaches coupled with computational insights), on the other hand, have helped in generating interesting and good inorganic catalysts. In this review, the recent advances in bifunctional catalysis employing MOFs are presented. In doing so, we have concentrated on the developments that happened during the past decade or so.
Keywords: Metal−organic frameworks, Lewis acid catalysis, Lewis basic catalysis, Bifunctional catalysis, Multifunctional catalysis, Tandem reactions, Deacetalization−Knoevenagel condensation reactions, CO2 fixation reactions, Nanoparticle supported MOFs
1. Introduction
Catalysis has been so widely woven into the fabric of life that the practice and pursuit of the subject transcends across different disciplines of science and engineering. The canvas of catalysis has been large and continuously growing over the years. It may be noted that there has been considerable success in discovering newer catalysts compared to obtaining greater insights over the known ones.1−3
For carrying out efficient catalysis and understanding the catalytic process, two different strategies exist toward the design of solid catalysts. In one approach, the individual steps for the overall reaction are considered and optimized, and the second approach focuses on the location of the active site within the structure. For one of the goals of preparing new compounds that could be potential catalysts, one needs to look for maximum surface area, precise catalytic reaction centers, and good activity, selectivity, and durability. The desiderata of designing a new catalyst need to encompass the following: (i) to be able to operate under mild conditions that are environmentally benign; (ii) reasonable freedom from restrictions imposed by diffusional considerations of molecules (reactants and products); (iii) possession of well-defined and specially separated reaction centers; and (iv) the scope to probe the mechanistic understanding of the catalytic action.
In the area of catalysis, the idea that the catalyst possesses bifunctional or multifunctional character is a desired property. There have been studies toward the multifunctional behavior of catalysts, especially those involving zeolites and mesoporous compounds.4 It is becoming clear that in seeking to create compounds with good surface areas and catalytic activity one needs to have solids that have pore diameters in the microporous region (∼20 Å).
Aluminosilicate zeolites have been the backbone of the study of catalysts and the science of catalysis over many decades. These compounds possess porosity in the microporous range of 4–20 Å. The discovery of metal–organic frameworks (MOFs) in the late 90s and its subsequent developments appear have caught the imagination of the catalyst community.5−10 The attractive features of many MOF compounds toward catalysis are as follows: (i) they permit free flow of reactants and products through the channels (pores) and cages; (ii) they have spatially distributed distinct catalytic sites; and (iii) they create the possibility for performing shape-selective, enantioselective, and regioselective reactions. The MOFs offer ease of synthesis coupled with a diverse range of compositional variations, which would be desirable toward heterogeneous catalysis. The MOFs offer the following advantages: (i) The MOF structures are flexible (breathable) and expand and contract by external stimuli;11−14 (ii) the metal as well as the ligand that forms the structures can be replaced by postsynthetic modifications to render them attractive toward many physical and chemical properties;15−21 (iii) the organic ligands are amenable for manipulations to create specific functionality in the compounds, which would be desirable toward organocatalysis;22−30 and (iv) MOFs can provide hydrophilic and hydrophobic environments which can be exploited toward specific catalysis.31−35
There have been many catalytic studies that were carried out employing MOFs. In most of the studies, the metal sites were always exploited for their Lewis-acidic behavior.24,36−38 Most of the earlier attempts toward catalysis employing MOFs were predominantly Lewis acid catalysis only.39−42 Brönsted acid catalysis was also attempted employing MOFs.43,44 Over the years it has been shown that the organic ligands in MOFs also catalyze many reactions, especially acting as a Lewis base.45−48 As the MOFs possess both the Lewis acidic as well as basic centers, bifunctional catalysis was attempted.49−52
The bifunctional MOFs offer advantages toward studying the cascade reactions. The cascade/domino/tandem reactions53,54 utilize at least two consecutive reactions and involve different chemical functionality available within the compound. The cascade process involves a set of reactions where the product(s) of the reaction(s) is consumed in a subsequent reaction. In these reactions, the isolation of intermediates is not required and the reaction(s) proceeds in a stepwise manner. The cascade reaction requires different catalytically active sites distributed over a large surface area and in a periodic manner. The MOFs with their large surface area with good pore size distribution and availability of functional groups would be ideally suited for such reactions.55−57
In this review, we focus on the recent developments on MOFs that offer multifunctionality toward heterogeneous catalysis. We have specifically given closer attention to the developments toward catalytic reactions that have been performed employing MOFs during the past decade or so. In this task, there may be a few oversights, which are not intentional.
2. Generation of Functionality in MOFs
Most of the MOF compounds possess Lewis acidity due to the metal centers—the strength of the acidity depends on the size of the metal ions, the oxidation state, and its coordination preferences. In addition to this, it may be possible to create acidity in MOFs by suitable postsynthetic modifications. Here, we outline a few such scenarios:
2.1. Lewis Acid Functionality
The acidity can be classified as Brönsted acid or Lewis acid—the former is the stronger acid compared to the latter. The MOFs that were explored toward acid catalysis are tabulated in Table 1. Lewis acidity in MOFs generally refers to an accessible metal site, with a low coordination number, which is also known as a coordinatively unsaturated metal site (CU). The coordinatively unsaturated metal sites can be achieved in situ by the removal of labile ligands bonded to the metal, which usually are the solvent molecules. A Zn MOF, {[Zn(BPBN)Cl]·5H2O}n (BPBN = 3,5-bis(4-oxo-4H-pyridin-1-yl)-benzonitrile) (Figure 1a) has been synthesized and has been utilized for the synthesis of naphthimidazole from 2,3-diaminonaphthalene and DMF at 120 °C with good yield (Figure 1b). The tetrahedrally coordinated Zn center acts as Lewis acid site and facilitates this reaction.58 UiO-66 (UiO = University of Oslo) is one of the MOFs which was studied toward the Lewis acid catalytic reaction. Zr-MOFs provide unsaturated metal sites, which could be exploited as Lewis acid centers in catalytic reactions. The open metal sites generally act as electron pair acceptors and accelerate the reaction process.59 Most of the earlier studies exploiting the Lewis acid functionality concentrated on cyanosilylation of imines.60 These are typically low temperature reactions, and in most cases the yield is >95%. Recently, lanthanide centers were explored as possible Lewis acid catalytic centers in the compound, [La2/3(qptca)1/2] (qptca = 1,1′:4′,1′′:4″,1‴:4′′′,1⁗-quinquephenyl]-2,2′′,2′′′′,5′′-tetracarboxylic acid), toward the Friedel–Crafts reaction. The alkylation of indole and pyrrole with β-nitrostyrene with a wide substrate scope gave the desired product with high yield and recyclability.61
Table 1. MOFs with Different Lewis Acidic Functionalities and Their Utilities toward Catalysisa.
| Sr. No. | MOF Compound | Lewis acidic metal site | Labile solvent | Catalytic reaction | Ref |
|---|---|---|---|---|---|
| 1 | Zr6-fBDC and Zr6-fBPDC | Zr4+ | H2O | Arene C–H Iodination | (258) |
| 2 | Zr6OTf-BTB | Zr4+ | - | Povarov Reactions | (259) |
| 3 | [Zn2(TBIB)2(HTCPB)2]·9DMF·19H2O | Zn2+ | - | Cycloaddition of CO2 | (260) |
| Friedländer Reaction | |||||
| 4 | MOF-525, PCN-222 and PCN-224 | Zr2+, Mn2+, Zn2+ | - | Cycloaddition of CO2 | (261) |
| 5 | M-NU-1008 | M = Zr4+, Hf, Th, Ce3+ | H2O | Cycloaddition of CO2 | (262) |
| 6 | ZIF-8 | Zn2+ | - | Epoxide Hydroxylation | (263) |
| 7 | Tb(BTC)(H2O)3(DMF)1.1 | Tb3+ | H2O, DMF | Synthesis of β-Aminoalcohols | (264) |
| 8 | Er(BTC)(H2O)·(DMF)1.1 | Er3+ | H2O, DMF | Hantzsch Coupling and Tetrahydro-4H-Chromene Synthesis | (265) |
| 9 | [Ba2(BDPO)(H2O)]·DMA | Ba2+ | H2O | Cycloaddition of CO2 | (266) |
| 10 | NH2-MIL-101/PAN | Cr3+ | - | Friedel–Crafts Acylation of Anisole | (267) |
| Esterification reaction | |||||
| 11 | Cu(II)-MOF | Cu2+ | H2O | Cycloaddition of CO2 | (268) |
| 12 | Zr6O4(OH)4(OAc)2.4[M(PNNNP)X]2.4 [M = Pd, Pt] | Zr4+, Pd(I), Pt(I) | - | Hydroamination of o-Alkynyl Aniline | (269) |
| 13 | [Zn2(iso)2(bpy)2] | Zn2+ | DMF | Cycloaddition of CO2 | (270) |
| 14 | [Zn(BPBA)Cl]·5H2O | Zn2+ | H2O | Cyclization of ortho-Substituted Diaminonaphthalene to Naphthimidazole | (58) |
| 15 | Ce-doping MIL-88A(Fe) | Ce3+, Fe3+ | - | Catalytic Ozonation | (271) |
| 16 | Pd(II)/UIO-66 (Zr), Pd(II)/MIL-101 (Cr) and Pd(II)/MOF-5 (Zn) | Pd2+, Cr3+, Zn2+ | H2O | CO Esterification to Dimethyl Carbonate | (272) |
| 17 | UiO-66 | Zr4+ | H2O | Aldose Sugars to Polyhydroxyalkyl and C-Glycosyl Furans | (273) |
| 18 | [Cd3(BDC)3(OPP)(DMF)2]·2DMA | Cd2+ | DMF | Hantzsch Reaction | (274) |
| 19 | Cu-BTC(MOF-199) | Cu2+ | - | Aerobic Oxidative Synthesis of Imines | (275) |
| 20 | MIL-101(Fe,Sc) | Fe3+, Sc3+ | - | Glucose to 5-Hydroxymethyl Furfural | (276) |
| 21 | Al-ITQ-Br, Al-ITQ-NO2, L-MOF-EB L-MOF-AB | Fe3+, Al3+ | - | Oxidation of Thiophenol to Diphenyldisulfide | (277) |
| 22 | [Eu(tctb)(H2O)] | Eu3+ | H2O | Diamines to Benzimidazoles | (278) |
| 23 | [M6(TATAB)4(DABCO)3(H2O)3]·12DMF·9H2O | M = Co2+, Ni2+ | H2O | Chemical Fixation of CO2 | (279) |
| 24 | MixUMCM-1-NH2. | Zn2+ | - | Aldol–Tishchenko Reaction | (280) |
| 25 | [In3(NIPH)3(HNIPH)(OH)2]·4H2O | In3+ | -H2O | Multicomponent Strecker Reactions | (281) |
| 26 | [Mn2(TDP)(H2O)2]·3H2O·3DMF | Mn2+ | -H2O | Chemical Fixation of CO2 | (282) |
| 27 | [Cu6(TADIPA)3(DABCO)(H2O)2(DMF)2]·13H2O [Cu6(TADIPA)3(H2O)6]·16H2O·8DMF | Cu2+ | -H2O, DMF, DMA | Chemical Fixation of CO2 | (283) |
| [H3O][Cu6(TPTA)3(DMA)4(COO)]·12H2O·7DMA | |||||
| [Cu6(C17O9N2H8)3(C6H12N2)(H2O)2(DMF)2]·3DMF·8H2O | |||||
| 28 | UiO-66-TA | Zr4+ | -H2O | Hydrogenation of Cinnamaldehyde | (284) |
| 29 | MIL-101(Cr)-LP | Cr3+ | - | Reduction of Imine | (285) |
| 30 | MIL-101(Cr) MOF | Cr3+ | - | glucose to fructose | (286) |
| 31 | [Zn3(Hbtc)2(atz)2]·CH3CN·2CH3OH [Co3(Hbtc)2(atz)2]·H2O·2DMF | Zn2+ Co2+ | - | Coupling of CO2 and Epoxides | (287) |
| 32 | [Zn(bix)]{V2O6}(V-Zn-MOF | Zn2+ | - | Cyanosilylation Reaction of Aldehydes | (288) |
| 33 | [Dy3(data)3·2DMF]·DMF | Dy3+ | DMF | Chemical Fixation of CO2 | (155) |
| 34 | Cu-MOF | Cu2+ | - | Catalytic CO2 Fixation | (289) |
| 35 | [Cd(bpp)(L)(H2O)]·DMF | Cd2+ | -H2O | Strecker Reaction | (290) |
| 36 | Cr-UiO-66-CAT | Zr4+ | - | Oxidation of Alcohols to Ketones | (85) |
H2fBDC = 2,3,5,6-tetrafluoro-1,4-benzenedicarboxylic acid; H2fBPDC = 2,2′,3,3′,5,5′,6,6′-octafluoro-4,4′-biphenyldicarboxylic acid; TBIB = 1,3,5-tri(1H-benzo[d]imidazol-1-yl)benzene); H3TCPB = 1,3,5-tris(4′-carboxyphenyl-)benzene; BTC = 1,3,5-benzenetricarboxylate; DMF = N,N-dimethylformamide; PAN = polyacrylonitrile; PNNNP = 2,6-(HNPAr2)2C5H3N; Ar = p-C6H4CO2–; X = Cl–, I–; iso = isophthalic acid; bpy = 4,4-dipyridyl; BPBA = 3,5-bis(4-oxo-4H-pyridin-1-yl)-benzoate; OPP = N,N′-(oxybis(4,1-phenylene))bis(1-(pyridin-4-yl)methanimine); H2BDC = terephthalic acid; H3tctb = tris(p-carboxylic acid)tridurylborane; H3TATAB = 4,4′,4″-s-triazine-1,3,5-triyl-tri-p-aminobenzoic acid; DABCO = 1,4-diazabicyclo[2.2.2]octane; BDC = 1,4-benzenedicarboxylate; ABDC = 2-amino-1,4-benzenedicarboxylate, btb = 4,4′,4″,-benzene-1,3,5-triyl-trisbenzoic acid; H2NIPH = 5-nitroisophthalic acid; H4TPTA = 1,1′,3′,1″-terphenyl-3,3′′,5,5′-tetracarboxylic acid; H4CBDA = 5,5′-(carbonylbis(azanediyl)) diisophthalic acid; TA = terephthalic acid; LP = Lewis pair; bix = 1,4-bis(imidazole-1-ylmethyl)benzene; 2,5-data = 2,5-diamino-terephthalate; H2L = 5-(1-oxo-2,3-dihydro-1H-inden-2-yl)isophthalic acid; H2L = 4,4′-(dimethylsilanediyl)bis-benzoic acid; bpp = 1,3-bis(4-pyridyl)propane.
Figure 1.

(a) 3D structure of the [Zn(BPBN)Cl]·5H2O MOF. (b) Synthesis of naphthimidazole in the presence of heterogeneous catalyst. Reproduced with permission from ref (58). Copyright 2020 Elsevier.
As an example of unusual Lewis acidity in MOFs, the example of easily modifiable nature of the ligand was exploited in preparing Pd-mono(thiocatecholato) units inside the MOF UiO-66.85 This modified MOF exhibited excellent regioselectivity toward the sp2 carbon, oxidation of alcohols to ketones, etc.85,86 A more interesting approach is to replace the ligand, which expands the MOF, allowing for enhanced catalytic activity.83 In this work, C2 symmetry ligands were exchanged for C3 symmetric, which causes defects in the overall structure, paving the way for better Lewis acid activity.87
2.2. Brönsted Acid Functionality
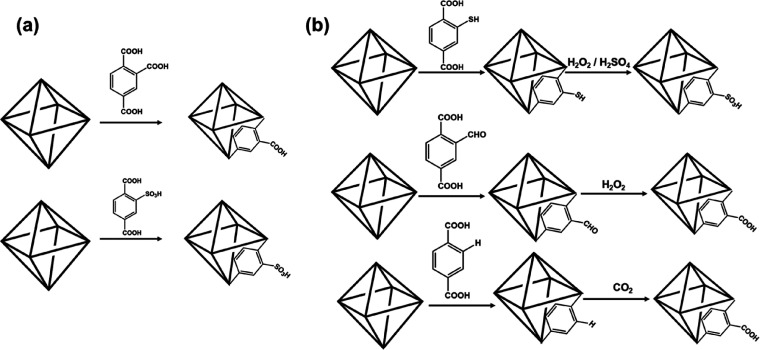
In the traditional framework compounds of aluminosilicate zeolites, metal phosphates, etc. Brönsted acidity was generated by manipulating the structure by having elements of different valencies.88−90 The charge compensating protons have been found to have strong Brönsted acid character. In MOFs, such a possibility is difficult to achieve, as the framework contains metal centers with fixed valences and organic ligands. There are examples of MOF compounds where elements of mixed valency exist as part of the structure.91,92 The Brönsted acidity in many MOFs, however, appears to arise out of the nonbonded acidic groups of the ligands (Scheme 1).43,72 The MOFs, thus, provide the versatility of having both the Lewis acidic as well as Brönsted acidic functionality within the same MOF.68,93−95 The many catalytic reactions that have been carried out employing Brönsted acid functionality in MOFs are listed in Table 2. In MIL-(Cr)-101-SO3H, the Cr3+ ions act as a Lewis acidic and the -SO3H groups act as the Brönsted acidic centers for the catalytic conversion of glucose.83
Scheme 1. Schematic Showing How the Additional Functionality Is Used in Generating Brönsted Acidity in MOFs: (a) Direct Synthesis; (b) via Postsynthetic Modification.
Table 2. MOFs with Brönsted Acidity and the Associated Catalysisa.
| Sr. No. | MOF | Brönsted acidic site | Catalytic reaction | Ref |
|---|---|---|---|---|
| 1 | PTA⊂MIL-101(Al)-NH2 | H3[PW12O40]·nH2O | Glucose Dehydration to 5-Hydroxymethylfurfural | (62) |
| 2 | H3PW12O40@Zr-MOF | -SO3H | Levulinic Acid to γ-Valerolactone | (63) |
| 3 | [(CH2COOH)2IM]HSO4@H-UiO-66 | [(CH2COOH)2IM]HSO4 | Biodiesel Synthesis | (64) |
| 4 | PO4/NU(eq) and PO4/NU(half) | -PO4 | Glucose to 5-Hydroxymethylfurfural | (65) |
| 5 | MIL-101(Cr)-SO3H | -SO3H | Cross-Dehydrogenative Coupling of C–H Bonds | (66) |
| 6 | CoFe2O4/MIL-88B(Fe)-NH2/(Py-Ps)PMo | -SO3H | Transesterification | (67) |
| 7 | MIL-101(Cr)-NH-CO-Pr-COOH | -COOH | Synthesis of Quinazolin-(4H)-1-one | (68) |
| 8 | MOF-808-SO4 | -SO4 | Dimerization of Isobutene (2-Methyl-1-propene) | (69) |
| 9 | [Zr6O4(OH)5.6(C9H3O6)2(HCOO)0.18(SO4)2.1](H2O)2 | -OH | Isobutene Dimerization | (70) |
| 10 | Hf-MOF-808 | μ3-OH | Meerwein–Ponndorf–Verley Reduction | (71) |
| Styrene Oxide Ring-Opening | ||||
| α-Pinene Oxide Isomerization | ||||
| 11 | MIL-100(Cr) and MIL-100(Fe) | -H2O-CO | Acetalization of Benzaldehyde with Methanol | (72) |
| 12 | PCN-222(Ni)-SO4 | SO42– | Tandem Semisynthesis of Artemisinin | (73) |
| 13 | Zr-MOF-808-S | OH–/H2O | Glycerol Dehydration | (74) |
| 14 | Al-MIL-53-RSO3HAl-MIL-53-ArSO3H | -SO3H | [4 + 2] Cycloaddition Reaction | (75) |
| 15 | UiO-66 (Zr, Hf), Zr-BTC | μ3-OH | Cycloaddition Reaction | (76) |
| 16 | MIL-100(Fe) (Lys-PM2) | Lys-PM2 | Conversion of Glucose to Levulinic Acid | (77) |
| 17 | [BSO3HMIm][HSO4](IRMOF-3) | [BSO3HMIm][HSO4] | Bligh–Dyer Method for Biodiesel Production | (78) |
| 18 | MIL-101(Cr)-SO3H | -SO3H | Methanolysis of Styrene Oxide | (79) |
| 19 | Cr3(μ3-O)(H2O)3(NDC(SO3H5/6)2)3(BUT-8(Cr)-SO3H | -SO3H | Esterification Reaction | (80) |
| 20 | MIL-IMAc-Br– | Br– | Cycloaddition of CO2 | (81) |
| 21 | (H4SiW12O40) (POM@MOF) | H4SiW12O40 | Glucose into 5-Hydroxymethylfurfural | (82) |
| 22 | MIL-(Cr)-101-SO3H | SO3H | Catalytic Conversion of Glucose | (83) |
| 23 | UiO-66(SO3H)2 | SO3H | Synthesis of Dihydro-2-oxypyrrole Derivatives | (84) |
PTA = phosphotungstic acid; Py-Ps = pyridine with 1,3-propanesultone; H4TADIPA = 5-5′-(1H-1,2,4-triazole-3,5-diyl) diisophthalic acid; Lys = lysine functionalized phosphotungstic acid; [BSO3HMIm][HSO4] = 1-butylsulfonate-3-methylimidazolium bisulfate; NDC(SO3H)22– = 4,8-disulfonaphthalene-2,6-dicarboxylatlate); IMAc = 1H-imidazole-1-acetic acid.
The acidic stability of the MOFs was exploited in generating new Brönsted acidity through postsynthetic modifications in UiO-66(SH)2.96 In this study, UiO-66(SH)2 was modified by treatment with H2O2 and H2SO4 to form UiO-66(SO3H)2, which was later found to be a good catalyst for synthesis of dihydro-2-oxypyrrole derivatives.84
2.3. Basic Functionalities in MOFs
It has been well established that Lewis acidities are much easier to generate in MOFs. In many of the traditional framework compounds, the basic functionality is generated when the extra-framework protons are the Brönsted acid sites and the framework oxygens and their conjugate form the basic sites.97,98 In addition, alkali and alkaline earth exchanged zeolites are also designated as basic.
An easier approach toward the generation of Lewis basicity is feasible in MOFs compared to the traditional framework of zeolites and aluminophosphates. In this approach, the functionality of organic ligands plays an important role in creating the basicity to the structure. More importantly the Lewis basic sites are spatially separated and, in a sense, can be considered as a single basic site, similar to the acid site, which many researchers have exploited.99,100
Thus, in MOFs the -NH2 group plays a crucial role as the Lewis basic site. In addition, researchers have also used nonbonded carboxyl (-COO–) and similar units as Lewis bases.101 The Lewis basic sites in MOFs and their utility toward catalysis are listed in Table 3. For example, IRMOF-1 (commonly known as MOF-5) is constructed from the Zn2+ ions and ligand BDC, while replacement of BDC with NH2-BDC yields IRMOF-3, which exhibits Lewis basic behavior toward the Knoevenagel condensation reaction.102,103 A range of metals viz., Zn,47 Zr,104 Al,105 Ti,106 and Cu107 were employed for the preparation of MOFs with NH2–BDC, which were found to be useful in base catalyzed Knoevenagel condensation reactions. ZIFs (zeolite imidazolate frameworks), which were formed by assembling Zn metal and imidazole linker, provide high stability, high surface area as well as sufficient basicity, arising out of the N atoms of the imidazole have been exploited toward base catalysis.108 Such ZIF compounds were also found to be good catalysts toward Knoevenagel condensation reaction at room temperature with ∼99% yield.109
Table 3. MOFs with Different Basic Functionalities and Their Utility toward Catalysisa.
| Sr. No. | MOF Compound | Ligand responsible for basicity | Catalytic reaction | Ref |
|---|---|---|---|---|
| 1 | [Cd(C16H10N2O8S)(H2O)] | -NH | Knoevenagel Condensation | (110) |
| 2 | [(Nd2(TATMA)2·4DMF·4H2O]n | -N | Knoevenagel Condensation | (111) |
| 3 | [H2N(CH3)2]·[Zn4(L)1.5(ad)3(H2O)2)]·4DMF | -NH2 | Knoevenagel Condensation | (112) |
| 4 | {[Zn2(D-CAM)2(L)]·MeOH·2H2O}n | -NH | Ring-Opening of Spiro-Epoxyoxindoles | (113) |
| 5 | Co2(bdda)1.5(OAc)1·5H2O | -NH | Henry Reactions | (114) |
| 6 | [Zn(OBA)(BPDB)0.5]n·2DMF | -N | Knoevenagel Condensation | (115) |
| 7 | [Y3(μ3-O)2(μ3OH)(H2O)2(BTCTBA)2]·2[(CH3)2NH2]·5DMF·C6H5Cl·4H2O | C=O–N–H | Knoevenagel Condensation | (116) |
| 8 | [Zn2(hfipbb)2(4-bpdh)]·0.5DMF and [Zn2(hfipbb)2(4-bpdb)]·2DMF | -N=N- | Knoevenagel Condensation | (117) |
| 9 | UiO-67-BPY@UiO-66 | -Nbpy | Knoevenagel Condensation | (118) |
| 10 | [Zn2(3-tpom)(L)2]·2H2O | -O | Strecker Reaction | (119) |
| 11 | [Cu2(L)(H2O)2]·(3DMF)(4H2O) | -N | Henry Reactions | (120) |
| 12 | [(CH3)2NH2+]2[Zn3((μ3-O))(L)2(H2O)]·4DMF·2H2O | -NH2 | Chemical Fixation of CO2 | (121) |
| Biginelli Reactions | ||||
| 13 | [Cu2(L)(H2O)2]·(5DMF)(4H2O) | -NH2 | Biginelli Reactions | (122) |
| 14 | [CoL(H2O)3]·2NO3 | -N | Knoevenagel Condensation | (123) |
| 15 | UiO-67@Fe | -Nbpy | Morita–Baylis–Hillman Reaction | (124) |
| 16 | ZIF-8, ZIF-67 | -Nim | Knoevenagel Condensation | (125) |
| 17 | MIL-125-NH2 | -NH2 | Plasticizers Syntheses | (126) |
| 18 | SB-Cu1 | -N | N-Arylation | (127) |
| 19 | NH2cCo-PYI1 and NH2cCo-PYI2 | -NH2 | Aldol and Knoevenagel Condensations | (128) |
Nic = nicotinamide; pic = picrate; H3dcp = 3,5-pyrazoledicarboxylic acid; DMA = N,N-dimethylacetamide; ad = adenine; H4L = 5,5′-(1,3,6,8-tetraoxobenzo phenanthroline-2,7-diyl)bis-1,3-benzenedicarboxylic acid; H3TATMA = 4,4,4″-s-triazine-1,3,5-triyltri-m-aminobenzoate; L = N′-(pyridin-4-ylmethylene)isonicotinohydrazide; bdda: 4,4′-[benzene-1,4-diylbis(methylidenenitrilo)] dibenzoic acid); H3BTCTBA = 4,4′,4″-[1,3,5-benzenetriyltris(carbonylimino)]trisbenzoic acid; 4-bpdb = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene; 4-bpdh = 2,5-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene; H2L = dicarboxylic acid 4,4′-(dimethylsilanediyl)bis-benzoic acid; 3-tpom = tetrakis(3-pyridyloxymethylene)methane; H4L = 5,5′-(piperazine-1,4-diyl)diisophthalic acid; L = 4-(trifluoromethyl)aniline, 1-bromo-3,5-dimethylbenzene; L = tris(4-(4H-1,2,4-triazol-4-yl)phenyl)amine; PYI = pyrrolidine-2-yl-imidazole.
3. Multifunctional Catalytic Centers in MOFs
From the above descriptions, it is clear that forming bifunctional catalytic sites in MOFs is facile. The metal sites can provide the Lewis acidity, and the Lewis basic sites can be generated from the carboxylate units, specific functional groups in the ligand. The acid–base pair can be spatially well separated and in the precise location to facilitate bifunctional catalysis.45,129−131 The bifunctionality available in MOFs can be utilized in multiple ways: (i) in organic reactions involving multiple steps without separation and purification of the intermediates in each step;132 (ii) in catalyzing multiple reactions simultaneously involving more than one reaction pathway; (iii) in promoting tandem catalysis involving reactions that proceed sequentially; and (iv) in catalyzing acid–base reactions. The bifunctionality in MOFs toward catalysis can be achieved by the following: (i) coordinatively unsaturated metal sites for Lewis acid functionality along with a suitable ligand that offers Lewis basic functionality; (ii) use of multifunctional ligands either during the formation of the MOFs or through postsynthetic modifications; and (iii) incorporation of metal nanoparticles within the MOF structure (Scheme S1). These techniques are popular in generating bifunctional MOF compounds, and there are other techniques that may also be utilized to generate bifunctionality in MOFs. The number of MOF compounds with bifunctionality has grown rapidly in recent years. This is not surprising, as the initial efforts during the development of MOFs were toward establishing newer framework compounds and exploring their physical properties.133−136 The emphasis on the utility of MOFs toward catalysis involving both the acidic as well as basic functionalities is summarized in Table 4. In addition to preparing MOFs with bifunctionality by careful choice of the ligands, it is also possible to generate bifunctionality by carrying out postsynthetic modifications. Examples of bi- or multifunctionality through postsynthetic modifications (PSMs) have been known.137,138 The PSM allows better control on the structure and fine-tuning of the functionality toward a particular catalytic activity.
Table 4. MOFs with Bi-/Multifunctionality and Their Use in Catalysisa.
| Sr. No. | MOF Compound | Reactive site | Catalytic reaction | Ref | |
|---|---|---|---|---|---|
| 1 | MIL-101(Cr)-N(CH2PO3H2)2 | Cr(III) Phosphonates, -NH2 | Synthesis of N-Amino-2-Pyridone and Pyrano [2,3-c]Pyrazole Derivatives | (139) | |
| 2 | [Zn2(TCA)(BIB)2.5]·(NO3) | Zn (II), NTCA | Cycloaddition of CO2 | (140) | |
| 3 | [Mn2(DPP)(H2O)3]·6H2O | Mn (II), -Npyridine | Cycloaddition of CO2 | (141) | |
| 4 | [Zn(1,4-NDCA)(3-BPDB)0.5]·(DMF)(MeOH) [Cd4(1,4-NDCA)4(3-BPDB)4]·2(DMF) | Zn(II)Cd(II), -N=N- | Friedländer Reaction | (142) | |
| Michael Addition | |||||
| 5 | [M3(5-CFIA)2(8H2O)]·H2O | Cd(II), Mn(II), -NH | Aldol Condensation | (143) | |
| β-Enamination Reactions | |||||
| 6 | [Zn(HL)2] | Zn (II), -Ntriazole | Knoevenagel Condensation | (50) | |
| 7 | [Zn15(L-NH2)6(HL-NH2)6(LNA)4(HLNA)2(μ3–OH)2] | Zn(II), -NH2 | Knoevenagel Condensation | (144) | |
| 8 | SulP1/SulP2-MOF-808(Hf) | Hf(IV), Phosphonate | Reductive Amination and Hydroaminomthylation Reactions | (145) | |
| 9 | ED/MIL-101(Cr) | Cr (III), -NH2 | Hantzsch Condensation Reaction | (146) | |
| 10 | Zn-Bp-BTC MOF | Zn(II), -Nbipyridine | Knoevenagel Condensation | (147) | |
| Multicomponent Reaction | |||||
| Benzimidazole Synthesis | |||||
| 11 | Lysine - (Zr)MOF-808 | Zr(IV),- NH2 | Henry Condensation and Friedel/Crafts Type Alkylation | (148) | |
| 12 | C32H40Fe2N2S4Zn | Zn(II), -NH2 | One Pot Synthesis of Chromene and Imidazopyrimidine Derivatives | (149) | |
| 13 | Co/Ni2(BTC)(OH)(4-TPT)2(H2O)·(DMA)0.5(H2O)2 | Co(II)Ni(II), Npyridyl | Oxidation–Knoevenagel Cascade Reaction | (150) | |
| 14 | Cu3TATAT | Cu(II), -NH, N | Aerobic Oxidation/Knoevenagel Condensation | (151) | |
| 15 | Hf/Zr MOF-808 | Hf(IV)Zr(IV) Defective -OH | Tandem N-Alkylation of Amines with Benzyl Alcohol | (152) | |
| 16 | PMoV2@DETA-MIL-101 | Cr(III)PMoV2, -NH2 | Aerobic Oxidation-Knoevenagel One-Pot Reaction | (153) | |
| 17 | CuI@UiO-67-IM | CuI | One-Pot Azide–Alkyne Cycloaddition | (154) | |
| 18 | [Dy3(data)3·2DMF]·DMF, NH2-TMU-73 | Dy(III), -NH2 | Solvent-Free Conversion of CO2 to Cyclic Carbonates | (155) | |
| 19 | Co-NDTz and Co-NDPhTz | Co(II), -Ntetrazole | Tandem Oxidation and CO2 Conversion Reactions | (156) | |
| 20 | [Cu2Br2(pypz)]n·nH2O | Cu(II), Br– | Homocoupling of Arylboronic Acids and Epoxidation of Olefins | (157) | |
| 21 | [Cd(PBA)(DMF)]·DMF | Cd(II), -NH | Cyanosilylation and Hydroboration | (158) | |
| 22 | Ni-DDIA | Ni(II)-COOH | Biginelli Reaction | (94) | |
| 23 | MIL-101(Cr)-NH–CO-Pr-COOH | Cr(III)-COOH | Synthesis of Quinazolin-(4H)-1-one Derivatives | (68) | |
| 24 | MIL-101(Cr)-SO3H | Cr(III)-SO3H | Hydrogenation of Imines | (159) | |
| 25 | Arg2PTA/ZIF-8 | Zn2+, -Nimidazole | Production of Biodiesel from Insect Lipid | (160) |
Abbreviations: data = 2,5-data = 2,5-diamino-terephthalate; MA = melamine; ED = ethylene diamine; DPP = 2,6-di(2,4-dicarboxyphenyl)-4-(pyridine-4-yl)pyridine; H8L = tetraphenylsilane tetrakis-4-phosphonic acid; H3TCA = tricarboxy triphenyl amine; BIB = 1,3-bis(imidazol-1-ylmethyl)benzene; H4L = 2,6-di(2,4-dicarboxyphenyl)-4-(pyridine-4-yl)pyridine); Bp = 4,4′-bipyridine; BTC = 1,3,5-benzene tricarboxylate; H2LNA = 2,6-naphthalenedicarboxylic acid; H2L-NH2 = 2,2′-diamino[1,1′-biphenyl]-4,4′-dicarboxylic acid; CSMCRI-10 = Central Salt & Marine Chemicals Research Institute; BuPh3P = (4-bromobutyl)triphenylphosphonium bromide; SIPA = 5-sulfoisopthalic acid; ABPY = 4,4′-azopyridine; 5-CFIA = 5-(carboxyformamido)isophthalic acid; BDC = 1,4-benzenedicarboxylate; A = acid; B = base; (BINDIH4) = N,N′-bis(5-isophthalic acid)naphthalenediimide; DATRZ = 3,5-diamino-1,2,4-triazole; pypz = bis[3,5-dimethyl-4-(4′-pyridyl)pyrazol-1-yl] methane; PBA = 5-(4-pyridin-3-yl-benzoylamino)isophthalic acid; H2NDTz = 2,6-naphthaleneditetrazole; H2NDPhTz = 2,6-bis(4-(1H-tetrazol-5-yl)phenyl)naphthalene; Arg = Arginine.
3.1. Examples of Catalysis Involving Acidic and Basic Centers
As listed in Table 4, there have been a number of studies involving bifunctionality in MOFs. In this section, we focus on a few select examples that characterize the bifunctionality in a MOF compound. The examples utilize both the acidic as well as basic functionality toward catalysis, but these reactions are carried out independently and not as tandem catalytic reactions. The tandem catalytic studies are dealt with separately.
The MOF [M3(C10H4O7N1)2(8H2O)]·H2O (M = Cd, Mn), was explored toward the formation of the β-enaminoester (Lewis acid catalyzed), and the -NH moiety present in the ligand was exploited toward the Claisen–Schmidt reactions (Lewis base catalyzed) (Figure 2a).143 The Claisen–Schmidt reaction is a classic base catalyzed aldol condensation reaction involving an aromatic aldehyde and a ketone forming conjugated β-hydroxy carbonyl compounds. The uncoordinated -NH group acts as the basic center and catalyzes the Claisen–Schmidt reaction (Figure 2b). The formation of β-enaminoesters is acid catalyzed, and the Cd2+ center acts as the Lewis acidic center and catalyzes the reaction between ethyl acetoacetate and aniline (Figure 2c).143 A two-dimensional (2D) MOF, [Cd(PBA)(DMF)]·DMF (Cd-PBA), (H2PBA = 5-(4-pyridin-3-yl-benzoylamino)-isophthalic acid), was found to be a catalyst for the base catalyzed (the N from the pyridine acts as the base center) Knoevenagel condensation reaction and the Lewis acid catalyzed (Cd centers) cyanosilylation of various aldehydes with trimethylsilyl cyanide.158
Figure 2.
(a) View of the structure of the MOF [Cd3(C10H4O7N1)2(8H2O)]·H2O with Cd centers (Lewis acidic) and an -NH moiety (Lewis basic). (b) Schematic of the possible reaction pathway for the base catalyzed aldol condensation reaction. (c) Schematic of the acid catalyzed enamine formation involving the Cd center. Reproduced with permission from ref (143). Copyright 2023 American Chemical Society.
There are many reports in the literature where only one of the functional groups (usually the Lewis basic functionality) was investigated toward catalytic studies. As mentioned earlier, in all the MOFs, the metal centers always act as Lewis acid centers. In most of the cases, it appears that the reaction of choice for the Lewis base catalyzed reaction is the Knoevenagel condensation (Table 3).
3.2. Tandem/Cascade Reaction
The chemical industries, generally, look to eliminate the number of steps in a chemical reaction process. Another impediment in many reactions is the need to isolate intermediates for further processing. Tandem/cascade reactions provide a possible alternative to reduce the need to isolate the intermediates and in that way also reduce the number of steps.161,162 To carry out such tandem reactions, it is necessary to have different catalytically active centers, preferably distributed uniformly, across the surface of the compound. It is even more important if the reaction requires both the acidic as well as basic functionalities. There is a need to replace multistep as well as salt forming chemical reactions. The approach that provides some success is the tandem reaction, where multiple reactions are combined in a sequential way to yield a single product. This approach is also known as “one-pot”, “domino”, and cascade reactions.53 There are many advantages in employing “tandem” reactions: (i) good atom economy; (ii) reduction of the formation of chemical wastes; (iii) reduction in the consumption of energy; (iv) no need to isolate any intermediates; etc. The tandem catalyses are known as concurrent tandem catalysis (CTC)53,163 and auto tandem catalysis (ATC).164 One of the important criteria in carrying out the tandem catalytic reactions is the compatibility of the catalyst toward the reactants, intermediates, and solvents. In addition, it is preferable to have the catalytically active centers separated spatially and in a periodic manner. It would be an added advantage if the catalyst can also host both acidic as well as basic catalytic centers. From the arguments as well as the descriptions above, the MOFs have positioned themselves to be an excellent candidate to investigate the multistep tandem/cascade reactions. The many cascade reactions that have been carried out using MOFs are summarized in Table 5. In this section, we provide select examples of such reactions. The reaction that was most studied as tandem reactions in MOFs is the deacetalization (acid catalyzed) followed by Knoevenagel condensation (base catalyzed).
Table 5. Summary of Tandem Deacetalization–Knoevenagel Reaction Employing Bifunctional MOFsa.
| Sr. No. | MOF compound | Reactive site | Reactants | Products | Reaction conditions | Ref |
|---|---|---|---|---|---|---|
| 1 | Yb-BDC-NH2Dy-BDC-NH2Sm-BDC-NH2 | Yb3+Dy3+Sm3+, -NH2 | BDA and MN | 2-benzylidenemalononitrile | DMSO-d6 (2 mL), catalyst (100 mg), 50 °C, 24 h | (291) |
| 2 | 3.1% Ru/UiO-66 | Ru2+, Zn2+ | BA and MN | 2-benzylidenemalononitrile | toluene (1.5 mL), O2 1 atm,100 °C | (292) |
| 3 | HNUST-8 | Cu2+, acylamide | BDA and MN | 2-benzylidenemalononitrile | DMSO, 50 °C, 48 h, 0.5 mol % HNUST-8 | (293) |
| 4 | HNUST-6 | Cu2+, acylamide | BDA and MN | 2-benzylidenemalononitrile | 0.5 mol % catalyst, DMSO, 50 °C, 48 h | (294) |
| 5 | [Zn5(L)4(OH)2(H2O)4]n·8n(DMF)·4n(H2O) | Zn2+, – NH | BDA and MN | 2-benzylidenemalononitrile | 1 mol % of catalyst 1 or 2, DMF (0.5 mL), 80 °C, 3 h | (295) |
| 6 | Cz-MOF-253-800 | Al3+, N | BD and MN | 2-benzylidenemalononitrile | 2 mL of toluene, and 5 mg of Pd/Cz-MOF-253–800, 80 °C, 17 h, 150 psi H2 | (296) |
| 7 | ZIF-8,UiO-66(Zr)-NH2,MIL-101(Cr)-NH2) | Zn2+, O2–, N–, -OH, -NH2 | BDA and MN | 2-benzylidenemalononitrile | 100 °C, 3 h in the Pickering emulsions consisting of water (3 mL)–toluene (2 mL) MOFs (50 mg) | (297) |
| 8 | CSMCRI-15 | Cd2+, -N=N–NH2 | BDA and MN | 2-benzylidenemalononitrile | 2 mol % catalyst, 4 h, 60 °C, solvent free | (298) |
| 9 | H-ZIF-8/Au@mSiO2 | Au, Zn2+, -N- | p-nitro BD and MN | 2-(4-nitrobenzylidene)malononitrile | catalyst (30 mg) and 2.0 mL tetrahydrofuran at 30 °C for 0.5 h | (299) |
| 10 | Hf-UiO-66-N2H3 | Hf, N2H3 | BD, MN | 2-benzylidenemalononitrile | ethanol (0.3 mL), catalyst (20 mg), RT, 4 h | (300) |
| 11 | NUC-29 | Cd2+, Npyridine | BDA, MN | 2-benzylidenemalononitrile | 1.0 mol %, based on the {Cd} center, DMSO 50 mL, 5 h, 70 °C | (301) |
| 12 | NUC – 53 | Zn2+, Npyridine | BDA, MN | 2-benzylidenemalononitrile | 0.3 mol %, DMSO 3 mL, 6h, 70 °C | (302) |
| 13 | UiO-67-(NH2)2 | Zr4+, -NH2 | BDA, MN | 2-benzylidenemalononitrile | ethanol (0.3 mL) and catalyst (15 mg) 10 h, 60 °C | (303) |
| 14 | IRA900(xOH)-MIL-101(Al)-NH2 | Al3+, -NH2 | BDA, MN | 2-benzylidenemalononitrile | solvent free, catalyst 0.3 g, 5 h at 110 °C | (304) |
| 15 | Cu(ABDC)(DMF) | Cu2+, -NH2 | BDA, MN | 2-benzylidenemalononitrile | d3-acetonitrile, 0.1 mol % catalyst, 24 h, 60 °C | (305) |
| 16 | Zr12BDC-NH2 | Zr4+, -NH2 | BDA, MN | 2-benzylidenemalononitrile | CDCl3 (1.5 mL), catalyst, 55 °C, 24 h, 100 mg | (306) |
| 17 | MIL-101(Cr)@PMF | Cr3+, -NH-NH2 | BDA, MN | 2-benzylidenemalononitrile | 30 mg catalyst, 12 h, 4 mL ethanol, 65 °C | (307) |
| 18 | [(CH3)2NH2]2[BaZn(TDP)(H2O)]·DMF·5H2O, (NUC-51) | Zn2+, Ba2+, -COOH, Npyridine | BDA, MN | 2-benzylidenemalononitrile | 1.0 mol % catalyst, 5 mL (DMSO) 4 h 70 °C | (308) |
| 19 | (Me2NH2)[InZn(TDP)(OH2)]·4DMF·4H2O (NUC-42) | Zn2+, In3+, −COOH, Npyridine | BDA, MN | 2-benzylidenemalononitrile | DMSO, 5 mL. 8h, 60 °C, 1 mol % catalyst | (309) |
| 20 | MIL-101(Cr)@MOF-867 | Cr3+, Zn2+, -C=N | BDA, MN | 2-benzylidenemalononitrile | 20 mg, 70 °C, 12 h, DMSO (4 mL) | (310) |
Abbreviations: BA = benzyl alcohol, MN = malononitrile; BDC-NH2 = 2-aminobenzenedicarboxylate; BDA = benzaldehyde dimethyl acetal; PDBAD = 4′,4‴-((pyridine-3,5-dicarbonyl)bis(azanediyl))bis[1,1′-biphenyl]-3,5-dicarboxylic acid; MOPBB = (5-methoxy-isophthaloyl)-bis(azanediyl)]diisophthalic acid; 1,4-BDC = 1,4-benzene dicarboxylate; BD = benzaldehyde; H3TCA = 4,4′,4′-tri- carboxytriphenylamine; DPA = (E)-1,2-di(pyridin-4-yl)diazene; 2-MeIm = 2-methylimidazole; 2- H2BDC-N2H3 = hydrazinyl-1,4-benzenedicarboxylic acid; H6TDP - 2,4,6-tris(2,4-dicarboxyphenyl)pyridine; ABDC = 2,2′-diamino-[1,1′-biphenyl]-4,4′-dicarboxylic acid; PMF = polymelamine formaldehyde.
3.2.1. Deacetalization–Knoevenagel Tandem Reaction
The functionalization of Cr-MIL-101, with amino and sulfo groups, through postsynthetic modifications, allowed the one-pot deacetalization–Knoevenagel tandem reaction.165 The general mechanistic pathway involves the acidic (Cr or SO3H) center to polarize the oxygen atom of the benzaldehyde dimethyl acetal to form benzaldehyde, which also increases the electrophilicity of the C center. The basic site helps in the nucleophile attack on the -C=O carbon, aiding the formation of the final product. The main outcome of this study appears to be the formation of the ammonium functionality, through proton transfer from the sulfonic acid to the amino group, which acts as the catalytic site. This approach of forming a zwitterionic form in a MOF could be an important development and would pave the way forward to carry out tandem reactions.165 The use of 5-sulfoisopthalic acid as the primary linker and 4,4′-azopyridine (I) and 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene (II) as the secondary linkers along with Cd2+ ions resulted in two different MOFs.166 Both the compounds were explored toward the one-pot tandem deacetalization–Knoevenagel condensation reactions due to the presence of Lewis acidic (Cd metal centers) as well as basic (azine, free pyridine, and uncoordinated sulfo oxygens) sites. This highlight of the study is in identifying the formation of aldehyde (Lewis acidic) as slower compared to the Knoevenagel reaction (Lewis basic). In the tandem reaction, the aldehyde formed by the Lewis acid catalysis was immediately consumed in the subsequent Knoevenagel reaction, which facilitates the forward reaction due to Le Chatelier’s principle.166 A detailed time dependent study clearly outlines the relative merits of the Lewis acidic as well as basic sites in the two-component stepwise cascade reaction (Figure 3).
Figure 3.

Kinetic study for the one-pot tandem deacetalization–-Knoevenagel reaction with I (a) and II (b) as a heterogeneous catalyst (solvent-free condition). Adapted with permission from ref (166). Copyright 2018 American Chemical Society.
The direct synthesis route was adapted in the preparation of PCN-700, where the Brönsted acidity was achieved by introducing H2TPDC-(COOH)2 [(1,1′,4′,1″-terphenyl)-2,2″,4,4″-tetracarboxylic acid] and the basicity by introducing H2BDC-NH2 (2-aminoterephthalic acid) in the framework (Figure 4).167 The modified compound, PCN-700-AB, was found to be a good catalyst toward the one-pot tandem reaction of benzaldehyde dimethyl acetal into benzylidene malononitrile. The spatial distribution of the acidic and basic sites in PCN-700-AB was found to effectively catalyze this cascade reaction. When the acidic sites were blocked by making an ester-CH3 group in the same MOF, PCN-MB (Figure S1), the tandem reaction yields were poorer.167 This clearly establishes the need to have both the acidic as well as the basic functionalities in the same compound for this reaction. A general scheme for this tandem reaction is given in Scheme 2.
Figure 4.
Structures of PCN-700, PCN-700-B, and PCN-700-AB. Hydrogen atoms are omitted for clarity. Reproduced with permission under a Creative Commons CC-BY 3.0 from ref (167). Copyright 2019 CCS Chemistry.
Scheme 2. Mechanism of the Deacetalization–Knoevenagel Condensation Reaction.
LA = Lewis acidic site; LB = Lewis basic site.
3.2.2. Other Tandem Reactions
Though the deacetalization–Knoevenagel reaction was the dominant cascade reaction investigated over many MOFs, there are other tandem reactions that have been explored as well (Table 6). For example, Cr-MOF (MIL-101-Cr) (Cr3(F)(H2O)2O[(O2C)C6H4(CO2)]3) was modified by PSM to generate MIL-101-SO3H-NH2, which was found to be a good catalyst toward the one-pot tandem catalytic reaction.168,169 The modified MOF was prepared through a postsynthetic route by sulfonation of the framework with chlorosulfonic acid in dichloromethane.170 This compound was found to exhibit catalytic activity toward a three-component condensation reaction between aromatic aldehydes, resorcinol, and malononitrile in aqueous medium, forming 2-amino-4H chromene by the Knoevenagel condensation reaction followed by the Michael reaction (Figure S2).170 The unmodified MOF, MIL-101-Cr, was found to give a lower yield of the desired product, and increased acidity by grafting -SO3H to the framework gave improved catalytic activity for the overall reaction (Scheme S2).
Table 6. Summary of Other Cascade Reactions Employing Bifunctional MOFsa.
| Reaction name | Sr. No. | MOF compound | Reactive site | Reactants | Product | Conditions | Ref |
|---|---|---|---|---|---|---|---|
| Multicomponent Hanstch Reaction | 1 | OMS-MIL-101(Cr) | Cr, NH2, | aromatic aldehydes, dimedone, β-ketoesters ammonium acetate | methyl 2,7,7-trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate | 4 mol % of cat., EtOH | (146) |
| 2 | MIL-101(Cr)-N(CH2PO3H2)2 | Cr -NH2, -PO3, -OH, O– | ethyl cyanoacetate or ethyl acetoacetate, hydrazine hydrate, malononitrile, aldehydes | N-amino-2-pyridone and pyrano [2,3-c]pyrazole | 5–10 mol % cat. | (139) | |
| Chromene Synthesis | 3 | Zn-Bp-BTC MOF | N, O–, -OH | benzaldehyde, malononitrile, and active methylene dimedone | 2-amino-4H-chromene | 0.06 mmol cat., ethanol | (147) |
| 4 | zinc(II)-L1/L2/L3/L4 | Zn, -NH2 | aldehyde malononitrile 1,3-diketone | chromene | EtOH (2 mL), RT, 2 h, air | (149) | |
| Henry Condensation–Fridel Craft Alkylation | 5 | (Zr)MOF-808 | Zr, lysine | benzaldehyde, nitromethane, indole | 1-(2-nitro-1-phenylethyl)-1H-indene | 25 mg cat., 30 °C | (148) |
| Oxidation–Knoevenagel Cascade Reaction | 6 | M2(BTC)(OH)(4-TPT)2(H2O)·(DMA)0.5(H2O) | Co, Ni, N | benzyl alcohol malononitrile | benzylidene | 0.25 mmol % cat., air, 400 μL CH3CN, n-dodecane, 12 h, 353 K | (150) |
| Ni3(BTC)2(4-TPT)2(H2O)6·1.5H2O | |||||||
| 7 | Cu3TATAT | Cu, N | benzyl alcohol malononitrile | benzylidene | 8 mol % cat., TEMPO (0.5 equiv), 5 mL CH3CN, 75 °C, 1 atm O2, 12 h | (151) | |
| 8 | H5PMo10V2O40@ MIL-101 | Mo, V, Cr | benzyl alcohol malononitrile | benzylidene | 0.5 mol % cat., toluene (1 mL) O2, sealed, 120 °C, 24 h | (153) | |
| Oxidation–imine Formation | 9 | SulP1MOF-808(Hf)-Ir/Rh | Rh/IrPO3, Hf | ketone, benzyl amine | N-(cyclopentylmethyl)aniline | 1.5 mol % catalyst in 5 mL toluene under 50 bar of H2 at 90 °C for 24 h | (145) |
| 10 | Zr/Hf-MOF-808 | Zr/Hf, defect -OH | aniline, benzyl alcohols | N,1-diphenylmethanimine | T = 120 °C, 0.6 mmol of catalyst and o-xylene as solvent, 2 h | (152) | |
| Knoevenagel Condensation–hydrogenation | 11 | IY-SO3H/Rh@S-ZIF-8 | -SO3H, Zn, N | p-nitrobenzaldehyde, malononitrile | 2-(4-aminobenzylidene) malononitrile | toluene (5 mL), 30 °C, 2 h; 2 MPa H2, 80 °C, 12 h | (221) |
PDEAEMA = poly[(2-diethylamino)ethyl methacrylate; IY = Integrated yolk; H6TATAT = 5,5′,5″-(1,3,5-triazine-2,4,6-triyl)tris(azanediyl)triisophthalate; AP = 2-aminopyridine; BTC = 1,3,5-benzene tricarboxylate; Bp = 4,4′-bipyridine; N-ferrocenylmethyl-N-butyl dithiocarbamate (L1); N-ferrocenylmethyl-N-ethylmorpholine dithiocarbamate (L2); N-ferrocenylmethyl-N-2-(diethylamino)ethylamine dithiocarbamate (L3); N-4-methoxybenzyl-N-3-methylpyridyl dithiocarbamate (L4).
The MOF Hf6(μ3-O)4(μ3-OH)4(HCO2)6((O2C)3C6H3)6/3, [MOF-808(Hf)], (Figure 5a) was modified postsynthetically to give rise to a bifunctional catalyst for the reductive amination of ketones. The compound MOF-808(Hf) was exchanged to incorporate sulfonated phenylphosphines without oxidation to give a ligand attached with the MOF (Figure 5b). This ligand (SulP1/-MOF-808(Hf), Figure 5b) complexes with Ir and Rh to give a bifunctional catalyst (SulP1/-MOF-808(Hf–Ir)), containing both the metal–phosphine complexes and the Lewis acidic framework (Hf metal sites). The metalated MOF is a good example of having possible a homogeneous catalyst anchored over a heterogeneous host, which helps in the tandem reductive amination and hydro aminomethylation reactions. The catalytic tandem reaction of functionalized acetophenones with benzyl amine derivatives under 50 bar of H2 at 90 °C gave the product N-(cyclopentylmethyl)aniline with good yield (>90%). The formation of the product involves Lewis acid catalysis of the aldehyde addition to the amines, forming the corresponding imines, which are reduced further by the Ir/Rh centers (Figure 5c).145 This work is reminiscent of the anchoring of chiral homogeneous noble metal catalysts within mesoporous MCM-41 toward superior performance in allylic amination and other reactions.2
Figure 5.

(a) Structure of MOF-808. (b) Schematic of the postsynthetic exchange of sulfonated phosphines for formate groups on MOF-808(Hf). (c) Scheme for the reductive amination reaction. Reproduced with permission from ref (145). Copyright 2018 John Wiley and Sons.
A chromium MOF, OMS-MIL-101(Cr) (OMS = open metal site), was reacted with ethylene diamine, which creates basic centers in addition to the Cr acidic centers (Figure 6a).146 This catalyst was useful toward multicomponent Hantzsch reactions. The condensation between aromatic aldehydes, dimedone, β-ketoesters, and ammonium acetate gave the product polyhydroquinoline with good yield (>98%) (Figure 6b).146 The mechanism proceeds via the activation of the aryl halides over the acidic Cr3+ sites, and the -NH2 group helps in activating the β-ketoesters.146 The grafting of lysine (2,6-diamino-hexanoic acid) to (Zr)MOF-808 allowed exploration of the multifunctionality by carrying out two sequential reactions: (i) Henry condensation (base catalyzed) and (ii) Friedel/Crafts type alkylation (acid catalyzed) in one-pot solvent-free conditions.148 In this cascade reaction, both grafted basic sites (aliphatic amino groups) and framework acid sites (coordinatively unsaturated Zr sites) were employed.
Figure 6.
(a) Schematic illustration for the preparation of ED/MIL-101(Cr). (b) Plausible mechanism for MIL-101(Cr)-NH2 catalysis of the Hantzsch reaction. Reproduced with permission from ref (146). Copyright 2018 John Wiley and Sons.
A detailed kinetic study indicated that the former step is slower than the later (Figure S3). The combination of naphthalene dicarboxylic acid as the primary ligand and 1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene (3-BPDB) as the secondary ligand gave three-dimensional structures of Zn and Cd. The Lewis acid character (Zn/Cd center) was employed toward the condensation of amino benzaldehyde and ketones (Friedländer reaction). The Lewis basic character (-N=N-) was used for the three-component condensation that involves Knoevenagel and Michael reactions.142 In this study, a cascade reaction of two distinct base catalyzed mechanisms has been investigated. Anchoring CuI over modified UiO-67 was found to be a good catalyst for the azide–alkyne cycloaddition reaction.154 The mechanism involves mediated alkyne interactions followed by the azide reaction forming the triazole derivatives. It is a different approach, as metal salts have been anchored over the MOFs instead of simple metal nanoparticles.
In a recent work, for the first time, a 4-step cascade reaction was carried out using the same strategy of spatially separated Lewis acid and base functionality in [Zn2(SDBA)(3-ATZ)] (SDBA = 4,4′-sulfonyldibenzoic acid; 3-ATZ = 3-amino 1,2,4-triazole) (Figure 7a).171 The different steps that were studied involve deacetalization (Lewis acid catalyzed), Henry, and Michael reactions (Lewis base catalyzed) (Scheme 3). This reaction was possible due to the presence of additional basic sites in the MOFs (primary amine, -NH2, and sulfonyl oxygen atoms). The four-step reaction consists of the following: the first reaction is the formation of an aldehyde (B) from the benzaldehyde dimethyl acetal (A) (Lewis acid catalyzed); the second reaction is between the nitroalkane and the aldehyde, forming (Henry reaction) 2-nitro-1-phenyl ethanol (C) (Lewis base catalyzed); the third reaction is the dehydration of (C) to give trans β-nitrostyrene (D) (Lewis base catalyzed); and finally reaction between nitromethane and (D) gives (1,3-dinitropropan-2-yl)benzene (E) (Scheme 3). The time dependent study clearly indicates that the dehydration of 2-nitro-1-phenyl ethanol to trans-β-nitrostyrene was the rate limiting step and that the Le Chatlier’s principle was in action for the reaction to proceed in the forward reaction. It is clearly a good example where the bifunctionality of the MOF was exploited toward a cascade reaction that involved multiple steps. The number of steps (3) in the base catalyzed reactions is more compared to that of the acid catalyzed one in this cascade reaction. The presence of the primary -NH2 group, which is a strong base, helped in this reaction171 (Figure 7b).
Figure 7.
(a, b) Connectivity between the Zn-ATZ layers through the acid ligand (SDBA) in I and II. (c, d) Time dependent study of the one-pot tandem four-step deacetalization–Henry–Michael reactions for I and II. Reprinted with permission from ref (171). Copyright 2023 American Chemical Society.
Scheme 3. Summary of the Four-Step Cascade Reaction Involving the Deacetalization–Henry–Michael Reaction.
Reproduced with permission from ref (171). Copyright 2023 American Chemical Society.
3.2.3. Oxidation–Knoevenagel/Amination Cascade Reaction
The compounds M(BTC)(OH)(4-TPT)2(H2O)·(DMA)0.5(H2O)2 (H3BTC = 1,3,5-tribenzoic acid; 4-TPT = 2,4,6-tris(4-pyridyl)-1,3,5-triazine; M = Co, Ni) and Ni3(BTC)2(4-TPT)2(H2O)6·1.5H2O have terminal H2O molecules coordinated to the metal centers (Co/Ni) which can be removed by heating (Figure 8a).150 This creates an open coordination site at the metal center, which can act as Lewis acidic center. The secondary ligand 4-TPT can be the Lewis basic center. The compounds were found to be good catalysts for the oxidation of benzyl alcohol to benzaldehyde (acid catalyzed) followed by reaction with malononitrile to give benzilidine malononitrile (base catalyzed) (Figure 8b).150 A similar reaction was also carried out over the Cu3TATAT MOF (H6TATAT = 5,5′,5″-(1,3,5-triazine-2,4,6-triyl) tris(azanediyl)triisophthalate) compound.151
Figure 8.
(a) Structure of MOF highlighting the acidic and basic centers. (b) Schematic of the oxidation–Knoevenagel condensation reaction. Reproduced with permission from ref (150). Copyright 2021 Royal Society of Chemistry.
The use of Zr/Hf-MOF-808 toward the synthesis of secondary amines was established by reacting anilines and benzyl alcohols. This reaction does not require any additional base and/or external H2.152 The reaction proceeds through the deprotonation of the alcohol by the metal center followed by the dehydrogenation to form benzaldehyde, which reacts with the amino group of the aniline, forming the final benzylaniline product (Scheme 4). There are some important observations in this reaction. The presence of a defective -OH group in the Hf-cluster metal center enhances the acidity and helps in the formation of benzaldehyde in the first step. The captured proton at the defective -OH group site also aids in the formation of the final product by reacting with the imine nitrogen formed through the condensation of the aldehyde and amine (Scheme 4). This is an interesting strategy where the proton is captured initially and later released during the final step to give the desired product.
Scheme 4. Possible Mechanism for the N-Alkylation Reaction of Aniline with Benzyl Alcohol to Form the N-Benzylaniline Product.

Adapted with permission under a Creative Commons (CC BY 4.0) from ref (152). Copyright 2021 American Chemical Society.
The encapsulation of polyoxometalate H5PMo10V2O40 (PMoV2) into the cages of an alkylamine-modified MIL-101 was employed for the aerobic oxidation–Knoevenagel one-pot tandem reaction (Scheme S3). The main observation in this reaction is that it does not use any noble metals for the aerobic oxidation of the alcohols.153
3.3. Anchoring Metal Nanoparticles toward Catalysis
The development of metal nanoparticles during the 90s and the associated advancements in nanoscience provide an important opportunity to explore multifunctionality in MOFs.172−174 It may be noted that the use of well distributed supported metal catalysis (e.g., Pd or Pt in Al2O3, SiO2, zeolites, and mesoporous silica) is one of the earlier examples of bifunctional catalysts.175−177 Many of the supported catalysts are useful toward the “spillover” reactions involving hydrogen.178 The noble metal and the support influence the electronic state of the metal along with its precise morphology. Today, it has been possible to prepare noble metal nanoparticles with controlled sizes and shapes,179−182 which may be of importance in heterogeneous catalysis. The noble metal nanoparticles can be stabilized by anchoring the particles through suitable functional groups—most notably the thiol (-SH) functionality. The usefulness of noble metal centers for diverse catalytic reactions has been known over many decades.183−187 Many of these earlier reactions are homogeneous catalytic reactions, where the recyclability of the catalyst would be difficult. In recent years, it has been possible to prepare atom precise metal nanoclusters and assemble them into extended structures.188−192 In addition, the ease of functionalizing the ligands that can be used in the preparation of MOFs opens up an interesting possibility toward anchoring metal nanoparticles at precise locations within the MOFs. The MOFs provide reasonable thermal and chemical stability and pore and channel sizes which can be gainfully employed to explore the usefulness of metal nanoparticles toward catalytic reactions. This approach would be similar to carrying out the homogeneous reactions within heterogeneous surroundings. Catalytic reactions of this nature were attempted by anchoring organometallic complexes directly into the mesoporous compounds, notably on MCM-41 and related ones.193−195
Most of the MOFs possess Lewis acidity, and the functional ligand provides the necessary additional reactive center (either Bronsted acidic or basic). These MOFs are already bifunctional and the anchoring of metal nanoparticles adds to the existing functionality. The main advantages of anchoring the metal nanoparticles are their small (nano) size and the availability of periodically placed metals over the surface of the MOFs, which would be useful toward many heterogeneous catalytic reactions. Much of the earlier work on such type of nanoparticle anchored MOFs was termed “ship in a bottle” catalysts.196,197 The usefulness of this approach was shown by anchoring Au nanoparticles in a MOF, Au@Cu(II)-MOF, toward a tandem oxidation–Knoevenagel condensation reaction that involves the conversion of benzyl alcohol to benzylidene malononitrile with good yield, conversion, and selectivity.198 Similarly, Pd@Ni-MOF was shown to be a good catalyst toward Suzuki coupling of aryl chlorides.199 It is notable that many reactions involving Suzuki coupling are carried out by employing aryl bromides and iodides and that the presence of Pd nanoparticles uniformly distributed in a heterogeneous environment (Pd@Ni-MOF) converts the aryl chlorides with good yield as well as recyclability.199 Many studies of this nature have been known in the literature.200−205 The important nanoparticle loaded MOFs and their utility in heterogeneous catalytic studies are listed in Table 7.
Table 7. Nanoparticle Loaded MOFs and Their Utility in Catalysisa.
| Sr. No. | MOF compound | Reactive site | Nanoparticle | Catalytic reaction | Ref |
|---|---|---|---|---|---|
| 1 | Au@ZIF-8 | Zn2+/-Nim | Au | hydrogenation of n-hexene | (311) |
| 2 | nFe3O4@Pd/ZIF-8@ZIF-8 | Zn2+/-Nim | Fe3O4/Pd | hydrogenation of styrene | (312) |
| 3 | UiO-66-biguanidine/Pd | Zr4+/-NH2 | Pd | Suzuki–Miyaura coupling | (313) |
| 4 | Ag@UiO-66-SH | Zr4+/-NH2 | Ag | three-component A3 coupling | (314) |
| 5 | AgPd@MIL-125-NH2-PDA | Cr3+/-NH2 | Ag-Pd | Suzuki coupling reaction | (315) |
| hydrogenation of aldehyde | |||||
| 6 | MOF-Pd NPs | Cu2+/-NH2 | Pd | aerobic oxidation of benzyl alcohol | (316) |
| 7 | Pt@UiO-66-NH2 | Zr4+/-NH2 | Pt | synthesis of nitrones | (173) |
| 8 | Au-NP/Ni-Cu MOF | Ni2+/Cu2+ | Au | chemical degradation of Rhodamine B | (317) |
| 9 | Au@Cu(II)-MOF | Cu2+/-N | Au | oxidation–condensation reactions | (198) |
| 10 | Co–MOF-74@Cu–MOF-74 | Co2+/Cu2+ | CoCu | 1, 4-diphenyl-1,3-butadiene from phenylacetylene | (209) |
| 11 | Pd(0)@UiO-68-AP | Zr4+/-N | Pd | oxidation–Knoevenagel condensation | (215) |
| 12 | Au/NH2–UiO-66 | Zr4+/-NH2 | Au | tandem reaction | (216) |
| 13 | Pd-Au@Mn(II)-MOF | Mn(II)/N | Pd-Au | alcohol to imines | (217) |
| 14 | Al-ITQ-SO3H/Pd | Al(III), SO3H | Pd | oxidation–acetalization | (219) |
| 15 | IY-SO3H/Rh@S-ZIF-8 | Zn(II)SO3H | Rh | Knoevenagel condensation–hydrogenation reaction | (221) |
| 16 | Pd@UiO-66(Hf) | Hf(IV) | Pd | Hantzsch reaction | (318) |
| 17 | Pd@NH2-UiO-66 | Zr(IV)/-NH2 | Pd | Suzuki coupling/asymmetric aldol condensation | (224) |
| 18 | Ni@ZrOF | Zr(IV)/ -NH2 | Ni | chemical fixation of CO2 | (319) |
| 19 | Au@[Zn14(L)6(O)2(H2O)3] | Zn(II) | Au | chemical fixation of CO2 | (249) |
| 20 | Ag(I)@MOF-NHC | Zn(II)/-N | Ag | chemical fixation of CO2 | (250) |
| 21 | Pt@MOF-5, Pt@UiO-66, Pt@UiO-66-NH2 | Zr(IV)/-NH2 | Pt | biomass valorization | (320) |
| 22 | [Zn(4-bpdh)]3DMF | -N | Pd | Sonogashira coupling reaction | (321) |
| 23 | Ag/UiO-66 | Zr4+ | Ag | 3,4-dihydropirimidin-2(1H)-one synthesis | (322) |
| 24 | Au/MOF-199 | Cu2+ | Au | A3-coupling reaction | (323) |
| 25 | metal/UiO-66 | Zr4+ | Pt, Pd, Ru | oxidation of volatile organic compound | (324) |
| 26 | Pd(0)@UiO-68-AP | Zr, -NH | Pd | oxidation–Knoevenagel cascade reaction | (215) |
| 27 | Au@NH2-UiO-66 | Zr, -NH2 | Au | oxidation–Knoevenagel cascade reaction | (216) |
| 28 | Pd-Au@Mn(II)-MOF | Mn, Npyr | Pd, Au | oxidation–imine/acetal formation cascade reaction | (217) |
| 29 | Pd@MIL-101 | Cr, N | Pd | oxidation–imine/acetal formation cascade reaction | (218) |
| 30 | Pd@Al-MOF | Al, −SO3H | Pd | oxidation–imine/acetal formation cascade reaction | (219) |
| 31 | Pd@PDEAEMA-g-UiO-66 | Zr, -NH2 | Pd | Knoevenagel condensation–hydrogenation | (220) |
| 32 | Pd/MIL-101-SO3H | -SO3H | Pd | hydrogenation esterification cascade reaction | (222) |
| 33 | Pd@UiO-66(Hf) | Zr | Pd | hydrogenation esterification cascade reaction | (223) |
OPNs = organic polymer networks; PDA = polydopamine; AP = aminopyridine; NHC = N-heterocyclic carbene; 4-bpdh = 2,5-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene.
As discussed above, anchoring of noble metal particles supported over the MOFs was found to be dominant in many catalytic reactions. There have been attempts at synthesizing 3d metal nanoparticles, at the expense of the MOF framework, by anchoring them over carbonized MOFs.206−208 It has been shown that MOFs when heated in an inert atmosphere (Ar atmosphere) or under vacuum at elevated temperatures form carbon with the metals distributed over the carbon. This carbonization process, in general, results in the formation of different forms of carbon such as amorphous carbon, graphitized carbon, or a mixture of both. Depending on the composition of the MOFs, the resulting metal nanoparticles can be either single nanoparticles or bimetallic nanoparticles supported over the amorphous carbon. It has been known that amorphous carbon can act as a good support toward many catalytic reactions.209−214 The bimetals supported over the carbonized MOFs can be exploited toward catalytic reactions where the differences in the catalytic activity of the different metals would be important.
This strategy was employed toward the preparation of Co-Cu bimetallic nanoparticles supported on carbon (Co-C@Cu-C) by pyrolyzing Co/Cu-MOF-74.209 This bimetallic catalyst was found to be a good catalyst for the conversion of phenylacetylene to 1,4-diphenyl-1,3-butadiene. This reaction involves C–C coupling as well as hydrogenation reactions, and the reaction proceeds over the Co centers. The possible mechanism for this cascade reaction is given in Scheme 5. Phenylacetylene is initially activated by metallic Cu NPs, forming Cu+-phenylacetylide complex on the surface of Cu NPs. The metallic Co NPs in Co-C@Cu-C help in the dissociation of NaBH4 to form Co-BH3 and CoH, which act as the active species for the hydrogenation of the phenylacetylene. The proposed mechanism also involves the migration the hydrides on Co nanoparticles to diffuse to interact with the adsorbed Cu+-phenylacetylide complex. The remaining hydrides in BH3(i-PrO)− dissociate over the metallic Co NPs, which helps in the spillover of hydrogen to react with the adsorbed Cu+-phenylacetylide complex. The in situ formed complex between CuI-phenylacetylide dimerizes to form 1, 4-diphenyl-1,3-butadiene.
Scheme 5. Co-MOF-74@Cu-MOF-74 Derived Bifunctional Co-C@Cu-C for One-Pot Production of 1,4-Diphenyl-1,3-butadiene from Phenylacetylene.

Reprinted with permission from ref (209). Copyright 2020 John Wiley and Sons.
The pyrolysis of a cobalt MOF, [Co(C14H8O6)(C10H8N2)2H2O)]·(C3H7NO), results in Co supported on amorphous/graphitized carbon. The Co nanoparticles were shown to be a good green catalyst for the selective reduction of nitroarenes to amines in the presence of the hydrazine as a hydrogen source.199 The Co metal particles supported on the carbon matrix help in the decomposition of hydrazine to produce the hydrogen in situ. The hydrogen spillover helps in the reduction of nitroaromatics to the aniline derivatives. The examples clearly support the spillover effect in employing metal nanoparticles toward hydrogenation reactions. A mixed precursor containing Cu, Co, and Ni introduced into MIL-101 was reduced in situ with NH3BH3 to give Cu@Co@Ni NPs inside MIL-101 pores. The compound Cu@Co@Ni/MOF catalyzes nitroarene hydrogenation in the presence of NH3BH3, which supplies the necessary hydrogen for the reaction.204
3.3.1. Cascade Reactions Involving Anchored Nanoparticles
A bifunctional Pd(0)@UiO-68-AP catalyst, prepared using the postsynthetic approach, catalyzes aerobic oxidation of benzyl alcohol by the Pd NPs followed by reaction with malononitrile via the Knoevenagel condensation, forming benzilidine malononitrile.215 The oxidation of the benzyl alcohol is promoted by the Pd catalyst whereas the Knoevenagel condensation is facilitated by the basic nitrogen center.215 A similar reaction was also carried out employing Au nanoparticles anchored over NH2-UiO-66,216 for the selective oxidation of primary alcohols in tandem with Knoevenagel condensation reactions. A Mn(II) MOF, (MnL2)·2CH3OH (L = 4,4,4-trifluoro-1-(4-(pyridin-4-yl)phenyl)butane-1,3-dione), with Pd-Au bimetallic alloy nanoparticles (Pd-Au@Mn(II)-MOF) was employed as a bifunctional heterogeneous catalyst for the one-pot tandem synthesis of imines from benzyl alcohols and aniline (Figure S4). The oxidation reaction was catalyzed by nanoparticles, and the imine formation was due to the N-containing ligand.217 Similar reactions have also been carried out with Pd nanoparticles (NPs) encapsulated in MIL-101.218 An aluminum MOF, Al-ITQ-SH, having thiol units was used to anchor Pd nanoparticles, and the thiol moieties were converted into sulfonic groups (Bro̷nsted acid).219 This compound catalyzes a one-pot, two-step oxidation–acetalization reaction where the oxidation of benzyl alcohol into benzaldehyde under an O2 atmosphere was catalyzed by Pd nanoparticles, followed by the acetalization of aldehydes employing the Brönsted acid functionality. A Pd NP loaded and pH-switchable polymer-grafted UiO-66-MOF, Pd@PDEAEMA-g-UiO-66, (PDEAEMA = poly[(2-diethylamino)ethyl methacrylate]), was employed toward a biphasic Knoevenagel condensation–hydrogenation cascade reaction using different atmospheres (Figure 9a). The Knoevenagel condensation step was carried out under air atmosphere, whereas the hydrogenation reaction was carried out in hydrogen atmosphere (Figure 9b).220
Figure 9.
(a) Schematic of synthesis of Pd@PDEAEMA-g-UiO-66 through PSM. Yellow balls: Pd nanoparticles. (b) Knoevenagel condensation followed by hydrogenation cascade reactions over the Pd@PDEAEMA-g-UiO-66 catalyst. Reproduced with permission from ref (220). Copyright 2017 American Chemical Society.
A bifunctional composite compound with a macro-/microporous ZIF-8 shell and rhodium nanoparticles anchored over sulfonated cross-linked polystyrene was prepared to carry out the Knoevenagel–hydrogenation reaction.221 The reactants containing the aldehyde and malononitrile in toluene solvent under a pressure of hydrogen (2 MPa) gave the benzilidene malononitrile product in near ∼100% yield. The composite catalyst was found to be robust with negligible leaching of the anchored Rh nanoparticles.221 Another reaction that utilizes nanoparticle supported over MOFs is the hydrogenation–esterification reaction. The hydrogenation is catalyzed by the noble metal nanoparticles, and the esterification reaction is catalyzed by the acidic sites. The compound MIL-101-SO3H was appended with Pd nanoparticles to give Pd/MIL-101-SO3H.222 This compound was active to convert furoic acid (FA) (biomass derived) into value chemicals such as ethyl tetrahydro-2-furoate (ETF). The FA was esterified with ethanol, forming ethyl furoate (EF), which was hydrogenated to give ETF (yield = 100%, selectivity >99%) (Scheme 6). A similar reaction has also been attempted over Pd@UiO-66(Hf).223
Scheme 6. Scheme of the Cascade Reaction Process from FA to ETF.
Reproduced with permission from ref (222). Copyright 2019 Royal Society of Chemistry.
The use of bifunctionality was shown elegantly by adding an l-proline functionality to -NH2-UiO-66, which also had Pd nanoparticles through PSM. This modified MOF was shown to be a chiral catalyst toward Suzuki coupling (Pd centered) followed by asymmetric aldol condensation (N centered). The reaction between 1,4-bromobenzaldehyde and phenylboronic acid in EtOH–H2O was catalyzed by Pd@NH2-UiO-66(pro) and K2CO3 at 80 °C.224
As described above, the overall activity of the MOFs has been enhanced by having the nanoparticles. Though most of the studies concentrated on noble metals, there have been efforts toward other transition metals as well. As can be noted, in most of the cases one finds a good synergistic and cooperative effect between the metal nanoparticles and the overall functionality of the MOFs. The reactions, in a way, replicate many of the well-established and known organometallic catalyses in these compounds. This effort paves the way for carrying out homogeneous catalysis under a heterogeneous environment. It is likely that many other reactions would also be attempted in the future to reap the benefits of having well dispersed nanomaterials in bifunctional MOFs. This approach really makes the MOF compounds truly multifunctional.
3.4. Fixation of Atmospheric CO2
One of the important issues of concern is the control of CO2 in the atmosphere. CO2 has been designated as an important greenhouse gas, and it is desirable to convert it to other useful chemicals.225−230 There have been attempts to convert the CO2 under atmospheric conditions, both through the catalytic route231−233 as well as electrochemically.234−236 The intense research, over the years, has involved conversion of CO2 into cyclic carbonate, carboxylation, reductive N-functionalization of various amines with CO2 to furnish N-formyl compounds, and carboxylative cyclization of propargyl amines and alcohols.237−241 In many of these attempts, organometallic compounds, nanoparticle grafted compounds, and others have been explored.242 There have been considerable efforts toward fixing CO2 at atmospheric pressures employing MOFs, notably bifunctional ones. Some of the important studies toward the fixation of CO2 employing MOFs are listed in Table 8.
Table 8. Summary of Atmospheric CO2 Fixation over MOFsa.
| Sr. No. | MOF | Reactive site | Reactant | Product | Reaction conditions | Ref |
|---|---|---|---|---|---|---|
| 1 | (I–)Meim-UiO-66 | Zr4+, I– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 0.1 MPa, 120 °C, 24 h | (248) |
| 2 | MIL-IL(A) | Cr3+, Br– | SO | 4-phenyl-1,3-dioxolan-2-one | 2 MPa, 110 °C, 2 h | (325) |
| MIL-IL(B) | ||||||
| 3 | MIL-IMAc-Br– | Cu2+-COOH, Br– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 0.5 MPa, 60 °C, 24 h | (81) |
| 4 | Mg-MOF-74 | Mg2+, Co2+, OH | SO | 4-phenyl-1,3-dioxolan-2-one | 2 MPa, 100 °C, 4 h | (326, 327) |
| Co-MOF-74 | ||||||
| 5 | UiO-66-NH2 | Zr4+, NH2 | SO | 4-phenyl-1,3-dioxolan-2-one | 2.0 MPa, 100 °C, 4 h | (328) |
| 6 | BIT-101 | Zn2+, COO | PO | 4-methyl-1,3-dioxolan-2-one | 3 MPa, 160 °C, 24 h | (329) |
| BIT-102 | ||||||
| BIT-103 | ||||||
| 7 | 1-NH2 | CONH, NH2 | ECH, PO | 4-(chloromethyl)-1,3-dioxolna-2-one-4-methyl-1,3-dioxolan-2-one | 0.1 MPa, 90 °C, 50 h, 3 MPa, 100 °C, 6 h | (330) |
| 8 | UMCM-1-NH2 | Zn4O, NH2 | PO | 4-methyl-1,3-dioxolan-2-one | 1.2 MPa, 120 °C, 24 h | (331) |
| 9 | Zn-TATAB | Zn2+, NH | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 atm, 100 °C, 16 h | (332) |
| 10 | Co/Ni-TATAB | Co2+, Ni2+, NH | ECHECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 bar, 80 °C, 15 h | (279) |
| 11 | Zn-BTC-2MeIm | Zn2+, 2MeIm | ECH, PO | 4-(chloromethyl)-1,3-dioxolan-2-one | 3.0 MPa, 100 °C, 6 h | (333) |
| 12 | Co(tp)(bpy) MOF-508a | Co2+, Zn2+, free pyridine N | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 MPa, 100 °C, 8 h | (334) |
| 13 | (Me2NH2)2·[Zn8(Ad)4(DABA)6O]·7DMF}n | Zn2+, Ad, and NH2 | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 bar, 100 °C, 24 h | (335) |
| 14 | ZIF-8 | Zn2+, Im | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 7 bar, 100 °C, 4 h | (336) |
| 15 | ZIF-90 | Zn2+, CHO | PO | 4-methyl-1,3-dioxolan-2-one | 2 MPa, 120 °C, 8 h | (337) |
| 16 | CZ-ZIF | Zn2+/Co2+, HmIm | ECHPO | 4-(chloromethyl)-1,3-dioxolan-2-one | 7 bar, 100 °C, 4 h | (338) |
| 17 | ZIF-67 | Co2+, HmIm | ECHPO | 4-(chloromethyl)-1,3-dioxolan-2-one | 10 bar, 120 °C, 6 h | (339) |
| 18 | Ti-ZIF | Ti4+, Im | SO | 4-phenyl-1,3-dioxolan-2-one | 1.72 bar, 100 °C, 8 h | (340) |
| 19 | MIL-101-P(n-Bu)3BrMIL-101-N(n Bu)3Br | Cr3+, Br– | POPO | 4-methyl-1,3-dioxolan-2-one | 2.0 MPa, 80 °C, 8 h | (341) |
| 20 | F-ZIF-90 | Zn2+, I– | AGE | 4-((allyloxy)methyl)-1,3-dioxolan-2-one | 1.17 MPa, 120 °C, 6 h | (342) |
| 21 | IL-ZIF-90 | Zn2+, I– | PO | 4-methyl-1,3-dioxolan-2-one | 1.0 MPa, 120 °C, 3 h | (343) |
| 22 | ZnTCPP⊂(Br)Etim-UiO-66 | Zn2+, Br– | AGE | 4-((allyloxy)methyl)-1,3-dioxolan-2-one | 1 bar, 140 °C, 14 h | (344) |
| 23 | Salen-Co(23%)⊂(Br–)Etim-UiO- 66 | Co3+, Br– | SO | 4-phenyl-1,3-dioxolan-2-one | 0.1 MPa, 120 °C,12 h | (345) |
| 24 | UiO-67-IL | Zr4+, Br– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 atm, 90 °C, 12 h | (346) |
| 25 | FJI-C10 | Cr3+, Br– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 bar, 80 °C, 12 h | (347) |
| 26 | IL@MIL101-SO3H(4) | Cr3+, Br– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 atm, 90 °C, 24 h | (348) |
| 27 | MIL-101-IMBr-6 | Cr3+, Br– | PO | 4-methyl-1,3-dioxolan-2-one | 0.8 MPa, 80 °C, 4 h | (349) |
| 28 | IL/MIL-101-NH2 | -COOH, NH2 | PO | 4-methyl-1,3-dioxolan-2-one | 1.3 MPa, 120 °C, 1 h | (350) |
| 29 | polyILs@MIL-101 | Cr3+, Br– | SO | 4-phenyl-1,3-dioxolan-2-one | 1 bar, 70 °C, 48 h | (351) |
| 30 | MIL-101-IP | Cr3+, Br– | PO | 4-methyl-1,3-dioxolan-2-one | 1 atm, 25 °C, 48 h | (352) |
| 31 | IL@ZIF-8(Zn/Co) | Zn2+, Co2+, Br– | SO | 4-phenyl-1,3-dioxolan-2-one | 1 atm, 100 °C, 24 h | (353) |
| 32 | F-IRMOF-3-2d | Zn4O, -NH2, I– | POPOPO | 4-methyl-1,3-dioxolan-2-one | 2 MPa, 140 °C, 1.5 h | (354) |
| F-IRMOF-3-4d | ||||||
| F-IRMOF-3-6d | ||||||
| 33 | F-IRMOF-3(MeI) | Zn-OH, I– | AGE | 4-((allyloxy)methyl)-1,3-dioxolan-2-one | 1.2 MPa, 120 °C, 6 h | (355) |
| F-IRMOF-3(BuI) | ||||||
| 34 | MIL-101-tmzOH-Br | Cr3+, OH–, Br– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 MPa, 80 °C, 2 h | (356) |
| 35 | 2MeIm@Co-BTC-x | Co2+, MeIm | ECHECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 3.0 MPa, 90 °C, 5 h, 3.0 MPa, 90 °C, 7 h | (357) |
| 36 | Cr-MIL-101-[BuPh3P]Br | Cr3+, Br– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 MPa, 80 °C, 2 h | (358) |
| 37 | [TMPyPMn(I)]4+(I–)4@ZIF-8 | Zn2+, Mn2+, I– | ECH | 4-(chloromethyl)-1,3-dioxolan-2-one | 1 bar, 100 °C, 36 h | (359) |
| 38 | [Zn(II)NMeTPyP]4+[I–]4@PCN- 224 | Zn2+, I– | ECHPO | 4-(chloromethyl)-1,3-dioxolan-2-one-4-methyl-1,3-dioxolan-2-one | 0.8 MPa, 90 °C, 24 h | (360) |
Abbreviations: ECH = epichlorohydrin; PO = propylene oxide; SO = styrene oxide; AGE = allyl glycidyl ether; dhtp = 2,5-dihydroxyterephthalate; NH2-BDC = 2-aminoterephthalate; aip = 5-aminoisophthalic acid; NIP = 5-nitroisophthalic acid; L = N4,N4′-di(pyridine-4-yl) biphenyl-4,4′-dicarboxamide; Im = imidazole; BTC = 1,3,5-benzene tricarboxylate; HmIm = 2-methylimidazole; ICA = imidazole 2-carboxaldehyde; TATAB = 4,4′,4″-s-triazine-1,3,5-triyl-tri-p-aminobenzoic acid; DABCO = 1,4-diazabicyclo[2.2.2]-octane; MIL = Materials Institute Lavoisier; UiO = University of Oslo; BIT = Beijing Institute of Technology; Hip = 5-hydroxyisophthalic acid; Bpy = 4,4′-bipyridine; BTB = 1,3,5-tris(4-carboxyphenyl)benzene; DMF = N,N′-dimethylformamide; Ad = adeninate; DABA = 2,2′-dimethyl- 4,4′-azobenzoate; 2-MeIm = 2-methylimidazole; ZIF = zeolitic imidazolate framework; 2-F-BIM = 2-(furan-2-yl)-1H-benzo[d]imidazole; TCPP = 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin; IL = ionic liquid; ICA = imidazole-2-carboxyaldehyde; Etim = ethyl imidazolium; polyILs = poly(ionic liquids); IP = ionic polymer; MeI = methyl iodide; BuI = butyl iodide; IRMOF = isoreticular metal–organic framework; IMAc = 1H-imidazole-1-acetic acid; PCN = Porous coordination network; Zn(II)NMeTPyP = 5,10,15,20-tetrakis(1-methylpyridinium-4-yl) zinc(II) porphyrin; [(Etim-H2BDC)+(Br)] = 2-(3-ethyl-imidazol-1-yl)-terephthalic acid; ImBDC = 2-(imidazole-1-yl)terephthalate; salen-Co(III) - N,N′-bis(3-carboxylsalicylidene)-1,2-cyclohexanediamino cobalt(III) acetate; [BuPh3P]Br = 4-(bromobutyl)triphenylphosphonium bromide; MPImBr = 1-methyl-3-propylimidazolium bromide; HmIm = 2-methylimidazole; 2-Br-BDC = 2-bromoterephthalate; L = (Br) allylium-2-bp functionalized biphenyl dicarboxylic acid.
Tetra(4-pyridyl)porphyrin (H2TPyP) was employed along with 4,4′-oxybis(benzoic acid) (H2OBA) to form a 2D compound, [Zn2(C40H24N8)0.5(C14H8O5)(DMA)](DMA)(H2O)6.243 The Zn2+ ions were ion-exchanged with the Cu2+ in a postsynthetic modification, and the Cu compound was employed for the cyclic carbonate formation reaction with tetra-n-tert-butylammonium bromide (TBAB) as a cocatalyst, which gave cyclic carbonates with high yield.243
A plausible reaction mechanism for the cycloaddition of CO2 to cyclic carbonates using bifunctional MOFs is given in Scheme 7. The zirconium-phosphonate MOF Zr(H4L) (H8L = tetraphenylsilane tetrakis-4-phosphonic acid) was shown to be a good catalyst toward the CO2/epoxide coupling reaction. The presence of a Bro̷nsted acid (protonated phosphonate) along with the Lewis acidic (Zr metal) functionality helped in catalyzing the reaction forming cyclic carbonates (Figure S5).244 A copper MOF, [Cu(2,5-BPTA)(bpy)(H2O)] (2,5-BPTA = 2,5-bis(prop-2-yn-1-yloxy)terephthalic acid and bpy = 4,4′-bipyridine, Figure 10a), was shown to catalyze CO2 to cyclic carbonates in the presence of TBAB as a cocatalyst.245 The Cu2+ ions acts as the Lewis acid centers to polarize the epoxide oxygen and increase the electrophilicity of the carbon. The Br– from the TBAB helps in the ring opening of the epoxides. The Cu2+ acts as the center for the subsequent binding of CO2 with the ring opened epoxide along with the elimination of the Br– by ring closing, forming the cyclic carbonates (Figure 10b).245 As noted in Table 8, the number of examples of the conversion of CO2 to cyclic carbonates are many. It appears that the presence of stronger acidic sites along with basic sites helps toward catalyzing this reaction. Lanthanide MOFs (Ln = Sm, Eu, Gd, Tb, Dy, Ho, Er, Yb; NTB = 4,4′,4″-nitrilotribenzoic acid; NMP = N-methylpyrrolidone) have also been shown to effectively catalyze the CO2 conversion.246 The compounds catalyze the reaction between CO2 and epichlorohydrin in the presence of n-Bu4NBr as the cocatalyst with a yield of 97%.246
Scheme 7. Plausible Reaction Mechanism for Cycloaddition of CO2 and Epoxides Using Bifunctional MOFs.

LA = Lewis acid; LB = Lewis base; X® = halide ions.
Figure 10.
(a) 2D layer of the [Cu(2,5-BPTA)(bpy)(H2O)] MOF. (b) Possible mechanism for the formation of cyclic carbonate. Reproduced with permission from ref (245). Copyright 2023 American Chemical Society.
In many of the cycloadditions of CO2 to epoxide, a cocatalyst is generally employed. The role of the cocatalysts such as TBAB, in the cycloaddition of CO2, is to enhance the catalytic activity by participating during the ring opening step. A zeolitic imidazole framework-78 (ZIF-78) was found to be a good catalyst (Figure 11) toward the CO2/propylene oxide (PO) cycloaddition, forming propylene carbonate (PC) in the absence of any cocatalyst. The compound ZIF-78 exhibits a strong basic framework due to the presence of imidazolate (-N) linkers with the Zn centers providing the Lewis acidity. This reaction, however, was carried out at an elevated temperature and pressure.247 The mechanism involves the formation of an adduct with propylene oxide and the Lewis acid centers, which is followed by the ring opening. The Lewis basic -NH groups attack on the O– ion, and CO2 forms the cyclic carbonates (Scheme 8)
Figure 11.
(a) Structure of ZIF-78, (b) Lewis acidic Zn center and Lewis basic N (Im) center. Reproduced with permission from ref (247). Copyright 2017 Elsevier.
Scheme 8. Proposed Catalytic Mechanism for the CO2/PO Cycloaddition Reaction Using the ZIF-78 Heterogeneous Catalyst.

Reproduced with permission from ref (247). Copyright 2017 Elsevier.
To date, several examples of heterogeneous bifunctional MOF catalysts have been established. The quaternary ammonium, phosphonium, imidazolium, triazolium, or pyridinium functionalized linkers can be successfully introduced as pendant groups on organic linkers, while preparing the MOFs. This would eliminate the need to use the cocatalyst during the cycloaddition of CO2 to carbonates. A N-containing ligand was introduced by the postsynthetic modification on UiO-66 to result in UiO-66-Py (pyridine) and UiO-67-Bpy (bipyridine) (Figure S6). N-Alkylation of the bipyridine and pyridine centers converts the neutral framework into an ionic one (R = Me, Et, nPr, iPr; X = I, Br, Cl) and gives rise to bifunctional behavior. This modified MOF catalyzes the cycloaddition of propylene oxide to CO2, with yields of ∼99% (Figure S6).51 A halide functionalized bifunctional imidazolium (Im) zirconium metal–organic framework (Zr-MOF), (I–)Meim-UiO-66, was shown to be an efficient and recyclable heterogeneous catalyst for the cycloaddition of CO2 with epoxides without the use of cocatalysts (Figure S7). The presence of ionic I– in the structure acts as the Lewis base, and the Bro̷nsted-acid (Zr-OH/Zr-OH2) helps in the formation of cyclic carbonate with 100% conversion of styrene oxide.248
The use of 2,6-di(1H-tetrazol-5-yl)naphthalene (H2NDTz) and 2,6-bis(4-(1H-tetrazol-5-yl)phenyl)naphthalene (H2NDPhTz) with cobalt gave two cobalt MOFs, which catalyze the epoxidation–carboxylation of styrene with CO2 in tandem fashion. The control experiments revealed the synergic effect between the oxidant and the MOF, as ∼4% of styrene oxide was obtained in the absence of catalyst. This work highlights the utility of nitrogen-rich heterocycles in MOFs for efficient development of bifunctional catalysts.156
In addition to utilizing the Lewis acidic and basic sites of MOFs toward CO2 fixation, it has been shown that the use of metal nanoparticles also catalyzes such reactions. Gold nanoparticles anchored over the MOF [Zn14(L)6(O)2(H2O)3] (L = 2,6-bis(2′,5′-dicarboxylphenyl)pyridine) were employed toward the cycloaddition of CO2 with epoxides with TBAB as the cocatalyst (Scheme 9).249 A bifunctional catalyst having Ag(I) ions grafted onto N-heterocyclic carbene (NHC) sites in a MOF was found to be good catalyst for the efficient fixation of CO2 with propargyl alcohols. The availability of catalytically active alkynophilic Ag(I) and CO2-philic NHC sites in the 1D pores of the MOF helped in the fixation of CO2 with propargyl alcohols (Scheme 10).250 Silver nanoparticles immobilized on MOFs were shown to effectively catalyze the carboxylation of terminal alkyne with CO2 to the corresponding carboxylic ester employing a porphyrin-based compound, [Zn3(C40H24N8)(C20H8N2O4)2(DEF)2](DEF)3 (DEF = N,N-diethylformamide).242 In this reaction Cs2CO3 acts as the cocatalyst. CO2 fixation was achieved in a modified ZIF-90 compound where the carboxylate functionality provided the necessary catalytic reactive center. The catalytic activity of the modified ZIF-90-C was found to be ∼100% at 50 °C for the fixation of CO2 in the presence of PhSiH3 as the H-donor and morpholine.238,251
Scheme 9. Tentative Mechanism for CO2 Cycloaddition with Epoxides.

TBAB = Bu4NBr. Reproduced with permission from ref (249). Copyright 2019 American Chemical Society.
Scheme 10. Proposed Mechanism for the Coupling of CO2 with Propargylic Alcohol Catalyzed by Ag(1)@MOF-NHC.

Reproduced with permission from ref (250). Copyright 2022 Royal Society of Chemistry.
The number of catalytic reactions described and discussed here is indicative of the potential that the MOFs provide as a catalyst. The examples given in this review are to highlight the utility of MOFs toward a particular reaction. For more detailed discussion about the catalytic reactions and their possible mechanism, readers may need to look at the original references.
4. Concluding Remarks
The modern world is driven by the need to have clean technology where wastages are minimized. In the area of applied catalysis, the emergence of zeolites with precise and quantifiable Brönsted acidity more or less replaced the need to have corrosive ligand acids (such as HF, H2SO4, etc.) toward alkylations and isomerization of hydrocarbons.252 The strategy to design new optimal catalyst, from the available knowledge of framework structures, with uniform pore/channel size having well dispersed catalytically active sites may require insights from computational design. Such an approach is likely to gain pace, and designer catalysts for a particular reaction would be feasible in the future. The flexible and ever ready synthetic chemist may be adept toward this new reality to reap the benefits. For the brighter side, as it is known in enzyme catalysis, “the placement of appropriate reactive groups in the right environment” may be enough for a designed catalyst.253
The many examples presented in this article already provide the needed enthusiasm to pursue the design strategy actively. The dual functionality that provides both the acidic as well as basic sites is unique to MOFs, as these can be achieved with reasonable ease. In addition, the “ship in a bottle” type catalysts are another facet of MOF compounds, where the electronic behavior of the participating moieties can be fine-tuned to achieve better catalytic behavior. MOF catalysis can be considered to be another variant of inorganic enzyme catalysis.254 Normally enzymes have a well-defined catalytic site (cavity) and molecular selectivity (shape and regio- or enantioselectivity). In MOFs, one finds the same features as exemplified in this article.
As highlighted in this article, many MOFs are useful in the synthesis of fine chemicals. The lack of stability at elevated temperatures and pressures would hinder the usage of MOFs in oil refining, petrochemical synthesis, etc. The development of MOFs as industrial catalysts needs to overcome the following requirements: (1) reproducibility and large-scale synthesis; (2) processability as extruded pellets and membranes; (3) quantifications of relative acidities; and (4) lack of quantifiable hydrophobicity and hydrophilicity. The number of MOFs that are stable and amenable to structural manipulations are limited to ZIFs, UiO, and the MIL family of compounds.255,256 There is considerable scope to expand this for catalysis.
The areas where MOFs can be useful could be in converting biomass as feedstocks in the synthesis of sugars and related products.257 Exploration of MOFs as supports and as composite materials also requires more attention. The structural defects that may be inherent in many MOFs need to be studied carefully, as some of the structural defects could be important for the overall catalytic activity. These are just a few of the aspects that may be worth investigating in the future so as to arrive at more robust and functional catalysts that are based on MOFs.
In addition, the use of computational approaches, especially in making newer and designer MOFs, would be beneficial. The various studies with respect to the MOF chemistry and structures indicate that they are on firm and sturdy foundations. This would be a boon for further growth in heterogeneous catalysis.
Acknowledgments
SN thanks the Science and Engineering research Board, (SERB) Govt. of India, for the award of a JC Bose national fellowship. SN and KM thank CSIR, Government of India, for the award of a research grant and a research fellowship.
Data Availability Statement
The data underlying this study are available in the published articles and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.3c00033.
Generation of different functionality in the MOF (Scheme 1), bifunctional modification of MOF-101 (Scheme S2), synthetic process of PMoV2@DETA-MIL-101 (Scheme S3), structure of PCN-700-MB and the steps in the synthetic procedure for the conversion of PCN-700 to PCN-700-MB (Figure S1), MIL-101(Cr)-SO3H catalyzed one-pot three component condensation reaction (Figure S2), simplified model of two consecutive reactions A → B → C for the calculation of kinetic rate constants (Figure S3), synthesis of Mn(II) MOF and Pd-Au@Mn(II) MOF (Figure S4), coordination environment in Zr(H4L) with thermal ellipsoids set at 50% probability (Figure S5), syntheses of ionic UiO-67-Bpy (bipyridine) and UiO-66-Py (pyridine) (Figure S6), syntheses of Meim-UiO-66 and (I–)Meim-UiO-66 via reticular chemistry and by a PSM method, respectively (Figure S7) (PDF)
Author Contributions
CRediT: Srinivasan Natarajan conceptualization, writing-review & editing; Krishna Manna writing-original draft.
The authors declare no competing financial interest.
Supplementary Material
References
- Thomas J. M. Turning Points in Catalysis. Angew. Chem., Int. Ed. Engl. 1994, 33 (9), 913–937. 10.1002/anie.199409131. [DOI] [Google Scholar]
- Thomas J. M. Design, Synthesis, and in Situ Characterization of New Solid Catalysts. Angew. Chemie Int. Ed. 1999, 38 (24), 3588–3628. . [DOI] [PubMed] [Google Scholar]
- Yang X.; Cao B.; Jiang D.; He S.; Yuan C.; Li H.; Naqvi S. R.; Wang S. Catalytic Pyrolysis of Guaiacol on Enteromorpha-Based Biochar: A Combination of Experiments and Density Functional Theory. Fuel Process. Technol. 2023, 239, 107527 10.1016/j.fuproc.2022.107527. [DOI] [Google Scholar]
- Thomas J. M.; Thomas J. W.. Principles and Practice of Heterogeneous Catalysis; John Wiley Sons, 2014. [Google Scholar]
- James S. L. Metal-Organic Frameworks. Chem. Soc. Rev. 2003, 32 (5), 276–288. 10.1039/b200393g. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science (80-.) 2013, 341 (6149), 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Miras H. N.; Vilà-Nadal L.; Cronin L. Polyoxometalate Based Open-Frameworks (POM-OFs). Chem. Soc. Rev. 2014, 43 (16), 5679–5699. 10.1039/C4CS00097H. [DOI] [PubMed] [Google Scholar]
- Natarajan S.; Mahata P. Metal–Organic Framework Structures – How Closely Are They Related to Classical Inorganic Structures?. Chem. Soc. Rev. 2009, 38 (8), 2304–2318. 10.1039/b815106g. [DOI] [PubMed] [Google Scholar]
- Natarajan S.; Mandal S. Open-Framework Structures of Transition-Metal Compounds. Angew. Chemie Int. Ed. 2008, 47 (26), 4798–4828. 10.1002/anie.200701404. [DOI] [PubMed] [Google Scholar]
- Zhou H.-C.; Long J. R.; Yaghi O. M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112 (2), 673–674. 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- Alhamami M.; Doan H.; Cheng C. H. A Review on Breathing Behaviors of Metal-Organic-Frameworks (MOFs) for Gas Adsorption. Materials (Basel). 2014, 7 (4), 3198–3250. 10.3390/ma7043198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaidi S. K.; Mohamed M. H.; Banerjee D.; Thallapally P. K. Flexibility in Metal–Organic Frameworks: A Fundamental Understanding. Coord. Chem. Rev. 2018, 358, 125–152. 10.1016/j.ccr.2017.11.022. [DOI] [Google Scholar]
- DeCoste J. B.; Peterson G. W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114 (11), 5695–5727. 10.1021/cr4006473. [DOI] [PubMed] [Google Scholar]
- Thallapally P. K.; Tian J.; Radha Kishan M.; Fernandez C. A.; Dalgarno S. J.; McGrail P. B.; Warren J. E.; Atwood J. L. Flexible (Breathing) Interpenetrated Metal–Organic Frameworks for CO2 Separation Applications. J. Am. Chem. Soc. 2008, 130 (50), 16842–16843. 10.1021/ja806391k. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Natarajan S.; Mani P.; Pankajakshan A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2021, 31 (4), 2006291. 10.1002/adfm.202006291. [DOI] [Google Scholar]
- Wang Z.; Cohen S. M. Postsynthetic Modification of Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38 (5), 1315–1329. 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]
- Tanabe K. K.; Wang Z.; Cohen S. M. Systematic Functionalization of a Metal–Organic Framework via a Postsynthetic Modification Approach. J. Am. Chem. Soc. 2008, 130 (26), 8508–8517. 10.1021/ja801848j. [DOI] [PubMed] [Google Scholar]
- Deria P.; Mondloch J. E.; Karagiaridi O.; Bury W.; Hupp J. T.; Farha O. K. Beyond Post-Synthesis Modification: Evolution of Metal–Organic Frameworks via Building Block Replacement. Chem. Soc. Rev. 2014, 43 (16), 5896–5912. 10.1039/C4CS00067F. [DOI] [PubMed] [Google Scholar]
- Kandiah M.; Usseglio S.; Svelle S.; Olsbye U.; Lillerud K. P.; Tilset M. Post-Synthetic Modification of the Metal–Organic Framework Compound UiO-66. J. Mater. Chem. 2010, 20 (44), 9848–9851. 10.1039/c0jm02416c. [DOI] [Google Scholar]
- Li J.; Huang J.-Y.; Meng Y.-X.; Li L.; Zhang L.-L.; Jiang H.-L. Zr- and Ti-Based Metal–Organic Frameworks: Synthesis, Structures and Catalytic Applications. Chem. Commun. 2023, 59 (18), 2541–2559. 10.1039/D2CC06948B. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Liao Y.; Zhao S.; Zhang X.; Liu Q.; Shi X. Research Progress in Metal–Organic Frameworks (MOFs) in CO2 Capture from Post-Combustion Coal-Fired Flue Gas: Characteristics, Preparation, Modification and Applications. J. Mater. Chem. A 2022, 10 (10), 5174–5211. 10.1039/D1TA07856A. [DOI] [Google Scholar]
- Xu C.; Fang R.; Luque R.; Chen L.; Li Y. Functional Metal–Organic Frameworks for Catalytic Applications. Coord. Chem. Rev. 2019, 388, 268–292. 10.1016/j.ccr.2019.03.005. [DOI] [Google Scholar]
- Paz F. A. A.; Klinowski J.; Vilela S. M. F.; Tome J. P. C.; Cavaleiro J. A. S.; Rocha J. Ligand Design for Functional Metal–Organic Frameworks. Chem. Soc. Rev. 2012, 41 (3), 1088–1110. 10.1039/C1CS15055C. [DOI] [PubMed] [Google Scholar]
- Chughtai A. H.; Ahmad N.; Younus H. A.; Laypkov A.; Verpoort F. Metal–Organic Frameworks: Versatile Heterogeneous Catalysts for Efficient Catalytic Organic Transformations. Chem. Soc. Rev. 2015, 44 (19), 6804–6849. 10.1039/C4CS00395K. [DOI] [PubMed] [Google Scholar]
- He H.; Sun F.; Aguila B.; Perman J. A.; Ma S.; Zhu G. A Bifunctional Metal–Organic Framework Featuring the Combination of Open Metal Sites and Lewis Basic Sites for Selective Gas Adsorption and Heterogeneous Cascade Catalysis. J. Mater. Chem. A 2016, 4 (39), 15240–15246. 10.1039/C6TA05098K. [DOI] [Google Scholar]
- Gulati S.; Vijayan S.; Mansi; Kumar S.; Harikumar B.; Trivedi M.; Varma R. S. Recent Advances in the Application of Metal-Organic Frameworks (MOFs)-Based Nanocatalysts for Direct Conversion of Carbon Dioxide (CO2) to Value-Added Chemicals. Coord. Chem. Rev. 2023, 474, 214853 10.1016/j.ccr.2022.214853. [DOI] [Google Scholar]
- Li B.; Chrzanowski M.; Zhang Y.; Ma S. Applications of Metal-Organic Frameworks Featuring Multi-Functional Sites. Coord. Chem. Rev. 2016, 307, 106–129. 10.1016/j.ccr.2015.05.005. [DOI] [Google Scholar]
- Li B.; Ma D.; Li Y.; Zhang Y.; Li G.; Shi Z.; Feng S.; Zaworotko M. J.; Ma S. Dual Functionalized Cages in Metal–Organic Frameworks via Stepwise Postsynthetic Modification. Chem. Mater. 2016, 28 (13), 4781–4786. 10.1021/acs.chemmater.6b01898. [DOI] [Google Scholar]
- Liu X.; Li J.; Li N.; Li B.; Bu X.-H. Recent Advances on Metal-Organic Frameworks in the Conversion of Carbon Dioxide. Chin. J. Chem. 2021, 39 (2), 440–462. 10.1002/cjoc.202000357. [DOI] [Google Scholar]
- Liu K.; Jiao S.; Zhao H.; Cao F.; Ma D. Hybridization of MOFs and Ionic POFs: A New Strategy for the Construction of Bifunctional Catalysts for CO2 Cycloaddition. Green Chem. 2021, 23 (4), 1766–1771. 10.1039/D0GC04425C. [DOI] [Google Scholar]
- Chen X.; Qian P.; Zhang T.; Xu Z.; Fang C.; Xu X.; Chen W.; Wu P.; Shen Y.; Li S.; Wu J.; Zheng B.; Zhang W.; Huo F. Catalyst Surfaces with Tunable Hydrophilicity and Hydrophobicity: Metal–Organic Frameworks toward Controllable Catalytic Selectivity. Chem. Commun. 2018, 54 (32), 3936–3939. 10.1039/C8CC00318A. [DOI] [PubMed] [Google Scholar]
- Liu L.; Tao Z.-P.; Chi H.-R.; Wang B.; Wang S.-M.; Han Z.-B. The Applications and Prospects of Hydrophobic Metal–Organic Frameworks in Catalysis. Dalt. Trans. 2021, 50 (1), 39–58. 10.1039/D0DT03635H. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy A.; Asiri A. M.; Garcia H. Catalysis by Metal–Organic Frameworks in Water. Chem. Commun. 2014, 50 (85), 12800–12814. 10.1039/C4CC04387A. [DOI] [PubMed] [Google Scholar]
- Li H.-C.; Liu W.-J.; Han H.-X.; Yu H.-Q. Hydrophilic Swellable Metal–Organic Framework Encapsulated Pd Nanoparticles as an Efficient Catalyst for Cr(vi) Reduction. J. Mater. Chem. A 2016, 4 (30), 11680–11687. 10.1039/C6TA03688K. [DOI] [Google Scholar]
- Ding M.; Jiang H.-L. Improving Water Stability of Metal–Organic Frameworks by a General Surface Hydrophobic Polymerization. CCS Chem. 2021, 3 (8), 2740–2748. 10.31635/ccschem.020.202000515. [DOI] [Google Scholar]
- Yadav A.; Kanoo P. Metal-Organic Frameworks as Platform for Lewis-Acid-Catalyzed Organic Transformations. Chem. – An Asian J. 2019, 14 (20), 3531–3551. 10.1002/asia.201900876. [DOI] [PubMed] [Google Scholar]
- He H.; Perman J. A.; Zhu G.; Ma S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12 (46), 6309–6324. 10.1002/smll.201602711. [DOI] [PubMed] [Google Scholar]
- Ji P.; Feng X.; Oliveres P.; Li Z.; Murakami A.; Wang C.; Lin W. Strongly Lewis Acidic Metal–Organic Frameworks for Continuous Flow Catalysis. J. Am. Chem. Soc. 2019, 141 (37), 14878–14888. 10.1021/jacs.9b07891. [DOI] [PubMed] [Google Scholar]
- Horike S.; Dincǎ M.; Tamaki K.; Long J. R. Size-Selective Lewis Acid Catalysis in a Microporous Metal-Organic Framework with Exposed Mn2+ Coordination Sites. J. Am. Chem. Soc. 2008, 130 (18), 5854–5855. 10.1021/ja800669j. [DOI] [PubMed] [Google Scholar]
- Alaerts L.; Séguin E.; Poelman H.; Thibault-Starzyk F.; Jacobs P. A.; De Vos D. E. Probing the Lewis Acidity and Catalytic Activity of the Metal–Organic Framework [Cu3(Btc)2] (BTC = Benzene-1,3,5-Tricarboxylate). Chem. – A Eur. J. 2006, 12 (28), 7353–7363. 10.1002/chem.200600220. [DOI] [PubMed] [Google Scholar]
- Schlichte K.; Kratzke T.; Kaskel S. Improved Synthesis, Thermal Stability and Catalytic Properties of the Metal-Organic Framework Compound Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73 (1), 81–88. 10.1016/j.micromeso.2003.12.027. [DOI] [Google Scholar]
- Tanabe K. K.; Cohen S. M. Modular, Active, and Robust Lewis Acid Catalysts Supported on a Metal–Organic Framework. Inorg. Chem. 2010, 49 (14), 6766–6774. 10.1021/ic101125m. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Yaghi O. M. Bro̷nsted Acidity in Metal–Organic Frameworks. Chem. Rev. 2015, 115 (14), 6966–6997. 10.1021/acs.chemrev.5b00221. [DOI] [PubMed] [Google Scholar]
- Li B.; Leng K.; Zhang Y.; Dynes J. J.; Wang J.; Hu Y.; Ma D.; Shi Z.; Zhu L.; Zhang D.; Sun Y.; Chrzanowski M.; Ma S. Metal–Organic Framework Based upon the Synergy of a Bro̷nsted Acid Framework and Lewis Acid Centers as a Highly Efficient Heterogeneous Catalyst for Fixed-Bed Reactions. J. Am. Chem. Soc. 2015, 137 (12), 4243–4248. 10.1021/jacs.5b01352. [DOI] [PubMed] [Google Scholar]
- Vermoortele F.; Ameloot R.; Vimont A.; Serre C.; De Vos D. An Amino-Modified Zr-Terephthalate Metal–Organic Framework as an Acid–Base Catalyst for Cross-Aldol Condensation. Chem. Commun. 2011, 47 (5), 1521–1523. 10.1039/C0CC03038D. [DOI] [PubMed] [Google Scholar]
- Wang X.-S.; Liang J.; Li L.; Lin Z.-J.; Bag P. P.; Gao S.-Y.; Huang Y.-B.; Cao R. An Anion Metal–Organic Framework with Lewis Basic Sites-Rich toward Charge-Exclusive Cationic Dyes Separation and Size-Selective Catalytic Reaction. Inorg. Chem. 2016, 55 (5), 2641–2649. 10.1021/acs.inorgchem.6b00019. [DOI] [PubMed] [Google Scholar]
- Gascon J.; Aktay U.; Hernandez-Alonso M. D.; van Klink G. P. M.; Kapteijn F. Amino-Based Metal-Organic Frameworks as Stable Highly Active Basic Catalysts. J. Catal. 2009, 261 (1), 75–87. 10.1016/j.jcat.2008.11.010. [DOI] [Google Scholar]
- Panchenko V. N.; Matrosova M. M.; Jeon J.; Jun J. W.; Timofeeva M. N.; Jhung S. H. Catalytic Behavior of Metal–Organic Frameworks in the Knoevenagel Condensation Reaction. J. Catal. 2014, 316, 251–259. 10.1016/j.jcat.2014.05.018. [DOI] [Google Scholar]
- Huang Y.-B.; Liang J.; Wang X.-S.; Cao R. Multifunctional Metal–Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46 (1), 126–157. 10.1039/C6CS00250A. [DOI] [PubMed] [Google Scholar]
- Liu F.; Kumar S.; Li S.; You H.; Ren P.; Zhao L. Bifunctional Design of Stable Metal-Organic Framework Bearing Triazole–Carboxylate Mixed Ligand: Highly Efficient Heterogeneous Catalyst for Knoevenagel Condensation Reaction under Mild Conditions. Catal. Commun. 2020, 142, 106032 10.1016/j.catcom.2020.106032. [DOI] [Google Scholar]
- Ji H.; Naveen K.; Lee W.; Kim T. S.; Kim D.; Cho D.-H. Pyridinium-Functionalized Ionic Metal–Organic Frameworks Designed as Bifunctional Catalysts for CO2 Fixation into Cyclic Carbonates. ACS Appl. Mater. Interfaces 2020, 12 (22), 24868–24876. 10.1021/acsami.0c05912. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Zhang J.; Huo H.; Wang Z.; Xu X.; Yang Y.; Lin K.; Fan R. One-Pot Synthesis of Bimetallic Metal–Organic Frameworks (MOFs) as Acid–Base Bifunctional Catalysts for Tandem Reaction. Catal. Sci. Technol. 2020, 10 (2), 315–322. 10.1039/C9CY01940E. [DOI] [Google Scholar]
- Wasilke J.-C.; Obrey S. J.; Baker R. T.; Bazan G. C. Concurrent Tandem Catalysis. Chem. Rev. 2005, 105 (3), 1001–1020. 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]
- Tietze L. F.; Beifuss U. Sequential Transformations in Organic Chemistry: A Synthetic Strategy with a Future. Angew. Chem., Int. Ed. Engl. 1993, 32 (2), 131–163. 10.1002/anie.199301313. [DOI] [Google Scholar]
- Liang J.; Huang Y.-B.; Cao R. Metal–Organic Frameworks and Porous Organic Polymers for Sustainable Fixation of Carbon Dioxide into Cyclic Carbonates. Coord. Chem. Rev. 2019, 378, 32–65. 10.1016/j.ccr.2017.11.013. [DOI] [Google Scholar]
- Ma D.; Li B.; Shi Z. Multi-Functional Sites Catalysts Based on Post-Synthetic Modification of Metal-Organic Frameworks. Chin. Chem. Lett. 2018, 29 (6), 827–830. 10.1016/j.cclet.2017.09.028. [DOI] [Google Scholar]
- Bhattacharjee S.; Chen C.; Ahn W.-S. Chromium Terephthalate Metal–Organic Framework MIL-101: Synthesis, Functionalization, and Applications for Adsorption and Catalysis. RSC Adv. 2014, 4 (94), 52500–52525. 10.1039/C4RA11259H. [DOI] [Google Scholar]
- Agarwal R. A.; De D. Selective CO2 Adsorption and Lewis Acid Catalytic Activity towards Naphthimidazole Synthesis by a Zn-MOF. Polyhedron 2020, 185, 114584 10.1016/j.poly.2020.114584. [DOI] [Google Scholar]
- Vermoortele F.; Bueken B.; Le Bars G.; Van de Voorde B.; Vandichel M.; Houthoofd K.; Vimont A.; Daturi M.; Waroquier M.; Van Speybroeck V.; Kirschhock C.; De Vos D. E. Synthesis Modulation as a Tool To Increase the Catalytic Activity of Metal–Organic Frameworks: The Unique Case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135 (31), 11465–11468. 10.1021/ja405078u. [DOI] [PubMed] [Google Scholar]
- Ohmori O.; Fujita M. Heterogeneous Catalysis of a Coordination Network: Cyanosilylation of Imines Catalyzed by a Cd(Ii)-(4,4′-Bipyridine) Square Grid Complex. Chem. Commun. 2004, 14, 1586–1587. 10.1039/B406114B. [DOI] [PubMed] [Google Scholar]
- Wu J.-Q.; Wu X.-Y.; Lu J.-M.; Shi Q.; Shao L.-X. Highly Active La(III)-Based Metal-Organic Framework as a Heterogeneous Lewis Acid Catalyst for Friedel-Crafts Alkylation. Chem. – A Eur. J. 2022, 28 (69), e202202441 10.1002/chem.202202441. [DOI] [PubMed] [Google Scholar]
- Rahaman M. S.; Tulaphol S.; Hossain M. A.; Jasinski J. B.; Sun N.; George A.; Simmons B. A.; Maihom T.; Crocker M.; Sathitsuksanoh N. Cooperative Bro̷nsted-Lewis Acid Sites Created by Phosphotungstic Acid Encapsulated Metal–Organic Frameworks for Selective Glucose Conversion to 5-Hydroxymethylfurfural. Fuel 2022, 310, 122459 10.1016/j.fuel.2021.122459. [DOI] [Google Scholar]
- Li J.; Zhao S.; Li Z.; Liu D.; Chi Y.; Hu C. Efficient Conversion of Biomass-Derived Levulinic Acid to γ-Valerolactone over Polyoxometalate@Zr-Based Metal–Organic Frameworks: The Synergistic Effect of Bro̷nsted and Lewis Acidic Sites. Inorg. Chem. 2021, 60 (11), 7785–7793. 10.1021/acs.inorgchem.1c00185. [DOI] [PubMed] [Google Scholar]
- Ye C.; Qi Z.; Cai D.; Qiu T. Design and Synthesis of Ionic Liquid Supported Hierarchically Porous Zr Metal–Organic Framework as a Novel Bro̷nsted–Lewis Acidic Catalyst in Biodiesel Synthesis. Ind. Eng. Chem. Res. 2019, 58 (3), 1123–1132. 10.1021/acs.iecr.8b04107. [DOI] [Google Scholar]
- Yabushita M.; Li P.; Islamoglu T.; Kobayashi H.; Fukuoka A.; Farha O. K.; Katz A. Selective Metal–Organic Framework Catalysis of Glucose to 5-Hydroxymethylfurfural Using Phosphate-Modified NU-1000. Ind. Eng. Chem. Res. 2017, 56 (25), 7141–7148. 10.1021/acs.iecr.7b01164. [DOI] [Google Scholar]
- Chen J.; Zhang Y.; Chen X.; Dai S.; Bao Z.; Yang Q.; Ren Q.; Zhang Z. Cooperative Interplay of Bro̷nsted Acid and Lewis Acid Sites in MIL-101(Cr) for Cross-Dehydrogenative Coupling of C–H Bonds. ACS Appl. Mater. Interfaces 2021, 13 (9), 10845–10854. 10.1021/acsami.0c20369. [DOI] [PubMed] [Google Scholar]
- Xie W.; Wang H. Synthesis of Heterogenized Polyoxometalate-Based Ionic Liquids with Brönsted-Lewis Acid Sites: A Magnetically Recyclable Catalyst for Biodiesel Production from Low-Quality Oils. J. Ind. Eng. Chem. 2020, 87, 162–172. 10.1016/j.jiec.2020.03.033. [DOI] [Google Scholar]
- Oudi S.; Oveisi A. R.; Daliran S.; Khajeh M.; Teymoori E. Bro̷nsted-Lewis Dual Acid Sites in a Chromium-Based Metal-Organic Framework for Cooperative Catalysis: Highly Efficient Synthesis of Quinazolin-(4H)-1-One Derivatives. J. Colloid Interface Sci. 2020, 561, 782–792. 10.1016/j.jcis.2019.11.056. [DOI] [PubMed] [Google Scholar]
- Trickett C. A.; Osborn Popp T. M.; Su J.; Yan C.; Weisberg J.; Huq A.; Urban P.; Jiang J.; Kalmutzki M. J.; Liu Q.; Baek J.; Head-Gordon M. P.; Somorjai G. A.; Reimer J. A.; Yaghi O. M. Identification of the Strong Bro̷nsted Acid Site in a Metal–Organic Framework Solid Acid Catalyst. Nat. Chem. 2019, 11 (2), 170–176. 10.1038/s41557-018-0171-z. [DOI] [PubMed] [Google Scholar]
- Liu P.; Redekop E.; Gao X.; Liu W.-C.; Olsbye U.; Somorjai G. A. Oligomerization of Light Olefins Catalyzed by Bro̷nsted-Acidic Metal–Organic Framework-808. J. Am. Chem. Soc. 2019, 141 (29), 11557–11564. 10.1021/jacs.9b03867. [DOI] [PubMed] [Google Scholar]
- Rojas-Buzo S.; Bohigues B.; Lopes C. W.; Meira D. M.; Boronat M.; Moliner M.; Corma A. Tailoring Lewis/Bro̷nsted Acid Properties of MOF Nodes via Hydrothermal and Solvothermal Synthesis: Simple Approach with Exceptional Catalytic Implications. Chem. Sci. 2021, 12 (29), 10106–10115. 10.1039/D1SC02833B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W.; Liu Y.; Li H.; Cui Y. Metal-Organic Frameworks as Solid Bro̷nsted Acid Catalysts for Advanced Organic Transformations. Coord. Chem. Rev. 2020, 420, 213400 10.1016/j.ccr.2020.213400. [DOI] [Google Scholar]
- Feng L.; Wang Y.; Yuan S.; Wang K.-Y.; Li J.-L.; Day G. S.; Qiu D.; Cheng L.; Chen W.-M.; Madrahimov S. T.; Zhou H.-C. Porphyrinic Metal–Organic Frameworks Installed with Bro̷nsted Acid Sites for Efficient Tandem Semisynthesis of Artemisinin. ACS Catal. 2019, 9 (6), 5111–5118. 10.1021/acscatal.8b04960. [DOI] [Google Scholar]
- Li X.; Huang L.; Kochubei A.; Huang J.; Shen W.; Xu H.; Li Q. Evolution of a Metal-Organic Framework into a Bro̷nsted Acid Catalyst for Glycerol Dehydration to Acrolein. ChemSusChem 2020, 13 (18), 5073–5079. 10.1002/cssc.202001377. [DOI] [PubMed] [Google Scholar]
- Li R.; Jiang Y.; Zhao J.; Ramella D.; Peng Y.; Luan Y. Development of a Bro̷nsted Acid Al–MIL-53 Metal–Organic Framework Catalyst and Its Application in [4 + 2] Cycloadditions. RSC Adv. 2017, 7 (55), 34591–34597. 10.1039/C7RA06201J. [DOI] [Google Scholar]
- Nguyen L. H. T.; Nguyen T. T. T.; Dang M.-H. D.; Tran P. H.; Doan T. L. H. Heterocyclic Reaction Inducted by Bro̷nsted–Lewis Dual Acidic Hf-MOF under Microwave Irradiation. Mol. Catal. 2021, 499, 111291 10.1016/j.mcat.2020.111291. [DOI] [Google Scholar]
- Qu H.; Liu B.; Gao G.; Ma Y.; Zhou Y.; Zhou H.; Li L.; Li Y.; Liu S. Metal-Organic Framework Containing Bro̷nsted Acidity and Lewis Acidity for Efficient Conversion Glucose to Levulinic Acid. Fuel Process. Technol. 2019, 193, 1–6. 10.1016/j.fuproc.2019.04.035. [DOI] [Google Scholar]
- Cheng J.; Mao Y.; Guo H.; Qian L.; Shao Y.; Yang W.; Park J.-Y. Synergistic and Efficient Catalysis over Bro̷nsted Acidic Ionic Liquid [BSO3HMIm][HSO4]–Modified Metal–Organic Framework (IRMOF-3) for Microalgal Biodiesel Production. Fuel 2022, 322, 124217 10.1016/j.fuel.2022.124217. [DOI] [Google Scholar]
- Mortazavi S.-S.; Abbasi A.; Masteri-Farahani M. Influence of SO3H Groups Incorporated as Bro̷nsted Acidic Parts by Tandem Post-Synthetic Functionalization on the Catalytic Behavior of MIL-101(Cr) MOF for Methanolysis of Styrene Oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 599, 124703 10.1016/j.colsurfa.2020.124703. [DOI] [Google Scholar]
- Dou Y.; Zhang H.; Zhou A.; Yang F.; Shu L.; She Y.; Li J.-R. Highly Efficient Catalytic Esterification in an – SO3H-Functionalized Cr(III)-MOF. Ind. Eng. Chem. Res. 2018, 57 (25), 8388–8395. 10.1021/acs.iecr.8b01239. [DOI] [Google Scholar]
- Ma D.; Zhang Y.; Jiao S.; Li J.; Liu K.; Shi Z. A Tri-Functional Metal–Organic Framework Heterogeneous Catalyst for Efficient Conversion of CO2 under Mild and Co-Catalyst Free Conditions. Chem. Commun. 2019, 55 (95), 14347–14350. 10.1039/C9CC08236K. [DOI] [PubMed] [Google Scholar]
- Lara-Serrano M.; Morales-delaRosa S.; Campos-Martin J. M.; Abdelkader-Fernández V. K.; Cunha-Silva L.; Balula S. S. One-Pot Conversion of Glucose into 5-Hydroxymethylfurfural Using MOFs and Bro̷nsted-Acid Tandem Catalysts. Adv. Sustain. Syst. 2022, 6 (5), 2100444. 10.1002/adsu.202270014. [DOI] [Google Scholar]
- Su Y.; Chang G.; Zhang Z.; Xing H.; Su B.; Yang Q.; Ren Q.; Yang Y.; Bao Z. Catalytic Dehydration of Glucose to 5-Hydroxymethylfurfural with a Bifunctional Metal-Organic Framework. AIChE J. 2016, 62 (12), 4403–4417. 10.1002/aic.15356. [DOI] [Google Scholar]
- Ghorbani-Vaghei R.; Azarifar D.; Daliran S.; Oveisi A. R. The UiO-66-SO3H Metal–Organic Framework as a Green Catalyst for the Facile Synthesis of Dihydro-2-Oxypyrrole Derivatives. RSC Adv. 2016, 6 (35), 29182–29189. 10.1039/C6RA00463F. [DOI] [Google Scholar]
- Fei H.; Shin J.; Meng Y. S.; Adelhardt M.; Sutter J.; Meyer K.; Cohen S. M. Reusable Oxidation Catalysis Using Metal-Monocatecholato Species in a Robust Metal–Organic Framework. J. Am. Chem. Soc. 2014, 136 (13), 4965–4973. 10.1021/ja411627z. [DOI] [PubMed] [Google Scholar]
- Fei H.; Cohen S. M. Metalation of a Thiocatechol-Functionalized Zr(IV)-Based Metal–Organic Framework for Selective C–H Functionalization. J. Am. Chem. Soc. 2015, 137 (6), 2191–2194. 10.1021/ja5126885. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Li J.-R.; Verdegaal W. M.; Liu T.-F.; Zhou H.-C. Isostructural Metal–Organic Frameworks Assembled from Functionalized Diisophthalate Ligands through a Ligand-Truncation Strategy. Chem. – A Eur. J. 2013, 19 (18), 5637–5643. 10.1002/chem.201203297. [DOI] [PubMed] [Google Scholar]
- Rabo J. A.; Gajda G. J. Acid Function in Zeolites: Recent Progress. Catal. Rev. 1989, 31 (4), 385–430. 10.1080/01614948909349936. [DOI] [Google Scholar]
- Blumenfeld A. L.; Fripiat J. J. Characterization of Bro̷nsted and Lewis Acidity in Zeolites by Solid-State NMR and the Recent Progress in the REDOR Technique. Magn. Reson. Chem. 1999, 37 (13), S118–S125. . [DOI] [Google Scholar]
- Weitkamp J. Zeolites and Catalysis. Solid State Ionics 2000, 131 (1), 175–188. 10.1016/S0167-2738(00)00632-9. [DOI] [Google Scholar]
- Masoomi M. Y.; Morsali A.; Dhakshinamoorthy A.; Garcia H. Mixed-metal MOFs: Unique Opportunities in Metal–Organic Framework (MOF) Functionality and Design. Angew. Chem. 2019, 131 (43), 15330–15347. 10.1002/ange.201902229. [DOI] [PubMed] [Google Scholar]
- Salama R. S.; Mannaa M. A.; Altass H. M.; Ibrahim A. A.; Khder A. E.-R. S. Palladium Supported on Mixed-Metal–Organic Framework (Co–Mn-MOF-74) for Efficient Catalytic Oxidation of CO. RSC Adv. 2021, 11 (8), 4318–4326. 10.1039/D0RA09970H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmutzki M. J.; Hanikel N.; Yaghi O. M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4 (10), eaat9180. 10.1126/sciadv.aat9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.-Y.; Chen Z.-Y.; Wei N.; Liu L.; Han Z.-B. Highly Efficient Cooperative Catalysis of Single-Site Lewis Acid and Bro̷nsted Acid in a Metal–Organic Framework for the Biginelli Reaction. Inorg. Chem. 2019, 58 (12), 7657–7661. 10.1021/acs.inorgchem.9b00816. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Sun L.; Feng D.; Zhou H.-C. An In Situ One-Pot Synthetic Approach towards Multivariate Zirconium MOFs. Angew. Chemie Int. Ed. 2016, 55 (22), 6471–6475. 10.1002/anie.201602274. [DOI] [PubMed] [Google Scholar]
- Phang W. J.; Jo H.; Lee W. R.; Song J. H.; Yoo K.; Kim B.; Hong C. S. Superprotonic Conductivity of a UiO-66 Framework Functionalized with Sulfonic Acid Groups by Facile Postsynthetic Oxidation. Angew. Chemie Int. Ed. 2015, 54 (17), 5142–5146. 10.1002/anie.201411703. [DOI] [PubMed] [Google Scholar]
- Huang M.; Kaliaguine S.; Auroux A.. Lewis Basic and Lewis Acidic Sites in Zeolites. In Studies in Surface Science and Catalysis; Elsevier, 1995; Vol. 97, pp 311–318. [Google Scholar]
- Schoonheydt R. A.; Geerlings P.; Pidko E. A.; Van Santen R. A. The Framework Basicity of Zeolites. J. Mater. Chem. 2012, 22 (36), 18705–18717. 10.1039/c2jm31366a. [DOI] [Google Scholar]
- Thomas J. M. The Ineluctable Need for in Situ Methods of Characterising Solid Catalysts as a Prerequisite to Engineering Active Sites. Chem.—Eur. J. 1997, 3 (10), 1557–1562. 10.1002/chem.19970031004. [DOI] [Google Scholar]
- Thomas J. M. On the Nature of Isolated Active Sites in Open-Structure Catalysts for the Aerial Oxidation of Alkanes. Top. Catal. 2001, 15, 85–91. 10.1023/A:1016697525123. [DOI] [Google Scholar]
- Bhadra B. N.; Ahmed I.; Lee H. J.; Jhung S. H. Metal-Organic Frameworks Bearing Free Carboxylic Acids: Preparation, Modification, and Applications. Coord. Chem. Rev. 2022, 450, 214237 10.1016/j.ccr.2021.214237. [DOI] [Google Scholar]
- Lee Y.-R.; Cho S.-M.; Ahn W.-S.; Lee C.-H.; Lee K.-H.; Cho W.-S. Facile Synthesis of an IRMOF-3 Membrane on Porous Al2O3 Substrate via a Sonochemical Route. Microporous Mesoporous Mater. 2015, 213, 161–168. 10.1016/j.micromeso.2015.04.021. [DOI] [Google Scholar]
- Zhu L.; Liu X.-Q.; Jiang H.-L.; Sun L.-B. Metal–Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117 (12), 8129–8176. 10.1021/acs.chemrev.7b00091. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Yao H.-F.; Xi F.-G.; Gao E.-Q. Amino-Functionalized Zr(IV) Metal–Organic Framework as Bifunctional Acid–Base Catalyst for Knoevenagel Condensation. J. Mol. Catal. A Chem. 2014, 390, 198–205. 10.1016/j.molcata.2014.04.002. [DOI] [Google Scholar]
- Srirambalaji R.; Hong S.; Natarajan R.; Yoon M.; Hota R.; Kim Y.; Ho Ko Y.; Kim K. Tandem Catalysis with a Bifunctional Site-Isolated Lewis Acid–Bro̷nsted Base Metal–Organic Framework, NH2-MIL-101(Al). Chem. Commun. 2012, 48 (95), 11650–11652. 10.1039/c2cc36678a. [DOI] [PubMed] [Google Scholar]
- Sun D.; Ye L.; Li Z. Visible-Light-Assisted Aerobic Photocatalytic Oxidation of Amines to Imines over NH2-MIL-125(Ti). Appl. Catal. B Environ. 2015, 164, 428–432. 10.1016/j.apcatb.2014.09.054. [DOI] [Google Scholar]
- Sun L.-B.; Li J.-R.; Park J.; Zhou H.-C. Cooperative Template-Directed Assembly of Mesoporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2012, 134 (1), 126–129. 10.1021/ja209698f. [DOI] [PubMed] [Google Scholar]
- Lee Y.-R.; Jang M.-S.; Cho H.-Y.; Kwon H.-J.; Kim S.; Ahn W.-S. ZIF-8: A Comparison of Synthesis Methods. Chem. Eng. J. 2015, 271, 276–280. 10.1016/j.cej.2015.02.094. [DOI] [Google Scholar]
- Tran U. P. N.; Le K. K. A.; Phan N. T. S. Expanding Applications of Metal–Organic Frameworks: Zeolite Imidazolate Framework ZIF-8 as an Efficient Heterogeneous Catalyst for the Knoevenagel Reaction. ACS Catal. 2011, 1 (2), 120–127. 10.1021/cs1000625. [DOI] [Google Scholar]
- Kundu T.; Manna K.; Jana A. K.; Natarajan S. A Luminescent Inorganic–Organic Hybrid, [Cd(C16H10N2O8S)(H2O)], for the Selective and Recyclable Detection of Chromates and Dichromates in Aqueous Solution. New J. Chem. 2019, 43 (33), 13263–13270. 10.1039/C9NJ03224J. [DOI] [Google Scholar]
- Guo F.; Yuan B.; Shi W. A Novel 2D Metal-Organic Framework with Lewis Basic Sites as a Heterogeneous Base Catalysis. Inorg. Chem. Commun. 2017, 86, 285–289. 10.1016/j.inoche.2017.11.007. [DOI] [Google Scholar]
- Zhang S.; He H.; Sun F.; Zhao N.; Du J.; Pan Q.; Zhu G. A Novel Adenine-Based Zinc(II) Metal-Organic Framework Featuring the Lewis Basic Sites for Heterogeneous Catalysis. Inorg. Chem. Commun. 2017, 79, 55–59. 10.1016/j.inoche.2016.11.011. [DOI] [Google Scholar]
- Patel P.; Patel U.; Parmar B.; Dadhania A.; Suresh E. Regioselective Ring-Opening of Spiro-Epoxyoxindoles by a Dual-Ligand Zinc-Based Metal–Organic Framework as an Efficient Heterogeneous Catalyst. ACS Appl. Nano Mater. 2022, 5 (3), 3712–3721. 10.1021/acsanm.1c04380. [DOI] [Google Scholar]
- Cirujano F. G.; Luque R.; Dhakshinamoorthy A. Metal-Organic Frameworks as Versatile Heterogeneous Solid Catalysts for Henry Reactions. Molecules 2021, 26 (5), 1445. 10.3390/molecules26051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi S. A. A.; Morsali A. Function–Structure Relationship in Metal–Organic Frameworks for Mild, Green, and Fast Catalytic C–C Bond Formation. Inorg. Chem. 2019, 58 (21), 14429–14439. 10.1021/acs.inorgchem.9b01819. [DOI] [PubMed] [Google Scholar]
- Qiao J.; Zhang B.; Yu X.; Zou X.; Liu X.; Zhang L.; Liu Y. A Stable Y(III)-Based Amide-Functionalized Metal–Organic Framework for Propane/Methane Separation and Knoevenagel Condensation. Inorg. Chem. 2022, 61 (8), 3708–3715. 10.1021/acs.inorgchem.1c03924. [DOI] [PubMed] [Google Scholar]
- Joharian M.; Morsali A.; Azhdari Tehrani A.; Carlucci L.; Proserpio D. M. Water-Stable Fluorinated Metal–Organic Frameworks (F-MOFs) with Hydrophobic Properties as Efficient and Highly Active Heterogeneous Catalysts in Aqueous Solution. Green Chem. 2018, 20 (23), 5336–5345. 10.1039/C8GC02367K. [DOI] [Google Scholar]
- Gong Y.; Yuan Y.; Chen C.; Zhang P.; Wang J.; Khan A.; Zhuiykov S.; Chaemchuen S.; Verpoort F. Enhancing Catalytic Performance via Structure Core-Shell Metal-Organic Frameworks. J. Catal. 2019, 375, 371–379. 10.1016/j.jcat.2019.06.031. [DOI] [Google Scholar]
- Gupta V.; Mandal S. K. A Microporous Metal–Organic Framework Catalyst for Solvent-Free Strecker Reaction and CO2 Fixation at Ambient Conditions. Inorg. Chem. 2020, 59 (7), 4273–4281. 10.1021/acs.inorgchem.9b03051. [DOI] [PubMed] [Google Scholar]
- Gupta A. K.; De D.; Bharadwaj P. K. A NbO Type Cu(Ii) Metal–Organic Framework Showing Efficient Catalytic Activity in the Friedländer and Henry Reactions. Dalt. Trans. 2017, 46 (24), 7782–7790. 10.1039/C7DT01595J. [DOI] [PubMed] [Google Scholar]
- Verma A.; De D.; Tomar K.; Bharadwaj P. K. An Amine Functionalized Metal–Organic Framework as an Effective Catalyst for Conversion of CO2 and Biginelli Reactions. Inorg. Chem. 2017, 56 (16), 9765–9771. 10.1021/acs.inorgchem.7b01286. [DOI] [PubMed] [Google Scholar]
- Gupta A. K.; De D.; Tomar K.; Bharadwaj P. K. A Cu(Ii) Metal–Organic Framework with Significant H2 and CO2 Storage Capacity and Heterogeneous Catalysis for the Aerobic Oxidative Amination of C(Sp3)–H Bonds and Biginelli Reactions. Dalt. Trans. 2018, 47 (5), 1624–1634. 10.1039/C7DT04006G. [DOI] [PubMed] [Google Scholar]
- Guo F.; Su C.; Fan Y.; Shi W.; Zhang X. Assembly of Two Self-Interpenetrating Metal–Organic Frameworks Based on a Trigonal Ligand: Syntheses, Crystal Structures, and Properties. Inorg. Chem. 2020, 59 (10), 7135–7142. 10.1021/acs.inorgchem.0c00596. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Zhu M.; Shang H.; Cheng Y.; Ramella D.; Zhu K.; Luan Y. UiO-67 Metal–Organic Framework Immobilized Fe3+ Catalyst for Efficient Morita–Baylis–Hillman Reaction. New J. Chem. 2022, 46 (7), 3199–3206. 10.1039/D1NJ04544J. [DOI] [Google Scholar]
- Amarante S. F.; Freire M. A.; Mendes D. T. S. L.; Freitas L. S.; Ramos A. L. D. Evaluation of Basic Sites of ZIFs Metal Organic Frameworks in the Knoevenagel Condensation Reaction. Appl. Catal. A Gen. 2017, 548, 47–51. 10.1016/j.apcata.2017.08.006. [DOI] [Google Scholar]
- Yuan B.; Wang Y.; Wang M.; Gou G.; Li L. Metal–organic Frameworks as Recyclable Catalysts for Efficient Esterification to Synthesize Traditional Plasticizers. Appl. Catal. A Gen. 2021, 622, 118212 10.1016/j.apcata.2021.118212. [DOI] [Google Scholar]
- Bagheri S.; Esfanidiary N.; Yliniemi J. Porous SB-Cu1 Two-Dimensional Metal-Organic Framework: The Green Catalyst towards CN Bond-Forming Reactions. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 637, 128202 10.1016/j.colsurfa.2021.128202. [DOI] [Google Scholar]
- Xing S.; Li J.; Niu G.; Han Q.; Zhang J.; Liu H. Chiral and Amine Groups Functionalized Polyoxometalate-Based Metal-Organic Frameworks for Synergic Catalysis in Aldol and Knoevenagel Condensations. Mol. Catal. 2018, 458, 83–88. 10.1016/j.mcat.2018.08.011. [DOI] [Google Scholar]
- Boronat M.; Climent M. J.; Corma A.; Iborra S.; Montón R.; Sabater M. J. Bifunctional Acid–Base Ionic Liquid Organocatalysts with a Controlled Distance Between Acid and Base Sites. Chem. – A Eur. J. 2010, 16 (4), 1221–1231. 10.1002/chem.200901519. [DOI] [PubMed] [Google Scholar]
- Climent M. J.; Corma A.; Iborra S.; Velty A. Designing the Adequate Base Solid Catalyst with Lewis or Bronsted Basic Sites or with Acid–Base Pairs. J. Mol. Catal. A Chem. 2002, 182–183, 327–342. 10.1016/S1381-1169(01)00501-5. [DOI] [Google Scholar]
- Climent M. J.; Corma A.; Guil-López R.; Iborra S.; Primo J. Use of Mesoporous MCM-41 Aluminosilicates as Catalysts in the Preparation of Fine Chemicals: A New Route for the Preparation of Jasminaldehyde with High Selectivity. J. Catal. 1998, 175 (1), 70–79. 10.1006/jcat.1998.1970. [DOI] [Google Scholar]
- Shi W.; Quan Y.; Lan G.; Ni K.; Song Y.; Jiang X.; Wang C.; Lin W. Bifunctional Metal–Organic Layers for Tandem Catalytic Transformations Using Molecular Oxygen and Carbon Dioxide. J. Am. Chem. Soc. 2021, 143 (40), 16718–16724. 10.1021/jacs.1c07963. [DOI] [PubMed] [Google Scholar]
- Farha O. K.; Hupp J. T. Rational Design, Synthesis, Purification, and Activation of Metal–Organic Framework Materials. Acc. Chem. Res. 2010, 43 (8), 1166–1175. 10.1021/ar1000617. [DOI] [PubMed] [Google Scholar]
- Rowsell J. L. C.; Yaghi O. M. Metal–Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73 (1), 3–14. 10.1016/j.micromeso.2004.03.034. [DOI] [Google Scholar]
- Rosseinsky M. J. Recent Developments in Metal–Organic Framework Chemistry: Design, Discovery, Permanent Porosity and Flexibility. Microporous Mesoporous Mater. 2004, 73 (1), 15–30. 10.1016/j.micromeso.2003.05.001. [DOI] [Google Scholar]
- Chen Z.; Kirlikovali K. O.; Li P.; Farha O. K. Reticular Chemistry for Highly Porous Metal–Organic Frameworks: The Chemistry and Applications. Acc. Chem. Res. 2022, 55 (4), 579–591. 10.1021/acs.accounts.1c00707. [DOI] [PubMed] [Google Scholar]
- Cohen S. M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112 (2), 970–1000. 10.1021/cr200179u. [DOI] [PubMed] [Google Scholar]
- Stock N.; Biswas S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112 (2), 933–969. 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- Babaee S.; Zarei M.; Sepehrmansourie H.; Zolfigol M. A.; Rostamnia S. Synthesis of Metal–Organic Frameworks MIL-101(Cr)-NH2 Containing Phosphorous acid Functional Groups: Application for the Synthesis of N-Amino-2-Pyridone and Pyrano [2,3-c]Pyrazole Derivatives via a Cooperative Vinylogous Anomeric-Based Oxidation. ACS Omega 2020, 5 (12), 6240–6249. 10.1021/acsomega.9b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C.; Zhou S.; Kang X.; Zhao Y.; Yan R.; Zhang Y.; Wen L. A Cationic Zinc–Organic Framework with Lewis Acidic and Basic Bifunctional Sites as an Efficient Solvent-Free Catalyst: CO2 Fixation and Knoevenagel Condensation Reaction. Inorg. Chem. 2018, 57 (17), 11157–11164. 10.1021/acs.inorgchem.8b01713. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Zhao Y.-Y.; Chen L.; Zhu C.-Y.; Li P.; Gao W.; Li J.-Y.; Zhang X.-M. Thermally Activated Bipyridyl-Based Mn-MOFs with Lewis Acid–Base Bifunctional Sites for Highly Efficient Catalytic Cycloaddition of CO2 with Epoxides and Knoevenagel Condensation Reactions. Dalt. Trans. 2023, 52 (12), 3671–3681. 10.1039/D3DT00043E. [DOI] [PubMed] [Google Scholar]
- Sarkar A.; Mistry S.; Natarajan S. Friedländer, Knoevenagel, and Michael Reactions Employing the Same MOF: Synthesis, Structure, and Heterogeneous Catalytic Studies of ([Zn(1,4-NDCA)(3-BPDB)0.5]·(DMF)(MeOH) and [Cd4(1,4-NDCA)4(3-BPDB)4]·2(DMF). J. Phys. Chem. C 2021, 125 (49), 27230–27240. 10.1021/acs.jpcc.1c08474. [DOI] [Google Scholar]
- Manna K.; Natarajan S. Highly Selective MOF-Based Turn-Off Luminescence Detection of Hg2+ Ions in an Aqueous Medium and Its Dual Functional Catalytic Activity toward Aldol Condensation and β-Enamination Reactions. Inorg. Chem. 2023, 62 (1), 508–519. 10.1021/acs.inorgchem.2c03679. [DOI] [PubMed] [Google Scholar]
- Huang G.-Q.; Chen J.; Huang Y.-L.; Wu K.; Luo D.; Jin J.-K.; Zheng J.; Xu S.-H.; Lu W. Mixed-Linker Isoreticular Zn(II) Metal–Organic Frameworks as Bro̷nsted Acid–Base Bifunctional Catalysts for Knoevenagel Condensation Reactions. Inorg. Chem. 2022, 61 (21), 8339–8348. 10.1021/acs.inorgchem.2c00941. [DOI] [PubMed] [Google Scholar]
- Prasad R. R. R.; Dawson D. M.; Cox P. A.; Ashbrook S. E.; Wright P. A.; Clarke M. L. A Bifunctional MOF Catalyst Containing Metal–Phosphine and Lewis Acidic Active Sites. Chem. – A Eur. J. 2018, 24 (57), 15309–15318. 10.1002/chem.201803094. [DOI] [PubMed] [Google Scholar]
- Rostamnia S.; Alamgholiloo H.; Jafari M. Ethylene Diamine Post-Synthesis Modification on Open Metal Site Cr-MOF to Access Efficient Bifunctional Catalyst for the Hantzsch Condensation Reaction. Appl. Organomet. Chem. 2018, 32 (8), e4370 10.1002/aoc.4370. [DOI] [Google Scholar]
- Madasamy K.; Kumaraguru S.; Sankar V.; Mannathan S.; Kathiresan M. A Zn Based Metal Organic Framework as a Heterogeneous Catalyst for C–C Bond Formation Reactions. New J. Chem. 2019, 43 (9), 3793–3800. 10.1039/C8NJ05953E. [DOI] [Google Scholar]
- Cirujano F. G.; Martín N.; Fu G.; Jia C.; De Vos D. Cooperative Acid–Base Bifunctional Ordered Porous Solids in Sequential Multi-Step Reactions: MOF vs. Mesoporous Silica. Catal. Sci. Technol. 2020, 10 (6), 1796–1802. 10.1039/C9CY02404B. [DOI] [Google Scholar]
- Anamika; Yadav C. L.; Drew M. G. B.; Kumar K.; Singh N. Ferrocene-Functionalized Dithiocarbamate Zinc(II) Complexes as Efficient Bifunctional Catalysts for the One-Pot Synthesis of Chromene and Imidazopyrimidine Derivatives via Knoevenagel Condensation Reaction. Inorg. Chem. 2021, 60 (9), 6446–6462. 10.1021/acs.inorgchem.1c00162. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Y.; Liu Q.; Zhang L.-Y.; Bao Y.-M.; Tan J.-Y.; Zhang N.; Zhang J.-Y.; Liu Z.-J. MOFs Assembled from C3 Symmetric Ligands: Structure, Iodine Capture and Role as Bifunctional Catalysts towards the Oxidation–Knoevenagel Cascade Reaction. Dalt. Trans. 2021, 50 (2), 647–659. 10.1039/D0DT03565C. [DOI] [PubMed] [Google Scholar]
- Miao Z.; Luan Y.; Qi C.; Ramella D. The Synthesis of a Bifunctional Copper Metal Organic Framework and Its Application in the Aerobic Oxidation/Knoevenagel Condensation Sequential Reaction. Dalt. Trans. 2016, 45 (35), 13917–13924. 10.1039/C6DT01690A. [DOI] [PubMed] [Google Scholar]
- Bohigues B.; Rojas-Buzo S.; Moliner M.; Corma A. Coordinatively Unsaturated Hf-MOF-808 Prepared via Hydrothermal Synthesis as a Bifunctional Catalyst for the Tandem N-Alkylation of Amines with Benzyl Alcohol. ACS Sustain. Chem. Eng. 2021, 9 (47), 15793–15806. 10.1021/acssuschemeng.1c04903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Abednatanzi S.; Chen H.; Liu Y.-Y.; Leus K.; Van Der Voort P. Bifunctional Noble-Metal-Free Catalyst for the Selective Aerobic Oxidation-Knoevenagel One-Pot Reaction: Encapsulation of Polyoxometalates into an Alkylamine-Modified MIL-101 Framework. ACS Appl. Mater. Interfaces 2021, 13 (20), 23558–23566. 10.1021/acsami.1c01621. [DOI] [PubMed] [Google Scholar]
- Hu Y.-H.; Wang J.-C.; Yang S.; Li Y.-A.; Dong Y.-B. CuI@UiO-67-IM: A MOF-Based Bifunctional Composite Triphase-Transfer Catalyst for Sequential One-Pot Azide–Alkyne Cycloaddition in Water. Inorg. Chem. 2017, 56 (14), 8341–8347. 10.1021/acs.inorgchem.7b01025. [DOI] [PubMed] [Google Scholar]
- Abazari R.; Sanati S.; Morsali A.; Kirillov A. M.; Slawin A. M. Z.; Carpenter-Warren C. L. Simultaneous Presence of Open Metal Sites and Amine Groups on a 3D Dy(III)-Metal–Organic Framework Catalyst for Mild and Solvent-Free Conversion of CO2 to Cyclic Carbonates. Inorg. Chem. 2021, 60 (3), 2056–2067. 10.1021/acs.inorgchem.0c03634. [DOI] [PubMed] [Google Scholar]
- Valverde-González A.; Borrallo-Aniceto M. C.; Díaz U.; Maya E. M.; Gándara F.; Sánchez F.; Iglesias M. Nitrogen-Rich Cobalt (II) MOFs as Efficient Bifunctional Catalysts for Single or Tandem Oxidation and CO2 Conversion Reactions. J. CO2 Util. 2023, 67, 102298 10.1016/j.jcou.2022.102298. [DOI] [Google Scholar]
- Parshamoni S.; Telangae J.; Sanda S.; Konar S. A Copper-Based Metal–Organic Framework Acts as a Bifunctional Catalyst for the Homocoupling of Arylboronic Acids and Epoxidation of Olefins. Chem. – An Asian J. 2016, 11 (4), 540–547. 10.1002/asia.201501084. [DOI] [PubMed] [Google Scholar]
- Hu L.; Hao G.-X.; Luo H.-D.; Ke C.-X.; Shi G.; Lin J.; Lin X.-M.; Qazi U. Y.; Cai Y.-P. Bifunctional 2D Cd(II)-Based Metal–Organic Framework as Efficient Heterogeneous Catalyst for the Formation of C–C Bond. Cryst. Growth Des. 2018, 18 (5), 2883–2889. 10.1021/acs.cgd.7b01728. [DOI] [Google Scholar]
- Chen J.; Zhang Z.; Bao Z.; Su Y.; Xing H.; Yang Q.; Ren Q. Functionalized Metal–Organic Framework as a Biomimetic Heterogeneous Catalyst for Transfer Hydrogenation of Imines. ACS Appl. Mater. Interfaces 2017, 9 (11), 9772–9777. 10.1021/acsami.7b00562. [DOI] [PubMed] [Google Scholar]
- Feng W.; Tie X.; Duan X.; Yan S.; Fang S.; Sun P.; Gan L.; Wang T. Covalent Immobilization of Phosphotungstic Acid and Amino Acid on Metal-Organic Frameworks with Different Structures: Acid-Base Bifunctional Heterogeneous Catalyst for the Production of Biodiesel from Insect Lipid. Renew. Energy 2023, 210, 26–39. 10.1016/j.renene.2023.03.120. [DOI] [Google Scholar]
- Behr A.; Vorholt A. J.; Ostrowski K. A.; Seidensticker T. Towards Resource Efficient Chemistry: Tandem Reactions with Renewables. Green Chem. 2014, 16 (3), 982–1006. 10.1039/C3GC41960F. [DOI] [Google Scholar]
- Vilches-Herrera M.; Domke L.; Börner A. Isomerization–Hydroformylation Tandem Reactions. ACS Catal. 2014, 4 (6), 1706–1724. 10.1021/cs500273d. [DOI] [Google Scholar]
- Zieliński G. K.; Grela K. Tandem Catalysis Utilizing Olefin Metathesis Reactions. Chem.—Eur. J. 2016, 22 (28), 9440–9454. 10.1002/chem.201505136. [DOI] [PubMed] [Google Scholar]
- Wang J.; Yang T.; Chen H.; Xu Y.-N.; Yu L.-F.; Liu T.; Tang J.; Yi Z.; Yang C.-G.; Xue W.; Yang F. The Synthesis and Antistaphylococcal Activity of 9, 13-Disubstituted Berberine Derivatives. Eur. J. Med. Chem. 2017, 127, 424–433. 10.1016/j.ejmech.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Liu H.; Xi F.-G.; Sun W.; Yang N.-N.; Gao E.-Q. Amino- and Sulfo-Bifunctionalized Metal–Organic Frameworks: One-Pot Tandem Catalysis and the Catalytic Sites. Inorg. Chem. 2016, 55 (12), 5753–5755. 10.1021/acs.inorgchem.6b01057. [DOI] [PubMed] [Google Scholar]
- Mistry S.; Sarkar A.; Natarajan S. New Bifunctional Metal–Organic Frameworks and Their Utilization in One-Pot Tandem Catalytic Reactions. Cryst. Growth Des. 2019, 19 (2), 747–755. 10.1021/acs.cgd.8b01327. [DOI] [Google Scholar]
- Hu X.-J.; Li Z.-X.; Xue H.; Huang X.; Cao R.; Liu T.-F. Designing a Bifunctional Bro̷nsted Acid–Base Heterogeneous Catalyst Through Precise Installation of Ligands on Metal–Organic Frameworks. CCS Chem. 2020, 2 (1), 616–622. 10.31635/ccschem.019.201900040. [DOI] [Google Scholar]
- Li B.; Zhang Y.; Ma D.; Li L.; Li G.; Li G.; Shi Z.; Feng S. A Strategy toward Constructing a Bifunctionalized MOF Catalyst: Post-Synthetic Modification of MOFs on Organic Ligands and Coordinatively Unsaturated Metal Sites. Chem. Commun. 2012, 48 (49), 6151–6153. 10.1039/c2cc32384b. [DOI] [PubMed] [Google Scholar]
- Lee Y.-R.; Chung Y.-M.; Ahn W.-S. A New Site-Isolated Acid–Base Bifunctional Metal–Organic Framework for One-Pot Tandem Reaction. RSC Adv. 2014, 4 (44), 23064–23067. 10.1039/c4ra02683g. [DOI] [Google Scholar]
- Saikia M.; Saikia L. Sulfonic Acid-Functionalized MIL-101(Cr) as a Highly Efficient Heterogeneous Catalyst for One-Pot Synthesis of 2-Amino-4H-Chromenes in Aqueous Medium. RSC Adv. 2016, 6 (19), 15846–15853. 10.1039/C5RA28135K. [DOI] [Google Scholar]
- Sarkar A.; Mistry S.; Bhattacharya S.; Natarajan S. Multistep Cascade Catalytic Reactions Employing Bifunctional Framework Compounds. Inorg. Chem. 2023, 62, 11142. 10.1021/acs.inorgchem.3c01243. [DOI] [PubMed] [Google Scholar]
- Luo S.; Zeng Z.; Zeng G.; Liu Z.; Xiao R.; Chen M.; Tang L.; Tang W.; Lai C.; Cheng M.; Shao B.; Liang Q.; Wang H.; Jiang D. Metal Organic Frameworks as Robust Host of Palladium Nanoparticles in Heterogeneous Catalysis: Synthesis, Application, and Prospect. ACS Appl. Mater. Interfaces 2019, 11 (36), 32579–32598. 10.1021/acsami.9b11990. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang B.; Tang L.; Goh T. W.; Qi S.; Volkov A.; Pei Y.; Qi Z.; Tsung C.-K.; Stanley L.; Huang W. Cooperative Multifunctional Catalysts for Nitrone Synthesis: Platinum Nanoclusters in Amine-Functionalized Metal–Organic Frameworks. Angew. Chemie Int. Ed. 2017, 56 (51), 16371–16375. 10.1002/anie.201710164. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy A.; Asiri A. M.; Garcia H. Metal Organic Frameworks as Versatile Hosts of Au Nanoparticles in Heterogeneous Catalysis. ACS Catal. 2017, 7 (4), 2896–2919. 10.1021/acscatal.6b03386. [DOI] [Google Scholar]
- Luo W.; Cao W.; Bruijnincx P. C. A.; Lin L.; Wang A.; Zhang T. Zeolite-Supported Metal Catalysts for Selective Hydrodeoxygenation of Biomass-Derived Platform Molecules. Green Chem. 2019, 21 (14), 3744–3768. 10.1039/C9GC01216H. [DOI] [Google Scholar]
- Bailie J. E.; Hutchings G. J.; O’Leary S. Supported Catalysts. Encycl. Mater. Sci. Technol. 2001, 8986–8990. 10.1016/B0-08-043152-6/01620-X. [DOI] [Google Scholar]
- Parker G. J. Guided-Wave Optical Communications: Materials. Encycl. Mater. Sci. Technol. 2001, 3703–3707. 10.1016/B0-08-043152-6/00660-4. [DOI] [Google Scholar]
- Xiong M.; Gao Z.; Qin Y. Spillover in Heterogeneous Catalysis: New Insights and Opportunities. ACS Catal. 2021, 11 (5), 3159–3172. 10.1021/acscatal.0c05567. [DOI] [Google Scholar]
- Rao C. N. R.; Kulkarni G. U.; Thomas P. J.; Edwards P. P. Metal Nanoparticles and Their Assemblies. Chem. Soc. Rev. 2000, 29 (1), 27–35. 10.1039/a904518j. [DOI] [Google Scholar]
- Rajumon M. K.; Prabhakaran K.; Rao C. N. R. Adsorption of Oxygen on (100), (110) and (111) Surfaces of Ag, Cu and Ni: An Electron Spectroscopic Study. Surf. Sci. 1990, 233 (1), L237–L242. 10.1016/0039-6028(90)90169-9. [DOI] [Google Scholar]
- Lingampalli S. R.; Ayyub M. M.; Magesh G.; Rao C. N. R. Photocatalytic Reduction of CO2 by Employing ZnO/Ag1-XCux/CdS and Related Heterostructures. Chem. Phys. Lett. 2018, 691, 28–32. 10.1016/j.cplett.2017.10.048. [DOI] [Google Scholar]
- Ayyappan S.; Gopalan R. S.; Subbanna G. N.; Rao C. N. R. Nanoparticles of Ag, Au, Pd, and Cu Produced by Alcohol Reduction of the Salts. J. Mater. Res. 1997, 12 (2), 398–401. 10.1557/JMR.1997.0057. [DOI] [Google Scholar]
- Osborn J. A.; Wilkinson G.; Mrowca J. J.. Tris(Triphenylphosphine)Halorhodium(I). Inorganic Syntheses; Inorganic Syntheses; 1967; pp 67–71. [Google Scholar]
- Knowles W. S. Asymmetric Hydrogenations (Nobel Lecture). Angew. Chemie Int. Ed. 2002, 41 (12), 1998–2007. . [DOI] [PubMed] [Google Scholar]
- Fihri A.; Bouhrara M.; Nekoueishahraki B.; Basset J.-M.; Polshettiwar V. Nanocatalysts for Suzuki Cross-Coupling Reactions. Chem. Soc. Rev. 2011, 40 (10), 5181–5203. 10.1039/c1cs15079k. [DOI] [PubMed] [Google Scholar]
- Bodkin J. A.; McLeod M. D. The Sharpless Asymmetric Aminohydroxylation. J. Chem. Soc. Perkin Trans. 1 2002, (24), 2733–2746. 10.1039/b111276g. [DOI] [Google Scholar]
- Hoyle C. E.; Bowman C. N. Thiol–Ene Click Chemistry. Angew. Chemie Int. Ed. 2010, 49 (9), 1540–1573. 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; Das A.; Das A. K.; Sheriff A.; Sunny K.; Nair A. S.; Bhandary S.; Bhowal R.; Chopra D.; Pathak B.; Yamazoe S.; Mandal S. Single Cu Atom Doping on Au11 Nanocluster: Its Implication toward Selectivity in C–C Coupling Reaction. Chem. Mater. 2023, 35 (4), 1659–1666. 10.1021/acs.chemmater.2c03293. [DOI] [Google Scholar]
- Mukherjee S.; Jayakumar D.; Mandal S. Insight into the Size Evolution Transformation Process of the Fcc-Based Au28(SR)20 Nanocluster. J. Phys. Chem. C 2021, 125 (22), 12149–12154. 10.1021/acs.jpcc.1c02259. [DOI] [Google Scholar]
- Pradeep T.; Anshup Noble Metal Nanoparticles for Water Purification: A Critical Review. Thin Solid Films 2009, 517 (24), 6441–6478. 10.1016/j.tsf.2009.03.195. [DOI] [Google Scholar]
- Tom R. T.; Nair A. S.; Singh N.; Aslam M.; Nagendra C. L.; Philip R.; Vijayamohanan K.; Pradeep T. Freely Dispersible Au@TiO2, Au@ZrO2, Ag@TiO2, and Ag@ZrO2 Core–Shell Nanoparticles: One-Step Synthesis, Characterization, Spectroscopy, and Optical Limiting Properties. Langmuir 2003, 19 (8), 3439–3445. 10.1021/la0266435. [DOI] [Google Scholar]
- Jain P.; Pradeep T. Potential of Silver Nanoparticle-Coated Polyurethane Foam as an Antibacterial Water Filter. Biotechnol. Bioeng. 2005, 90 (1), 59–63. 10.1002/bit.20368. [DOI] [PubMed] [Google Scholar]
- Maschmeyer T.; Rey F.; Sankar G.; Thomas J. M. Heterogeneous Catalysts Obtained by Grafting Metallocene Complexes onto Mesoporous Silica. Nature 1995, 378 (6553), 159–162. 10.1038/378159a0. [DOI] [Google Scholar]
- Chen J.; Li Q.; Xu R.; Xiao F. Distinguishing the Silanol Groups in the Mesoporous Molecular Sieve MCM-41. Angew. Chem., Int. Ed. Engl. 1996, 34 (23–24), 2694–2696. 10.1002/anie.199526941. [DOI] [Google Scholar]
- Oldroyd R. D.; Thomas J. M.; Maschmeyer T.; MacFaul P. A.; Snelgrove D. W.; Ingold K. U.; Wayner D. D. M. The Titanium (Iv)-Catalyzed Epoxidation of Alkenes by Tert-Alkyl Hydroperoxides. Angew. Chem., Int. Ed. Engl. 1996, 35 (23–24), 2787–2790. 10.1002/anie.199627871. [DOI] [Google Scholar]
- Ploetz E.; Engelke H.; Lächelt U.; Wuttke S. The Chemistry of Reticular Framework Nanoparticles: MOF, ZIF, and COF Materials. Adv. Funct. Mater. 2020, 30 (41), 1909062. 10.1002/adfm.201909062. [DOI] [Google Scholar]
- Wang T.; Gao L.; Hou J.; Herou S. J. A.; Griffiths J. T.; Li W.; Dong J.; Gao S.; Titirici M.-M.; Kumar R. V.; Cheetham A. K.; Bao X.; Fu Q.; Smoukov S. K. Rational Approach to Guest Confinement inside MOF Cavities for Low-Temperature Catalysis. Nat. Commun. 2019, 10 (1), 1340. 10.1038/s41467-019-08972-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-S.; Jin F.-Z.; Ma H.-C.; Li X.-B.; Liu M.-Y.; Kan J.-L.; Chen G.-J.; Dong Y.-B. Au@Cu(II)-MOF: Highly Efficient Bifunctional Heterogeneous Catalyst for Successive Oxidation–Condensation Reactions. Inorg. Chem. 2016, 55 (13), 6685–6691. 10.1021/acs.inorgchem.6b00925. [DOI] [PubMed] [Google Scholar]
- Manna K.; Suresh Kumar B.; Maity T.; Natarajan S. C–C Coupling of Aryl Chlorides and Reduction of Nitroarenes to Amines Employing Recyclable Heterogeneous Green Catalysts. ChemNanoMat 2022, 8 (6), e202200081 10.1002/cnma.202200081. [DOI] [Google Scholar]
- Hu P.; Morabito J. V.; Tsung C.-K. Core–Shell Catalysts of Metal Nanoparticle Core and Metal–Organic Framework Shell. ACS Catal. 2014, 4 (12), 4409–4419. 10.1021/cs5012662. [DOI] [Google Scholar]
- Yang Q.; Xu Q.; Jiang H.-L. Metal–Organic Frameworks Meet Metal Nanoparticles: Synergistic Effect for Enhanced Catalysis. Chem. Soc. Rev. 2017, 46 (15), 4774–4808. 10.1039/C6CS00724D. [DOI] [PubMed] [Google Scholar]
- Moon H. R.; Lim D.-W.; Suh M. P. Fabrication of Metal Nanoparticles in Metal–Organic Frameworks. Chem. Soc. Rev. 2013, 42 (4), 1807–1824. 10.1039/C2CS35320B. [DOI] [PubMed] [Google Scholar]
- Rösler C.; Fischer R. A. Metal–Organic Frameworks as Hosts for Nanoparticles. CrystEngComm 2015, 17 (2), 199–217. 10.1039/C4CE01251H. [DOI] [Google Scholar]
- Sun J.-L.; Chen Y.-Z.; Ge B.-D.; Li J.-H.; Wang G.-M. Three-Shell Cu@Co@Ni Nanoparticles Stabilized with a Metal–Organic Framework for Enhanced Tandem Catalysis. ACS Appl. Mater. Interfaces 2019, 11 (1), 940–947. 10.1021/acsami.8b18584. [DOI] [PubMed] [Google Scholar]
- Jana A. K.; Hota R.; Natarajan S. Palladium Nanoparticles Encapsulated in [M(C19H11N2O2)2·H2O] (M = Co and Mn) as a Potential Catalyst for the Homocoupling of Aryl Halides. Cryst. Growth Des. 2016, 16 (12), 6992–6999. 10.1021/acs.cgd.6b01211. [DOI] [Google Scholar]
- Parmeggiani C.; Matassini C.; Cardona F. A Step Forward towards Sustainable Aerobic Alcohol Oxidation: New and Revised Catalysts Based on Transition Metals on Solid Supports. Green Chem. 2017, 19 (9), 2030–2050. 10.1039/C7GC00406K. [DOI] [Google Scholar]
- Dhakshinamoorthy A.; Garcia H. Catalysis by Metal Nanoparticles Embedded on Metal–Organic Frameworks. Chem. Soc. Rev. 2012, 41 (15), 5262–5284. 10.1039/c2cs35047e. [DOI] [PubMed] [Google Scholar]
- Ndolomingo M. J.; Bingwa N.; Meijboom R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55 (15), 6195–6241. 10.1007/s10853-020-04415-x. [DOI] [Google Scholar]
- Cai J.; Zhuang Y.; Chen Y.; Xiao L.; Zhao Y.; Jiang X.; Hou L.; Li Z. Co–MOF-74@Cu–MOF-74 Derived Bifunctional Co–C@Cu–C for One-Pot Production of 1, 4-Diphenyl-1, 3-Butadiene from Phenylacetylene. ChemCatChem. 2020, 12 (24), 6241–6247. 10.1002/cctc.202001140. [DOI] [Google Scholar]
- Hao M.; Qiu M.; Yang H.; Hu B.; Wang X. Recent Advances on Preparation and Environmental Applications of MOF-Derived Carbons in Catalysis. Sci. Total Environ. 2021, 760, 143333 10.1016/j.scitotenv.2020.143333. [DOI] [PubMed] [Google Scholar]
- Wen M.; Cui Y.; Kuwahara Y.; Mori K.; Yamashita H. Non-Noble-Metal Nanoparticle Supported on Metal–Organic Framework as an Efficient and Durable Catalyst for Promoting H2 Production from Ammonia Borane under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8 (33), 21278–21284. 10.1021/acsami.6b04169. [DOI] [PubMed] [Google Scholar]
- Konnerth H.; Matsagar B. M.; Chen S. S.; Prechtl M. H. G.; Shieh F.-K.; Wu K. C.-W. Metal-Organic Framework (MOF)-Derived Catalysts for Fine Chemical Production. Coord. Chem. Rev. 2020, 416, 213319 10.1016/j.ccr.2020.213319. [DOI] [Google Scholar]
- Pérez-Mayoral E.; Godino-Ojer M.; Matos I.; Bernardo M. Opportunities from Metal Organic Frameworks to Develop Porous Carbons Catalysts Involved in Fine Chemical Synthesis. Catalysts 2023, 13 (3), 541. 10.3390/catal13030541. [DOI] [Google Scholar]
- Fu Y.; Zhai X.; Wang S.; Shao L.; Bai X.-J.; Su Z.-S.; Liu Y.-L.; Zhang L.-Y.; Chen J.-Y. Fabrication of Metal Nanoparticle Composites by Slow Chemical Reduction of Metal–Organic Frameworks. Inorg. Chem. 2021, 60 (21), 16447–16454. 10.1021/acs.inorgchem.1c02277. [DOI] [PubMed] [Google Scholar]
- Li Y.-A.; Yang S.; Liu Q.-K.; Chen G.-J.; Ma J.-P.; Dong Y.-B. Pd(0)@UiO-68-AP: Chelation-Directed Bifunctional Heterogeneous Catalyst for Stepwise Organic Transformations. Chem. Commun. 2016, 52 (39), 6517–6520. 10.1039/C6CC01194B. [DOI] [PubMed] [Google Scholar]
- Hinde C. S.; Webb W. R.; Chew B. K. J.; Tan H. R.; Zhang W.-H.; Hor T. S. A.; Raja R. Utilisation of Gold Nanoparticles on Amine-Functionalised UiO-66 (NH2-UiO-66) Nanocrystals for Selective Tandem Catalytic Reactions. Chem. Commun. 2016, 52 (39), 6557–6560. 10.1039/C6CC02169G. [DOI] [PubMed] [Google Scholar]
- Chen G.-J.; Ma H.-C.; Xin W.-L.; Li X.-B.; Jin F.-Z.; Wang J.-S.; Liu M.-Y.; Dong Y.-B. Dual Heterogeneous Catalyst Pd–Au@Mn(II)-MOF for One-Pot Tandem Synthesis of Imines from Alcohols and Amines. Inorg. Chem. 2017, 56 (1), 654–660. 10.1021/acs.inorgchem.6b02592. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Y.; Li J.-X.; Ding L.-L.; Liu L.; Wang S.-M.; Han Z.-B. Palladium Nanoparticles Encapsulated in the MIL-101-Catalyzed One-Pot Reaction of Alcohol Oxidation and Aldimine Condensation. Inorg. Chem. 2018, 57 (21), 13586–13593. 10.1021/acs.inorgchem.8b02206. [DOI] [PubMed] [Google Scholar]
- Moreno J. M.; Velty A.; Díaz U. MOFs Based on 1D Structural Sub-Domains with Bro̷nsted Acid and Redox Active Sites as Effective Bi-Functional Catalysts. Catal. Sci. Technol. 2020, 10 (11), 3572–3585. 10.1039/D0CY00235F. [DOI] [Google Scholar]
- Jiang W.-L.; Fu Q.-J.; Yao B.-J.; Ding L.-G.; Liu C.-X.; Dong Y.-B. Smart PH-Responsive Polymer-Tethered and Pd NP-Loaded NMOF as the Pickering Interfacial Catalyst for One-Pot Cascade Biphasic Reaction. ACS Appl. Mater. Interfaces 2017, 9 (41), 36438–36446. 10.1021/acsami.7b12166. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Feng L.; Wu C.; Wang X.; Zhang X. Synthesis of 3D-Ordered Macro/Microporous Yolk–Shelled Nanoreactor with Spatially Separated Functionalities for Cascade Reaction. ACS Appl. Mater. Interfaces 2019, 11 (37), 33978–33986. 10.1021/acsami.9b11578. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen L.; Li Y.; Li H.; Xie Z.; Kuang Q.; Zheng L. Palladium NPs Supported on Sulfonic Acid Functionalized Metal–Organic Frameworks as Catalysts for Biomass Cascade Reactions. Dalt. Trans. 2019, 48 (17), 5515–5519. 10.1039/C9DT00348G. [DOI] [PubMed] [Google Scholar]
- Kulkarni B. B.; Kanakikodi K. S.; Maradur S. P. Synergistic Catalytic Activity of Core-Shell Pd@UiO-66(Hf) MOF Catalyst for the One-Pot Hydrogenation-Esterification of Furfural. Microporous Mesoporous Mater. 2022, 343, 112147 10.1016/j.micromeso.2022.112147. [DOI] [Google Scholar]
- Cheng L.; Zhao K.; Zhang Q.; Li Y.; Zhai Q.; Chen J.; Lou Y. Chiral Proline-Decorated Bifunctional Pd@NH2-UiO-66 Catalysts for Efficient Sequential Suzuki Coupling/Asymmetric Aldol Reactions. Inorg. Chem. 2020, 59 (12), 7991–8001. 10.1021/acs.inorgchem.0c00065. [DOI] [PubMed] [Google Scholar]
- Federsel C.; Jackstell R.; Beller M. State-of-the-Art Catalysts for Hydrogenation of Carbon Dioxide. Angew. Chemie Int. Ed. 2010, 49 (36), 6254–6257. 10.1002/anie.201000533. [DOI] [PubMed] [Google Scholar]
- Dalton D. M.; Rovis T. C–H Carboxylation Takes Gold. Nat. Chem. 2010, 2 (9), 710–711. 10.1038/nchem.815. [DOI] [PubMed] [Google Scholar]
- Tundo P.; Selva M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35 (9), 706–716. 10.1021/ar010076f. [DOI] [PubMed] [Google Scholar]
- Yoshida S.; Fukui K.; Kikuchi S.; Yamada T. Silver-Catalyzed Preparation of Oxazolidinones from Carbon Dioxide and Propargylic Amines. Chem. Lett. 2009, 38 (8), 786–787. 10.1246/cl.2009.786. [DOI] [Google Scholar]
- Sakakura T.; Kohno K. The Synthesis of Organic Carbonates from Carbon Dioxide. Chem. Commun. 2009, 11, 1312–1330. 10.1039/b819997c. [DOI] [PubMed] [Google Scholar]
- Federsel C.; Jackstell R.; Beller M. Moderne Katalysatoren Zur Hydrierung von Kohlendioxid. Angew. Chem. 2010, 122 (36), 6392–6395. 10.1002/ange.201000533. [DOI] [Google Scholar]
- Maeda C.; Miyazaki Y.; Ema T. Recent Progress in Catalytic Conversions of Carbon Dioxide. Catal. Sci. Technol. 2014, 4 (6), 1482–1497. 10.1039/c3cy00993a. [DOI] [Google Scholar]
- Huang K.; Sun C.-L.; Shi Z.-J. Transition-Metal-Catalyzed C–C Bond Formation through the Fixation of Carbon Dioxide. Chem. Soc. Rev. 2011, 40 (5), 2435–2452. 10.1039/c0cs00129e. [DOI] [PubMed] [Google Scholar]
- Paddock R. L.; Nguyen S. T. Chemical CO2 Fixation: Cr (III) Salen Complexes as Highly Efficient Catalysts for the Coupling of CO2 and Epoxides. J. Am. Chem. Soc. 2001, 123 (46), 11498–11499. 10.1021/ja0164677. [DOI] [PubMed] [Google Scholar]
- Yaashikaa P. R.; Kumar P. S.; Varjani S. J.; Saravanan A. A Review on Photochemical, Biochemical and Electrochemical Transformation of CO2 into Value-Added Products. J. CO2 Util. 2019, 33, 131–147. 10.1016/j.jcou.2019.05.017. [DOI] [Google Scholar]
- Qiao Y.; Yi J.; Wu S.; Liu Y.; Yang S.; He P.; Zhou H. Li-CO2 Electrochemistry: A New Strategy for CO2 Fixation and Energy Storage. Joule 2017, 1 (2), 359–370. 10.1016/j.joule.2017.07.001. [DOI] [Google Scholar]
- Li H.; Opgenorth P. H.; Wernick D. G.; Rogers S.; Wu T.-Y.; Higashide W.; Malati P.; Huo Y.-X.; Cho K. M.; Liao J. C. Integrated Electromicrobial Conversion of CO2 to Higher Alcohols. Science (80-.) 2012, 335 (6076), 1596. 10.1126/science.1217643. [DOI] [PubMed] [Google Scholar]
- Zhu K.; Li Y.; Li Z.; Liu Y.; Wu H.; Li H. In Situ Activation of COOH-Functionalized ZIF-90-Enabled Reductive CO2N-Formylation. Chem. Commun. 2022, 58 (91), 12712–12715. 10.1039/D2CC04643A. [DOI] [PubMed] [Google Scholar]
- Li Z.; Li H.; Yang S. Carboxylate-Functionalized Zeolitic Imidazolate Framework Enables Catalytic N-Formylation Using Ambient CO2. Adv. Sustainable Syst. 2022, 6 (3), 2100380. 10.1002/adsu.202100380. [DOI] [Google Scholar]
- Gao Z.; Liang L.; Zhang X.; Xu P.; Sun J. Facile One-Pot Synthesis of Zn/Mg-MOF-74 with Unsaturated Coordination Metal Centers for Efficient CO2 Adsorption and Conversion to Cyclic Carbonates. ACS Appl. Mater. Interfaces 2021, 13 (51), 61334–61345. 10.1021/acsami.1c20878. [DOI] [PubMed] [Google Scholar]
- Chiusoli G. P.; Costa M.; Gabriele B.; Salerno G. Sequential Reaction of Carbon Dioxide and Carbon Monoxide with Acetylenic Amines in the Presence of a Palladium Catalyst. J. Mol. Catal. A Chem. 1999, 143 (1), 297–310. 10.1016/S1381-1169(98)00397-5. [DOI] [Google Scholar]
- Shi M.; Nicholas K. M. Palladium-Catalyzed Carboxylation of Allyl Stannanes. J. Am. Chem. Soc. 1997, 119 (21), 5057–5058. 10.1021/ja9639832. [DOI] [Google Scholar]
- Dutta G.; Jana A. K.; Singh D. K.; Eswaramoorthy M.; Natarajan S. Encapsulation of Silver Nanoparticles in an Amine-Functionalized Porphyrin Metal–Organic Framework and Its Use as a Heterogeneous Catalyst for CO2 Fixation under Atmospheric Pressure. Chem. – An Asian J. 2018, 13 (18), 2677–2684. 10.1002/asia.201800815. [DOI] [PubMed] [Google Scholar]
- Dutta G.; Jana A. K.; Natarajan S. Chemical Fixation of CO2 and Other Heterogeneous Catalytic Studies by Employing a Layered Cu-Porphyrin Prepared Through Single-Crystal to Single-Crystal Exchange of a Zn Analogue. Chem. – An Asian J. 2018, 13 (1), 66–72. 10.1002/asia.201701384. [DOI] [PubMed] [Google Scholar]
- Gao C.-Y.; Ai J.; Tian H.-R.; Wu D.; Sun Z.-M. An Ultrastable Zirconium-Phosphonate Framework as Bifunctional Catalyst for Highly Active CO2 Chemical Transformation. Chem. Commun. 2017, 53 (7), 1293–1296. 10.1039/C6CC08773F. [DOI] [PubMed] [Google Scholar]
- Manna K.; Kumar R.; Sundaresan A.; Natarajan S. Fixing CO2 under Atmospheric Conditions and Dual Functional Heterogeneous Catalysis Employing Cu MOFs: Polymorphism, Single-Crystal-to-Single-Crystal (SCSC) Transformation and Magnetic Studies. Inorg. Chem. 2023, 62 (34), 13738–13756. 10.1021/acs.inorgchem.3c01245. [DOI] [PubMed] [Google Scholar]
- Si X.; Pan X.; Xue J.; Yao Q.; Huang X.; Duan W.; Qiu Y.; Su J.; Cao M.; Li J. Robust Acid–Base Ln-MOFs: Searching for Efficient Catalysts in Cycloaddition of CO2 with Epoxides and Cascade Deacetalization–Knoevenagel Reactions. RSC Adv. 2022, 12 (52), 33501–33509. 10.1039/D2RA06545B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-F.; Huang K.-W.; Ko B.-T.; Lin K.-Y. A. Bifunctional ZIF-78 Heterogeneous Catalyst with Dual Lewis Acidic and Basic Sites for Carbon Dioxide Fixation via Cyclic Carbonate Synthesis. J. CO2 Util. 2017, 22, 178–183. 10.1016/j.jcou.2017.10.005. [DOI] [Google Scholar]
- Liang J.; Chen R.-P.; Wang X.-Y.; Liu T.-T.; Wang X.-S.; Huang Y.-B.; Cao R. Postsynthetic Ionization of an Imidazole-Containing Metal–Organic Framework for the Cycloaddition of Carbon Dioxide and Epoxides. Chem. Sci. 2017, 8 (2), 1570–1575. 10.1039/C6SC04357G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-L.; Yang G.-P.; Cheng S.; Qian J.; Fan D.; Wang Y.-Y. Facile Incorporation of Au Nanoparticles into an Unusual Twofold Entangled Zn(II)-MOF with Nanocages for Highly Efficient CO2 Fixation under Mild Conditions. ACS Appl. Mater. Interfaces 2019, 11 (50), 47437–47445. 10.1021/acsami.9b17348. [DOI] [PubMed] [Google Scholar]
- Das R.; Parihar V.; Nagaraja C. M. Strategic Design of a Bifunctional Ag(i)-Grafted NHC-MOF for Efficient Chemical Fixation of CO2 from a Dilute Gas under Ambient Conditions. Inorg. Chem. Front. 2022, 9 (11), 2583–2593. 10.1039/D2QI00479H. [DOI] [Google Scholar]
- Zhu K.; Li Y.; Li Z.; Liu Y.; Wu H.; Li H. In Situ Activation of COOH-Functionalized ZIF-90-Enabled Reductive CO 2 N-Formylation. Chem. Commun. 2022, 58 (91), 12712–12715. 10.1039/D2CC04643A. [DOI] [PubMed] [Google Scholar]
- Flanigen E. M.; Jansen J. C.; van Bekkum H.. Introduction to Zeolite Science and Practice; Studies in Surface Science and Catalysis; Elsevier Science, 1991. [Google Scholar]
- Knowles J. R. Enzyme Catalysis: Not Different, Just Better. Nature 1991, 350 (6314), 121–124. 10.1038/350121a0. [DOI] [PubMed] [Google Scholar]
- Wright P. A.; Thomas J. M.; Cheetham A. K.; Nowak A. K. Localizing Active Sites in Zeolitic Catalysts: Neutron Powder Profile Analysis and Computer Simulation of Deuteropyridine Bound to Gallozeolite-L. Nature 1985, 318 (6047), 611–614. 10.1038/318611a0. [DOI] [Google Scholar]
- Yang D.; Gates B. C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9 (3), 1779–1798. 10.1021/acscatal.8b04515. [DOI] [Google Scholar]
- Bavykina A.; Kolobov N.; Khan I. S.; Bau J. A.; Ramirez A.; Gascon J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120 (16), 8468–8535. 10.1021/acs.chemrev.9b00685. [DOI] [PubMed] [Google Scholar]
- Herbst A.; Janiak C. MOF Catalysts in Biomass Upgrading towards Value-Added Fine Chemicals. CrystEngComm 2017, 19 (29), 4092–4117. 10.1039/C6CE01782G. [DOI] [Google Scholar]
- Ji P.; Drake T.; Murakami A.; Oliveres P.; Skone J. H.; Lin W. Tuning Lewis Acidity of Metal–Organic Frameworks via Perfluorination of Bridging Ligands: Spectroscopic, Theoretical, and Catalytic Studies. J. Am. Chem. Soc. 2018, 140 (33), 10553–10561. 10.1021/jacs.8b05765. [DOI] [PubMed] [Google Scholar]
- Feng X.; Song Y.; Lin W. Dimensional Reduction of Lewis Acidic Metal–Organic Frameworks for Multicomponent Reactions. J. Am. Chem. Soc. 2021, 143 (21), 8184–8192. 10.1021/jacs.1c03561. [DOI] [PubMed] [Google Scholar]
- Agarwal R. A.; Gupta A. K.; De D. Flexible Zn-MOF Exhibiting Selective CO2 Adsorption and Efficient Lewis Acidic Catalytic Activity. Cryst. Growth Des. 2019, 19 (3), 2010–2018. 10.1021/acs.cgd.8b01462. [DOI] [Google Scholar]
- Epp K.; Semrau A. L.; Cokoja M.; Fischer R. A. Dual Site Lewis-Acid Metal-Organic Framework Catalysts for CO2 Fixation: Counteracting Effects of Node Connectivity, Defects and Linker Metalation. ChemCatChem. 2018, 10 (16), 3506–3512. 10.1002/cctc.201800336. [DOI] [Google Scholar]
- Lyu J.; Zhang X.; Li P.; Wang X.; Buru C. T.; Bai P.; Guo X.; Farha O. K. Exploring the Role of Hexanuclear Clusters as Lewis Acidic Sites in Isostructural Metal–Organic Frameworks. Chem. Mater. 2019, 31 (11), 4166–4172. 10.1021/acs.chemmater.9b00960. [DOI] [Google Scholar]
- Xiao J.-D.; Lu S.-M.; Jia G.-Q.; Wang Q.-N.; Li C. Relation Between Coordination and Lewis-Acid Property of MOF-Derived Mononuclear Zn(II) Catalyst Toward Epoxide Hydroxylation. ChemCatChem. 2021, 13 (24), 5236–5242. 10.1002/cctc.202101340. [DOI] [Google Scholar]
- Karimi M.; Hajiashrafi T.; Heydari A.; Azhdari Tehrani A. Terbium–Organic Framework as Heterogeneous Lewis Acid Catalyst for β-Aminoalcohol Synthesis: Efficient, Reusable and Green Catalytic Method. Appl. Organomet. Chem. 2017, 31 (12), e3866 10.1002/aoc.3866. [DOI] [Google Scholar]
- Hajiashrafi T.; Karimi M.; Heydari A.; Tehrani A. A. Erbium-Organic Framework as Heterogeneous Lewis Acid Catalysis for Hantzsch Coupling and Tetrahydro-4H-Chromene Synthesis. Catal. Lett. 2017, 147 (2), 453–462. 10.1007/s10562-016-1913-4. [DOI] [Google Scholar]
- Li X.-Y.; Ma L.-N.; Liu Y.; Hou L.; Wang Y.-Y.; Zhu Z. Honeycomb Metal–Organic Framework with Lewis Acidic and Basic Bifunctional Sites: Selective Adsorption and CO2 Catalytic Fixation. ACS Appl. Mater. Interfaces 2018, 10 (13), 10965–10973. 10.1021/acsami.8b01291. [DOI] [PubMed] [Google Scholar]
- Hassan H. M. A.; Alhumaimess M. S.; Kamel M. M.; Alsohaimi I. H.; Aljaddua H. I.; Aldosari O. F.; Algamdi M. S.; Mohamed R. M. K.; El-Aassar M. R. Electrospinning NH2-MIL-101/PAN Nanofiber Mats: A Promising Catalyst with Lewis Acidic and Basic Bifunctional Sites for Organic Transformation Reactions. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 642, 128659. 10.1016/j.colsurfa.2022.128659. [DOI] [Google Scholar]
- Gupta A. K.; Guha N.; Krishnan S.; Mathur P.; Rai D. K. A Three-Dimensional Cu(II)-MOF with Lewis Acid–base Dual Functional Sites for Chemical Fixation of CO2 via Cyclic Carbonate Synthesis. J. CO2 Util 2020, 39, 101173. 10.1016/j.jcou.2020.101173. [DOI] [Google Scholar]
- Reiner B. R.; Mucha N. T.; Rothstein A.; Temme J. S.; Duan P.; Schmidt-Rohr K.; Foxman B. M.; Wade C. R. Zirconium Metal–Organic Frameworks Assembled from Pd and Pt PNNNP Pincer Complexes: Synthesis, Postsynthetic Modification, and Lewis Acid Catalysis. Inorg. Chem. 2018, 57 (5), 2663–2672. 10.1021/acs.inorgchem.7b03063. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zou M.; Li Q.; Dai W.; Wang D.; Zhang S.; Li B.; Yang L.; Luo S.; Luo X. Thermally Activated Construction of Open Metal Sites on a Zn-Organic Framework: An Effective Strategy to Enhance Lewis Acid Properties and Catalytic Performance for CO2 Cycloaddition Reactions. Appl. Surf. Sci. 2022, 572, 151408. 10.1016/j.apsusc.2021.151408. [DOI] [Google Scholar]
- Yu D.; Wang L.; Yang T.; Yang G.; Wang D.; Ni H.; Wu M. Tuning Lewis Acidity of Iron-Based Metal-Organic Frameworks for Enhanced Catalytic Ozonation. Chem. Eng. J. 2021, 404, 127075 10.1016/j.cej.2020.127075. [DOI] [Google Scholar]
- Xu Y.-P.; Wang Z.-Q.; Tan H.-Z.; Jing K.-Q.; Xu Z.-N.; Guo G.-C. Lewis Acid Sites in MOFs Supports Promoting the Catalytic Activity and Selectivity for CO Esterification to Dimethyl Carbonate. Catal. Sci. Technol. 2020, 10 (6), 1699–1707. 10.1039/C9CY02330E. [DOI] [Google Scholar]
- Ronaghi N.; Shade D.; Moon H. J.; Najmi S.; Cleveland J. W.; Walton K. S.; France S.; Jones C. W. Modulation and Tuning of UiO-66 for Lewis Acid Catalyzed Carbohydrate Conversion: Conversion of Unprotected Aldose Sugars to Polyhydroxyalkyl and C-Glycosyl Furans. ACS Sustain. Chem. Eng. 2021, 9 (34), 11581–11595. 10.1021/acssuschemeng.1c04463. [DOI] [Google Scholar]
- Rouhani F.; Morsali A. Highly Effective Bro̷nsted Base/Lewis Acid Cooperative Catalysis: A New Cd Metal–Organic Framework for the Synthesis of Hantzsch 1,4-DHPs at Ambient Temperature. New J. Chem. 2017, 41 (24), 15475–15484. 10.1039/C7NJ01509G. [DOI] [Google Scholar]
- Venu B.; Shirisha V.; Vishali B.; Naresh G.; Kishore R.; Sreedhar I.; Venugopal A. A Cu-BTC Metal–Organic Framework (MOF) as an Efficient Heterogeneous Catalyst for the Aerobic Oxidative Synthesis of Imines from Primary Amines under Solvent Free Conditions. New J. Chem. 2020, 44 (15), 5972–5979. 10.1039/C9NJ05997K. [DOI] [Google Scholar]
- Pertiwi R.; Oozeerally R.; Burnett D. L.; Chamberlain T. W.; Cherkasov N.; Walker M.; Kashtiban R. J.; Krisnandi Y. K.; Degirmenci V.; Walton R. I. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts. 2019, 9, 437. 10.3390/catal9050437. [DOI] [Google Scholar]
- Moreno J. M.; Velty A.; Díaz U.; Corma A. Synthesis of 2D and 3D MOFs with Tuneable Lewis Acidity from Preformed 1D Hybrid Sub-Domains. Chem. Sci. 2019, 10 (7), 2053–2066. 10.1039/C8SC04372H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyshkanov S.; Nguyen T. N.; Ebrahim F. M.; Stylianou K. C.; Dyson P. J. In Situ Formation of Frustrated Lewis Pairs in a Water-Tolerant Metal-Organic Framework for the Transformation of CO2. Angew. Chemie Int. Ed. 2019, 58 (16), 5371–5375. 10.1002/anie.201901171. [DOI] [PubMed] [Google Scholar]
- Ugale B.; Kumar S.; Dhilip Kumar T. J.; Nagaraja C. M. Environmentally Friendly, Co-Catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks. Inorg. Chem. 2019, 58 (6), 3925–3936. 10.1021/acs.inorgchem.8b03612. [DOI] [PubMed] [Google Scholar]
- Bauer G.; Ongari D.; Xu X.; Tiana D.; Smit B.; Ranocchiari M. Metal–Organic Frameworks Invert Molecular Reactivity: Lewis Acidic Phosphonium Zwitterions Catalyze the Aldol-Tishchenko Reaction. J. Am. Chem. Soc. 2017, 139 (50), 18166–18169. 10.1021/jacs.7b10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.; Zhang P.; Xu J.; Qi H.; Sun J.; Jing S.; Chen X.; Fan Y.; Wang L. An In-Based 3D Metal-Organic Framework as Heterogeneous Lewis Acid Catalyst for Multi-Component Strecker Reactions. Inorg. Chim. Acta 2018, 479, 165–171. 10.1016/j.ica.2018.04.037. [DOI] [Google Scholar]
- Chen H.; Fan L.; Hu T.; Zhang X. Template-Induced {Mn2}–Organic Framework with Lewis Acid–Base Canals as a Highly Efficient Heterogeneous Catalyst for Chemical Fixation of CO2 and Knoevenagel Condensation. Inorg. Chem. 2021, 60 (10), 7276–7283. 10.1021/acs.inorgchem.1c00352. [DOI] [PubMed] [Google Scholar]
- Gu J.; Sun X.; Liu X.; Yuan Y.; Shan H.; Liu Y. Highly Efficient Synergistic CO2 Conversion with Epoxide Using Copper Polyhedron-Based MOFs with Lewis Acid and Base Sites. Inorg. Chem. Front. 2020, 7 (22), 4517–4526. 10.1039/D0QI00938E. [DOI] [Google Scholar]
- Xu H.; Chen M.; Ji M. Solid Lewis Acid-Base Pair Catalysts Constructed by Regulations on Defects of UiO-66 for the Catalytic Hydrogenation of Cinnamaldehyde. Catal. Today 2022, 402, 52–59. 10.1016/j.cattod.2022.03.001. [DOI] [Google Scholar]
- Niu Z.; Bhagya Gunatilleke W. D. C.; Sun Q.; Lan P. C.; Perman J.; Ma J.-G.; Cheng Y.; Aguila B.; Ma S. Metal-Organic Framework Anchored with a Lewis Pair as a New Paradigm for Catalysis. Chem. 2018, 4 (11), 2587–2599. 10.1016/j.chempr.2018.08.018. [DOI] [Google Scholar]
- Guo Q.; Ren L.; Kumar P.; Cybulskis V. J.; Mkhoyan K. A.; Davis M. E.; Tsapatsis M. A Chromium Hydroxide/MIL-101(Cr) MOF Composite Catalyst and Its Use for the Selective Isomerization of Glucose to Fructose.. Angew. Chemie - Int. Ed. 2018, 57 (18), 4926–4930. 10.1002/anie.201712818. [DOI] [PubMed] [Google Scholar]
- Gao X.; Liu M.; Lan J.; Liang L.; Zhang X.; Sun J. Lewis Acid–Base Bifunctional Crystals with a Three-Dimensional Framework for Selective Coupling of CO2 and Epoxides under Mild and Solvent-Free Conditions. Cryst. Growth Des. 2017, 17 (1), 51–57. 10.1021/acs.cgd.6b01132. [DOI] [Google Scholar]
- Tian H.; Liu S.; Zhang Z.; Dang T.; Lu Y.; Liu S. Highly Stable Polyoxovanadate-Based Zn–MOF with Dual Active Sites as a Solvent-Free Catalyst for C–C Bond Formation. ACS Sustain. Chem. Eng. 2021, 9 (12), 4660–4667. 10.1021/acssuschemeng.1c00389. [DOI] [Google Scholar]
- Ansari S. N.; Kumar P.; Gupta A. K.; Mathur P.; Mobin S. M. Catalytic CO2 Fixation over a Robust Lactam-Functionalized Cu(II) Metal Organic Framework. Inorg. Chem. 2019, 58 (15), 9723–9732. 10.1021/acs.inorgchem.9b00684. [DOI] [PubMed] [Google Scholar]
- Gupta V.; Mandal S. K. Design and Construction of a Chiral Cd(II)-MOF from Achiral Precursors: Synthesis, Crystal Structure and Catalytic Activity toward C–C and C–N Bond Forming Reactions. Inorg. Chem. 2019, 58 (5), 3219–3226. 10.1021/acs.inorgchem.8b03307. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wang Y.; Liu L.; Wei N.; Gao M.-L.; Zhao D.; Han Z.-B. Robust Bifunctional Lanthanide Cluster Based Metal–Organic Frameworks (MOFs) for Tandem Deacetalization–Knoevenagel Reaction. Inorg. Chem. 2018, 57 (4), 2193–2198. 10.1021/acs.inorgchem.7b03084. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chang L.; Yang Z.; Shi Y.; Long C.; Han J.; Zhang B.; Qiu X.; Li G.; Tang Z. Facile Synthesis of Ultrathin Metal-Organic Framework Nanosheets for Lewis Acid Catalysis. Nano Res. 2019, 12 (2), 437–440. 10.1007/s12274-018-2235-1. [DOI] [Google Scholar]
- Zheng B.; Luo X.; Wang Z.; Zhang S.; Yun R.; Huang L.; Zeng W.; Liu W. An Unprecedented Water Stable Acylamide-Functionalized Metal–Organic Framework for Highly Efficient CH4/CO2 Gas Storage/Separation and Acid–Base Cooperative Catalytic Activity. Inorg. Chem. Front. 2018, 5 (9), 2355–2363. 10.1039/C8QI00662H. [DOI] [Google Scholar]
- Wang Z.; Luo X.; Zheng B.; Huang L.; Hang C.; Jiao Y.; Cao X.; Zeng W.; Yun R. Highly Selective Carbon Dioxide Capture and Cooperative Catalysis of a Water-Stable Acylamide-Functionalized Metal–Organic Framework. Eur. J. Inorg. Chem. 2018, 2018 (11), 1309–1314. 10.1002/ejic.201701404. [DOI] [Google Scholar]
- Karmakar A.; Soliman M. M. A.; Rúbio G. M. D. M.; Guedes Da Silva M. F. C.; Pombeiro A. J. L. Synthesis and Catalytic Activities of a Zn(Ii) Based Metallomacrocycle and a Metal–Organic Framework towards One-Pot Deacetalization-Knoevenagel Tandem Reactions under Different Strategies: A Comparative Study. Dalt. Trans. 2020, 49 (24), 8075–8085. 10.1039/D0DT01312A. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang B.; Fang Y.; Sun W.; Qi Z.; Pei Y.; Qi S.; Yuan P.; Luan X.; Goh T. W.; Huang W. Metal–Organic-Framework-Derived Carbons: Applications as Solid-Base Catalyst and Support for Pd Nanoparticles in Tandem Catalysis.. Chem. – A Eur. J. 2017, 23 (18), 4266–4270. 10.1002/chem.201605852. [DOI] [PubMed] [Google Scholar]
- Bao Y.-S.; Liu W.; Dong Z.-L.; Xing Z.-Q.; Yang M.; Cui Y.-H.; Meng L.-X.; Li L.-C.; Xu X.-M.; Han Z.-B.; Zhang Y.-Y. Metal–Organic Frameworks as an Efficient Pickering Interfacial Catalyst for the Deacetalization-Knoevenagel Tandem Reaction. New J. Chem. 2023, 47 (18), 8906–8912. 10.1039/D3NJ00788J. [DOI] [Google Scholar]
- Seal N.; Neogi S. Lewis Acid-Base Integrated Robust Metal-Organic Framework and Reconfigurable Composite for Solvent-Free Biginelli Condensation and Tandem Catalysis with Size Selectivity. Mater. Today Chem. 2022, 26, 101064. 10.1016/j.mtchem.2022.101064. [DOI] [Google Scholar]
- Wang X.; Hou Y.; Wang X.; Guo Y.; Zhang X. Integration of Au Nanoparticles and Metal-Organic Frameworks in Hollow Double-Shelled Nanoreactor for Efficient Tandem Catalysis. Appl. Surf. Sci. 2023, 608, 155123. 10.1016/j.apsusc.2022.155123. [DOI] [Google Scholar]
- Das A.; Anbu N.; Dhakshinamoorthy A.; Biswas S. A Highly Catalytically Active Hf(IV) Metal-Organic Framework for Knoevenagel Condensation. Microporous Mesoporous Mater. 2019, 284, 459–467. 10.1016/j.micromeso.2019.04.057. [DOI] [Google Scholar]
- Lv H.; Zhang Z.; Fan L.; Gao Y.; Zhang X. A Nanocaged Cadmium-Organic Framework with High Catalytic Activity on the Chemical Fixation of CO2 and Deacetalization-Knoevenagel Condensation. Microporous Mesoporous Mater. 2022, 335, 111791. 10.1016/j.micromeso.2022.111791. [DOI] [Google Scholar]
- Chen H.; Liu S.; Lv H.; Qin Q.-P.; Zhang X. Nanoporous {Y2}-Organic Frameworks for Excellent Catalytic Performance on the Cycloaddition Reaction of Epoxides with CO2 and Deacetalization–Knoevenagel Condensation. ACS Appl. Mater. Interfaces 2022, 14 (16), 18589–18599. 10.1021/acsami.2c02929. [DOI] [PubMed] [Google Scholar]
- Das A.; Anbu N.; SK M.; Dhakshinamoorthy A.; Biswas S. Highly Active Bisamino Functionalized Zr(IV)-UiO-67 Metal-Organic Framework for Cascade Catalysis. Eur. J. Inorg. Chem. 2020, 2020 (29), 2830–2834. 10.1002/ejic.202000399. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Zhang J.; Wang Z.; Huo H.; Jiang Y.; Xu X.; Lin K. Ion-Exchange Fabrication of Hierarchical Al-MOF-Based Resin Catalysts for the Tandem Reaction. ACS Appl. Mater. Interfaces 2020, 12 (32), 36159–36167. 10.1021/acsami.0c09544. [DOI] [PubMed] [Google Scholar]
- Nicks J.; Zhang J.; Foster J. A. Tandem Catalysis by Ultrathin Metal–Organic Nanosheets Formed through Post-Synthetic Functionalisation of a Layered Framework. Chem. Commun. 2019, 55 (60), 8788–8791. 10.1039/C9CC02061F. [DOI] [PubMed] [Google Scholar]
- Peh S. B.; Cheng Y.; Zhang J.; Wang Y.; Chan G. H.; Wang J.; Zhao D. Cluster Nuclearity Control and Modulated Hydrothermal Synthesis of Functionalized Zr12 Metal–Organic Frameworks. Dalt. Trans. 2019, 48 (21), 7069–7073. 10.1039/C8DT05060K. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Y.; Zhou M.-L.; Cui Y.-H.; Yang M.; Bao Y.-S.; Ye Y.; Tian D.-M.; Liu L.-Y.; Han Z.-B. Polymelamine Formaldehyde-Coated MIL-101 as an Efficient Dual-Functional Core–Shell Composite to Catalyze the Deacetalization–Knoevenagel Tandem Reaction. Inorg. Chem. 2022, 61 (35), 13678–13684. 10.1021/acs.inorgchem.1c03948. [DOI] [PubMed] [Google Scholar]
- Lv H.; Fan L.; Chen H.; Zhang X.; Gao Y. Nanochannel-Based {BaZn}–Organic Framework for Catalytic Activity on the Cycloaddition Reaction of Epoxides with CO2 and Deacetalization-Knoevenagel Condensation. Dalt. Trans. 2022, 51 (9), 3546–3556. 10.1039/D1DT04231A. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhang Z.; Hu T.; Zhang X. Nanochannel {InZn}–Organic Framework with a High Catalytic Performance on CO2 Chemical Fixation and Deacetalization–Knoevenagel Condensation. Inorg. Chem. 2021, 60 (21), 16429–16438. 10.1021/acs.inorgchem.1c02262. [DOI] [PubMed] [Google Scholar]
- Yang M.; Bao Y.-S.; Zhou M.-L.; Wang S.; Cui Y.-H.; Liu W.; Li L.-C.; Meng L.-X.; Zhang Y.-Y.; Han Z.-B. An Efficient Bifunctional Core–Shell MIL-101(Cr)@MOF-867 Composite to Catalyze Deacetalization–Knoevenagel Tandem Reaction. Catal. Lett. 2023, 153, 3561. 10.1007/s10562-022-04259-x. [DOI] [Google Scholar]
- Yang Q.; Liu W.; Wang B.; Zhang W.; Zeng X.; Zhang C.; Qin Y.; Sun X.; Wu T.; Liu J.; Huo F.; Lu J. Regulating the Spatial Distribution of Metal Nanoparticles within Metal-Organic Frameworks to Enhance Catalytic Efficiency. Nat. Commun. 2017, 8 (1), 14429. 10.1038/ncomms14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y.; Mao Y.; Shi S.; Wan M.; Ma C.; Wang S.; Chen C.; Zhao D.; Zhang N. Fabrication of Magnetic Pd/MOF Hollow Nanospheres with Double-Shell Structure: Toward Highly Efficient and Recyclable Nanocatalysts for Hydrogenation Reaction. ACS Appl. Mater. Interfaces 2019, 11 (35), 32251–32260. 10.1021/acsami.9b07864. [DOI] [PubMed] [Google Scholar]
- Veisi H.; Abrifam M.; Kamangar S. A.; Pirhayati M.; Saremi S. G.; Noroozi M.; Tamoradi T.; Karmakar B. Pd Immobilization Biguanidine Modified Zr-UiO-66 MOF as a Reusable Heterogeneous Catalyst in Suzuki–Miyaura Coupling. Sci. Rep. 2021, 11 (1), 21883. 10.1038/s41598-021-00991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparna R. K.; Mukherjee S.; Rose S. S.; Mandal S. Silver Nanoparticle-Incorporated Defect-Engineered Zr-Based Metal–Organic Framework for Efficient Multicomponent Catalytic Reactions. Inorg. Chem. 2022, 61 (41), 16441–16447. 10.1021/acs.inorgchem.2c02542. [DOI] [PubMed] [Google Scholar]
- Mishra B.; Ghosh D.; Tripathi B. P. Finely Dispersed AgPd Bimetallic Nanoparticles on a Polydopamine Modified Metal Organic Framework for Diverse Catalytic Applications. J. Catal. 2022, 411, 1–14. 10.1016/j.jcat.2022.03.009. [DOI] [Google Scholar]
- Taher A.; Susan M. A. B. H.; Begum N.; Lee I.-M. Amine-Functionalized Metal–Organic Framework-Based Pd Nanoparticles: Highly Efficient Multifunctional Catalysts for Base-Free Aerobic Oxidation of Different Alcohols. New J. Chem. 2020, 44 (44), 19113–19121. 10.1039/D0NJ04138F. [DOI] [Google Scholar]
- Nabi S.; Sofi F. A.; Rashid N.; Ingole P. P.; Bhat M. A. Au-Nanoparticle Loaded Nickel-Copper Bimetallic MOF: An Excellent Catalyst for Chemical Degradation of Rhodamine B. Inorg. Chem. Commun. 2020, 117, 107949 10.1016/j.inoche.2020.107949. [DOI] [Google Scholar]
- Zhou Y.-X.; Chen Y.-Z.; Hu Y.; Huang G.; Yu S.-H.; Jiang H.-L. MIL-101-SO3H: A Highly Efficient Bro̷nsted Acid Catalyst for Heterogeneous Alcoholysis of Epoxides under Ambient Conditions.. Chem. – A Eur. J. 2014, 20 (46), 14976–14980. 10.1002/chem.201404104. [DOI] [PubMed] [Google Scholar]
- Singh M.; Solanki P.; Patel P.; Mondal A.; Neogi S. Highly Active Ultrasmall Ni Nanoparticle Embedded Inside a Robust Metal–Organic Framework: Remarkably Improved Adsorption, Selectivity, and Solvent-Free Efficient Fixation of CO2. Inorg. Chem. 2019, 58 (12), 8100–8110. 10.1021/acs.inorgchem.9b00833. [DOI] [PubMed] [Google Scholar]
- Liu X.; Liu Z.; Wang R. Functionalized Metal-Organic Framework Catalysts for Sustainable Biomass Valorization. Adv. Polym. Technol. 2020, 2020, 1201923. 10.1155/2020/1201923. [DOI] [Google Scholar]
- Kiani A.; Alinezhad H.; Ghasemi S. Versatile and an Efficient Sonogashira Coupling Reaction Catalyzed with Modified Pd-Functionalized TMU-16 as a Novel and Reusable Nanocatalyst. J. Organomet. Chem. 2021, 950, 121975. 10.1016/j.jorganchem.2021.121975. [DOI] [Google Scholar]
- Salama R. S.; El-Sayed E.-S. M.; El-Bahy S. M.; Awad F. S. Silver Nanoparticles Supported on UiO-66 (Zr): As an Efficient and Recyclable Heterogeneous Catalyst and Efficient Adsorbent for Removal of Indigo Carmine. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127089. 10.1016/j.colsurfa.2021.127089. [DOI] [Google Scholar]
- Jiang Y.; Zhang X.; Dai X.; Zhang W.; Sheng Q.; Zhuo H.; Xiao Y.; Wang H. Microwave-Assisted Synthesis of Ultrafine Au Nanoparticles Immobilized on MOF-199 in High Loading as Efficient Catalysts for a Three-Component Coupling Reaction. Nano Res. 2017, 10 (3), 876–889. 10.1007/s12274-016-1341-1. [DOI] [Google Scholar]
- Li J.; Xu Z.; Wang T.; Xie X.; Li D.; Wang J.; Huang H.; Ao Z. A Versatile Route to Fabricate Metal/UiO-66 (Metal = Pt, Pd, Ru) with High Activity and Stability for the Catalytic Oxidation of Various Volatile Organic Compounds. Chem. Eng. J. 2022, 448, 136900. 10.1016/j.cej.2022.136900. [DOI] [Google Scholar]
- Dai W.; Mao P.; Liu Y.; Zhang S.; Li B.; Yang L.; Luo X.; Zou J. Quaternary Phosphonium Salt-Functionalized Cr-MIL-101: A Bifunctional and Efficient Catalyst for CO2 Cycloaddition with Epoxides. J. CO2 Util. 2020, 36, 295–305. 10.1016/j.jcou.2019.10.021. [DOI] [Google Scholar]
- Yang D.-A.; Cho H.-Y.; Kim J.; Yang S.-T.; Ahn W.-S. CO2 Capture and Conversion Using Mg-MOF-74 Prepared by a Sonochemical Method. Energy Environ. Sci. 2012, 5 (4), 6465–6473. 10.1039/C1EE02234B. [DOI] [Google Scholar]
- Cho H.-Y.; Yang D.-A.; Kim J.; Jeong S.-Y.; Ahn W.-S. CO2 Adsorption and Catalytic Application of Co-MOF-74 Synthesized by Microwave Heating. Catal. Today 2012, 185 (1), 35–40. 10.1016/j.cattod.2011.08.019. [DOI] [Google Scholar]
- Kim J.; Kim S.-N.; Jang H.-G.; Seo G.; Ahn W.-S. CO2 Cycloaddition of Styrene Oxide over MOF Catalysts. Appl. Catal. A Gen. 2013, 453, 175–180. 10.1016/j.apcata.2012.12.018. [DOI] [Google Scholar]
- Huang X.; Chen Y.; Lin Z.; Ren X.; Song Y.; Xu Z.; Dong X.; Li X.; Hu C.; Wang B. Zn-BTC MOFs with Active Metal Sites Synthesized via a Structure-Directing Approach for Highly Efficient Carbon Conversion. Chem. Commun. 2014, 50 (20), 2624–2627. 10.1039/C3CC49187K. [DOI] [PubMed] [Google Scholar]
- Song L.; Chen C.; Chen X.; Zhang N. Isomorphic MOFs Functionalized by Free-Standing Acylamide and Organic Groups Serving as Self-Supported Catalysts for the CO2 Cycloaddition Reaction. New J. Chem. 2016, 40 (3), 2904–2909. 10.1039/C5NJ03169A. [DOI] [Google Scholar]
- Babu R.; Kathalikkattil A. C.; Roshan R.; Tharun J.; Kim D.-W.; Park D.-W. Dual-Porous Metal Organic Framework for Room Temperature CO2 Fixation via Cyclic Carbonate Synthesis. Green Chem. 2016, 18 (1), 232–242. 10.1039/C5GC01763G. [DOI] [Google Scholar]
- Han Y. H.; Zhou Z. Y.; Tian C. B.; Du S. W. A Dual-Walled Cage MOF as an Efficient Heterogeneous Catalyst for the Conversion of CO2 under Mild and Co-Catalyst Free Conditions. Green Chem. 2016, 18 (14), 4086–4091. 10.1039/C6GC00413J. [DOI] [Google Scholar]
- Kumar S.; Karthikeyan S.; Lee A. F.. G-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. 10.3390/catal8020074. [DOI] [Google Scholar]
- Song X.; Wu Y.; Pan D.; Zhang J.; Xu S.; Gao L.; Wei R.; Zhang J.; Xiao G. Dual-Linker Metal-Organic Frameworks as Efficient Carbon Dioxide Conversion Catalysts. Appl. Catal. A Gen. 2018, 566, 44–51. 10.1016/j.apcata.2018.08.011. [DOI] [Google Scholar]
- He H.; Zhu Q.; Zhao J.; Sun H.; Chen J.; Li C.; Du M. Rational Construction of an Exceptionally Stable MOF Catalyst with Metal-Adeninate Vertices toward CO2 Cycloaddition under Mild and Cocatalyst-Free Conditions. Chem.—Eur. J. 2019, 25 (49), 11474–11480. 10.1002/chem.201901471. [DOI] [PubMed] [Google Scholar]
- Miralda C. M.; Macias E. E.; Zhu M.; Ratnasamy P.; Carreon M. A. Zeolitic Imidazole Framework-8 Catalysts in the Conversion of CO2 to Chloropropene Carbonate. Acs Catal. 2012, 2 (1), 180–183. 10.1021/cs200638h. [DOI] [Google Scholar]
- Tharun J.; Mathai G.; Kathalikkattil A. C.; Roshan R.; Won Y.; Cho S. J.; Chang J.; Park D. Exploring the Catalytic Potential of ZIF-90: Solventless and Co-Catalyst-Free Synthesis of Propylene Carbonate from Propylene Oxide and CO2. ChemPlusChem. 2015, 80 (4), 715–721. 10.1002/cplu.201402395. [DOI] [PubMed] [Google Scholar]
- Kuruppathparambil R. R.; Babu R.; Jeong H. M.; Hwang G.-Y.; Jeong G. S.; Kim M.-I.; Kim D.-W.; Park D.-W. A Solid Solution Zeolitic Imidazolate Framework as a Room Temperature Efficient Catalyst for the Chemical Fixation of CO 2. Green Chem. 2016, 18 (23), 6349–6356. 10.1039/C6GC01614F. [DOI] [Google Scholar]
- Kuruppathparambil R. R.; Jose T.; Babu R.; Hwang G.-Y.; Kathalikkattil A. C.; Kim D.-W.; Park D.-W. A Room Temperature Synthesizable and Environmental Friendly Heterogeneous ZIF-67 Catalyst for the Solvent Less and Co-Catalyst Free Synthesis of Cyclic Carbonates. Appl. Catal. B Environ. 2016, 182, 562–569. 10.1016/j.apcatb.2015.10.005. [DOI] [Google Scholar]
- Verma S.; Baig R. B. N.; Nadagouda M. N.; Varma R. S. Titanium-Based Zeolitic Imidazolate Framework for Chemical Fixation of Carbon Dioxide. Green Chem. 2016, 18 (18), 4855–4858. 10.1039/C6GC01648K. [DOI] [Google Scholar]
- Ma D.; Li B.; Liu K.; Zhang X.; Zou W.; Yang Y.; Li G.; Shi Z.; Feng S. Bifunctional MOF Heterogeneous Catalysts Based on the Synergy of Dual Functional Sites for Efficient Conversion of CO 2 under Mild and Co-Catalyst Free Conditions. J. Mater. Chem. A 2015, 3 (46), 23136–23142. 10.1039/C5TA07026K. [DOI] [Google Scholar]
- Jose T.; Hwang Y.; Kim D.-W.; Kim M.-I.; Park D.-W. Functionalized Zeolitic Imidazolate Framework F-ZIF-90 as Efficient Catalyst for the Cycloaddition of Carbon Dioxide to Allyl Glycidyl Ether. Catal. Today 2015, 245, 61–67. 10.1016/j.cattod.2014.05.022. [DOI] [Google Scholar]
- Tharun J.; Bhin K.-M.; Roshan R.; Kim D. W.; Kathalikkattil A. C.; Babu R.; Ahn H. Y.; Won Y. S.; Park D.-W. Ionic Liquid Tethered Post Functionalized ZIF-90 Framework for the Cycloaddition of Propylene Oxide and CO 2. Green Chem. 2016, 18 (8), 2479–2487. 10.1039/C5GC02153G. [DOI] [Google Scholar]
- Liang J.; Xie Y.-Q.; Wu Q.; Wang X.-Y.; Liu T.-T.; Li H.-F.; Huang Y.-B.; Cao R. Zinc Porphyrin/Imidazolium Integrated Multivariate Zirconium Metal–Organic Frameworks for Transformation of CO2 into Cyclic Carbonates. Inorg. Chem. 2018, 57 (5), 2584–2593. 10.1021/acs.inorgchem.7b02983. [DOI] [PubMed] [Google Scholar]
- Liu T.-T.; Liang J.; Xu R.; Huang Y.-B.; Cao R. Salen-Co (III) Insertion in Multivariate Cationic Metal–Organic Frameworks for the Enhanced Cycloaddition Reaction of Carbon Dioxide. Chem. Commun. 2019, 55 (28), 4063–4066. 10.1039/C8CC10268F. [DOI] [PubMed] [Google Scholar]
- Ding L.-G.; Yao B.-J.; Jiang W.-L.; Li J.-T.; Fu Q.-J.; Li Y.-A.; Liu Z.-H.; Ma J.-P.; Dong Y.-B. Bifunctional Imidazolium-Based Ionic Liquid Decorated UiO-67 Type MOF for Selective CO2 Adsorption and Catalytic Property for CO2 Cycloaddition with Epoxides. Inorg. Chem. 2017, 56 (4), 2337–2344. 10.1021/acs.inorgchem.6b03169. [DOI] [PubMed] [Google Scholar]
- Liang J.; Xie Y.-Q.; Wang X.-S.; Wang Q.; Liu T.-T.; Huang Y.-B.; Cao R. An Imidazolium-Functionalized Mesoporous Cationic Metal–Organic Framework for Cooperative CO 2 Fixation into Cyclic Carbonate. Chem. Commun. 2018, 54 (4), 342–345. 10.1039/C7CC08630J. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Huang H.; Vardhan H.; Aguila B.; Zhong C.; Perman J. A.; Al-Enizi A. M.; Nafady A.; Ma S. Facile Approach to Graft Ionic Liquid into MOF for Improving the Efficiency of CO2 Chemical Fixation. ACS Appl. Mater. Interfaces 2018, 10 (32), 27124–27130. 10.1021/acsami.8b08914. [DOI] [PubMed] [Google Scholar]
- Liu D.; Li G.; Liu H. Functionalized MIL-101 with Imidazolium-Based Ionic Liquids for the Cycloaddition of CO2 and Epoxides under Mild Condition. Appl. Surf. Sci. 2018, 428, 218–225. 10.1016/j.apsusc.2017.09.040. [DOI] [Google Scholar]
- Wang T.; Song X.; Luo Q.; Yang X.; Chong S.; Zhang J.; Ji M. Acid-Base Bifunctional Catalyst: Carboxyl Ionic Liquid Immobilized on MIL-101-NH2 for Rapid Synthesis of Propylene Carbonate from CO2 and Propylene Oxide under Facile Solvent-Free Conditions. Microporous Mesoporous Mater. 2018, 267, 84–92. 10.1016/j.micromeso.2018.03.011. [DOI] [Google Scholar]
- Ding M.; Jiang H. L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. 10.1021/acscatal.7b03404. [DOI] [Google Scholar]
- Aguila B.; Sun Q.; Wang X.; O'Rourke E.; Al-Enizi A. M.; Nafady A.; Ma S. Lower Activation Energy for Catalytic Reactions through Host–Guest Cooperation within Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2018, 57 (32), 10107–10111. 10.1002/anie.201803081. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Jia X.; Huang H.; Guo X.; Qiao Z.; Zhong C. Solvent-Free Mechanochemical Route for the Construction of Ionic Liquid and Mixed-Metal MOF Composites for Synergistic CO 2 Fixation. J. Mater. Chem. A 2020, 8 (6), 3180–3185. 10.1039/C9TA10409G. [DOI] [Google Scholar]
- Zhou X.; Zhang Y.; Yang X.; Zhao L.; Wang G. Functionalized IRMOF-3 as Efficient Heterogeneous Catalyst for the Synthesis of Cyclic Carbonates. J. Mol. Catal. A Chem. 2012, 361, 12–16. 10.1016/j.molcata.2012.04.008. [DOI] [Google Scholar]
- Kim Y.-J.; Park D.-W. Functionalized IRMOF-3: An Efficient Heterogeneous Catalyst for the Cycloaddition of Allyl Glycidyl Ether and CO2. J. Nanosci. Nanotechnol. 2013, 13 (3), 2307–2312. 10.1166/jnn.2013.6878. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Sun W.; Yang N.; Li P.; Gong T.; Sun W.; Sui Q.; Gao E. A Facile and Versatile “Click” Approach Toward Multifunctional Ionic Metal–Organic Frameworks for Efficient Conversion of CO2. ChemSusChem 2019, 12 (10), 2202–2210. 10.1002/cssc.201802990. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Song X.; Xu S.; Zhang J.; Zhu Y.; Gao L.; Xiao G. 2-Methylimidazole Modified Co-BTC MOF as an Efficient Catalyst for Chemical Fixation of Carbon Dioxide. Catal. Lett. 2019, 149, 2575–2585. 10.1007/s10562-019-02874-9. [DOI] [Google Scholar]
- Wu Y.; Song X.; Li S.; Zhang J.; Yang X.; Shen P.; Gao L.; Wei R.; Zhang J.; Xiao G. 3D-Monoclinic M–BTC MOF (M= Mn, Co, Ni) as Highly Efficient Catalysts for Chemical Fixation of CO2 into Cyclic Carbonates. J. Ind. Eng. Chem. 2018, 58, 296–303. 10.1016/j.jiec.2017.09.040. [DOI] [Google Scholar]
- Bahadori M.; Marandi A.; Tangestaninejad S.; Moghadam M.; Mirkhani V.; Mohammadpoor-Baltork I. Ionic Liquid-Decorated MIL-101 (Cr) via Covalent and Coordination Bonds for Efficient Solvent-Free CO2 Conversion and CO2 Capture at Low Pressure. J. Phys. Chem. C 2020, 124 (16), 8716–8725. 10.1021/acs.jpcc.9b11668. [DOI] [Google Scholar]
- He H.; Zhu Q.; Zhang C.; Yan Y.; Yuan J.; Chen J.; Li C.; Du M. Encapsulation of an Ionic Metalloporphyrin into a Zeolite Imidazolate Framework in Situ for CO2 Chemical Transformation via Host–Guest Synergistic Catalysis. Chem.—Asian J. 2019, 14 (7), 958–962. 10.1002/asia.201900021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published articles and its Supporting Information.