Abstract
In this review we highlight the contributions of passive experiments that address important exercise related questions in integrative physiology and medicine. Passive experiments differ from active experiments in that passive experiments involve no planned human intervention to generate observations and test hypotheses. Experiments of nature and natural experiments are two types of passive experiments. Experiments of nature include research participants with rare genetic or acquired conditions that facilitate exploration of specific physiological mechanisms. In this way, experiments of nature are parallel to classical “knockout” animal models among human research participants. Natural experiments are gleaned from data sets that allow population-based questions to be addressed. An advantage of both types of passive experiments is that more extreme and/or prolonged exposures to physiological and behavioral stimuli are possible in humans. In this review, we discuss a number of key passive experiments that have generated foundational medical knowledge or mechanistic physiological insights related to exercise. Both natural experiments and experiments of nature will be essential to generate and test hypotheses about the limits of human adaptability to stressors like exercise.
1 ∣. Introduction
Most review articles cover a topic or narrow range of topics and attempt to synthesize what is known about the topic under discussion, and perhaps chart future directions and testable hypotheses for the field (286, 309). In this review we instead want to discuss some medical and physiological insights related to exercise and humans that stem from what Claude Bernard, the founder of modern physiology, described as “passive” experimentation (28, 247, 260, 262, 279, 333). Claude Bernard used the term passive to indicate that, in contrast to “active” experiments, no planned human intervention is involved in the observations (28, 262). As detailed below, we will primarily discuss two categories of passive experiments— Experiments of Nature and Natural Experiments:
“First, a distinction in usage needs to be emphasized. Experiments of Nature usually refer to single changes (in an organism) that illustrate a biological function, whereas Natural Experiments usually describe an environmental change or behavioral difference that affects a population.”
N. Paneth (personal communication)
The general strategy of presentation for this review includes twelve sections, beginning with this introduction followed by definitions and conceptual foundations in the next section. In the following nine sections, we categorically describe physiological insights gleaned from passive experimentation, and the ultimate section is a summary and call to better integrate data from all available sources of evidence. Some of the sections are brief, while others are longer, and our selection of topics was based on our personal interests and their relevance to exercise. The intriguing and dramatic nature of the observations along with their historical significance also played a role in the topics we chose to cover in this review. Finally, we hope that both the general and specific concepts we seek to highlight are interesting and provocative enough to overcome the admittedly and purposefully idiosyncratic nature of the review.
2 ∣. Conceptual Foundations and Definitions
Some conceptual foundations related to passive experiments were discussed in the previous section. Briefly, passive experiments are generated from data or experimental models that require little or no active intervention by the investigator. A key point that will be highlighted throughout this review is that passive experiments can allow far more prolonged or extreme exposures to a physiological or behavioral stimuli than traditional active experimentation. With this information as a background, we will discuss our motivations for a review on the topic of passive experiments. Next, definitions of key terminology are provided to enhance our conceptual framework.
2.1 ∣. Motivations to Highlight Passive Experimentation
First, during The Pandemic, members of our research group and collaborators from around the world repurposed their scientific expertise to study convalescent plasma as a treatment for coronavirus disease 2019 (COVID-19) (26, 34, 48-54, 63, 104, 114-116, 119, 176, 177, 183-185, 191, 192, 194, 202, 220, 225, 266, 277, 297, 319, 322, 323, 349). The idea - based on a long line of evidence beginning with the Nobel Prize Laureate Emil Adolf van Behring in 1890 - was that antibodies in plasma donated by recovered patients could be administered to those with active disease, speed their recovery and reduce the chances of death (222, 223, 297). Central to our multifaceted demonstration of convalescent plasma efficacy as a treatment for COVID-19 was a series of observations made in small numbers of “Experiments of Nature" (323, 349). Patients participating in these “Experiments of Nature” had rare conditions and were unable to generate endogenous antibodies in response to SARS-CoV-2—the causative agent of COVID-19. Our interest in these COVID-19 related experiments of nature stemmed from our backgrounds as integrative physiologists. We were well aware of how observations from rare patients and unusual groups of human subjects have shed important light on the physiological responses to and the health-related consequences of exercise (80, 141-143, 175, 180, 371). Other observations that contributed to our pandemic-based thinking about convalescent plasma were population based, including the classic series of studies on cardiovascular mortality in sedentary London bus drivers versus the physically active London bus conductors (154, 249-253, 268, 269). Thus, our minds were open to lessons that both experiments of nature and natural experiments might provide about convalescent plasma and COVID-19.
Second, in an era of “big data” there is great interest in what sorts of knowledge can be gleaned from natural experiments (76, 77, 86, 87, 201). Thus, it is important to frame questions and explore the possibility of accounting for putative factors associated with an outcome of interest and gain insight into causation. Perhaps the most well-known early natural experiment was the 1854 Broad Street cholera outbreak in which John Snow inferred that cholera was water-borne and identified the source of the cholera outbreak (a public water pump) by showing the strong association between the locations of death and illness in clusters around a public water pump (280, 329, 354). More recent examples of natural experiments associated with ‘big data’ include the effect of smoking bans on rates of myocardial infarction (312), the effects of military service on lifetime earnings (9), and the impact of nuclear weapons on biological tissue (82, 144). The power of natural experiments was highlighted with the award of the 2021 Nobel Prize in Economic Science to Card, Angrist and Imbens for their natural experiments on topics like the relationships between changes in the minimum wage and unemployment (45). At the experiment of nature level - rare patients - with very low cholesterol levels who were part of a much larger heart health observational cohort were instrumental in developing cholesterol lowering drugs that target the PCSK9 system (62).
Third, while active experimental approaches like randomized controlled trials in clinical medicine or interventional studies in physiology are a powerful paradigm to isolate variables of interest and reduce experimental bias, there are some studies that for logistical or ethical reasons cannot be done on humans. As an extreme example, it seems unlikely that anyone would advocate the creation of human knockout models. On a larger scale, it would be both logistically and ethically challenging to conduct a decades long randomized (and blinded) study on something like cigarette smoking and health outcomes. Additionally, outcomes from highly controlled interventional studies are sometimes not confirmed with real world data. For example, some medical treatments that show efficacy in randomized controlled trials fail to generate a signal of improved outcomes in insurance claims data (310). A more physiologically based real world data vs. lab experimental example are the improvements in race performance associated with next generation running shoes designed to improve running economy. Laboratory-based data suggests these shoes might reduce the energetic cost of running by ~4% (18, 156, 165, 166, 168) which would may translate to improvement in marathon performance by ~3 minutes (190). By contrast a comparison of race results in the same elite runners before and after they started using the improved shoes indicates that the improvement in performance is ~1% or ~1 minute for men (320). Finally, in many fields of science like astronomy and meteorology, active experiments are generally not practicable on many topics. And of course, the insights that led Darwin to develop the theory of evolution were all “observational”.
2.2 ∣. Definitions
Passive Experiment
A scientific question that is posed and addressed using data or an experimental model that did not require an intentional intervention by the investigator.
Experiment of Nature
A form of passive experimentation based on observations made (typically in humans) with rare genetic or acquired conditions that allow specific physiological mechanisms to be explored and hypotheses to be tested.
Natural Experiment
A form of passive experimentation based on observations made in human populations with differing exposures to some environmental or behavioral stimulus that allow specific biomedical outcomes to be explored and hypotheses to be tested.
Exercise
“A type of physical activity that involves planned, structured, and repetitive bodily movement done to maintain or improve one or more components of physical fitness,” as defined by US Centers for Disease Control and Prevention (CDC).
Occupational activity
“Activity undertaken as part of one’s employment. This does not include exercise or physical activities engaged in at employer sponsored gyms or other facilities,” as defined by CDC.
Physical activity
“Any bodily movement that is produced by the contraction of skeletal muscle and that substantially increases energy expenditure,” as defined by CDC.
Cardiorespiratory fitness
We define cardiorespiratory fitness as the (maximum) capacity of the cardiovascular (heart and blood vessels) and respiratory (lungs) systems to supply oxygen-rich blood to the active skeletal muscles and the capacity of the muscles to use oxygen to produce energy for movement (36). This is typically assessed by measuring either the peak workload obtained on an incremental cycle or treadmill test. It can also include gas exchange based measurements of made during incremental cycling or treadmill exercise which are noted by a leveling or plateauing of oxygen consumption despite an increase in workload.
Exercise versus physical activity
The studies featured in this paper will use varying combinations of intentional exercise and occupational or leisure time physical activity as inputs that are related to acute or chronic outcomes of interest. Intentional exercise could be an acute exercise test of some sort with a measured physiological response or health outcome of interest linked to the results of the test. Exercise can also refer to structured training over many months or years for the purposes of recreation, fitness, health and/or athletic competition. As defined earlier, physical activity is a broader term that typically encompasses exercise but also includes things like active transportation via walking or cycling, recreational activities like walking the dog, and occupational physical activity. While the motivations for exercise and physical activity may vary, the important similarity is that both refer to voluntary contractions of skeletal muscles and the associated physiological responses and health consequences.
Some studies also link fitness, typically assessed via graded exercise testing to outcomes of interest. Others use duration of activity along with categories like light, moderate, or intense to describe the dose of exercise used in a comparison. While individuals with higher fitness levels can be more active and/or exercise more, it is possible that at least some people in the high fitness category have inherently high so-called intrinsic fitness (239). An unresolved issue is whether untrained people with generally higher levels of fitness are more active and that contributes to their increased fitness and peak exercise capacity. The other possibility is that people with higher intrinsic fitness are just more willing and able to be active.
Fast versus slow twitch muscles
Skeletal muscle fibers represent a broad continuum of physiological characteristics. Skeletal muscle fibers are classically categorically classified as “fast twitch” and “slow twitch” based on glycolytic histochemical profile, properties of the innervating motor neuron, and intrinsic contractile properties of the fiber (98, 102, 206, 314, 340). Slow twitch muscle fibers (type 1 muscle fibers or ‘red’ muscles) are characterized by: (i) expression of myosin heavy chain 1 (MHC 1), and (ii) “slow” oxidative metabolism. Fast twitch muscle fibers (type 2 muscle fibers or ‘white’ muscles) are characterized by: (i) expression of myosin heavy chain 2 (MHC II), and (ii) “fast” glycolytic metabolism.
Group III and IV afferents
Thinly or unmyelinated sensory afferents in tissues that respond to mechanical and chemical stimuli. In the case of exercise such afferents respond to the mechanical and metabolic effects of contraction in skeletal muscles. They can evoke both sensations perceived by the subject and autonomic responses to skeletal muscle contractions (133, 241).
Central Command
A feed forward signal related to the motor effort associated with contractions. It can increase respiration, heart rate and blood pressure in response to exercise (133, 241).
2.3 ∣. Overview of the article
With the history, rationale, and key definitions outlined above as a background, we believe that sharing with others some of the insights that passive experiments have taught physiologists and others interested in exercise will be useful to a wider audience. To do this we will first review two iconic passive experiments related to physical activity and exercise. We will then highlight how both experiments of nature and natural experiments have provided insights into key physiological responses and health outcomes associated exercise and physical activity. Besides the scientific insights that come from these studies, they serve as exemplars of the two categories of passive experiments. Figure 1 is a conceptual representation of the passive experimental paradigm. Importantly, in a world where the epistemological biomedical hierarchy is dominated by randomized interventional trials and more recently “big data” subjected to analysis by so-called “artificial intelligence” - it is essential to acknowledge that both “small N” and large passive experiments and observational studies can be paradigm shifting.
Figure 1. Conceptualization of passive experiments within wider evaluation framework.
A conceptual model displaying different types of experiment based on allocation of an intervention (null, natural, or experimental) and assignment to an intervention (randomized or not). Although randomized experiments are generally considered to be least susceptible to bias, passive experiments (both natural experiments and experiments of nature) enable the evaluation of changes to a system that are difficult or implausible to manipulate experimentally. Adapted from de Vocht et al (86) and Remler and Van Ryzin (294).
The general strategy of presentation of the next sections of this article is divided by topic area. We begin with two formative and classic examples of passive experiments which informed the overall aim of this articles— classic examples of an experiment of nature in patients with McArdle’s Disease and a natural experiment on physical activity and human health. These examples will be used to highlight the major forms of passive experiments outlined above. In the subsequent sections we will review important insights such experiments have provided in additional areas, including sections: (i) too much exercise; (ii) exercise and vascular adaptations; (iii) physical activity, exercise and body weight; (iv) maximal oxygen uptake and aging; (v) blood pressure; (vi) fast and slow twitch muscle; and (vii) high hemoglobin-oxygen affinity and hypoxia.
Some of these areas of emphasis will include more extensive explorations of related topics. We will highlight articles that have in some way been hypothesis generating, changed thinking, or challenged dogma related to the physiological responses to and health outcomes associated with exercise and physical activity. Many of the studies featured in this review have just a few patients or research participants. Several articles represent many more research participants and include a report on the incidence of atrial fibrillation in more than 50,000 finishers of the long-distance Vasaloppet cross country ski race held annually in Sweden (6, 7, 107, 338). Table 1 lists key papers and includes one or more that are central to each major areas covered in this review. The papers featured in Table 1 were selected because they have been cited more than 250 times, or because they have so clearly delineated a key physiological principal.
Table 1.
Notable passive experiments associated with foundational medical knowledge or mechanistic physiological insights related to exercise.
| Source | Reference | Cited By, n |
Study Type | Participants, n |
Concept(s) | Representative Figure |
|---|---|---|---|---|---|---|
| Hagberg et al, 1982 | 142 | 328 | Experiment of Nature | 30 | Anaerobic threshold and McArdle's disease | 2 |
| Morris et al, 1953 | 252 | 2678 | Natural Experiment | ~31,000 | Occupational physical activity and cardiovascular disease | 3 |
| Paffenbarger et al, 1978 | 274 | 2637 | Natural Experiment | ~17,000 | Physical activity and cardiovascular disease | -- |
| Feldman et al, 2015 | 111 | 63 | Natural Experiment | ~38,000 | Cardiorespiratory fitness and risk of death | 4 |
| Farahmand et al, 2003 | 107 | 98 | Natural Experiment | ~75,000 | Risk of death among cross-country skiers | -- |
| Currens and White, 1961 | 80 | 176 | Natural Experiment | 1 | Large coronary arties in a lifelong Champion runner | 5 |
| Haskell et al, 1993 | 152 | 243 | Natural Experiment | 22 | Improved vascular function in ultramarathon runners | -- |
| Mayer et al, 1956 | 231 | 502 | Natural Experiment | 213 | Occupational physical activity, caloric consumption, and body weight | 6 |
| Westerterp et al, 1986 | 370 | 289 | Natural Experiment | 4 | Caloric consumption during Tour de France cycling | -- |
| Williams et al, 2005 | 374 | 34 | Experiment of Nature | 70 | Physical activity and body composition among twins | 7 |
| Bathgate et al, 2018 | 23 | 31 | Experiment of Nature | 2 | Discordant physical activity among twins | 8 |
| Fontana et al, 2004 | 118 | 1028 | Natural Experiment | 36 | Caloric restriction and cardiovascular disease | -- |
| Coyle et al, 1984 | 75 | 568 | Natural Experiment | 7 | Cardiovascular plasticity and detraining | -- |
| Trappe et al, 2013 | 351 | 112 | Natural Experiment | 15 | Cardiorespiratory fitness and aging | 9 |
| Heath et al, 1981 | 155 | 658 | Natural Experiment | 50 | Cardiorespiratory fitness and aging | 10 |
| Trappe et al, 1996 | 352 | 312 | Natural Experiment | 53 | Cardiorespiratory fitness and aging | -- |
| McGuire et al, 2001 | 237 | 436 | Natural Experiment | 5 | Cardiorespiratory fitness and bed rest | 11 |
| Booth, 1989 | 35 | 9 | Natural Experiment | -- | Cardiorespiratory fitness and aging | 12 |
| Alam and Smirk, 1938 | 4 | 64 | Experiment of Nature | 1 | Blood pressure reflex and exercise | 13 |
| Pryor et al, 1990 | 291 | 107 | Experiment of Nature | 13 | Muscle sympathethic nerve activity and McArdle's disease | -- |
| Marshall et al, 1961 | 230 | 116 | Natural Experiment | 7 | Blood pressure during exercise and sympathetic nervous system | 14 |
| Dominelli et al, 2020 | 92 | 22 | Experiment of Nature | 25 | Hemoglobin-oxygen affinity and hypoxia | 15 |
Major advantages of natural experiments are that both the duration and “dose” of a given exercise intervention or physical activity behavior can be much greater than those used in controlled interventional studies. This can allow a more complete exploration of the limits of the relevant biological adaptations. Additionally, rare experiment of nature patients can serve as “human knockout” models to better understand important physiological mechanisms.
Finally, we note that there are two main reasons exercise is of scientific interest. The first is its benefits to human health. The second reason is because the stresses associated with exercise test the magnitude and mechanisms underpinning numerous acute and chronic adaptive responses in an array of tissues and organ systems. In both cases passive experiments of both types have been instrumental to advancing our understanding.
3 ∣. McArdle’s Disease and the anaerobic threshold - an experiment of nature.
The experiment of nature exercise studies that informed our mindset related to the value of experiments of nature emanated from a study on patients with McArdle’s disease (142)— a rare genetic, metabolic disorder also known as glycogen storage disease type V (311). Patients with McArdle’s disease have a severe deficiency or absence of the key enzyme that catalyzes and regulates breakdown of skeletal muscle glycogen (myophosphorylase), and thus do not generate lactate and the associated acidosis in skeletal muscle during heavy exercise (41). Since the first description by Brian McArdle in 1951 (233), studies of patients with McArdle’s disease have provided transformative insights into the regulation of the respiration and autonomic nervous systems during exercise (296).
In this context, one of the dominant ideas over the last 100 plus years in integrative physiology has been the “anaerobic threshold” concept (20, 25, 39, 40, 65, 85, 96, 109, 162, 200, 290, 305, 361-365). The “anaerobic threshold” concept is associated with more than 6000 publications with the rates of publications also continuing to increase over time. Of note, although many remember the groundbreaking study by Wasserman, Whipp, Koyal and Beaver in 1973 (365), there were many landmark studies in the 1970s and 1980s from a group in Germany that have gone underappreciated because many of the studies were published only in German or not indexed on MEDLINE database (357, 358). The “anaerobic threshold” concept is based on the idea that during heavy exercise oxygen delivery to the contracting skeletal muscles is inadequate, leading to skeletal muscle hypoxia (140). This hypoxia then stimulates a greater reliance on “anaerobic" metabolism which leads to the subsequent accumulation of arterial lactate and hydrogen ion (40). This accumulation of arterial lactate and hydrogen ion contributes to the non-linear rise ventilation seen with exercise levels above ~60% of in untrained subjects and ~80% of in highly trained endurance athletes. There are numerous nomenclature systems related to the anaerobic threshold concept, including the lactate threshold which focuses on the exercise intensity above which there is a progressive rise in blood lactate levels. This exercise intensity is highly correlated with the fraction of that can be sustained during competitive running, especially for events lasting several hours (108, 179).
An important general point is that while hypoxia in exercising muscles can certainly stimulate anaerobic metabolism, there is little evidence for skeletal muscle hypoxia even during heavy exercise (66). Skeletal muscle mitochondria can operate at very low partial pressures of oxygen (167, 295). Thus, the contemporary idea is that increases in skeletal muscle lactic acid levels, and the related rise in blood lactate, occur when the delivery of pyruvate to the mitochondria via glycolysis exceeds the ability of the mitochondria to metabolize it (164). This explains in part why the increase in skeletal muscle mitochondrial content caused by endurance exercise training can reduce lactate levels at a given workload (67, 110, 164).
The specific part of the anaerobic threshold concept that was advanced by studies enrolling patients with McArdle’s disease was the idea that skeletal muscle acidosis, hydrogen ion and/or a fall in pH in the blood contributed to the nonlinear rise in ventilation seen during heavy exercise. As described by James Hagberg in a biographical review of the anaerobic threshold (140), these seminal studies were made possible by the close proximity and overlapping scientific interests of the Human Exercise Physiology Laboratory and the Neuromuscular Disease Center— both at Washington University in St Louis. In addition to basic studies, the Neuromuscular Disease Center performed clinical research and provided care to patients with rare neuromuscular diseases. The proximity of the labs facilitated conversations between Hagberg and colleagues, who were physiologists in the Exercise Lab, and investigators in the Neuromuscular Disease Center. This ultimately led to collaborative studies designed to assess exercise responses in patients with McArdle’s disease.
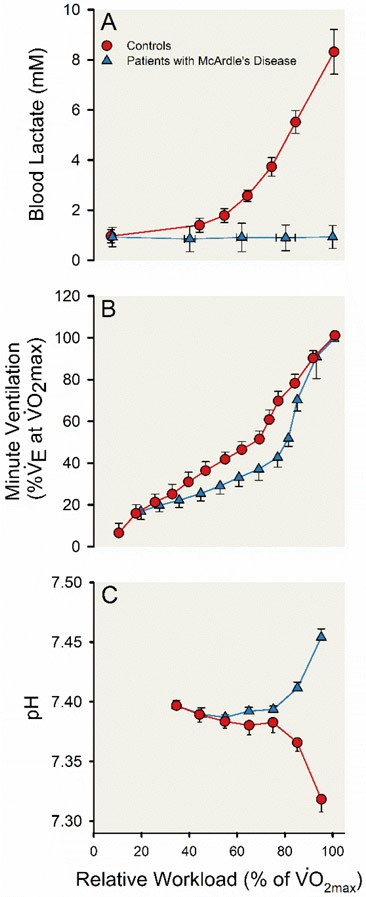
When classic incremental exercise and gas exchange testing was performed on four patients with McArdle’s disease and 26 controls, the patients with McArdle’s disease demonstrated the normal nonlinear rise in ventilation during heavy exercise. This rise in ventilation occurred although no increase in blood lactate levels were seen in patients with McArdle’s disease. There was, however, an increase in arterial pH during exercise among patients with McArdle’s disease — likely associated with the unexplained relative hyperventilation among these patients. These observations challenged the causal link between skeletal muscle lactate production, changes in arterial pH, and the non-linear rise in ventilation seen during heavy exercise that were central to the anaerobic threshold paradigm. Findings from this pioneering and perhaps serendipitous work (142) are displayed in Figure 2.
Figure 2. Physiological responses during progressive incremental exercise for control participants and participants with McArdle’s disease.
Line charts displaying the increase in blood lactate (A) and minute ventilation (; B) and change in arterial blood pH (C) during progressive incremental exercise until volitional exhaustion. The progressive incremental exercise test included four minutes of light exercise at a workload corresponding to 30% maximal aerobic capacity () on a bicycle ergometer followed by regular increases in workload every minute designed to elicit an approximate 10% increase in until a pedal frequency of 50-60 revolutions per minute could no longer be maintained. Twenty-six control participants (red lines and circles) and four participants with McArdle disease (blue lines and triangles) are represented. Symbols represent group means and the error bars represent standard error. Adapted from Hagberg et al 1982 (142).
One caveat of these observations is that exercise capacity is very low in patients with McArdle’s disease, and these patients also have unusually high cardiac output responses to exercise with a so-called hyperdynamic circulation (219). Thus, it is conceivable that these factors might contribute to the nonlinear rise in ventilation during heavy exercise in patients with McArdle’s disease. However, glycogen depletion studies in normal healthy control subjects, which limit the availability of glucose in skeletal muscle for the formation of lactate during heavy exercise, show that the depleted subjects experience a typical nonlinear rise in ventilation during heavy exercise (158). Importantly, these individuals have normal values and otherwise normal cardiovascular responses to exercise.
The ventilatory anaerobic threshold has some utility as practical measure to diagnose pathophysiological responses to exercise in patients with conditions like congestive heart failure, and it (or several similar indices) may also be useful in the context developing training plans for elite athletes. However, the mechanistic links central to the hypothesis are clearly not obligatory based on the compelling observations in the four patients with McArdle’s disease made by Hagberg and colleagues. Patients with McArdle’s disease will be discussed again in the section on blood pressure regulation and the sympathoexcitatory role of acid sensing receptors and channels in skeletal muscle afferent nerves (105, 291).
Section Summary
Patients with McArdle’s disease show a normal, non-linear increase in ventilation and arterial pH during heavy incremental exercise in the absence of a rise in blood lactate concentrations. This experiment of nature is important because it challenged that there was an obligatory and causal link between the non-linear rise in minute ventilation during heavy exercise and arterial blood lactate levels.
4 ∣. Physical Activity, human health, and the natural experiment paradigm.
The natural experiment study that informed our mindset emanated from observations on occupational physical activity and heart disease in British civil servants reported in the decade following World War II. The idea that exercise (or more generally physical activity) is good for human health dates to antiquity (33, 226). For example, both Hippocrates and Galen suggested that a dearth of physical exercise was detrimental to health (269). However, by the 20th century a diametrically opposite view – that exercise was dangerous – was widespread (151, 214). Based on comparisons of mortality between American college men, giants of both cardiology and science postulated that athletes had “demonstrably larger” hearts and shorter life expectancy than scholars (151, 282, 285, 303). In as much as exercise was dangerous, several weeks of complete bed rest was prescribed for patients with acute myocardial infarction (214). However, the classic studies of Morris and colleagues on the incidence of cardiovascular disease among civil servants in the United Kingdom changed the prevailing opinion on the “dangers” of exercise (154, 249-253, 268, 269).
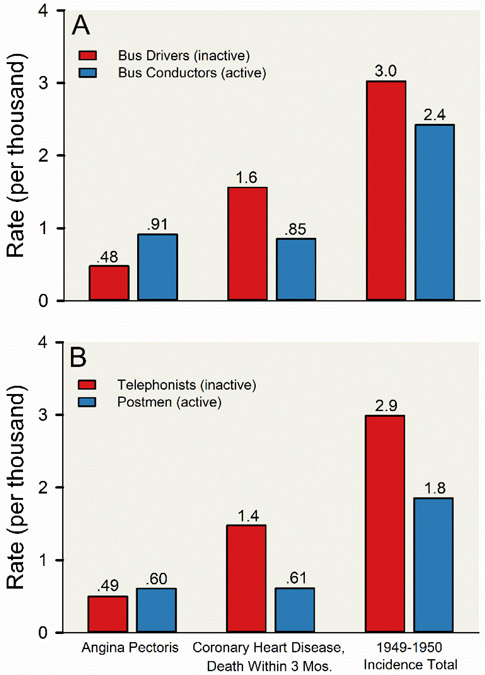
The design of these natural experiments was straightforward— examine groups of civil servants who entered the workforce with similar demographic and health backgrounds but were subsequently exposed to different levels of occupational physical activity. Perhaps the most well-known of these seminal studies is the study comparing coronary heart disease and occupational physical activity among about 31,000 transport workers in London (251, 252). The bus drivers primarily sat during occupational activities while the bus conductors systematically walked up and down the stairs of the prototypical London double decker buses collecting fairs from passengers. Other occupational groups studied by Morris and colleagues included individuals involved in clerical work (telephonists) compared to postal workers who walked while delivering the mail. As shown in Figure 3, these studies demonstrate that the incidence of death from coronary heart disease was reduced by ~50% in the “active” civil servants compared to “inactive” civil servants (252). Of note, a later report in the same population showed that the uniform size was bigger in the drivers than conductors even when the joined the transport service (154). This difference in uniform size raises the possibility that conductors were less active and less fit from a young age, and there was selection bias in the cohorts. It also highlights that selection bias is one of the main challenges of some natural experiments (195).
Figure 3. Total incidence of coronary heart disease and associated clinical presentations among male civil servants in the United Kingdom.
Vertical bar charts displaying the total incidence coronary heart- disease and associated clinical presentations among about 31,000 male civil servants aged 35 to about 60 years employed by the London Transport Executive (A) and the Civil Service (B). In this natural experiment conducted in 1949 to 1950, all ill-health related occupational absences and retirements and the associated medical causes were recorded, and medical causes assigned to any code associated with coronary heart disease (code numbers 420 to 434) underwent detailed scrutiny. Civil servants included in these observations were homogenous for demographic and health backgrounds and were subsequently exposed to highly divergent levels of occupational physical activity. Among the civil servants observed in this natural experiment, red vertical bars represent employees who primarily sat during occupational activities (bus drivers [A] and telephonists [B]) and blue vertical bars represent employees who systematically walked during occupational activities (bus conductors [A] and postmen [B]). The groups of employees with high levels of occupational physical activity (bus conductors and postmen) had lower incidence of coronary heart disease and greater incidence of angina pectoris compared to groups of employees with low levels of occupational physical activity (bus drivers and telephonists). Adapted from Morris et al 1953 (252).
Effect sizes similar to those reported by Morris and colleagues have been remarkably consistent across numerous population cohorts over the last 70 years (38, 163, 186, 199, 326, 334, 347). Additionally, using similar approaches, Paffenbarger and colleagues demonstrated dose response relationships between protection against cardiovascular disease and high levels of both occupational and recreational physical activity (37, 211-213, 267, 271-275, 325). Importantly, the natural experiment approach to study the relationship between physical activity or exercise and cardiovascular disease is ideal because the outcomes of interest take decades to emerge (214, 265). Thus, randomizing a large group of otherwise similar volunteers to a sedentary lifestyle versus a physically active lifestyle would be both logistically challenging and - given the population-based data - ethically questionable (278, 279, 281). Would an Institutional Review Board approve a study that required years of intentional sedentary behavior?
The fundamental observations from the natural experiments outlined above have stimulated many lines of scientific investigation on the causal factors linking physical activity and the risk of cardiovascular disease. These scientific investigations range from interventional studies in humans, studies in animal models and observations at the genetic and cellular level. However, taken together a major theme is that either high levels of physical activity —via occupational physical activity, leisure time physical activity, or structured exercise programs — improve cardiovascular and metabolic health (24, 83, 145, 214, 248, 270, 327).
Why and how are exercise and physical activity protective? The standard answer relates to the effects of these behaviors on traditional risk factors such as blood pressures, lipids, and diabetes that are causally associated with cardiovascular disease. However, the effects of exercise and physical activity on cardiovascular risk are greater than might be expected based on their effects on the traditional risk factors enumerated above (182). In several of the following sections we will consider mechanistic evidence from both natural experiments and experiments of nature related to the health benefits of exercise.
Section Summary
The London transport workers study is a natural experiment that provided the first clear evidence that physical activity was protective against cardiovascular disease. The work of Morris and colleagues transformed the field of physical activity and exercise related population health studies. It also led to countless studies that sought to understand the biological mechanisms responsible for the protective effects of physical activity and exercise on human health.
5 ∣. Too much exercise?
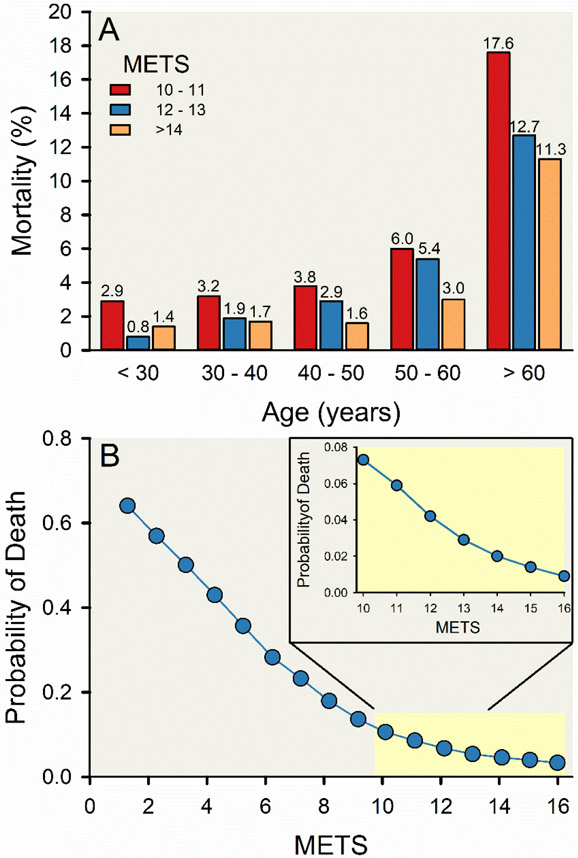
Numerous population-based studies that have intellectual roots in the studies of Morris and Paffenbarger have shown that exercise or physical activity reduces the risk of both cardiovascular disease and all-cause mortality (31, 32, 182, 196, 198, 209, 210, 256, 261, 359, 369). Many studies demonstrate a dose response relationship, such that more exercise or physical activity confers a greater benefit for risk of cardiovascular disease or mortality, but there is also evidence of a “ceiling effect” with diminishing risk reduction with very high levels of exercise or physical activity (10, 215, 246, 360). Likewise, there is an inverse relationship between cardiovascular fitness measured during incremental exercise and mortality as shown in Figure 4 (111). More recently, there is evidence that extremely high levels of exercise might contribute to an increase in risk of cardiovascular disease and mortality (292, 315). The notion that high exercise doses may be associated with increased mortality, or is perhaps even more risky than sedentarism for health, is controversial and has received much attention in the popular media (308). In contrast, there is also substantial evidence of exercise-related survival benefits in extreme endurance exercisers compared to the general population (134), including data from a large cohort of Scandinavian endurance athletes (189).
Figure 4. Negative association between cardiorespiratory fitness and risk of all-cause mortality.
Vertical bar charts displaying a graded decrease in risk of all-cause mortality with increasing cardiorespiratory fitness groups 30 years of age and older, stratified by 10-year age group and categorical cardiorespiratory fitness level (A). Line and scatter plot displaying a reduction in risk of all-cause mortality with higher cardiorespiratory fitness (expressed in metabolic equivalent units [METs]; B). Cardiorespiratory fitness was estimated using a maximal treadmill stress test following the standardized Bruce protocol (43) in about 70,000 people who underwent physician-referred stress testing in hospitals affiliated with Henry Ford Health System as part of the Henry Ford Exercise Testing Project (3). These figures represent a group of about 38,000 adults (about 14,000 women) free of known cardiovascular disease with high cardiorespiratory fitness levels (≥ 10 METs) were followed for 11.5 years on average for all-cause mortality. Adapted from Feldman et al 2015 (111).
A notable natural experiment on the “too much exercise” question is a series of studies including ~50,000 men and ~25,000 women finishers of the long-distance Vasaloppet cross-country ski races (6, 106, 107, 146). Health outcomes were compared to similar aged subjects using the comprehensive Swedish national health records database. Participants with faster and more race finishes had reduced all cause and cardiovascular mortality. The reductions in mortality were also of a similar relative magnitude as those seen by Morris more than 60 years earlier. Because both arduous training for years is required to complete such a race and very high fitness is required to complete it with a fast time, these data provide powerful evidence to counter the too much exercise hypothesis. However, there was also a modest increased risk of atrial fibrillation in separate analysis of the fittest and fastest male skiers compared to slower and less frequent finishers and also the control cohort (6). The association between increased risk of atrial fibrillation and extremely high levels of regular endurance exercise has been consistently observed, although the causal mechanisms are not fully elucidated (2, 17, 60, 157, 187, 243, 245, 292). Plausible explanations for the apparent increase in atrial fibrillation include exercise induced fibrosis of the atria and high vagal tone. Another explanation is that the fastest and more frequent finishers were more aware of any atrial fibrillation they might experience and sought medical attention for it.
Section Summary
The data from cross country skiers refutes the too much exercise and increased mortality hypothesis. It shows that the dose response relationship between fitness, physical activity and mortality extends to people in the very highest categories of both. It is also notable because many women were included. In the next two sections we will touch on additional evidence relevant to the health benefits of exercise and physical activity and also relevant to the too much exercise hypothesis.
6 ∣. Exercise and Vascular Adaptations
At the time of the early epidemiological studies on exercise and cardiovascular disease, it was generally thought that atherosclerotic blockages of the coronary arteries played a key role in this pathophysiology of what is termed “coronary heart disease”. This general proposition has been refined over the last 30 years to include the idea that an acute coronary artery thrombosis, in addition to atherosclerotic narrowing of the coronary arteries, triggers an acute “heart attack” or an Acute Coronary Syndrome. Such events damage heart muscle and can evoke life threatening arrhythmias.
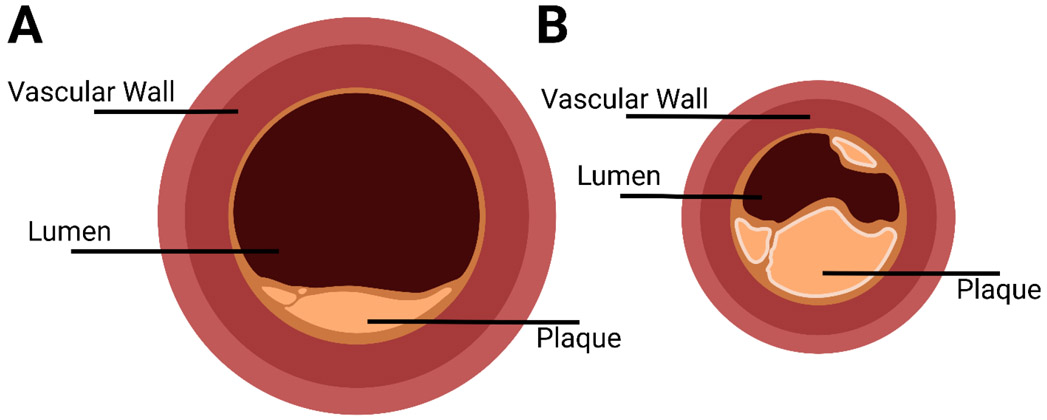
An autopsy study performed on seven-time Boston Marathon Champion and Olympic Bronze medalist (1924 Paris Olympics) Clarence “Mr. Marathon” DeMar (1888-1958) represents a remarkable experiment of nature which provides key insights on the effects of lifelong endurance exercise on the coronary arteries. DeMar had continued to train after his elite, competitive career was over finishing his last Boston marathon at age 65 and was one of the first well-known lifelong athletes. An autopsy performed after his death from cancer at age 70 demonstrated that DeMar had extremely large coronary arteries. While his coronary arteries had significant atherosclerotic plaque, their lumens had diameters that were estimated to be two or three times the normal size (80). Anatomic findings of DeMar’s coronary arteries are represented in Figure 5.
Figure 5. Extremely large coronary arteries in a lifelong endurance exerciser.
Diagrammatic representation of the left main coronary artery 0.5 cm from the ostium from an autopsy study of a lifelong champion runner (Clarence “Mr. Marathon” DeMar; A) and a “control” patient who died at a similar age (B). A lifetime of endurance exercise training was associated with a very large coronary artery lumen diameter in DeMar. Redrawn from photographs in Currens and White (80). Created with BioRender.com.
Subsequent autopsy data reported in 1972 on Masai tribesmen in Kenya who had died traumatic deaths confirmed the observations from DeMar’s autopsy (228). The physically active and fit Masai, who ate a surprisingly atherogenic diet, also had very large coronary arteries and atherosclerotic plaque. These observations indicate that exercise can cause remodeling of the coronary arteries, a phenomenon that has been confirmed in many studies using many models (136, 138, 197, 204, 205). These experiments of nature also demonstrate that exercise is not absolutely protective against the development of atherosclerosis, an important secondary insight.
In the 1990s, ideas about exercise, endothelial function, and improved coronary artery vasodilation had both emerged and converged. A natural experiment by Haskell and colleagues showed that coronary arteries of 11 male ultramarathon runners dilated more compared to coronary arteries of 11 physically inactive men (controls) when nitroglycerin was administered via intracoronary infusion (152). This observation extends the earlier experiments of nature data on DeMar and the Masai by showing that high levels of lifelong physical activity and exercise were associated with both bigger coronary arteries and increased vasodilatory capacity. While atherosclerotic lesions can be present among lifelong habitual exercisers, larger coronary vessels and improved vasodilator and coronary artery function are almost certainly protective (160, 218, 284). This observation also provides evidence to refute the “too much exercise” and cardiovascular health hypothesis discussed previously. These data also align with the more general observation that professional athletes have improved survival compared with nonathletic counterparts (61, 229, 313).
Before we turn our attention to the next example of how informative both natural experiments and experiments of nature can be, two caveats on the larger question of exercise and vascular function are worth mentioning. The first is the discovery of Endothelial Derived Relaxing Factor (EDRF) in 1978 and subsequently nitric oxide. EDRF was discovered by accident when the vascular endothelium was apparently not removed from isolated blood vessels being used in studies of vascular pharmacology and unexpected relaxation vs. contractions were seen in response to muscarinic agonists. Up to that time the endothelium was routinely denuded from blood vessels in pharmacology studies to ensure that the drugs of interest reached the vascular smooth muscle. Thus, what turned out to be a serendipitous finding opened a whole new subfield of vascular biology, new insights into pathophysiology, and new targets for therapy (121-132).
One of the first demonstrations that some of the beneficial effects of exercise might be associated with EDRF came from studies that compared the vasodilator responses to brief periods of ischemia (reactive hyperemia) in both forearms of tennis players. The primary finding of these studies was that the vasodilator responses in the trained, dominant forearm were greater than those vasodilator responses in the non-dominant (less trained) arm (331). This within subject finding was consistent with the idea that exercise and/or chronic physical activity could cause the blood vessels to remodel and become larger. However, in a later study, interarterial infusions of acetylcholine in the brachial arteries of the trained and untrained forearms of tennis players showed similar responses (137). This finding was consistent with the idea that in response to training an increase in shear stress initially causes temporary endothelial adaptations that recede as the vasculature anatomically remodels and a given level of blood flow evokes less shear stress (136). So, while training clearly improves endothelial function, its effect on vascular remodeling needs to be considered when interpreting results of studies that use drugs to assess EDRF mediated mechanisms.
Passive experiments of both types have provided critical insights showing that exercise can have profoundly beneficial effects on health, cause vascular remodeling in the coronary and peripheral circulations, and that the too much exercise hypothesis has serious limitations. The studies highlighted above have the advantage of extremely long-term exposures to the exercise stimulus in contrast to the weeks or months long exposures typically employed in interventional exercise training studies. As noted earlier, the duration of exposure and extreme dose of the exposure are some of the advantages of both natural experiments and experiments of nature. The differences in forearm vascular physiology in the dominant and non-dominant arms of tennis players have the added advantage of a within subject comparison.
Section Summary
The autopsy observations made in Clarence “Mr. Marathon” DeMar and subsequent experiments of nature and natural experiments show that exercise can cause marked remodeling of the blood vessels in the heart and trained skeletal muscles. They also show training improves vasodilator function. These findings explain many of the health benefits of exercise and physical activity. They have informed a wide range of investigations on related topics using approaches that range from molecular to population based.
7 ∣. Physical Activity, Exercise and Body Weight
Because many of the health benefits of exercise and physical activity may operate by preventing weight gain or buffering the negative metabolic effects of obesity, this section focuses on body weight. Both natural experiments and experiments of nature indicate the exercise and physical activity can be profoundly protective against the development of obesity (78, 255).
Increases in body weight and the incidence of obesity at the individual and population level are major public health problems (64, 188, 318, 353). The increase in prevalence of overweight and obesity are mainly attributed to changes in food environment and food systems, primarily increased availability and accessibility of relatively low-cost, high-calorie food products and intense marketing of such food products (64, 339, 355). Simultaneously, the energy expenditure needed for daily life has rapidly declined in most middle- and high-income countries because of modernization and associated increases in automation, mechanization, urbanization, motorization, and computerization (120, 135). In short, the modern world is often described as an obesogenic environment (203, 339).
For those interested in the role of exercise and physical activity on body weight regulation two competing perspectives seem to have emerged. The first is that you can't out exercise a bad diet. The second was summarized in the cult classic novel Once A Runner on elite distance running and it states “If the furnace is hot enough you can burn anything, even Big Macs.” (283) In general, the idea that you can't out exercise a bad diet is the predominant perspective, but is there evidence to the contrary? In this section, we summarize data from several passive experiments, mostly natural experiments, showing that under some circumstances high levels of voluntary exercise or physical activity can have profound effects on both body weight and health. Again, these studies include exposures to physical activity and exercise that are both greater and perhaps much longer in duration than typically employed in interventional studies.
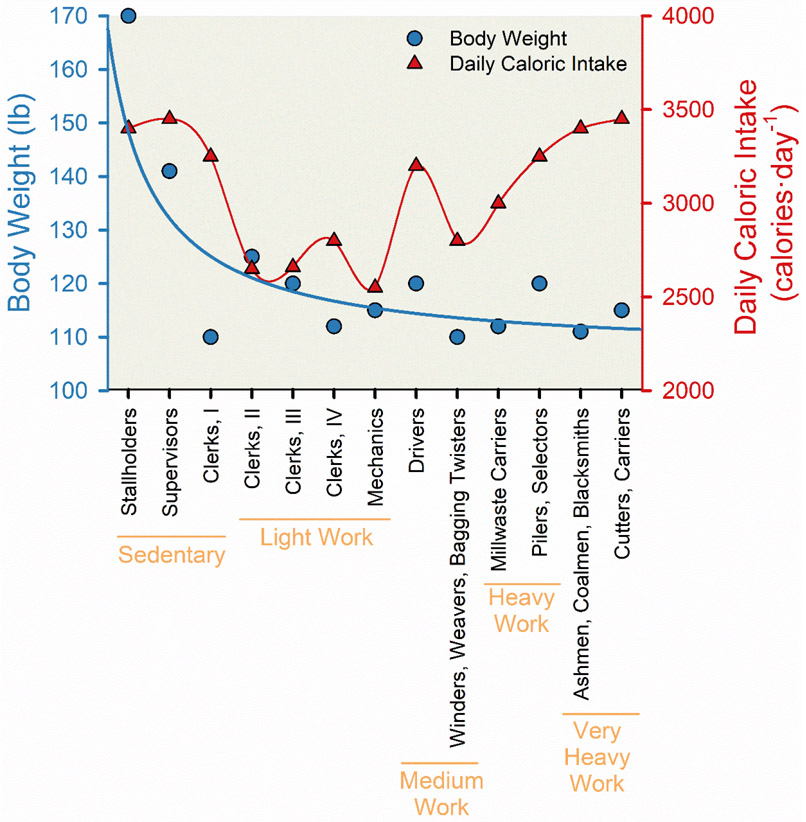
The classic natural experiment by Jean Mayer and colleagues helped describing the interplay between occupational physical activity, caloric consumption, and body weight predicted that “mechanized, urbanized modern living… [may] be a major factor in the increased incidence of obesity” (169, 231). Jean Mayer (1920-1993) was one of the most influential nutrition researchers of the 20th century. In the 1950s he studied workers at a jute processing plant in India (231). Jute is plant-based fiber component used as a raw material to manufacture rope. Mayer described U-shaped relationship between caloric consumption and body weight (Figure 6). The most physically active manual laborers had high caloric consumption and low body weights. The sedentary managers had similarly high caloric consumption but much higher body weights. Workers who got some physical activity at work seem to have both a low body weight and lower caloric consumption.
Figure 6. Association between occupational physical activity and both body weight and daily caloric intake.
Line charts displaying the reduction in body weight (blue circles and line) and “U-shaped” relationship in daily caloric intake (red triangles and line) with greater occupational physical activity among 213 employees of a jute processing plant in West Bengal, India. In this natural experiment conducted in the 1950s, food intake was assessed via dietary interviews, weight was measured using portable scales, and occupational physical activity was stratified into broad activity-based categories using both oxygen consumption and surveys to assess physical demand associated with occupation. Categories of occupational physical activity listed from least to most activity included: sedentary work (stallholders, supervisors, and clerks I), light work (clerks II, clerks III, clerks IV, and mechanics), medium work (drivers, winders, weavers, and bagging twisters), heavy work (millwaste carriers, pilers, and selectors), and very heavy work (ashmen, coalmen, blacksmiths, cutters, and carriers). Adapted from Mayer et al (231).
There are many potential interpretations of the relationship noted in Figure 6. The most obvious is that for those doing the heaviest labor, physical activity mitigated the effects of calories consumed on body weight. An additional observation from the medium physical activity occupations suggests that moderate levels of physical activity prevent weight gain by improving the regulation of appetite. Likewise, the old order Amish who live a non-mechanized agricultural lifestyle which includes heavy manual labor and kilometers per day of walking also have very low rates of obesity (21, 22). These very low rates of obesity were observed in spite of eating a high fat, high calorie diet. Likewise, participants of the Tour de France cycle more than 3,000 km during the 3 weeks of the race and consume nearly 5500 calories per day, and these very lean athletes struggle to maintain their body weight (370).
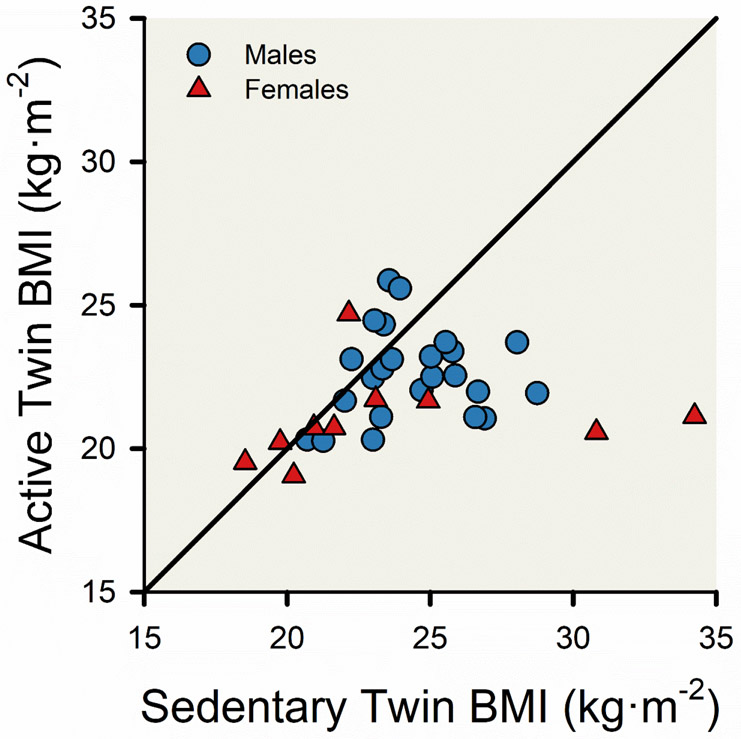
The National Runners study is an Observational cohort that includes tens of thousands of participants who exercise by running or walking regularly. There are a number of identical twin pairs in the study, including 35 (10 female pairs) that had a twin who is inactive. Although studies of twin pairs are most often associated with estimating ‘heritability’ and genetic studies, twin studies offer a powerful tool to study casual inference associated with divergent lifestyles or environmental factors (47, 232, 236). Williams and colleagues compared body mass index (BMI) values for 35 pairs of twins discordant for exercise—one twin was highly active and the corresponding twin was much less active (374). The active twins ran about 60 km per week. Williams and colleagues found that especially among the higher BMI twin pairs, years of physical activity (running) prevented the active twin from becoming obese (BMI>30 kg·m−2), see Figure 7. So, while there is evidence from a variety of sources that genetics contribute to body weight and obesity, this study shows that high levels of habitual physical activity can override the genetic contributions to body weight regulation.
Figure 7. Null association between body mass index (BMI) of active and sedentary monozygotic twins.
The scatter plot displays the BMI of 10 pairs of female twins (red triangles) and 25 pairs of male twins (blue circles). The monozygotic twins were identified using data from the National Runners’ Health Study (372, 373), and the active twin is defined as running more weekly miles than the sedentary twin by at least 25 weekly miles for males and 20 weekly miles for females. The black line represents the line of equality and a hypothetical line depicting identical BMI values between pairs of twins. Adapted from Williams et al 2005 (374).
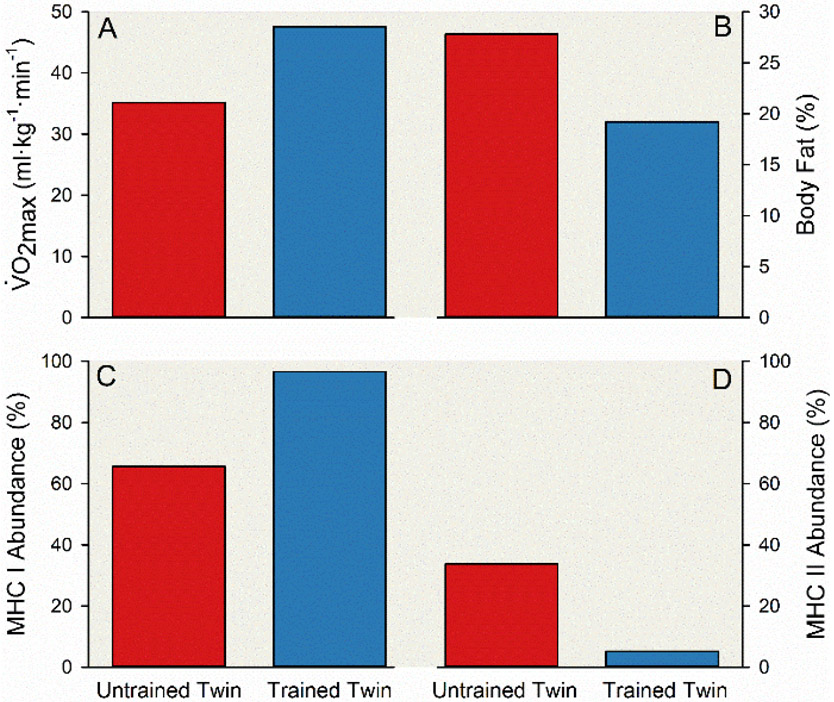
This observation has been supported recently by a remarkable case report of 52-year-old identical twin brothers highly divergent for physical activity (23). The active twin had been doing high volume endurance exercise for 30 years including participation in Ironman distance triathlons, and the inactive twin had refrained from regular exercise throughout his adulthood. This twin pair had divergent physiological adaptations to these discordant exercise habits, including greater cardiorespiratory fitness, lower body fat, and greater “slow twitch” muscle fibers in the active twin—see Figure 8. Additionally, at a molecular level, the influence of key genetic variants implicated in obesity are only seen in cohorts born after World War II when they were more likely to be exposed to a high calorie, low physical activity environment (304).
Figure 8. Divergent physiological adaptations among a pair of male monozygotic twins with 30 years of discordant exercise habits.
Vertical bar charts displaying differences in maximal aerobic capacity (; A), body fat (B), and myosin heavy chain (MHC) isoform composition of vastus lateralis muscle fibers (C & D) between two 52-year-old male monozygotic twins. The trained twin (blue bars) regularly engaged in various modes of endurance exercise including running ~40,000 miles from July 1993 to June 2015. Conversely, the untrained twin (red bars) refrained from regular exercise other than normal activities of daily living. was estimated using a maximal graded cycling exercise test with an open-circuit indirect calorimeter. The trained twin initiated the test with a workload of 125 W and the workload was increased 25 W each minute until volitional task failure, and the untrained twin initiated the test with a workload of 110 W and the workload was increased 15 to 25 W each minute until volitional task failure (A). Body composition was assessed with dual energy x-ray absorptiometry (B). A muscle biopsy from the vastus lateralis was obtained using the standard Bergström technique (16, 254), and muscle fiber composition was classified by myosin heavy chain protein (MHC) isoform using both single fibers and homogenized samples via standard SDS-PAGE methods (C & D). Adapted from Bathgate et al (23).
Another important observation related to the interactions between exercise, diet and health demonstrates that, among low fit subjects, there is an inverse relationship between mortality and a measurement known as the “healthy eating index”. This relationship is absent in the fittest subjects and is consist with the idea that perhaps it is possible to “out exercise” an unhealthy diet (90, 159). More recent data from a very large cohort gleaned from the UK biobank shows the profound beneficial effects exercise and physical activity can have on mortality even in those with suboptimal dietary habits. The protective effects diet alone in the absence of exercise and physical activity were quite modest (90).
While there are innumerable diet and exercise studies using the passive experiment paradigms, two more seem especially provocative on the topic of weight loss. First the National Weight Control Registry (http://www.nwcr.ws/) is an observational study of (formerly) obese individuals who have lost more 30 or more pounds (~14kg) and maintained the weight loss for at least one year (55-57, 81, 161, 193, 264, 293, 348, 376). Successful “biggest losers" have a number of habits that permit them to maintain their weight loss over many years. Among the habits associated with weight maintenance is roughly an hour a day of exercise (276, 375). The second is data on individuals who have undergone long periods of voluntary caloric restriction (118). When individuals who have undergone voluntary caloric restriction for many years are compared to controls and matched habitual exercisers at least some members of the caloric restriction cohort had abnormal glucose tolerance (117). These findings indicate that even an extreme diet may not generate the beneficial effects of exercise on metabolic health.
The above studies can be summarized as offering evidence that high levels of exercise and physical activity modulate the effects of diet on body weight and health. Exercise and physical activity might also improve the regulation of appetite. While it is perhaps unreasonable to expect many people to engage in the extreme levels of behavior highlighted in this section, the observations summarized above do make key points about the biology underpinning the interactions of diet and exercise. Because of the extreme nature of behaviors that can be framed as interventions, they also show the value of both natural experiments and experiments of nature.
Section Summary
The natural experiments and experiments of nature related to physical activity, exercise and diet show the profound impact that physical activity and exercise can have on body weight. This impact can override genetic factors that might contribute to increased body weight. It can also contribute to sustained weight loss and protect health when an unhealthy diet is consumed.
8 ∣. Maximal Oxygen Uptake and Aging
This section is our longest section and covers two related areas maximal oxygen uptake () and the effects of aging on it. We opted for a single section on this topic because observations in aging humans, especially masters athletes provide mechanistic insights into the both the physiological determinants of and healthy human aging (14, 94, 113, 150, 153, 155, 173, 174, 179, 207, 208, 288, 321, 341, 343-346, 351, 352). Additionally, because exercise capacity is also predictive on longevity as noted previously, the physiological determinants of are of interest in that context.
As noted previously, physical activity or exercise quantified in terms of duration per day or week along with an estimate of intensity is a powerful predictor of all-cause mortality. Additionally, peak exercise capacity which can include gas exchange based measurements of maximal oxygen uptake is also a predictor of all-cause mortality and fitter individuals have much lower risk of death for both cardiovascular and all causes. The relationship between physical activity and fitness can be complicated and while the two are not synonymous they are almost certainly related (239). Thus, what do natural experiments and especially experiments of nature tell us about peak exercise capacity and the physiological determinants of maximal oxygen consumption and how they change with aging?
represents the maximum ability of the cardiovascular and respiratory systems to deliver oxygen from the air to the exercising skeletal muscles. Normal values are ~40 and ~35 ml/kg/min in healthy young men and women, respectively and there is typically a 10% per decade reduction in starting in the fourth decade of life (173, 351), see Figure 9. Although endurance exercise training can cause marked increases in , there is debate about trainability as some individuals do not respond to a few months of training. However, it appears that if the exercise stimulus is great enough and for a long enough duration, almost all previously (otherwise healthy) sedentary humans can experience marked and sometimes dramatic increases in their (15).
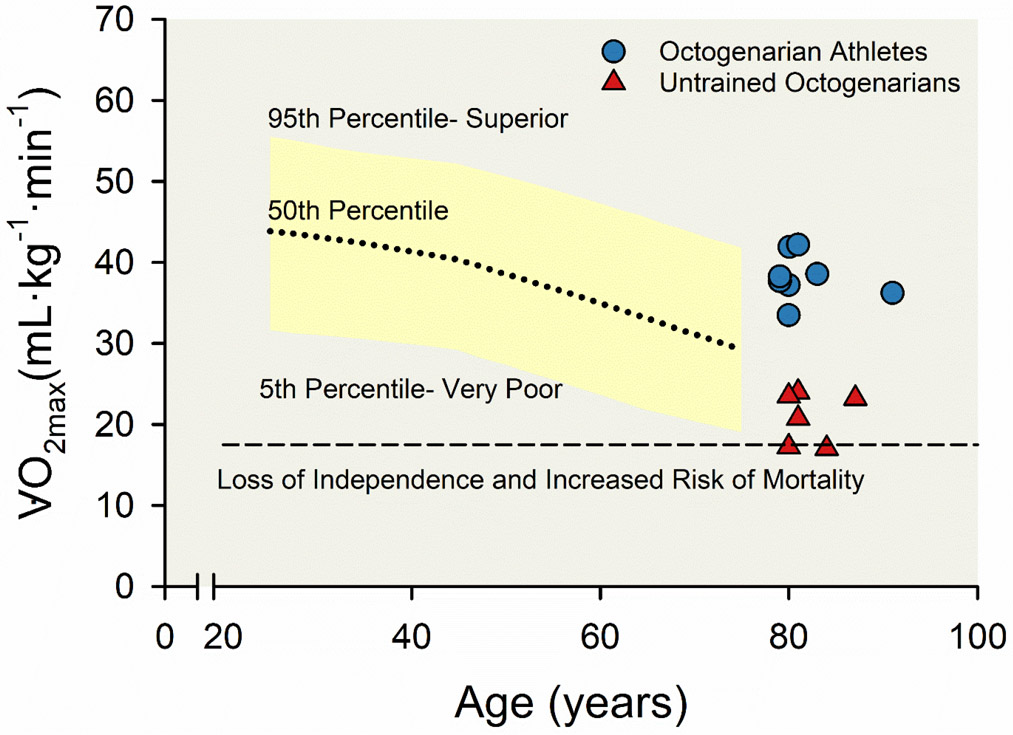
Figure 9. Age-related decline in maximal aerobic capacity () among a large, representative cohort, nine octogenarian lifelong athletes, and six healthy untrained octogenarians.
Plots displaying the decline in across the human lifespan among men, including normative values (yellow area fill) from about 45,000 healthy men from the Cooper Institute in Dallas, Texas (5) and data from Trappe and colleagues (351) representing nine octogenarian lifelong athletes (blue circles) and six healthy untrained octogenarians (red triangles). The dashed line represents the prognostic exercise capacity (5 metabolic equivalents (METs) or 17.5 mL·kg−1·min−1) associated with both a loss of independent lifestyle and increased risk of mortality, as described by Myers and colleagues (257). Adapted from Trappe et al 2013 (351).
Why is predictive of all-cause mortality is of interest. Individuals with high intrinsic fitness - high levels of fitness in the absence of training - may just be more physiologically robust or resilient. They might also be more physically active, and because peak exercise capacity expressed on a body weight basis, they might also be lighter. Additionally, the types of physical activity and exercise training required to increase maximum oxygen uptake improve vascular function, metabolic health, and can have a positive influence on the autonomic nervous system (182).
The major physiological determinants of are now generally agreed to be a peak cardiac output along with red cell mass or the closely related total body hemoglobin (224). In most individuals, except patients with pulmonary conditions and some elite athletes, the respiratory system does not limit . Importantly, during heavy exercise the vast majority (80%) of cardiac output is delivered to the exercising skeletal muscles, and these muscles extract a high fraction of the arterial oxygen content, for use by the mitochondria. With training there are increases in skeletal muscle mitochondria content as well as muscle capillary density. While both adaptations can improve oxygen extraction, the limiting factors are still dominated by total oxygen delivery to the contracting muscles because it is not possible to extract what has not been delivered (178).
Now for some insights on that come from experiments of nature. The first observation comes from the classic 1930s study “New Records in Human Power” (302). In this study measurements of oxygen consumption during heavy exercise were performed on several champion runners. The measurements were made in ways analogous to modern protocols to assess . The athletes studied included the world record holder in the mile Glenn Cunningham and the first man to break 9 minutes for two miles (~3.2 km), Don Lash. Lash is important for another reason because he continued to train throughout life and was one of the first master athletes to be studied.
The study was performed at the Harvard Fatigue Lab and several luminaries in integrative physiology including Robinson and Dill (who were authors on the classic 1930s study) (350). Of note, Cunningham & Lash both had values of ~75 mL·kg−1·min−1. These values are in the range seen in contemporary Champions and they were achieved via very limited training regimens by modern standards (170). However, their training included what would be described in current terms as very “high intensity” duration repetitions of up to a few minutes in duration (149). These observations are some of the first clear demonstrations suggesting that limited periods of very high intensity training can result in impressive values for in individuals who with inherent biological talent.
The next study that is of interest comes from a study on Eero Mäntyranta, a Finnish cross-country skier, multiple-time World Champion, and Olympic medalist. This individual was a rare genetic “human knock out” with a truncated erythropoietin receptor leading to chronically high levels of endogenous erythropoietin and the resulting increased red blood cell mass and total body hemoglobin. While there is limited data on Mäntyranta he was competitively active when laboratory data on issues related to red cell mass, hemoglobin and were being explored in Scandinavia (100, 101). Data from these studies ultimately informed the subsequently banned practice of blood doping were.
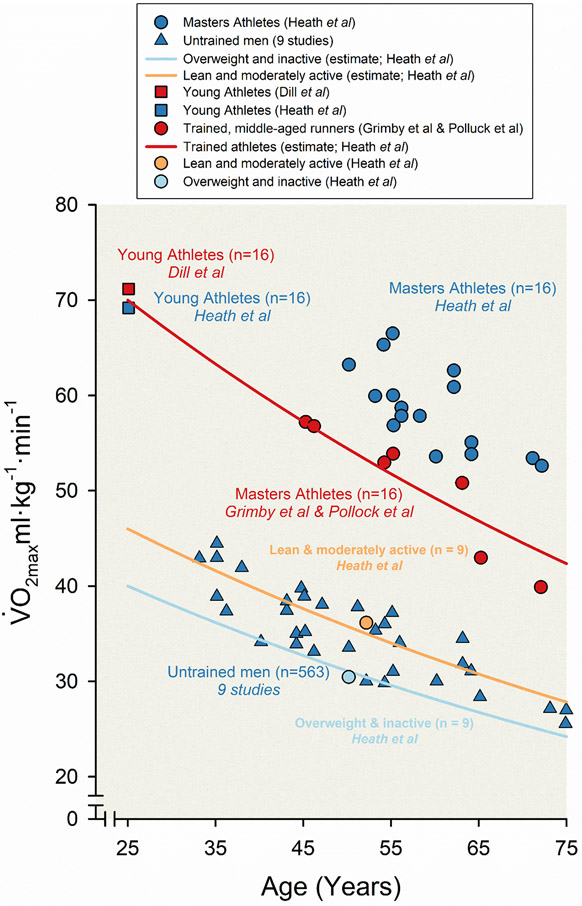
As mentioned earlier declines roughly 10% per decade starting in the fourth decade of life. What happens to this decline in individuals who train regularly? In 1981 Heath and colleagues reported values measured in 16 elite master athlete runners (all males with a mean age of 59) and compared them to matched younger runners (155). This study exploited the early 1970s running boom and the fact that at least a few individuals who had been elite athletes in their youth (like Don Lash mentioned earlier) had either continued to train or had resumed training in middle age for fitness and health purposes. There were also some elite masters runners who had taken up the sport later. As shown in Figure 10, the values in the elite master athletes were far higher in comparison to the older controls and the data indicated that the decline in with aging might be limited to ~5% per decade. Additionally, the estimated O2 pulse, an index of oxygen consumption per heartbeat was similar in the younger and older runners indicating that cardiac stroke volume was likely similarly high in the two groups. Thus, the lower values in the master vs. younger athletes was almost all explained by the well-known age-related reduction in maximum heart rate (342). A decline in maximum heart rate, if there was no increase in stroke volume, would lead to a reduction in maximum cardiac output.
Figure 10. Age-related decline in maximal aerobic capacity () among both untrained and trained men.
Scatter and line plots displaying the decline in across the human lifespan from an amalgam of studies (8, 13, 27, 30, 42, 84, 88, 89, 139, 242, 289, 299, 300). In these experiments of nature, maximal aerobic capacity () was estimated using standardized, graded, maximal stress testing among champion male athletes with very high levels of physical activity and sedentary men with no formal training across the lifespan. Four sets of data from Heath and colleagues are represented, displaying the decline in between 16 highly trained male Masters endurance athletes (blue circles), 16 well-trained young athletes (blue square), nine lean and moderately active middle-aged men (orange circle), and nine overweight and inactive middle-aged men (light blue circle). Lines representing the estimated decline in among leaner and/or moderately active untrained men (orange line) and overweight and/or physically inactive men (light blue line) from Heath and colleagues are plotted based on two assumptions: (i) declines at a rate of 9% per decade beginning at 25 years of age, and (ii) average was 46 mL·kg−1·min−1 for untrained group A and 40 mL·kg−1·min−1 at 25 years of age. The line representing the estimated decrease in among trained men (red line) is plotted based on a presumed decline in of 9% per decade and a value of 70 mL·kg−1min·−1 at 25 years of age. Data from nine studies including 563 untrained men across the lifespan is represented as blue triangles. Data associated with 16 highly trained young male endurance athletes from Dill and colleagues is represented as red square. Data associated with well-trained middle-aged and older runners from both Grimby and colleagues and Pollock and colleagues are represented as red circles. Adapted from Heath et al 1981 (155).
An interesting observation on this topic comes from the great rowing champion Eskild Ebbesen who won three Olympic gold and two bronze medals over five Olympiads (1996-2012). His was measured over more than 20 years remained remarkably constant (~5.5 L·min−1 from age 25-40) even though his maximum heart rate fell 20 beats per minute from age 19 to 40 (263). The duration of a 2000m rowing competition (~6-7 minutes) means that it occurs at a pace and power output that evokes . These observations suggests that Ebbesen’s stroke volume increased to compensate for his reduced maximum heart rate. Likewise, acute reductions in maximum heart rate caused by administration of beta-blocking drugs in younger endurance athletes can cause a compensatory increase in stroke volume (181). The findings in Ebbesen and the beta-blocked athletes are consistent with the idea that the ventricles of young subjects are highly compliant. More recently, it has been shown that the ventricular compliance of lifelong endurance athletes is also maintained in comparison to sedentary and recreationally active subjects of the same age (29).
Because master athletes start with higher values, there is some evidence that the percentage decline they experience with aging can be greater than in sedentary humans (113, 288). However, this finding might be confounded by any reductions in the frequency, intensity or duration of training by the master athletes. Longitudinal data supporting that prolonged intense endurance exercise training can limit the fall comes from Costill and colleagues who studied 53 elite distance runners in their twenties and then invited these individuals back to the lab more than two decades later (68-74, 352). Their mean age was ~50 years and some continued to train very hard, and these individuals showed the ~6% decline in similar to the cross sectional data from Heath and colleagues (155). There were more marked declines in individuals who engaged in fitness related activities and a much larger drop seen in individuals who did not engage in any physical activity or exercise.
Likewise, in another cohort of 135 committed Master athletes the decline in performance over ~8 years was greater than the minimal rate of only 5-6 % per decade suggested by the Heath data and age specific record performances (155, 173). However, among the subgroup of male athletes who continued to perform high intensity training and maintained their lean body mass the decline in was in fact minimal. In the women hormone replacement therapy was associated with a blunted decline in . That continued high levels of training and maintenance of muscle mass can slow the decline in with aging have been extended to even older athletes with observations made on men who had been elite Swedish cross-country skiers and remained highly active into their 80s and case reports in elite runners (298, 351).
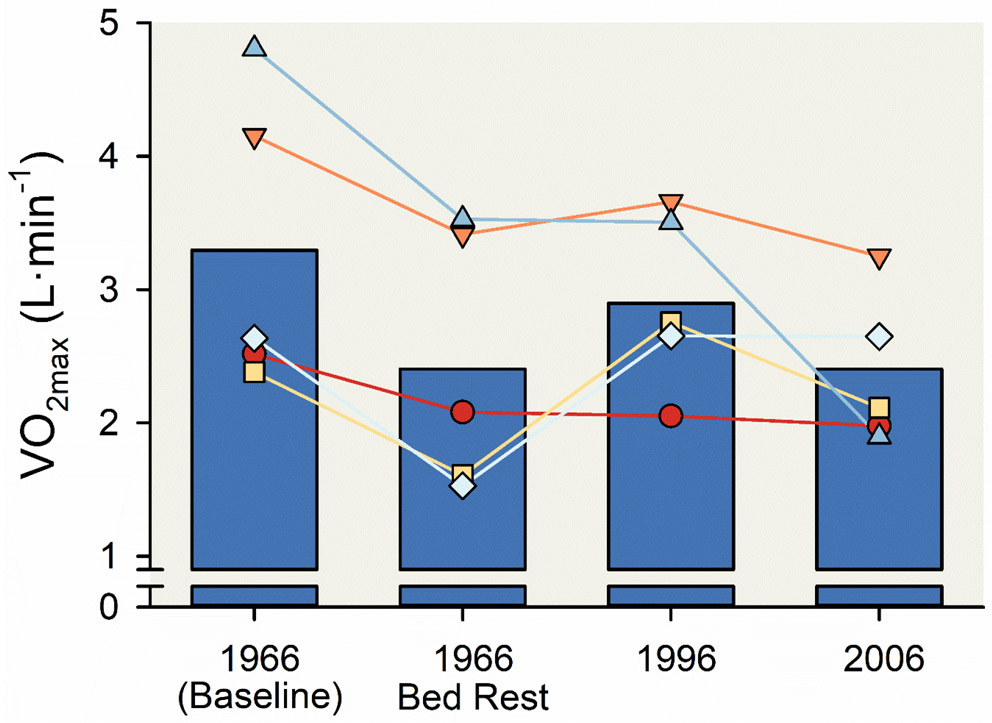
There are some additional experiment of nature observations associated with exercise training (and detraining) that add to the insights enumerated above. The first is that even very brief periods of physical inactivity can cause dramatic reductions in . The classic Dallas Bed Rest Study showed that only three weeks of bedrest in young men can cause marked reductions in . When the same five volunteers who participated in the original 1966 study were studied 30 and 40 years later, the decline in their over four decades was similar to the decline caused by 3 weeks of bed rest 40 years earlier (235, 237, 238, 307), see Figure 11. This led the authors to speculate that three weeks of inactivity equals 30 years of aging. This provocative assertion highlights the negative effects of inactivity on exercise capacity and almost certainly health and life expectancy.
Figure 11. Reduction in maximal aerobic capacity () associated with three weeks of bed rest.
Vertical bar charts displaying the reduction in associated with three weeks of bed rest among five 20-year-old men. After retraining in 1966 returned to baseline. Of note, the reduction in after three weeks of bed rest was similar the reduction in after 30 or 40 years of aging. was estimated using a graded, maximal stress test following a standardized protocol— the test was initiated with a two-minute interval of exercise with a workload of 60 W and the workload was increased 30 W every two minutes until volitional task failure. Line and scatter plots represent data from individual participants. Adapted from McGavock et al (235).
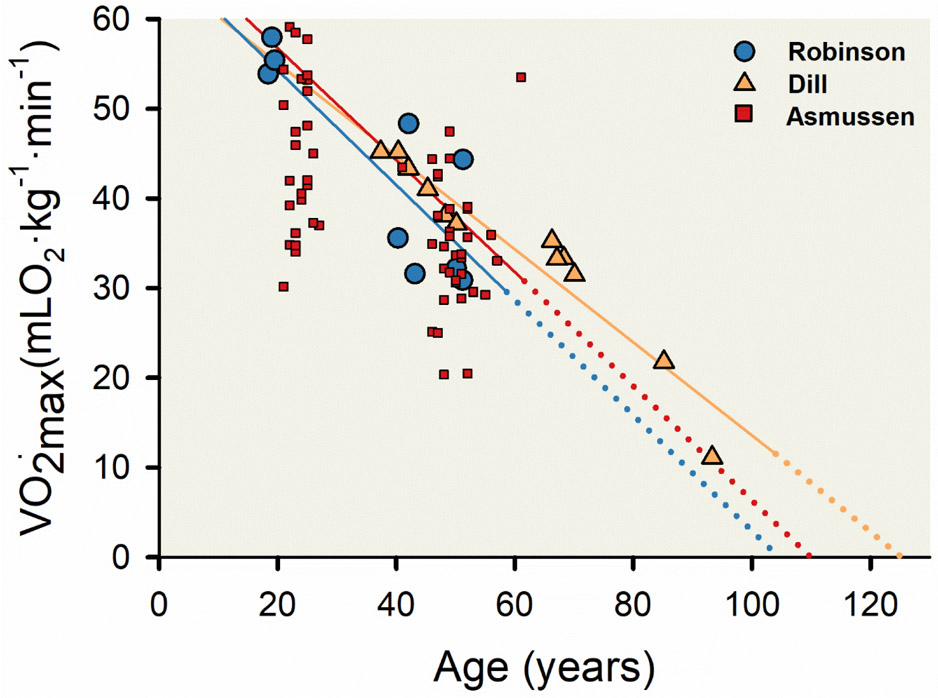
An especially stimulating experiment of nature came in the form of a short letter published in the Journal of Applied physiology in 1989 by Frank Booth (35). He plotted serial measurements of available over many decades on the noted physiologists Dill, Asmussen and Robinson (11, 79, 301), see Figure 12. He noted that the decline was close to the 10% per decade discussed earlier. Booth also speculated that the ongoing decline later in life was due in part to a loss of skeletal muscle mass as opposed to a reduced oxygen delivery capacity by the cardiovascular system. This decline in muscle mass may explain the accelerated declines in record performances seen by elite master athletes after the 8th decade of life. Of note Dill lived to be 95 and continued to participate as an experimental subject into his late eighties (377). He commented that in his 80's and 90's it was more difficult for him to maintain his muscle mass. He also noted that in his early 90s his was declining and approaching his basal metabolic rate and speculated that he would likely die at about the time his maximal oxygen uptake and basal metabolic rate converged. (MJJ attended a lecture Dill gave in the early 1980s when he was a student at the University of Arizona. He heard this remarkable scientist comment on his muscle mass and make this provocative statement on the limits of his life expectancy.)
Figure 12. Association of maximal aerobic capacity () and human aging.
Three linear regression lines and corresponding scatter plots displaying the decline in across the human lifespan from three longitudinal studies—Asmussen et al [red line and symbols; (11)], Robinson et al [blue line and symbols; (301)], and Dill et al [orange line and symbols; (79, 258)].The solid portion of each regression line represents data as collected, and the dashed portion of each regression line represents a theoretical association extracted from each linear regression to its intercept on the x-axis. Data from Asmussen and colleagues represents 23 men who had their measured twice, the first between ages 21 and 27 years and the second between ages 41 and 61 years. Data from Robinson and colleagues represents three men who had their measured three times at ages 18 to 19 years, 40 to 43 years, and 50 to 51 years. Data from Dill and colleagues represents 12 measurements of D.B. Dill over a 56-year period. Linear regression equations of best fit and associated Pearson correlation coefficients were y = 69.37 - 0.6315x, r = 0.57 (Asmussen et al); y = 67.19 - 0.6458x, r = 0.83 (Robinson et al); and y = 65.28 – 0.519x, r = 0.96 (Dill et al). Adapted from Booth FW 1989 (35).
Section Summary
The collection of observations from both natural experiments and experiments of nature reviewed in this section indicate that it is possible for middle aged and older humans to have high values as a result of training. Additionally, continued training can delay and blunt the decline in with aging. Such activity should then translate into a better position on the exercise capacity vs. risk of death curve shown in Figure 4. The interaction of muscle mass, training, and exercise capacity highlighted by Booth’s observation in 1989 deserve continued attention and there is a vital need for more data in women.
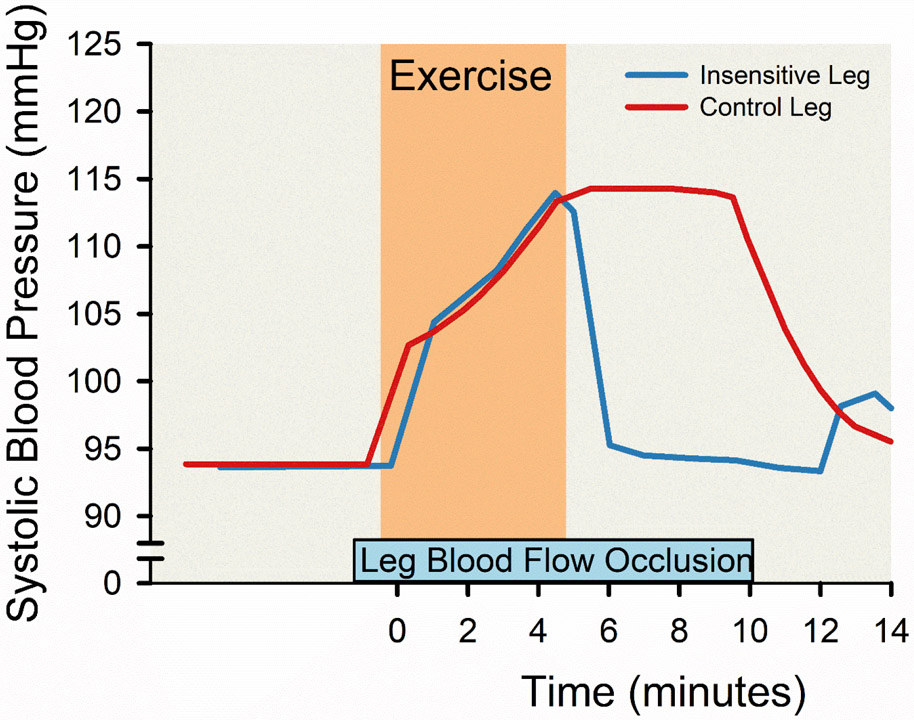
9 ∣. Blood Pressure
Blood pressure rises with exercise. The physiological mechanisms responsible for this rise have been of interest since the dawn of experimental physiology in the late 19th and early 20th centuries (200, 241). The current iteration of these ideas includes a so-called Central Command signal or a feed forward message to the brainstem cardiovascular centers that is related to the motor effort required to activate the skeletal muscles (241). There are also ideas about baroreceptor resetting, and feedback (known as the exercise pressor response) from the contracting skeletal muscles themselves. In the late 1930s Alam and Smirk working at the British medical school in Cairo conducted a series of studies and developed a paradigm to study the role of both Central Command and feedback from contracting muscles that is with us today. The basic idea is that a volunteer subject performs exercise (usually a handgrip or leg extension) while heart rate and blood pressure are measured (4). A pneumatic cuff is inflated to a pressure above systolic blood pressure around the upper arm or thigh to occlude the circulation in the exercising muscles. The occlusion can be implemented throughout contractions or just prior to stopping them leading to a period of post-exercise ischemia. The idea is that any sustained increases in blood pressure or heart rate that are seen during post-exercise ischemia are evoked by the stimulation sensory afferents in the contracting muscles – the exercise pressor response. By contrast any changes in heart rate or blood pressure that occur when voluntary contractions start, or stop are thought to be caused by the modulation of Central Command. This basic paradigm has been used in countless human studies and versions of it have been adapted to study these mechanisms in animal models.
With, for example, a static handgrip there is an immediate rise in heart rate and blood pressure with the onset of exercise that increases as the exercise becomes fatiguing. With post exercise ischemia the increase in blood pressure is sustained after contractions stop, but heart rate returns to baseline. These fundamental observations led to the idea that heart rate was primarily under the control of central command and that feedback from the active skeletal muscles contributed to large increases in blood pressure during fatiguing exercise (356). Importantly, the role of feedback from the contracting muscles was clearly demonstrated when Alam and Smirk studied a single volunteer (Figure 13) who had normal motor function to his exercising leg but a neurological lesion that rendered that leg insensitive (4). This individual did not show the sustained increases in blood pressure seen in normal subjects during periods of post-exercise ischemia.
Figure 13. Absent blood pressure raising reflex emanating from leg muscles during volitional exercise in a participant with a spinal cord lesion.
Line charts displaying the changes in arterial blood pressure associated with about five minutes of volitional exercise with concurrent occlusion of leg blood flow using suprasystolic sphygmomanometer cuff around the proximal thigh in a participant with a unilateral spinal cord lesion. The spinal cord lesion was associated with unilateral sensory loss on the right leg (insensitive leg; blue line) with no loss of sensation in the left leg (control leg; red line) and normal muscle power in both lower limbs. Adapted from Alam and Smirk 1938 (4).
A large body of work has flowed from these observations including classic studies using microneurography showing that skeletal muscle afferents are essential to evoke increases in efferent muscle sympathetic nerve activity during exercise. That these sensory afferents are stimulated by skeletal muscle acidosis comes from another experiment of nature showing that patients with McArdle’s disease, who are unable to produce lactic acid in their contracting skeletal muscles, have no rise in muscle sympathetic nerve activity during fatiguing static handgrip exercise (105, 291). Studies in animal models have identified a number of acid sensitive channels and receptors that stimulate group III and especially group IV in the active skeletal muscles to evoke the reflex increases in blood pressure and MSNA noted above. These studies have also demonstrated that in pathophysiological conditions like congestive heart failure and hypertension augment the activation of these afferents and contribute to the excessive sympathetic activation seen in these conditions (241). Of note, one of the most cited papers in the history of Integrative physiology shed important light on this topic in the early 1970s (234).
Smirk, the senior author of the 1937 and 1938 papers, went on to a distinguished career as a clinical investigator and leading expert on the regulation and pathophysiology of blood pressure in humans. He did this after WW II while Professor of Medicine at the University of Otago on the South Island of New Zealand (97). In what was then an extremely isolated environment, he was the first to systematically treat hypertension with autonomic blocking drugs and reduce the morbidity and mortality from this condition. For his efforts in developing novel treatments for hypertension he was Knighted, and his 1957 book High Arterial Pressure remains a classic (332). He also developed an early version of a clinical research unit that included research nurses and pharmacists.
The insights from Alam and Smirk come from studies using primarily static exercise and include intentional periods of ischemia. A key question then is what is the role of sympathetic activity to skeletal muscle during dynamic large muscle mass exercise like cycling or running? This is important for several reasons including the fact that in comparison to “athletic" animals like dogs and horses, humans have relatively small hearts (324). With the discovery in the 1980s that human skeletal muscle blood flow could be very high, it became clear that at least some vasoconstriction of the resistance vessels in the contracting skeletal muscles was required to regulate blood pressure during exercise. This is part of what Rowell described as the Sleeping Giant hypothesis (172, 306). The idea is that perhaps 10 of the ~30 kg of skeletal muscle in a healthy young male might be active and maximally dilated during heavy whole body exercise. If so, the blood flow demands of the active muscle (~300mL·100g−1·min−1) would exceed the ability of the heart to generate sufficient cardiac output to maintain arterial pressure (much less raise it) during exercise. Thus, the vasodilator capacity of skeletal muscle was a Sleeping Giant.
Much of the Sleeping Giant hypothesis has been generated based on hemodynamic calculations and observations showing that there can be vasoconstriction in contracting skeletal muscles. However, in the early 1960s Marshall, Schirger and Shepherd studied six patients who had undergone surgical thoraco-lumbar surgical sympathectomy (230). This procedure was done to control severe hypertension in these patients in an era when drug therapy for high blood pressure was in its infancy. The sympathectomy patients studied had normal heart rate responses to exercise because the autonomic innervation to their hearts remained intact. They also had intact baroreceptors. At rest they suffered from orthostatic symptoms when they stood up, so the exercise studies were conducted in the supine position. Remarkably, during supine exercise (Figure 14) blood pressure fell during even mild exercise in these patients. To ensure that there was adequate venous return to the heart, the patients also exercised during 15-degree head down tilt and blood pressure still fell. The absence of sympathetic innervation to the exercising leg muscles in these patients was almost certainly responsible for the hypotensive responses during exercise. The findings clearly anticipate the Sleeping Giant hypothesis of Rowell. They also validate the much later observations that the vast increases in skeletal muscle blood flow possible during exercise need continued regulation by the sympathetic nervous system so that blood pressure can be maintained during whole body exercise in humans - especially while upright.
Figure 14. Paradoxical reduction in blood pressure during supine exercise in a participant who had undergone sympathectomy to treat hypertension.
Line chart displaying the paradoxical reduction in arterial blood pressure associated with two minutes of supine cycling exercise which, in a separate experimental exercise, was not attenuated by a 15-degree head down tilt designed to facilitate cardiac filling. These data represent a 44-year-old man who had undergone thoracolumbar sympathectomy for essential hypertension. Blood pressure was recorded from the radial artery using a Statham strain-gage transducer. Adapted from Marshall et al (230).
One last unique observation about the blood pressure responses to exercise are the incredibly high values seen when healthy humans engage in heavy weightlifting with a large mass of muscle. McDougall and colleagues made direct intra-arterial blood pressure measurements while five experienced strength trained, in all subjects engaged in heavy weightlifting (227). Values as high as 480/300 mmHg were recorded in one subject performing leg presses, and systolic blood pressures greater than 300 mmHg were not uncommon. There was also a rhythmic waxing and waning of the pressure as the subject performed a Valsalva maneuver with each effort and blood pressure rose progressively over the 10 or more repetitions. This data is a reminder of the extreme physiological responses that can occur during exercise. It also shows the magnitude of the rise in blood pressure that can be generated by the mechanisms discussed earlier. Such data are also extremely impressive to clinicians familiar with the risks associated with even modest increases in blood pressure and can help better define what normal responses to acute physiological stress.
Section Summary
The four experiments of nature summarized above have made major contributions to understanding autonomic nervous system regulation of blood pressure during exercise. Acid sensitive afferents in the contracting muscles contribute to the rise in blood pressure during exercise via a reflex increase in MSNA. An increase in MSNA is required to regulate blood pressure during whole body dynamic exercise, and dramatic increases in blood pressure occur during heavy weightlifting. These observations have informed numerous basic and clinical studies on blood pressure regulation during exercise.
10 ∣. Fast and Slow Twitch Muscles
In the section on body composition, identical twins divergent for physical activity have been discussed. One of the most extreme examples are a pair of brothers who were studied in their 50s. One had been sedentary while the other had been participating in endurance activities including Ironman distance triathlons for decades. These brothers had the expected differences in , body composition and other phenotypic traits associated with lifelong endurance exercise training in comparison to inactivity. However, this twin pair also underwent skeletal muscle biopsy testing which provided new insights into the limits of muscle plasticity in humans (23), see Figure 8.
Muscle plasticity is an umbrella term used to describe changes in muscle phenotype due to adaptations caused by use or disuse (287). For example, skeletal muscle hypertrophy in response to resistance exercise training or compensatory overload is a form of muscle plasticity. In response to endurance exercise training the mitochondrial content of the muscles subject to training can double, and capillary density can increase (164). At the other end of the spectrum is disuse atrophy and the reduced vascular capacity seen with immobilization or denervation.
Most species have one or more forms of fast and slow-twitch skeletal muscle fibers that can be distinguished on the basis of biochemical analysis and by the physiological properties of the muscle fibers and their parent motor neurons (44, 330). Fast twitch muscle fibers contain fast skeletal muscle myosin isoforms, and slow-twitch skeletal muscles contain slower isoforms of myosin. In response to endurance exercise training, it is well known that the mitochondrial content of both fast and slow skeletal muscles can increase. In some circumstances mitochondrial content in trained fast-twitch skeletal muscles can equal or even exceed that seen in untrained slow skeletal muscle fibers. There can also be subtle shifts in myosin isoform, but dramatic changes in the percentage of skeletal muscle fibers categorized as fast of slow in response to training have generally not been observed (1, 112, 171, 240). However, the training interventions have typically been for less than six months.
More extreme interventions in animals like cross-innervation of predominately fast and slow muscles, or chronic electrical stimulation can cause dramatic shifts in skeletal muscle fiber type especially from fast to slow twitch (287). In this context, the twin brothers described above, who had 30 years of divergent physical activity including very prolonged endurance exercise training by the active brother, demonstrated dramatic differences in their skeletal muscle fiber type. The sedentary brother had ~35% fast-twitch fibers and ~65% slow-twitch fibers. By contrast the active brother had almost no fast-twitch fibers and nearly 100% slow-twitch fibers. This change in fiber type with exercise training is far more dramatic than previously thought possible. It opens new questions about skeletal muscle plasticity in humans. It is also a topic that perhaps can only be explored in experiments of nature who have extreme differences in physical activity for years or even decades as seen in the twin brothers highlighted in Figure 8.
A hint that this sort of extreme plasticity was possible was seen in the detraining study of Coyle and colleagues (59, 75). In seven subjects who had undergone many years of prolonged intense endurance exercise training prior to detraining some of the endurance training adapted properties of the skeletal muscle fibers seen in these subjects showed little change. In the one subject who had only been training for a year more obvious changes were seen. These observations show the limits of the idea that skeletal muscle fiber type is predominantly genetically determined (147, 330).
In the context of genetic determinism, it does appear that either one or two copies of R ACTN3 gene variant is far more common – nearly ubiquitous - in elite power and speed athletes. This observation being perhaps the most notable genotype/phenotype associations found to date in the field of human performance. However, at least one world class long jumper with a personal best of >8m has been identified with two copies of the XX variant (221).
Section Summary
The experiments of nature highlighted in this section raise important questions about the limits of skeletal muscle plasticity. Extensive fiber type transitions towards the slow twitch phenotype may be possible in humans with years of prolonged intense endurance exercise training. Additionally, while at least one copy of R variant for ACTN3 is nearly ubiquitous in elite speed or power athletes it is apparently not obligatory.
11 ∣. High Hemoglobin-Oxygen Affinity and Hypoxia
Hypoxia in conjunction with exercise is one of the most extreme physiological challenges humans confront. One of the acute adaptations to hypoxia that is thought to be beneficial is an acute right shift in the oxygen hemoglobin dissociation curve to facilitate the unloading of oxygen at the tissues (12, 216, 217). While this may be beneficial to hypoxic tissues, it may also limit the ability of hemoglobin to bind oxygen in the lung (366). This could contribute to further desaturation of arterial blood and thus limit oxygen delivery to the periphery. By contrast, many birds and mammals adapted evolutionarily to life at high-altitude have left shifted oxygen hemoglobin dissociation curves on the basis of structural differences in their heme proteins (19, 244, 259, 316, 317, 335-337, 368). These evolutionary adaptations in animals raise the possibility that in high altitude environments it may be advantageous to load more oxygen at the lung via a left shift in the oxygen hemoglobin dissociation curve and rely on either increases in skeletal muscle perfusion or peripheral extraction in the exercising muscles to off-load it.
To test this hypothesis in humans, individuals with rare hemoglobin variants that are left shifted have served as experiments of nature and been studied at high altitude and during hypoxia. In the 1970s two brothers with hemoglobin Minneapolis with a P50 of 16mmHg (oxygen partial pressure associated with 50% hemoglobin saturation) were compared to relatives with normal P50 values (26 mmHg). The left shifted individuals better maintained their low altitude exercise capacity measured in Minneapolis (250m) when studied at high altitude in Colorado (3,100m). To further study this topic, our group has performed several studies on exercise-related effects among people with high hemoglobin-oxygen affinity (91-93, 328, 367). We recently performed exercise studies in normoxia and hypoxia (FiO2=0.15, simulating ~3000m of terrestrial elevation) in a family (N=11) with hemoglobin Malmo, and one individual with hemoglobin San Diego (92). The P50 in these individuals was ~16 mmHg. Figure 15 shows that individuals with left shifted hemoglobin had a far smaller reduction (~4% decline) in their during hypoxia than healthy controls (~12% decline) with normal P50.
Figure 15. Absent reduction in maximal aerobic capacity () during hypoxic exercise (relative to normoxic exercise) among people with high affinity hemoglobin variants.
The reduction in during hypoxic exercise (fraction of inspired oxygen = 0.15) compared to normoxic exercise (fraction of inspired oxygen = 0.21) displayed as line and scatter plots (A) and vertical bar charts (B) among 11 people with high affinity hemoglobin variants (HAH; blue triangles) and 14 controls with normal affinity hemoglobin (red circles). was estimated using a stepwise maximal graded exercise test on a bicycle ergometer. Adapted from Dominelli et al (92).
One potential confound with these left-shifted individuals is that they also have very high hemoglobin concentrations of 18 or 19 g/dL as opposed to the normal values of 13-15 g·dL−1. Thus, it is possible that their relatively preserved exercise capacity during hypoxia is due in part to the polycythemia. However, the decline while breathing hypoxic gas was highly related to the fall in O2 saturation each subject experienced suggesting that the differences in the P50 of the hemoglobin in the left shifted subjects was causal.
Patients with rare hemoglobin variants can also be used to test the hypothesis that the kidney serves as a “crito-meter” and that oxygen delivery to the kidney plays a role in the maintenance / set point for hemoglobin concentration. Figure 16 is a plot of P50 versus hemoglobin concentration coming from blank subjects with a wide variety of hemoglobin variants. Individuals with right shifts in the oxygen hemoglobin dissociation curve are relatively anemic, individuals who are left shifted have higher values for hemoglobin and hematocrit and are polycythemic. This is consistent with the idea that the kidney is a “crito-meter” because the easier offloading of oxygen to renal tissues facilitated by right shift hemoglobin would suppress erythropoietin production in the kidney (46, 95, 99, 103, 148). By contrast left shifted hemoglobin would limit O2 unloading and stimulate increase in erythropoietin release.
Figure 16. Association between hemoglobin oxygen affinity and hemoglobin concentration.
Linear correlation line and corresponding scatterplot displaying the negative association between a metric of hemoglobin oxygen affinity (P50) and hemoglobin concentration in humans. Data represent 50 people with high affinity hemoglobin variants (blue triangles), six controls with normal affinity hemoglobin (red circles), and 11 people with low affinity hemoglobin variants. Because females have hemoglobin concentration values of 1 to 2 g·dL−1 lower than males, hemoglobin concentrations were increased by 10% among females to obtain comparable data. Symbols with white outline represent females and symbols with black outline represent males. Adapted from Shepherd et al (328).
One other experiment of nature finding in humans relevant to exercise and hypoxia that deserves mention comes from observations of increased pulmonary diffusing capacity (a marker of lung surface area) in individuals who were born in Leadville Colorado (58, 335). Leadville natives had much higher pulmonary diffusing capacity compared to individuals who had moved to altitude later in life. This is consistent with the idea that lifelong exposure to high altitude evokes structural changes in the lung to facilitate oxygen transport from the alveoli to the desaturated blood in the pulmonary capillaries.
Section Summary
The experiments of nature highlighted in this section show that in humans a left shift in the oxygen-hemoglobin dissociation curve may be beneficial during hypoxia by protecting arterial O2 saturation. This is in contrast to the standard teaching that a right shift in the oxygen-hemoglobin dissociation is beneficial during hypoxia. The increased pulmonary diffusing capacity seen in life long high-altitude residents demonstrates that marked structural adaptations are possible in the lung. These adaptations are far beyond those seen in shorter time frame interventional studies.
12 ∣. Overall Summary
In this review we have we have highlighted important insights related to how humans respond to exercise and physical activity. These insights all stem from what Claude Bernard described as Passive Experiments. This means the data was generated without the active intervention of the experimenter. We have further pointed out that passive experiments can include natural experiments — such as the series of studies among bus drivers and bus conductors that demonstrated high levels of occupational physical activity are associated with a reduction in both risk of coronary heart disease and mortality. The category of passive experiments known as experiments of nature have been critical in establishing the role of key physiological mechanisms engaged in the short- and long-term responses to exercise. While passive experiments can have many features in common with prospective studies, they can have the added benefit of a more prolonged, intense, and extreme exposure to an environmental or behavioral input of interest compared to a prospective experiment. They also allow investigators to determine if selected mechanisms are obligatory for a given physiological response to occur. As investigators seek to understand the limits of human adaptability to stressors like exercise, both natural experiments and experiments of nature will be essential to generate and test hypotheses about the limits of this adaptability.
Acknowledgements
The authors thank both Wyatt Pruter and Ashley Jones for their assistance in editing the article, organizing the references, and constructing the figures. In addition, we acknowledge BioRender for graphical support in preparation of figures. This work was supported by NIH grants (R-35-HL-139854 to MJJ; K01-HL-148144 to S.E.B.; F32-HL-154320 to J.W.S.) and the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2022-05293 and DGECR-2022-00320 to S.A.K.). The article is dedicated to the continuing legacy of Charles M. Tipton and John O. Holloszy.
References
- 1.Adams GR, and Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol 2: 2829–2870, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, and Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 103: 1572–1577, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mallah MH, Keteyian SJ, Brawner CA, Whelton S, and Blaha MJ. Rationale and design of the Henry Ford Exercise Testing Project (the FIT project). Clin Cardiol 37: 456–461, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam M, and Smirk F. Unilateral loss of a blood pressure raising, pulse accelerating, reflex from voluntary muscle due to a lesion of the spinal cord. Clin Sci 3: 247–252, 1938. [Google Scholar]
- 5.American College of Sports Medicine. ACSM's Exercise Testing and Prescription. Wolters Kluwer Health, 2017. [Google Scholar]
- 6.Andersen K, Farahmand B, Ahlbom A, Held C, Ljunghall S, Michaelsson K, and Sundstrom J. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 34: 3624–3631, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Andersen K, Hallmarker U, James S, and Sundstrom J. Long-Distance Skiing and Incidence of Hypertension: A Cohort Study of 206 889 Participants in a Long-Distance Cross-Country Skiing Event. Circulation 141: 743–750, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Andersen KL, and Hermansen L. Aerobic work capacity in middle-aged Norwegian men. J Appl Physiol 20: 432–436, 1965. [DOI] [PubMed] [Google Scholar]
- 9.Angrist JD. Lifetime Earnings and the Vietnam Era Draft Lottery: Evidence from Social Security Administrative Records. The American Economic Review 80: 313–336, 1990. [Google Scholar]
- 10.Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, and Matthews CE. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 175: 959–967, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asmussen E, and Mathiasen P. Some physiologic functions in physical education students re-investigated after twenty-five years. J Am Geriatr Soc 10: 379–387, 1962. [DOI] [PubMed] [Google Scholar]
- 12.Aste-Salazar H, and Hurtado A. The affinity of hemoglobin for oxygen at sea level and at high altitudes. American Journal of Physiology-Legacy Content 142: 733–743, 1944. [Google Scholar]
- 13.Astrand I Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl 49: 1–92, 1960. [PubMed] [Google Scholar]
- 14.Astrand PO. J.B. Wolffe Memorial Lecture. "Why exercise?". Med Sci Sports Exerc 24: 153–162, 1992. [PubMed] [Google Scholar]
- 15.Bacon AP, Carter RE, Ogle EA, and Joyner MJ. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One 8: e73182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagley JR, McLeland KA, Arevalo JA, Brown LE, Coburn JW, and Galpin AJ. Skeletal Muscle Fatigability and Myosin Heavy Chain Fiber Type in Resistance Trained Men. J Strength Cond Res 31: 602–607, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, Marti B, and Attenhofer Jost CH. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J 29: 71–78, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Barnes KR, and Kilding AE. A Randomized Crossover Study Investigating the Running Economy of Highly-Trained Male and Female Distance Runners in Marathon Racing Shoes versus Track Spikes. Sports Med 49: 331–342, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Bartels H, Hilpert P, Barbey K, Betke K, Riegel K, Lang EM, and Metcalfe J. Respiratory Functions of Blood of the Yak, Llama, Camel, Dybowski Deer, and African Elephant. Am J Physiol 205: 331–336, 1963. [DOI] [PubMed] [Google Scholar]
- 20.Bassett DR Jr. Scientific contributions of A. V. Hill: exercise physiology pioneer. J Appl Physiol (1985) 93: 1567–1582, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Bassett DR Jr., Tremblay MS, Esliger DW, Copeland JL, Barnes JD, and Huntington GE. Physical activity and body mass index of children in an old order Amish community. Med Sci Sports Exerc 39: 410–415, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Bassett DR, Schneider PL, and Huntington GE. Physical activity in an Old Order Amish community. Med Sci Sports Exerc 36: 79–85, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Bathgate KE, Bagley JR, Jo E, Talmadge RJ, Tobias IS, Brown LE, Coburn JW, Arevalo JA, Segal NL, and Galpin AJ. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur J Appl Physiol 118: 2097–2110, 2018. [DOI] [PubMed] [Google Scholar]
- 24.Batty GD, and Lee IM. Physical activity and coronary heart disease. BMJ 328: 1089–1090, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaver WL, Wasserman K, and Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60: 2020–2027, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Belov A, Huang Y, Villa CH, Whitaker BI, Forshee R, Anderson SA, Eder A, Verdun N, Joyner MJ, Wright SR, Carter RE, Hung DT, Broad Institute C-AT, Homer M, Hoffman C, Lauer M, and Marks P. Early administration of COVID-19 convalescent plasma with high titer antibody content by live viral neutralization assay is associated with modest clinical efficacy. Am J Hematol 97: 770–779, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benestad AM. Trainability of old men. Acta Med Scand 178: 321–327, 1965. [DOI] [PubMed] [Google Scholar]
- 28.Bernard C, Greene HC, Henderson LJ, and Cohen IB. An Introduction to the Study of Experimental Medicine. Dover Publications, 1957. [Google Scholar]
- 29.Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B, and Levine BD. Impact of lifelong exercise "dose" on left ventricular compliance and distensibility. J Am Coll Cardiol 64: 1257–1266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binkhorst RA, Pool J, van Leeuwen P, and Bouhuys A. Maximum oxygen uptake in healthy nonathletic males. Int Z Angew Physiol 22: 10–18, 1966. [DOI] [PubMed] [Google Scholar]
- 31.Blair SN, Kohl HW 3rd, Barlow CE, Paffenbarger RS Jr., Gibbons LW, and Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 273: 1093–1098, 1995. [PubMed] [Google Scholar]
- 32.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH, and Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Blair SN, Kohl HW, Gordon NF, and Paffenbarger RS Jr. How much physical activity is good for health? Annu Rev Public Health 13: 99–126, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, Pekosz A, Lau B, Wesolowski A, Katz L, Shan H, Auwaerter PG, Thomas D, Sullivan DJ, Paneth N, Gehrie E, Spitalnik S, Hod EA, Pollack L, Nicholson WT, Pirofski LA, Bailey JA, and Tobian AA. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 130: 2757–2765, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth FW. VO2max limits. J Appl Physiol (1985) 67: 1299–1300, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Booth FW, Roberts CK, and Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brand RJ, Paffenbarger RS Jr., Sholtz RI, and Kampert JB. Work activity and fatal heart attack studied by multiple logistic risk analysis. Am J Epidemiol 110: 52–62, 1979. [DOI] [PubMed] [Google Scholar]
- 38.Breslow L, and Buell P. Mortality from coronary heart disease and physical activity of work in California. J Chronic Dis 11: 421–444, 1960. [DOI] [PubMed] [Google Scholar]
- 39.Brooks GA. The "Anaerobic Threshold" Concept Is Not Valid in Physiology and Medicine. Med Sci Sports Exerc 53: 1093–1096, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 17: 22–34, 1985. [PubMed] [Google Scholar]
- 41.Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell Metab 27: 757–785, 2018. [DOI] [PubMed] [Google Scholar]
- 42.Bruce RA, and Hornsten TR. Exercise stress testing in evaluation of patients with ischemic heart disease. Prog Cardiovasc Dis 11: 371–390, 1969. [DOI] [PubMed] [Google Scholar]
- 43.Bruce RA, Kusumi F, and Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85: 546–562, 1973. [DOI] [PubMed] [Google Scholar]
- 44.Burke RE. Revisiting the notion of 'motor unit types'. Prog Brain Res 123: 167–175, 1999. [PubMed] [Google Scholar]
- 45.Card D, Angrist JD, and Imbens GW. Press Release: The Prize in Economic Sciences 2021. NobelPrizeorg 2021. [Google Scholar]
- 46.Cargill WH, and Hickam JB. The Oxygen Consumption of the Normal and the Diseased Human Kidney. J Clin Invest 28: 526–532, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carmelli D, Fabsitz RR, Swan GE, Reed T, Miller B, and Wolf PA. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol 151: 452–458, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Casadevall A, Dragotakes Q, Johnson PW, Senefeld JW, Klassen SA, Wright RS, Joyner MJ, Paneth N, and Carter RE. Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality. Elife 10: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casadevall A, Grossman BJ, Henderson JP, Joyner MJ, Shoham S, Pirofski LA, and Paneth N. The Assessment of Convalescent Plasma Efficacy against COVID-19. Med (N Y) 1: 66–77, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casadevall A, Henderson JP, Joyner MJ, and Pirofski LA. SARS-CoV-2 variants and convalescent plasma: reality, fallacies, and opportunities. J Clin Invest 131: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casadevall A, Joyner MJ, and Pirofski LA. Implications of Coronavirus Disease 2019 (COVID-19) Antibody Dynamics for Immunity and Convalescent Plasma Therapy. Clin Infect Dis 73: e540–e542, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casadevall A, Joyner MJ, and Pirofski LA. A Randomized Trial of Convalescent Plasma for COVID-19-Potentially Hopeful Signals. JAMA 324: 455–457, 2020. [DOI] [PubMed] [Google Scholar]
- 53.Casadevall A, Joyner MJ, and Pirofski LA. SARS-CoV-2 viral load and antibody responses: the case for convalescent plasma therapy. J Clin Invest 130: 5112–5114, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casadevall A, Pirofski LA, and Joyner MJ. The Principles of Antibody Therapy for Infectious Diseases with Relevance for COVID-19. mBio 12: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catenacci VA, Grunwald GK, Ingebrigtsen JP, Jakicic JM, McDermott MD, Phelan S, Wing RR, Hill JO, and Wyatt HR. Physical activity patterns using accelerometry in the National Weight Control Registry. Obesity (Silver Spring) 19: 1163–1170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catenacci VA, Odgen L, Phelan S, Thomas JG, Hill J, Wing RR, and Wyatt H. Dietary habits and weight maintenance success in high versus low exercisers in the National Weight Control Registry. J Phys Act Health 11: 1540–1548, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catenacci VA, Ogden LG, Stuht J, Phelan S, Wing RR, Hill JO, and Wyatt HR. Physical activity patterns in the National Weight Control Registry. Obesity (Silver Spring) 16: 153–161, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerny FC, Dempsey JA, and Reddan WG. Pulmonary gas exchange in nonnative residents of high altitude. J Clin Invest 52: 2993–2999, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chi MM, Hintz CS, Coyle EF, Martin WH 3rd, Ivy JL, Nemeth PM, Holloszy JO, and Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol 244: C276–287, 1983. [DOI] [PubMed] [Google Scholar]
- 60.Claessen G, Colyn E, La Gerche A, Koopman P, Alzand B, Garweg C, Willems R, Nuyens D, and Heidbuchel H. Long-term endurance sport is a risk factor for development of lone atrial flutter. Heart 97: 918–922, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Clarke PM, Walter SJ, Hayen A, Mallon WJ, Heijmans J, and Studdert DM. Survival of the fittest: retrospective cohort study of the longevity of Olympic medallists in the modern era. BMJ 345: e8308, 2012. [DOI] [PubMed] [Google Scholar]
- 62.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, and Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 37: 161–165, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Cohn CS, Estcourt L, Grossman BJ, Pagano MB, Allen ES, Bloch EM, Casadevall A, Devine DV, Dunbar NM, Foroutan F, Gniadek TJ, Goel R, Gorlin J, Joyner MJ, Metcalf RA, Raval JS, Rice TW, Shaz BH, Vassallo RR, Winters JL, Beaudoin G, and Tobian AAR. COVID-19 convalescent plasma: Interim recommendations from the AABB. Transfusion 61: 1313–1323, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, and Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 377: 13–27, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conconi F, Ferrari M, Ziglio PG, Droghetti P, and Codeca L. Determination of the anaerobic threshold by a noninvasive field test in runners. J Appl Physiol Respir Environ Exerc Physiol 52: 869–873, 1982. [DOI] [PubMed] [Google Scholar]
- 66.Connett RJ, Gayeski TE, and Honig CR. Lactate accumulation in fully aerobic, working, dog gracilis muscle. Am J Physiol 246: H120–128, 1984. [DOI] [PubMed] [Google Scholar]
- 67.Constable SH, Favier RJ, McLane JA, Fell RD, Chen M, and Holloszy JO. Energy metabolism in contracting rat skeletal muscle: adaptation to exercise training. Am J Physiol 253: C316–322, 1987. [DOI] [PubMed] [Google Scholar]
- 68.Costill DL. Metabolic responses during distance running. J Appl Physiol 28: 251–255, 1970. [DOI] [PubMed] [Google Scholar]
- 69.Costill DL. Physiology of marathon running. JAMA 221: 1024–1029, 1972. [PubMed] [Google Scholar]
- 70.Costill DL, Branam G, Eddy D, and Sparks K. Determinants of Marathon running success. Int Z Angew Physiol 29: 249–254, 1971. [DOI] [PubMed] [Google Scholar]
- 71.Costill DL, and Fox EL. Energetics of marathon running. Medicine and science in sports 1: 81–86, 1969. [Google Scholar]
- 72.Costill DL, Thomason H, and Roberts E. Fractional utilization of the aerobic capacity during distance running. Med Sci Sports 5: 248–252, 1973. [PubMed] [Google Scholar]
- 73.Costill DL, and Winrow E. A comparison of two middle-aged ultramarathon runners. Res Q 41: 135–139, 1970. [PubMed] [Google Scholar]
- 74.Costill DL, and Winrow E. Maximal oxygen intake among marathon runners. Arch Phys Med Rehabil 51: 317–320, 1970. [PubMed] [Google Scholar]
- 75.Coyle EF, Martin WH 3rd, Sinacore DR, Joyner MJ, Hagberg JM, and Holloszy JO. Time course of loss of adaptations after stopping prolonged intense endurance training. J Appl Physiol Respir Environ Exerc Physiol 57: 1857–1864, 1984. [DOI] [PubMed] [Google Scholar]
- 76.Craig P, Cooper C, Gunnell D, Haw S, Lawson K, Macintyre S, Ogilvie D, Petticrew M, Reeves B, Sutton M, and Thompson S. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health 66: 1182–1186, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craig P, Katikireddi SV, Leyland A, and Popham F. Natural Experiments: An Overview of Methods, Approaches, and Contributions to Public Health Intervention Research. Annu Rev Public Health 38: 39–56, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crane M, Bohn-Goldbaum E, Grunseit A, and Bauman A. Using natural experiments to improve public health evidence: a review of context and utility for obesity prevention. Health Res Policy Syst 18: 48, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunningham DJC. The Regulation of Human Respiration. Wiley (Periodicals), 1963. [Google Scholar]
- 80.Currens JH, and White PD. Half a century of running. Clinical, physiologic and autopsy findings in the case of Clarence DeMar ("Mr. Marathon"). N Engl J Med 265: 988–993, 1961. [DOI] [PubMed] [Google Scholar]
- 81.Daeninck E, and Miller M. What can the National Weight Control Registry teach us? Curr Diab Rep 6: 401–404, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Darby SC, Olsen JH, Doll R, Thakrar B, Brown PD, Storm HH, Barlow L, Langmark F, Teppo L, and Tulinius H. Trends in childhood leukaemia in the Nordic countries in relation to fallout from atmospheric nuclear weapons testing. BMJ 304: 1005–1009, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das P, and Horton R. Rethinking our approach to physical activity. Lancet 380: 189–190, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Davies CT. Limitations to the prediction of maximum oxygen intake from cardiac frequency measurements. J Appl Physiol 24: 700–706, 1968. [DOI] [PubMed] [Google Scholar]
- 85.Davis JA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 17: 6–21, 1985. [PubMed] [Google Scholar]
- 86.de Vocht F, Katikireddi SV, McQuire C, Tilling K, Hickman M, and Craig P. Conceptualising natural and quasi experiments in public health. BMC Med Res Methodol 21: 32, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dee EC, Paguio JA, Yao JS, Stupple A, and Celi LA. Data science to analyse the largest natural experiment of our time. BMJ Health Care Inform 27: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dehn MM, and Bruce RA. Longitudinal variations in maximal oxygen intake with age and activity. J Appl Physiol 33: 805–807, 1972. [DOI] [PubMed] [Google Scholar]
- 89.Dill DB, Robinson S, and Ross JC. A longitudinal study of 16 champion runners. J Sports Med Phys Fitness 7: 4–27, 1967. [PubMed] [Google Scholar]
- 90.Ding D, Van Buskirk J, Nguyen B, Stamatakis E, Elbarbary M, Veronese N, Clare PJ, Lee IM, Ekelund U, and Fontana L. Physical activity, diet quality and all-cause cardiovascular disease and cancer mortality: a prospective study of 346 627 UK Biobank participants. Br J Sports Med 2022. [DOI] [PubMed] [Google Scholar]
- 91.Dominelli PB, Baker SE, Wiggins CC, Stewart GM, Sajgalik P, Shepherd JRA, Roberts SK, Roy TK, Curry TB, Hoyer JD, Oliveira JL, Foster GE, and Joyner MJ. Dissociating the effects of oxygen pressure and content on the control of breathing and acute hypoxic response. J Appl Physiol (1985) 127: 1622–1631,2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dominelli PB, Wiggins CC, Baker SE, Shepherd JRA, Roberts SK, Roy TK, Curry TB, Hoyer JD, Oliveira JL, and Joyner MJ. Influence of high affinity haemoglobin on the response to normoxic and hypoxic exercise. J Physiol 598: 1475–1490, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dominelli PB, Wiggins CC, Roy TK, Secomb TW, Curry TB, and Joyner MJ. The Oxygen Cascade During Exercise in Health and Disease. Mayo Clin Proc 96: 1017–1032, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donato AJ, Tench K, Glueck DH, Seals DR, Eskurza I, and Tanaka H. Declines in physiological functional capacity with age: a longitudinal study in peak swimming performance. J Appl Physiol (1985) 94: 764–769, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donnelly S Why is erythropoietin made in the kidney? The kidney functions as a 'critmeter' to regulate the hematocrit. Adv Exp Med Biol 543: 73–87, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Douglas CG, and Haldane JS. The regulation of normal breathing. J Physiol 38: 420–440, 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doyle AE. Sir Horace Smirk. Pioneer in drug treatment of hypertension. Hypertension 17: 247–250, 1991. [DOI] [PubMed] [Google Scholar]
- 98.Duchateau J, and Enoka RM. Distribution of motor unit properties across human muscles. J Appl Physiol (1985) 132: 1–13, 2022. [DOI] [PubMed] [Google Scholar]
- 99.Edwards A, and Kurtcuoglu V. Renal blood flow and oxygenation. Pflugers Arch 474: 759–770, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ekblom B, Goldbarg AN, and Gullbring B. Response to exercise after blood loss and reinfusion. J Appl Physiol 33: 175–180, 1972. [DOI] [PubMed] [Google Scholar]
- 101.Ekblom B, and Hermansen L. Cardiac output in athletes. J Appl Physiol 25: 619–625, 1968. [DOI] [PubMed] [Google Scholar]
- 102.Enoka RM. Morphological features and activation patterns of motor units. J Clin Neurophysiol 12: 538–559, 1995. [DOI] [PubMed] [Google Scholar]
- 103.Epstein FH. Oxygen and renal metabolism. Kidney Int 51: 381–385, 1997. [DOI] [PubMed] [Google Scholar]
- 104.Estcourt LJ, Cohn CS, Pagano MB, Iannizzi C, Kreuzberger N, Skoetz N, Allen ES, Bloch EM, Beaudoin G, Casadevall A, Devine DV, Foroutan F, Gniadek TJ, Goel R, Gorlin J, Grossman BJ, Joyner MJ, Metcalf RA, Raval JS, Rice TW, Shaz BH, Vassallo RR, Winters JL, and Tobian AAR. Clinical Practice Guidelines From the Association for the Advancement of Blood and Biotherapies (AABB): COVID-19 Convalescent Plasma. Ann Intern Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fadel PJ, Wang Z, Tuncel M, Watanabe H, Abbas A, Arbique D, Vongpatanasin W, Haley RW, Victor RG, and Thomas GD. Reflex sympathetic activation during static exercise is severely impaired in patients with myophosphorylase deficiency. J Physiol 548: 983–993, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farahmand B, Hallmarker U, Brobert GP, and Ahlbom A. Acute mortality during long-distance ski races (Vasaloppet). Scand J Med Sci Sports 17: 356–361, 2007. [DOI] [PubMed] [Google Scholar]
- 107.Farahmand BY, Ahlbom A, Ekblom O, Ekblom B, Hallmarker U, Aronson D, and Brobert GP. Mortality amongst participants in Vasaloppet: a classical long-distance ski race in Sweden. J Intern Med 253: 276–283, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Farrell PA, Wilmore JH, Coyle EF, Billing JE, and Costill DL. Plasma lactate accumulation and distance running performance. Med Sci Sports 11: 338–344, 1979. [PubMed] [Google Scholar]
- 109.Faude O, Kindermann W, and Meyer T. Lactate threshold concepts: how valid are they? Sports Med 39: 469–490, 2009. [DOI] [PubMed] [Google Scholar]
- 110.Favier RJ, Constable SH, Chen M, and Holloszy JO. Endurance exercise training reduces lactate production. J Appl Physiol (1985) 61: 885–889, 1986. [DOI] [PubMed] [Google Scholar]
- 111.Feldman DI, Al-Mallah MH, Keteyian SJ, Brawner CA, Feldman T, Blumenthal RS, and Blaha MJ. No evidence of an upper threshold for mortality benefit at high levels of cardiorespiratory fitness. J Am Coll Cardiol 65: 629–630, 2015. [DOI] [PubMed] [Google Scholar]
- 112.Fitts RH, and Widrick JJ. Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24: 427–473, 1996. [PubMed] [Google Scholar]
- 113.Fitzgerald MD, Tanaka H, Tran ZV, and Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol (1985) 83: 160–165, 1997. [DOI] [PubMed] [Google Scholar]
- 114.Focosi D, Franchini M, Joyner MJ, and Casadevall A. Are convalescent plasma stocks collected during former COVID-19 waves still effective against current SARS-CoV-2 variants? Vox Sang 117: 641–646, 2022. [DOI] [PubMed] [Google Scholar]
- 115.Focosi D, Franchini M, Pirofski LA, Burnouf T, Fairweather D, Joyner MJ, and Casadevall A. COVID-19 Convalescent Plasma Is More than Neutralizing Antibodies: A Narrative Review of Potential Beneficial and Detrimental Co-Factors. Viruses 13: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Focosi D, Franchini M, Pirofski LA, Burnouf T, Paneth N, Joyner MJ, and Casadevall A. COVID-19 Convalescent Plasma and Clinical Trials: Understanding Conflicting Outcomes. Clin Microbiol Rev e0020021, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fontana L, Klein S, and Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr) 32: 97–108, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fontana L, Meyer TE, Klein S, and Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 101: 6659–6663, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Franchini M, Focosi D, Casadevall A, Joyner MJ, and Perotti C. Convalescent plasma for COVID-19. TSUNAMI is not the final word. Eur J Intern Med 97: 116–118, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frank LD, Andresen MA, and Schmid TL. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prev Med 27: 87–96, 2004. [DOI] [PubMed] [Google Scholar]
- 121.Furchgott RF. The 1989 Ulf von Euler lecture. Studies on endothelium-dependent vasodilation and the endothelium-derived relaxing factor. Acta Physiol Scand 139: 257–270, 1990. [DOI] [PubMed] [Google Scholar]
- 122.Furchgott RF. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA 276: 1186–1188, 1996. [PubMed] [Google Scholar]
- 123.Furchgott RF. Endothelium-Derived Relaxing Factor: Discovery, Early Studies, and Identifcation as Nitric Oxide (Nobel Lecture). Angew Chem Int Ed Engl 38: 1870–1880, 1999. [DOI] [PubMed] [Google Scholar]
- 124.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep 19: 235–251, 1999. [DOI] [PubMed] [Google Scholar]
- 125.Furchgott RF. An historical survey and prospects of research on EDRF. Nihon Heikatsukin Gakkai Zasshi 23: 435–440, 1987. [DOI] [PubMed] [Google Scholar]
- 126.Furchgott RF. Introduction to EDRF research. J Cardiovasc Pharmacol 22 Suppl 7: S1–2, 1993. [PubMed] [Google Scholar]
- 127.Furchgott RF. Nitric oxide: from basic research on isolated blood vessels to clinical relevance in diabetes. An R Acad Nac Med (Madr) 115: 317–331, 1998. [PubMed] [Google Scholar]
- 128.Furchgott RF. A research trail over half a century. Annu Rev Pharmacol Toxicol 35: 1–27, 1995. [DOI] [PubMed] [Google Scholar]
- 129.Furchgott RF. Robert F. Furchgott: a pharmacologist's pharmacologist. Mol Interv 4: 74–78, 2004. [DOI] [PubMed] [Google Scholar]
- 130.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res 53: 557–573, 1983. [DOI] [PubMed] [Google Scholar]
- 131.Furchgott RF, and Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 132.Furchgott RF, and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 133.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E, and Lucia A. Elite athletes live longer than the general population: a meta-analysis. Mayo Clin Proc 89: 1195–1200, 2014. [DOI] [PubMed] [Google Scholar]
- 135.Giskes K, van Lenthe F, Avendano-Pabon M, and Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev 12: e95–e106, 2011. [DOI] [PubMed] [Google Scholar]
- 136.Green DJ, Hopman MT, Padilla J, Laughlin MH, and Thijssen DH. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol Rev 97: 495–528, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Green DJ, Maiorana A, O'Driscoll G, and Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Green DJ, and Smith KJ. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb Perspect Med 8: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grimby G, and Saltin B. Physiological analysis of physically well-trained middle-aged and old athletes. Acta Med Scand 179: 513–526, 1966. [DOI] [PubMed] [Google Scholar]
- 140.Hagberg J A Personal Biography of a Physiological Misnomer: The Anaerobic Threshold. Int J Sports Med 43: 391–400, 2022. [DOI] [PubMed] [Google Scholar]
- 141.Hagberg JM, Coyle EF, Baldwin KM, Cartee GD, Fontana L, Joyner MJ, Kirwan JP, Seals DR, and Weiss EP. The historical context and scientific legacy of John O. Holloszy. J Appl Physiol (1985) 127: 277–305, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hagberg JM, Coyle EF, Carroll JE, Miller JM, Martin WH, and Brooke MH. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol 52: 991–994, 1982. [DOI] [PubMed] [Google Scholar]
- 143.Hagberg JM, King DS, Rogers MA, Montain SJ, Jilka SM, Kohrt WM, and Heller SL. Exercise and recovery ventilatory and VO2 responses of patients with McArdle's disease. J Appl Physiol (1985) 68: 1393–1398, 1990. [DOI] [PubMed] [Google Scholar]
- 144.Haines A, and Hartog M. Doctors and the test ban: 25 years on. BMJ 297: 408–411, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hallal PC, and Pratt M. Physical activity: moving from words to action. Lancet Glob Health 8: e867–e868, 2020. [DOI] [PubMed] [Google Scholar]
- 146.Hallmarker U, Lindback J, Michaelsson K, Arnlov J, Asberg S, Wester P, Hellberg D, Lagerqvist B, and James S. Survival and incidence of cardiovascular diseases in participants in a long-distance ski race (Vasaloppet, Sweden) compared with the background population. Eur Heart J Qual Care Clin Outcomes 4: 91–97, 2018. [DOI] [PubMed] [Google Scholar]
- 147.Hamel P, Simoneau JA, Lortie G, Boulay MR, and Bouchard C. Heredity and muscle adaptation to endurance training. Med Sci Sports Exerc 18: 690–696, 1986. [PubMed] [Google Scholar]
- 148.Hansell P, Welch WJ, Blantz RC, and Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol 40: 123–137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hargiss HW. Glenn Cunningham Trains https://www.oberheide.org/hargiss/1928%20KU/352/Glenn%20Cunningham1.htm.
- 150.Harridge SD, and Lazarus NR. Physical Activity, Aging, and Physiological Function. Physiology (Bethesda) 32: 152–161, 2017. [DOI] [PubMed] [Google Scholar]
- 151.Hartley PH, and Llewellyn GF. Longevity of Oarsmen. Br Med J 1: 657–662, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, and Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation 87: 1076–1082, 1993. [DOI] [PubMed] [Google Scholar]
- 153.Hawkins SA, Marcell TJ, Victoria Jaque S, and Wiswell RA. A longitudinal assessment of change in VO2max and maximal heart rate in master athletes. Med Sci Sports Exerc 33: 1744–1750, 2001. [DOI] [PubMed] [Google Scholar]
- 154.Heady JA, Morris JN, and Raffle PA. Physique of London busmen; epidemiology of uniforms. Lancet 271: 569–570, 1956. [DOI] [PubMed] [Google Scholar]
- 155.Heath GW, Hagberg JM, Ehsani AA, and Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol Respir Environ Exerc Physiol 51: 634–640, 1981. [DOI] [PubMed] [Google Scholar]
- 156.Hebert-Losier K, Finlayson SJ, Driller MW, Dubois B, Esculier JF, and Beaven CM. Metabolic and performance responses of male runners wearing 3 types of footwear: Nike Vaporfly 4%, Saucony Endorphin racing flats, and their own shoes. J Sport Health Sci 11: 275–284, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heidbuchel H, Anne W, Willems R, Adriaenssens B, Van de Werf F, and Ector H. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol 107: 67–72, 2006. [DOI] [PubMed] [Google Scholar]
- 158.Heigenhauser GJ, Sutton JR, and Jones NL. Effect of glycogen depletion on the ventilatory response to exercise. J Appl Physiol Respir Environ Exerc Physiol 54: 470–474, 1983. [DOI] [PubMed] [Google Scholar]
- 159.Heroux M, Janssen I, Lam M, Lee DC, Hebert JR, Sui X, and Blair SN. Dietary patterns and the risk of mortality: impact of cardiorespiratory fitness. Int J Epidemiol 39: 197–209, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, and Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart 84: 383–389, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hill JO, Wyatt H, Phelan S, and Wing R. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J Nutr Educ Behav 37: 206–210, 2005. [DOI] [PubMed] [Google Scholar]
- 162.Hollmann W 42 years ago--development of the concepts of ventilatory and lactate threshold. Sports Med 31: 315–320, 2001. [DOI] [PubMed] [Google Scholar]
- 163.Holloszy JO. The Epidemiology of Coronary Heart Disease: National Differences and the Role of Physical Activity. J Am Geriatr Soc 11: 718–725, 1963. [DOI] [PubMed] [Google Scholar]
- 164.Holloszy JO, and Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 165.Hoogkamer W, Kipp S, Frank JH, Farina EM, Luo G, and Kram R. A Comparison of the Energetic Cost of Running in Marathon Racing Shoes. Sports Med 48: 1009–1019, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hoogkamer W, Kipp S, and Kram R. The Biomechanics of Competitive Male Runners in Three Marathon Racing Shoes: A Randomized Crossover Study. Sports Med 49: 133–143, 2019. [DOI] [PubMed] [Google Scholar]
- 167.Hoppeler H, Vogt M, Weibel ER, and Fluck M. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol 88: 109–119, 2003. [DOI] [PubMed] [Google Scholar]
- 168.Hunter I, McLeod A, Valentine D, Low T, Ward J, and Hager R. Running economy, mechanics, and marathon racing shoes. J Sports Sci 37: 2367–2373, 2019. [DOI] [PubMed] [Google Scholar]
- 169.Johnson ML, Burke BS, and Mayer J. Relative importance of inactivity and overeating in the energy balance of obese high school girls. Am J Clin Nutr 4: 37–44, 1956. [DOI] [PubMed] [Google Scholar]
- 170.Jones AM, Kirby BS, Clark IE, Rice HM, Fulkerson E, Wylie LJ, Wilkerson DP, Vanhatalo A, and Wilkins BW. Physiological demands of running at 2-hour marathon race pace. J Appl Physiol (1985) 130: 369–379, 2021. [DOI] [PubMed] [Google Scholar]
- 171.Jorgenson KW, Phillips SM, and Hornberger TA. Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review. Cells 9: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Joyner MJ. Feeding the sleeping giant: muscle blood flow during whole body exercise. J Physiol 558: 1, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Joyner MJ. Physiological limiting factors and distance running: influence of gender and age on record performances. Exerc Sport Sci Rev 21: 103–133, 1993. [PubMed] [Google Scholar]
- 174.Joyner MJ. Physiological limits to endurance exercise performance: influence of sex. J Physiol 595: 2949–2954, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Joyner MJ, Baker SE, Senefeld JW, Klassen SA, and Wiggins CC. Experiments of nature and within species comparative physiology. Comp Biochem Physiol A Mol Integr Physiol 253: 110864, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO, Shepherd JRA, Rea RF, Whelan ER, Clayburn AJ, Spiegel MR, Baker SE, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, van Buskirk CM, Winters JL, Stubbs JR, van Helmond N, Butterfield BP, Sexton MA, Diaz Soto JC, Paneth NS, Verdun NC, Marks P, Casadevall A, Fairweather D, Carter RE, and Wright RS. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc 95: 1888–1897, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, van Helmond N, Verdun NC, Marks P, van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS, Fairweather D, Wright RS, and Casadevall A. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 384: 1015–1027, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Joyner MJ, and Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Joyner MJ, and Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol 586: 35–44, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Joyner MJ, and Dempsey JA. Physiological Redundancy and the Integrative Responses to Exercise. Cold Spring Harb Perspect Med 8: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Joyner MJ, Freund BJ, Jilka SM, Hetrick GA, Martinez E, Ewy GA, and Wilmore JH. Effects of beta-blockade on exercise capacity of trained and untrained men: a hemodynamic comparison. J Appl Physiol (1985) 60: 1429–1434, 1986. [DOI] [PubMed] [Google Scholar]
- 182.Joyner MJ, and Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joyner MJ, Klompas AM, Klassen SA, Senefeld JW, Fairweather D, Wright RS, and Carter RE. In Reply-How Safe Is COVID-19 Convalescent Plasma? Mayo Clin Proc 96: 2281–2282, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Joyner MJ, Paneth NS, Senefeld JW, Fairweather D, Bruno KA, Wright RS, Carter RE, and Casadevall A. Concerns about estimating relative risk of death associated with convalescent plasma for COVID-19. Nat Med 28: 51–52, 2022. [DOI] [PubMed] [Google Scholar]
- 185.Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, Carter RE, Klompas AM, Wiggins CC, Shepherd JR, Rea RF, Whelan ER, Clayburn AJ, Spiegel MR, Johnson PW, Lesser ER, Baker SE, Larson KF, Ripoll JG, Andersen KJ, Hodge DO, Kunze KL, Buras MR, Vogt MN, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Van Buskirk CM, Winters JL, Stubbs JR, Paneth NS, Verdun NC, Marks P, and Casadevall A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 130: 4791–4797, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kahn HA. The relationship of reported coronary heart disease mortality to physical activity of work. Am J Public Health Nations Health 53: 1058–1067, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Karjalainen J, Kujala UM, Kaprio J, Sarna S, and Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ 316: 1784–1785, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kelly T, Yang W, Chen CS, Reynolds K, and He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 32: 1431–1437, 2008. [DOI] [PubMed] [Google Scholar]
- 189.Kettunen JA, Kujala UM, Kaprio J, Backmand H, Peltonen M, Eriksson JG, and Sarna S. All-cause and disease-specific mortality among male, former elite athletes: an average 50-year follow-up. Br J Sports Med 49: 893–897, 2015. [DOI] [PubMed] [Google Scholar]
- 190.Kipp S, Kram R, and Hoogkamer W. Extrapolating Metabolic Savings in Running: Implications for Performance Predictions. Front Physiol 10: 79, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S, Grossman BJ, Henderson JP, Musser J, Salazar E, Hartman WR, Bouvier NM, Liu STH, Pirofski LA, Baker SE, van Helmond N, Wright RS, Fairweather D, Bruno KA, Wang Z, Paneth NS, Casadevall A, and Joyner MJ. The Effect of Convalescent Plasma Therapy on Mortality Among Patients With COVID-19: Systematic Review and Meta-analysis. Mayo Clin Proc 96: 1262–1275, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klassen SA, Senefeld JW, Senese KA, Johnson PW, Wiggins CC, Baker SE, van Helmond N, Bruno KA, Pirofski LA, Shoham S, Grossman BJ, Henderson JP, Wright RS, Fairweather D, Paneth NS, Carter RE, Casadevall A, and Joyner MJ. Convalescent Plasma Therapy for COVID-19: A Graphical Mosaic of the Worldwide Evidence. Front Med (Lausanne) 8: 684151, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Klem ML, Wing RR, McGuire MT, Seagle HM, and Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr 66: 239–246, 1997. [DOI] [PubMed] [Google Scholar]
- 194.Klompas AM, van Helmond N, Juskewitch JE, Pruthi RK, Sexton MA, Soto JCD, Klassen SA, Senese KA, van Buskirk CM, Winters JL, Stubbs JR, Hammel SA, Joyner MJ, and Senefeld JW. Coagulation profile of human COVID-19 convalescent plasma. Sci Rep 12: 637, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Knapp EA, Bennett WL, Wilson RF, Zhang A, Tseng E, Cheskin LJ, Bass EB, Kharrazi H, and Stuart EA. Methods and Risks of Bias in Natural Experiments in Obesity: Opportunities for the Future Informed by a Systematic Review. Obesity (Silver Spring) 27: 1950–1957, 2019. [DOI] [PubMed] [Google Scholar]
- 196.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, and Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. [DOI] [PubMed] [Google Scholar]
- 197.Koller A, Laughlin MH, Cenko E, de Wit C, Toth K, Bugiardini R, Trifunovits D, Vavlukis M, Manfrini O, Lelbach A, Dornyei G, Padro T, Badimon L, Tousoulis D, Gielen S, and Duncker DJ. Functional and structural adaptations of the coronary macro- and microvasculature to regular aerobic exercise by activation of physiological, cellular, and molecular mechanisms: ESC Working Group on Coronary Pathophysiology and Microcirculation position paper. Cardiovasc Res 118: 357–371, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kraus WE, Powell KE, Haskell WL, Janz KF, Campbell WW, Jakicic JM, Troiano RP, Sprow K, Torres A, Piercy KL, and Physical Activity Guidelines Advisory C. Physical Activity, All-Cause and Cardiovascular Mortality, and Cardiovascular Disease. Med Sci Sports Exerc 51: 1270–1281, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kristal-Boneh E, Silber H, Harari G, and Froom P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study). Eur Heart J 21: 116–124, 2000. [DOI] [PubMed] [Google Scholar]
- 200.Krogh A, and Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47: 112–136, 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood) 33: 1163–1170, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kunze KL, Johnson PW, van Helmond N, Senefeld JW, Petersen MM, Klassen SA, Wiggins CC, Klompas AM, Bruno KA, Mills JR, Theel ES, Buras MR, Golafshar MA, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, Verdun NC, Marks P, Paneth NS, Fairweather D, Wright RS, van Buskirk CM, Winters JL, Stubbs JR, Senese KA, Pletsch MC, Buchholtz ZA, Rea RF, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Lesser ER, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Casadevall A, Carter RE, and Joyner MJ. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat Commun 12: 4864, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lake A, and Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health 126: 262–267, 2006. [DOI] [PubMed] [Google Scholar]
- 204.Laughlin MH, McAllister RM, Jasperse JL, Crader SE, Williams DA, and Huxley VH. Endothelium-medicated control of the coronary circulation. Exercise training-induced vascular adaptations. Sports Med 22: 228–250, 1996. [DOI] [PubMed] [Google Scholar]
- 205.Laughlin MH, Oltman CL, and Bowles DK. Exercise training-induced adaptations in the coronary circulation. Med Sci Sports Exerc 30: 352–360, 1998. [DOI] [PubMed] [Google Scholar]
- 206.Lavin KM, Coen PM, Baptista LC, Bell MB, Drummer D, Harper SA, Lixandrao ME, McAdam JS, O'Bryan SM, Ramos S, Roberts LM, Vega RB, Goodpaster BH, Bamman MM, and Buford TW. State of Knowledge on Molecular Adaptations to Exercise in Humans: Historical Perspectives and Future Directions. Compr Physiol 12: 3193–3279, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lazarus NR, and Harridge SDR. Declining performance of master athletes: silhouettes of the trajectory of healthy human ageing? J Physiol 595: 2941–2948, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lazarus NR, Lord JM, and Harridge SDR. The relationships and interactions between age, exercise and physiological function. J Physiol 597: 1299–1309, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, Rosengren A, Wei L, Yang W, Chuangshi W, Huaxing L, Nair S, Diaz R, Swidon H, Gupta R, Mohammadifard N, Lopez-Jaramillo P, Oguz A, Zatonska K, Seron P, Avezum A, Poirier P, Teo K, and Yusuf S. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 390: 2643–2654, 2017. [DOI] [PubMed] [Google Scholar]
- 210.Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, and Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 64: 472–481, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee IM, and Paffenbarger RS Jr. Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol 151: 293–299, 2000. [DOI] [PubMed] [Google Scholar]
- 212.Lee IM, and Paffenbarger RS Jr. How much physical activity is optimal for health? Methodological considerations. Res Q Exerc Sport 67: 206–208, 1996. [DOI] [PubMed] [Google Scholar]
- 213.Lee IM, Paffenbarger RS Jr., and Hsieh CC. Time trends in physical activity among college alumni, 1962-1988. Am J Epidemiol 135: 915–925, 1992. [DOI] [PubMed] [Google Scholar]
- 214.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, and Lancet Physical Activity Series Working G. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380: 219–229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee IM, and Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 33: S459–471; discussion S493-454, 2001. [DOI] [PubMed] [Google Scholar]
- 216.Lenfant C, and Sullivan K. Adaptation to high altitude. N Engl J Med 284: 1298–1309, 1971. [DOI] [PubMed] [Google Scholar]
- 217.Lenfant C, Torrance J, English E, Finch CA, Reynafarje C, Ramos J, and Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest 47: 2652–2656, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Levine BD, and Mitchell JH. 'Ultra' coronary arteries: bigger and better? Circulation 87: 1402–1404, 1993. [DOI] [PubMed] [Google Scholar]
- 219.Lewis SF, Haller RG, Cook JD, and Blomqvist CG. Metabolic control of cardiac output response to exercise in McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol 57: 1749–1753, 1984. [DOI] [PubMed] [Google Scholar]
- 220.Li M, Beck EJ, Laeyendecker O, Eby Y, Tobian AAR, Caturegli P, Wouters C, Chiklis GR, Block W, McKie RO, Joyner MJ, Wiltshire TD, Dietz AB, Gniadek TJ, Shapiro AJ, Yarava A, Lane K, Hanley DF, Bloch EM, Shoham S, Cachay ER, Meisenberg BR, Huaman MA, Fukuta Y, Patel B, Heath SL, Levine AC, Paxton JH, Anjan S, Gerber JM, Gebo KA, Casadevall A, Pekosz A, and Sullivan DJ. Convalescent plasma with a high level of virus-specific antibody effectively neutralizes SARS-CoV-2 variants of concern. Blood Adv 6: 3678–3683, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lucia A, Olivan J, Gomez-Gallego F, Santiago C, Montil M, and Foster C. Citius and longius (faster and longer) with no alpha-actinin-3 in skeletal muscles? Br J Sports Med 41: 616–617, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, and Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med 38: e66–73, 2010. [DOI] [PubMed] [Google Scholar]
- 223.Luke TC, Kilbane EM, Jackson JL, and Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 145: 599–609, 2006. [DOI] [PubMed] [Google Scholar]
- 224.Lundby C, Montero D, and Joyner M. Biology of VO2 max: looking under the physiology lamp. Acta Physiol (Oxf) 220: 218–228, 2017. [DOI] [PubMed] [Google Scholar]
- 225.Ma T, Wiggins CC, Kornatowski BM, Hailat RS, Clayburn AJ, Guo WL, Johnson PW, Senefeld JW, Klassen SA, Baker SE, Bruno KA, Fairweather D, Wright RS, Carter RE, Li C, Joyner MJ, and Paneth NS. The Role of Disease Severity and Demographics in the Clinical Course of COVID-19 Patients Treated With Convalescent Plasma. Front Med (Lausanne) 8: 707895, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.MacAuley D A history of physical activity, health and medicine. J R Soc Med 87: 32–35, 1994. [PMC free article] [PubMed] [Google Scholar]
- 227.MacDougall JD, Tuxen D, Sale DG, Moroz JR, and Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 58: 785–790, 1985. [DOI] [PubMed] [Google Scholar]
- 228.Mann GV, Spoerry A, Gray M, and Jarashow D. Atherosclerosis in the Masai. Am J Epidemiol 95: 26–37, 1972. [DOI] [PubMed] [Google Scholar]
- 229.Marijon E, Tafflet M, Antero-Jacquemin J, El Helou N, Berthelot G, Celermajer DS, Bougouin W, Combes N, Hermine O, Empana JP, Rey G, Toussaint JF, and Jouven X. Mortality of French participants in the Tour de France (1947-2012). Eur Heart J 34: 3145–3150, 2013. [DOI] [PubMed] [Google Scholar]
- 230.Marshall RJ, Schirger A, and Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation 24: 76–81, 1961. [DOI] [PubMed] [Google Scholar]
- 231.Mayer J, Roy P, and Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr 4: 169–175, 1956. [DOI] [PubMed] [Google Scholar]
- 232.McAdams TA, Rijsdijk FV, Zavos HMS, and Pingault JB. Twins and Causal Inference: Leveraging Nature's Experiment. Cold Spring Harb Perspect Med 11: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McArdle B Myopathy due to a defect in muscle glycogen breakdown. Clin Sci 10: 13–35, 1951. [PubMed] [Google Scholar]
- 234.McCloskey DI, and Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.McGavock JM, Hastings JL, Snell PG, McGuire DK, Pacini EL, Levine BD, and Mitchell JH. A forty-year follow-up of the Dallas Bed Rest and Training study: the effect of age on the cardiovascular response to exercise in men. J Gerontol A Biol Sci Med Sci 64: 293–299, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.McGue M, Osler M, and Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci 5: 546–556, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, and Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 104: 1350–1357, 2001. [PubMed] [Google Scholar]
- 238.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, and Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation 104: 1358–1366, 2001. [PubMed] [Google Scholar]
- 239.McGuire KA, and Ross R. Incidental physical activity is positively associated with cardiorespiratory fitness. Med Sci Sports Exerc 43: 2189–2194, 2011. [DOI] [PubMed] [Google Scholar]
- 240.McKendry J, Stokes T, McLeod JC, and Phillips SM. Resistance Exercise, Aging, Disuse, and Muscle Protein Metabolism. Compr Physiol 11: 2249–2278, 2021. [DOI] [PubMed] [Google Scholar]
- 241.Mitchell JH. Abnormal cardiovascular response to exercise in hypertension: contribution of neural factors. Am J Physiol Regul Integr Comp Physiol 312: R851–R863, 2017. [DOI] [PubMed] [Google Scholar]
- 242.Mitchell JH, Sproule BJ, and Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest 37: 538–547, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, Rebato C, and Elosua R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace 10: 618–623, 2008. [DOI] [PubMed] [Google Scholar]
- 244.Monge C, and Leon-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev 71: 1135–1172, 1991. [DOI] [PubMed] [Google Scholar]
- 245.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, Pare C, Azqueta M, and Sanz G. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J 23: 477–482, 2002. [DOI] [PubMed] [Google Scholar]
- 246.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, and Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 9: e1001335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Morabia A Claude Bernard was a 19th century proponent of medicine based on evidence. J Clin Epidemiol 59: 1150–1154, 2006. [DOI] [PubMed] [Google Scholar]
- 248.Morris JN. Exercise versus heart attack: questioning the consensus? Res Q Exerc Sport 67: 216–220, 1996. [DOI] [PubMed] [Google Scholar]
- 249.Morris JN, Chave SP, Adam C, Sirey C, Epstein L, and Sheehan DJ. Vigorous exercise in leisure-time and the incidence of coronary heart-disease. Lancet 1: 333–339, 1973. [DOI] [PubMed] [Google Scholar]
- 250.Morris JN, Everitt MG, Pollard R, Chave SP, and Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 2: 1207–1210, 1980. [DOI] [PubMed] [Google Scholar]
- 251.Morris JN, Heady JA, Raffle PA, Roberts CG, and Parks JW. Coronary heart-disease and physical activity of work. Lancet 262: 1111–1120; concl, 1953. [DOI] [PubMed] [Google Scholar]
- 252.Morris JN, Heady JA, Raffle PA, Roberts CG, and Parks JW. Coronary heart-disease and physical activity of work. Lancet 262: 1053–1057, 1953. [DOI] [PubMed] [Google Scholar]
- 253.Morris JN, Kagan A, Pattison DC, and Gardner MJ. Incidence and prediction of ischaemic heart-disease in London busmen. Lancet 2: 553–559, 1966. [DOI] [PubMed] [Google Scholar]
- 254.Murach KA, Bagley JR, McLeland KA, Arevalo JA, Ciccone AB, Malyszek KK, Wen Y, and Galpin AJ. Improving human skeletal muscle myosin heavy chain fiber typing efficiency. J Muscle Res Cell Motil 37: 1–5, 2016. [DOI] [PubMed] [Google Scholar]
- 255.Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, and Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 33: 29–36, 2009. [DOI] [PubMed] [Google Scholar]
- 256.Myers J Cardiology patient pages. Exercise and cardiovascular health. Circulation 107: e2–5, 2003. [DOI] [PubMed] [Google Scholar]
- 257.Myers J, Prakash M, Froelicher V, Do D, Partington S, and Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801,2002. [DOI] [PubMed] [Google Scholar]
- 258.Myhre LG, Oddershede I, Dill DB, and Yousef MK. Cardiac output during rest and exercise in desert heat. Med Sci Sports 11: 234–238, 1979. [PubMed] [Google Scholar]
- 259.Natarajan C, Jendroszek A, Kumar A, Weber RE, Tame JRH, Fago A, and Storz JF. Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet 14: e1007331, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Noble D Claude Bernard, the first systems biologist, and the future of physiology. Exp Physiol 93: 16–26, 2008. [DOI] [PubMed] [Google Scholar]
- 261.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, and Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil 15: 239–246, 2008. [DOI] [PubMed] [Google Scholar]
- 262.Normandin S Claude Bernard and an introduction to the study of experimental medicine: "physical vitalism," dialectic, and epistemology. J Hist Med Allied Sci 62: 495–528, 2007. [DOI] [PubMed] [Google Scholar]
- 263.Nybo L, Schmidt JF, Fritzdorf S, and Nordsborg NB. Physiological characteristics of an aging Olympic athlete. Med Sci Sports Exerc 46: 2132–2138, 2014. [DOI] [PubMed] [Google Scholar]
- 264.Ogden LG, Stroebele N, Wyatt HR, Catenacci VA, Peters JC, Stuht J, Wing RR, and Hill JO. Cluster analysis of the national weight control registry to identify distinct subgroups maintaining successful weight loss. Obesity (Silver Spring) 20: 2039–2047, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ogilvie D, Adams J, Bauman A, Gregg EW, Panter J, Siegel KR, Wareham NJ, and White M. Using natural experimental studies to guide public health action: turning the evidence-based medicine paradigm on its head. J Epidemiol Community Health 74: 203–208, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ordaya EE, Abu Saleh OM, Stubbs JR, and Joyner MJ. Vax-Plasma in Patients With Refractory COVID-19. Mayo Clin Proc 97: 186–189, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Paffenbarger RS, and Hale WE. Work activity and coronary heart mortality. N Engl J Med 292: 545–550, 1975. [DOI] [PubMed] [Google Scholar]
- 268.Paffenbarger RS Jr. Jerry Morris: pathfinder for health through an active and fit way of life. Br J Sports Med 34: 217, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Paffenbarger RS Jr., Blair SN, and Lee IM. A history of physical activity, cardiovascular health and longevity: the scientific contributions of Jeremy N Morris, DSc, DPH, FRCP. Int J Epidemiol 30: 1184–1192, 2001. [DOI] [PubMed] [Google Scholar]
- 270.Paffenbarger RS Jr., and Hyde RT. Exercise in the prevention of coronary heart disease. Prev Med 13: 3–22, 1984. [DOI] [PubMed] [Google Scholar]
- 271.Paffenbarger RS Jr., Hyde RT, Wing AL, and Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 314: 605–613, 1986. [DOI] [PubMed] [Google Scholar]
- 272.Paffenbarger RS Jr., Laughlin ME, Gima AS, and Black RA. Work activity of longshoremen as related to death from coronary heart disease and stroke. N Engl J Med 282: 1109–1114, 1970. [DOI] [PubMed] [Google Scholar]
- 273.Paffenbarger RS Jr., and Wing AL. Chronic disease in former college students. X. The effects of single and multiple characteristics on risk of fatal coronary heart disease. Am J Epidemiol 90: 527–535, 1969. [DOI] [PubMed] [Google Scholar]
- 274.Paffenbarger RS Jr., Wing AL, and Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 108: 161–175, 1978. [DOI] [PubMed] [Google Scholar]
- 275.Paffenbarger RS Jr., Wolf PA, Notkin J, and Thorne MC. Chronic disease in former college students. I. Early precursors of fatal coronary heart disease. Am J Epidemiol 83: 314–328, 1966. [DOI] [PubMed] [Google Scholar]
- 276.Paixao C, Dias CM, Jorge R, Carraca EV, Yannakoulia M, de Zwaan M, Soini S, Hill JO, Teixeira PJ, and Santos I. Successful weight loss maintenance: A systematic review of weight control registries. Obes Rev 21: e13003, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Paneth N, Casadevall A, Pirofski LA, Henderson JP, Grossman BJ, Shoham S, and Joyner MJ. WHO covid-19 drugs guideline: reconsider using convalescent plasma. BMJ 376: o295, 2022. [DOI] [PubMed] [Google Scholar]
- 278.Paneth N, and Joyner M. The use of observational research to inform clinical practice. J Clin Invest 131: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Paneth N, Joyner MJ, and Casadevall A. Finding evidence for treatment decisions in a pandemic. Trends Mol Med 28: 536–541, 2022. [DOI] [PubMed] [Google Scholar]
- 280.Paneth N, Vinten-Johansen P, Brody H, and Rip M. A rivalry of foulness: official and unofficial investigations of the London cholera epidemic of 1854. Am J Public Health 88: 1545–1553, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Paneth NS, Joyner MJ, and Casadevall A. The fossilization of randomized clinical trials. J Clin Invest 132: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Park RJ. High-protein diets, "damaged hearts," and rowing men: antecedents of modern sports medicine and exercise science, 1867-1928. Exerc Sport Sci Rev 25: 137–169, 1997. [PubMed] [Google Scholar]
- 283.Parker JL. Once a Runner: A Novel. Scribner, 2009. [Google Scholar]
- 284.Parker JL, Oltman CL, Muller JM, Myers PR, Adams HR, and Laughlin MH. Effects of exercise training on regulation of tone in coronary arteries and arterioles. Med Sci Sports Exerc 26: 1252–1261, 1994. [PubMed] [Google Scholar]
- 285.Parnell RW. Some notes on physique and athletic training, with special reference to heart size. Br Med J 1: 1292–1295, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pautasso M Ten simple rules for writing a literature review. PLoS Comput Biol 9: e1003149, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pette D Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol (1985) 90: 1119–1124, 2001. [DOI] [PubMed] [Google Scholar]
- 288.Pimentel AE, Gentile CL, Tanaka H, Seals DR, and Gates PE. Greater rate of decline in maximal aerobic capacity with age in endurance-trained than in sedentary men. J Appl Physiol (1985) 94: 2406–2413, 2003. [DOI] [PubMed] [Google Scholar]
- 289.Pollock ML, Miller HS Jr., and Wilmore J. Physiological characteristics of champion American track athletes 40 to 75 years of age. J Gerontol 29: 645–649, 1974. [DOI] [PubMed] [Google Scholar]
- 290.Poole DC, Rossiter HB, Brooks GA, and Gladden LB. The anaerobic threshold: 50+ years of controversy. J Physiol 599: 737–767, 2021. [DOI] [PubMed] [Google Scholar]
- 291.Pryor SL, Lewis SF, Haller RG, Bertocci LA, and Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's disease). J Clin Invest 85: 1444–1449, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rao P, Hutter AM Jr., and Baggish AL. The Limits of Cardiac Performance: Can Too Much Exercise Damage the Heart? Am J Med 131: 1279–1284, 2018. [DOI] [PubMed] [Google Scholar]
- 293.Raynor DA, Phelan S, Hill JO, and Wing RR. Television viewing and long-term weight maintenance: results from the National Weight Control Registry. Obesity (Silver Spring) 14: 1816–1824, 2006. [DOI] [PubMed] [Google Scholar]
- 294.Remler DK, and Van Ryzin GG. Research Methods in Practice: Strategies for Description and Causation. SAGE Publications, 2021. [Google Scholar]
- 295.Richmond KN, Burnite S, and Lynch RM. Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol 273: C1613–1622, 1997. [DOI] [PubMed] [Google Scholar]
- 296.Riley M, Nicholls DP, Nugent AM, Steele IC, Bell N, Davies PM, Stanford CF, and Patterson VH. Respiratory gas exchange and metabolic responses during exercise in McArdle's disease. J Appl Physiol (1985) 75: 745–754, 1993. [DOI] [PubMed] [Google Scholar]
- 297.Ripoll JG, van Helmond N, Senefeld JW, Wiggins CC, Klassen SA, Baker SE, Larson KF, Murphy BM, Andersen KJ, Ford SK, Casadevall A, and Joyner MJ. Convalescent Plasma for Infectious Diseases: Historical Framework and Use in COVID-19. Clin Microbiol Newsl 43: 23–32, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Robinson AT, Watso JC, Babcock MC, Joyner MJ, and Farquhar WB. Record-Breaking Performance in a 70-Year-Old Marathoner. N Engl J Med 380: 1485–1486, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Robinson S Experimental studies of physical fitness in relation to age. Arbeitsphysiologie 10: 251–323, 1938. [Google Scholar]
- 300.Robinson S, Dill DB, Robinson RD, Tzankoff SP, and Wagner JA. Physiological aging of champion runners. J Appl Physiol 41: 46–51, 1976. [DOI] [PubMed] [Google Scholar]
- 301.Robinson S, Dill DB, Ross JC, Robinson RD, Wagner JA, and Tzankoff SP. Training and physiological aging in man. Fed Proc 32: 1628–1634, 1973. [PubMed] [Google Scholar]
- 302.Robinson S, Edwards HT, and Dill DB. New Records in Human Power. Science 85: 409–410, 1937. [DOI] [PubMed] [Google Scholar]
- 303.Rook A An investigation into the longevity of Cambridge sportsmen. Br Med J 1: 773–777, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rosenquist JN, Lehrer SF, O'Malley AJ, Zaslavsky AM, Smoller JW, and Christakis NA. Cohort of birth modifies the association between FTO genotype and BMI. Proc Natl Acad Sci U S A 112: 354–359, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rossiter HB. The "Anaerobic Threshold" Concept Is Valid in Physiology and Medicine. Med Sci Sports Exerc 53: 1089–1092, 2021. [DOI] [PubMed] [Google Scholar]
- 306.Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876-2003): cycles of revision and new vision. J Appl Physiol (1985) 97: 384–392, 2004. [DOI] [PubMed] [Google Scholar]
- 307.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr., Wildenthal K, and Chapman CB. Response to exercise after bed rest and after training. Circulation 38: VII1–78, 1968. [PubMed] [Google Scholar]
- 308.Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, Fiuza-Luces C, Garatachea N, and Lucia A. Strenuous Exercise Worse Than Sedentarism? J Am Coll Cardiol 65: 2673–2674, 2015. [DOI] [PubMed] [Google Scholar]
- 309.Sanders DA. How to write (and how not to write) a scientific review article. Clin Biochem 81: 65–68, 2020. [DOI] [PubMed] [Google Scholar]
- 310.Sanoff HK, Chang Y, Lund JL, O'Neil BH, and Dusetzina SB. Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma. Oncologist 21: 1113–1120, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Santalla A, Nogales-Gadea G, Ortenblad N, Brull A, de Luna N, Pinos T, and Lucia A. McArdle disease: a unique study model in sports medicine. Sports Med 44: 1531–1544, 2014. [DOI] [PubMed] [Google Scholar]
- 312.Sargent RP, Shepard RM, and Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ 328: 977–980, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sarna S, Sahi T, Koskenvuo M, and Kaprio J. Increased life expectancy of world class male athletes. Med Sci Sports Exerc 25: 237–244, 1993. [PubMed] [Google Scholar]
- 314.Schiaffino S, and Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531,2011. [DOI] [PubMed] [Google Scholar]
- 315.Schnohr P, O'Keefe JH, Marott JL, Lange P, and Jensen GB. Dose of jogging and long-term mortality: the Copenhagen City Heart Study. J Am Coll Cardiol 65: 411–419, 2015. [DOI] [PubMed] [Google Scholar]
- 316.Scott GR, Hawkes LA, Frappell PB, Butler PJ, Bishop CM, and Milsom WK. How bar-headed geese fly over the Himalayas. Physiology (Bethesda) 30: 107–115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Scott GR, and Milsom WK. Control of breathing and adaptation to high altitude in the bar-headed goose. Am J Physiol Regul Integr Comp Physiol 293: R379–391, 2007. [DOI] [PubMed] [Google Scholar]
- 318.Seidell JC, and Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab 66 Suppl 2: 7–12, 2015. [DOI] [PubMed] [Google Scholar]
- 319.Senefeld JW, Casadevall A, and Joyner MJ. Convalescent plasma to deliver therapeutic antibodies against COVID-19. Trends Mol Med 28: 435–436, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Senefeld JW, Haischer MH, Jones AM, Wiggins CC, Beilfuss R, Joyner MJ, and Hunter SK. Technological advances in elite marathon performance. J Appl Physiol (1985) 130: 2002–2008, 2021. [DOI] [PubMed] [Google Scholar]
- 321.Senefeld JW, and Hunter SK. Are masters athletic performances predictive of human aging in men and women? Mov Sport Sci/Sci Mot 5–12, 2019. [Google Scholar]
- 322.Senefeld JW, Johnson PW, Kunze KL, Bloch EM, van Helmond N, Golafshar MA, Klassen SA, Klompas AM, Sexton MA, Diaz Soto JC, Grossman BJ, Tobian AAR, Goel R, Wiggins CC, Bruno KA, van Buskirk CM, Stubbs JR, Winters JL, Casadevall A, Paneth NS, Shaz BH, Petersen MM, Sachais BS, Buras MR, Wieczorek MA, Russoniello B, Dumont LJ, Baker SE, Vassallo RR, Shepherd JRA, Young PP, Verdun NC, Marks P, Haley NR, Rea RF, Katz L, Herasevich V, Waxman DA, Whelan ER, Bergman A, Clayburn AJ, Grabowski MK, Larson KF, Ripoll JG, Andersen KJ, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Buchholtz ZA, Pletsch MC, Wright K, Greenshields JT, Joyner MJ, Wright RS, Carter RE, and Fairweather D. Access to and safety of COVID-19 convalescent plasma in the United States Expanded Access Program: A national registry study. PLoS Med 18: e1003872, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Senefeld JW, Klassen SA, Ford SK, Senese KA, Wiggins CC, Bostrom BC, Thompson MA, Baker SE, Nicholson WT, Johnson PW, Carter RE, Henderson JP, Hartman WR, Pirofski LA, Wright RS, Fairweather L, Bruno KA, Paneth NS, Casadevall A, and Joyner MJ. Use of convalescent plasma in COVID-19 patients with immunosuppression. Transfusion 61: 2503–2511,2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Senefeld JW, Shepherd JRA, Baker SE, and Joyner MJ. Sex-based limits to running speed in the human, horse and dog: The role of sexual dimorphisms. FASEB J 35: e21562, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sesso HD, Paffenbarger RS Jr., and Lee IM. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation 102: 975–980, 2000. [DOI] [PubMed] [Google Scholar]
- 326.Shapiro S, Weinblatt E, Frank CW, and Sager RV. Incidence of coronary heart disease in a population insured for medical care (HIP): myocardial infarction, angina pectoris, and possible myocardial infarction. Am J Public Health Nations Health 59 Suppl 6: 1–101, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shephard RJ. Exercise in coronary heart disease. Sports Med 3: 26–49, 1986. [DOI] [PubMed] [Google Scholar]
- 328.Shepherd JRA, Dominelli PB, Roy TK, Secomb TW, Hoyer JD, Oliveira JL, and Joyner MJ. Modelling the relationships between haemoglobin oxygen affinity and the oxygen cascade in humans. J Physiol 597: 4193–4202, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shiode N, Shiode S, Rod-Thatcher E, Rana S, and Vinten-Johansen P. The mortality rates and the space-time patterns of John Snow's cholera epidemic map. Int J Health Geogr 14: 21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Simoneau JA, and Bouchard C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J 9: 1091–1095, 1995. [DOI] [PubMed] [Google Scholar]
- 331.Sinoway LI, Musch TI, Minotti JR, and Zelis R. Enhanced maximal metabolic vasodilatation in the dominant forearms of tennis players. J Appl Physiol (1985) 61: 673–678, 1986. [DOI] [PubMed] [Google Scholar]
- 332.Smirk FH. High Arterial Pressure. Blackwell Scientific Publications, 1957. [Google Scholar]
- 333.Snow J. On the Mode of Communication of Cholera. Edinb Med J 1: 668–670, 1856. [PMC free article] [PubMed] [Google Scholar]
- 334.Spain DM, and Bradess VA. Occupational physical activity and the degree of coronary atherosclerosis in "normal" men. A postmortem study. Circulation 22: 239–242, 1960. [DOI] [PubMed] [Google Scholar]
- 335.Storz JF. Evolution. Genes for high altitudes. Science 329: 40–41, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Storz JF. Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? J Exp Biol 219: 3190–3203, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Storz JF, Scott GR, and Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol 213: 4125–4136, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Svedberg N, Sundstrom J, James S, Hallmarker U, Hambraeus K, and Andersen K. Long-Term Incidence of Atrial Fibrillation and Stroke Among Cross-Country Skiers. Circulation 140: 910–920, 2019. [DOI] [PubMed] [Google Scholar]
- 339.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, and Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378: 804–814, 2011. [DOI] [PubMed] [Google Scholar]
- 340.Talbot J, and Maves L. Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip Rev Dev Biol 5: 518–534, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Tanaka H Aging of Competitive Athletes. Gerontology 63: 488–494, 2017. [DOI] [PubMed] [Google Scholar]
- 342.Tanaka H, Monahan KD, and Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. [DOI] [PubMed] [Google Scholar]
- 343.Tanaka H, and Seals DR. Age and gender interactions in physiological functional capacity: insight from swimming performance. J Appl Physiol (1985) 82: 846–851, 1997. [DOI] [PubMed] [Google Scholar]
- 344.Tanaka H, and Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol 586: 55–63, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tanaka H, and Seals DR. Invited Review: Dynamic exercise performance in Masters athletes: insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol (1985) 95: 2152–2162, 2003. [DOI] [PubMed] [Google Scholar]
- 346.Tanaka H, Tarumi T, and Rittweger J. Aging and Physiological Lessons from Master Athletes. Compr Physiol 10: 261–296, 2019. [DOI] [PubMed] [Google Scholar]
- 347.Taylor HL, Klepetar E, Keys A, Parlin W, Blackburn H, and Puchner T. Death rates among physically active and sedentary employees of the railroad industry. Am J Public Health Nations Health 52: 1697–1707, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Thomas JG, Bond DS, Phelan S, Hill JO, and Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med 46: 17–23, 2014. [DOI] [PubMed] [Google Scholar]
- 349.Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, Flora DB, Griffiths EA, Gulati AP, Hwang C, Koshkin VS, Papadopoulos EB, Robilotti EV, Su CT, Wulff-Burchfield EM, Xie Z, Yu PP, Mishra S, Senefeld JW, Shah DP, Warner JL, Covid, and Cancer C. Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19. JAMA Oncol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tipton CM. Contemporary exercise physiology: fifty years after the closure of Harvard Fatigue Laboratory. Exerc Sport Sci Rev 26: 315–339, 1998. [PubMed] [Google Scholar]
- 351.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, and Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) 114: 3–10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Trappe SW, Costill DL, Vukovich MD, Jones J, and Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol (1985) 80: 285–290, 1996. [DOI] [PubMed] [Google Scholar]
- 353.Tsur AM, and Twig G. The actual burden of obesity-accounting for multimorbidity. Lancet Diabetes Endocrinol 10: 233–234, 2022. [DOI] [PubMed] [Google Scholar]
- 354.Tulchinsky TH. John Snow, Cholera, the Broad Street Pump; Waterborne Diseases Then and Now. Case Studies in Public Health 77–99, 2018. [Google Scholar]
- 355.Vandevijvere S, Chow CC, Hall KD, Umali E, and Swinburn BA. Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull World Health Organ 93: 446–456, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Victor RG, Seals DR, and Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest 79: 508–516, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Wackerhage H Contributions by the Cologne group to the development of lactate exercise testing and anaerobic threshold concepts in the 1970s and 1980s. J Physiol 599: 1713–1714, 2021. [DOI] [PubMed] [Google Scholar]
- 358.Wackerhage H, Gehlert S, Schulz H, Weber S, Ring-Dimitriou S, and Heine O. Lactate Thresholds and the Simulation of Human Energy Metabolism: Contributions by the Cologne Sports Medicine Group in the 1970s and 1980s. Front Physiol 13: 899670, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Warburton DE, Nicol CW, and Bredin SS. Health benefits of physical activity: the evidence. CMAJ 174: 801–809, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wasfy MM, and Baggish AL. Exercise Dose in Clinical Practice. Circulation 133: 2297–2313, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Wasserman K, Beaver WL, and Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 81: II14–30, 1990. [PubMed] [Google Scholar]
- 362.Wasserman K, and Koike A. Is the anaerobic threshold truly anaerobic? Chest 101: 211S–218S, 1992. [DOI] [PubMed] [Google Scholar]
- 363.Wasserman K, and McIlroy MB. Detecting the Threshold of Anaerobic Metabolism in Cardiac Patients during Exercise. Am J Cardiol 14: 844–852, 1964. [DOI] [PubMed] [Google Scholar]
- 364.Wasserman K, Stringer WW, Casaburi R, Koike A, and Cooper CB. Determination of the anaerobic threshold by gas exchange: biochemical considerations, methodology and physiological effects. Z Kardiol 83 Suppl 3: 1–12, 1994. [PubMed] [Google Scholar]
- 365.Wasserman K, Whipp BJ, Koyl SN, and Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol 35: 236–243, 1973. [DOI] [PubMed] [Google Scholar]
- 366.Webb KL, Dominelli PB, Baker SE, Klassen SA, Joyner MJ, Senefeld JW, and Wiggins CC. Influence of High Hemoglobin-Oxygen Affinity on Humans During Hypoxia. Front Physiol 12: 763933, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Webb KL, Elshaer AN, Dominelli PB, Senefeld JW, Hammer SM, Baker SE, Shepherd JRA, Roy TK, Joyner MJ, and Wiggins CC. Muscle oxygenation during normoxic and hypoxic cycling exercise in humans with high-affinity haemoglobin. Exp Physiol 107: 854–863, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Weber RE, Jessen TH, Malte H, and Tame J. Mutant hemoglobins (alpha 119-Ala and beta 55-Ser): functions related to high-altitude respiration in geese. J Appl Physiol (1985) 75: 2646–2655, 1993. [DOI] [PubMed] [Google Scholar]
- 369.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, and Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378: 1244–1253, 2011. [DOI] [PubMed] [Google Scholar]
- 370.Westerterp KR, Saris WH, van Es M, and ten Hoor F. Use of the doubly labeled water technique in humans during heavy sustained exercise. J Appl Physiol (1985) 61: 2162–2167, 1986. [DOI] [PubMed] [Google Scholar]
- 371.Whipp BJ. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol 55: 1638–1639, 1983. [DOI] [PubMed] [Google Scholar]
- 372.Williams PT. High-density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Engl J Med 334: 1298–1303, 1996. [DOI] [PubMed] [Google Scholar]
- 373.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners' Health Study. Arch Intern Med 157: 191–198, 1997. [PMC free article] [PubMed] [Google Scholar]
- 374.Williams PT, Blanche PJ, and Krauss RM. Behavioral versus genetic correlates of lipoproteins and adiposity in identical twins discordant for exercise. Circulation 112: 350–356, 2005. [DOI] [PubMed] [Google Scholar]
- 375.Wing RR, and Hill JO. Successful weight loss maintenance. Annu Rev Nutr 21: 323–341, 2001. [DOI] [PubMed] [Google Scholar]
- 376.Wyatt HR, Grunwald GK, Mosca CL, Klem ML, Wing RR, and Hill JO. Long-term weight loss and breakfast in subjects in the National Weight Control Registry. Obes Res 10: 78–82, 2002. [DOI] [PubMed] [Google Scholar]
- 377.Yousef MK, Dill DB, Vitez TS, Hillyard SD, and Goldman AS. Thermoregulatory responses to desert heat: age, race and sex. J Gerontol 39: 406–414, 1984. [DOI] [PubMed] [Google Scholar]