Abstract
Cryptosporidium parvum is an important diarrhea-causing protozoan parasite of immunocompetent and immunocompromised hosts. Immunoglobulin A (IgA) has been implicated in resistance to mucosal infections with bacteria, viruses, and parasites, but little is known about the role of IgA in the control of C. parvum infection. We assessed the role of IgA during C. parvum infection in neonatal mice. IgA-secreting hybridomas were developed by using Peyer’s patch lymphocytes from BALB/c mice which had been orally inoculated with viable C. parvum oocysts. Six monoclonal antibodies (MAbs) were selected for further study based on indirect immunofluorescence assay reactivity with sporozoite and merozoite pellicles and the antigen (Ag) deposited on glass substrate by gliding sporozoites. Each MAb was secreted in dimeric form and recognized a 23-kDa sporozoite Ag in Western immunoblots. The Ag recognized comigrated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis with P23, a previously defined neutralization-sensitive zoite pellicle Ag. MAbs were evaluated for prophylactic or therapeutic efficacy against C. parvum, singly and in combinations, in neonatal BALB/c mice. A combination of two MAbs given prophylactically prior to and 12 h following oocyst challenge reduced the number of intestinal parasites scored histologically by 21.1% compared to the numbers in mice given an isotype-matched control MAb (P < 0.01). Individual MAbs given therapeutically in nine doses over a 96-h period following oocyst challenge increased efficacy against C. parvum infection. Four MAbs given therapeutically each reduced intestinal infection 34.4 to 42.2% compared to isotype-matched control MAb-treated mice (P < 0.05). One MAb reduced infection 63.3 and 72.7% in replicate experiments compared to isotype-matched control MAb-treated mice (P < 0.0001). We conclude that IgA MAbs directed to neutralization-sensitive P23 epitopes may have utility in passive immunization against murine C. parvum infection.
Since the first case of human cryptosporidiosis was described in 1976, the coccidian parasite Cryptosporidium parvum has become recognized as an important diarrhea-causing agent worldwide (13, 41). Immunocompromised individuals such as AIDS patients are particularly susceptible and exhibit an unrelenting infection which may progress to death (13, 41). No commercially available antiparasite chemotherapy is consistently effective in treating such patients (6). Passive immunization with antibodies (Abs) against whole C. parvum organisms has variable efficacy in immunocompromised or neonatal hosts (1, 5, 9, 12, 24, 25, 31, 37, 39, 40, 42). Recovery from and resistance to cryptosporidiosis require principally cellular, but also humoral, immune components in immunocompetent hosts (31, 45). Despite anti-C. parvum Ab responses, AIDS patients with cryptosporidiosis fail to clear the infection (4). However, the relative success of orally administered Abs to immunodeficient hosts suggests that passive humoral immunization can control intestinal C. parvum infection (5, 24, 25, 31, 32, 39, 40, 42, 45).
Although C. parvum is a mucosal pathogen, the role of immunoglobulin A (IgA) during infection has only recently received attention. IgA to 15- to 17-, 23-, 26-, and 33-kDa antigens of C. parvum sporozoites has been detected in intestinal washes and serum from infected humans and mice (4, 30, 36). In addition, the level of parasite-specific IgA in serum, saliva, and feces was higher in AIDS patients with chronic C. parvum infection than in uninfected AIDS patients or normal individuals (4, 10, 14). While some studies concluded that IgA has little or no protective effect against cryptosporidiosis (4, 10), IgA responses to neutralization-sensitive epitopes have not been evaluated in such patients (31). Epitope specificity of Abs is clearly important in neutralization of C. parvum zoites (reviewed in reference 31). The epitope specificity of secretory IgA responses in AIDS patients may be defective; Abs against neutralization-sensitive epitopes either may not be generated or may be insufficient to control C. parvum in the presence of cellular and/or other immune dysfunctions (14).
IgA has been associated with resistance to a number of mucosal pathogens (8, 22, 23, 29, 44). For example, treatment with IgA MAbs specific to rotavirus, Sendai virus, Vibrio cholerae, or Salmonella typhimurium controlled otherwise lethal challenges in mice (8, 19, 22, 44). Because IgA conferred protection against these mucosal pathogens and Ag-specific IgA responses occur in hosts with cryptosporidiosis, we hypothesized that IgA directed to neutralization-sensitive epitopes may be useful in passive immunization against C. parvum. To determine whether IgA can influence the course of C. parvum infection, we produced dimeric IgA MAbs to P23, a previously defined C. parvum Ag containing neutralization-sensitive epitopes (1, 3, 21, 26). Here we report that dimeric anti-P23 IgA MAbs have efficacy against intestinal C. parvum infection in neonatal mice.
MATERIALS AND METHODS
Parasite isolation and Ag preparation.
The Iowa isolate of C. parvum (originally obtained from H. Moon, Ames, Iowa) was used in the present study and maintained by passage in 2-day-old Holstein bull calves (2, 33). Oocysts were isolated from feces by sequential centrifugation involving discontinuous sucrose and isopycnic Percoll gradients as previously described (2). Oocysts were stored in 2.5% (wt/vol) KCr2O7 at 4°C for up to 3 months prior to use.
Prior to excystation, oocysts were washed with sterile phosphate-buffered saline (PBS) containing 1.75% (wt/vol) sodium hypochlorite, followed by sterile PBS (4°C). Oocysts were then incubated (45 min, 37°C) in Hanks’ balanced salt solution containing 0.1% (wt/vol) taurocholic acid. Excysted sporozoites were isolated by DEAE-cellulose anion-exchange chromatography as previously described (33). For use in mouse immunization, enzyme-linked immunosorbent assays (ELISAs), and Western immunoblotting, sporozoites were disrupted by freeze-thaw cycles and sonication (20 10-s pulses, 1-min intervals) in lysis buffer (50 mM Tris, 5.0 mM EDTA, 0.1 mM Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK], 5.0 mM iodoacetamide, 1.0 mM phenylmethylsulfonyl fluoride, 0.01 mM leupeptin, 0.01 mM pepstatin, 1.0% (wt/vol) octyl glucoside) and then centrifuged (10,000 × g, 30 min) to remove the insoluble fraction. For mouse immunizations and ELISA, the supernatant was dialyzed against PBS (12,000 to 14,000 molecular weight exclusion limit, 4°C) and the protein concentration was determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). Sporozoite Ag was stored at −80°C prior to use.
Merozoites were isolated by Percoll density gradient centrifugation of intestinal contents from neonatal BALB/c mice at 65 h postinoculation with oocysts (32).
IgA MAb production and characterization.
Six-week-old BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) were housed in microisolator cages and maintained with 12-h photoperiod cycles. Mice were inoculated per os twice at 3-week intervals with 5 × 104 viable C. parvum oocysts. Three weeks following the second oocyst inoculation, mice were injected intraperitoneally with 5 μg of sporozoite Ag in PBS. Sera were screened by ELISA and indirect immunofluorescence assay (IFA) as described below to determine anti-C. parvum IgA antibody titers. Positive sera were stored at −80°C until used as controls in immunoassays.
Four days after the final immunization, mice were euthanized by CO2 inhalation. Peyer’s patches were then dissected and removed aseptically, pooled, and incubated (1 h, 37°C) in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini Bioproducts), 0.1% (wt/vol) collagenase type IV (Sigma), and 100 U of penicillin, 100 μg streptomycin, and 0.25 μg of amphotericin B (Sigma) per ml. Peyer’s patches were then transferred into a glass homogenizer and gently disrupted. Peyer’s patch cells were fused with SP2/O myeloma cells and resuspended in monocyte-macrophage-thymocyte-conditioned medium as previously described (11). Hybridomas producing IgA antibodies were identified by ELISA. Briefly, plates were coated (12 h, 4°C) with an affinity-purified goat anti-mouse IgA Ab (5 μg/ml; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) and blocked with 1% (wt/vol) nonfat dry milk in PBS (30 min, 21°C). Hybridoma supernatants were added to plates, which were then incubated (2 h, 37°C), washed, and incubated (2 h, 37°C) with a peroxidase-labelled goat anti-mouse IgA Ab (Kirkegaard & Perry). Following washing, the plates were developed with 2,2′-azino-di(3-ethylbenzthiazoline sulfate) (ABTS; Kirkegaard & Perry) and A410 was determined with an ELISA reader (Dynatech 700; Dynatech Laboratories, Inc., Chantilly, Va.). Control wells were incubated with 0.5-μg/ml monomeric IgA (IgA-producing myeloma MOPC-318; Sigma) or sera from fusion mice (1:10 to 1:1,600 dilution in PBS) and processed identically. Hybridomas secreting IgA were then assessed for sporozoite specificity by ELISA and IFA as follows.
ELISA plates were coated with sporozoite Ag (5 μg/ml of carbonate buffer), blocked, and incubated with IgA-positive hybridoma supernatants. Immune serum and IgA Ab MOPC-318 were included as controls. Plates were then washed, incubated with peroxidase-labelled goat anti-mouse IgA Ab (Kirkegaard & Perry), and developed with ABTS. Hybridomas secreting C. parvum-specific IgA were assayed further by IFA to identify patterns of binding to sporozoites and merozoites. For IFA, purified sporozoites or merozoites were gently heat fixed in multiwell glass slides that had previously been treated with poly-l-lysine (0.01%, wt/vol; Sigma). Hybridoma supernatants, along with immune and preimmune control sera (1:10 dilution), were added to individual wells and incubated (40 min, 37°C) in a humidified chamber. Following washing, each well was incubated sequentially (40 min, 37°C) with a biotinylated rat anti-mouse IgA MAb (Pharmingen, San Diego, Calif.) and streptavidin-fluorescein (Pharmingen) and then examined with an Olympus epifluorescence microscope. Following IFA evaluation, antisporozoite and antimerozoite IgA-secreting hybridomas were cloned three times by limiting dilution. A single IgA-secreting hybridoma (92.07t) not recognizing C. parvum was selected for use as an isotype control and cloned. Cloned hybridomas were adapted to serum-free medium (Ultradoma; Biowhittaker) and then cryopreserved. Ascites was produced for all IgA MAbs as previously described (11). The IgA MAb ascites titer against C. parvum sporozoites was determined by IFA. The IgA MAb concentration in ascites was determined by capture ELISA using standard concentrations of IgA MOPC-318 and linear regression analysis (35).
To determine the molecular weights of sporozoite Ags recognized by anti-C. parvum MAbs, Western immunoblotting was performed as follows. Soluble sporozoite Ag was boiled (1 min) in sample buffer (62.5 mM Tris [pH 6.8], 2.0% [wt/vol] sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol, 5% [vol/vol] 2-mercaptoethanol, 0.001% [wt/vol] bromophenol blue), separated by 5 to 20% gradient SDS-polyacrylamide gel electrophoresis (2 μg of Ag per lane), and transferred (4.5 h, 30 mV) to nitrocellulose. Molecular mass standards consisted of myosin (200 kDa), β-galactosidase (116 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (69 kDa), ovalbumin (46 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (21.5 kDa), and lysozyme (14.3 kDa) (Bio-Rad, Hercules, Calif.). Nitrocellulose membranes were blocked (3% [wt/vol] nonfat dry milk) and incubated separately (2 h, 37°C) with supernatant-derived anti-C. parvum IgA MAbs, an isotype-matched control Ab (MOPC-318 or MAb 92.07t), or anti-C. parvum P23 MAb C6B6 (IgG1) (1, 3). Following washing, nitrocellulose lanes were incubated (2 h, 37°C) with affinity-purified, peroxidase-labelled goat anti-mouse IgA or IgG Abs (Kirkegaard & Perry), washed, and developed with 4-chloronaphthol.
The monomeric-polymeric state of IgA MAbs was evaluated by Western immunoblotting. Briefly, fetal bovine serum-free hybridoma supernatants were separated by nonreducing 3 to 15% gradient SDS-polyacrylamide gel electrophoresis and electrotransferred to nitrocellulose membranes (22). Membranes were incubated (2 h, 37°C) with peroxidase-labelled rat anti-mouse IgA MAb (Pharmingen) and developed with 4-chloronaphthol.
Experimental design. (i) Prophylactic effect of IgA MAbs on intestinal infection.
Six anti-C. parvum IgA MAbs (G9H4, H8H6, H8H2, F3H6, H8H12, and G9H9) were evaluated for a prophylactic effect against a C. parvum oocyst challenge in neonatal BALB/c mice (Harlan/Bioproducts for Science, Indianapolis, Ind.) as follows. Groups of 10 5- to 6-day-old BALB/c mice were inoculated with 104 oocysts per mouse intragastrically by using a blunt, curved 30-gauge needle. Four hours prior to challenge and 12 h postchallenge, mice received combinations of ascites containing IgA MAbs G9H4 and H8H6, H8H2 and F3H6, or H8H12 and G9H9. Control groups received isotype-matched control MAb 92.07t ascites or no treatment. The rationale for selection of MAb combinations was based on preliminary data obtained from in vitro MAb binding inhibition assays to define epitope specificity. MAbs selected for combinations appeared to recognize distinct P23 epitopes in these assays (11a). MAbs were administered intraperitoneally (125 μg of each MAb in a 100-μl volume) and orally (125 μg of each MAb in a 100 μl volume) at each treatment. Each mouse received a total, cumulative MAb combination dose of 1,000 μg. At 96 h postchallenge, mice were euthanized by CO2 inhalation. The ileum (one-inch section) and cecum were collected, fixed in 10% neutral buffered formalin, and processed for histopathology. Infections were scored histologically as previously described (33).
(ii) Treatment effect of IgA MAbs on intestinal infection.
To determine treatment effect, groups of 10 5- to 6-day-old BALB/c mice were inoculated with oocysts as described above and treated with a single MAb as follows. At the time of oocyst inoculation, 3 h later, and every 12 h thereafter until necropsy at 96 h, mice received 100 μl of ascites containing 100 μg of individual IgA MAb G9H4, H8H6, H8H2, F3H6, H8H12, or G9H9 per os (total, cumulative MAb dose of 900 μg in nine doses). Control mice were treated identically with MAb 92.07t. At 96 h postchallenge, mice were euthanized by CO2 inhalation. The cecum and 1-in. portions of the proximal colon, the ileum, and the terminal jejunum were removed, fixed longitudinally in 10% neutral buffered formalin, and processed for histopathologic evaluation. Infection scores were determined histologically as previously described (33).
Mean infection scores for test and control groups in each experiment were analyzed by Student’s one-tailed t test for significant differences (35).
RESULTS
Mucosal immunization with C. parvum results in a preferential Peyer’s patch IgA response to P23.
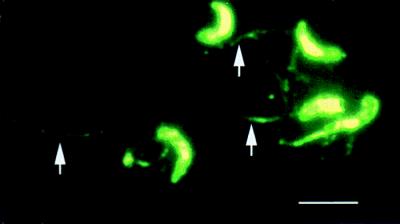
From 20 fusions, six anti-C. parvum IgA MAb-producing hybridomas (G9H4, H8H6, H8H2, F3H6, H8H12, and G9H9) were selected for further study based on their strong reactivity with sporozoites by ELISA and IFA. By IFA, each MAb bound diffusely to the pellicle of both sporozoites (Fig. 1) and merozoites (data not shown). In addition, each MAb also bound to antigen trails deposited on a glass substrate over the course traveled by sporozoites during gliding motility (Fig. 1).
FIG. 1.
Sporozoites stained by IFA with IgA MAb G9H4. Note the reactivity of MAb G9H4 with the sporozoite pellicle and Ag trails (arrows). The staining patterns of MAbs G9H4, H8H12, G9H9, H8H2, F3H6, and H8H6 were the same. Bar, 5 μm.
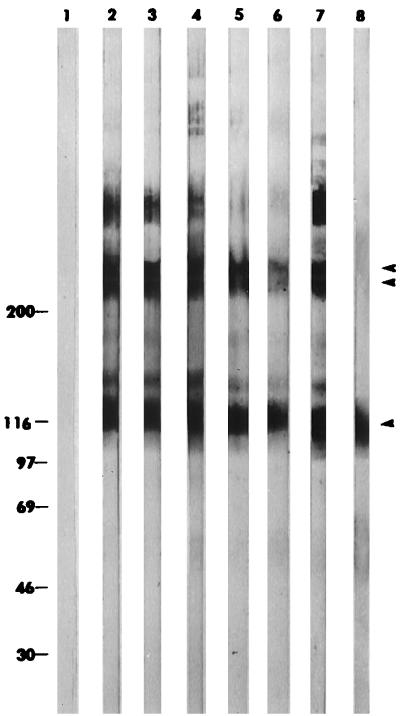
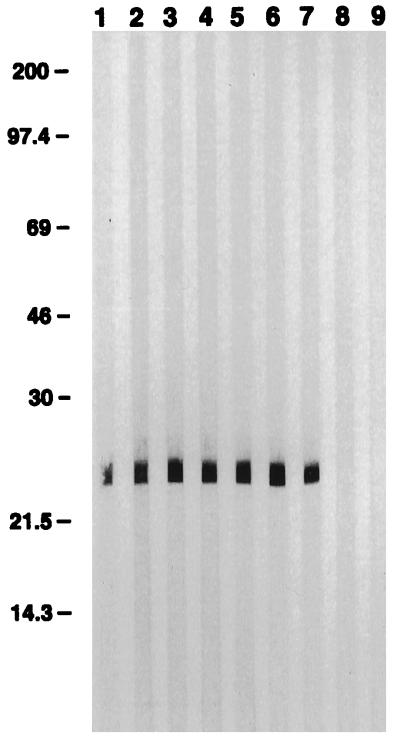
Hybridomas maintained in serum-free medium secreted IgA MAbs in dimeric form, as demonstrated by Western immunoblot analysis of supernatants separated under nonreducing conditions (Fig. 2). Each MAb contained monomers and polymers. All six MAbs recognized a 23-kDa C. parvum sporozoite molecule in a Western immunoblot (Fig. 3). This molecule comigrated with P23, previously defined by MAb C6B6 (1, 3).
FIG. 2.
Western immunoblot demonstrating the monomeric-polymeric states of anti-C. parvum IgA MAbs. Lanes: 1, SP2/O hybridoma supernatant; 2, F3H6; 3, G9H4; 4, G9H9; 5, H8H2; 6, H8H6; 7, H8H12; 8, MOPC-318 monomeric IgA control. Double bands typical of dimeric IgA at >200 kDa are indicated by the paired arrowheads. IgA monomers migrating at 116 to 120 kDa are indicated by the single arrowhead. The values on the left are molecular masses in kilodaltons.
FIG. 3.
Western immunoblot demonstrating reactivity of anti-C. parvum IgA MAbs with sporozoite P23. Lanes: 1, F3H6; 2, H8H2; 3, H8H6; 4, H8H12; 5, G9H4; 6, G9H9; 7, C6B6; 8, MOPC-318 monomeric IgA control; 9, dimeric IgA isotype-matched control MAb 92.07t. The values on the left are molecular masses in kilodaltons.
IgA MAbs significantly reduce C. parvum infection in mice.
A combination of MAbs G9H4 and H8H6 administered prophylactically to mice significantly reduced the mean infection score compared to that of mice receiving an isotype-matched control MAb (P = 0.008; 21.1% reduction) (Table 1). A combination of MAbs H8H2 and F3H6 or H8H12 and G9H9 did not significantly reduce infection (Table 1). Because neutralization of C. parvum by MAbs is dependent on time and MAb concentration (27, 31), a second experiment was performed in which the number and duration of treatments with individual MAbs were increased. In the second experiment, all MAb treatments were given per os.
TABLE 1.
Prophylactic effect of oral and intraperitoneal administration of anti-P23 IgA MAb combinations against C. parvum challenge in neonatal mice
| Treatment | Mean infection score ± SDa | P valueb |
|---|---|---|
| None | 5.9 ± 1.2 | >0.5 |
| 92.07t | 5.2 ± 0.8 | |
| G9H4 + H8H6 | 4.1 ± 0.9 | 0.008 |
| H8H12 + G9H9 | 5.0 ± 0.7 | >0.25 |
| H8H2 + F3H6 | 4.8 ± 1.6 | >0.25 |
Scores are based on ileum and cecum infection levels (0, absence of infection; 1, 1 to 33% of mucosa parasitized; 2, 34 to 66% of mucosa parasitized; 3, greater than 66% of mucosa parasitized). Cumulative scores that included both intestinal regions were calculated for each mouse. The maximum cumulative score per mouse was 6.
Compared to C. parvum-challenged mice given isotype-matched control IgA MAb 92.07t.
Individual anti-P23 IgA MAbs H8H2, F3H6, G9H9, and H8H6 significantly reduced mean intestinal infection scores (range, 34.4 to 42.2%) compared to those of isotype-matched control MAb-treated mice (Table 2). MAb G9H4 reduced infection 63.3 and 72.7% in replicate experiments compared to isotype-matched control MAb-treated mice (P < 0.0001; Table 2). MAb H8H12 did not reduce the mean infection score (Table 2).
TABLE 2.
Treatment effect of multiple oral doses of individual anti-P23 IgA MAbs on C. parvum infection in neonatal mice
| Treatment per os | Mean infection score ± SDa (P value)b
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| 92.07t | 9.0 ± 1.0 | 8.8 ± 0.9 |
| H8H2 | 5.2 ± 1.1 (<0.001) | NDc |
| H8H12 | 9.6 ± 2.0 (>0.5) | ND |
| F3H6 | 5.8 ± 2.0 (<0.025) | 5.4 ± 1.1 (<0.05) |
| G9H9 | 5.2 ± 1.8 (<0.005) | ND |
| G9H4 | 3.3 ± 1.1 (<0.0001) | 2.4 ± 1.8 (<0.0001) |
| H8H6 | 5.9 ± 1.8 (<0.005) | ND |
Scores are based on infection levels in the terminal jejunum, ileum, cecum, and proximal colon (0, absence of infection; 1, 1 to 33% of mucosa parasitized; 2, 34 to 66% of mucosa parasitized; 3, greater than 66% of mucosa parasitized). Cumulative scores that included each intestinal region were calculated for each mouse. The maximum cumulative score per mouse was 12.
Compared to C. parvum-infected mice treated with isotype-matched control IgA MAb 92.07t per os.
ND, not done.
DISCUSSION
The anti-C. parvum P23 IgA MAbs presented herein were produced following a combination of oral and intraperitoneal immunizations. One or both of these routes of immunization preferentially induced an anti-P23 IgA response in Peyer’s patches. Other studies have suggested that P23 is immunodominant based on serum IgG responses following infection (21, 31). The six IgA MAbs selected recognized surface P23 on sporozoites and merozoites, as well as in sporozoite Ag deposited in trails. Sporozoite Ag trails have been identified with MAbs against GP15 (16, 38) and P23 (3, 38) by IFA. Ags deposited in trails may be important functional target Ags because they are deposited during locomotion and are shed during invasion of host cells (3, 16, 38). Spleen-derived MAbs against GP15 (monomeric IgA) and P23 (IgG1) have been shown to decrease infection levels in mouse models, indicating that GP15 and P23 contain neutralization-sensitive epitopes (1, 26, 31, 37). Because P23 is conserved among geographically diverse bovine and human C. parvum isolates (26), present in both infectious zoite stages, deposited during zoite motility, and known to contain neutralization-sensitive epitopes, it may be a biologically relevant Ag which can be targeted for immunological intervention. Results of the present study support the relevance of P23 and suggest that dimeric IgA targeted to this Ag may be a functional mucosal immune response to infection.
While Ab responses to C. parvum have been described in humans, mice, sheep, cattle, and other mammals, relatively few studies have examined mucosal IgA responses (4, 10, 14, 30, 36; reviewed in reference 31). IgA directed to a 15- to 17-kDa C. parvum antigen has been observed in the intestines and sera of infected mice (30). Anti-C. parvum IgA has been demonstrated in the serum and feces of AIDS patients with cryptosporidiosis (4, 10). The saliva of human immunodeficiency virus-positive pre-AIDS patients who cleared a C. parvum infection had higher titers of specific IgA than did that of AIDS patients with persistent cryptosporidiosis, suggesting that C. parvum-specific secretory IgA may contribute to recovery from cryptosporidiosis (14). Results presented here support this hypothesis.
The utility of Abs in the control of intestinal cryptosporidiosis is exemplified by passive immunotherapy studies with polyclonal Abs and MAbs (reviewed in reference 31). Anti-C. parvum MAbs, and polyclonal Abs in hyperimmune hen egg yolk and secretory IgG1-rich hyperimmune bovine colostrum, have been efficacious in the control of intestinal cryptosporidiosis in immunologically immature or immunocompromised rodent models (1, 5, 9, 12, 25, 26, 32, 34, 37). Hyperimmune polyclonal Ab preparations have had variable efficacy against intestinal C. parvum infection in immunocompromised humans (24, 39, 40, 42).
Hepatobiliary cryptosporidiosis is a common complication in AIDS patients having persistent intestinal C. parvum infections (6, 7, 15, 20, 28, 41, 43). While oral Ab-based immunotherapy has shown efficacy against intestinal infection, this approach is unlikely to be effective against hepatobiliary cryptosporidiosis (5, 25). Further, hepatobiliary cryptosporidiosis may provide a reservoir of infection which can contribute to relapse of intestinal infection following clearance with Ab-based immunotherapy (39, 40). Parenterally administered dimeric IgA migrates to hepatobiliary and other extraintestinal mucosal sites which are inaccessible to orally administered Abs. Additionally, IgA acquires a secretory component during migration, thereby enhancing its resistance to the harsh mucosal environment (17, 23). These properties may confer a therapeutic advantage on IgA-based passive immunization against C. parvum infection. Studies assessing the delivery and efficacy of anti-C. parvum dimeric IgA MAbs in hepatobiliary cryptosporidiosis are in progress in our laboratories. In preliminary studies, significant anti-C. parvum IgA MAb concentrations and reduction of infection have been observed in the intestinal tract following parenteral anti-C. parvum IgA MAb administration in mice with cryptosporidiosis (11a).
IgA prevents access of pathogens to mucosal surfaces, precipitates pathogen agglutination and clearance, and effectively protects animals against otherwise lethal infections (8, 17, 19, 22, 29, 44). Results presented here demonstrate that dimeric IgA MAbs can reduce infection by an opportunistic intestinal protozoan parasite. While cellular immunity is required to overcome C. parvum infection in immunocompetent patients (31, 45), IgA directed to neutralization-sensitive zoite epitopes may have utility in passive immunization against cryptosporidiosis in immunocompromised hosts.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AI 30233 and AI 39203 from the National Institutes of Health, Bethesda, Md., and IgX Ltd., Buckinghamshire, England.
MAb C6B6 was kindly provided by Charles R. Sterling (University of Arizona, Tucson) and Michael Arrowood (Centers for Disease Control and Prevention, Atlanta, Ga.). Excellent technical assistance was provided by Patricia Figuli, John D. Palting, Nannete C. Westhof, Rebecca C. Langer, Brian Curran, and Jennifer Hensel.
REFERENCES
- 1.Arrowood M J, Mead J R, Mahrt J L, Sterling C R. Effects of immune colostrum and orally administered antisporozoite monoclonal antibodies on the outcome of Cryptosporidium parvum infections in neonatal mice. Infect Immun. 1989;57:2283–2288. doi: 10.1128/iai.57.8.2283-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 3.Arrowood M J, Sterling C R, Healey M C. Immunofluorescent microscopical visualization of trails left by gliding Cryptosporidium parvum sporozoites. J Parasitol. 1991;77:315–317. [PubMed] [Google Scholar]
- 4.Banhamou Y, Kapal N, Hoang C, Matta H, Meillet D, Magne D, Raphael M, Gentilini M, Opolon P, Gobert J G. Inefficacy of intestinal secretory immune response to Cryptosporidium in acquired immunodeficiency syndrome. Gastroenterology. 1995;108:627–635. doi: 10.1016/0016-5085(95)90433-6. [DOI] [PubMed] [Google Scholar]
- 5.Bjorneby J M, Hunsaker B D, Riggs M W, Perryman L E. Monoclonal antibody immunotherapy in nude mice persistently infected with Cryptosporidium parvum. Infect Immun. 1991;59:1172–1176. doi: 10.1128/iai.59.3.1172-1176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blagburn B L, Soave R. Prophylaxis and chemotherapy. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 111–128. [Google Scholar]
- 7.Bouche H, Housset C, Dumont J, Carnot F, Menu Y, Aveline B, Belghiti J, Boboc B, Erlinger S, Berthelot P, Pol S. AIDS-related cholangitis: diagnostic features and courses in 15 patients. J Hepatol. 1993;17:34–39. doi: 10.1016/s0168-8278(05)80518-5. [DOI] [PubMed] [Google Scholar]
- 8.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 9.Cama V A, Sterling C R. Hyperimmune hens as a novel source of anti-Cryptosporidium antibodies suitable for passive immune transfer. J Protozool. 1991;38:42s–43s. [PubMed] [Google Scholar]
- 10.Cozon G, Biron F, Jeannin M, Cannella D, Revillard J P. Secretory IgA antibodies to Cryptosporidium parvum in AIDS patients with chronic cryptosporidiosis. J Infect Dis. 1994;169:696–699. doi: 10.1093/infdis/169.3.696. [DOI] [PubMed] [Google Scholar]
- 11.Enriquez F J, Bradley-Dunlop D, Joens L. Increased proportion of antigen-specific antibody-producing hybridomas following an in vitro immunization with in vivo immunized mouse spleen cells. Hybridoma. 1991;10:745–751. doi: 10.1089/hyb.1991.10.745. [DOI] [PubMed] [Google Scholar]
- 11a.Enriquez, F. J. Unpublished data.
- 12.Fayer R, Perryman L E, Riggs M W. Hyperimmune bovine colostrum neutralizes Cryptosporidium sporozoites and protects mice against oocyst challenge. Infect Immun. 1989;75:151–153. [PubMed] [Google Scholar]
- 13.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 1–42. [Google Scholar]
- 14.Flanigan T P. Human immunodeficiency virus infection and cryptosporidiosis: protective immune responses. Am J Trop Med Hyg. 1994;50:S29–S35. [PubMed] [Google Scholar]
- 15.Godwin T A. Cryptosporidiosis in the acquired immunodeficiency syndrome: a study of 15 autopsy cases. Hum Pathol. 1991;22:1215–1224. doi: 10.1016/0046-8177(91)90103-v. [DOI] [PubMed] [Google Scholar]
- 16.Gut J M, Nelson R G. Cryptosporidium parvum sporozoites deposit trails of 11A5 antigen during gliding locomotion and shed 11A5 antigen during invasion of MDCK cells in vitro. J Eukaryot Microbiol. 1994;41:42S. [PubMed] [Google Scholar]
- 17.Kraehenbuhl J P, Neutra M. Transepithelial transport and mucosal defense: secretion of IgA. Trends Curr Biol. 1992;2:170–174. doi: 10.1016/0962-8924(92)90036-m. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood D N J, Weber J N. Parasite infections in AIDS. Parasitol Today. 1989;5:310–315. doi: 10.1016/0169-4758(89)90121-x. [DOI] [PubMed] [Google Scholar]
- 19.Mazanec M B, Nedruc J G, Lamm M E. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J Virol. 1987;61:2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGowan I. The natural history of cryptosporidial diarrhoea in HIV-infected patients. AIDS. 1993;7:349–354. doi: 10.1097/00002030-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Mead J R, Arrowood M J, Sterling C R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988;74:135–143. [PubMed] [Google Scholar]
- 22.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M. Monoclonal secretory IgA protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neutra M R, Michetti P, Kraehenbuhl J P. Secretory immunoglobulin A. Induction, biogenesis and function. In: Johnson L R, editor. Physiology of the gastrointestinal tract. New York, N.Y: Raven Press; 1994. pp. 685–708. [Google Scholar]
- 24.Nord J M P, DiJohn D, Tzipori S, Tacket C O. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS. 1990;4:581–584. doi: 10.1097/00002030-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Perryman L E, Bjorneby J M. Immunotherapy of cryptosporidiosis in immunodeficient animal models. J Protozool. 1991;38:98S–100S. [PubMed] [Google Scholar]
- 26.Perryman L E, Jasmer D P, Riggs M W, Bohnet S G, McGuire T C, Arrowood M J. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 27.Perryman L E, Riggs M W, Mason P H, Fayer R. Kinetics of Cryptosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect Immun. 1990;58:257–259. doi: 10.1128/iai.58.1.257-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen C. Cryptosporidiosis in patients infected with the human immunodeficiency virus. Clin Infect Dis. 1992;15:903–909. doi: 10.1093/clind/15.6.903. [DOI] [PubMed] [Google Scholar]
- 29.Renegar K B, Small P A J. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 30.Reperant J M, Naciri M, Chardes T, Bout D T. Immunological characterization of a 17-kDa antigen from Cryptosporidium parvum recognized early by mucosal IgA antibodies. Fed Eur Microbiol Soc Microbiol Lett. 1992;99:7–14. doi: 10.1016/0378-1097(92)90280-2. [DOI] [PubMed] [Google Scholar]
- 31.Riggs M W. Immunology: host response and development of passive immunotherapy and vaccines. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press, Inc.; 1997. pp. 129–162. [Google Scholar]
- 32.Riggs M W, Cama V A, Leary H L, Jr, Sterling C R. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect Immun. 1994;62:1927–1939. doi: 10.1128/iai.62.5.1927-1939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riggs M W, Perryman L E. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun. 1987;55:2081–2087. doi: 10.1128/iai.55.9.2081-2087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riggs M W, Stone A L, Yount P A, Langer R C, Arrowood M J, Bentley D L. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol. 1997;158:1787–1795. [PubMed] [Google Scholar]
- 35.Snedecor G W, Cochran W G. Statistical methods. Ames: Iowa State University Press; 1980. pp. 3–459. [Google Scholar]
- 36.Tarazona R, Lally N C, Dominguez-Carmona M, Blewett D A. Characterization of IgA responses in mice infected with Cryptosporidium parvum. Int J Parasitol. 1997;27:417–423. doi: 10.1016/s0020-7519(96)00189-0. [DOI] [PubMed] [Google Scholar]
- 37.Tilley M, Upton S E, Fayer R, Barta J R, Chrisp C E, Freed P S, Blagburn B L, Anderson B C, Barnard S M. Identification of a 15-kilodalton surface glycoprotein on sporozoites of Cryptosporidium parvum. Infect Immun. 1991;59:1002–1007. doi: 10.1128/iai.59.3.1002-1007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilley M, Upton S J. Both CP15 and CP25 are left as trails behind gliding sporozoites of Cryptosporidium parvum (apicomplexa) Fed Eur Microbiol Soc Microbiol Lett. 1994;120:275–278. doi: 10.1111/j.1574-6968.1994.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 39.Tzipori S, Roberton D, Chapman C. Remission of diarrhoea due to cryptosporidiosis in an immunodeficient child treated with hyperimmune bovine colostrum. Br Med J. 1986;293:1276–1277. doi: 10.1136/bmj.293.6557.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzipori S, Roberton D, Cooper D A, White L. Chronic cryptosporidial diarrhoea and hyperimmune cow colostrum. Lancet. 1987;ii:344–347. doi: 10.1016/s0140-6736(87)90944-5. [DOI] [PubMed] [Google Scholar]
- 41.Ungar B L P. Cryptosporidiosis in humans (Homo sapiens) In: Dubey J P, Fayer R, Speer C A, editors. Cryptosporidiosis in man and animals. Baton Rouge, La: CRC Press, Inc.; 1990. pp. 59–82. [Google Scholar]
- 42.Ungar B L P, Ward D J, Fayer R, Quinn C A. Cessation of Cryptosporidium associated diarrhea in an acquired immunodeficiency syndrome patient after treatment with hyperimmune bovine colostrum. Gastroenterology. 1990;98:486–489. doi: 10.1016/0016-5085(90)90842-o. [DOI] [PubMed] [Google Scholar]
- 43.Vakil N B, Schwartz S M, Buggy B P, Brummitt C F, Kherellah M, Letzer D M, Gilson I H, Jones P G. Biliary cryptosporidiosis in HIV-infected people after the waterborne outbreak of cryptosporidiosis in Milwaukee. N Engl J Med. 1996;334:19–23. doi: 10.1056/NEJM199601043340104. [DOI] [PubMed] [Google Scholar]
- 44.Winner L S, Mack J, Weltzin R A, Mekalanos J J, Kraehenbuhl J P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zu S X, Fang G D, Fayer R, Guerrant R L. Cryptosporidiosis: pathogenesis and immunology. Parasitol Today. 1992;8:24–27. doi: 10.1016/0169-4758(92)90307-n. [DOI] [PubMed] [Google Scholar]