Abstract
Headache is a common symptom of influenza infection; however, its causes and consequences remain uncertain. In this manuscript, we analyzed which demographic and clinical factors were associated with the presence of headache during the course of influenza infection and whether patients with headache had a different prognosis, evaluated by need of hospitalization, sick leave or school absenteeism. The influence study (NCT05704335) was an observational study that analyzed data routinely collected from the Health Sentinel Network between 2010 and 2020. During the study period, 7832 cases were considered, among which, 5275 (67.4%) reported headache. The presence of headache was independently associated with myalgia (2.753; 95%CI: 2.456–3.087, P < 0.001), asthenia (OR: 1.958; 95%CI: 1.732–2.214, P < 0.001), shivering (OR: 1.925; 95%CI: 1.718–2.156, P < 0.001), nasopharyngeal erythema (OR: 1.505; 95%CI: 1.293–1.753, P < 0.001), fever (OR: 1.469; 95%CI: 1.159–1.861; P = 0.001), sudden onset of symptoms (OR: 1.380; 95%CI: 1.120–1.702, p = 0.004), female sex (OR: 1.134; 95%CI: 1.023–1.257, P = 0.018), and gastrointestinal symptoms (OR: 1.169; 95%CI: 1.039–1.315; P = 0.01). Patients with headache had a sex and age adjusted lower odds of being referred to the hospital (OR: 0.463; 95%CI: 0.264–0.812, P = 0.007) and a higher odd of having a sick leave and/or school absenteeism (absenteeism (OR: 1.342; 95%CI: 1.190–1.514, P < 0.001). In conclusion, the presence of headache seems associated with symptoms caused by the innate immune response. These findings support a headache pathophysiology linked with the innate immune response. Due to the potential negative consequences and its treatable nature, clinicians should systematically evaluate it and, whenever necessary, treat it too.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01728-z.
Keywords: Headache disorders, Virus diseases, Migraine, Infections, Public Health, Vaccines
Introduction
Influenza viruses are negative-sense, single-stranded RNA viruses of the Orthomixoviridae family. They are responsible for acute respiratory infections (ARI) of the upper and lower respiratory tract [1]. Despite the existence of an effective vaccine, influenza accounts for approximately 145,000 deaths per year in the world [2].
Headache is a highly prevalent symptom of influenza, reported by 30–60% of patients [3–5]. It contributes to the disability caused by influenza, causing work and school absenteeism. Prior studies have reported that 91.7% Influenza patients miss at least one workday due to the disease [6], and 39% children miss at least one school day due to the disease [7]. Indirect costs account for about 8 billion United States Dollars (USD) per year, out of the total 11.2 USD annual cost attributed to Influenza [8].
The pathophysiology of headache in acute viral infections is unclear. Some authors argue that it is caused by the innate immune response and the cytokine release [9]. Recent studies point out that some patients with primary headache disorders exhibit higher level of selected cytokines and interleukins [10]. This hypothesis could also be supported if the prevalence of headache would be higher in patients with other systemic symptoms well known to be originated by cytokines, such as fever, myalgia or malaise [4]. In the case of other respiratory viruses, such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the presence of headache was associated to the presence of other symptoms, such as myalgia, fever or malaise [11, 12].
The objectives of this study are: 1) to analyze which demographic and clinical factors are associated with the presence of headache during the course of influenza infection; 2) to assess whether patients with headache during the course of influenza infection have a different prognosis than patients without headache, evaluated by need of hospitalization, frequency of medical leave or school absenteeism.
Methods
Study design
The influenCEF study is an observational analytic study that aims to characterize the frequency, phenotype, risk factors, duration, and pathophysiology of headache as a symptom of influenza infection. In this manuscript, we report the data from the risk-factors part of the study, with case–control study design. The study protocol was registered in ClinicalTrials.gov (NCT05704335). The study was conducted and reported in accordance with the Strengthening in the Reporting in Observational Studies in Epidemiology [13]. East Valladolid Ethics Review Board approved the study (PI 22–2884).
Study setting
The study setting was Castile and León region, the largest region of Spain, in the northwest area of the country. The study used data from the Health Sentinel Network of Castile and Leon (Red Centinela Sanitaria de Castilla y León, RCSCYL), in collaboration with the Department of Microbiology of the Hospital Clínico Universitario de Valladolid, and the National Influenza Center of Valladolid.
Study period
A total of ten consecutives surveillance seasons were investigated, from the 2010–2011 season to the 2019–2020 season. An influenza surveillance season is defined as the calendar weeks during which influenza is most likely to circulate at detectable levels (as per sentinel surveillance systems), based on historical knowledge, and usually corresponds to the period between the 40th week of the year until the 20th week of the year after [14]. The reason why the subsequent seasons were not considered was the COVID-19 pandemic, which impacted in the epidemiology and clinical presentation of Influenza, and the change in the study procedures, changing from an in-paper evaluation of patients to a digital registry in electronic health records.
Study procedures
The study was done using the data routinely collected by the RCSCYL. The Health Sentinel Network of Castile and Leon participates in the Influenza Comprehensive Surveillance Program (Programa de Vigilancia Integrada de la Gripe, PVIG) since the 1996–1997 season, which on 2020–2021 became the Acute Respiratory Infections Comprehensive Surveillance Program (Programa de Vigilancia Integrada de las Infecciones Respiratorias Agudas, VIGIRA) [15]. It is part of the Acute Respiratory Infections Surveillance System (Sistema de Vigilancia de Infección Respiratoria Aguda, SiVIRA) of the Carlos III Health Institute, which is allied to the European Influenza Surveillance Network (EISN) of the European Centre for Disease Prevention and Control (ECDC). This study includes information from the PVIG program, which records patients attended by general practitioners and primary care pediatricians involved in the sentinel network in primary care settings because of an influenza syndrome. All patients who meet the definition criteria of influenza-like illness (ILI) [16] are registered as clinical cases. A nasopharyngeal swab sample is collected from a random sample of all clinical cases, and the diagnosis is confirmed by a multiplex RT-PCR system (Luminex NxTag Respiratory Panel-Luminex; FilmArray Respiratory Panel 2.1. plus; GenexPert Flu/RSV Cepheid), depending on the responsible laboratory. Samples positive to influenza are then sent to the National Influenza Centre for diagnostic confirmation and influenza subtyping, by specific CDC RT-PCR. Active monitoring is done by ca. 100 healthcare providers, including general practitioners, pediatricians, and nurses, covering a population that ranged between 27,461 (season 2015–2016) and 35,081 (season 2017–2018) (supplementary appendix) [17]. Each provider gathers systematically a series of variables in an in-paper questionnaire, at the moment of the patient evaluation. All healthcare providers had been trained in advance in the study variables and data collection, with annual update meetings to refresh all the study procedures.
Study population
In the present study, patients were included if they fulfilled the ILI criteria [16]: 1) acute respiratory illness with onset during the last seven days; 2) presence of at least one of the following symptoms: fever or low-grade fever, malaise, headache, or myalgia; 3) presence of at least one of the following respiratory symptoms: cough, odynophagia, or dyspnea.
Study variables
A series of variables are consistently collected by the VIGIRA system, including the season, a series of demographic variables, prior medical history, clinical variables, microbiological variables, and consequences of the disease. No additional variables were obtained. Demographic variables included sex at birth, age at the moment of the infection (stratified into the following age groups: 0–4, 5–14, 15–44, 45–64, 65–74 and 75 or older), and vaccination status at the moment of the infection. Variables related to the prior medical history included prior history of cardiovascular disorders, diabetes mellitus, chronic obstructive pulmonary disease (COPD), prior history of cancer, chronic kidney disease, chronic hepatic disease, or degree three obesity (body mass index > 40). Clinical variables assessed the onset of the disease within 48 h, the sudden onset of the symptoms, the epidemiological contact with another person infected by Influenza, and the presence of fever, shivering, asthenia, myalgia, cough, dyspnea, nasopharyngeal erythema, headache, and gastrointestinal symptoms. Microbiological variables included the oropharyngeal swab test obtention, the result of the test, and the influenza subtype and lineage. Last, the consequences of the disease were assessed in terms of need of hospital referral; the medical leave, in the case of adult patients; work absenteeism, in the case of children, adolescent and youth patients,
Statistical analysis
Qualitative and ordinal variables are described as frequency and percentage and quantitative variables as mean and standard deviation (SD) or median and inter-quartile range. In the hypothesis testing between cases and controls, two-tailed chi-squared test and Fisher exact test were used in the evaluation of qualitative variables. P value was considered statistically significant if < 0.05.
To assess which variables were associated with the presence of headache, first, a direct comparison between the frequency of each variable within cases and controls was done. Second, a logistic regression analysis was done. First, a univariable logistic regression was done, with “headache” as the dependent variable, to evaluate the strength of the association with influenza. Second, all the variables that presented a P value < 0.2 were included into a multivariable regression analysis by an “enter” method. Third, the observed P values were adjusted for multiple comparisons by False Discovery Rate, according to the Benjamini Hochberg procedure [18].
To evaluate whether headache was associated with a different prognosis, in terms of need of hospital referral, medical leave, or school absenteeism, three regression analyses were conducted, with these variables as dependent variables. Headache was inserted as the only independent variable, and afterwards, all the demographic variables or these that could influence the results, based on the prior literature (age or sex), were included in a multivariable regression, to assess whether headache remained as statistically significant.
In all the regression analyses, odds ratios (OR) and their corresponding 95% confidence intervals (CI) were estimated for all covariates using the backward strategy. Multicollinearity was assessed by the variance inflation factor (VIF) and was deemed as critical when VIF was > 5. Due to the study design and the study procedures, there were no missing data in the database. Compensation for multiple comparisons was done through the procedure of False Discovery Rate with the process of Benjamini-Hochberg [18].
Results
During the study period, a total of 7832 cases were considered. The number of cases per season varied between 505 (2013–2014 season) and 1146 (2017–2018 season) (Supplementary Table 2).
Prevalence of headache, and age-adjusted prevalence
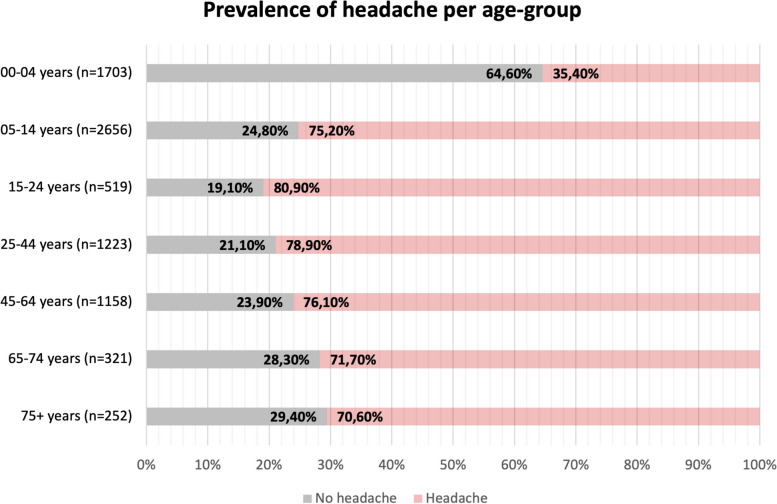
The prevalence of headache in the entire sample was 5275/7832 (67.4%). Headache had a prevalence > 70% in all age groups except in patients aged 00–04 years, where only 35.4% patients reported it. The highest prevalence was observed in patients aged 15–44 years. Figure 1 shows the prevalence of headache per age-group.
Fig. 1.
Prevalence of headache per age-group
Differences between patients with and without headache
Patients with headache were more frequently female. Table 1 shows the differences between patients with and without headache. Regarding prior medical history, disorders that were more prevalent within patients with headache included diabetes (+ 1.6%; P < 0.001), and COPD (+ 0.5; P = 0.034). All the clinical symptoms were more frequent within patients with headache. There were no differences in the microbiological variables. Concerning the consequences of the infection, patients with headache were referred to the hospital less frequently (-0.5%, P = 0.006) but required a medical leave more often (+ 4.2%, P < 0.001).
Table 1.
Differences between patients with and without headache
| Variable | Entire study sample (n = 7832) | Patients without headache (n = 2557) | Patients with headache (n = 5275) | P value |
|---|---|---|---|---|
| Female sex | 3856 (49.2%) | 1221 (47.8%) | 2635 (50.0%) | 0.068 |
| Cardiovascular disorders | 193 (2.5%) | 64 (2.5%) | 129 (2.4%) | 0.878 |
| Diabetes | 211 (2.7%) | 41 (1.6%) | 170 (3.2%) | < 0.001 |
| COPD | 79 (1.0%) | 17 (0.7%) | 62 (1.2%) | 0.034 |
| Cancer | 65 (0.8%) | 16 (0.6%) | 49 (0.9%) | 0.165 |
| Kidney disorders | 21 (0.3%) | 3 (0.1%) | 18 (0.3%) | 0.072 |
| Hepatic disorders | 14 (0.2%) | 4 (0.2%) | 10 (0.2%) | 0.745 |
| Obesity | 67 (0.9%) | 15 (0.6%) | 52 (1.0%) | 0.072 |
| Vaccination | 337 (4.3%) | 112 (4.4%) | 225 (4.3%) | 0.814 |
| Onset within 48 h | 4573 (58.4%) | 1398 (54.7%) | 3175 (60.2%) | < 0.001 |
| Epidemiological contact | 2964 (37.8%) | 884 (34.6%) | 2080 (39.4%) | < 0.001 |
| Sudden onset | 7325 (93.5%) | 2308 (90.3%) | 5017 (95.1%) | < 0.001 |
| Fever | 7427 (94.8%) | 2361 (92.3%) | 5066 (96.0%) | < 0.001 |
| Shivering | 5129 (65.5%) | 1188 (46.5%) | 3941 (74.7%) | < 0.001 |
| Asthenia | 6009 (76.7%) | 1548 (60.5%) | 4461 (84.6%) | < 0.001 |
| Myalgia | 5248 (67.0%) | 1158 (45.3%) | 4090 (77.5%) | < 0.001 |
| Cough | 6695 (85.5%) | 2135 (83.5%) | 4560 (86.4%) | 0.001 |
| Dyspnea | 736 (9.4%) | 208 (8.1%) | 528 (10.0%) | 0.008 |
| Nasopharyngeal erythema | 6838 (87.3%) | 2128 (83.2%) | 4710 (89.3%) | < 0.001 |
| Gastrointestinal symptoms | 2123 (27.1%) | 628 (24.6%) | 1495 (28.3%) | < 0.001 |
| Oropharyngeal swab obtention | 2113 (27.0%) | 668 (26.1%) | 1445 (27.4%) | 0.235 |
| Confirmed infection | 1194/2113 (56.5%) | 376/668 (56.3%) | 818/1445 (56.6%) | 0.985 |
| A subtype | 809/1194 (67.8%) | 257/376 (68.4%) | 552/818 (67.5%) | 0.764 |
| B subtype | 385/1194 (32.2%) | 119/376 (31.6%) | 266/818 (32.5%) | 0.823 |
| Hospital referral | 49 (0.6%) | 25 (1.0%) | 24 (0.5%) | 0.006 |
| Scholar absenteeism | 2205/6624 (33.3%) | 765/2315 (33.0%) | 1440/4309 (33.4%) | 0.759 |
| Medical leave | 591 (7.5%) | 120 (4.7%) | 471 (8.9%) | < 0.001 |
COPD Chronic obstructive pulmonary disease. Differing denominators indicate subgroup analyses
Factors associated with the presence of headache
In the univariate regression analysis, diabetes, COPD, and all the clinical variables were associated with headache (Table 2). In the multivariable regression, after adjusting for multiple comparisons, the variables that were associated with the presence of headache (Table 2) were female sex (OR: 1.134; 95% CI: 1.023–1.257, P = 0.034), sudden onset of the headache (OR: 1.359; 95% CI: 1.100–1.680, P = 0.010), the presence of fever (OR: 1.500; 95% CI: 1.180–1.907, P = 0.003), shivering (OR: 1.920; 95% CI: 1.713 – 2.152, P < 0.001), asthenia (OR: 1.955; 95% CI: 1.728 – 2.212, P < 0.001), myalgia (OR: 2.723; 95% CI: 2.427 – 3.055, P < 0.001), nasopharyngeal erythema (OR: 1.494; 95% CI: 1.280 – 1.745, P < 0.001) and gastrointestinal symptoms (OR: 1.174; 95% CI: 1.043 – 1.321, P = 0.018).
Table 2.
Univariable regression analysis of the variables associated with the presence of headache. Differing denominators indicate subgroup analyses
| Variable | Type of analysis | Odds ratio | 95% CI lower bound | 95% CI upper bound | P value | FDR-adjusted P value |
|---|---|---|---|---|---|---|
| Female sex | Univariable | 1.092 | 0.994 | 1.200 | 0.068 | |
| Multivariable | 1.134 | 1.023 | 1.257 | 0.017 | 0.034 | |
| Cardiovascular disorders | Univariable | 0.976 | 0.721 | 1.323 | 0.878 | |
| Diabetes | Univariable | 2.044 | 1.448 | 2.884 | < 0.001 | |
| Multivariable | 1.211 | 0.841 | 1.743 | 0.303 | 0.428 | |
| COPD | Univariable | 1.777 | 1.037 | 3.045 | 0.036 | |
| Multivariable | 1.209 | 0.685 | 2.138 | 0.514 | 0.633 | |
| Cancer | Univariable | 1.489 | 0.845 | 2.623 | 0.168 | |
| Multivariable | 1.050 | 0.574 | 1.923 | 0.873 | 0.873 | |
| Kidney disorders | Univariable | 2.915 | 0.858 | 9.905 | 0.086 | |
| Multivariable | 1.922 | 0.529 | 6.985 | 0.321 | 0.428 | |
| Hepatic disorders | Univariable | 1.212 | 0.38 | 3.869 | 0.745 | |
| Obesity | Univariable | 1.687 | 0.948 | 3.003 | 0.075 | |
| Multivariable | 1.171 | 0.628 | 2.185 | 0.620 | 0.709 | |
| Smoking habit | Univariable | 1.163 | 0.91 | 1.486 | 0.228 | |
| Vaccination | Univariable | 0.973 | 0.771 | 1.226 | 0.814 | |
| Onset within 48 h | Univariable | 1.253 | 1.139 | 1.379 | < 0.001 | |
| Epidemiological contact | Univariable | 1.232 | 1.117 | 1.36 | < 0.001 | |
| Multivariable | 1.070 | 0.960 | 1.193 | 0.220 | 0.352 | |
| Sudden onset | Univariable | 2.098 | 1.751 | 2.514 | < 0.001 | |
| Multivariable | 1.359 | 1.100 | 1.680 | 0.004 | 0.010 | |
| Fever | Univariable | 2.012 | 1.646 | 2.460 | < 0.001 | |
| Multivariable | 1.500 | 1.180 | 1.907 | 0.001 | 0.003 | |
| Shivering | Univariable | 3.404 | 3.082 | 3.76 | < 0.001 | |
| Multivariable | 1.920 | 1.713 | 2.152 | < 0.001 | < 0.001 | |
| Asthenia | Univariable | 3.572 | 3.203 | 3.983 | < 0.001 | |
| Multivariable | 1.955 | 1.728 | 2.212 | < 0.001 | < 0.001 | |
| Myalgia | Univariable | 4.170 | 3.768 | 4.614 | < 0.001 | |
| Multivariable | 2.723 | 2.427 | 3.055 | < 0.001 | < 0.001 | |
| Cough | Univariable | 1.261 | 1.106 | 1.437 | 0.001 | |
| Multivariable | 0.984 | 0.846 | 1.144 | 0.834 | 0.873 | |
| Dyspnea | Univariable | 1.256 | 1.062 | 1.486 | 0.008 | |
| Multivariable | 0.831 | 0.691 | 0.999 | 0.049 | 0.087 | |
| Nasopharyngeal erythema | Univariable | 1.681 | 1.468 | 1.925 | < 0.001 | |
| Multivariable | 1.494 | 1.280 | 1.745 | < 0.001 | < 0.001 | |
| Gastrointestinal symptoms | Univariable | 1.215 | 1.09 | 1.354 | < 0.001 | |
| Multivariable | 1.174 | 1.043 | 1.321 | 0.008 | 0.018 | |
| Sample | Univariable | 1.067 | 0.959 | 1.187 | 0.235 | |
| Confirmed infection | Univariable | 1.01 | 0.842 | 1.212 | 0.914 | |
| A subtype | Univariable | 1.046 | 0.895 | 1.223 | 0.573 | |
| B subtype | Univariable | 1.088 | 0.872 | 1.358 | 0.456 |
CI Confidence interval, COPD Chronic obstructive pulmonary disease, FDR False discovery rate. Values in bold denotes statistical signification
Consequences of the presence of headache
In the analysis of the implications of the presence of headache, the presence of headache was associated with a lower odds of hospital referral (OR: 0.463; 95% CI: 0.264–0.812, P = 0.007), even after adjusting for sex and age (OR: 0.404; 95% CI: 0.224–731, P = 0.003). In the case of medical leave, the presence of headache was associated with a higher odd of medical leave (OR: 1.991; 95% CI: 1.620–2.447, P < 0.001) but no higher school absenteeism (OR: 1.017; 95% CI: 0.914–1.132, P = 0.759). When adjusted for sex and age, patients with headache had higher odds of medical leave / school absenteeism (OR: 1.342; 95% CI: 1.190–1.514, P < 0.001).
Discussion
In the present study, the factors associated with the presence of headache in patients with influenza were explored. For this, a series of variables were compared between patients with and without headache, including demographic variables, prior medical history of patients, clinical variables and microbiological variables. A subsequent regression analysis was done, to adjust of all the possible confounders. We observed that headache as an influenza symptom was associated with myalgia, asthenia, shivering, nasopharyngeal erythema, fever, sudden onset of symptoms and gastrointestinal symptoms, and with female sex, as the only demographic variable. Second, we explored whether the consequences of the infection were different in patients with headache, and we observed a 60% lower odds of hospital referral and 34% higher odds of medical leave or school absenteeism.
The first striking finding was the high prevalence of headache, which reached two-thirds of the entire study sample. This prevalence seems higher than the observed prevalence in prior studies on Influenza, which observed that 32.4% and 60% out of 37 and 279 patients reported headache during the course of the disease [4, 5]. When compared with SARS-CoV-2, a meta-analysis that included 9573 studies and 28,438 COVID-19 survivors reported a prevalence at onset or hospital admission of 47.1% (95% CI: 35.8 – 58.6%) [19], however, in one study in which neurologists worked at the emergency room and included all the consecutive patients with COVID-19 that were attended by them, the prevalence of headache reached 74.6% of patients [20]. The prevalence was not similar in all age-groups, being higher in patients aged 15–64 years. This has two main implications, related to the pathophysiology and disease consequences. The higher prevalence in younger patients could be related both with the immune-senescence that may exist in old patients [21]. In young children, the verbal description of some symptoms may be challenging, and despite present, headache may not be properly characterized or reported [22]. On the other hand, given the negative consequences of headache, its peak prevalence in people within the most active years, in terms of scholar performance and work, may be particularly relevant [23].
The differences between patients with and without headache should be contextualized. Despite statistically significant, most of these were within the range of 5–10% difference between groups, which may not be that clinically relevant. Given the large sample size, the study had enough power to detect small differences. The exception to this were myalgia, shivering and asthenia, which were 32%, 28% and 24% more frequent within patients with headache, respectively. These symptoms have been associated with the innate immune response and the release of inflammatory mediators [24, 25]. The symptoms that were independently associated with the presence of headache supported the study hypothesis that headache is likely caused by the immune response and the cytokine and interleukin release. Despite this observation results novel in the case of Influenza, prior studies have reported that headache in patients with SARS-CoV-2 is associated with a higher frequency of systemic symptoms [9, 12, 26].
Headache could be associated with a more benign disease course. In the case of SARS-CoV-2, patients with headache had a better prognosis, in terms of mortality or intensive-care admission [9, 11, 27]. Due to this study design, there was no information about patients’ mid-and-long term prognosis, and the only variable that could act as surrogate marker of patients’ outcome was the need of hospital referral. Patients with headache were referred to the hospital 60% less frequently, after adjusting for age and sex, which could support a more benign disease presentation [28].
On the other hand, headache is a disabling symptom, and patients with headache had 34% higher odd of missing work or school. Some aspects, such as the use of symptomatic drugs, including over-the-counter treatments, was not assessed, so we could not evaluate whether these patients had managed the disease adequately [29]. Healthcare providers and even the entire population must be aware of the optimal management of influenza, including drugs that may alleviate the symptoms, the annual vaccination of the vulnerable and exposed population, and the epidemiological measures to minimize the spread of the disease [30, 31]. In our sample, 38% of the cases reported that they suspected the source of their contagion, emphasizing the relevance of isolation measures [32].
The findings of this study should be interpreted in the context of its strengths and limitations. Data was consistently collected by healthcare providers who collaborated with the health sentinel network, carrying out active monitoring. This ensured consistency of data, including the adequacy of study definitions, the representativeness of study participants, and the same method for both cases and controls. The possible effect of patients’ age and sex was anticipated as a possible confounder, and it was controlled to increase the between-group comparability. The main limitation was the selected number of variables that were studied, that did not include some aspects that could be relevant, such as prior history of headache disorders, prior history of headache attributed to acute viral infections. There was no info about the headache phenotype or the presence of other symptoms that are typically associated to headache, such as photo/phonophobia and/or nausea. Patients were evaluated only once, and therefore, there was no information about the patients’ outcome, in terms of mortality, need of intensive-care admission, or need of oxygen therapy. Future studies should consider a longitudinal design to explore this missing piece of the puzzle.
In conclusion, headache is a prevalent symptom of influenza. The presence of headache was independently associated to myalgia, asthenia, shivering, nasopharyngeal erythema, fever, sudden onset of symptoms, male sex, and gastrointestinal symptoms. Patients with headache had a lower odds of hospital referral and a higher odd of medical leave or school absenteeism. These findings support a headache pathophysiology linked with the innate immune response, highlighting the importance of an adequate treatment of it.
Supplementary Information
Additional file 1: Supplementary table 1. Surveyed population per age-group. Supplementary table 2. Number of cases per season during the entire study period.
Acknowledgements
The authors acknowledge all the members and participants of the Sentinel Network of Castile and Leon (Red Centinela Sanitaria de Castilla y Leon) and the members of the Public Health Headquarters at the Regional Health Administration (Dirección General de Salud Pública de la Consejería de Sanidad de la Junta de Castilla y Leon).
The authors declare that no funds, grant, or other support were received during the preparation of this manuscript. No authors had a financial relationship to the work.
Authors’ contributions
D.G-A, I.S-M and AL.G-P designed the study L.S-L, Y.G-O, A.S-M obtained the dataL.S-L, D.G-A and I.S-M analized the dataL.S-L, D.G-A and I.S-M wrote the main manuscript text. All authors reviewed the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ivan Sanz-Muñoz and Ángel Luis Guerrero-Peral contributed equally to this work.
References
- 1.Uyeki TM, Hui DS, Zambon M, Wentworth DE, Monto AS. Influenza. Lancet. 2022;400:693–706. doi: 10.1016/S0140-6736(22)00982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2017;2019(7):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capitani E, Montomoli E, Camarri A, Bova G, Capecchi PL, Mercone A, Nante N, Manini I. Epidemiological and virological surveillance of severe acute respiratory infections in the 2019/2020 season in Siena, Tuscany. Italy J Prev Med Hyg. 2021;62:E782–E788. doi: 10.15167/2421-4248/jpmh2021.62.3.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eccles R. Mechanisms of symptoms of the common cold and influenza. Br J Hosp Med (Lond) 2007;68(2):71–75. doi: 10.12968/hmed.2007.68.2.22824. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen CJ, Quinn JV, Rogan DT, Yang S. Factors associated with influenza in an emergency Department setting. J Emerg Med. 2019;56:478–483. doi: 10.1016/j.jemermed.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Galante M, Garin O, Sicuri E, Cots F, García-Altés A, Ferrer M, Dominguez À, Alonso J. Health services utilization, work absenteeism and costs of pandemic influenza A (H1N1) 2009 in Spain: a multicenter-longitudinal study. Plos One. 2012;7:e31696. doi: 10.1371/journal.pone.0031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjos SA, Irving SA, Meece JK, Belongia EA. Elementary school-based influenza vaccination: evaluating impact on respiratory illness absenteeism and laboratory-confirmed influenza. Plos One. 2013;8:e72243. doi: 10.1371/journal.pone.0072243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36:3960–3966. doi: 10.1016/j.vaccine.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Trigo J, García-Azorín D, Sierra-Mencía Á, Tamayo-Velasco Á, Martínez-Paz P, Tamayo E, Guerrero AL, Gonzalo-Benito H. Cytokine and interleukin profile in patients with headache and COVID-19: a pilot, CASE-control, study on 104 patients. J Headache Pain. 2021;22:51. doi: 10.1186/s10194-021-01268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musubire AK, Cheema S, Ray JC, Hutton EJ, Matharu M. Cytokines in primary headache disorders: a systematic review and meta-analysis. J Headache Pain. 2023;24:36. doi: 10.1186/s10194-023-01572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Membrilla JA, de Lorenzo Í, Sastre M, Díaz de Terán J, Headache as a Cardinal Symptom of Coronavirus Disease A cross-sectional study. Headache. 2019;2020(60):2176–2191. doi: 10.1111/head.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trigo J, García-Azorín D, Planchuelo-Gómez Á, Martínez-Pías E, Talavera B, Hernández-Pérez I, Valle-Peñacoba G, Simón-Campo P, de Lera M, Chavarría-Miranda A, López-Sanz C, Gutiérrez-Sánchez M, Martínez-Velasco E, Pedraza M, Sierra Á, Gómez-Vicente B, Arenillas JF, Guerrero ÁL. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. 2020;21:94. doi: 10.1186/s10194-020-01165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Plos Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Europe. WHO Regional Office for Europe guidance for sentinel influenza surveillance in humans. May 2011. Available at: https://www.euro.who.int/__data/assets/pdf_file/0020/90443/E92738.pdf accessed the 08 July 2022.
- 15.Council of Castile and Lion. counseling of Health. Program of Surveillance integrated of the infections respiratory treble (VIGIRA) of Castile and Lion. Protocol 2021/2022 (v3.1). Available in: https://www.saludcastillayleon.es/profesionales/es/sentinels/nuevo-programa-vigilancia-integrada-ira-red-sentinel-sanit.files/1788984-Programa%20VIGIRA%20version1.0.pdf accessed the 08 July 2022.
- 16.Publications Office of the European Union. 2008 / 426 / EC: Commission decision of 28 April 2008 amending Decision 2002 / 253 / EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119 / 89 / EC of the European Parliament of the Council (notified under document number C(2008) 1589). Available at: https://op.europa.eu/en/publication-detail/-/publication/3e53de24-26d6-4645-b9ab-3931f3874c9e Accessed the 06 February of 2023.
- 17.Sentinel Sanitary Network of Castile Y Lion. Address General of Health Public. Report of Program of surveillance of the ARI (VIGIRA). Week 2022/23 (of 6 to the 12 of June). Available in: https://www.saludcastillayleon.es/profesionales/es/sentinels/informacion-semanal-infecciones-respiratory-acute-anger.files/2182300-VIGIRA%20Informe%20semanal%202022–23.pdf Accessed the 06 February of 2023.
- 18.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-de-Las-Peñas C, Navarro-Santana M, Gómez-Mayordomo V, Cuadrado ML, García-Azorín D, Arendt-Nielsen L, Plaza-Manzano G. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a meta-analysis of the current literature. Eur J Neurol. 2021;28:3820–3825. doi: 10.1111/ene.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caronna E, Ballvé A, Llauradó A, Gallardo VJ, Ariton DM, Lallana S, LópezMaza S, OlivéGadea M, Quibus L, Restrepo JL, Rodrigo-Gisbert M, Vilaseca A, Hernandez Gonzalez M, Martinez Gallo M, Alpuente A, Torres-Ferrus M, PujolBorrell R, Alvarez-Sabin J, Pozo-Rosich P. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–1421. doi: 10.1177/0333102420965157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between immunosenescence and age-related diseases. Semin Immunopathol. 2020;42:545–557. doi: 10.1007/s00281-020-00806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bondesson E, Olofsson T, Caverius U, Schelin MEC, Jöud A. Consultation prevalence among children, adolescents and young adults with pain conditions: a description of age- and gender differences. Eur J Pain. 2020;24:649–658. doi: 10.1002/ejp.1518. [DOI] [PubMed] [Google Scholar]
- 23.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betakova T, Kostrabova A, Lachova V, Turianova L. Cytokines induced during influenza virus infection. Curr Pharm Des. 2017;23:2616–2622. doi: 10.2174/1381612823666170316123736. [DOI] [PubMed] [Google Scholar]
- 25.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallardo VJ, Shapiro RE, Caronna E, Pozo-Rosich P. The relationship of headache as a symptom to COVID-19 survival: a systematic review and meta-analysis of survival of 43,169 inpatients with COVID-19. Headache. 2022;62:1019–1028. doi: 10.1111/head.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampaio Rocha-Filho PA, Albuquerque PM, Carvalho LCLS, Dandara Pereira Gama M, Magalhães JE. Headache, anosmia, ageusia and other neurological symptoms in COVID-19: a cross-sectional study. J Headache Pain. 2022;23(1):2. doi: 10.1186/s10194-021-01367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Rubio A, Platero L, Eiros Bouza JM. Seasonal influenza in Spain: Clinical and economic burden and vaccination programmes. Med Clin (Barc) 2019;153:16–27. doi: 10.1016/j.medcli.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Klepser ME. Socioeconomic impact of seasonal (epidemic) influenza and the role of over-the-counter medicines. Drugs. 2014;74:1467–1479. doi: 10.1007/s40265-014-0245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729–1737. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 31.Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A, BaloxavirMarboxil Investigators Group Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KEE, Ghedin E. Quantifying between-Host Transmission in Influenza virus infections. Cold Spring Harb Perspect Med. 2020;10:a038422. doi: 10.1101/cshperspect.a038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table 1. Surveyed population per age-group. Supplementary table 2. Number of cases per season during the entire study period.