Abstract
Background
If any benefit is to be derived from the use of the health-related quality of life (HRQoL) questionnaires in chronic kidney disease (CKD) patients, they should be validated and culturally adapted to the target population. We aimed to critically appraise the psychometric properties of HRQoL questionnaires used in African populations with CKD.
Methods
Web of Science, Embase, PubMed and PsycINFO databases were searched. Psychometric validation studies of HRQoL questionnaires reporting at least one psychometric property of the COSMIN checklist in CKD African population, published up to October 16, 2023 were included and independently assessed for methodological quality and level of measurement properties by using the COSMIN methodology.
Results
From 1163 articles, 5 full-text were included. Only the Kidney Disease Quality-of-Life questionnaire was translated and cross-culturally adapted for studies of patients with CKD. Internal consistency was of doubtful quality in 4 studies and very good in 1. Its measurement was sufficient in 1 study and insufficient in 4. Test–retest reliability was of doubtful quality in 4 studies. Its measurement was sufficient in 3 studies and insufficient in 1. Structural validity was of inadequate quality in 1 study and very good quality in 1. Its measurement was sufficient in both. Construct validity was of inadequate quality in all studies. Their measurement was insufficient in 4 studies and sufficient in 1.
Conclusions
This review highlighted that only one HRQoL questionnaire used in studies of African populations with CKD underwent a small number of cultural adaptations and psychometric validations, generally of poor methodological quality. HRQoL validation studies in African CKD populations are needed to better take advantage of the benefits in patient care, population health management, and research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03482-5.
Keywords: Quality of life, Psychometric, Chronic kidney disease, Africa, Systematic review
Introduction
Chronic kidney disease (CKD) is a global public health problem because of its prevalence, severity [1], complexity [2] and high cost of management [3, 4]. The incidence and prevalence of CKD has been increasing over the years [5]. Africa is affected by the double burden of infectious diseases and chronic non-communicable diseases including CKD [6, 7].
Health-related quality of life (HRQoL), a multidimensional concept, is an important clinical endpoint for patients, healthcare providers, and funding partners [8, 9]. CKD patients have to deal with significant lifestyle changes that affect their HRQoL in all its dimensions. HRQoL is negatively affected by CKD glomerular filtration category G3 to G5, with or without kidney replacement therapy [10]. HRQoL is worse in the CKD population than in the general population [10, 11]. The physical component score of an HRQoL survey (0–100 scale) was found to be 42.6, 40.3, and 34.8, respectively (the higher the score, the better the quality of life), in patients with moderate CKD, in those with advanced CKD, and in those on dialysis [10].
HRQoL and symptoms (a dimension of HRQoL) can be assessed using validated, self-administered questionnaires characterized as patient-reported outcome measures (PROMs) [12]. These instruments strongly depend on the sociocultural context of the target population. Thus, they must be culturally adapted following published guidelines [13] and validated on the basis of classical or modern psychometric properties. Several instruments measure HRQoL in CKD. Some are generic, such as the Medical Outcome Survey 36-item Short Form [14], the Nottingham Health Profile [15], the World Health Organization Quality of Life [16]. Others are specific to kidney disease, such as the Kidney Disease Quality of Life (KDQOL) [17, 18], the Kidney Disease Questionnaire [19], the CHOICE Health Experience Questionnaire [20], the Dialysis Symptom Index [21], the Modified Edmonton Symptom Assessment System [22], and the End-Stage Renal Disease Symptom Checklist-Transplant Module [23].
If any benefit is to be derived from the use of these questionnaires, they should be validated and culturally adapted to the target population.
The COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) is a methodological quality assessment of psychometric studies [24]. COSMIN recommends that 3 quality domains of HRQoL instruments (reliability, validity, and responsiveness) be assessed for systematic reviews [24].
There are few systematic reviews of the psychometric properties of HRQoL assessment, and they include few studies conducted in Africa. Two systematic reviews [17, 18] of the psychometric properties of HRQoL assessment worldwide included only 2 African studies [25, 26]. Several studies of the assessment of HRQoL in CKD have been conducted in the African continent, sometimes in areas where the KDQOL is not culturally adapted, such as Senegal [27, 28]. To our knowledge, no systematic review of the psychometric properties of HRQoL assessment has been conducted in Africa. A systematic review targeting exclusively African literature, including a manual search of African researchers can enable to increase the sensitivity of the detection of validation studies. We hypothesize that few psychometric validation studies of HRQoL questionnaires in CKD have been conducted in Africa despite the use of these questionnaires in HRQoL assessment in Africa CKD population.
The aim of the present systematic review was to identify, critically appraise and summarize the psychometric properties of instruments measuring HRQoL in CKD and their cross-cultural adaptation in African populations living with CKD using the COSMIN methodology.
Methods
Study design
This was a systematic review using the COSMIN recommendations for systematic reviews of PROMs [29] and the COSMIN methodology for assessing the quality of psychometric studies [24]. The results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [30] (see Supplementary file 1: Additional file 1). The review protocol was prepared but has not been registered beforehand.
Search methods
The following databases were searched for the literature review: Web of Science, Embase, PubMed/MEDLINE, PsycINFO. A manual search was also performed using the references of the various articles or with fellow African researchers through social media (Twitter, WhatsApp). The search was limited to all articles in French and English and published up to October16, 2023. The search equations were constructed from the following keywords: construct, population, instrument name, and psychometric properties. For the PubMed and Embase search, the PubMed filters for searching psychometric studies were used [31] (see Supplementary file 1: Additional file 2).
Eligibility criteria
Studies were selected according to the following criteria: Type of participant: African population living with CKD G1 to G5, dialysis patients or kidney transplant recipients; measurement instrument: any measurement instrument for HRQoL in CKD; type of study: psychometric validation studies reporting at least one psychometric property of the COSMIN checklist, and published in English or French. We excluded conference or congress abstracts, editorials, clinical cases, reviews, theses and commentaries.
Selection of the studies
One author (MF) removed duplicates and selected articles on the basis of titles and abstracts by using the Rayyan tools website (https://www.rayyan.ai/). Then 2 independent authors (MF, FG) evaluated the full text and resolved discrepancies by consensus.
Methodological quality assessment of the included studies
Two independent authors (MF, FG) assessed the quality of the included studies using the COSMIN Risk of Bias assessment checklist [24]. The 2 authors discussed discrepancies to reach consensus. The checklist included 10 boxes for content (development of PROMs, content validity), internal structure (structural validity, internal consistency, cross-cultural validity/invariance of the measure), and measurement properties (reproducibility, measurement error, criterion validity, hypothesis testing for construct validity, responsiveness). The process of translating the questionnaire was evaluated (back translation, expert committee, cognitive debriefing). Each study was scored as “very good quality “, “adequate quality “, “doubtful quality “, or “inadequate quality “.
Measurement property assessment of each study
The psychometric properties for each study were scored on the basis of criteria for good measurement properties (Table 1) [29]. Each score was reported as sufficient (+), insufficient (-), or indeterminate (?) and was evaluated by 2 authors (MF, FG). The following properties were assessed: reliability (internal consistency, test–retest reliability); validity (content validity, construct validity, cross-cultural validity/measurement invariance, measurement error, criterion validity, hypothesis testing for construct validity); and responsiveness.
Table 1.
Criteria for good measurement properties according to the checklist [29]
| Measurement property | Ratinga | Criteria |
|---|---|---|
| Structural validity | + |
CTT: CFA: CFI or TLI or comparable measure > 0.95 OR RMSEA < 0.06 OR SRMR < 0.08b IRT/Rasch: No violation of unidimensionalityc: CFI or TLI or comparable measure > 0.95 OR RMSEA < 0.06 OR SRMR < 0.08 AND no violation of local independence: residual correlations among the items after controlling for the dominant factor < 0.20 OR Q3’s < 0.37 AND no violation of monotonicity: adequate looking graphs OR item scalability > 0.30 AND adequate model fit: IRT: χb > 0.01 Rasch: infit and outfit mean squares ≥ 0.5 and ≤ 1.5 OR Z‐ standardized values > ‐2 and < 2 |
| ? | CTT: Not all information for ‘ + ’ reported IRT/Rasch: Model fit not reported | |
| - | Criteria for ‘ + ’ not met | |
| Internal consistency | + | Criteria for “At least low evidenced for sufficient structural validitye “ not met |
| ? | Criteria for “At least low evidenced for sufficient structural validitye “ not met | |
| - | At least low evidenced for sufficient structural validitye AND Cronbach’s alpha(s) < 0.70 for each unidimensional scale or subscalef | |
| Reliability | + | ICC or weighted Kappa ≥ 0.70 |
| ? | ICC or weighted Kappa not reported | |
| - | ICC or weighted Kappa < 0.70 | |
| Measurement error | + | SDC or LoA < MICe |
| ? | MIC not defined | |
| - | SDC or LoA > MICe | |
| Hypotheses testing for construct validity | + | The result is in accordance with the hypothesisg |
| ? |
No hypothesis defined (by the review team) The result is not in accordance with the hypothesisg |
|
| - | The result is in accordance with the hypothesisg | |
| Cross‐cultural validity\measurement invariance | + | No important differences found between group factors (such as age, gender, language) in multiple group factor analysis OR no important DIF for group factors (McFadden’s Rb < 0.02) |
| ? | No multiple group factor analysis OR DIF analysis performed | |
| - | Important differences between group factors OR DIF was found | |
| Criterion validity | + | Correlation with gold standard ≥ 0.70 OR AUC ≥ 0.70 |
| ? |
Not all information for ‘ + ’ reported Correlation with gold standard < 0.70 OR AUC < 0.70 |
|
| - | Correlation with gold standard ≥ 0.70 OR AUC ≥ 0.70 | |
| Responsiveness | + | The result is in accordance with the hypothesisg OR AUC ≥ 0.70 |
| ? | No hypothesis defined (by the review team) | |
| - | The result is not in accordance with hypothesisg or AUC < 0.70 |
AUC area under the receiver operating characteristic curve, CFA confirmatory factor analysis, CFI comparative fit index, CTT classical test theory, DIF differential item functioning, ICC intraclass correlation coefficient, IRT item response theory, LoA limits of agreement, MIC minimal important change, RMSEA root mean square error of approximation, SEM standard error of measurement, SDC smallest detectable change, SRMR standardized root mean residuals, TLI Tucker‐Lewis index
a“ + “ = sufficient, “ – “ = insufficient, “? “ = indeterminate
bTo rate the quality of the summary score, the factor structures should be equal across studies
cunidimensionality refers to a factor analysis per subscale, and structural validity refers to a factor analysis of a (multidimensional) patient‐reported outcome measure
dAs defined by grading the evidence according to the GRADE approach
eThis evidence may come from different studies
fThe criterion Cronbach alpha < 0.95 was deleted because this is relevant in the development phase of a PROM but not when evaluating an existing PROM
gThe results of all studies should be taken together and then decided if 75% of the results are in accordance with the hypotheses
Data extraction and synthesis
One author (MF) extracted data by using a predefined Excel sheet. The extracted data related to the population (sample size, sex, age, target population) and characteristics of the questionnaire (type of administration, language, country).
The synthesis of the psychometric properties of the studies was performed in accordance with COSMIN recommendations [24, 29, 32].
Results
Description of the included studies
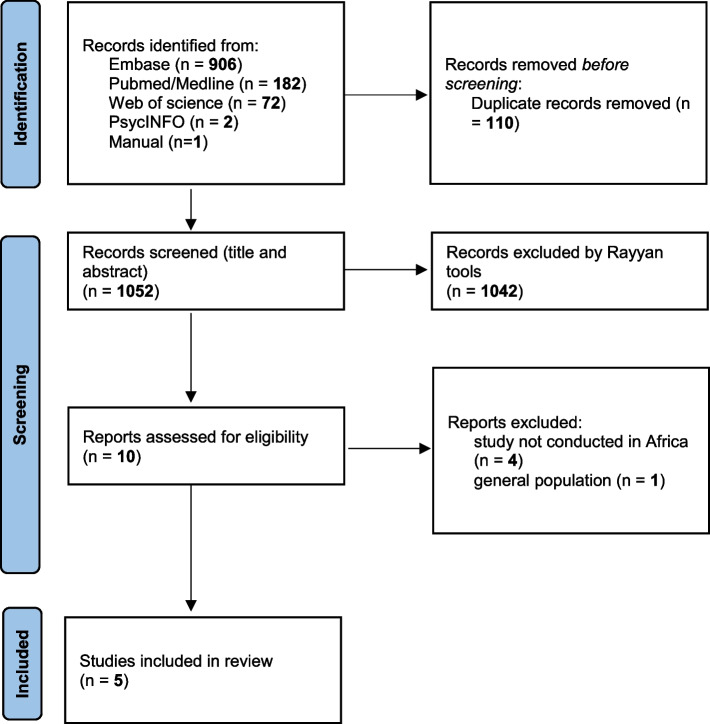
As a result of the literature search, 1163 articles were screened. After removing duplicates, 1052 articles were evaluated for relevance according to the title and/or the abstract. Ten full-text articles were screened, and finally 5 full-text articles were included in the review. The PRISMA flow chart of the study selection is presented in Fig. 1.
Fig. 1.
PRISMA flow chart diagram
The 5 studies evaluated only one questionnaire, the KDQOL. It was validated in all studies and applied to patients with CKD G3 to G5 who were undergoing dialysis (haemodialysis or peritoneal dialysis) or who had renal transplantation. The sample sizes of the 5 studies ranged from 80 to 363 (Table 2). Two studies reported a mean (± standard deviation) age of 43.9 ± 14.2 and 48 ± 14.7 years [26, 33]; 2 others reported a median (interquartile range) age of 46 (35–58) and 54 (42–60) [34, 35]. Among 3 studies, the proportion of males ranged from 54 to 64% [33–35]. One study reported a predominance of women [26]. The studies were conducted in Morocco, Egypt, Ethiopia, Sudan, and Uganda. The original English version of KDQOL was translated into Arabic, Amharic, and Luganda. Backward translation and expert committee were used in all studies and cognitive debriefing in 4 [26, 33–35].
Table 2.
Characteristics of the included studies
| Author, year of publication | Bouidida et al. 2014 [26] | Abd ElHafeez et al. 2012 [34] | Gebrie et al. 2022 [33] | Elamin et al. 2019 [25] | Bagasha et al. 2022 [35] |
|---|---|---|---|---|---|
| KDQOL (item number) | 79 | 36 | 36 | 36 | 79 |
| Country | Morocco | Egypt | Ethiopia | Sudan | Uganda |
| Sample | 80 | 100 | 292 | 144 | 364 |
| Population | HD = 62; PD = 18 | CKD at G3-G4 | HD | HD = 62; KT = 82 | CKD G5 in CC or HD |
| Men (%) or sex ratio | 0.7 | 54 | 64 | NR | 60 |
| Age (years)a | 43.9 (14.2) | 54 (42–60) | 48 ± 14.7 | NR | 46 (35–58) |
| Literacy (%) | 28 | 24 | 26 | NR | NR |
| Cognitive debriefing | Yes | Yes | Yes | No | Yes |
| Language | Morocco (Arabic) | Arabic | Amharic | Arabic | Luganda |
| Backward translation | Yes | Yes | Yes | Yes | Yes |
| Expert committee | Yes | Yes | Yes | Yes | Yes |
KDQOL kidney disease quality of life, HD haemodialysis, PD peritoneal dialysis, CKD chronic kidney disease, KT kidney transplant, CC conservative care, NR not reported
amean ± SD or median (interquartile range)
Methodological quality of the included studies
The methodological quality of each study is presented in Table 3. No study reported PROM development, measurement error, cross-cultural validity, criterion validity, or responsiveness.
Table 3.
Methodological quality of the included studies
| Author, year of publication | Structural validity | Internal consistency | Test–retest reliability | Hypotheses testing for construct validity |
|---|---|---|---|---|
| Bouidida et al. 2014 [26] | NR | Doubtful | Doubtful | Inadequate |
| Abd ElHafeez et al. 2012 [34] | Inadequate | Inadequate | Doubtful | Inadequate |
| Gebrie et al. 2022 [33] | Very good | Very good | Doubtful | Inadequate |
| Elamin et al. 2019 [25] | NR | Doubtful | Doubtful | Inadequate |
| Bagasha et al. 2022 [35] | NR | Doubtful | NR | Inadequate |
Patient reported outcomes measure (PROM) development, Measurement error, cross-cultural validity, criterion validity, and responsiveness were not reported in any of the studies
NR not reported
Reliability
Internal consistency was assessed in all 5 studies. It was rated as very good in one study [33], inadequate in one study [33] and doubtful in others because structural validity (unidimensionality of scales or sub-scales) was not reported in these studies.
Test–retest reliability was assessed in 4 studies [25, 26, 33, 34]. It was rated doubtful in all studies because the article did not clarify whether patients were stable or what was the time interval used.
The intraclass correlation (ICC) was calculated, but the model and formula were not described in 2 studies [26, 33] and the ICC model was derived from a two-way random effects model in 1 study [25]. The time interval was 10 to 14 days in 2 study [26], and 2 weeks in 2 studies [25, 34].
Validity
Structural validity was assessed in 2 studies. It was rated inadequate in one study [34] because the sample size included fewer than 5 times the number of items, and very good in one study [33].
Hypothesis testing for construct validity was assessed in all studies. Known group validity was assessed in all studies and convergent validity in 4 [25, 26, 33, 34]. Hypothesis testing for construct validity was rated inadequate in 4 studies [25, 26, 34, 35] because the comparator had insufficient measurement properties or no information on these measurement properties. These studies used the physical component and mental component scores of the Arabic version of KDQOL-36 to be validated, the Arabic version of the Depression Anxiety Stress Scale-21 (DASS-21), and overall health rate as comparator. It was rated inadequate in 1 study [33] because the correlation of the score with that of the comparator instrument (5-level EuroQol 5-dimensional questionnaire [EQ-5D-5L] and the EuroQol Visual Analogue Scale [EQ-VAS]) was assessed in only 3 dimensions of the KDQOL.
Measurement property assessment of the included studies
Structural validity, internal consistency, test–retest reliability, and hypothesis testing for construct validity were reported in the included studies. Measurement error and cross-cultural validity/measurement invariance, criterion validity and responsiveness were not reported in any study. The measurement properties of each study are in Table 4.
Table 4.
Measurement properties reported in the included studies and summary of the results
| Author, year of publication | Bouidida et al. 2014 [26] | Abd ElHafeez et al. 2012 [34] | Gebrie et al. 2022 [33] | Elamin et al. 2019 [25] | Bagasha et al. 2022 [35] |
|---|---|---|---|---|---|
| Structural validity | NR | (+) | (+) | NR | NR |
|
KMO = 0.73 Bartlett’s test: p < 0.001 |
RMSEA = 0.085[0.064–0.095]; CFI = 0.854; TLI = 0.838; SRMR = 0.067 |
||||
| Internal consistency | (-) | (-) | (+) | (-) | (-) |
| Cronbach’s alpha = 0.38–0.89 | Cronbach’s alpha = 0.23–0.95 | Cronbach’s alpha = 0.81–0.91 | Cronbach’s alpha = 0.66–0.86 | Cronbach’s alpha = 0.41–0.96 | |
| Test–retest reliability | (-) | (+) | (+) | (+) | NR |
| ICC = 0.67–0.90 | ICC = 0.79–0.95 | ICC = 0.90–0.96 | ICC = 0.74–0.98 | ||
| Hypotheses testing for construct validity | (-) | (-) | (+) | (-) | (-) |
Measurement error, cross-cultural validity, criterion validity, and responsiveness were not reported in any of the 5 studies
( +) sufficient, (–) insufficient, (?) indeterminate, NR not reported, KMO Kaiser–Meyer–Olkin, ICC intraclass correlation coefficient, CFI comparative fit index, RMSEA root mean square error of approximation, SRMR standardized root mean residuals, TLI Tucker‐Lewis index
Reliability
Internal consistency: One study was rated sufficient [33] and 4 were rated insufficient because the subscale values of Cronbach’s alpha were < 0.70 [25, 26, 34, 35].
Test–retest reliability: Test–retest reliability was assessed with the ICC in 4 studies [25, 26, 33, 34]: it was rated sufficient in 3 studies [25, 33, 34] and insufficient in 1 because of ICC < 0.7 [26].
Validity
Structural validity was assessed with confirmatory factor analysis in 1 study [33] and exploratory factor analysis in 1 study [34]. It was rated sufficient in these 2 studies because of standardized root mean squared residual < 0.08 [33] and the Kaiser–Meyer–Olkin (KMO) was above the recommended value of 0.60 [34].
Hypothesis testing for construct validity: This was rated insufficient in 4 studies [25, 26, 34, 35] because the comparator had insufficient measurement properties or no information on these measurement properties. It was rated sufficient in 1 study [33]. The correlation of scores for 3 sub-dimensions of the KDQOL-36 (symptoms/problem, effect and burden of kidney disease) with those of comparator instruments (EQ-5D-5L and EQ-VAS) was > 0.5 [33].
Discussion
This is the first systematic review to evaluate the psychometric properties of HRQoL questionnaires used in African patients with CKD by using the COSMIN checklist. We found a small number of studies of cultural adaptation and psychometric properties of an HRQoL questionnaire in African populations. All studies used the KDQOL questionnaire. The KDQOL-36, the most frequently used PROM in nephrology and adapted in several populations, consist of 5 dimensions, 2 are generic (“physical component summary” and “mental component summary”) and 3 are specific to kidney disease, including the “symptoms and problems lists”, “effect of kidney disease” and “burden of kidney disease”. The five dimensions are summarized by a score ranging from 0 to 100 (the lower the score, the more impaired HRQoL). These studies were generally of poor methodological quality according to the COSMIN checklist. Many psychometric properties, such as PROM development, structural validity, measurement error, cross-cultural validity, criterion validity, and responsiveness, were not reported in these studies.
In line with our findings, Aiyegbusi et al. [17] and Yangoz et al. [18], reported a small number of studies of cultural adaptation and psychometric properties of the KDQOL in African populations. Yet, several studies have assessed the HRQoL of African CKD patients with this questionnaire [15, 17, 36–41]. In this context, does this questionnaire really measure what it is supposed to measure? Is this questionnaire culturally adapted to the context of Africa? If any benefit is to be derived from the use of PROMs, they must actually measure what they are intended to measure (validation), produce consistent results (reliability), and capture all aspects of the construct(s) being studied that matter to the target population (adaptation) [18, 42]. African researchers must first validate and culturally adapt these questionnaires in their context before using them to obtain a reliable assessment of HRQoL in CKD. For longitudinal studies, responsiveness must be assessed in validation and cultural adaptation studies.
Good quality assessment of the consequences of CKD and the disability, like HRQoL, provides a better understanding of patient’s experience and the impact of their chronic disease and comorbidities, a better consideration of these consequences, an outcome of interest in clinical research and a better management, beyond the management of biological symptoms.
According to COSMIN guidelines, we found several methodological issues with the HRQoL questionnaire assessed in this review: no information on the clinical course of the patients or the time interval in test–retest reliability, failure to perform confirmatory factor analysis for construct validity, small sample sizes, unspecified missing data, lack of clear hypotheses for construct validity, no information on the psychometric properties of the comparators in construct validity, and no information on measurement error and responsiveness. Similar issues were reported by Aiyegbusi et al. [18]. A limitation of the evaluation of the methodological quality with COSMIN is an element of subjectivity because particular appreciations are left to the raters.
Despite these methodological issues, the KDQOL may have a potential utility in clinical practice in some African countries. The use of an HRQoL questionnaire in clinical practice has several benefits, including enhancing the communication between the patient and the clinician [43], facilitating reporting of serious adverse events [44], helping renal teams with the development of strategies to improve the HRQoL of CKD patients [45], and integrating routinely collected clinical and laboratory data (big PRO data) with several opportunities in patient care, population health management, and research [46]. Routine collection of HRQoL may allow for better management of quality of life including symptoms. These findings confirm the need to plan similar studies, but with adaptation of the questionnaire to the cultural context of the population, in order to have a better vision of the different aspects of the HRQoL of the patient with CKD and with these elements to outline a strategy to improve the condition of the patients. African researchers have a lot of work to do in terms of research, and must necessarily equip themselves with good instruments for assessment HRQoL.
The main strength of this systematic review is the use of the international COSMIN guidelines [29]. The use of PubMed/MEDLINE filters to build the search equation is also of value [31]. However, the study also has limitations. First, the included studies did not adequately report their assessments for a number of measurement properties. Second, because of the same questionnaire (KDQOL) studied in all studies and the small number of studies, we could not summarize the evidence and grade the quality of the evidence by using the GRADE approach recommended by COSMIN.
Conclusion
Our review highlighted the publication of only a small number of HRQoL questionnaire validation studies, all with KDQOL, in African CKD populations. These studies are of poor methodological quality according to the COSMIN checklist. HRQoL validation studies in African CKD populations are needed to better take advantage of the benefits of such outcome in patient care, population health management, and research.
Supplementary Information
Supplementary file 1: Additional file 1. PRISMA 2020 for Abstracts Checklist. Additional file 2. PRISMA checklist. Additional file 3. Search equations.
Acknowledgements
The authors thank Dr Carole Ayav and Laura Smales for contributions.
Abbreviations
- COSMIN
COnsensus-based Standards for the selection of health status Measurement INstruments
- HRQoL
Health-Related Quality of Life
- CKD
Chronic Kidney Disease
- PROM
Patient-Reported Outcome Measures
- KDQOL
Kidney Disease Quality of Life
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- ICC
Intraclass Correlation
- DASS-21
Depression Anxiety Stress Scale-21
- EQ-5D-5L
5-Level EuroQol 5-dimensional questionnaire
- EQ-VAS
EuroQol Visual Analogue Scale
Authors’ contributions
MF drafted the article. All authors were involved in revising the article critically for important intellectual content, and all authors approved the final version to be submitted for publication. Study conception and design: MF, FG, AF. Literature search: MF. Article selection: MF, FG. Articles evaluation: MF, FG.
Authors’ information
MoF is a nephrologist, researcher at Cheikh Anta Diop University (UCAD) of Dakar, Senegal. MoF is a PhP student in epidemiology at the Lorraine University, Nancy, France and at Cheikh Anta Diop University (UCAD) of Dakar.
Funding
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Manns BJ, Klarenbach SW, James MT, Ravani P, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1:e184852. doi: 10.1001/jamanetworkopen.2018.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. US renal data system 2013 annual data report. Am J Kidney Dis. 2014;63(1 Suppl):A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24:1478–1483. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19:125. doi: 10.1186/s12882-018-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seck SM, Doupa D, Gueye L, Dia CA. Prevalence of chronic kidney disease and associated factors in senegalese populations: a community-based study in saint-louis. Nephrourol Mon. 2014;6:e19085. doi: 10.5812/numonthly.19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mclaren S, Jhamb M, Unruh M. Using patient-reported measures to improve outcomes in kidney disease. Blood Purif. 2021;50:649–654. doi: 10.1159/000515640. [DOI] [PubMed] [Google Scholar]
- 9.Wyld MLR, Morton RL, Clayton P, Wong MG, Jardine M, Polkinghorne K, et al. The impact of progressive chronic kidney disease on health-related quality-of-life: a 12-year community cohort study. Qual Life Res. 2019;28:2081–2090. doi: 10.1007/s11136-019-02173-1. [DOI] [PubMed] [Google Scholar]
- 10.Legrand K, Speyer E, Stengel B, Frimat L, Ngueyon Sime W, Massy ZA, et al. Perceived health and quality of life in patients with ckd, including those with kidney failure: findings from national surveys in France. Am J Kidney Dis. 2020;75:868–878. doi: 10.1053/j.ajkd.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Wee H-L, Seng BJJ, Lee JJ, Chong KJ, Tyagi P, Vathsala A, et al. Association of anemia and mineral and bone disorder with health-related quality of life in Asian pre-dialysis patients. Health Qual Life Outcomes. 2016;14:94. doi: 10.1186/s12955-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvert MJ, Freemantle N. Use of health-related quality of life in prescribing research. Part 1: why evaluate health-related quality of life? J Clin Pharm Ther. 2003;28:513–21. doi: 10.1046/j.0269-4727.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- 13.Epstein J, Santo RM, Guillemin F. A review of guidelines for cross-cultural adaptation of questionnaires could not bring out a consensus. J Clin Epidemiol. 2015;68:435–441. doi: 10.1016/j.jclinepi.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SM, McEwen J, McKenna SP. Measuring health status: a new tool for clinicians and epidemiologists. J R Coll Gen Pract. 1985;35:185–188. [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasz W, Piotr S. A trial of objective comparison of quality of life between chronic renal failure patients treated with hemodialysis and renal transplantation. Ann Transplant. 2003;8:47–53. [PubMed] [Google Scholar]
- 17.Aiyegbusi OL, Kyte D, Cockwell P, Marshall T, Gheorghe A, Keeley T, et al. Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with chronic kidney disease: a systematic review. PLoS One. 2017;12:e0179733. doi: 10.1371/journal.pone.0179733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yangöz ŞT, Turan Kavradim S, Özer Z, Boz İ. Psychometric properties of the kidney disease quality of life-36 instrument: a systematic review using COSMIN methodology. Nurs Health Sci. 2021;23:792–806. doi: 10.1111/nhs.12877. [DOI] [PubMed] [Google Scholar]
- 19.Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron. 1992;60:302–306. doi: 10.1159/000186769. [DOI] [PubMed] [Google Scholar]
- 20.Wu AW, Fink NE, Cagney KA, Bass EB, Rubin HR, Meyer KB, et al. Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE health experience questionnaire. Am J Kidney Dis. 2001;37:11–21. doi: 10.1053/ajkd.2001.20631. [DOI] [PubMed] [Google Scholar]
- 21.Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, Levenson DJ, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the dialysis symptom index. J Pain Symptom Manage. 2004;27:226–240. doi: 10.1016/j.jpainsymman.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69:1621–1625. doi: 10.1038/sj.ki.5000184. [DOI] [PubMed] [Google Scholar]
- 23.Franke GH, Reimer J, Kohnle M, Luetkes P, Maehner N, Heemann U. Quality of life in end-stage renal disease patients after successful kidney transplantation: development of the ESRD symptom checklist - transplantation module. Nephron. 1999;83:31–39. doi: 10.1159/000045470. [DOI] [PubMed] [Google Scholar]
- 24.Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, et al. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res. 2018;27:1171–9. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elamin S, E Elbasher AH, E Ali SE, Abu-Aisha H. Arabic translation, adaptation, and validation of the kidney disease quality of life short-form 36. Saudi J Kidney Dis Transpl. 2019;30:1322–32. doi: 10.4103/1319-2442.275476. [DOI] [PubMed] [Google Scholar]
- 26.Bouidida B, Rhou H, Ezaitouni F, Ouzeddoun N, Bayahia R, Elhajji K, et al. Translation, cultural adaptation and validation of the kidney disease quality of life-short form 1.3 in an African country. Transplant Proc. 2014;46:1295–301. doi: 10.1016/j.transproceed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Yaya K, Moustapha CM, Mohamed SS, Tall LA, Maria F, Christian H, et al. Quality of life of patients on peritoneal dialysis in Dakar: a Senegalese single centre experience. Open J of Nephrol. 2016;6:37–42. doi: 10.4236/ojneph.2016.62005. [DOI] [Google Scholar]
- 28.Cisse MM, Ka EF, Gueye S, Tall AOL, Faye M, Niang A, et al. Quality of life in hemodialysis patients in Dakar: differences for the tropics? Med Sante Trop. 2012;22:198–202. doi: 10.1684/mst.2012.0057. [DOI] [PubMed] [Google Scholar]
- 29.Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1147–1157. doi: 10.1007/s11136-018-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–964):W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 31.Terwee CB, Jansma EP, Riphagen II, de Vet HCW. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18:1115–1123. doi: 10.1007/s11136-009-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terwee CB, Prinsen CAC, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, et al. COSMIN standards and criteria for evaluating the content validity of health-related patient-reported outcomes measures: a Delphi study. Qual Life Res. 2018;27(5):1159–70. doi: 10.1007/s11136-018-1829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebrie MH, Asfaw HM, Bilchut WH, Lindgren H, Wettergren L. Psychometric properties of the kidney disease quality of life-36 (KDQOL-36) in Ethiopian patients undergoing hemodialysis. Health Qual Life Outcomes. 2022;20:24. doi: 10.1186/s12955-022-01932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abd ElHafeez S, Sallam SA, Gad ZM, Zoccali C, Torino C, Tripepi G, et al. Cultural adaptation and validation of the “Kidney Disease and Quality of Life-Short Form (KDQOL-SFTM) version 13” questionnaire in Egypt. BMC Nephrol. 2012;13:170. doi: 10.1186/1471-2369-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagasha P, Naitala R, Namukwaya E, Leng M, Katabira E, Namisango E. Cultural adaptation and validation of the Kidney Disease and Quality of Life-Short Form (KDQOL-SFTM) version 1.3 questionnaire in Uganda. Afr J Nephrol. 2022;25:116–35. [Google Scholar]
- 36.Bagasha P, Namukwaya E, Leng M, Kalyesubula R, Mutebi E, Naitala R, et al. Comparison of the health-related quality of life of end stage kidney disease patients on hemodialysis and non-hemodialysis management in Uganda. BMC Palliat Care. 2021;20:52. doi: 10.1186/s12904-021-00743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okpechi IG, Nthite T, Swanepoel CR. Health-related quality of life in patients on hemodialysis and peritoneal dialysis. Saudi J Kidney Dis Transpl. 2013;24:519. doi: 10.4103/1319-2442.111036. [DOI] [PubMed] [Google Scholar]
- 38.Masina T, Chimera B, Kamponda M, Dreyer G. Health related quality of life in patients with end stage kidney disease treated with haemodialysis in Malawi: a cross sectional study. BMC Nephrol. 2016;17:61. doi: 10.1186/s12882-016-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannor EK, Norman BR, Adusei KK, Sarfo FS, Davids MR, Bedu-Addo G. Quality of life among patients with moderate to advanced chronic kidney disease in Ghana - a single centre study. BMC Nephrol. 2019;20:122. doi: 10.1186/s12882-019-1316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Nigatu Y, Araya T, Assefa Z, Dereje N. Health related quality of life (HRQOL) of patients with End Stage Kidney Disease (ESKD) on hemodialysis in Addis Ababa, Ethiopia: a cross-sectional study. BMC Nephrol. 2021;22:280. doi: 10.1186/s12882-021-02494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shumbusho G, Hategeka C, Vidler M, Kabahizi J, McKnight M. Health related quality of life of patients undergoing in-centre hemodialysis in Rwanda: a cross sectional study. BMC Nephrol. 2022;23:345. doi: 10.1186/s12882-022-02958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims, guidance for industry. 2009. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Cited 2023 May 03.
- 43.Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 44.Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelstein FO, Wuerth D, Finkelstein SH. Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney Int. 2009;76:946–952. doi: 10.1038/ki.2009.307. [DOI] [PubMed] [Google Scholar]
- 46.Calvert M, Thwaites R, Kyte D, Devlin N. Putting patient-reported outcomes on the ‘Big data road map’. J R Soc Med. 2015;108:299–303. doi: 10.1177/0141076815579896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1: Additional file 1. PRISMA 2020 for Abstracts Checklist. Additional file 2. PRISMA checklist. Additional file 3. Search equations.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].