Abstract
Actinobacillus actinomycetemcomitans leukotoxin (Ltx) is a member of the repeats-in-toxin (RTX) family of pore-forming toxins and kills human immune cells. Currently, it remains unclear whether toxin-mediated killing of target cells involves the induction of necrosis or apoptosis. Therefore, the goal of this investigation was to determine whether Ltx is capable of causing apoptotic cell death in toxin-sensitive promyelocytic HL-60 cells. Multiparameter flow cytometric analysis of toxin-treated cells stained with Hoechst 33258 (or 33342) and 7-aminoactinomycin D allowed us to identify four populations: viable cells, early apoptotic cells, late apoptotic and/or secondarily necrotic cells, and a final population that was composed of cellular debris. Compared with control cells, HL-60 cells treated with Ltx exhibited a gradual decrease in forward light scatter with a coincident increase in side light scatter, indicative of a decrease in cell size and organelle condensation, respectively. Additional experiments demonstrated that Ltx-treated cells showed evidence of internucleosomal DNA fragmentation and phosphatidylserine translocation. The results of our studies clearly demonstrate that Ltx can kill HL-60 cells by inducing apoptosis. We hypothesize that elimination of acute inflammatory cells via this mechanism plays a critical role in the pathogenesis of diseases caused by A. actinomycetemeomitans.
Actinobacillus actinomycetemcomitans is a gram-negative, facultatively anaerobic bacterium that has been implicated as an etiologic agent of numerous human diseases, such as endocarditis, meningitis, osteomyelitis, and localized juvenile periodontitis (LJP). One of the potential virulence factors produced by A. actinomycetemcomitans is a 116-kDa leukotoxin (Ltx), a member of the repeats-in-toxin (RTX) family of pore-forming bacterial toxins, which includes Escherichia coli alpha-hemolysin and the cytotoxins synthesized by Actinobacillus pleuropneumoniae (20, 24, 26, 30). The common structural feature of the RTX toxins is a repeat domain containing multiple copies of the consensus sequence GGXGXDX(L/I/V/W/Y/F)X, which is critical to maintaining the cytolytic activity of these proteins. Whereas most members of the RTX family affect a wide variety of cell types from different species, Ltx-mediated killing is limited to a subset of human leukocytes. Recent work from our laboratory suggests that Ltx sensitivity is based on cell surface expression of a β2-integrin, lymphocyte function-associated antigen I (LFA-1) (19). Cell types known to express LFA-1 are lymphocytes, monocytes, and neutrophils, all of which play essential roles in the host defense against bacterial infection. Binding of Ltx to LFA-1 has been hypothesized to facilitate insertion of the toxin into the cell membrane and subsequent pore formation. Elimination of LFA-1-expressing cells via this mechanism may allow A. actinomycetemcomitans to evade detection by the host immune system and suggests that Ltx plays a key role in the development of diseases caused by this organism.
The mechanism by which A. actinomycetemcomitans Ltx kills target cells remains ambiguous. To date, two mechanisms of eukaryotic cell death have been identified: necrosis and apoptosis. Necrotic cell death has classically been considered a passive process resulting from physical injury. In contrast, cells undergoing apoptosis play an active role in their own demise, whereby either extracellular stimuli or genetic preprogramming activates a cascade of intracellular events resulting in ultrastructural and biochemical alterations. Cells exposed to Ltx are thought to undergo osmotic lysis secondary to pore formation in the cell membrane (9, 23, 45, 46). This paradigm, a process that would be consistent with necrosis, is based on the results of studies conducted with toxin-sensitive target cells, including polymorphonuclear leukocytes, macrophages, and a promyelocytic cell line, HL-60. In contrast, when peripheral blood lymphocytes (27) and NK cells (42) were exposed to relatively high doses of toxin for extended periods of time, very few cells showed evidence of cell death when assessing by vital staining. These cell types were therefore considered to be resistant to Ltx-mediated cytotoxicity. However, when examined by electron microscopy and flow cytometry, the cells exhibited alterations that were suggestive of the induction of apoptosis. More recently, the RTX leukotoxin produced by Pasteurella haemolytica was found to cause marked cytoplasmic blebbing and nuclear condensation, hallmarks of apoptosis, in bovine leukocytes (43). Additionally, Khelef and coworkers (15) reported that exposure to another RTX toxin, the Bordetella pertussis adenylate cyclase-hemolysin, resulted in alterations of nuclear morphology and DNA fragmentation in murine macrophages. These observations suggest that some RTX toxins are capable of inducing the morphologic changes indicative of apoptotic cell death.
The focus of this report is to further define the mechanism(s) by which A. actinomycetemcomitans Ltx kills human target cells. In this study, we have utilized several methods to determine whether exposure to the toxin induces apoptosis or an alternative form of cell death in Ltx-sensitive HL-60 cells. The results of our study provide morphologic, biochemical, and molecular evidence that Ltx does induce apoptosis in HL-60 cells. Specifically, Ltx-treated cells exhibited alterations consistent with apoptosis, including annexin binding, increased staining with Hoechst dyes, and internucleosomal DNA fragmentation.
MATERIALS AND METHODS
Cells.
The Ltx-sensitive human promyelocytic leukemia cell line HL-60 (ATCC CCL 240; American Type Culture Collection, Bethesda, Md.), was used for all experiments. Cells were grown in RPMI 1640 medium (GibcoBRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum, 0.1 mM minimal essential medium nonessential amino acids, 1× minimal essential medium vitamin solution, 2 mM l-glutamine, and 50 μg of gentamicin per ml. Cultures were maintained at 37°C under 7% CO2.
Preparation of recombinant Ltx.
Briefly, E. coli transformed with the pOTSNco12 vector (39, 40) containing the A. actinomycetemcomitans Ltx ltxC and ltxA genes (18) was grown at 37°C in Luria-Bertani medium supplemented with 0.1 mg of ampicillin per ml. After the cultures had grown to an optical density at 550 nm of 0.4, nalidixic acid (Sigma, St. Louis, Mo.) was added to a final concentration of 0.06 mg/ml to induce expression of the ltx genes. Induced cultures were grown for an additional 4 h, at which time the bacteria were pelleted, washed with cold phosphate-buffered saline (PBS), aliquoted, and stored at −20°C. Immediately prior to use, bacterial pellets were resuspended in RPMI 1640 and sonicated for 40 s with a Virsonic 300 sonicator (VirTis, Gardiner, N.Y.). The suspensions were spun at 14,000 × g for 5 min such that the leukotoxic activity was retained in the supernatants. Sonicates prepared from E. coli transformed with the pOTSNco12 vector lacking the ltx genes were used as experimental controls (referred to as vector control supernatant). In some experiments, Ltx was heat inactivated by incubating the sonicate at 60°C for 1 h.
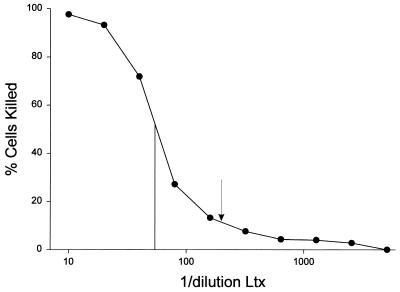
Fresh sonicates containing the recombinant Ltx were prepared for each experiment. Leukotoxic activity was determined by measuring vital-dye uptake. Briefly, 2 × 105 HL-60 cells were incubated in 100 μl of vector control supernatant or serial dilutions of Ltx-containing supernatants at 37°C for 1 h. The cells were then placed on ice, 100 μl of trypan blue (0.4%) was added to each well, and the surviving cells were counted with a hemocytometer. Cells in at least four fields were counted in triplicate, and results were averaged for each dilution assayed. Percent lysis was calculated by dividing the number of surviving cells by the number of cells in the negative controls and subtracting this value from 100. Ltx-mediated killing of HL-60 cells was found to occur in a dose-dependent fashion (Fig. 1). Heat treatment (60°C for 1 h) or preincubation of sonicates with the Ltx-specific monoclonal antibody 107 resulted in elimination of leukotoxic activity (data not shown). HL-60 cells treated with sonicates prepared from mock-transformed E. coli were 100% viable. One unit of leukotoxic activity (Ltx unit) was defined as the dilution of Ltx that killed 50% of 2 × 105 HL-60 cells in 1 h. For all subsequent experiments, cells were treated with 0.2 Ltx unit unless mentioned otherwise.
FIG. 1.
Dose dependence of Ltx-mediated cytolysis of HL-60 cells. Leukotoxic activity was determined by incubating HL-60 cells with serial dilutions of Ltx-containing supernatants at 37°C for 1 h and determining the numbers of surviving cells. Percent lysis was calculated by dividing the number of surviving cells by the number of cells in the negative controls (HL-60 cells incubated with vector control supernatants) and subtracting the result from 100. To standardize experiments, 1 U of Ltx activity was defined as the dilution of sonicate that killed 50% of the HL-60 cells after a 1-h incubation (1.0 Ltx unit was equivalent to a 1:60 dilution [vertical line] in this particular experiment). The data show that toxin-mediated cytolysis is a dose-dependent phenomenon. For all subsequent experiments, cells were treated with 0.2 Ltx unit unless mentioned otherwise. This was equivalent to the dilution that resulted in killing of 10% of HL-60 cells after a 1-h incubation (arrow).
Flow cytometry (fluorescence-activated cell sorting).
All of the flow cytometry experiments were analyzed with a FacstarPlus flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
(i) Analysis of Hoechst and 7-AAD staining.
Differential staining with specific fluorochromes can be used to distinguish cells undergoing apoptosis from vital and necrotic cells (4). Therefore, staining of Ltx-treated HL-60 cells with Hoechst 33258 (or 33342) and 7-aminoactinomycin D (7-AAD) (Sigma) was evaluated as previously described (5, 35, 41). HL-60 cells (106) were cultured in the presence or absence of Ltx. Hoechst 33258 or 33342 (1 μg/ml; Sigma) was then added to each sample, and the tubes were incubated at 37°C for 7 min. The tubes were immediately placed on ice, 7-AAD (1 μg/ml) was added, and the tubes were incubated for 10 min, after which the cells were analyzed by flow cytometry. Hoechst fluorescence was excited at 310 nm, and emitted light (50 mW) was detected with a 424/44-nm bandpass filter and logarithmic amplification. 7-AAD fluorescence was assayed simultaneously with a second laser (488 nm, 250 mW) and detected with a 660/20-nm bandpass filter and linear amplification.
(ii) Evaluation of light scatter.
Morphologic changes associated with different mechanisms of cell death affect the light scatter properties of a given cell type. Therefore, the forward light scatter (FSC) and side (90°) light scatter (SSC) properties of cells treated with Ltx or vector control supernatants were determined with each fluorescence experiment. Light scatter measurements were done with linear amplification.
(iii) Quantitation of DNA fragmentation.
DNA fragmentation was measured by the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-fluorescein isothiocyanate [dUTP-FITC] nick end labeling) assay with the In Situ Cell Death Detection Kit (Boehringer Mannheim, Indianapolis, Ind.). Briefly, aliquots of 106 HL-60 cells were treated with 0.2 Ltx unit for 2 to 24 h, washed twice with PBS, and fixed by incubation with 4% paraformaldehyde for 30 min on ice. The cells were washed twice, permeabilized by treatment with 0.1% Triton in 0.1% citric acid on ice for 2 min, and then washed. The labeling reaction was carried out by adding solutions containing terminal deoxynucleotidyl transferase, dUTP-FITC, and unlabeled nucleotides (according to the manufacturer’s instructions) and incubating the tubes at 37°C for 1 h. The samples were washed twice and finally resuspended in PBS for flow cytometric analysis. dUTP-FITC fluorescence was excited with a laser operating at 488 nm (250 mW) and detected with a 530/30-nm bandpass filter with logarithmic amplification.
(iv) Evaluation of alterations in cell membrane asymmetry.
Translocation of phosphatidylserine (PS) from the inner leaflet to the outer leaflet of the plasma membrane has been observed in many cell types undergoing apoptosis. To determine whether Ltx-mediated killing of HL-60 cells involves a similar perturbation in plasma membrane asymmetry, we measured PS translocation by binding of annexin-FITC to toxin-treated cells, using the Apoptosis Detection Kit (R&D Systems, Minneapolis, Minn.) (16). This protein is known to bind PS in a Ca2+-dependent fashion. Briefly, 106 HL-60 cells were treated with Ltx, washed twice with cold PBS, and stained according to the manufacturer’s recommendations. 7-AAD was used to determine alterations in membrane permeability. For flow cytometry, fluorescence was excited with a laser emitting light at 488 nm and 250 mW. Annexin-FITC was detected with a 530/20-nm bandpass filter and 7-AAD was detected with a 660/20-nm bandpass filter, as previously described (44).
Transmission electron microscopy (TEM).
Aliquots of HL-60 cells (106) were incubated with 0.2 Ltx unit at 37°C for 18 h. Cells (4 × 106) were then pooled into a single tube and stained with Hoechst 33342 and 7-AAD as described above. A Becton Dickinson FacstarPlus flow cytometer was used to sort the cells on the basis of Hoechst 33342 and 7-AAD fluorescence. This gave rise to four populations of cells with the following phenotypes: Hoechst 33342dim/7-AADdim, Hoechst 33342bright/7-AADdim, Hoechst 33342bright/7-AADbright, and Hoechst 33342dim/7-AADbright. Cells corresponding to each population were collected in Haemoline (BioChem Pharma, Allentown, Pa.), transferred to microcentrifuge tubes, pelleted, and fixed in 1% osmium tetroxide (in distilled H2O). A total of 4 × 107 cells were sorted in order to collect 2 × 106 cells representative of each of the individual populations. After dehydration through a series of graded alcohol and propylene oxide solutions, the cells were infiltrated with Epon (epoxy resin) and polymerized. Ultrathin sections were cut, recovered on Formvar-coated copper grids, stained with uranyl acetate and lead citrate, and then examined with a 100 CXII transmission electron microscope (Jeol, Tokyo, Japan) operated at 80 kV.
Scanning electron microscopy (SEM).
HL-60 cells (2 × 106) were incubated with 0.2 Ltx unit for 18 h at 37°C and then transferred to microcentrifuge tubes, pelleted, and treated with Karnovsky’s fixative. The fixed cells were distributed on a 0.2-μm-pore-size filter (Nuclepore Corp., Pleasanton, Calif.) clamped between a sintered glass support and a 15-ml column. The cells were dehydrated by adding a series of graded ethanol solutions to the filtration system and then slowly dried over the course of 24 h by evaporation from 5.0 ml of Freon. The filter was removed from the filtration apparatus and mounted on an aluminum stub, after which the cells were gold sputter coated. A T330A scanning electron microscope (Jeol) was used to analyze and photograph the cells.
Analysis of DNA fragmentation by agarose gel electrophoresis.
Induction of DNA fragmentation following exposure of HL-60 cells to Ltx was evaluated according to the method of Wolfe et al. (50). Briefly, 106 HL-60 cells were treated with Ltx for 18 h at 37°C and then transferred to microcentrifuge tubes. The cells were washed twice with cold PBS, and the pellet was resuspended in 15 μl of sterile distilled H2O and vortexed. Ten microliters of RNase solution (50 mg/ml) and 9 μl of loading buffer (0.02% bromophenol blue and 40% glycerol in 1× Tris-borate-EDTA [TBE]) were added, and the tubes were incubated at room temperature for 30 min. A 1.8% agarose running gel with a digestion gel (0.8% agarose in 1× TBE containing 2% sodium dodecyl sulfate and 50 μg of proteinase K per ml) above the sample wells was then prepared. The samples and standards (123-bp ladder [GibcoBRL]) were loaded into the appropriate wells. The gel was run at 2 V/cm for 1 h and then at 10 V/cm for 2 h. The gel was stained with 0.5 μg of ethidium bromide per ml in distilled H2O for 30 min, destained for 3 h, and photographed.
Statistical analysis.
Student’s t test was used to compare the effects of Ltx and vector control (pOTS) supernatants on Hoechst and 7-AAD staining of HL-60 cells.
RESULTS
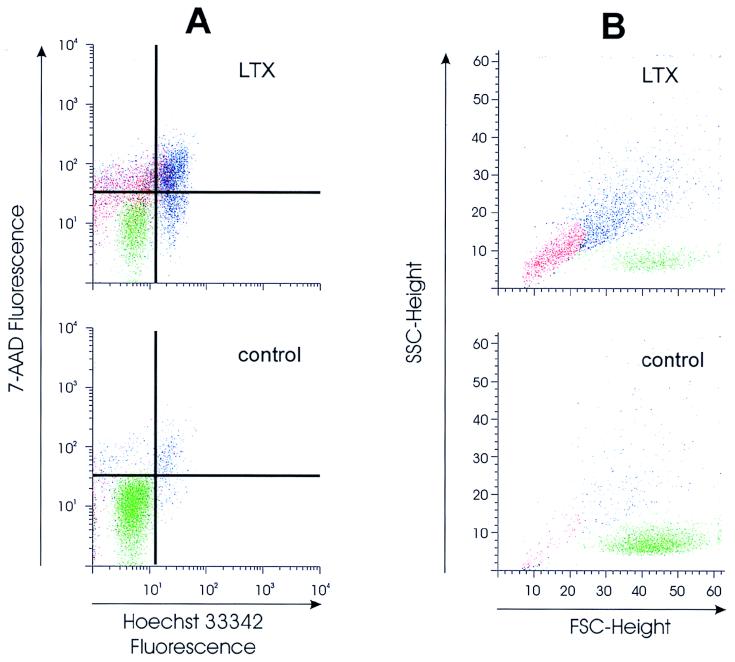
The ability of Ltx to induce apoptosis in HL-60 cells was assessed by multiparameter flow cytometric analysis following staining with Hoechst 33342 and 7-AAD. This method was chosen because it has been shown to be a reliable technique for discriminating between apoptosis and necrosis in a variety of cell types (5, 35, 41). As shown in Fig. 2A, cells treated with vector control supernatant were observed within a discrete population that exhibited minimal staining with either dye (lower left quadrant). In contrast, quadrant analysis of the same cells following an 18-h exposure to Ltx resulted in the generation of four populations. Viable cells (43.3%) exhibited dim fluorescence for both stains (lower left quadrant), similar to that observed for control cells. Apoptotic cells appeared as two populations. The first population contained cells in the early stages of apoptosis that were characterized as Hoechst 33342bright/7-AADdim (10.4%) and appeared in the lower right quadrant. The second population was composed of cells in the late stages of apoptosis and undergoing secondary necrosis that were Hoechst 33342bright/7-AADbright (29.9%). These cells were detected in the upper right quadrant. The remaining cells (16.4%) stained with only 7-AAD and were located in the upper left quadrant.
FIG. 2.
Multiparameter flow cytometric analysis of Ltx-treated HL-60 cells. Cells were exposed to Ltx (0.2 Ltx unit) or vector control supernatant for 18 h and then stained with Hoechst 33342 and 7-AAD. Cellular fluorescence (A) and light scatter (B) were then analyzed by flow cytometry. Based on the levels of blue and red fluorescence, four populations were observed following toxin treatment: Hoechstdim/7-AADdim (vital cells, lower left quadrant), Hoechstbright/7-AADdim (apoptotic cells, lower right quadrant), Hoechstbright/7-AADbright (cells in the late stages of apoptosis with secondary necrosis, upper right quadrant), and Hoechstdim/7-AADbright (cellular debris, upper left quadrant). Analytical gates were then applied to these populations in order to correlate their light scatter and Hoechst and 7-AAD staining characteristics (B). Cells treated with vector control supernatant were detected within a single discrete population (representing 90.6% of the cells) that demonstrated relatively high FSC (MCF = 44.2) and low SSC (MCF = 7.4). A similar, albeit smaller, population of viable Hoechstdim/7-AADdim cells was observed following toxin treatment (green, 43.3% of the cells). Apoptotic cells (Hoechstbright/7-AADdim and Hoechstbright/7-AADbright) were contained within a single population (blue, 40.3% of the cells) that exhibited a decrease in FSC (MCF = 33.0) and an increase in SSC (MCF = 20.0) relative to viable cells. The Hoechstdim/7-AADbright population (red, 16.4% of the cells) exhibited a decrease in FSC (MCF = 14.5) and virtually identical SSC (MCF = 7.8) compared to viable cells, suggesting that it was composed of cellular debris as opposed to intact cells. These results are representative of those from four experiments; at least 10,000 cells were analyzed per experiment.
To further assess the fate of HL-60 cells following exposure to Ltx, the morphology of toxin-treated cells was examined. Cells undergoing apoptosis exhibit distinctive changes in their size and compaction which can be measured by fluorescence-activated cell sorter analysis as alterations in FSC and SSC, respectively. An initial comparison of the light scatter patterns of toxin-treated HL-60 cells and control cells is shown in Fig. 2B. Analysis of control cells showed a single discrete population of cells that exhibited relatively high FSC and low SSC (mean channel fluorescence [MCF] values of 44.2 and 7.4, respectively). In comparison, three populations were observed following treatment with Ltx. The first group of cells exhibited light scatter properties that were virtually identical to those of control cells (FSC MCF = 43.8 and SSC MCF = 7.7). The second population was composed of cells that demonstrated decreased FSC (MCF = 33.0) and increased SSC (MCF = 20.0) relative to the control cells. Such changes are indicative of a reduction in cell size and organelle condensation, both of which are consistent with the morphological alterations associated with apoptosis. The final population resulting from toxin treatment exhibited a decrease in FSC (MCF = 14.5) without a change in SSC (MCF = 7.8) compared with the control cells. The extremely low FSC of this population suggested that it was composed of cellular fragments as opposed to intact cells. To relate these morphologic parameters to the Hoechst and 7-AAD staining characteristics of toxin-treated cells, analytical gates were applied to each of the three populations identified by light scatter (Fig. 2). The population with relatively high FSC and low SSC was found to be composed of viable Hoechst 33342dim/7-AADdim cells (green). Cells in the early stages of apoptosis (Hoechst 33342bright/7-AADdim), as well as those in the late phases of apoptosis and undergoing secondary necrosis (Hoechst 33342bright/7-AADbright cells), were localized within the population that showed low FSC and high SSC (blue). The population that exhibited low FSC and low SSC contained predominantly Hoechst 33342dim/7-AADbright cells (red).
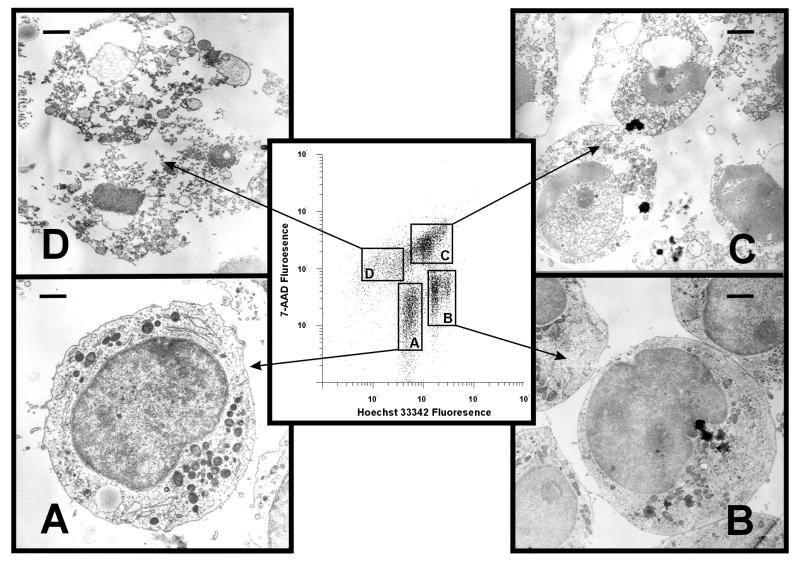
Ultrastructural characterization of cells representative of the individual populations was achieved through cell sorting and subsequent evaluation by TEM (Fig. 3). Hoechst 33342dim/7-AADdim cells (gate A) exhibited ultrastructural properties consistent with normal HL-60 cells. Cells in this population possessed well-defined plasma membranes and contained intact organelles with no evidence of nuclear condensation (Fig. 3A). In contrast, the Hoechst 33342bright/7-AADdim cells (gate B) represented cells in the early stages of apoptosis. The cells were smaller and the cytoplasm appeared less granular than for control cells. Cytoplasmic organelles, such as the Golgi apparatus and rough endoplasmic reticulum, were noted far less frequently in the Hoechst 33342bright/7-AADdim cells than in Hoechst 33342dim/7-AADdim or untreated controls cells. The Hoechst 33342bright/7-AADdim cells also contained sharply circumscribed intracytoplasmic electron-dense masses and occasional blebbing of their plasma membranes (Fig. 3B), neither of which was present in untreated cells. The nuclei of these cells were less granular than those of Hoechst 33342dim/7-AADdim cells. Hoechst 33342bright/7-AADbright cells (gate C) showed the chromatin clumping that is typical of apoptotic cells. A large number of these cells also showed disruption of their plasma membranes. Finally, the Hoechst 33342dim/7-AADbright population (gate D) was composed almost exclusively of cellular debris that was likely generated during secondary necrosis. A majority of the structures (Fig. 3D) did not contain nuclear material, explaining their failure to stain with Hoechst 33342.
FIG. 3.
TEM of HL-60 cells exposed to Ltx. HL-60 cells were incubated with 0.2 Ltx unit for 18 h and then stained and sorted on the basis of Hoechst and 7-AAD fluorescence. A dual-parameter plot analysis was performed on these cells; four sort windows (center panel; gates A, B, C and D) were set, and cells were sorted and analyzed by TEM. (A) Cell representative of the Hoechstdim/7-AADdim population. These cells had ultrastructural features identical to those of untreated HL-60 cells. (B) Cells typical of those found in the Hoechstbright/7-AADdim population, a majority of which exhibited morphologic characteristics typical of cells in the early stages of apoptosis. These included a decrease in cell size, intact cell membranes, and flocculation of the nucleus. (C) Cells derived from the Hoechstbright/7-AADbright population, demonstrating that these cells had undergone chromatin condensation, nuclear fragmentation, loss of plasma membrane integrity, and significant cytoplasmic vacuolization. These are characteristic features of cells undergoing the late stages of apoptosis and/or secondary necrosis. (D) Hoechstdim/7-AADbright population, composed of cellular debris resulting from the degeneration of apoptotic cells via secondary necrosis. Magnification, 5 mm = 1 μm. Bars, 7 mm.
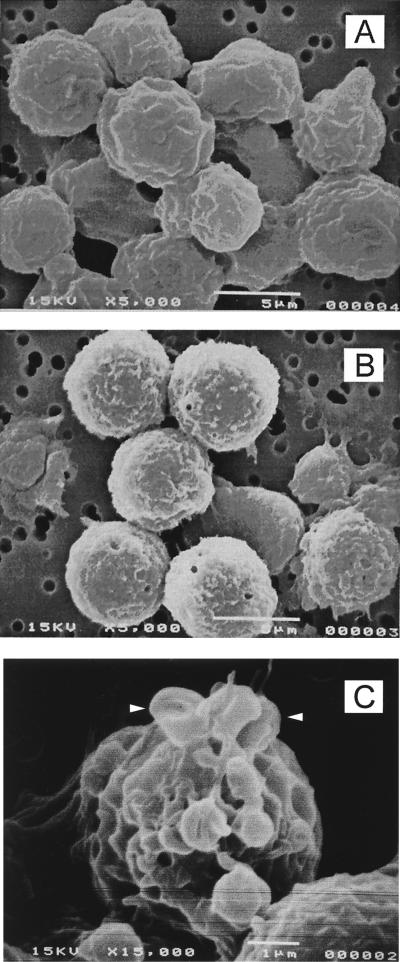
SEM confirmed that HL-60 cells treated with Ltx showed morphologic features indicative of apoptosis. Compared to cells treated with control supernatants, approximately 25% of cells exposed to 0.2 Ltx unit for 18 h appeared to be markedly shrunken (Fig. 4A and B). These cells were devoid of the villous projections found on the surfaces of control cells. Additionally, many of the toxin-treated cells exhibited perturbations of their plasma membranes as evidenced by membrane budding (Fig. 4C, arrowheads). Taken together, TEM and SEM analyses clearly demonstrated that Ltx-treated cells possessed many of the morphologic characteristics commonly observed in cells undergoing apoptosis.
FIG. 4.
SEM of HL-60 cells exposed to Ltx. HL-60 cells were incubated with vector control (pOTS) supernatant (A) or 0.2 Ltx unit (B and C) for 18 h and then fixed, processed, and analyzed by SEM. Relative to controls, toxin-treated cells exhibited smoothing of the cell surface and a decrease in cell size (B). At a higher magnification (C), membrane budding (arrowheads) was detected in cells exposed to Ltx.
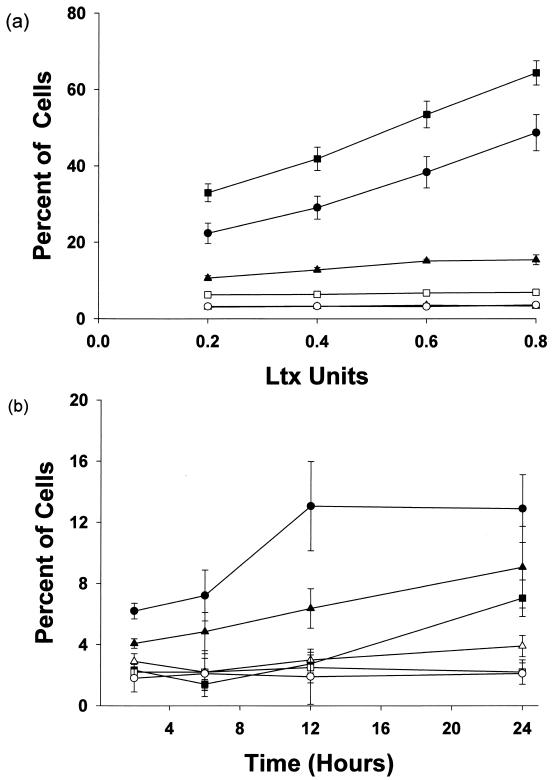
The frequency of apoptotic cells was directly related to the Ltx dose as evidenced by the dose-dependent increase in the numbers of Hoechstbright/7-AADdim and Hoechstbright/ 7-AADbright cells in the toxin-treated group relative to cells exposed to vector control supernatant (Fig. 5a). The increases in the numbers of Hoechstbright/7-AADdim and Hoechstbright/ 7-AADbright cells following treatment with Ltx (at all doses tested) were found to be statistically significant compared to control values (P < 0.01). These effects were completely inhibited by heating the toxin at 60°C for 1 h (data not shown).
FIG. 5.
Ltx-induced alterations in Hoechst and 7-AAD staining of HL-60 cells. (a) HL-60 cells were incubated with increasing doses of Ltx (closed symbols) or appropriate dilutions of vector control supernatant (open symbols) for 18 h. The cells were then stained with Hoechst 33258 and 7-AAD and analyzed by flow cytometry. The percentages of Hoechstbright/7-AADdim (triangles), Hoechstbright/7-AADbright (circles), and total apoptotic (squares) cells are indicated. The data represent the means and standard errors of the means from three separate experiments. The increased numbers of Hoechstbright/7-AADdim and Hoechstbright/7-AADbright cells detected following toxin treatment were statistically significant at all doses tested compared to control cells (P < 0.01). The data suggest that noncytolytic doses of toxin were capable of inducing apoptosis in HL-60 cells. (b) The kinetics of Ltx-induced alterations in Hoechst and 7-AAD staining of HL-60 cells were evaluated over a 24-h time course. Cells were incubated with 0.2 Ltx unit (closed symbols) or an appropriate dilution of vector control supernatant (open symbols) for 2, 6, 12, or 24 h and then stained with Hoechst 33342 and 7-AAD and analyzed by flow cytometry. The percentages of Hoechstbright/7-AADdim (triangles), Hoechstbright/7-AADbright (circles), and Hoechstdim/7-AADbright (squares) cells are indicated. The data represent the means and standard errors of the means from two separate experiments. These results suggest that Ltx-treated cells initially became Hoechstbright/7-AADdim and subsequently took up 7-AAD as they entered the later stages of apoptosis.
Time course experiments were conducted to evaluate the kinetics of Ltx-induced apoptosis. There was a steady increase in the number of Hoechstbright/7-AADdim cells following treatment with 0.2 Ltx unit over the course of 24 h (Fig. 5b). In contrast, the number of Hoechstbright/7-AADbright cells plateaued at 12 h. Relative to cellular debris, we detected an increase in the number of Hoechstdim/7-AADbright events starting at the 12-h time point. These data suggest that following exposure to Ltx, HL-60 cells initially became Hoechstbright/7-AADdim and subsequently took up 7-AAD as the cells entered the later stages of apoptosis. Ultimately, some of the Hoechstbright/7-AADbright cells disintegrated and entered the Hoechstdim/7-AADbright pool. This observation explains the plateauing that was observed with respect to the number of Hoechstbright/7-AADbright cells that was seen at 12 h. It therefore appears that the Hoechstbright/7-AADbright compartment reaches a state of equilibrium at 12 h due to a constant influx of cells from the Hoechstbright/7-AADdim pool and an efflux of cells into the Hoechstdim/7-AADbright pool. The generation of apoptotic cells exhibited the same kinetics when HL-60 cells were treated with 0.4 or 0.6 Ltx unit (data not shown). Treatment with doses of toxin exceeding 0.8 Ltx unit resulted in the disintegration of a majority of the cells by the 2-h time point.
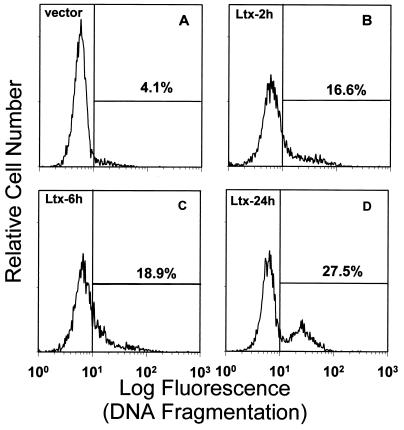
In addition to characteristic changes in morphology, cells undergoing apoptosis also exhibit fragmentation of their genomic DNA. Two different methods were used to document DNA fragmentation in Ltx-treated cells. Initially, the TUNEL assay (Fig. 6) was used to evaluate cells that had been treated with 0.2 Ltx unit for 2, 6, or 24 h. After treatment with vector control supernatant for 24 h, 4.1% of HL-60 cells were TUNEL positive (Fig. 6A). Following a 2-h exposure to Ltx, 16.6% of HL-60 cells were TUNEL positive (Fig. 6B). The percentage of positive cells increased over time to a maximum of 27.5% after 24 h (Fig. 6D). Toxin-induced DNA fragmentation was dose dependent and heat inhibitable (data not shown). The results of these experiments showed that minimally cytolytic doses of Ltx were capable of inducing DNA fragmentation in HL-60 cells.
FIG. 6.
Flow cytometric analysis of Ltx-induced DNA fragmentation in HL-60 cells. HL-60 cells were incubated with vector control (pOTS) supernatant (A) for 24 h or with 0.2 Ltx unit for 2, 6, or 24 h (B through D, respectively) and then labeled by the TUNEL reaction. The cells were then analyzed on a flow cytometer, and the data are plotted as the relative amount of DNA fragmentation (log fluorescence) versus relative cell number. In this representative experiment, 27.5% of the cells treated with 0.2 Ltx unit for 24 h were TUNEL positive, while 4.1% of vector control supernatant-treated cells were positive. The frequency of cells exhibiting DNA cleavage increased with time: 16.6 and 18.9% were TUNEL positive following 2- and 6-h exposures to Ltx, respectively. These results are representative of those from three experiments; at least 10,000 cells were analyzed per experiment.
To distinguish between the ordered generation of DNA fragments that occurs during apoptosis and the random cleavage that is seen in the latter stages of necrosis, agarose gel electrophoresis (Fig. 7) was utilized. HL-60 cells were treated with a range of doses of toxin for 3 h and then analyzed by the method of Wolfe et al. (50). DNA laddering characteristic of apoptosis was observed in cells treated with 0.8, 0.4, or 0.2 Ltx unit (Fig. 7, lanes 1, 2, and 3, respectively). Untreated HL-60 cells (Fig. 7, lane 4) and cells exposed to supernatant prepared from uninduced E. coli (lanes 5 and 6) exhibited intact genomic DNA. In the toxin-treated cells, DNA degradation that was not due to internucleosomal cleavage could be seen; however, this was not unexpected. From our dose-response and time course experiments, we knew that 15 to 20% of the cells treated with 0.2 Ltx unit for 18 h were in the late stages of apoptosis (undergoing secondary necrosis). At this stage in apoptosis, there is random cleavage of the DNA that is most likely responsible for the smearing seen in Fig. 7. In contrast, the bands composing the ladder represent the state of the DNA in the 10 to 15% of the cells that had not yet entered the late stages of the cell death process. Using pulsed-field gel electrophoresis, we were unable to detect the larger fragments of DNA (50 to 300 kb) observed in some models of apoptosis (data not shown) (34, 49). This observation indicated that Ltx induced rapid cleavage of HL-60 DNA into the smaller fragments seen with conventional agarose gel electrophoresis.
FIG. 7.
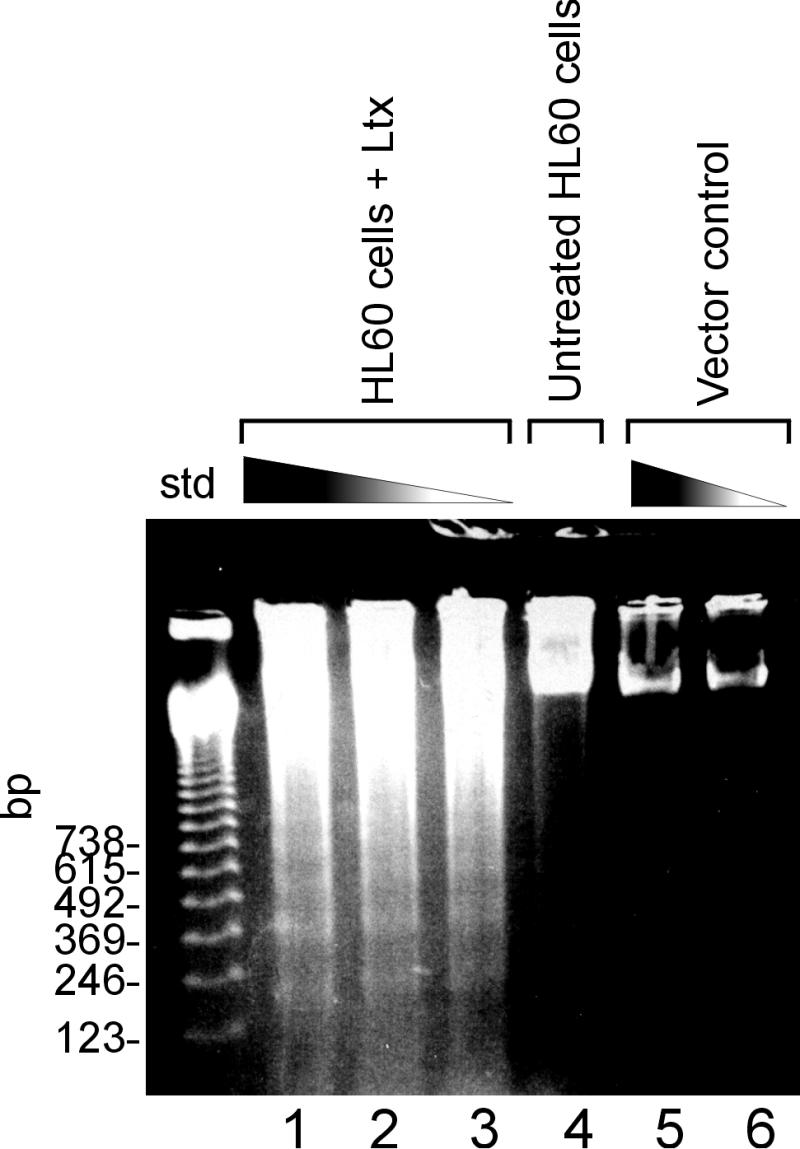
Electrophoretic analysis of DNA from HL-60 cells exposed to Ltx. HL-60 cells (106) were treated with Ltx for 18 h at 37°C and then transferred to microcentrifuge tubes. Samples were then prepared for agarose gel electrophoresis according to the method of Wolfe et al. (50) by using a 1.8% agarose running gel containing a digestion gel (0.8% agarose in 1× TBE containing 2% sodium dodecyl sulfate and 50 μg of proteinase K per ml) above the sample wells. The samples and standards (std) (123-bp ladder [GibcoBRL]) were loaded into the appropriate wells, and the gel was run at 2 V/cm for 1 h and then at 10 V/cm for 2 h. DNA laddering was detected in cells treated with 0.8, 0.4, and 0.2 Ltx unit (lanes 1, 2, and 3, respectively). Untreated HL-60 cells (lane 4) and cells exposed to supernatant prepared from uninduced E. coli (lanes 5 and 6) exhibited intact genomic DNA.
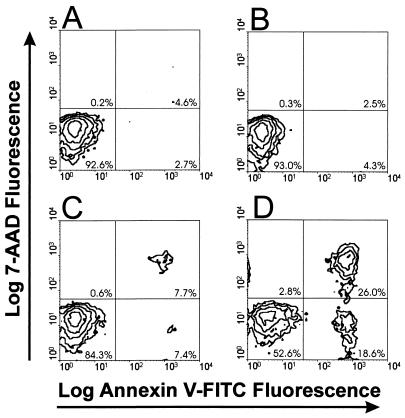
Finally, the ability of Ltx to induce translocation of phosphatidylserine (PS) from the inner to the outer leaflet of the plasma membrane was evaluated. Ltx-treated cells were incubated with FITC-labeled annexin V and subsequently analyzed by flow cytometry. In these experiments (Fig. 8), cells were also stained with 7-AAD to rule out the possibility that annexin V was binding to PS within the inner leaflet of the cell membrane secondary to changes in membrane permeability. After incubation of HL-60 cells with control supernatant for 18 h, 2.7% of cells bound annexin V-FITC and excluded 7-AAD (Fig. 8A), while 18.6% of the cells treated with 0.3 Ltx unit were FITCbright/7-AADdim (Fig. 8D). These results indicated that Ltx did induce the externalization of PS in HL-60 cells. This effect was found to be dose dependent. In this representative experiment, 4.3% of the cells treated with 0.075 Ltx unit and 7.4% of those treated with 0.15 Ltx unit were FITCbright/7-AADdim (Fig. 8B and C, respectively). The numbers of apoptotic cells detected by this technique were in agreement with those measured in our other assays. There was a simultaneous dose-dependent increase in the number of HL-60 cells that bound annexin V and were 7-AADbright. Time course experiments failed to detect necrotic cells (FITCdim/7-AADbright), suggesting that the FITCbright/7-AADbright population is composed of cells in the late stages of apoptosis (data not shown). Additionally, the onset of PS externalization occurred following a 10-min exposure of HL-60 cells to the toxin. It therefore appeared that translocation of PS was an early event in Ltx-induced apoptosis. This observation is consistent with the kinetics seen in other models of apoptotic cell death (4, 28).
FIG. 8.
Ltx-induced alterations in HL-60 cell membrane asymmetry. HL-60 cells were incubated with vector control (pOTS) supernatant (A), 0.075 Ltx unit (B), 0.15 Ltx unit (C), or 0.3 Ltx unit (D) for 18 h. Cells were then stained with fluoresceinated annexin V and 7-AAD and analyzed by flow cytometry. A total of 2.7% of HL-60 cells incubated with vector control supernatant for 18 h bound annexin V-FITC and excluded 7-AAD (A), whereas 18.6% of the cells treated with 0.3 Ltx unit (D) exhibited this phenotype. These results indicate that Ltx did induce the externalization of PS in HL-60 cells without altering permeability to 7-AAD. This effect was found to be dose dependent, as 4.3% of cells treated with 0.075 Ltx unit and 7.4% of those treated with 0.15 Ltx unit were FITCbright/7-AADdim (B and C, respectively). There was also a dose-dependent increase in the number of cells within the FITCbright/7-AADbright population, which represented cells undergoing late apoptosis and/or secondary necrosis. These data are representative of those from three experiments; at least 10,000 cells were analyzed per experiment.
DISCUSSION
Although it is well established that pore-forming bacterial toxins have potent cytotoxic activity, the mechanism(s) by which they kill cells is not clearly defined. Previous reports have shown that killing of polymorphonuclear leukocytes and HL-60 cells by A. actinomycetemcomitans Ltx, a member of the RTX family of pore-forming toxins, was rapid and showed minimal evidence of apoptosis (27, 29, 42, 47). When T-lymphocyte and NK-cell intoxication was analyzed, the kinetics of cell death were more protracted and the cells showed both morphologic and cytometric evidence of apoptosis (27, 42). In the current study, we have decreased the toxin dosage and utilized sensitive histologic, molecular, and flow cytometric techniques to determine if, in fact, HL-60 cells can undergo apoptotic cell death following exposure to Ltx. Analysis of toxin-treated cells by electron microscopy revealed numerous alterations in cell morphology that were indicative of the induction of apoptosis. These included a generalized decrease in cell size, chromatin condensation, and the generation of electron-dense amorphous intracytoplasmic bodies (7, 8, 13, 32). Agarose gel electrophoresis was used to show that the toxin induced internucleosomal DNA degradation, one of the hallmarks of apoptosis. The extent of Ltx-induced DNA fragmentation was found to be both time and dose dependent as determined by using the TUNEL assay. Minimal DNA cleavage was detected at early time points following addition of the toxin, which is consistent with this being a relatively late event in the apoptotic process. Most notable was the observation that significant fragmentation was detected in cells treated with doses of toxin that elicited minimal uptake of trypan blue. Historically, cells excluding such vital dyes were considered to be viable. Our current results suggest that many of these cells are actually undergoing apoptotic cell death despite having relatively intact cell membranes.
In addition to changes in morphology and DNA fragmentation, cells undergoing apoptosis display other subtle molecular and biochemical alterations. These include (i) perturbations in the structure of their plasma membranes that affect the maintenance of the asymmetry of the lipid bilayer, (ii) changes in cellular staining with specific fluorochromes, and (iii) dissipation of mitochondrial transmembrane potentials (reviewed by Kroemer et al. [17]). HL-60 cells exposed to low doses of Ltx bound fluoresceinated annexin V while excluding 7-AAD. This is due to a change in cell membrane asymmetry that results in exposure of PS residues within the inner leaflet of the membrane to the extracellular environment and is again associated with apoptosis (4, 16). We failed to detect significant numbers of cells that stained with 7-AAD and did not bind annexin, a phenotype characteristic of necrotic cells. Following exposure of cells to Ltx, we observed statistically significant increases in the numbers of Hoechstbright/7-AADdim and Hoechstbright/7-AADbright cells relative to controls. These cell populations are representative of cells in the early and late stages of apoptosis, respectively (38). Time course experiments showed that Ltx-treated cells initially became Hoechstbright/7-AADdim and ultimately contributed to the Hoechstbright/7-AADbright pool as they entered the later stages of apoptosis. These observations are in agreement with our annexin and 7-AAD data, which taken together show that under the conditions of our studies, Ltx induces apoptosis in HL-60 cells.
While the ultrastructural features of Hoechst 33342dim/7-AADdim cells were identical to those of untreated control cells, Hoechst 33342bright/7-AADdim cells were comparatively smaller and showed a distinct loss in nuclear granularity. A decrease in cytoplasmic organelles, including the Golgi apparatus and rough endoplasmic reticulum, was also noted in the Hoechst 33342bright/7-AADdim cells compared to normal cells or untreated controls. These cells did not show evidence of the classical nuclear changes, such as chromatin condensation, suggesting that they were in the very early stages of apoptotic cell death. The decreased cell size and presence of electron-dense masses in the cytoplasm of these cells may explain the differences we noted in the light scatter analysis of this population relative to normal cells.
Ultrastructural analysis of the Hoechst 33342bright/7-AADbright population revealed margination of the chromatin in many of the cells. The nuclear changes observed in these cells are indicative of the cells being in the later stages of apoptosis (5, 35, 41). These cells also showed significant plasma membrane disruption. This finding was not expected, since examination of unsorted Ltx-treated cells via TEM did not show any drastic membrane changes in cells that exhibited chromatin clumping (data not presented). We believe that sorting cells in the later stages of apoptosis is responsible for the poor condition of some of the Hoechst 33342bright/7-AADbright cells. The plasma membranes of these cells have been weakened by PS transposition and most likely are susceptible to mechanical disruption as a consequence of either the actual cell sorting or the centrifugation steps that must accompany this procedure.
Our studies support an emerging concept that certain bacterial toxins are capable of inducing apoptosis in target cells and that they do this through a variety of mechanisms (3). For example, several toxins are capable of entering cells and directly activating the central apoptotic cell death pathway. B. pertussis adenylate cyclase-hemolysin enters the cytoplasm of murine macrophages and elicits a very rapid increase in cyclic AMP (cAMP) (15). The elevation in intracellular cAMP is believed to result in the induction of apoptosis via a cascade of events initiated by the activation of cAMP-dependent protein kinase I (15, 22, 48). Other bacterial toxins, such as Corynebacterium diphtheriae diphtheria toxin (2, 31), Pseudomonas aeruginosa exotoxin (31), and Shigella dysenteriae Shiga toxin (14), are capable of inhibiting protein synthesis, a process that is also associated with the induction of apoptosis. In contrast, Staphylococcus aureus enterotoxin B does not gain entry into target cells but binds to Vβ elements of the T-cell antigen receptor (10, 25). This interaction is believed to activate a signal transduction pathway(s) that triggers apoptosis in murine thymocytes.
Recent evidence indicates that some pore-forming toxins are able to generate morphologic and biochemical alterations that are consistent with apoptosis in susceptible target cells (11, 12, 15, 27, 42, 43). Staphylococcus aureus alpha-toxin, the major cytolysin produced by this organism, has been shown to induce apoptosis in a dose-dependent fashion (11, 12). At high concentrations, the toxin is thought to nonspecifically adsorb to the surfaces of target cells and cause the formation of large-diameter pores that facilitate a massive influx of Ca2+ and loss of ATP that result in necrotic cell death. In contrast, at low doses, alpha-toxin is believed to specifically interact with a receptor molecule on the surface of toxin-sensitive cells, resulting in the formation of small-diameter pores. The pores formed in this manner are too narrow to allow the inward flow of Ca2+ but do permit passage of smaller molecules, including monovalent ions. In this system, an uncontrolled influx of Na+ resulting from treatment with low doses of alpha-toxin has been implicated as playing a key role in the activation of apoptosis in human T cells. The ability of RTX toxins to induce apoptosis also appears to be dose dependent. Initially, we found that relatively high doses of Ltx induced very rapid target cell death with little morphologic or cytometric evidence of apoptosis. As demonstrated in the current report, when the toxin concentrations were decreased, cells clearly exhibited the characteristics of apoptosis.
We believe that the differences that were detected following exposure to high versus low doses of Ltx may be a reflection of variations in the mechanism of transmembrane pore assembly. Although details of the structure of the RTX channels are unresolved, homology modeling (21) has been used to construct a molecular model of the pore-forming domain of Ltx (residues 150 to 405) based on the coordinates of the colicin pore (36). This region of the Ltx is predicted to contain nine α helices. Helices 1 through 7 are amphipathic, and helices 8 and 9 are hydrophobic. The hydrophilic residues would presumably line the lumen of the aqueous channel, while the hydrophobic helices move from the protein core to the lipid bilayer upon membrane insertion. To date, the number of Ltx molecules that associate to form the transmembrane channel is unknown. We hypothesize that two or more Ltx molecules could oligomerize to form the channel assembly. A logical extension of this reasoning suggests that at high doses, progressive oligomerization of Ltx molecules leads to fusion of transmembrane pores, which could be responsible for extremely rapid destruction of the cell membrane with little time for activation of the central apoptotic death pathway. However, at lower Ltx doses, transmembrane pores would be smaller and fewer such that cells, although gravely injured, could survive for much longer times. After pore formation, previously demonstrated toxin-induced increases in intracellular Ca2+ levels would lead to perturbations that are consistent with apoptosis (4, 43), including (i) collapse of the electrochemical gradients within the cell, (ii) DNA fragmentation, and (iii) cytoskeletal reorganization leading to the formation of surface membrane blebs. We are currently attempting to demonstrate the presence of oligomeric Ltx channels and to define their structure and assembly.
The results of the current study clearly demonstrate that low doses (less than 0.5 Ltx unit) of A. actinomycetemcomitans Ltx kill HL-60 cells via an apoptotic mechanism. Previous work from our laboratory suggested a similar effect of the toxin on human T and NK cells (27, 42). Based on these observations, we propose that exposure to Ltx in situ results in the elimination of host immune cells through the induction of apoptosis and thereby facilitates bacterial colonization of the subgingival microenvironment. Clinical evidence to support this hypothesis comes from patients with LJP. A. actinomycetemcomitans is considered to be an etiologic agent of this aggressive form of periodontal disease. Patients suffering from LJP classically present with a paucity of gingival inflammation despite the significant extent of alveolar bone destruction characteristic of the disease (1, 33). Additionally, externalization of PS by cells undergoing apoptosis has been proposed to serve as a recognition element for their elimination by phagocytic cells (6, 37). This may indirectly enhance A. actinomycetemcomitans colonization and invasion by diverting the “attention” of phagocytes away from the bacterial organisms. In conclusion, elimination and/or subversion of key inflammatory cells by Ltx-induced apoptosis is likely to play a central role in the pathogenesis of LJP and other diseases caused by A. actinomycetemcomitans. Continued study of Ltx-induced apoptosis in HL-60 cells will allow us to characterize the cellular machinery involved in this process and ultimately provide unique insight relative to the biology of this and other RTX toxins.
ACKNOWLEDGMENTS
Support for this research was provided from grants DE12305 and DE09517 from the National Institute of Dental Research.
Flow cytometry analysis was performed at the University of Pennsylvania School of Dental Medicine Flow Cytometry Facility. We thank Sylvia Decker (electron microscopy) and Sugandha Datar (flow cytometry) for expert assistance with the studies and James D. Lear (School of Medicine, University of Pennsylvania) for helpful comments and criticisms in preparation of the manuscript.
REFERENCES
- 1.Baer P N. The case for periodontosis as a clinical entity. J Periodontol. 1971;42:516–520. doi: 10.1902/jop.1971.42.8.516. [DOI] [PubMed] [Google Scholar]
- 2.Chang M P, Bramhall J, Graves S, Bonavida B, Wisnieski B J. Internucleosomal DNA cleavage precedes diphtheria toxin-induced cytolysis. Evidence that cell lysis is not a simple consequence of translation inhibition. J Biol Chem. 1989;264:15261–15267. [PubMed] [Google Scholar]
- 3.Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 4.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 5.Dive C, Gregory C D, Phipps D J, Evans D L, Milner A E, Wyllie A H. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992;133:275–285. doi: 10.1016/0167-4889(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 6.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 7.Harmon B V, Takano Y S, Winterford C M, Gobe G C. The role of apoptosis in the response of cells and tumours to mild hyperthermia. Int J Rad Biol. 1991;59:489–501. doi: 10.1080/09553009114550441. [DOI] [PubMed] [Google Scholar]
- 8.Huynh P N, Loria R M. Contrasting effects of alpha- and beta-androstenediol on oncogenic myeloid cell lines in vitro. J Leukocyte Biol. 1997;62:258–267. doi: 10.1002/jlb.62.2.258. [DOI] [PubMed] [Google Scholar]
- 9.Iwase M, Korchak H M, Lally E T, Berthold P, Taichman N S. Lytic effects of Actinobacillus actinomycetemcomitans leukotoxin on human neutrophil cytoplasts. J Leukocyte Biol. 1992;52:224–227. doi: 10.1002/jlb.52.2.224. [DOI] [PubMed] [Google Scholar]
- 10.Jenkinson E J, Kingston R, Smith C A, Williams G T, Owen J J. Antigen-induced apoptosis in developing T cells: a mechanism for negative selection of the T cell receptor repertoire. Eur J Immunol. 1989;19:2175–2177. doi: 10.1002/eji.1830191132. [DOI] [PubMed] [Google Scholar]
- 11.Jonas D, Schultheis B, Klas C, Krammer P H, Bhakdi S. Cytocidal effects of Escherichia coli hemolysin on human T lymphocytes. Infect Immun. 1993;61:1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann S H, Desnoyers S, Ottaviano Y, Davidson N E, Poirier G G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 14.Keenan K P, Sharpnack D D, Collins H, Formal S B, O’Brien A D. Morphologic evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986;125:69–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koopman G, Reutelingsperger C P, Kuijten G A, Keehnen R M, Pals S T, van Oers M H J. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 17.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 18.Lally E T, Golub E E, Kieba I R. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J Biol Chem. 1994;269:31289–31295. [PubMed] [Google Scholar]
- 19.Lally E T, Kieba I, Sato A, Green D R, Rosenbloom J, Korostoff J, Wang J F, Shenker B J, Ortlepp S, Robinson M K, Billings P C. RTX toxins recognize a β2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 20.Lally E T, Kieba I R, Demuth D R, Rosenbloom J, Golub E E, Taichman N S. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989;159:256–262. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- 21.Lally E T, Kieba I R, Golub E E, Lear J D, Tanaka J C. Structure/function aspects of Actinobacillus actinomycetemcomitans leukotoxin. J Periodontol. 1996;67:298–308. doi: 10.1902/jop.1996.67.3s.298. [DOI] [PubMed] [Google Scholar]
- 22.Lanotte M, Riviere J B, Hermouet S, Houge G, Vintermyr O K, Gjertsen B T, Doskeland S O. Programmed cell death (apoptosis) is induced rapidly and with positive cooperativity by activation of cyclic adenosine monophosphate-kinase I in a myeloid leukemia cell line. J Cell Physiol. 1991;146:73–80. doi: 10.1002/jcp.1041460110. [DOI] [PubMed] [Google Scholar]
- 23.Lear J D, Furblur U G, Lally E T, Tanaka J C. Actinobacillus actinomycetemcomitans leukotoxin forms large conductance, voltage-gated ion channels when incorporated into planar lipid bilayers. Biochim Biophys Acta. 1995;1238:34–41. doi: 10.1016/0005-2736(95)00086-i. [DOI] [PubMed] [Google Scholar]
- 24.Lian C J, Rosendal S, MacInnes J I. Molecular cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989;57:3377–3382. doi: 10.1128/iai.57.11.3377-3382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y S, Lei H Y, Low T L, Shen C L, Chou L J, Jan M S. In vivo induction of apoptosis in immature thymocytes by staphylococcal enterotoxin B. J Immunol. 1992;149:1156–1163. [PubMed] [Google Scholar]
- 26.Lo R Y, Shewen P E, Strathdee C A, Greer C N. Cloning and expression of the leukotoxin gene of Pasteurella haemolytica A1 in Escherichia coli K-12. Infect Immun. 1985;50:667–671. doi: 10.1128/iai.50.3.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangan D F, Taichman N S, Lally E T, Wahl S M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArthur W P, Tsai C C, Baehni P C, Genco R J, Taichman N S. Leukotoxic effects of Actinobacillus actinomycetemcomitans. Modulation by serum components. J Periodontal Res. 1981;16:159–170. doi: 10.1111/j.1600-0765.1981.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 30.Mobley H L, Chippendale G R, Swihart K G, Welch R A. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun. 1991;59:2036–2042. doi: 10.1128/iai.59.6.2036-2042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto H, Bonavida B. Diphtheria toxin- and Pseudomonas A toxin-mediated apoptosis. ADP ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alpha. J Immunol. 1992;149:2089–2094. [PubMed] [Google Scholar]
- 32.Nakamura S, Takeshima M, Nakamura Y, Ohtake S, Matsuda T. Induction of apoptosis in HL-60 leukemic cells by anticancer drugs in combination with anti-Fas monoclonal antibody. Anticancer Res. 1997;17:173–179. [PubMed] [Google Scholar]
- 33.Novak M J, Novak K F. Early-onset periodontitis. Curr Opin Periodontol. 1996;3:45–58. [PubMed] [Google Scholar]
- 34.Oberhammer F, Wilson J W, Dive C, Morris I D, Hickman J A, Wakeling A E, Walker P R, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ormerod M G, Collins M K, Rodriguez-Tarduchy G, Robertson D. Apoptosis in interleukin-3-dependent haemopoietic cells. Quantification by two flow cytometric methods. J Immunol Methods. 1992;153:57–65. doi: 10.1016/0022-1759(92)90305-d. [DOI] [PubMed] [Google Scholar]
- 36.Parker M W, Tucker A D, Tsernoglou D, Pattus F. Insights into membrane insertion based on studies of colicins. Trends Biochem Sci. 1990;15:126–129. doi: 10.1016/0968-0004(90)90205-p. [DOI] [PubMed] [Google Scholar]
- 37.Savill J S, Fadok V A, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 38.Schmid I, Uittenbogaart C H, Giorgi J V. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994;15:12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- 39.Shatzman A, Gross T L, Rosenberg M. Expression using vectors with phage λ regulatory sequences. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman D, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1994. pp. 16.3.1–16.3.11. [Google Scholar]
- 40.Shatzman A R, Rosenberg M. Expression, identification, and characterization of recombinant gene products in Escherichia coli. Methods Enzymol. 1987;152:661–673. doi: 10.1016/0076-6879(87)52072-9. [DOI] [PubMed] [Google Scholar]
- 41.Shenker B J, Datar S, Mansfield K, Shapiro I M. Induction of apoptosis in human T-cells by organomercuric compounds: a flow cytometric analysis. Toxicol Appl Pharmacol. 1997;143:397–406. doi: 10.1006/taap.1997.8111. [DOI] [PubMed] [Google Scholar]
- 42.Shenker B J, Vitale L A, Keiba I, Harrison G, Berthold P, Lally E T. Flow cytometric analysis of the cytotoxic effects of Actinobacillus actinomycetemcomitans leukotoxin on human natural killer cells. J Leukocyte Biol. 1994;55:153–160. doi: 10.1002/jlb.55.2.153. [DOI] [PubMed] [Google Scholar]
- 43.Stevens P K, Czuprynski C J. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro. Infect Immun. 1996;64:2687–2694. doi: 10.1128/iai.64.7.2687-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strathdee C A, Lo R Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taichman N S, Iwase M, Korchak H, Berthold P, Lally E T. Membranolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. J Periodontal Res. 1991;26:258–260. doi: 10.1111/j.1600-0765.1991.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 46.Taichman N S, Iwase M, Lally E T, Shattil S J, Cunningham M E, Korchak H M. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J Immunol. 1991;147:3587–3594. [PubMed] [Google Scholar]
- 47.Taichman N S, Wilton J M. Leukotoxicity of an extract from Actinobacillus actinomycetemcomitans for human gingival polymorphonuclear leukocytes. Inflammation. 1981;5:1–12. doi: 10.1007/BF00910774. [DOI] [PubMed] [Google Scholar]
- 48.Vintermyr O K, Gjertsen B T, Lanotte M, Doskeland S O. Microinjected catalytic subunit of cAMP-dependent protein kinase induces apoptosis in myeloid leukemia (IPC-81) cells. Exp Cell Res. 1993;206:157–161. doi: 10.1006/excr.1993.1132. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe H, Kanbe K, Shinozaki T, Hoshino H, Chigira M. Apoptosis of a fibrosarcoma induced by protein-free culture involves DNA cleavage to large fragments but not internucleosomal fragmentation. Int J Cancer. 1995;62:191–198. doi: 10.1002/ijc.2910620214. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe J T, Pringle J T, Cohen G M. Assays for measurements of DNA fragmentation during apoptosis. In: Colter T G, Martin S J, editors. Techniques in apoptosis: a user’s guide. London, United Kingdom: Portland Press Ltd.; 1996. pp. 51–70. [Google Scholar]