Abstract
Shigella flexneri is a triggering agent for reactive arthritis in HLA-B27-susceptible individuals. Considering the intracellular multiplication of bacteria, it seems likely that bacterial peptides may be presented by the major histocompatibility complex (MHC) class I pathway. To examine this hypothesis, we infected HLA-B*2705- and/or human β2-microglobulin-transfected murine L-cell lines with M90T, an invasive strain of S. flexneri. Bacterial infection induced no detectable modifications in the biosynthesis and expression level of HLA-B27, as assessed by immunoprecipitation, Northern blot analysis, and flow cytometry. Using confocal microscopy, we observed that bacterial infection induced a clustering of HLA-B27 molecules during macropinocytosis and before bacterial dissemination from cell to cell. Peptides naturally bound to HLA-B27 molecules were acid eluted from infected cells and separated by high-performance liquid chromatography. Major differences were observed in high-performance liquid chromatography profiles and in the nature of peptides presented following bacterial infection. Although most of the antigens presented were not accessed by Edman degradation, we obtained two sequences partially homologous to bacterial proteins. These peptides lacked the major HLA-B27 peptide anchor (Arg) at position 2, and one had an unusual length of 14 amino acids. These data suggest that alterations in the peptide presentation by HLA-B27 occur during infection, which could be relevant to the pathogenesis of HLA-B27-related arthritis.
One major function of major histocompatibility complex (MHC) class I molecules is to present peptides to CD8+ T lymphocytes. Antigen presentation by MHC class I molecules usually requires the introduction or the presence of the source antigen in the cytoplasm of the presenting cell (8). This antigen presentation pathway plays a central role in antibacterial immunity. Facultative intracellular bacteria such as listeriae, which have the ability to gain access to the cytoplasm after lysing the phagosome, and yersiniae, which inject proteins in the cytoplasm, generate epitopes presented to MHC class I-restricted cytotoxic T lymphocytes (CTL) (10, 34). Cells infected with the obligate intracellular pathogen Chlamydia could be killed in vitro by specific CD8+ T lymphocytes (1, 15), and specific MHC class I-restricted CTL against Salmonella have been induced in mice (39).
HLA-B27 is one of the most studied MHC class I molecules because of its strong association with a group of inflammatory rheumatic autoimmune diseases called spondyloarthropathies (17). Bacterial (Salmonella, Shigella, Yersinia, and Chlamydia) infections can trigger reactive arthritis (ReA) in genetically susceptible people. The description of HLA-B27-restricted CD8+ T cells with specificity for arthritogenic bacteria in synovial fluids from ReA patients provides a link between the bacterial infection and HLA association, supporting the so-called arthritogenic peptide model (11). These data are supported by results obtained for germ-free HLA-B27 transgenic rats in which the introduction of commensal bacteria is sufficient to induce a ReA-like disease (37).
Shigella flexneri, a gram-negative bacterium, is the etiological agent of bacillary dysentery. Certain aspects of the infection process have been studied in vitro, using mammalian epithelial cells or fibroblasts. After cellular entry, bacteria lyse the vacuole and gain access to the cytoplasm, where they divide and spread from cell to cell. The intracellular lifestyle of Shigella may result in the MHC class I presentation of bacterial peptides. As the role of Shigella in triggering ReA has been underestimated for a long time (24), the effect of this bacterial infection on HLA-B27 biosynthesis and peptide presentation has never been studied directly.
In the present work, we attempted to obtain new insights into the role of Shigella infection on the B27 expression, localization, and antigenic presentation. We report data suggesting that bacterial infection may modify the localization of mature HLA-B27 molecules and change the repertoire of HLA-B27-presented peptides.
MATERIALS AND METHODS
Bacterial strains and cell lines.
Two S. flexneri bacterial strains were used: M90T, an invasive serotype 5 isolate that carries the virulence plasmid pWR100; and BS176, an avirulent strain cured of pWR100. Bacteria were routinely grown in Trypticase soy broth in a shaker at 37°C or maintained on Congo red plates as previously described (19). The mouse L-cell lines stably transfected with human β2-microglobulin (β2m; 7.3.13) or with both HLA-B27 (B*2705 subtype) and human β2m (JT1) were a gift from J. Taurog (36). They were selected in hypoxanthine-aminopterin-thymidine medium and grown in 5% CO2 at 37°C in Dulbecco modified Eagle medium (DMEM) with 4.5 g of d-glucose per liter supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum (Seromed, Berlin, Germany). The HLA-B27 expression level was checked periodically by flow cytometry.
Bacterial infection of fibroblasts.
The technique for bacterial infection of fibroblasts was adapted from the original protocol (27). Semiconfluent cells were overlaid with S. flexneri grown to exponential phase, washed, and resuspended in DMEM (multiplicity of infection of about 300). To promote adherence of bacteria and allow infection of cells, plates were centrifuged for 10 min at 2,000 × g and incubated at 37°C without CO2 for various amounts of time. Cells were then washed three times with Earle’s balanced salt solution and incubated in the presence of DMEM containing 50 μg of gentamicin per ml at 37°C for 1 h before further analysis. Bacterial infection was quantitated by visualizing Giemsa-stained cells and by conducting plate counts to detect live intracellular bacteria. In a typical experiment, 14 × 107 CFU were obtained 2.5 h after infection of 106 cells at a multiplicity of infection of 300.
Indirect fluorescence staining and flow cytometry.
For surface staining, 5 × 105 cells were incubated with 1 μg of HLA-specific monoclonal antibodies (MAbs) B1.23.2 (38), W6/32 (22), HC10 (33), ME1 (38), TM1 (38), and Marb3 or Marb4 (40) in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) for 60 min on ice, washed in PBS–0.1% BSA, and then stained with fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 goat anti-mouse immunoglobulins (GAMIg; Sigma) for 60 min on ice. After being washed twice, samples were analyzed (104 events) on a FACScan (Becton Dickinson, Mountain View, Calif.) fluorescence-activated cell sorter (FACS). The same protocol was used for intracellular labeling of MHC class I molecules except that cells were first fixed (3.7% paraformaldehyde in PBS) and then permeabilized with a PBS solution containing 0.05% saponin and 1% BSA. Bacterial infection was assessed by using a polyclonal rabbit antiserum raised against S. flexneri lipopolysaccharide 5a (FlexV) revealed with FITC-conjugated anti-rabbit immunoglobulins (Molecular Probes Europe BV, Leyden, The Netherlands).
Fluorescent staining and confocal fluorescence microscopy.
About 5 × 105 cells were seeded on a coverslip the day before infection. Infected cell monolayers and controls were fixed with 3.7% paraformaldehyde in PBS for 20 min and permeabilized with 0.1% saponin–1% BSA in PBS for 10 min. For detection of both HLA-B27 and bacteria, double staining was performed with 1 to 2 μg of MAb ME1, B1.23.2, or HC10 (revealed with a GAMIg) followed by a 1:150 dilution of FlexV (visualized with an anti-rabbit Texas red-conjugated immunoglobulin; Amersham). For staining F-actin, coverslips were incubated with BODIPY FL phallacidin (Molecular Probes). Each incubation was performed at room temperature for 20 min. The nucleus was localized by using 4,6′-diamidino-2-phenylindole (DAPI) staining (Molecular Probes). Preparations were mounted on glycerol containing DABCO (1.4 diazabicyclo[2.2.2]octane; Sigma) and analyzed by using a Bio-Rad MRC 1024 confocal imaging system (Bio-Rad Microscience Ltd., Hertfordshire, United Kingdom) and an inverted Diaphot 300 Nikon microscope. Images were collected by using an oil immersion lens (60×; NA 1.4 plan Apochromat). Each image represented a single section for which the confocal system was adjusted to allow a field depth of about 0.4 μm. For FITC and Texas red excitations, a krypton-argon ion laser (15 mW) (Ion Laser Technology Inc., Salt Lake City, Utah) operating at 488 or 568 nm was used. For DAPI, an argon UV ion laser (250 mW) (Coherent Enterprise, Palo Alto, Calif.) operating at 363 nm was used. Images were merged and pseudo-colored in green for FITC, red for Texas Red, and blue for DAPI. Final images were recorded with a Polaroid Cl-3000 Digital Palette or a Tektronix Phaser 350.
Immunoprecipitation of HLA-B27.
JT1 cells (2.8 × 106 per well) were infected in DMEM containing 0.3 mM methionine for 60 min as described above, washed, and overlaid with DMEM lacking methionine, supplemented with 50 to 300 μCi of [35S]methionine and 50 μg of gentamicin per ml. Cells were incubated at 37°C for 60 min, then washed once in Earle’s balanced salt solution, collected with a scraper, and washed twice in PBS. Cells were lysed in 1% Nonidet P-40–Tris-NaCl buffer. The lysate was precleared by incubation with 5 μl of mouse serum overnight at 4°C followed by the addition of 60 μl of 25% (vol/vol) protein A-Sepharose CL-4B (Pharmacia, Uppsala, Sweden). The supernatant was collected, and the incorporated radioactivity was determined in a Beckman scintillation counter in order to have similar amounts of labeled proteins per sample (equivalent to 8 × 105 cpm). HLA-B27 molecules were detected by incubation with 3 μg of MAb B1.23.2 for 90 min, followed by immunoprecipitation with 40 μl of protein A-Sepharose solution for 20 min. Pellets were washed four times with 0.1% Nonidet P-40 in Tris-buffered saline, supplemented once with 150 mM NaCl and supplemented twice with 0.3 M NaCl. Samples were boiled in sample buffer, analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and autoradiographed.
RNA isolation and analysis.
JT1 cells were infected as described above for 30, 60, or 90 min, and total RNA was extracted by using an RNAzol extraction kit (Bioprobe Systems, Montreuil, France). Thirty micrograms of total RNA was loaded on a 1% agarose-formaldehyde gel and transferred to a Hybond N membrane (Amersham). The transcripts were hybridized with an HLA-B locus-specific probe (32) previously labeled with [32P]dCTP (Megaprime kit; Amersham). HLA-B transcripts were revealed and quantified by autoradiography (Kodak X-Omat film). Results were normalized by reference to a murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe.
HLA-B27 purification, peptide characterization, and in vitro binding assay.
HLA-B27 molecules were purified from 2 × 109 M90T-infected JT1 cells and 3 × 109 noninfected cells. The procedure for HLA purification has already been described (4). HLA-B27 molecules were captured on cyanogen bromide-activated Sepharose 4B columns (Pharmacia) coupled to MAb B1.23.2 and were eluted under basic conditions (50 mM diethylamine, pH 11.5). The amount of purified HLA-B27 molecules was assessed by SDS-PAGE analysis. Peptides were acid eluted (0.1% trifluoroacetic acid [TFA], pH 2), separated from HLA molecules through a 5-kDa-cutoff filter (Millipore) and submitted to reversed-phase high-performance liquid chromatography (HPLC). The gradient consisted of a stable step containing a 98:2 mixture of 0.05% TFA in H2O–0.05% TFA in acetonitrile for 5 min followed by a 75-min linear increase to 35% acetonitrile–0.05% TFA. Edman degradation from pooled and individual sequences was performed on an Applied Biosystems Inc. (Foster City, Calif.) 473-A or 494 protein sequencer. Synthetic peptides were prepared by solid-phase synthesis (Neosystem, Strasbourg, France). In vitro binding assays were performed on TAP-deficient T2 cells transfected with HLA-B*2705 according to previously described protocols (4).
RESULTS
Stable biosynthesis and expression of HLA-B27 molecules during JT1 infection by S. flexneri.
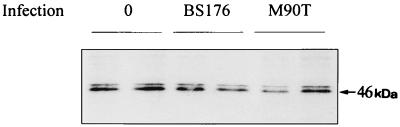
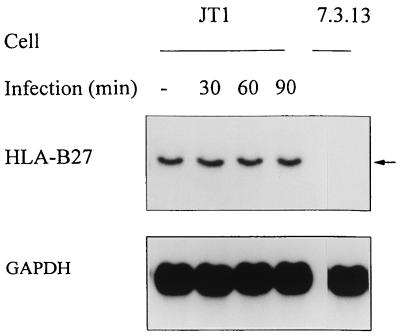
Immunoprecipitation of HLA-B27 molecules with MAb B1.23.2 showed no difference in the biosynthesis of the heavy chain (46 kDa) in M90T-infected cells compared to noninfected cells from 60 to 120 min postinfection (Fig. 1). Northern blot analysis of HLA-B27 mRNA levels during S. flexneri infection indicated no quantitative modification in the transcripts (Fig. 2). These data indicate that the presence of S. flexneri in fibroblasts did not significantly influence the transcription or translation level of HLA-B27.
FIG. 1.
Immunoprecipitation of HLA-B27 molecules from noninfected and infected JT1 cells. JT1 cells were infected either with the invasive strain S. flexneri M90T or with the noninvasive strain BS176. Sixty minutes after infection, cells were washed and incubated for a further 60 min with [35S]methionine; 2.8 × 106 cells were lysed, and the lysate was precleared before immunoprecipitation using MAb B1.23.2, specific for HLA-B,C. The gel was loaded in duplicate.
FIG. 2.
Northern blot analysis of HLA-B27 mRNA in infected JT1 cells. JT1 cells were either mock infected or infected with the invasive strain S. flexneri M90T. At indicated times postinfection, total RNA was extracted, separated by electrophoresis on an agarose-formaldehyde gel, and transferred to a membrane. The membrane was sequentially hybridized with a 0.5-kb HLA-B7 probe which recognizes HLA-B27 RNA and then with a GAPDH probe. The cell line 7.3.13, expressing the human β2m only, was used as a control. The HLA-B27 transcript is designated by an arrow.
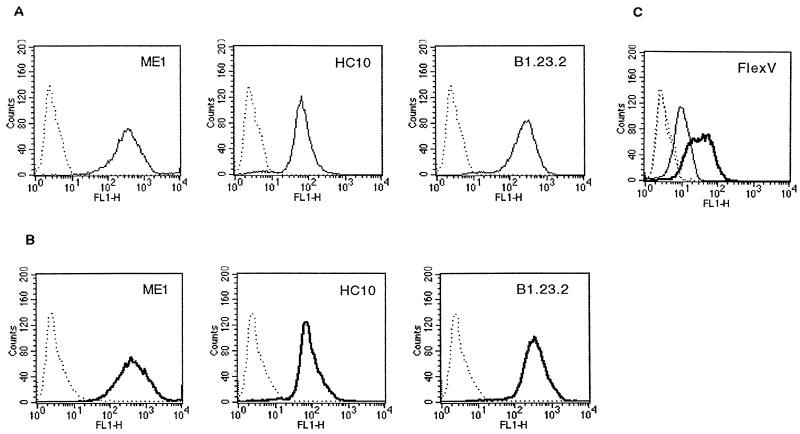
We examined intracellular and membrane expression of HLA-B27 molecules by using indirect fluorescence staining of permeabilized JT1 cells followed by FACS analysis (Fig. 3). According to the limits of cytometry detection, MAbs specific for different epitopes of the HLA-B27 molecule, as well as HC10, which stains empty class I heavy chains, did not show any modification of the HLA-B27 intracellular or membrane expression level by 2.5 h postinfection. Moreover, HLA-B27 surface expression assessed by MAb B1.23.2, ME1, TM1, Marb3, or Marb4 was not modified at 1.5, 2, and 3 h after infection with M90T (data not shown).
FIG. 3.
FACS analysis of total HLA-B27 expression in JT1 cells. JT1 cells were either mock infected (A) or infected with the invasive strain S. flexneri M90T for 2.5 h (B) and permeabilized before labeling with MAbs B1.23.2, ME1, and HC10. Infection was controlled by using a polyclonal antibody (FlexV) raised against S. flexneri serotype 5 (C). Specific staining in noninfected and infected cells is represented by light and bold lines, respectively; nonspecific staining is represented by dotted lines. Fluorescence intensity is expressed on the FL1 x axis, and the relative number of cells is shown on the y axis.
Bacterial infection induces a redistribution of mature HLA-B27 molecules.
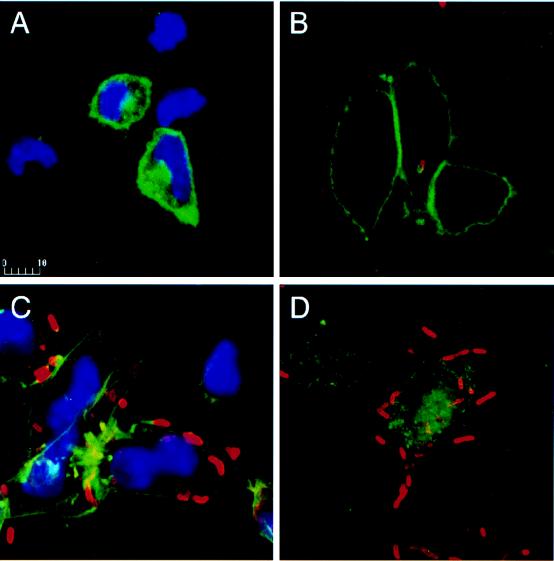
To determine whether bacterial infection influences the HLA-B27 localization, indirect fluorescent staining followed by confocal microscopy analysis was performed. In uninfected permeabilized JT1 cells, mature HLA-B27 molecules detected by ME1 were expressed at the cell surface and in the cytoplasm (Fig. 4A). As early as 15 min after infection, some mature HLA-B27 molecules from the cell surface were internalized by macropinocytosis and found around the bacteria (Fig. 4B). Later in the course of infection (2.5 h postinfection), some mature HLA-B27 molecules were redistributed behind the moving bacteria (Fig. 4C). Similar results were obtained with the HLA-B,C allele-specific MAb B1.23.2, indicating the epitope independence of the phenomenon. As staining of F-actin in infected HeLa cells is known to reveal a trail at one bacterial pole, we searched for a close localization of F-actin and HLA, using double staining of F-actin and mature HLA-B27 in JT1-infected cells. These molecules colocalized in cellular extensions. The specific recognition of HLA-B27 in these transfectants was confirmed by the absence of ME1 staining in infected control fibroblasts (data not shown). Visualization of HLA-B27 unfolded heavy chains with HC10 in JT1 fibroblasts showed that these molecules were essentially cytoplasmic. Staining of immature HLA-B27 molecules in protrusions induced by bacteria was much lower than their staining in the cytoplasm (Fig. 4D).
FIG. 4.
Confocal fluorescence analysis of HLA-B27 molecules in JT1 cells infected with S. flexneri M90T. JT1 cells were either mock infected (A) or infected with the invasive strain M90T (B to D) and were fixed in paraformaldehyde (3.7% in PBS) at 15 min postinfection (B) or at 2.5 h postinfection and permeabilized (C and D). Cells were stained first with MAb B1.23.2 (A to C) or HC10 (D) coupled to FITC-conjugated GAMIg and then with FlexV coupled to Texas red-conjugated goat anti-rabbit immunoglobulins. Nuclei were stained with DAPI (A and C).
HLA-B27-presented antigens are modified during bacterial infection.
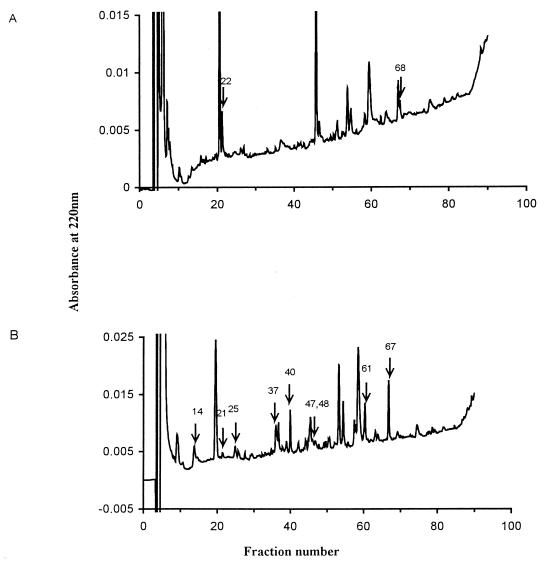
To study the HLA-B27 peptide profile consequent to bacterial infection, 250 μg of HLA-B27 molecules extracted from 2 × 109 JT1-infected cells and the same amount extracted from 3 × 109 noninfected cells were purified. Bound peptides were acid eluted and separated by HPLC. Two representative HPLC fractionations are shown in Fig. 5. Profiles from infected cells were more complex than those from the control cells, suggesting that bacterial infection modifies the HLA-B27-eluted material detected at 220 nm. The major individual specific peaks indicated by arrows and a pool of the remaining specific peaks were submitted to Edman degradation. Pool sequencing from uninfected cells showed a main Arg residue at position 2, accounting for 40% of the signal, and an Arg, Tyr, Phe, or Leu signal at the C terminus of the peptide, in agreement with previous reports of the HLA-B27 motif (4, 26). Eight of 11 individual HPLC fractions from infected cells did not give readable sequences, although the peak level absorbancy at 220 nm would have been adequate for sequencing HLA-B27-eluted peptides (4), for instance, in peaks 14, 61, and 67. Three individual sequences were obtained (Table 1) and were unusual in that they did not have the typical Arg B27 anchor residue at position 2 (HPLC fraction 37) or had an unusual length (HPLC fraction 47). Two peptides partially matched bacterial sequences identified in protein databases. HPLC fractions 37 and 47 were homologous to a sequence from the cystein aminopeptidase of Lactobacillus delbrueckii (14) and from an antigen described as the preabsorbing antigen of Streptococcus (45), respectively. The latter peptide had an unusual size of 14 amino acids (aa). To assess the ability of these unusual peptides to fit into the HLA-B27 binding pocket, we used an in vitro peptide binding assay based on the stabilization and refolding of empty class I molecules expressed on TAP-deficient T2-B27 transfected cell lines (4). Among the three synthetic peptides tested (Table 1), none was able to stabilize HLA-B27, in contrast to B*2705 endogenous control peptides (data not shown).
FIG. 5.
HPLC profiles of peptides eluted from HLA-B27 molecules purified from uninfected (A) or S. flexneri M90T-infected (B) JT1 cells. The separation was performed on a C18 column with an increasing gradient of acetonitrile in 0.05% TFA. Fractions sequenced by Edman NH2-terminal degradation are indicated by arrows.
TABLE 1.
Individual peptide sequences obtained from HLA-B27 molecules from S. flexneri M90T-infected JT1 cells
| HPLC fraction | Sequence | Homologya | Protein (aa) | Source | Synthetic peptide |
|---|---|---|---|---|---|
| 37 | XVFLPEEVK(K) | KAFLPEDVK(K) | Cysteine aminopeptidase (415–423) | Lactobacillus delbrueckii | KVFLPEEVK |
| SSFLPEEEK(K) | Adenosine deaminase hydrolase (329–337) | Mouse | |||
| 47 | XXPEAASAPGSGNR | YDPEAASAPGDGD | Preabsorbing antigen (1–13) | Streptococcus sp. | YDPEAASAPGSGNR |
| 48 | XRPEAAMDF | DRPEAAMDF | Hypothetical 50.2-kDa protein (335–343) | Saccharomyces cerevisiae | GRPEAAMDF |
Search for protein sequence homology was performed in the NBRF-PIR database. Identical amino acids are underlined.
DISCUSSION
S. flexneri has developed a range of strategies to escape the host immune response. On one hand, it induces rapid apoptosis in infected macrophages which are capable of presenting bacterial antigens via MHC class I (46). On the other hand, S. flexneri induces its own uptake by nonprofessional phagocytic cells such as epithelial cells and fibroblasts, in which it divides in the cytoplasm and perpetuates itself without lysing the host cell (18).
The interplay between arthritis-causing bacteria and HLA-B27 is a complex event (29). Although a decrease in the gram-negative bacterial infection rate in murine L cells has been described (13), we did not find such difference, at least by microscopic observation of stained infected cells (data not shown). The persistence of microbes inside the synovium of ReA patients has been documented for Chlamydia (24) and more recently for Salmonella (20). Increased survival of Salmonella enteritidis has been reported for the human U937 cell line (16) and for HLA-B*2705-transfected murine L cells (41) compared to that seen in untransfected cells. Bacterial products such as lipopolysaccharide may also be detected inside ReA synovial membranes and fluid (9). Various effects of bacterial infection on HLA-B27 biosynthesis and expression have been reported: a decrease in the synthesis of HLA-B27 molecules has been described for Yersinia enterocolitica-infected human monocytes (44), and Salmonella or Yersinia infection may induce alternative splicing of HLA-B27 mRNA, leading to a soluble form of the molecule (12). In our conditions, S. flexneri infection did not affect the expression level of HLA-B27 molecules. The induction of NF-κB, which could activate MHC class I transcription, has been found after S. flexneri infection (5), but we did not observe any increase of HLA-B27 mRNA expression. Mature HLA-B27 molecules were localized by confocal microscopy in association with the actin tails that allow bacterial infection of adjacent cells. The proximity of bacteria and HLA-B27 molecules may provide an environment suitable for the modification of HLA-B27 by secreted bacterial products. For instance, modifications of antigenic peptides could occur in compartments where bacteria are able to secrete proteases such as SepA (2) or oxydoreductases (42). The localization of class I molecules in bacterium-containing vacuoles has been described in the cases of Salmonella (23) and Chlamydia (21). The need of an acidic compartment and of cytoskeletal rearrangements for the processing of bacterial peptides via MHC class I and class II molecules has also been reported (35). Fusion of these vacuoles with MHC class II-expressing lysosomes (21) could allow mixing of both classes of antigen-presenting molecules and antigenic peptides under conditions (acidic pH and protease environment) usually not encountered by class I molecules during biosynthesis. This could explain the recovery from the class I molecules of longer peptides as in the case of class II-eluted peptides. Further analysis of subcellular compartments during the course of infection could clarify this point.
Among the many hypotheses that could explain the association of spondylarthropathies with HLA-B27 and the induction of disease by bacterial infection, it has been proposed that HLA-B27 may present a bacterium-derived peptide able to break the immune tolerance against naturally presented endogenous peptides. Along with this hypothesis, an HLA-B27-derived peptide sharing homologies with bacterial sequences has been defined by sequence comparisons (28) and found to be naturally presented by HLA-B27 (4). To confirm this “shared epitope” hypothesis, one must isolate bacterium-derived sequences directly from purified HLA-B27 molecules in infected cells. Similar to the findings presented here, profiles of peptides presented by HLA-B27 were modified during Salmonella infection (25). We also observed that S. flexneri infection altered the HPLC peptide profile in infected HLA-B27 fibroblasts. The data obtained indicated several unusual features. Eight of 11 peaks were not accessed by Edman sequencing, suggesting that the peptides eluted from infected cells did not have the same characteristics as high-affinity endogenous peptides presented by HLA-B27 (4). Peptides could be modified in a way impairing Edman degradation, for instance, by the presence of a formylmethionine amino acid at the first position of the bacterial peptide or through the engagement of cysteines in disulfide bonds. Two sequences shared a high degree of similarity with bacterial sequences but lacked HLA-B27 canonical anchor residues or had an unusual length. The ability of HLA-B27 to naturally present peptides of an unusual length, up to 33 aa (40), or lacking the peptide binding motif (6, 31) could be a specific behavior of this molecule related to its association with disease. Another hypothesis is that the HLA-B27 B pocket that accommodates the second residue of the peptide could be directly modified, subsequently changing the repertoire of bound peptides. Compared to other HLA molecules, B27 contains a cysteine-reactive residue (Cys 67) susceptible to modifications (43). Among the amino acids that they synthesize, members of the family Enterobacteriaceae provide a cysteine-reactive metabolite, homocysteine, that could alter the binding of endogenous peptides presented by HLA-B27 in vitro (3) and induce specific CTL (7). HLA-B27-restricted homocysteine-specific CTL have been found in ReA or ankylosing spondylitis patients (7).
As a whole, the ability of HLA-B27 to handle unusual peptides, especially in the case of bacterial infection as shown in this work, could be directly relevant to the pathogenesis of HLA-B27-associated diseases.
ACKNOWLEDGMENTS
This work was supported in part by the Association de Recherches sur la Polyathrite and BIOMED European Concerted Action.
We thank M. Rathman for critical reading of the manuscript, and we thank J. d’Alayer and M. Davi for performing the automated Edman degradation peptide sequencing.
REFERENCES
- 1.Beatty P R, Stephens R S. CD8+ T lymphocyte-mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588–4595. [PubMed] [Google Scholar]
- 2.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 3.Boisgérault, F., D. Charron, and A. Toubert. 1997. Unpublished data.
- 4.Boisgérault F, Tieng V, Stolzenberg M C, Dulphy N, Khalil I, Tamouza R, Charron D, Toubert A. Differences in endogenous peptides presented by HLA-B*2705 and B*2703 allelic variants. Implications for susceptibility to spondylarthropathies. J Clin Invest. 1996;98:2764–2770. doi: 10.1172/JCI119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer R B, Collaco C R, Niesel D W, Herzog N K. Shigella flexneri invasion of HeLa cells induces NF-κB DNA-binding activity. Infect Immun. 1993;61:4427–4433. doi: 10.1128/iai.61.10.4427-4433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frumento G, Harris P E, Gawinowicz M A, Suciu-Foca N, Pernis B. Sequence of a prominent 16-residue self-peptide bound to HLA-B27 in a lymphoblastoid cell line. Cell Immunol. 1993;152:623–626. doi: 10.1006/cimm.1993.1318. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Wordsworth P, McMichael A J, Kyaw M M, Seifert M, Rees D, Dougan G. Homocysteine modification of HLA antigens and its immunological consequences. Eur J Immunol. 1996;26:1443–1450. doi: 10.1002/eji.1830260707. [DOI] [PubMed] [Google Scholar]
- 8.Germain R N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 9.Granfors K, Jalkanen S, Toivanen P, Koski J, Lindberg A F. Bacterial lipopolysaccharide in synovial fluid cells in Shigella triggered reactive arthritis. J Rheumatol. 1992;19:500. [PubMed] [Google Scholar]
- 10.Harty J T, Bevan M J. CD8+ T cells specific for a single monamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann E, Yu D T Y, Meyer zum Büschenfelde K, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 12.Huang F, Yamaguchi A, Tsuchiya N, Ikawa T, Tamura N, Virtala M, Granfors K, Yasaei P, Yu D T Y. Induction of alternative splicing of HLA-B27 by bacterial invasion. Arthritis Rheum. 1997;40:694–703. doi: 10.1002/art.1780400414. [DOI] [PubMed] [Google Scholar]
- 13.Kapasi K, Inman R D. HLA-B27 expression modulates gram-negative bacterial invasion into transfected L cells. J Immunol. 1992;148:3554–3559. [PubMed] [Google Scholar]
- 14.Klein J R, Henrich B, Plapp R. Cloning and nucleotide sequence analysis of the Lactobacillus delbrueckii ssp. lactis DSM7290 cysteine aminopeptidase gene pepC. FEMS Microbiol Lett. 1994;124:291–300. doi: 10.1111/j.1574-6968.1994.tb07299.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuon W, Lauster R, Böttcher U, Koroknay A, Ulbrecht M, Hartmann M, Grolms M, Ugrinovic S, Braun J, Weiss E H, Sieper J. Recognition of chlamydial antigen by HLA-B27-restricted cytotoxic T cells in HLA-B*2705 transgenic CBA (H-2k) mice. Arthritis Rheum. 1997;40:945–954. doi: 10.1002/art.1780400524. [DOI] [PubMed] [Google Scholar]
- 16.Laitio P, Virtala M, Salmi M, Pelliniemi L J, Yu D T Y, Granfors K. HLA-B27 modulates intracellular survival of Salmonella enteritidis in human monocytic cells. Eur J Immunol. 1997;27:1331–1338. doi: 10.1002/eji.1830270606. [DOI] [PubMed] [Google Scholar]
- 17.Lopez de Castro J A. HLA-B27 and HLA-B73 polymorphism and its role on antigenicity, peptide presentation, and disease susceptibility. Clin Rheumatol. 1996;15:67–71. doi: 10.1007/BF03342650. [DOI] [PubMed] [Google Scholar]
- 18.Mantis N, Prévost M C, Sansonetti P J. Analysis of epithelial cell stress response during infection by Shigella flexneri. Infect Immun. 1996;64:2474–2482. doi: 10.1128/iai.64.7.2474-2482.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurelli A T, Baudry B, d’Hauteville H, Hale T L, Sansonetti P J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikkari S, Möttönen T, Saario R, Yli-Kerttula U, Leirisalo-Repo M, Laitio P, Toivanen P. Abstracts of the 60th National Meeting of the American College of Rheumatology 1996. Orlando, Fla: American College of Rheumatology; 1996. Demonstration of Salmonella DNA in the synovial fluid in reactive arthritis, abstr. 950; p. S185. [Google Scholar]
- 21.Ojcius D M, Hellio R, Dautry-Varsat A. Distribution of endosomal, lysosomal, and major histocompatibility complex markers in a monocytic cell line infected with Chlamydia psittaci. Infect Immun. 1997;65:2437–2442. doi: 10.1128/iai.65.6.2437-2442.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parham P, Barnstable C S, Bodmer W F. Use of monoclonal antibody (W6/32) in structural studies of HLA-A, B, C antigens. J Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- 23.Portillo F, Pucciarelli M, Jefferies W, Finlay B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 24.Rahman M U, Akhtar Cheema M, Schumacher R H, Hudson A P. Molecular evidence for the presence of Chlamydia in the synovium of patients with Reiter’s syndrome. Arthritis Rheum. 1992;35:521–529. doi: 10.1002/art.1780350506. [DOI] [PubMed] [Google Scholar]
- 25.Ringrose, J. H., B. A. Yard, A. Muijers, C. J. P. Boog, and T. E. W. Feltkamp. 1996. Comparison of peptides eluted from the groove of HLA-B27 from Salmonella infected and non-infected cells. Clin. Rheumatol. 15(Suppl 1):74–78. [DOI] [PubMed]
- 26.Rötzschke O, Falk K, Stevanovic S, Gnau V, Jung G, Rammensee H G. Dominant aromatic/aliphatic C-terminal anchor in HLA-B*2702 and B*2705 peptide motifs. Immunogenetics. 1994;39:74–77. doi: 10.1007/BF00171803. [DOI] [PubMed] [Google Scholar]
- 27.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scofield R H, Kurien B, Gross T, Warren W L, Harley J B. HLA-B27 binding of peptide from its own sequence and similar peptides from bacteria: implications for spondylarthropathies. Lancet. 1995;345:1542–1544. doi: 10.1016/s0140-6736(95)91089-1. [DOI] [PubMed] [Google Scholar]
- 29.Sieper J, Braun J, Wu P, Hauer R, Laitko S. The possible role of Shigella in sporadic enteric reactive arthritis. Br J Rheumatol. 1993;32:582–585. doi: 10.1093/rheumatology/32.7.582. [DOI] [PubMed] [Google Scholar]
- 30.Sieper J, Kingsley G. Recent advances in the pathogenesis of reactive arthritis. Immunol Today. 1996;17:160–163. doi: 10.1016/0167-5699(96)80612-8. [DOI] [PubMed] [Google Scholar]
- 31.Simmons W A, Summerfield S G, Roopenian D C, Slaughter C A, Zuberi A R, Gaskell S J, Bordoli R S, Hoyes J, Moomaw C R, Colbert R A, Leong L Y W, Butcher G W, Hammer R E, Taurog J D. Novel HY peptide antigens presented by HLA-B27. J Immunol. 1997;159:2750–2759. [PubMed] [Google Scholar]
- 32.Sodoyer R, Nguyen C, Strachan T, Santoni M J, Damotte M, Trucy J, Jordan B R. Allelism in the HLA class I multigene family. Ann Inst Pasteur. 1997;1360:71–84. doi: 10.1016/s0769-2625(85)80040-4. [DOI] [PubMed] [Google Scholar]
- 33.Stam N J, Spits H, Ploegh H. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 34.Starnbach M N, Bevan M J. Cells infected with Yersinia present an epitope to class I MHC-restricted CTL. J Immunol. 1994;153:1603–1612. [PMC free article] [PubMed] [Google Scholar]
- 35.Svensson M, Stockinger B, Wick M J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 36.Taurog J D, el-Zaatari F A. In vitro mutagenesis of HLA-B27. Substitution of an unpaired cysteine residue in the α 1 domain causes loss of antibody-defined epitopes. J Clin Invest. 1988;82:987–992. doi: 10.1172/JCI113708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhou M, Fernandez-Sueiro J L, Balish E, Hammer R E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toubert A, Raffoux C, Boretto J, Sire J, Sodoyer R, Thurau S R, Amor B, Colombani J, Lemonnier F A, Jordan B R. Epitope mapping of HLA-B27 and HLA-B7 antigens by using intradomain recombinants. J Immunol. 1988;141:2503–2509. [PubMed] [Google Scholar]
- 39.Turner S J, Carbone F R, Strugnell R A. Salmonella typhimurium DaroA DaroD mutants expressing a foreign recombinant protein induce specific major histocompatibility complex class I-restricted cytotoxic T lymphocytes in mice. Infect Immun. 1993;61:5374–5380. doi: 10.1128/iai.61.12.5374-5380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban R G, Chicz R M, Lane W S, Strominger J L, Rehm A, Kenter M J H, UytdeHaag F G, Ploegh H, Uchanska-Ziegler B, Ziegler A. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc Natl Acad Sci USA. 1994;91:1534–1538. doi: 10.1073/pnas.91.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virtala M, Kirveskari J, Granfors K. HLA-B27 modulates the survival of Salmonella enteritidis in transfected L cells, possibly by impaired nitric oxide production. Infect Immun. 1997;65:4236–4242. doi: 10.1128/iai.65.10.4236-4242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Disulfide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc Natl Acad Sci USA. 1995;92:4927–4931. doi: 10.1073/pnas.92.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whelan M A, Archer J A. Chemical reactivity of an HLA-B27 thiol group. Eur J Immunol. 1993;23:3278–3285. doi: 10.1002/eji.1830231233. [DOI] [PubMed] [Google Scholar]
- 44.Wuorela M, Jalkanen S, Kirveskari J, Granfors K. Yersinia enterocolitica O:3 alters the expression of serologic HLA-B27 epitopes on human monocytes. Infect Immun. 1997;65:2060–2066. doi: 10.1128/iai.65.6.2060-2066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshizawa N, Oshima S, Sagel I, Shimizu J, Treser G. Role of a streptococcal antigen in the pathogenesis of acute poststreptococcal glomerulonephritis. Characterization of the antigen and a proposed mechanism for the disease. J Immunol. 1992;148:3110–3116. [PubMed] [Google Scholar]
- 46.Zychlinsky A, Prévost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]