Abstract
Advances in telemedicine have allowed physicians to provide care in areas that were previously geographically or practically inaccessible. Roughly 70% of all US hospital have less than 200 bed capacity and nearly 50% have fewer than 100 beds. These smaller hospitals often do not have specialists available for bedside patient care, making them potential beneficiaries of telemedicine medical specialty services. In 2005, the American Stroke Association proposed implementing telemedicine services in effort to increase access to acute stroke care in neurologically underserved areas such as small hospitals. Tele-stroke services have since become established across the country and are now utilized by approximately 30% of US hospitals. By reducing the time between presentation and evaluation by a stroke specialist, tele-stroke programs have successfully increased patient access to life-saving treatment with tissue-plasminogen activator (t-PA) treatments. This change has been especially profound remote and underserved community hospitals. However in the evaluation of acute vision loss, an area where ophthalmology and stroke care overlap, increased reliance on tele-stroke services has contributed to some unique challenges. Acute vision has a complex differential and is commonly a result of conditions other than stroke. When tele-stroke services are engaged for the evaluation of acute vision loss, the neurologist is asked to make medical decisions without complete information about the eye. This situation can expose patients to costly or inappropriate testing, unnecessary hospitalizations, or lead to delayed diagnosis and treatment of non-neurologic conditions of the eye. The goal of this paper is to provide an overview of the overlap between stroke and vision loss, highlight the challenges inherent in using tele-stroke in evaluating acute vision loss and to offer our comments on how increased communication between emergency medicine, ophthalmology, and neurology services can ensure that patients with vision loss receive the highest standard of care in all hospitals.
Keywords: telemedicine, tele-stroke, acute vision loss, tele-neurology, CRAO
Introduction
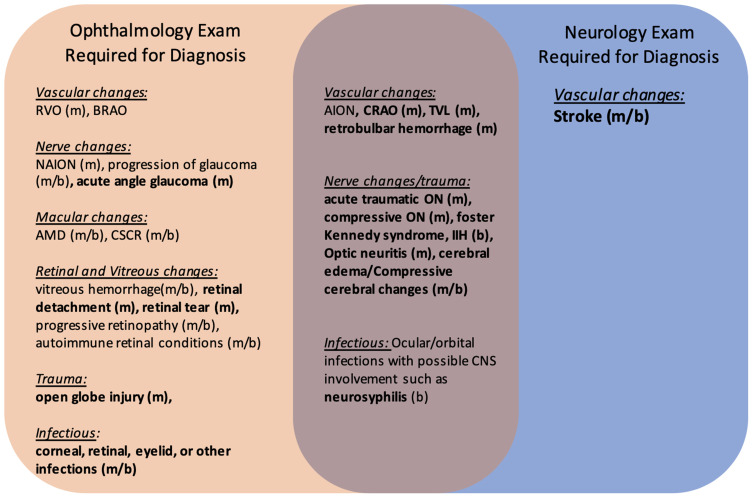
Visual function is determined by the health and relationship between the periocular structures, the globe, and the visual pathways in the brain. A disruption in any of these components can lead to changes or decrease in vision. Of the many etiologies that can lead to acute vision loss, some require a full ophthalmic exam for diagnosis and management, others require a neurologic evaluation, and some may require both (Figure 1).
Figure 1.
Common Etiologies of Acute Vision Loss Presenting to the Emergency Department.
Notes: Bolded conditions are those that require management in the emergency setting. Those that benefit from/require neuroimaging in the acute setting include systemic vasculitis (usually GCA), optic neuritis, CRAO, compressive ON, acute traumatic ON, IIH, infections of the CNS and eye such as neurosyphilis, and stroke. Of these, only cerebral strokes and CRAO require stroke protocol as standard of care. (m) Commonly Monocular. (b) Commonly Binocular. Bolded: Require Emergent management.
Abbreviations: RVO, Retinal vein occlusion; BRAO, Branch retinal artery occlusion; NAION, Non-arteritic ischemic optic neuropathy; AMD, Age-related macular degeneration; CSCR, Central serous choroidal retinopathy; CRAO, Central retinal artery occlusion; TVL, Transient vision loss; ON, Optic neuropathy; IIH, Idiopathic intracranial hypertension.
Tele-stroke services and are utilized by approximately 30% of US hospitals.1 As ophthalmologists, we have noticed an increasing incidence of people presenting to emergency departments with acute vision loss who are assessed by tele-stroke services, receive a full stroke workup, and are later diagnosed with conditions that could have been diagnosed on ophthalmic exam. In these cases, a timely ophthalmic exam could have prevented unnecessary, costly, or invasive testing, as well as often eliminating the need for a hospital admission. We postulate that the increased reliance on tele-stoke services without a simultaneous increase in accessibility of ophthalmic specialty services contribute to this.
The window for treating eligible patients in cases of ischemic stroke with tissue plasminogen activator (tPA) is within 3–4.5 hours of the onset of symptoms.2,3 Therefore, when a patient presents to the emergency department with signs of possible acute cerebral stroke, the priority is acting fast to rule in or out the stroke diagnosis. In the case of acute vision loss, a possible symptom of cerebral stroke or a retinal arterial occlusion, an emergent ophthalmic exam can rule in or out many of the non-neurologic causes for the vision change (see Figure 1). Below, we review the overlap between stroke and visual loss. We will then discuss challenges inherent in both in-person and tele-medicine ophthalmic exams and end with comments on how a multi-specialty approach incorporating tele-ophthalmic technologies into tele-stroke protocols can help us provide the highest quality of care for all patients regardless of resource status of the hospital.
Stroke and Vision Loss – Current Management and Implications
Our aim in the following section is to not to provide a comprehensive review of stroke management but instead to focus on recommendations for those in whom an arterial occlusion is the suspected etiology of vision loss in the context of increasing availability of and reliance on tele-stroke programs.
Cerebral Ischemia and Vision Loss
The spectrum of visual dysfunction resulting from cerebral stroke is varied and large. Ischemia of the lateral geniculate body (LGB), optic radiations, or occipital lobe can manifest as homonymous or incongruous quadrantinopias, sectoranopias or scotomas.4 Occipital pole ischemia can cause a complete hemianopia, whereas bilateral posterior infarctions can result in total blindness without the patient recognition of the loss, called Anton’s Syndrome. Midbrain infarctions, on the other hand, can result in ptosis or ophthalmoplegia due to cranial nerve palsies.
Notably, strokes are not the only cerebral causes for visual loss or change. Other causes include demyelinating disease, mass effects, infectious and inflammatory etiologies, toxicities, metabolic disturbances such as hepatic encephalopathy, seizures and migraines.5 These cerebral causes for vision changes should be considered, especially when the vision change is bilateral.
It is important to note that though vision loss can be the only presenting symptom of cerebral stroke,6 it is exceedingly rare for stroke-related vision loss to be present without other stroke symptoms. Even in conjunction with other symptoms, vision changes are one of the presenting symptoms in only 24–26% of stroke patients.7,8 Interestingly, even though 68–92% of patients diagnosed with an acute stroke are subsequently found to have an associated visual impairment, only 40% of multidisciplinary stroke teams report objective findings of ocular impairment during the emergent care9 and at least equally many patients remain unaware of their vision deficits even after formal visual testing.10,11 The discrepancy between the vision changes reported by the patients and their physicians in the emergency setting and the later objective vision findings is likely multifactorial, including subjective difficulty in identifying incongruous or small visual field deficits and the low sensitivity of confrontational visual field testing performed in the emergency setting.12
Implications
Vision change is rarely the sole presenting symptom of a cerebral stroke. If a patient presents with vision changes in concurrence with other symptoms or signs consistent with stroke, they should be triaged, evaluated and managed like any other stroke suspect. Patients with acute and painless vision changes should be assessed with confrontational visual field testing for pathognomonic cerebral ischemic patterns of visual loss such as homonymous hemianopia. If visual field defects are not found and the patient has no other signs or symptoms that suspicious for cerebral stroke or CRAO, the patient can be referred for outpatient management by ophthalmology, preferably within 24 hours of discharge. Patients diagnosed with acute cerebral stroke with concurrent vision changes can similarly be referred to outpatient ophthalmology for a full ophthalmic exam and serial visual field tests after receiving acute stroke care and discharge from the hospital.
Retinal Artery Occlusions
Retinal ischemia can result from an occlusion of the central retinal artery (CRAO), a retinal artery branch (branch retinal artery occlusion, BRAO), the posterior ciliary artery, or rarely due to an occlusion of the ophthalmic artery. An acute CRAO can often be identified on fundoscopic exam by the classic finding of retinal whitening with a cherry red foveal spot. However, this sign classically fades over a period of weeks, and prior retinal ischemic events may have subtle fundus findings at best. Moreover, the retinal lipid accumulation in Tay-Sachs disease or macular infarction from trauma or diabetic retinopathy can also present with a cherry-red spot without the presence of arterial occlusion.
Retinal artery occlusions are more common in elderly patients but remain overall rare, estimated to have the annual incidence of around 2 per 100000 persons.13 It is estimated that 92–95% of retinal artery occlusions occur due to thromboembolic disease, while 5–8% occur in association with vasculitis, typically giant cell arteritis (GCA).14,15
Retinal ischemia can be transient, with resolution of the occluding embolus before permanent ischemic damage occurs such that the symptoms and signs of ischemia resolve by the time the patient is evaluated. Transient vision loss (TVL) due to temporary retinal arterial occlusion (also called amaurosis fugax) is almost exclusively monocular, with an estimated incidence of 1.5/100,00 people per year in the third decade of life, increasing to 32/100,000 in the seventh decade of life.16 Binocular transient vision loss due to arterial occlusions is rare and is almost always associated with severe occlusive disease of the internal carotid artery (ICA), aortic arch, or vertebral circulation with occipital lobe ischemia. In patients younger than 45 years old, transient monocular vision loss (TMVL) is more likely to be secondary to vasospasm or migraine as opposed to arterial occlusions.
Recognition and treatment of the underlying vasculopathic risk factors in patients with permanent or transient retinal ischemia can significantly reduce a patient’s risk for future stroke. Emergent diagnosis and treatment for the underlying cause acute retinal ischemia can decrease the risk for subsequent stroke by 80%.17
The European Assessment Group for Lysis in the Eye (EAGLE) study found that 40% of their patients with CRAO had ipsilateral carotid artery stenosis of 70% or greater.18 Of those experiencing transient monocular vision loss due to retinal artery occlusions, ~10 to 15% develop a new stroke within 90 days, half of which occur within the first 48 hours.19 Because those with acute retinal artery occlusions are at a higher risk for subsequent cerebral strokes, the American Heart Association/American Stroke Association (AHA/ASA) recommends emergent neuroimaging for patients with confirmed or highly suspected retinal arterial occlusions.20 MRI scanning has demonstrated evidence of cerebral ischemia in up to 4% of patients with transient monocular vision loss due to transient retinal ischemia, 25%-40 of those with branch retinal artery occlusions and 30–33% with central retinal artery occlusions.21,22
Treatment of retinal artery occlusions is controversial and traditionally understood to be unsuccessful. Conservative treatment modalities include ocular massage, inhalation of carbogen, hyperbaric oxygen, anterior chamber needle decompressions, and pharmacologic reduction of intraocular pressure, all thought to increase chances of clot dislodgement and reinstatement of normal arterial flow. The benefit of these treatments is mostly theoretical, cited in case reports with mixed outcomes.23
Though it is not the standard of care at this time, there is growing interest in treating acute central retinal arterial occlusions with intravascular thrombolysis therapies. In 2010, the European Assessment Group for Lysis in the Eye (EAGLE) randomized controlled trial found that treating CRAO in people with symptoms for 20 hours or less with tPA did not improve visual outcomes and was associated with a significantly increased risk of symptomatic intracranial hemorrhage.24 However, a 2020 meta-analysis that incorporated newer studies demonstrated a strong effect with treatment of CRAO with intravenous tPA within 4.5 hours of onset.25 In light of this, in 2021 the American Heart Association/American Stroke Association (AHA/ASA) published a scientific statement emphasizing that treatment with IV tPA may be effective for CRAO if administered in the same time-window that applies for cerebral ischemic strokes.14 Thus currently, treatment with intra-arterial tPA may be considered if patients with CRAO without contraindications for IV tPA present to specialized centers within 4–6 hours of visual loss.
Implications
If suspicion for CRAO or transient monocular vision loss from temporary retinal arterial occlusion is high, consider emergent neuroimaging based on AHA/ASA recommendations. In those patients with microvascular or hypercoagulable risk factors, an episode of TMVL should trigger an investigation for thromboembolic disease or hypercoagulable state. If a patient’s transient vision changes have resolved, the patient’s vision and ocular exam can be followed on an outpatient basis by ophthalmology.
If suspicion for CRAO or TMVL from arterial occlusion is high in a patient above the age of 50, we recommend obtaining a CBC, ESR, CRP, as well as taking a thorough history to assess for clinical symptoms of GCA (Box 1). When the clinical suspicion for GCA is high, immediate treatment with high dose IV steroids is indicated to prevent contralateral visual loss as well as prevent further morbidity, followed by a temporal artery biopsy within two weeks of starting treatment. Missing the diagnosis of a vasculitis is a common error in this population and if left untreated, can result in many systemic, neurologic, and ophthalmologic complications.
Box 1.
Common Giant Cell Arteritis Symptoms
| Difficulty Chewing/ Jaw claudication |
| Low grade fevers |
| New onset temporal or occipital headaches |
| Temporal artery pain/Scalp tenderness |
| Fatigue and weight loss |
| Diplopia |
| Eye, ear, or neck pain |
| Episodes of transient vision loss |
| Abdominal pain |
| Dizziness |
| Muscle aches that persist throughout the day |
In emergency centers with in-person neurologic and ophthalmologic specialty coverage, a patient with isolated acute onset painless monocular loss would receive an emergent ophthalmologic examination. In settings without access to emergent in-person or telemedicine-assisted ophthalmologic examinations, our recommendation is to perform an ultrasound of the eye to look for evidence of vitreous opacities or retinal detachment that could explain the vision loss. If the patient is younger than 50 years of age, the pupillary and the external examination of the eye is unremarkable, and the patient has no vasculopathic risk factors and exhibits no signs or symptoms suspicious for stroke, they should be considered for an outpatient ophthalmologic evaluation.
Challenges of the Ophthalmic Exam in the Emergency Setting
In-Person Emergency Ophthalmic Examination
A funduscopic exam can rule in or out numerous causes of acute, painless vision loss, including vitreous hemorrhage, intraretinal hemorrhage, retinal detachment, advanced glaucoma, venous or arterial occlusions, and macular changes from diseases such as age-related macular degeneration, polypoidal choroidal vasculopathy, or central serous choroidal retinopathy (Figure 1.)
In tertiary care centers, stroke specialists can rely on information gained from an expert ophthalmologic exam when deciding whether to pursue stroke protocols, treatment, or hospital admission for patients with acute vision loss. The question emerges – why are not fundus examinations a routine part of the emergency room exam in patients who present with acute vision loss? The challenge in obtaining a fundus exam in emergency departments is multifold – it requires specialty equipment, significant technical skill, and the knowledge to interpret the findings. Few medical professionals feel comfortable with performing a fundoscopic examination, as the examination technique is not included in the standard medical school curriculum and can take years to master. Ophthalmology has not been a required element in medical schools for over two decades. Though ED providers and neurologists may be taught how to perform basic fundoscopic exams in their residency training programs, in reality these exams are rarely performed by non-ophthalmologists. One study found that of 350 patients who presented to the emergency department with signs and symptoms warranting an examination of the ocular fundus, only 14% had a funduscopic examination performed by an emergency physician.26 Of these patients, 12.6% were later found to have fundus findings that would have changed the course of management in the ED if they were noted at the time. Notably, none of these findings were detected on the funduscopic examination by the emergency physicians. In the writers’ experience, this study reflects of the reality of the field - The ability to perform and interpret the fundoscopic exam remains something outside of the scope of the majority of providers who are not trained in ophthalmology or optometry.
The challenge of obtaining a full ophthalmic exam in the emergency setting is compounded by the growing demand for ophthalmologists for the aging population. In 2016, the Health Resources and Services Administration estimated that the number of available ophthalmologists would fall short of the demand by more than 6000 physicians by 2025.27 Roughly 70% of all US hospital have less than 200 bed capacity and nearly 50% have fewer than 100 beds.28 Ophthalmologists are rarely on staff in small hospitals, and much less available for in-house emergency evaluations. In 104 of surveyed facilities in Florida, nonrural hospitals reported a median of 1 ophthalmologist available on call compared with a median of 0 at rural hospitals.29 In California, only 49% of rural facilities and 74% of nonrural facilities30 reported having emergency ophthalmology coverage. In both states, 50–73% of the facilities or practitioners surveyed rated teleophthalmology as having high or very high value.29
Telemedicine Emergency Ophthalmic Examination
The development of new technologies to obtain and analyze many parts of the ophthalmic examination is changing the landscape of ophthalmology and the way ophthalmologists can participate in multi-disciplinary teams to triage patients in the emergency room setting. Various tools have been developed to enable non-ocular specialists to acquire critical information about the ocular system and facilitate remote evaluations by ocular specialists (Table 1). These developments have the potential to reduce the reliance on in-person ophthalmic specialty care, which is particularly important in rural and resource poor healthcare settings.
Table 1.
Teleophthalmologic Tools in Published Literature
| Visual Acuity | ||
|---|---|---|
| Technology | Concluding Statement | Reference |
| Smartphone-based | The mean differences between the smartphone-based test and the ETDRS chart and the smartphone-based test and Snellen acuity data were 0.07 (95% CI, 0.05–0.09) and 0.08 (95% CI, 0.06–0.10) logMAR, respectively. | Brady CJ, Eghrari AO, Labrique AB. Smartphone-Based Visual Acuity Measurement for Screening and Clinical Assessment. JAMA. 2015;314(24):2682–2683.31 doi:10.1001/jama.2015.15855 |
| Tablet-based | The resulting MAVERIC vision demonstrated good repeatability and good agreement with the gold-standard near chart measures. | Aslam TM, Parry NR, Murray IJ, et al. Development and testing of an automated computer tablet-based method for self-testing of high and low contrast near visual acuity in ophthalmic patients. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):891–899.32 doi:10.1007/s00417-016-3293-2 |
| Web-based | The difference in VA between the index and reference tests was not significant, with a mean difference of 0.02 (SD 0.12) logMAR (P=0.09). | Claessens J, van Egmond J, Wanten J, Bauer N, Nuijts R, Wisse R. The Accuracy of a Web-Based Visual Acuity Self-assessment Tool Performed Independently by Eye Care Patients at Home: Method Comparison Study. JMIR Form Res. 2023;7:e41045.33 doi:10.2196/41045 |
| Web-based | Mean difference between dBSCVA measured with Democritus Digital Acuity & Reading Test (DDART) and conventional charts ranged between −0.84 and +0.85 symbols, without exceeding the 2.5-symbol noninferiority margin. | Labiris G, Panagiotopoulou EK, Delibasis K, et al. Validation of a web-based distance visual acuity test [published online ahead of print, 2023 Feb 24]. J Cataract Refract Surg. 2023;49(7):666-671.34 doi:10.1097/j.jcrs.0000000000001176 |
| Pupillary exam | ||
| Technology | Concluding Statement | Reference |
| Smartphone-based | Excellent agreement between pupil responses recorded using the iPhone-based Sensitometer test and infrared camera. | McAnany JJ, Smith BM, Garland A, Kagen SL. iPhone-based Pupillometry: A Novel Approach for Assessing the Pupillary Light Reflex. Optom Vis Sci. 2018;95(10):953–958.35 doi:10.1097/OPX.0000000000001289 |
| Web app format | The tool is sensitive enough to detect both locomotor-induced and stimulus-evoked pupillary changes, and its output is comparable to state-of-The-art commercial devices. (MEYE: Web-browser Tool) MEYE is available at: www.pupillometry.it | Mazziotti R, Carrara F, Viglione A, et al. MEYE: Web App for Translational and Real-Time Pupillometry [published correction appears in eNeuro. 2022 Apr 13;9(2):]. eNeuro. 2021;8(5):ENEURO.0122–21.2021. 36 doi:10.1523/ENEURO.0122–21.2021 |
| Extraocular movements | ||
| Technology | Concluding Statement | Reference |
| Smartphone-Based | The time required to produce nine-directional ocular photographs using the newly devised 9Gaze application was shorter compared to that of the conventional method. | Goseki T, Kunimi K, Shioya N, et al. New device for taking nine-directional ocular photographs: “9Gaze” application. J Eye Mov Res. 2022;15(1):10.16910/jemr.15.1.5.37 doi:10.16910/jemr.15.1.5 |
| IOP | ||
| Technology | Summary | Reference |
| Contact lens sensor | The Triggerfish CLS is safe and well tolerated, and provides reproducible results.The may only provide data on relative changes in intraocular pressure rather than absolute intraocular pressure. | Dunbar GE, Shen BY, Aref AA. The Sensimed Triggerfish contact lens sensor: efficacy, safety, and patient perspectives. Clin Ophthalmol. 2017;11:875–882.38 doi:10.2147/OPTH.S109708 |
| Smartphone-Based Tonometry | The smartphone tonometer results correlated best with Goldmann applanation tonometry (R2=0.67, p<0.001). | Wu Y, Luttrell I, Feng S, et al. Development and validation of a machine learning, smartphone-based tonometer. Br J Ophthalmol. 2020;104(10):1394–1398.39 doi:10.1136/bjophthalmol-2019-315446 |
| Slit Lamp Exam | ||
| Technology | Summary | Reference |
| Smartphone attachment | Available to Purchase. | https://www.digitaleyecenter.com/product/universal-slit-lamp-adapter-smartphone-tablet/ |
| Smartphone-Based Attachments | The differences between the mean Vertical Cup-to-Disc Ratio estimations obtained by each techniques were not statistically significant. | Russo A, Mapham W, Turano R, et al. Comparison of Smartphone Ophthalmoscopy With Slit-Lamp Biomicroscopy for Grading Vertical Cup-to-Disc Ratio. J Glaucoma. 2016;25(9):e777-e781.40 doi:10.1097/IJG.0000000000000499 |
| Smartphone Attachment | Comparison of images from a traditional slit lamp and study smartphone slit lamp show that the quality of the images is comparable. | Truong, P., Phan, A., Truong, B. et al A smartphone attachment for remote ophthalmic slit lamp examinations. Microsyst Technol 26, 3403–3407 (2020).41 https://doi.org/10.1007/s00542-020-04894-7 |
| Fundus Exam | ||
| Technology | Concluding Statement | Reference |
| Smartphone adapter | Images obtained by a nurse using a smartphone coupled to the open retinoscope and subsequently assessed by an ophthalmologist showed a high diagnostic performance for eye posterior pole pathologies. | González-Márquez F, Luque-Romero L, Ruiz-Romero MV, et al. Remote ophthalmology with a smartphone adapter handled by nurses for the diagnosis of eye posterior pole pathologies during the COVID-19 pandemic. J Telemed Telecare. 2023;29(6):474–483.42 doi:10.1177/1357633X21994017 |
| Smartphone attachment | This optical attachment is a promising, inexpensive, and valuable alternative to the direct ophthalmoscope, potentially eliminating problems of poor exam skills and inexperienced observer bias | Russo A, Morescalchi F, Costagliola C, Delcassi L, Semeraro F. A Novel Device to Exploit the Smartphone Camera for Fundus Photography. J Ophthalmol. 2015;2015:823139.43 doi:10.1155/2015/823139 |
| Smartphone-Based Fundus Imaging | Fundoscopic images obtained by using smartphones have substantial agreement with gold standards for clinical or photographic exams. | Vilela MA, Valença FM, Barreto PK, Amaral CE, Pellanda LC. Agreement between retinal images obtained via smartphones and images obtained with retinal cameras or fundoscopic exams - systematic review and meta-analysis. Clin Ophthalmol. 2018;12:2581–2589.44 Published 2018 Dec 11. doi:10.2147/OPTH.S182022 |
| Smartphone-Based Imaging Device | This widefield imaging device can view the retina beyond the posterior pole with a FOV of 65° in one shot. | Sivaraman A, Nagarajan S, Vadivel S, et al. A Novel, Smartphone-Based, Teleophthalmology-Enabled, Widefield Fundus Imaging Device With an Autocapture Algorithm. Transl Vis Sci Technol. 2021;10(12):21.45 doi:10.1167/tvst.10.12.21 |
| Smartphone-Based Fundus Photography | The imaging produced high-quality, wide-field retinal images. | Kim TN, Myers F, Reber C, et al. A Smartphone-Based Tool for Rapid, Portable, and Automated Wide-Field Retinal Imaging. Transl Vis Sci Technol. 2018;7(5):21.46 doi:10.1167/tvst.7.5.21 |
| Visual Field | ||
| Technology | Concluding Statement | Reference |
| Head-Mounted Device | VRVF was generally reliable in a telehealth setting for individuals with normal eyes. | Danielle McLaughlin, Hounsh Munshi, Eleonore Savatovsky, Elizabeth Vanner, Ta Chen Chang, Alana L Grajewski; Visual Field Testing in a Telehealth Setting: Remote Perimetry Using a Head-Mounted Device in Normal Eyes. Invest. Ophthalmol. Vis. Sci. 2022;63(7):1265 – A0405.47 |
| Tablet-Based Testing | There was good concordance between VFs measured at home and in the clinic (r = 0.94, P < 0.001). | Jones PR, Campbell P, Callaghan T, et al. Glaucoma Home Monitoring Using a Tablet-Based Visual Field Test (Eyecatcher): An Assessment of Accuracy and Adherence Over 6 Months. Am J Ophthalmol. 2021;223:42–52.48 doi:10.1016/j.ajo.2020.08.039 |
| Virtual Reality-Based Perimetry | The VR system was found to slightly underestimate VF defects in glaucoma subjects (1.4 dB). | Stapelfeldt J, Kucur SS, Huber N, Höhn R, Sznitman R. Virtual Reality-Based and Conventional Visual Field Examination Comparison in Healthy and Glaucoma Patients. Transl Vis Sci Technol. 2021;10(12):10.49 doi:10.1167/tvst.10.12.10 |
| Web-Based Confrontation Visual Field | The home-based VF test exhibits a reasonable receiver operating characteristic curve when compared to the Humphrey perimeter. | Tsapakis S, Papaconstantinou D, Diagourtas A, et al. Home-based visual field test for glaucoma screening comparison with Humphrey perimeter. Clin Ophthalmol. 2018;12:2597–2606.50 doi:10.2147/OPTH.S187832 |
| Tablet-Based Visual Field Testing | Home monitoring of VFs is viable for some patients and may provide clinically useful data. | Jones PR, Campbell P, Callaghan T, et al. Glaucoma Home Monitoring Using a Tablet-Based Visual Field Test (Eyecatcher): An Assessment of Accuracy and Adherence Over 6 Months. Am J Ophthalmol. 2021;223:42–52.48 doi:10.1016/j.ajo.2020.08.039 |
| Tablet-Based Visual Field Testing | Melbourne Rapid Fields (MRF) perimeties have a strong correlation to the Humphrey Field Analyzer. | Kong YX, He M, Crowston JG, Vingrys AJ. A Comparison of Perimetric Results from a Tablet Perimeter and Humphrey Field Analyzer in Glaucoma Patients. Transl Vis Sci Technol. 2016;5(6):2.51 doi:10.1167/tvst.5.6.2 |
| OCT | ||
| Technology | Concluding Statement | Reference |
| Handheld device | A handheld optical coherence tomography angiography (OCTA) system using a 100-kHz swept-source laser. | Yang J, Liu L, Campbell JP, Huang D, Liu G. Handheld optical coherence tomography angiography. Biomed Opt Express. 2017;8(4):2287–2300.52 doi:10.1364/BOE.8.002287 |
| Low cost OCT | Low-Cost portable device, demonstrated capability to image ex vivo porcine eye and murine skin as well as in vivo mouse retina | Kim S, Crose M, Eldridge WJ, Cox B, Brown WJ, Wax A. Design and implementation of a low-cost, portable OCT system. Biomed Opt Express. 2018;9(3):1232–1243.53 doi:10.1364/BOE.9.001232 |
Multiple studies have shown that by sending digital images of ophthalmic studies to remotely located ophthalmologists, tele-ophthalmology can be used to diagnose patients with retinal disease, glaucoma, and other conditions usually managed in the non-emergent setting.54 Teleophthalmology services used for emergency triaging also show promise. A prospective, observational study of 129 patients presenting to the Massachusetts Eye and Ear Infirmary emergency ward compared the diagnosis and urgency designation of ophthalmologists completing remote assessment to the gold standard in-person assessment. The study found a positive predictive value of an urgent telemedical triage of 0.93 (95% CI 0.88, 0.99) and a negative predictive value of a non-urgent telemedical triage of 0.67 (95% CI 0.56, 0.77).55
However, current integration of teleophthalmology into telestroke protocols for assessment of acute vision loss remains suboptimal. One review article found that of the 90 discrete teleophthalmology services described in the literature spanning from 1997 to 2020, only 6 pertained to emergency/trauma teleophthalmology care (n=6; 4.5%) and none of these specifically addressed stroke or integration into stroke care.56
Future Directions
It is our opinion that of the various technologies used to evaluate the ocular system in the emergency setting, technology enabling a fundus examination would be the most instrumental for remote triaging of acute vision loss. Non-mydriatic cameras that document the appearance of the ocular fundus are relatively easy to use and can greatly enhance the ability of practitioners to assess for non-stroke causes of acute vision loss. Several reports have published success in using nonmydriatic high-resolution digital fundus photography for obtaining fundus photos in non-ophthalmic settings such as emergency departments57,58. We believe that incorporating fundus photography with the capability of remote evaluation by ocular specialists in a telestroke consult would make a material difference in the triaging of acute vision loss.
Perhaps the most notable advantage of incorporating tele-ophthalmological evaluations into stroke-protocols for acute vision loss would be for patients presenting with acute CRAO to centers without in-person emergency ophthalmology coverage. In these cases, an urgent tele-ophthalmological evaluation and diagnosis of CRAO could help initiate transfer to a hospital with tPA capabilities where the patient may be eligible for treatment.
Artificial intelligence holds further promise for the utilization of tele-ophthalmology in a tele-stroke consult. Most tele-ophthalmology systems rely on point of care acquisition of ocular exam by non-ocular specialists and remote evaluation of the data by ocular specialists. Machine learning could aid diagnosis of ophthalmologic causes of acute vision loss even in the absence of immediate interpretation by ocular specialists. These technologies can also serve a role in diagnosis and management of patients with neurologic disease without vision loss. One study found that machine learning using retinal microvasculature features extracted from OCT-A and fundus images have the potential to diagnose acute cerebral stroke from retinal evaluation59. Using portable technologies, this could speed a diagnosis of stroke while a patient is in transit to the hospital, reducing death and disability.
Tele-ophthalmology, including evaluation of the retinal imaging, may play a role in the evaluation of and management of neurologic disease even in the absence of a diagnosis of ophthalmologic causes of vision loss. A study looking at 257 patients presenting to the emergency room with focal neurologic deficits found that the finding of arteriolar narrowing with non-mydriatic fundus imaging was an independent predictor of cerebrovascular disease overall and a diagnosis of transient ischemic attack, after controlling for the ABCD2 score and diffusion weighted imaging.58
Conclusions
In our efforts to expand patient access to specialty care through telemedicine, we must remember that our goal is to extend the reach of the medical care while ensuring noninferiority to traditional bedside treatment.60 Stroke workup is inappropriate for patients with evidence of other etiologies for their acute vision loss Working up presumed arterial occlusions without ruling out more common non-stroke causes for visual loss could lead to unnecessary testing and preclude interventions of other critical ophthalmic conditions in a timely fashion. To provide the best care for patients, common ophthalmic causes of acute vision loss should be ruled out quickly and efficiently before pursuing a stroke workup or treatment for suspected arterial occlusions. While a full ophthalmic exam is ideally performed in person at the time of the patient’s presentation for acute vision loss, tele-ophthalmology can facilitate a timely diagnosis. This is particularly important in the case of possible CRAO, where a stroke workup and even emergent thrombolytic treatment may be indicated. Few hospital centers to date offer tPA treatment to patients with CRAO. As the role of tPA treatment in patients with CRAO is better understood and may become standard of care, stroke services may depend on the integration of teleophthalmology to provide the highest quality care.
Implementing emerging telemedicine technologies to increase the communication between emergency medicine, ophthalmology, and neurology will allow practitioners to provide better care to patients with acute vision loss regardless of location.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.v RJ, Wilcock AD, Schwamm LH, et al. Assessment of telestroke capacity in US hospitals. JAMA Neurol. 2020;77(8):1035–1037. doi: 10.1001/JAMANEUROL.2020.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wechsler LR, Demaerschalk BM, Schwamm LH, et al. Telemedicine quality and outcomes in stroke: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2017;48(1):e3–e25. doi: 10.1161/STR.0000000000000114 [DOI] [PubMed] [Google Scholar]
- 3.Müller-Barna P, Schwamm LH, Haberl RL. Telestroke increases use of acute stroke therapy. Curr Opin Neurol. 2012;25(1):5–10. doi: 10.1097/WCO.0B013E32834D5FE4 [DOI] [PubMed] [Google Scholar]
- 4.Pula JH, Yuen CA. Eyes and stroke: the visual aspects of cerebrovascular disease. Stroke Vasc Neurol. 2017;2(4):210–220. doi: 10.1136/SVN-2017-000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biousse V, Newman NJ. Chapter 29 Eye syndromes and the neuro‐ophthalmology of stroke. Handb Clin Neurol. 2008;93:595–611. doi: 10.1016/S0072-9752(08)93029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltaziak K, Szpringer A, Czarnek-Chudzik A, et al. Quadrantanopia as the only symptom of post-COVID stroke in the occipital pole Case report. Medicine. 2021;100:44. doi: 10.1097/MD.0000000000027542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yew KS, Cheng E. Acute Stroke Diagnosis; 2009. Available from: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf. Accessed September 24, 2022.
- 8.Stuart-Shor EM, Wellenius GA, Dellolacono DM, Mittleman MA. Gender differences in presenting and prodromal stroke symptoms. Stroke. 2009;40(4):1121–1126. doi: 10.1161/STROKEAHA.108.543371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowe FJ; VIS Group2 3. Accuracy of referrals for visual assessment in a stroke population. Eye. 2011;25(2):161. doi: 10.1038/EYE.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijesundera C, Vingrys AJ, Wijeratne T, Crewther SG. Acquired visual deficits independent of lesion site in acute stroke. Front Neurol. 2020;11:705. doi: 10.3389/FNEUR.2020.00705/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe FJ, Wright D, Brand D, et al. A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. Biomed Res Int. 2013;2013:1–12. doi: 10.1155/2013/719096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LN, Baloh FG. The accuracy of confrontation visual field test in comparison with automated perimetry. J Natl Med Assoc. 1991;83(10):895. [PMC free article] [PubMed] [Google Scholar]
- 13.Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820–823.e2. doi: 10.1016/J.AJO.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.mac GB, Bao B, Schrag M, et al. Management of central retinal artery occlusion a scientific statement from the American heart association. Stroke. 2021;52:282–294. doi: 10.1161/STR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009;116(6):1188. doi: 10.1016/J.OPHTHA.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petzold A, Islam N, Hu HH, Plant GT. Embolic and nonembolic transient monocular visual field loss: a clinicopathologic review. Surv Ophthalmol. 2013;58(1):42–62. doi: 10.1016/J.SURVOPHTHAL.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 17.Coutts SB. Diagnosis and management of transient ischemic attack. Continuum. 2017;23(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol. 2019;200:271–272. doi: 10.1016/J.AJO.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 19.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167(22):2417–2422. doi: 10.1001/ARCHINTE.167.22.2417 [DOI] [PubMed] [Google Scholar]
- 20.Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597–1607. doi: 10.1016/j.ophtha.2018.03.054 [DOI] [PubMed] [Google Scholar]
- 21.Zhang LY, Zhang J, Kim RK, et al. Risk of acute ischemic stroke in patients with monocular vision loss of vascular etiology. J Neuroophthalmol. 2018;38(3):328–333. doi: 10.1097/WNO.0000000000000613 [DOI] [PubMed] [Google Scholar]
- 22.Fallico M, Lotery AJ, Longo A, et al. Risk of acute stroke in patients with retinal artery occlusion: a systematic review and meta-analysis. Eye. 2019;34(4):683–689. doi: 10.1038/s41433-019-0576-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma R, Newman N, Biousse V. Conservative treatments for acute nonarteritic central retinal artery occlusion: do they work? Taiwan J Ophthalmol. 2021;11(1):16. doi: 10.4103/TJO.TJO_61_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117(7). doi: 10.1016/J.OPHTHA.2010.03.061 [DOI] [PubMed] [Google Scholar]
- 25.Mac Grory B, Nackenoff A, Poli S, et al. Intravenous fibrinolysis for central retinal artery occlusion: a cohort study and updated patient-level meta-analysis. Stroke. 2020;51:2018–2025. doi: 10.1161/STROKEAHA.119.028743/FORMAT/EPUB [DOI] [PubMed] [Google Scholar]
- 26.Bruce BB, Lamirel C, Wright DW, et al. Nonmydriatic ocular fundus photography in the emergency department supplementary material refer to web version on PubMed central for supplementary material. N Engl J Med. 2011;364(4):387–389. doi: 10.1056/NEJMc1009733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Workforce H. National and regional projections of supply and demand for surgical specialty practitioners: 2013-2025 the national center for health workforce analysis (the National Center) informs public and private-sector decision-making on the U national and regional projections of supply and demand for surgical specialty practitioners: 2013–2025 2; 2016.
- 28.Sorra J, Famolaro T, Naomi Dyer Yount M, Alan Smith S, Wilson S, Helen Liu M. Hospital Survey on Patient Safety Culture: 2014 User Comparative Database Report Part II: appendix A-Overall Results by Hospital Characteristics Appendix B-Overall Results by Respondent Characteristics Part III: appendix C-Trending Results by Hospital Characteristics Appendix D-Trending Results by Respondent Characteristics; 2014. Available from: www.ahrq.gov. Accessed January 17, 2024.
- 29.Tauber J, Ayoub S, Shah P, et al. Assessing the demand for teleophthalmology in Florida emergency departments. Telemed E-Health. 2020;26(12):1500–1506. doi: 10.1089/TMJ.2019.0260 [DOI] [PubMed] [Google Scholar]
- 30.Wedekind L, Sainani K, Pershing S. Supply and perceived demand for teleophthalmology in triage and consultations in California emergency departments. JAMA Ophthalmol. 2016;134(5):537–543. doi: 10.1001/JAMAOPHTHALMOL.2016.0316 [DOI] [PubMed] [Google Scholar]
- 31.Brady CJ, Eghrari AO and Labrique AB. Smartphone-based visual acuity measurement for screening and clinical assessment. JAMA. 2015;314(24):2682–2683. doi: 10.1001/jama.2015.15855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslam TM, Parry NR, Murray IJ, et al. Development and testing of an automated computer tablet-based method for self-testing of high and low contrast near visual acuity in ophthalmic patients. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):891–899. doi: 10.1007/s00417-016-3293-2 [DOI] [PubMed] [Google Scholar]
- 33.Claessens J, van Egmond J, Wanten J, Bauer N, Nuijts R, Wisse R. The accuracy of a web-based visual acuity self-assessment tool performed independently by eye care patients at home: method comparison study. JMIR Form Res. 2023;7:.e41045. doi: 10.2196/41045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labiris G, Panagiotopoulou, EK, Delibasis, K et al . Validation of a web-based distance visual acuity test. J Cataract Refract Surg. 2023;49(7): 666–671. doi: 10.1097/j.jcrs.0000000000001176 [DOI] [PubMed] [Google Scholar]
- 35.McAnany JJ, Smith BM, Garland A, Kagen SL. iPhone-based pupillometry: a novel approach for assessing the pupillary light reflex. Optom Vis Sci. 2018;95(10):953–958. doi: 10.1097/OPX.0000000000001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazziotti R, Carrara F, Viglione A, et al. MEYE: web app for translational and real-time pupillometry. eNeuro. 2021;8(5):ENEURO.0122–21.2021. doi: 10.1523/ENEURO.0122-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goseki T, Kunimi K, Shioya N, et al. New device for taking nine-directional ocular photographs: “9Gaze” application. JEMR. 2022;15(1):10.16910/jemr.15.1.5. doi: 10.16910/jemr.15.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunbar GE, Shen B, Aref A. The Sensimed Triggerfish contact lens sensor: efficacy, safety, and patient perspectives. OPTH. 2017;11:875–882. doi: 10.2147/OPTH.S109708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Luttrell I, Feng S, et al. Development and validation of a machine learning, smartphone-based tonometer. Br J Ophthalmol. 2020;104(10):1394–1398. doi: 10.1136/bjophthalmol-2019-315446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russo A, Mapham W, Turano R, et al. Comparison of smartphone ophthalmoscopy with Slit-Lamp Biomicroscopy for grading vertical cup-to-disc ratio. J Glaucoma.2016;25(9):e777–e781. doi: 10.1097/IJG.0000000000000499 [DOI] [PubMed] [Google Scholar]
- 41.Truong P, Phan A, Truong B, Suen B, Melles G, Talke F. A smartphone attachment for remote ophthalmic slit lamp examinations. Microsyst Technol. 2020;26(11):3403–3407. doi: 10.1007/s00542-020-04894-7 [DOI] [Google Scholar]
- 42.González-Márquez F, Luque-Romero L, Ruiz-Romero MV, et al. Remote ophthalmology with a smartphone adapter handled by nurses for the diagnosis of eye posterior pole pathologies during the COVID-19 pandemic. J Telemed Telecare. 2023;29(6):474–483. doi: 10.1177/1357633X21994017 [DOI] [PubMed] [Google Scholar]
- 43.Russo A, Morescalchi F, Costagliola C, Delcassi L, Semeraro F. A novel device to exploit the Smartphone Camera for Fundus Photography. J Ophthalmol. 2015;2015:1–5. doi: 10.1155/2015/823139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilela MA, Valença FM, Barreto PK, Amaral CE, Pellanda LC. Agreement between retinal images obtained via smartphones and images obtained with retinal cameras or fundoscopic exams – systematic review and meta-analysis. OPTH. 2018. 12:2581–2589. doi: 10.2147/OPTH.S182022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivaraman A, Nagarajan S, Vadivel S, et al. A novel, smartphone-based, teleophthalmology-enabled, widefield fundus imaging device with an autocapture algorithm. Trans Vis Sci Tech. 2021;10(12):21. doi: 10.1167/tvst.10.12.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim TN, Myers, F, Reber, C et al . A smartphone-based tool for rapid, portable, and Automated Wide-Field Retinal Imaging. Trans Vis Sci Tech. 2018;7(5):21. doi: 10.1167/tvst.7.5.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaughlin D, Munshi H, Savatovsky E, Vanner E, Chang TA, Grajewski AL. Visual Field Testing in a Telehealth Setting: Remote Perimetry Using a Head-Mounted Device in Normal Eyes. Invest Ophthalmol Vis Sci. 2022;63(7):1265 – A0405. [Google Scholar]
- 48.Jones PR, Campbell P, Callaghan T, et al. Glaucoma home monitoring using a Tablet-Based Visual Field Test (eyecatcher): an assessment of accuracy and adherence over 6 months. Am J Ophthalmol. 2021; 223:42–52. doi: 10.1016/j.ajo.2020.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stapelfeldt J, Kucur ŞS, Huber N, Höhn R, Sznitman R. Virtual reality–based and conventional visual field examination comparison in healthy and glaucoma patients. Trans Vis Sci Tech. 2021;10(12):10. doi: 10.1167/tvst.10.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsapakis S, Papaconstantinou D, Diagourtas A, et al . Home-based visual field test for glaucoma screening comparison with Humphrey perimeter. Clin Ophthalmol. 2018;12:2597–2606. doi: 10.2147/OPTH.S187832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong YX, He M, Crowston JG, Vingrys AJ. A comparison of perimetric results from a tablet perimeter and Humphrey field analyzer in glaucoma patients. Trans Vis Sci Tech. 2016;5(6):2. doi: 10.1167/tvst.5.6.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Liu L, Campbell JP, Huang D, Liu G. Handheld optical coherence tomography angiography. Biomed Opt Express. 2017;8(4):2287. doi: 10.1364/BOE.8.002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Crose M, Eldridge WJ, Cox B, Brown WJ, Wax A. Design and implementation of a low-cost, portable OCT system. Biomed Opt Express. 2018;9(3):1232. doi: 10.1364/BOE.9.001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sommer AC, Blumenthal EZ. Telemedicine in ophthalmology in view of the emerging COVID-19 outbreak. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2341–2352. doi: 10.1007/S00417-020-04879-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meshkin RS, Armstrong GW, Hall NE, Rossin EJ, Hymowitz MB, Lorch AC. Effectiveness of a telemedicine program for triage and diagnosis of emergent ophthalmic conditions. Eye. 2022;37(2):325–331. doi: 10.1038/s41433-022-01940-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh L, Hong SC, Chalakkal RJ, Ogbuehi KC. A systematic review of current teleophthalmology services in New Zealand compared to the four comparable countries of the United Kingdom, Australia, United States of America (USA) and Canada. Clin Ophthalmol. 2021;15:4015. doi: 10.2147/OPTH.S294428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. Neurologist. 2012;18(6):350. doi: 10.1097/NRL.0B013E318272F7D7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vuong LN, Thulasi P, Biousse V, et al. Ocular fundus photography of patients with focal neurologic deficits in an emergency department. Neurology. 2015;85(3):256–262. doi: 10.1212/WNL.0000000000001759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pachade S, Coronado I, Abdelkhaleq R, et al. Detection of stroke with retinal microvascular density and self-supervised learning using OCT-A and Fundus imaging. J Clin Med. 2022;11(24):7408. doi: 10.3390/JCM11247408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kummervold PE, Johnsen JAK, Skrøvseth SO, Wynn R. Using noninferiority tests to evaluate telemedicine and E-health services: systematic review. J Med Internet Res. 2012;14(5):e132. doi: 10.2196/JMIR.2169 [DOI] [PMC free article] [PubMed] [Google Scholar]