Abstract
Purpose
The diversity and composition of the oral and gut microbiota of depressed rats were analyzed to explore the microbiological etiology of major depressive disorder (MDD).
Methods
The depressed rat model was established by inducing chronic unpredictable mild stress (CUMS). After the establishment of the model, body weight measurements and behavioral tests were conducted. The diversity and composition of oral and gut microbiota were analyzed using 16SrRNA sequencing.
Results
There were significant differences in the alpha and beta diversity of the oral microbiota of rats in the CUMS and control groups. The top three most abundant genera in the oral microbiota were Rothia, Psychrobacter, and Streptococcus. Linear discriminant analysis effect size (LEfSe) analysis showed that the abundance of Rothia decreased and that of Psychrotrophs increased in the CUMS group, and the differences were statistically significant. The top three most abundant genera in the gut microbiota were Lactobacillus, Ruminococcus and Oscillospira. LEfSe analysis showed that the abundance of Ruminococcus decreased in the CUMS group, and the difference was statistically significant. Spearman correlation analysis was performed to analyze the differential microbiota and depression-like behavior, which showed that differential microbiota significantly correlated with body weight, total distance traveled, average speed, and number of rearing. Spearman correlation analysis of oral and gut differential microbiota demonstrated a strong positive correlation between Facklamia in the oral cavity and Enterococcus, Streptococcus in the intestine (r=0.64–0.73, P<0.01); along with a strong negative correlation between Desulfovibrio in the oral cavity and Enterococcus, Turicibacter in the intestine(r=−0.51-−0.72, P<0.05).
Conclusion
Significant differences were observed in the diversity and composition of oral and gut microbiota between the CUMS depression model and control groups. Modulating the oral and gut microbiota may have positive effects on MDD.
Keywords: major depressive disorder, oral microbiota, gut microbiota, 16SrRNA sequencing
Introduction
The human microbiota is a diverse, complex, and symbiotic aggregate of microorganisms residing at various body sites like skin, oral cavity, gastrointestinal tract, respiratory tract, and urogenital tract.1 The oral cavity and gut are the two largest microbial habitats in the human body. An increasing number of studies have shown that the microbiota is involved in various physiological processes such as metabolism, neurotransmission, blood circulation, and immune response. The human microbiota reportedly plays a major role in maintaining the overall health. Moreover, microbial dysbiosis has been linked to obesity, malnutrition, neurological disorders, behavioral disorders, and cancer.2
Major depressive disorder (MDD) is a common mental illness characterized by significant and persistent depression, loss of interest, and anhedonia.3 Recent epidemiological surveys have shown that more than 300 million people worldwide suffer from MDD,4 and approximately 1 million people die from MDD every year. Depressive disorders seriously affect the physical and mental health of patients and are a major burden on the global society.5,6 Previous studies have reported that the occurrence and development of MDD are associated with multiple factors such as neurotransmitters, endocrine, imaging, and immunity. However, the pathogenesis of MDD still remains unclear. In recent years, research on microbiota has brought a new perspective to the study of mechanisms of depressive disorders. There is substantial evidence that gut microbiota play an important role in the occurrence and development of MDD through the gut-brain axis,7–9 and that imbalance in gut microbiota can result in the occurrence of MDD.10,11 Additionally, previous studies have shown that probiotics can alleviate MDD by modulating gut microbiota.12,13
The oral cavity is the second largest microbiome after the gut tract. Studies have elucidated a relationship between oral microorganisms and diseases such as diabetes,14 premature birth,15 cardiovascular diseases,16 and Alzheimer’s disease.17 However, only a few studies have investigated the role of oral microbiota in MDD. Studies have shown that oral administration of Porphyromonas gingivalis can change the composition of gut microbiota by reducing the gut barrier function and modulating the gut immune system.18 Accumulating evidence shows that oral microorganisms can change the gut microbiota by invading the intestine and resulting in microecological imbalance.19 Therefore, delving into the study of oral microbiota is also a potential research direction and would be of great value in elucidating the etiology of MDD.
The rodent model is a key tool for studying the biological mechanisms of mental illness. Its advantage is that it can nullify the influence of sex, age, diet, and genetics. Moreover, it maintains a highly controlled environment and reduces the variation among individuals in terms of oral and gut microbiota.20 Chronic unpredictable mild stress (CUMS) is a rodent model of depression. It is currently the most commonly used, reliable and effective model.21 In this study, a CUMS depression rat model was constructed, and 16SrRNA sequencing method was used to compare the compositional differences in the oral and gut microbiota of rats between the CUMS and control groups to provide a certain reference for elucidating the microbiological etiology of MDD.
Materials and Methods
Animals
A total of 20 male adult Sprague-Dawley (SD) rats (6 weeks old), weighing 180±20 g, were purchased from Beijing Vitong Lever Experimental Animal Technology Co., Ltd. [license number: SCXK (Beijing) 2021–0006]. Rats were raised in SPF-level animal rooms under controlled temperature (22±2 °C) and humidity (50%–60%), with a 12:12-h light: dark cycle. Real-time ventilation was used to keep the air fresh.
Animal Grouping and CUMS Procedure
The rats were randomly divided into two groups (n = 10/group): control and CUMS. Rats were individually housed in cages, and the CUMS model was established in accordance with the methods described in a previous study.21,22 The rats in the model group were exposed to CUMS to induce depressive-like symptoms. Stimulation methods included cold stimulation, heat stimulation, binding, noise stimulation, foreign body stimulation, tail clamping stimulation, dark stimulation, light stimulation, strobe light stimulation, moist padding, tilting the cage, fasting and food prohibition. One to two stimulation methods were randomly selected every day for a total of 28 days, and the methods were not repeated for three consecutive days.
Body Weight and Behavioral Tests
Body weight(g) of the 20 rats was measured before and after modeling. The rats were subjected to sucrose preference test (SPT) and open field test (OFT) to evaluate depressive-like behaviors.
SPT
The SPT is mainly used to evaluate anhedonia in rats.23 Reduced sucrose preference is generally considered an indicator of anhedonia, which is a core symptom of depression. Prior to the test, all the rats were trained to consume 1% sucrose solution for two days, and on the third day, an SPT test was conducted. For the test, the rats were given free access to one bottle with 100 mL of pure water and another bottle with 100 mL of sucrose solution. The positions of the two water bottles were alternated every 12 h to avoid position preference. At the beginning and the end of the 24 h test, the solution weights were measured, and the sucrose preference percentage was calculated using the following formula: Sucrose preference rate (%) = sucrose consumption/water + solution consumption × 100%.
OFT
The OFT was used to assess the locomotor activity and exploratory behavior of the rats.23 The OFT-100 open field experiment system consists of a box measuring 100 cm × 100 cm × 40 cm in dimension. Each rat was individually placed at the center of the box and observed for 5 min with an infrared camera. The total distance traveled(cm), average speed(cm/s), and number of rearing of each rat were recorded.
Sample Collection
After conducting the behavioral tests, the rats were anesthetized, and oral samples were collected using cotton swabs from the back of the tongue to the palate, buccal mucosa, upper and lower vestibules, and floor of the mouth for 30s. Fresh fecal samples from the cecum of each rat were collected using sterile cotton swabs, placed in a sterile sampling tube, and quickly transferred to a −80°C freezer.
16S rRNA Gene Amplicon Sequencing
Total genomic DNA samples were extracted using the OMEGA Soil DNA Kit (M5635-02), following the manufacturer’s instructions and stored at −20 °C before performing any further analysis. The quantity and quality of extracted DNAs were measured using a NanoDrop NC2000 spectrophotometer and agarose gel electrophoresis, respectively. PCR amplification of the bacterial 16S rRNA genes V3–V4 region was performed using the forward primer 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and the reverse primer 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The PCR components comprised: 5 μL buffer (5×), 0.25 μL Fast pfu DNA Polymerase (5U/μL), 2 μL (2.5 mM) dNTPs, 1 μL (10 uM) of each Forward and Reverse primer, 1 μL DNA Template, and 14.75 μL ddH2O. Thermal cycling consisted of initial denaturation at 98 °C for 5 min, followed by 25 cycles consisting of denaturation at 98 °C for 30s, annealing at 53 °C for 30s, and extension at 72 °C for 45s, with a final extension of 5 min at 72 °C.
Bioinformatics Analysis
Operational taxonomic unit (OTU) clustering was performed using QIIME 2 software (V2019.1),24 and the OTU representative sequences were compared with the Greengenes database for species information annotation. Alpha and beta diversity metrics were calculated using QIIME 2 (V2019.1). Beta diversity analysis was performed using principal coordinate analysis (PCoA) based on Jaccard distance. Linear discriminant analysis effect size (LEfSe) was used to identify the differential genera between two groups. Spearman correlation analysis was used to assess the correlation, and data are shown as a heat map using the GenesCloud program (https://www.genescloud.cn/chart/CorHeatmap).
Statistical Analysis
Statistical analysis and graphing of behavioral data were performed using GraphPad Prism 8.0. When the data satisfied the normal distribution and the variances were homogeneous, an unpaired t -test was used. When the data did not satisfy the normal distribution, non-parametric test was used. Their correlation was evaluated using Spearman correlation analysis, and P value <0.05 was considered statistically significant.
Results
Body Weight and Behavioral Tests
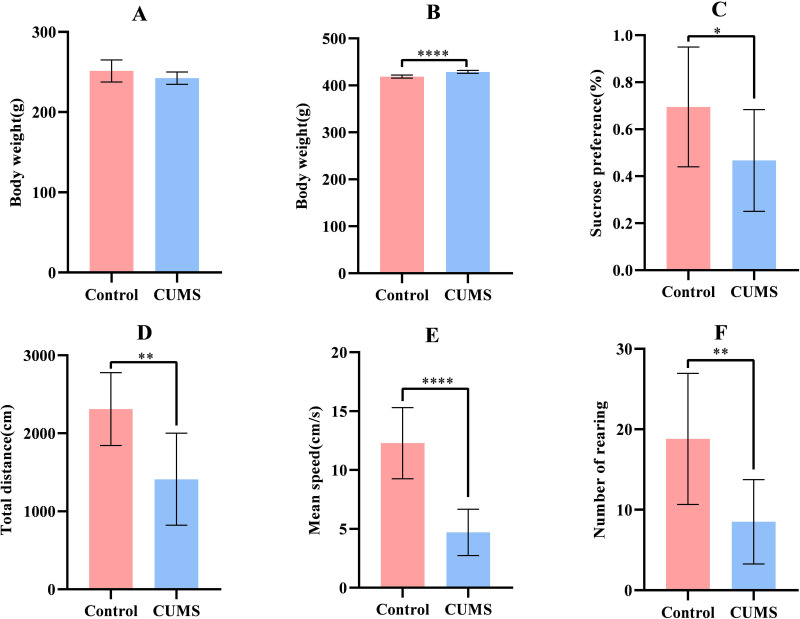
There was no significant difference in terms of body weight between the control and CUMS groups before modeling(P= 0.0882; Figure 1A). After modeling, rats in the CUMS group showed significant weight gain compared to rats in the control group (P<0.0001; Figure 1B). The preference coefficient for sugar water in SPT significantly decreased in the CUMS group (P=0.0454; Figure 1C) in comparison to the control group. In the context of OFT, the total distance(P = 0.0014; Figure 1D), average speed (P<0.0001; Figure 1E), and number of rearing (P= 0.0034; Figure 1F) of the CUMS group was found to be significantly decreased than that of the control group.
Figure 1.
Comparison of body weight and behavioral tests between in the two groups. (A) Baseline body weight of rats; (B) Body weight of rats after modeling; (C) Sugar water preference coefficient of rats; (D)Total distance of rats; (E) Average speed of rats; (F) Number of rearing of rats; (n=10), (*P<0.05, **P<0.01, ****P<0.0001).
The above results show that the CUMS group showed obvious depression-like symptoms, mainly anhedonia, reduced locomotor and exploratory behaviors, indicating that our depression model was successfully established.
Analysis of Alpha and Beta Diversity of Oral and Gut Microbiota in the Two Groups
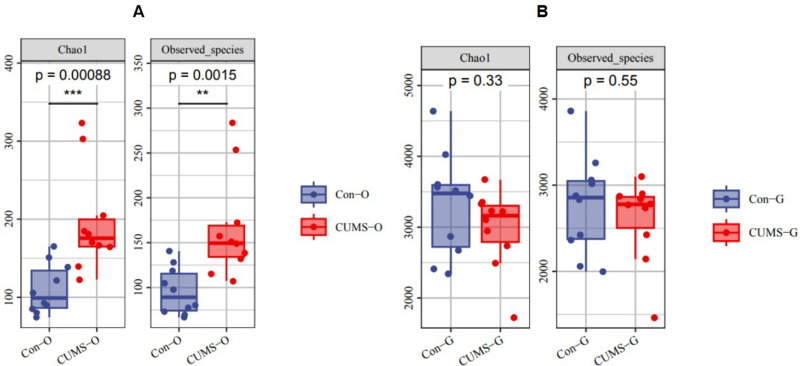
In the alpha diversity analysis, the Chao1 index and Observed species analysis of the oral microbiota showed that there were significant differences in these two indexes between the control and CUMS groups (Chao1: P < 0.001, Observed Species: P < 0.01). However, no significant differences were observed in the gut microbiota (Figure 2).
Figure 2.
Difference of alpha diversity in the two groups. (A) Differences in alpha diversity of oral flora in the two groups. (B) Differences in alpha diversity of gut flora in the two groups. (O stands for oral flora, G stands for gut flora; Chao1 and Observed species represent the bacterial richness; the larger the value is, the higher the bacterial richness), (n=10), (**P<0.01,***P<0.001).
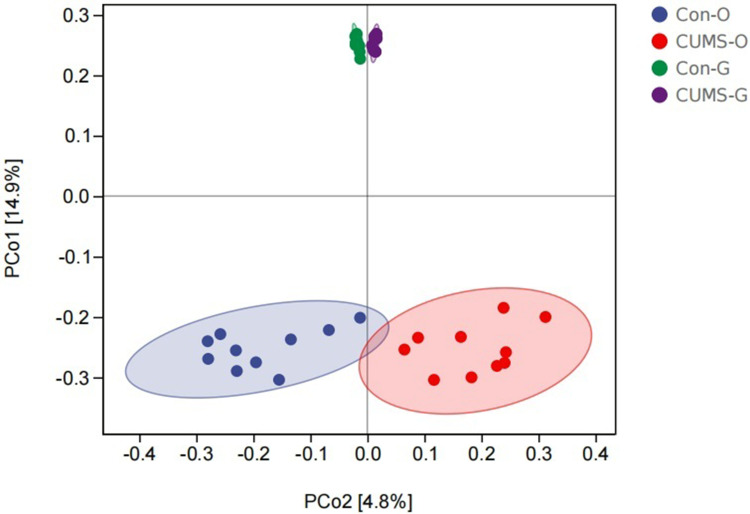
In the beta diversity analysis, there was a significant separation in the oral and gut microbiota between the CUMS and control groups, indicating the rich diversity of microbiota composition (Figure 3).
Figure 3.
Difference of beta diversity in the two groups using principal coordinate analysis (PCoA) based on Jaccard distance. The closer the projection distance of the two points on the coordinate axis, the more similar the community composition. (O stands for oral flora, G stands for gut flora), (n=10).
Analysis of Oral and Gut Microbiota Composition in the Two Groups
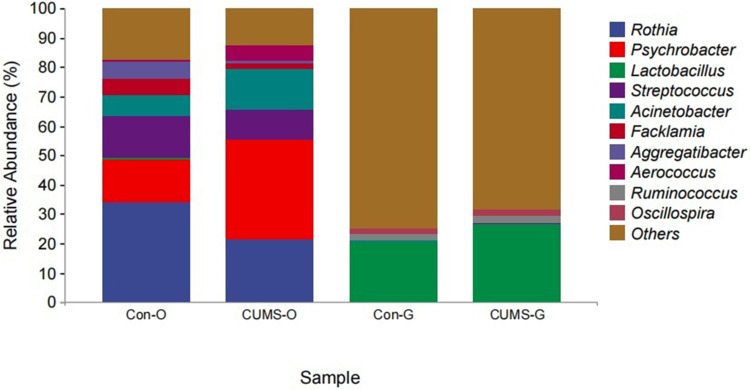
The top three most abundant bacterial genus within the oral cavity included Rothia (control group 34.06% vs CUMS group 21.28%), Psychrobacter (control group 14.78% vs CUMS group 34.02%), and Streptococcus (control group 14.43% vs CUMS group 9.79%) (Figure 4). LEfSe analysis showed that the abundance of Rothia decreased and that of Psychrotrophs increased in the CUMS group, and the difference was statistically significant.
Figure 4.
The composition and relative abundance of oral and gut flora at the genus level in the two groups. (O stands for oral flora, G stands for gut flora).
The top three most abundant bacterial genus in the gut microbiota included Lactobacillus (control group 26.62% vs CUMS group 20.27%), Ruminococcus (control group 2.64% vs CUMS group 2.51%) and Oscillospira (control group 2.29% vs CUMS group 1.72%) (Figure 4). LEfSe analysis showed that the abundance of Ruminococcus reduced in the CUMS group, and the difference was statistically significant.
LEfSe Analysis of Oral and Gut Microbiota in the Two Groups
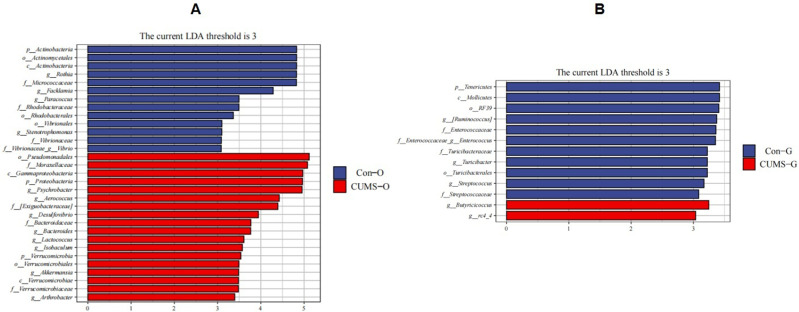
At the genus levels, Psychrobacter, Aerococcus, Desulfovibrio, Bacteroides, Lactococcus, Isobaculum, Akkermansia, and Arthrobacter showed higher abundance in the oral microbiota of the CUMS group, while Rothia, Facklamia, Paracoccus, Stenotrophomonas, and Vibrio showed lower abundance (Figure 5A).
Figure 5.
Taxonomic biomarkers found by LEfSe in the two groups. (A)Taxonomic biomarkers found by LEfSe in oral flora. (B)Taxonomic biomarkers found by LEfSe in gut flora. (O stands for oral flora, G stands for gut flora).
In the gut microbiota of the CUMS group, Butyricicoccus and rc4_4 showed higher abundance, while Ruminococcus, Enterococcus, Streptococcus and Turicibacter showed lower abundance (Figure 5B). All of the above differences were found to be statistically significant (P < 0.05).
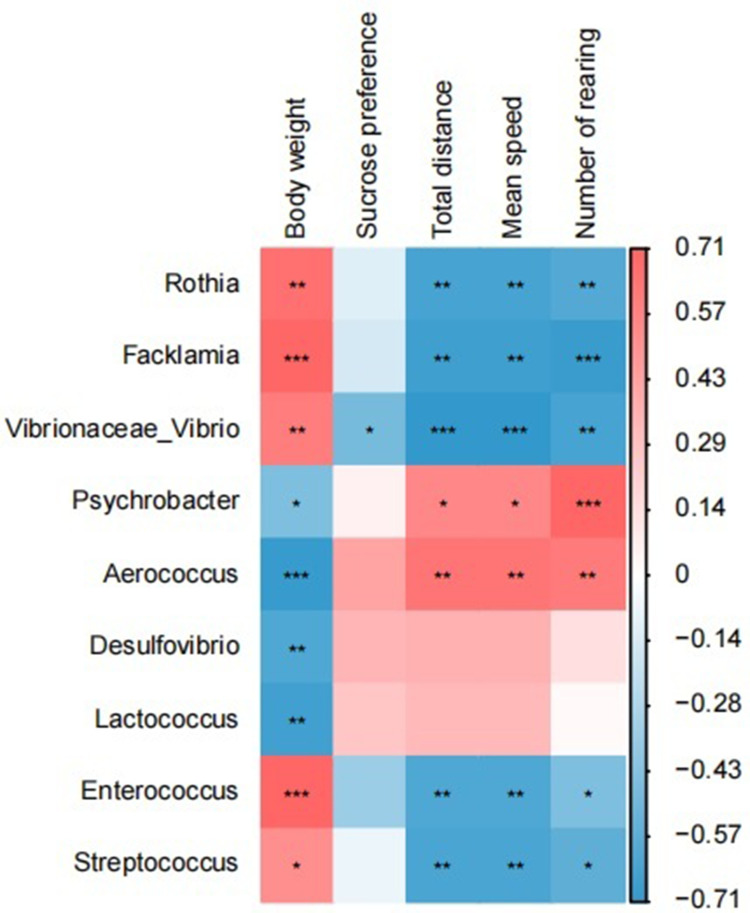
Correlation Analysis Between Oral and Gut Differential Microbiota and Depressive-Like Behavior
Spearman correlation analysis was performed on the differential microbiota and depressive-like behavior. The results showed that Rothia, Facklamia, Vibrio, Enterococcus, and Streptococcus positively correlated with body weight and negatively with total distance traveled, average speed, and number of rearing. Psychrobacter and Aerococcus positively correlated with the total distance traveled, average speed, and number of rearing, while negatively with body weight. Desulfovibrio, Bacteroides and Lactococcus positively correlated with body weight (P < 0.05) (Figure 6). The Spearman correlation coefficient and P value are shown in Table 1.
Figure 6.
Heat map of correlation between oral-gut differential flora and depressive-like behavior. (Red indicates positive correlation and blue indicates negative correlation), (*P<0.05, **P<0.01, ***P<0.001).
Table 1.
The Spearman Correlation Coefficient Value and P value
| Rothia | Facklamia | Vibrionaceae_Vibrio | Psychrobacter | Aerococcus | Desulfovibrio | Lactococcus | Enterococcaceae | Streptococcus | Turicibacter | rc4_4 | Body Weight | Sucrose Preference | Total Distance | Mean Speed | Number of Rearing | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rothia | 1 | |||||||||||||||
| Facklamia | 0.829** | 1 | ||||||||||||||
| Vibrionaceae_Vibrio | 0.630** | 0.713** | 1 | |||||||||||||
| Psychrobacter | −0.869** | −0.711** | −0.507* | 1 | ||||||||||||
| Aerococcus | −0.684** | −0.824** | −0.724** | 0.547* | 1 | |||||||||||
| Desulfovibrio | −0.544* | −0.464* | −0.536* | 0.162 | 0.589** | 1 | ||||||||||
| Lactococcus | −0.378 | −0.441 | −0.450* | 0.058 | 0.423 | 0.495* | 1 | |||||||||
| Enterococcaceae | 0.558* | 0.643** | 0.709*** | −0.438 | −0.705*** | −0.508* | −0.43 | 1 | ||||||||
| Streptococcus | 0.489* | 0.732*** | 0.448* | −0.543* | −0.672** | −0.107 | −0.295 | 0.620** | 1 | |||||||
| Turicibacter | 0.405 | 0.423 | 0.574** | −0.229 | −0.677** | −0.719*** | −0.139 | 0.452* | 0.171 | 1 | ||||||
| rc4_4 | −0.206 | −0.346 | −0.499* | 0.244 | 0.171 | 0.071 | 0.317 | −0.493* | −0.271 | 0.006 | 1 | |||||
| Body weight | 0.659** | 0.702*** | 0.602** | −0.445* | −0.695*** | −0.609** | −0.659** | 0.708*** | 0.517* | 0.321 | −0.394 | 1 | ||||
| Sucrose preference | −0.108 | −0.143 | −0.471* | 0.063 | 0.423 | 0.337 | 0.263 | −0.344 | −0.071 | −0.39 | 0.29 | −0.295 | 1 | |||
| Total distance | −0.638** | −0.669** | −0.711*** | 0.552* | 0.636** | 0.36 | 0.325 | −0.610** | −0.623** | −0.321 | 0.323 | −0.608** | 0.209 | 1 | ||
| Mean speed | −0.638** | −0.669** | −0.711*** | 0.552* | 0.636** | 0.36 | 0.325 | −0.610** | −0.623** | −0.321 | 0.323 | −0.608** | 0.209 | 1.000** | 1 | |
| Number of rearing | −0.599*** | −0.688** | −0.635** | 0.713*** | 0.609** | 0.15 | 0.022 | −0.448* | −0.561* | −0.367 | 0.256 | −0.452* | 0.232 | 0.682** | 0.682** | 1 |
Notes: *P<0.05, **P<0.01, ***P<0.001.
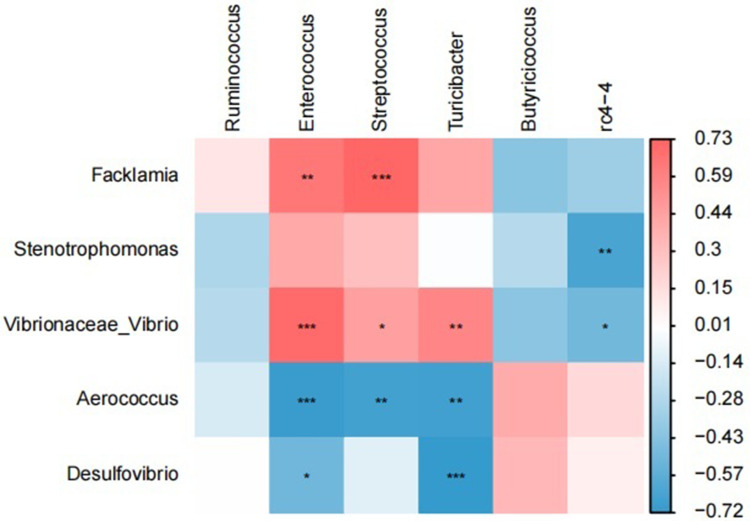
Correlation Analysis Between Oral and Gut Differential Microbiota
Spearman correlation analysis of oral and gut differential microbiota demonstrated a strong positive correlation between Facklamia in the oral cavity and Enterococcus, Streptococcus in the intestine (r = 0.64–0.73, P < 0.01); and a strong negative correlation between Desulfovibrio in the oral cavity and Enterococcus, Turicibacter in the intestine (r=−0.51-−0.72, P < 0.05) (Figure 7). The Spearman correlation coefficient and P value are listed in Table 1.
Figure 7.
Heat map of correlation between oral differential flora and gut differential flora. (Red indicates positive correlation and blue indicates negative correlation), (*P<0.05, **P<0.01, ***P<0.001).
Discussion
Characteristics of Gut Microbiota in Model Rats with CUMS-Induced Depression
Microbiota play a vital role in human health. Recent studies have shown that gut microbiota can affect the host brain function and behavior through the gut-brain axis.7,8 In the present study, we found that the relative abundance of Ruminococcus, Enterococcus, Streptococcus and Turicibacter in the gut microbiota of the CUMS group was reduced. Cheung et al reported that the relative abundance of Anaerostipes, Blotella, Clostridium, Clostridium, Parabacteroidetes, Coralibacter, and Streptococcus increased in patients with MDD, while that of Bifidobacterium, Microbacterium, Faecalibacterium and Ruminococcus decreased.7 Sanada et al conducted a meta-analysis of 10 studies and showed that the relative abundance of Faecalibacterium, Ruminococcus, Bifidobacterium, and Escherichia reduced in patients with MDD.25 The results of the two abovementioned studies are partly similar to our findings. Studies have also shown that Candida, Streptococcus, Escherichia, and Enterococcus can produce 5-hydroxytryptamine (5-HT), which is an inhibitory neurotransmitter and the reduction of which can lead to the occurrence of MDD.26 Gut microbiota and their products can directly affect the brain nervous system through the enteric nervous system and blood flow, and also indirectly mediate the occurrence of MDD through systemic inflammation.27
Characteristics of Oral Microbiota in Model Rats with CUMS-Induced Depression
The oral cavity harbors the second largest microbiome after the gut tract and is close to the brain, making it more likely to cause damage to the cranial nerves. Wingfield et al examined the composition of the salivary microbiota of patients with MDD and found that the abundance of Rothia, Haemophilus, Prevotella, Treponema, and Neisseria was significantly depleted in comparison to the control group.28 This study found significant differences in the α and β diversity of oral microbiota between the control and CUMS group. The CUMS group had the highest content of Rothia in the oral microbiota. Rothia belongs to the phylum Firmicutes, class Clostridiales, order Clostridiales, and family Lachnospiraceae. It is a Gram-positive, obligate anaerobic bacterium and a natural colonizer of the oral cavity. The Rothia genus comprises several species, including R. dentocariosa, R. mucilaginosa and R. aeria. Rothia can lead to bloodstream infections, endocarditis or other infections in immunocompromised patients. Ranga et al found that the R. mucilaginosa in the oral cavity produces enterobactin, which inhibits the growth of cariogenic Streptococcus mutans and pathogenic Staphylococcus aureus by chelating iron ions, which can cause oral microbiota imbalance.29 Rothia can also colonize the gastrointestinal tract and produce short-chain fatty acids. Studies have confirmed that Rothia in the intestine plays an important role as an anti-inflammatory bacterium in MDD.30–33
Interactions Between Oral and Gut Microbiota
In addition, we also found a correlation between the differential oral and gut microbiota, suggesting that the oral-gut microbiota axis may be involved in the pathogenesis of MDD. Previous studies have shown that there is a significant correlation between the microbial species present in the saliva and feces, and both have the same dynamics.34,35 Valles-Colomer et al studied more than 9700 human metagenomes and determined the human-to-human transmission of oral and gut microbiota, suggesting that the oral-gut microbiota axis plays an important role in the related diseases.36 Jun Qian et al found that periodontitis salivary microbiota can exacerbate depressive-like behavior by directly affecting the host gut microbiota,37 through oral-gut microbiota axis. Although the mouth and intestines are anatomically connected by the gastrointestinal tract, in health, physical and chemical barriers (such as stomach acid and bile acids) separate the mouth from the intestines. However, impairment of the oral-gut barrier can lead to translocation and communication between the organs. Oral microbiota can translocate to the gut, and gut microbiota can also spread to the oral cavity. This two-way interaction can change the ecosystem in both environments. Therefore, oral microbiota and their products can affect the brain function directly via the facial nervous system and bloodstream, or indirectly by being involved in gut dysbiosis and systemic inflammation.
Conclusion
In order to eliminate the influence of factors such as human sex, age, diet, and genetics, this study was the first to explore the characteristics of oral and gut microbiota of depressed rats exposed to CUMS, which was innovative. However, this analysis was limited to a cross sectional and did not take into account any changes in oral-gut microbiota dysbiosis and MDD over time. We found that the oral and gut microbiota of CUMS group were disordered, and the disordered microbiota may be the cause of MDD. In addition, we also found differences between the oral and gut microbiota, but there was also a degree of connection. Both may be involved in the occurrence of MDD, and modulating oral and gut microbiota may provide new options for treatment of MDD.
Acknowledgments
We would like to express our appreciation and gratitude to all of the participants in this study. The authors also acknowledge Miss Yanyan Zhang in Department of Psychiatry, First Hospital of Shanxi Medical University who has contributed to the research program.
Funding Statement
This study was financially supported by the National Natural Science Youth Fund Project (82201691), and the Science and Technology Innovation Project in Higher Education Institutions of Shanxi Province(2023L477).
Ethics Statement
This study complies with the Chinese National Laboratory Animal-Guidelines for Ethical Review of Animal Welfare (GB/T35892-2018) and was approved by the Medical Ethics Committee of First Hospital of Shanxi Medical University. The approval number is DWYJ-2023-148.
Disclosure
All authors declare that they have no conflicts of interest for this work.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith K. Mental health: a world of depression. Nature. 2014;515(7526):181. doi: 10.1038/515180a [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Agostino D, Wu YT, Daskalopoulou C, Hasan MT, Huisman M, Prina M. Global trends in the prevalence and incidence of depression: a systematic review and meta-analysis. J Affect Disord. 2021;281:235–243. doi: 10.1016/j.jad.2020.12.035 [DOI] [PubMed] [Google Scholar]
- 5.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139(1):81–132. doi: 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbert J, Lucassen PJ. Depression as a risk factor for Alzheimer’s disease: genes, steroids, cytokines and neurogenesis - What do we need to know. Front Neuroendocrinol. 2016;41:153–171. doi: 10.1016/j.yfrne.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic Review of Gut Microbiota and Major Depression. Front Psychiatry. 2019;10:34. doi: 10.3389/fpsyt.2019.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 9.Singh N, Singh V, Rai SN, Mishra V, Vamanu E, Singh MP. Deciphering the gut microbiome in neurodegenerative diseases and metagenomic approaches for characterization of gut microbes. Biomed Pharmacother. 2022;156:113958. doi: 10.1016/j.biopha.2022.113958 [DOI] [PubMed] [Google Scholar]
- 10.Xie A, Ensink E, Li P, et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov Disord. 2022;37(8):1644–1653. doi: 10.1002/mds.29128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Li J, Gui X, et al. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol Psychiatry. 2020;25(11):2759–2772. doi: 10.1038/s41380-020-0729-1 [DOI] [PubMed] [Google Scholar]
- 12.Vamanu E, Rai SN. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases. 2021;9(3). doi: 10.3390/diseases9030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogaru IA, Puiu MG, Manea M, Dionisie V. Current Perspectives on Pharmacological and Non-Pharmacological Interventions for the Inflammatory Mechanism of Unipolar Depression. Brain Sci. 2022;12(10). doi: 10.3390/brainsci12101403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsha TE, Prince Y, Davids S, et al. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J Dent Res. 2020;99(6):658–665. doi: 10.1177/0022034520913818 [DOI] [PubMed] [Google Scholar]
- 15.Gómez LA, De Avila J, Castillo DM, et al. Porphyromonas gingivalis Placental Atopobiosis and Inflammatory Responses in Women With Adverse Pregnancy Outcomes. Front Microbiol. 2020;11:591626. doi: 10.3389/fmicb.2020.591626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Cui J, Liu Y, Chen K, Huang L, Liu Y. Oral, Tongue-Coating Microbiota, and Metabolic Disorders: a Novel Area of Interactive Research. Front Cardiovasc Med. 2021;8:730203. doi: 10.3389/fcvm.2021.730203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dioguardi M, Crincoli V, Laino L, et al. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: a Systematic Review. J Clin Med. 2020;9(2). doi: 10.3390/jcm9020495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato T, Yamazaki K, Nakajima M, et al. Oral Administration of Porphyromonas gingivalis Alters the Gut Microbiome and Serum Metabolome. mSphere. 2018;3(5). doi: 10.1128/mSphere.00460-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488–500. doi: 10.1007/s13238-018-0548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Timberlake MA, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109. doi: 10.1016/j.pnpbp.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–116. doi: 10.1016/j.neubiorev.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Huang J, Xiong Y, Zhang X, Lin Y, Liu Z. Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis. Nutrients. 2021;14(1). doi: 10.3390/nu14010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang M, Jiang P, Li H, et al. Antidepressant-like effect of n-3 PUFAs in CUMS rats: role of tPA/PAI-1 system. Physiol Behav. 2015;139:210–215. doi: 10.1016/j.physbeh.2014.11.054 [DOI] [PubMed] [Google Scholar]
- 24.Bolyen E, Rideout JR, Dillon MR, et al. Author Correction: reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(9):1091. doi: 10.1038/s41587-019-0252-6 [DOI] [PubMed] [Google Scholar]
- 25.Sanada K, Nakajima S, Kurokawa S, et al. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2020;266:1–13. doi: 10.1016/j.jad.2020.01.102 [DOI] [PubMed] [Google Scholar]
- 26.Almeida C, Oliveira R, Soares R, Barata P. Influence of gut microbiota dysbiosis on brain function: a systematic review. Porto Biomed J. 2020;5(2). doi: 10.1097/j.pbj.0000000000000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narengaowa KW, Lan F, Awan UF, Qing H, Ni J. The Oral-Gut-Brain AXIS: the Influence of Microbes in Alzheimer’s Disease. Front Cell Neurosci. 2021;15:633735. doi: 10.3389/fncel.2021.633735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingfield B, Lapsley C, McDowell A, et al. Variations in the oral microbiome are associated with depression in young adults. Sci Rep. 2021;11(1):15009. doi: 10.1038/s41598-021-94498-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uranga CC, Arroyo P, Duggan BM, Gerwick WH, Edlund A. Commensal Oral Rothia mucilaginosa Produces Enterobactin, a Metal-Chelating Siderophore. mSystems. 2020;5(2). doi: 10.1128/mSystems.00161-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. doi: 10.1111/1462-2920.13589 [DOI] [PubMed] [Google Scholar]
- 31.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12(2):304–314. doi: 10.1111/j.1462-2920.2009.02066.x [DOI] [PubMed] [Google Scholar]
- 32.Khan MT, Duncan SH, Stams AJ, van Dijl JM, Flint HJ, Harmsen HJ. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012;6(8):1578–1585. doi: 10.1038/ismej.2012.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singhal S, Dian D, Keshavarzian A, Fogg L, Fields JZ, Farhadi A. The role of oral hygiene in inflammatory bowel disease. Dig Dis Sci. 2011;56(1):170–175. doi: 10.1007/s10620-010-1263-9 [DOI] [PubMed] [Google Scholar]
- 35.Bashan A, Gibson TE, Friedman J, et al. Universality of human microbial dynamics. Nature. 2016;534(7606):259–262. doi: 10.1038/nature18301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valles-Colomer M, Blanco-Míguez A, Manghi P, et al. The person-to-person transmission landscape of the gut and oral microbiomes. Nature. 2023;614(7946):125–135. doi: 10.1038/s41586-022-05620-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian J, Lu J, Cheng S, et al. Periodontitis salivary microbiota exacerbates colitis-induced anxiety-like behavior via gut microbiota. NPJ Biofilms Microbiomes. 2023;9(1):93. doi: 10.1038/s41522-023-00462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]