Abstract
Infection of fibroblast-like synovial cells with Chlamydia trachomatis (serotype D strain IC Cal 8) in culture induced the secretion of beta interferon (IFN-β). Chlamydial infection inhibited IFN-γ-induced expression of HLA-DR antigen in the cells. Addition of IFN-β antibody directly to infected cultures mitigated HLA-DR inhibition, suggesting involvement of produced IFN-β.
Chlamydia trachomatis, an obligate intracellular parasite, is a frequent cause of sexually transmitted diseases and a known triggering agent of reactive arthritis. Although chlamydiae cannot be cultivated from the joint chlamydial antigens, DNA and RNA have been detected in synovial tissue of reactive arthritis patients (11, 14, 16). In recent studies, chlamydial RNA and atypical forms of chlamydiae were identified by in situ hybridization and gold labeling immunoelectron microscopy within fibroblasts and macrophages in subintimal layers of the synovial membrane (5, 20). These findings support the hypothesis of inapparent chlamydial infection in reactive arthritis that may be associated with the persistence of C. trachomatis in a noncultivable state within synovial cells (SC).
C. trachomatis has a biphasic growth cycle. Infectious elementary bodies (EBs) enter the host cell and differentiate into larger reticulate bodies (RBs). These RBs divide by binary fission within the expanding endosome, resulting in development of an intracellular chlamydial inclusion. After a period of growth, RBs reorganize into new infectious EBs that are released by host cell lysis or exocytosis.
It has been suggested that persistent chlamydial infections are associated with reversible alterations of the chlamydial growth cycle (3). Interferons in particular have been implicated in restriction of facultative and obligate intracellular bacteria. Chlamydia-specific T lymphocytes of the Th1 subset which produce gamma interferon (IFN-γ) were identified in the synovial fluid of patients with reactive arthritis (12, 25). In several permanent cell lines and in epithelial cell cultures, IFN-γ treatment arrests chlamydiae at the EB stage or induces atypical RBs that do not differentiate into new EBs (9). In addition, IFN-α and IFN-β were found to inhibit intracellular chlamydial growth (21). IFN-γ is an inducer of major histocompatibility complex class II (MHC II) molecules on several cell types that are not conventional antigen-presenting cells. IFN-β can inhibit IFN-γ-induced MHC II expression and may function as a modulator of localized immune responses in inflammation (6). In healthy joints, synovial fibroblasts do not express MHC II molecules, but synoviocytes of patients with rheumatoid arthritis show an abundant MHC II expression and are able to act as antigen-presenting cells (7, 8). The synovial fibroblast possibly represents a cell type in which chlamydiae can persist in reactive arthritis. In nonprofessional phagocytes, the chlamydial inclusion does not fuse with endosomes and lysosomes. Lysosomal markers are absent within the chlamydial vacuole (13). This may result in a failure of antigen processing and presentation by MHC II molecules. Moreover, a chlamydial infection may modulate the IFN-γ-induced MHC II expression in these cells.
In this study, we investigated whether fibroblast-like SC produce IFN-β in response to C. trachomatis infection in culture and whether IFN-β can inhibit chlamydial growth in these cells. Furthermore, we evaluated the influence of C. trachomatis infection on IFN-γ-induced expression of HLA-DR molecules in fibroblast-like SC.
Human SC cultures were established from synovial biopsies obtained during meniscusectomies and arthroscopies of traumatic joint disease patients as previously described (22). Briefly, the tissue specimens were dissected into small pieces and digested in Iscove modified Dulbecco medium (IMDM; Biochrom, Berlin, Germany) containing 2 mg of collagenase type II (Biochrom) per ml. The cells were grown in IMDM supplemented with 30% fetal calf serum (FCS; Biochrom) together with 100 U of penicillin per ml and 100 μg of streptomycin (Sigma, Deisenhofen, Germany) per ml. Synoviocytes that were used during passages 4 to 12 were characterized as fibroblast-like cells by staining with monoclonal antibody to prolylhydroxylase (clone 5B5; Dako, Hamburg, Germany [22]).
High-titer stocks of C. trachomatis serotype D strain IC Cal 8 (obtained from the Institute of Ophthalmology, London, United Kingdom) were propagated in McCoy cell monolayers in serum-free medium SF-3 (Cytogen, Berlin, Germany) containing 1 μg of cycloheximide per ml (4). Infected cells were collected in phosphate-buffered saline (PBS) with 0.2 M sucrose and 2% FCS 48 h after infection and lysed by sonication. The suspension was centrifuged at 800 × g for 10 min to remove cell debris. Supernatants were stored at −70°C. Infectivity titers were quantified by titrating the number of inclusion-forming units (IFU) per ml in McCoy cells. These titers were used to determine the multiplicity of infection (MOI) for SC.
SC were grown in 11-mm-diameter culture tubes containing a glass coverslip (Sarstedt, Nürnbrecht, Germany). Cultures were checked for Mycoplasma contamination by DNA staining (bisbenzimidazole; Biochrom). Chlamydial stocks were diluted in PBS, and an inoculum of 0.2 ml was added to the culture tubes. SC monolayers (8 × 104 to 10 × 104 cells/tube) were infected by centrifugation at 4,000 × g at 37°C for 1 h at different MOIs (IFU per cell). After the inoculum was decanted, the cells were washed in medium to remove nonadsorbed chlamydiae and further incubated with 0.5 ml of IMDM containing 10% FCS but no antibiotics. For mock-infected cultures, synoviocytes were centrifuged with a harvest of uninfected McCoy cells. Culture supernatants of infected and mock-infected cells were collected, centrifuged at 14,000 × g for 5 min, and stored at −70°C.
Interferon was determined by a microtiter method based on the inhibition of the cytopathic effect of vesicular stomatitis virus (VSV) on human WISH cells (1). Briefly, serial dilutions of samples and IFN-β standard (Biochrom) were incubated on human WISH cells (ATCC CCL 25) for 20 h at 37°C and then challenged with VSV. Virus inhibition was measured by a colorimetric MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay 24 h later (19). To identify IFN-β in the bioassay, culture supernatants were preincubated with 100 nU of polyclonal sheep antibody to human IFN-β (Chemicon, Harrow, United Kingdom) per ml at room temperature for 1 h.
The effect of IFN-β on the production of infectious chlamydiae was tested in a growth yield assay. Confluent SC monolayers were incubated in IMDM supplemented with 10% FCS and IFN-β (50 to 200 U/ml). After 24 h, the cells were infected at an MOI of 1. After 72 h, infected monolayers were scraped from the coverslips into 0.5 ml of saccharose phosphate buffer (PBS with 0.2 M saccharose and 2% FCS) and briefly sonicated to release chlamydiae. Monolayers of McCoy cells were infected with serial dilutions of the disrupted SC suspensions and incubated in serum-free SF-3 medium containing 1 μg of cycloheximide per ml for 48 h. Chlamydial inclusions were visualized by immunofluorescence staining with fluorescein isothiocyanate (FITC)-conjugated antibody to C. trachomatis major outer membrane protein (MOMP) (Syva Microtrak C. trachomatis culture confirmation test; Behring Diagnostics, Inc., Marburg, Germany) to determine the titer of IFU per milliliter.
To induce expression of HLA-DR molecules, mock-infected SC and cells infected at an MOI of 5 were incubated in IMDM with 10% FCS and 10 U of IFN-γ per ml for 48 h. To determine an influence of IFN-β on IFN-γ-induced HLA-DR expression, infected cells were treated with 10 U of IFN-γ per ml and 100 nU of polyclonal sheep antibody to human IFN-β per ml. In control tubes, mock-infected cells were treated with 50 U of IFN-β per ml and 10 U of IFN-γ per ml for 48 h.
HLA-DR and Chlamydia antigens were detected by double immunofluorescence staining. Cells on coverslips were fixed in acetone for 30 min and air dried. The cells were incubated with monoclonal mouse antibody to human HLA-DR (clone DK 22; Dako) at a dilution of 1:50 in PBS containing 1% bovine serum albumin (BSA) at room temperature for 60 min. The coverslips were washed in PBS and incubated with biotinylated rabbit anti-mouse immunoglobulin G Fab2 fragment (Dako) at a dilution of 1:400 in PBS with 1% BSA for 1 h. After being washed with PBS, the cells were incubated with RPE-conjugated streptavidin (Dako) diluted 1:20 in PBS with 1% BSA for 30 min. To visualize chlamydial inclusions, the cells were incubated with FITC-conjugated antibody to C. trachomatis MOMP and Evans blue. After 30 min, the cells were washed again, mounted in PBS, and examined under a fluorescence microscope with excitation at 490 nm. The percentage of cells expressing HLA-DR molecules was determined by examining about 200 cells per coverslip.
For flow cytometric analysis, the cells were detached by use of EDTA (0.2 mM), washed twice in PBS, and incubated with phycoerythrin-conjugated monoclonal antibody to HLA-DR (Becton Dickinson, Hamburg, Germany) at room temperature for 20 min. Two additional washes were performed, and labeled cells were analyzed with a FACScan (Becton Dickinson) flow cytometer and CELL QUEST software. A total of 10,000 cells was scored in each sample.
In culture supernatants of Chlamydia-infected fibroblast-like synoviocytes, interferon activity was found by the VSV inhibition assay, whereas mock-infected cells did not release biologically active interferon (Tables 1 and 2). Interferon in culture supernatants was characterized as IFN-β, because specific antibody to IFN-β completely neutralized the activities. Maximal levels of IFN-β activity were detected at 48 to 72 h after infection (Table 2).
TABLE 1.
IFN-β release from fibroblast-like SC infected with various doses of C. trachomatis serotype Da
| Subject no.b | IFN-β (U/ml)c
|
|||
|---|---|---|---|---|
| Mock infected | MOI
|
|||
| 5 | 10 | 50 | ||
| 1 | —d | 11 (2) | 18 (5) | 28 (2) |
| 2 | —d | 14 (5) | 26 (7) | 41 (8) |
Supernatants were collected at 48 h after infection.
Experiments were performed with cell cultures established from different patients.
The data represent the means (standard deviations) of two wells.
—, not detectable.
TABLE 2.
Time course of IFN-β production by SC infected with C. trachomatis serotype Da
| Subject no.b | IFN-β (U/ml)c at h after infection:
|
|||
|---|---|---|---|---|
| 24 | 48d | 72 | 96 | |
| 1 | 9 (1) | 28 (6) | 26 (7) | 16 (4) |
| 2 | 8 (1) | 24 (8) | 20 (4) | 11 (2) |
| 3 | 26 (4) | 37 (5) | 44 (11) | 18 (4) |
Cells were infected at an MOI of 20.
Experiments were performed with cell cultures established from different patients.
The data represent the means (standard deviations) of two wells.
The mean values at 48 h were compared to the mean values of 24 h (paired t test, P ≤ 0.03). The mean paired difference was calculated from the paired differences of the mean values of subjects 1, 2, and 3.
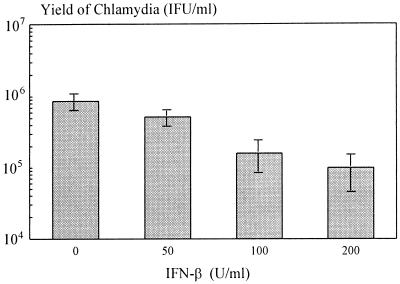
Infection of fibroblast-like SC with C. trachomatis resulted in intracellular growth characterized by the development of inclusion bodies. Infection at an MOI of 1 resulted in about 17% inclusion-positive cells and in production of new infectious chlamydiae. Treating the cells with IFN-β had a slight effect on chlamydial growth. IFN-β (200 U/ml) caused an eightfold reduction of chlamydial yield (Fig. 1).
FIG. 1.
Effect of IFN-β on chlamydial yield in SC. Values are the means and standard deviations of three different experiments.
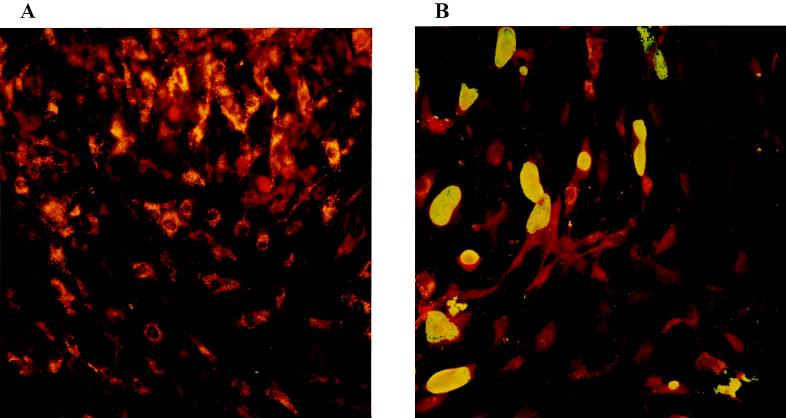
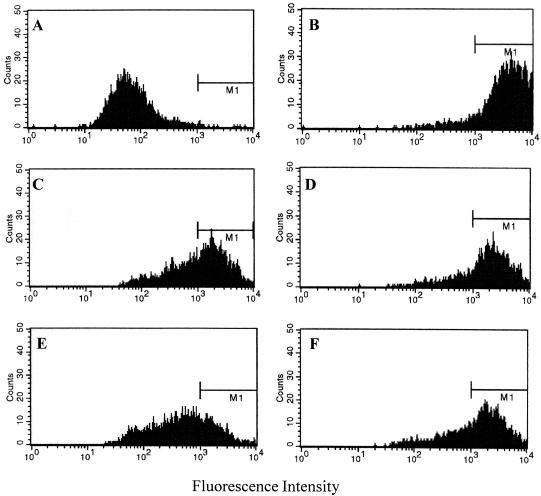
Synovial fibroblasts did not express HLA-DR in mock-infected and Chlamydia-infected cultures. Incubation with a low dose of IFN-γ (10 U/ml) induced expression of HLA-DR in about 90% of the cells (Table 3 and Fig. 2A). When synoviocytes were infected with C. trachomatis IC Cal 8 and then incubated with IFN-γ, the percentage of HLA-DR-positive cells was reduced in comparison to that for mock-infected cultures (Table 3 and Fig. 2B). The percentage of Chlamydia-mediated inhibition of IFN-γ-induced HLA-DR expression was 45% when the cells were infected at an MOI of 5. The inhibition depended on the infectious dose. At lower MOIs of 1 and 2, the number of HLA-DR-expressing cells did not vary between infected and mock-infected cultures. It is known that IFN-β can counter the stimulatory effect of IFN-γ on expression of MHC II antigen in several cell types (6). Coincubation of synoviocytes with IFN-β (50 U/ml) and IFN-γ (10 U/ml) for 2 days reduced the expression of HLA-DR (Table 3). The percentage of inhibition was 30%. Addition of anti-IFN-β antibody directly to infected cultures mitigated but did not abolish IFN-γ-induced HLA-DR expression (Table 3). The percentage of HLA-DR inhibition was reduced to 10%. In infected cultures, the percentage of HLA-DR-positive cells did not significantly differ between cells with a chlamydial inclusion and cells without an inclusion. Since acetone fixation of cells can destroy surface antigens, unfixed cells were stained with HLA-DR antibody and analyzed by fluorescence-activated cell sorting. In reference to negative controls, a boundary for HLA-DR-positive cells was defined at a fluorescence intensity of 103 (Fig. 3A and B). Chlamydial infection at an MOI of 5 or 10 reduced the percentage of DR-expressing cells from 92 to 60 or 28%, respectively (Fig. 3C and E). This effect was mitigated by neutralizing IFN-β activity in the cultures (Fig. 3D and F).
TABLE 3.
HLA-DR expression in fibroblast-like SC stimulated with IFN-γ and infected with C. trachomatis serotype D
| Infection type | Treatment | HLA-DR-positive cells (%)a |
|---|---|---|
| Mock | Medium | —b |
| Mock | 10 U of IFN-γ per ml | 91 (5) |
| Mock | 10 U of IFN-γ per ml plus 50 U of IFN-β per ml | 61 (7)c |
| C. trachomatis (MOI of 5) | 10 U of IFN-γ per ml | 50 (15)c |
| C. trachomatis (MOI of 5) | 10 U of IFN-γ per ml plus 100 nU of anti-IFN-β per ml | 79 (4)d |
Average number (standard deviation) of four separate experiments.
—, not detectable.
Significantly different from values for mock-infected cells after IFN-γ stimulation (Welch t test, P ≤ 0.01).
Significantly different from values for infected cells after IFN-γ stimulation (Welch t test, P ≤ 0.01).
FIG. 2.
Expression of HLA-DR molecules in C. trachomatis-infected cultures of SC detected by immunofluorescence staining. (A) Incubation with 10 U of IFN-γ per ml for 48 h induces HLA-DR molecules as indicated by the granular staining of SC. Cells were stained for HLA-DR by indirect immunofluorescence with an RPE-conjugated secondary antibody. Magnification, ca. ×200. (B) Following chlamydial infection and incubation with IFN-γ, a smaller number of SC show positive staining for HLA-DR antigen. Chlamydial inclusions were stained with FITC-conjugated antibody to MOMP. Magnification, ca. ×200.
FIG. 3.
Reduction of IFN-γ-induced HLA-DR expression on synovial fibroblasts after infection with C. trachomatis. (A) Mock-infected cells incubated in culture medium without IFN-γ (0.4% positive cells; total mean fluorescence intensity [MFI] = 97). (B) Mock-infected cells stimulated with 10 U of IFN-γ per ml (92% positive cells; MFI = 4,190). (C) Chlamydia-infected cells (MOI of 5) stimulated with 10 U of IFN-γ per ml (59% positive cells; MFI = 1,658). (D) Chlamydia-infected cells (MOI of 5) treated with IFN-γ (10 U/ml) and IFN-β antibody (100 nU/ml) (77% positive cells; MFI = 2,366). (E) Chlamydia-infected cells (MOI of 10) stimulated with 10 U of IFN-γ per ml (28% positive cells; MFI = 830). (F) Chlamydia-infected cells (MOI of 10) treated with IFN-γ (10 U/ml) and IFN-β antibody (100 nU/ml) (68% positive cells; MFI = 2,026). Flow cytometric analysis was performed after 48 h of incubation of cell cultures.
Fibroblast-like SC represent a cell type in which bacteria may persist in reactive arthritis. Investigations of Yersinia enterocolitica-induced arthritis have shown that yersiniae can invade and persist in synoviocytes in culture (15). In this work, we have reported that synoviocytes can also be infected with C. trachomatis. Infection of fibroblast-like cells resulted in production of IFN-β, which has a slight inhibitory effect on the production of infectious chlamydiae in these cells. The interferon-induced inhibition of chlamydial growth is characterized by the induction of indoleamine 2,3-dioxygenase, which catalyzes the degradation of tryptophan to kynurenine (2). Tryptophan is an essential amino acid, and a depletion of its intracellular pool is responsible for alterations in the growth cycle of Chlamydia (2). It has been reported that IFN-γ strongly stimulates indoleamine 2,3-dioxygenase activity in synoviocytes, while IFN-β has a weak stimulatory effect (18). This observation may explain the minor effect of IFN-β on chlamydial growth in synoviocytes. The in vivo mechanisms of chlamydial persistence have not been defined. IFN-γ produced by Chlamydia-reactive T lymphocytes might contribute to an inapparent infection of the synovial membrane in reactive arthritis (12, 25).
IFN-γ induced the expression of HLA-DR molecules in fibroblast-like synoviocytes. An IFN-γ concentration of 10 U/ml was sufficient to induce HLA-DR expression in about 90% of the cells and corresponds to levels found in the synovial fluid of patients with chronic arthritis (10). The expression of HLA-DR antigen on synovial fibroblasts in rheumatic diseases was repeatedly described. In rheumatoid arthritis, osteoarthritis, and traumatic damage, HLA-DR is expressed not only on macrophages but also on fibroblasts of the synovial membrane (24). Furthermore, it has been shown that synovial fibroblasts can possess an antigen-presenting capacity. Mycobacterium tuberculosis-reactive CD4 T cells that were isolated from synovial fluid of rheumatoid arthritis patients could be stimulated by IFN-γ-treated synovial fibroblasts as antigen-presenting cells (7). Infection of fibroblast-like SC with C. trachomatis significantly reduced the IFN-γ-induced expression of HLA-DR molecules. IFN-β was identified as a counterregulatory cytokine in MHC II expression (6). When infected cells were simultaneously incubated with IFN-γ and with a neutralizing antibody to IFN-β, the inhibition of HLA-DR expression was mitigated. We conclude that endogenously induced IFN-β is involved in HLA-DR inhibition. These results are consistent with in vitro studies on human cytomegalovirus-infected endothelial cells (23). The molecular mechanism of this antagonism between IFN-γ and IFN-β has not been fully elucidated. IFN-γ-induced MHC II transcription depends on class II transactivator. IFN-β acts in part by reducing the functional competence of class II transactivator for transactivating MHC II promoters (17).
Besides professional antigen-presenting cells and T lymphocytes, synovial fibroblasts probably play an important role in the immunopathogenesis of reactive arthritis. When Chlamydia-infected cells do not express HLA-DR antigen, the ability of the immune system to detect these cells may be impaired.
Acknowledgments
This study was sponsored by grants from the Bundesministerium für Forschung und Technologie, Germany (BMBF 01ZZ9104).
We thank W. Lungershausen (University Clinic of Jena) for providing synovial tissue and J. Hacker (Institut für Molekulare Infektionsbiologie, University of Würzburg) for helpful discussion.
REFERENCES
- 1.Armstrong J A. Cytopathic effect inhibition assay for interferon: microculture plate assay. Methods Enzymol. 1981;78:381–387. doi: 10.1016/0076-6879(81)78145-x. [DOI] [PubMed] [Google Scholar]
- 2.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benes S. Spread and persistence of infection with a trachoma biovar strain of Chlamydia trachomatis in multiplying and nonmultiplying McCoy cells. Sex Transm Dis. 1990;17:1–6. [PubMed] [Google Scholar]
- 5.Beutler A M, Schumacher H R, Jr, Whittum-Hudson J A, Salameh W A, Hudson A P. In situ hybridization for detection of inapparent infection with Chlamydia trachomatis in synovial tissue of a patient with Reiter’s syndrome. Am J Med Sci. 1995;310:206–213. doi: 10.1097/00000441-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 7.Boots A M H, Wimmers-Bertens J M M, Rijuders A W M. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology. 1994;82:268–274. [PMC free article] [PubMed] [Google Scholar]
- 8.Burmester G R, Jahn B, Rohner P, Zacher J, Winchester R J, Kalden J R. Differential gene expression of Ia antigens by rheumatoid synovial lining cells. J Clin Invest. 1987;80:595–604. doi: 10.1172/JCI113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De La Maza L M, Plunkett M J, Carlson E J, Peterson E M, Czarniecki C W. Ultrastructural analysis of the anti-chlamydial activity of recombinant murine interferon-γ Exp. Mol Pathol. 1987;47:13–25. doi: 10.1016/0014-4800(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 10.Firestein G S, Zvaifler N J. Peripheral blood and synovial fluid monocytes activation in inflammatory arthritis. Arthritis Rheum. 1987;30:863–871. doi: 10.1002/art.1780300803. [DOI] [PubMed] [Google Scholar]
- 11.Hammer M, Nettelnbreker E, Hopf S, Schmitz E, Pörschke K, Zeidler H. Chlamydial rRNA in the joints of patients with Chlamydia-induced arthritis and undifferentiated arthritis. Clin Exp Rheumatol. 1992;10:63–66. [PubMed] [Google Scholar]
- 12.Hassell A B, Reynolds D J, Deacon M, Gaston J S H, Pearce J H. Identification of T-cell stimulatory antigens of Chlamydia trachomatis using synovial fluid-derived T-cell clones. Immunology. 1993;79:513–519. [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzen R A, Scidmore M A, Rockey D D, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes R A, Hyder E, Treharne J D, Keat A C S. Intraarticular chlamydial antigen and inflammatory arthritis. Q J Med. 1991;80:575–588. [PubMed] [Google Scholar]
- 15.Huppertz H-I, Heesemann J. Experimental Yersinia infection of human synovial cells: persistence of live bacteria and generation of bacterial antigen deposits including “ghosts”, nucleic acid-free bacterial rods. Infect Immun. 1996;64:1484–1487. doi: 10.1128/iai.64.4.1484-1487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa H, Ohno O, Yamasaki K, Synichi I, Hirohata K. Arthritis presumably caused by Chlamydia in Reiter syndrome. Case report with electron microscopic studies. J Bone Joint Surg. 1986;68:777–779. [PubMed] [Google Scholar]
- 17.Lu H T, Riley J L, Babcock G T, Huston M, Stark G R, Boss J M, Ransohoff R M. Interferon (IFN) beta acts downstream of IFN-gamma-induced class II transactivator messenger RNA accumulation to block major histocompatibility complex class II gene expression and requires the 48-kD DNA-binding protein, ISGF-3-gamma. J Exp Med. 1995;182:1517–1525. doi: 10.1084/jem.182.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malone D G, Dolan P W, Brown R R, Kalayoglu M V, Arend R A, Byrne G I, Ozaki Y. Interferon γ induced production of indoleamine 2,3-dioxygenase in cultured synovial cells. J Rheumatol. 1994;21:1011–1019. [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Nanagara R, Li F, Beutler A, Hudson A, Schumacher H R. Alteration of Chlamydia trachomatis biologic behavior in synovial membranes: suppression of surface antigen production in reactive arthritis and Reiter’s syndrome. Arthritis Rheum. 1995;38:1410–1417. doi: 10.1002/art.1780381008. [DOI] [PubMed] [Google Scholar]
- 21.Paguirigan A M, Byrne G I, Becht S, Carlin J M. Cytokine-mediated indoleamine 2,3-dioxygenase induction in response to Chlamydia infection in human macrophage cultures. Infect Immun. 1994;62:1131–1136. doi: 10.1128/iai.62.4.1131-1136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rödel J, Straube E, Lungershausen W, Roth A, Groh A. Primary cultivation of human synovial cells from nonrheumatic synovial tissue and fluid. Exp Toxicol Pathol. 1996;48:243–247. doi: 10.1016/S0940-2993(96)80006-6. [DOI] [PubMed] [Google Scholar]
- 23.Sedmak D D, Chaiwiriyakul S, Knight D A, Waldmann W J. The role of interferon beta in human cytomegalovirus-mediated inhibition of HLA-DR induction on endothelial cells. Arch Virol. 1995;140:11–26. doi: 10.1007/BF01309727. [DOI] [PubMed] [Google Scholar]
- 24.Shiozawa S, Shiozawa K, Fujita T. Presence of HLA-DR antigen on synovial type A and B cells: an immunoelectron microscopic study in rheumatoid arthritis, osteoarthritis and normal traumatic joints. Immunology. 1983;50:587–593. [PMC free article] [PubMed] [Google Scholar]
- 25.Simon A K, Seipelt E, Wu P, Wenzel B, Braun J, Sieper J. Analysis of cytokine profiles in synovial T cell clones from chlamydial reactive arthritis patients: predominance of the Th1 subset. Clin Exp Immunol. 1993;94:122–126. doi: 10.1111/j.1365-2249.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]