Abstract
The incidence and mortality rates of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, are gradually increasing worldwide. Numerous studies have demonstrated that N-methyl-D-aspartic acid receptor (NMDAR)-mediated excitotoxicity contributes to neurodegenerative diseases. Ifenprodil, a subtype-selective NMDAR antagonist, showed strong therapeutic potential. However, it suffers from low oral bioavailability and off-target side effects. In this study, natural compounds were identified for selective inhibition of GluN1/GluN2B NMDAR of human. We obtained a set of natural compounds (n = 81,366) from COCONUT, TIPdb, PAMDB, CMNPD, YMDB, and NPAtlas databases, and then virtually screened by an ifenprodil-structure-based pharmacophore model and molecular docking. The top 100 compounds were selected for binding affinity prediction via batch drug–target affinity (BatchDTA). Then, the top 50 compounds were analyzed by absorption, distribution, metabolism, excretion, toxicity (ADMET). Molecular dynamics involving molecular mechanics/position-Boltzmann surface area (MM-PBSA) calculation were performed to further screening. The top 15 compounds with strong binding affinity and ifenprodil, a proven GluN2B-selective NMDAR antagonist, were subjected to molecular dynamic simulations (100 ns), root-mean-square deviation (RMSD), root-mean-square fluctuation (RMSF), radius of gyration (Rg), H-bonds, solvent accessible surface area (SASA), principal component analysis (PCA), and binding free energy calculations. Based on these analyses, one possible lead compound carrying positive charges (CNP0099440) was identified, with great binding affinity and less off-target activity by contrast to ifenprodil. CNP0099440 has great potential to be a GluN1/GluN2B NMDAR antagonist candidate and can be further detected via in vitro and in vivo experiments.
Keywords: NMDAR, pharmacodynamics, protein–molecule interaction, molecular dynamics, MM-PBSA
Impact Statement
Neurodegenerative diseases are closely associated with the largest growing treatment challenges especially for the aged people in modern society. As for lacking efficacious treatment options for Alzheimer’s disease (AD), new approaches and new drugs are quite necessary. NMDAR was proved inducing the excitotoxicity and aggravating the diseases. A high-throughput method was used in this study to screen novel inhibitors against subunit-selective GluN1/GluN2B NMDAR from Natural Product Databases and molecular docking, BatchDTA, HelixADMET, molecular dynamics, and MM-PBSA studies were relatively employed to verify the feasibility. CNP0099440 was finally identified as a potential novel inhibitor better than ifenprodil in terms of greater binding affinity and less off-target activity. This research laid solid foundation for future experiments both in vitro and in vivo and had potential to be one powerful substitute for ifenprodil in future clinical treatment.
Introduction
Neurodegenerative diseases (NDDs) cause a set of critical health problems on the aging society, with increasing incidence and mortality rates. The most known NDDs, including Alzheimer’s disease (AD), Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease, are mainly due to the persistent injury of neuronal function 1 Although the etiologies of NDDs seem to be distinct, they may act upon similar molecular mechanism. Food and Drug Administration has approved that N-methyl-D-aspartic acid receptors (NMDARs) are a common target for treating multiple NDDs, especially for AD. 2 Besides, only five therapies are approved to treat the symptoms of cognitive caused by AD in the United States, namely three cholinesterase inhibitors (donepezil, galantamine, and rivastigmine), one N-methyl-D-aspartate receptor antagonist, and one fixed-dose combination with donepezil and memantine for severe dementia. More and more therapeutic agents under development have failed to find novel methods to treat it, which leads AD becoming the least well-served therapeutic areas for drug treatments. 3 Thus, it is quite necessary to discover more novel antagonists against those target proteins as mentioned above by utilizing pharmacophore-based technique, which has been successfully and extensively applied for virtual screening, contributing a lot to multiple modern computer-aided drug design workflows. What’s more, it has been regarded as a valuable tool for communication between chemists specializing in computer and medicine. 4
Here, NMDARs, as special glutamate-gated ion channels, play important roles in brain physiological and pathological processes,5,6 while glutamate is the principal excitatory neurotransmitter in the central nervous system and is the agonist of ionotropic glutamate receptors (iGluRs). 7 There are seven subunits altogether in NMDARs including GluN1, GluN2A-D, and GluN3A-B. Glutamate-gated ion channels are usually blocked by Mg2+ at the resting state. Mg2+ can be removed by activated α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs) when glutamate is released from presynaptic sites, and then, NMDARs are activated. Multiple Na+ and Ca2+ subsequently flux into the cell, playing a critical role for physiological processes in neurons and initiating ischemic cell death. 8 Accumulating evidence has supported that hyperactive NMDARs induced excitotoxicity is a potential mechanism of neurodegeneration occurred in NDDs. 9 That is mainly because redundant calcium will overload inside the neurons, causing serious excitotoxicity and triggering a series of downstream prodeath signaling events. 8 Thus, NMDAR inhibitors are able to reduce NMDAR overexcitation induced by glutamate and prevent nerve cell apoptosis, treating AD and resistant depression, alleviating certain psychotic symptoms of schizophrenia, and reducing hyperalgesia and allodynia induced by nerve injury and diabetic neuropathy. 10 Multiple NMDAR antagonists have already been reported such as phencyclidine, ketamine, and dizocilpine. 11 Even though there are multiple NMDAR antagonists approved for NDD treatment, there is still a high demand for high selective NMDAR agents due to serious side effects of nonselective NMDAR inhibitors. 12 Compared with nonselective NMDAR antagonists, subunit-selective NMDAR antagonists hold stronger therapeutic potential for battling NDDs. 13 Among the subunit-selective NMDAR-targeting drugs, GluN1/GluN2B-selective NMDAR antagonists are the most promising. 14 Ifenprodil tartrate is the first discovered GluN1/GluN2B-selective NMDAR antagonist. 13 However, Ifenprodil tartrate suffers from low oral bioavailability and off-target side effects.
This study aimed to identify lead compounds serving as novel GluN2B-selective NMDAR antagonists via pharmacophore model. It is known that natural products are an important source of drug candidates. Thus, we screened a set of natural compounds from six natural compound databases through a constructed single-ligand-based pharmacophore model; analyzed potential activities via molecular docking, batch drug–target affinity (BatchDTA), and helixADMET; and verified the binding affinity and dynamic changing situations through molecular dynamics (MM) and molecular mechanics/position-Boltzmann surface area (MM-PBSA) studies. The whole selection workflow was depicted in Figure 1(A).
Figure 1.

(A) Workflow of identifying the potential lead compound specific to GluN2B NMDAR and (B) affinity scores for all 100 compounds marked in purple dots, and ifenprodil highlighted in red.
Materials and methods
Target protein preparation
The crystal structure of NMDAR subunit GluN1 and GluN2B (PDB ID: 5EWJ) was retrieved from RCSB Protein Data Bank (RCSB-PDB). The protein structure was modeled and validated using the SWISS-MODEL. 15 The protein preparation was in the Maestro of Schrödinger 2022.3 software. The environment was set at a 7.0 pH environment for optimization of the hydrogen-bonding network, and optimized potentials for liquid simulations 3 (OPLS3) force field was selected for a geometry optimization to a maximum root-mean-square deviation (RMSD) of 0.30 Å. Waters were removed with less than three H-bonds to nonwaters.
Ligand preparation
We retrieved 81,366 compounds from six natural compound databases, including COCONUT (n = 10,000), TIPdb (n = 8856), PAMDB (n = 4552), CMNPD (n = 31,561), YMDB (n = 2025), and NPAtlas (n = 33,372).16–21 Then, all the compounds were prepared using LigPrep2.4. 22 The possible states of each structure input were generated at a 6.8–7.2 pH without generating tautomer. Chiralities were determined and geometrically minimized from three-dimensional structure. The OPLS3 force field was chosen for the optimization to produce the low-energy isomer of the molecule. 23 A new database named “Natural Compounds” was eventually constructed via Create Phase Database in Maestro.
Pharmacophore-based virtual screening
The pharmacophore model named “5ewj-QEL-NMDZ1_XENLA.phypo” was freely downloaded from ePharmaLib (http://www.pharmbioinf.uni-freiburg.de/epharmalib) and visualized in Maestro. Five types of pharmacophore features were generated in the model, including two H-bond donors (HBD) (D3 and D4), two aromatic rings (R8 and R7), and one hydrophobic group (H5), as shown in Supplemental Figure S1(A). The screening criteria were fitting four out of five pharmacophore features.
Molecular docking
To investigate the molecular interaction of compounds and target protein, Glide (standard precision [SP] and extra precision [XP]) was performed.24,25 A 12 Å-binding pocket was generated for both Glide SP and XP on the basis of a centroid of co-crystallized native molecule “ifenprodil.” Then, all 2293 candidates met the pharmacophore-standard selection were first docked by Glide SP and the top 200 compounds ranked according to the docking scores of Glide SP were then selected to be docked by Glide XP, which was performed to secure more accurate ligand–protein configurations.
Ligand–target affinity estimation via BatchDTA
Deep neural networks (DNNs) are extensively employed for drug–target affinity (DTA) estimation due to their efficiency and accuracy. The affinity of ligands and proteins is a pivotal parameter for screening potential drug candidate. 26 BatchDTA is an improved platform that successfully alleviates the influence of the batch effects and far overweighs the other methods. It could be done through PaddleHelix platform (https://paddlehelix.baidu.com/app/drug/admet/train). The top 100 candidates were estimated their ligand–target affinity via deep learning-based BatchDTA as previous instruction. 27
Drug-likeness analysis
The estimation of pharmacokinetic properties is integral to discover and exploit hit-to-lead drugs, and ADMET in silico prediction belongs to a pivotal component of pharmaceutical R&D. 28 A total of 10 principal descriptors were used to evaluate the drug-likeness of top 50 screened compounds, including molecular weight (MW), fraction csp3 (Frac.Csp3), H-bond acceptors (HBA), HBD, ring count (nRING), aromatic ring count (nRot), molar refractivity (MR), topological polar surface area (TPSA), lipid–water partition coefficient (logP), and water solubility (logS). All descriptors were calculated using the PaddleHelix platform (https://paddlehelix.baidu.com/app/drug/admet/train). 29
MD simulation
The 100 ns MD simulations were performed via the GROningen MAchine for Chemical Simulations software (2021.3) for predicting the stability of 16 NMDAR–ligand complexes and native protein. 30 Acpype server was used to generate the parameters and topology files for each molecule, which can be obtained through the website (https://www.bio2byte.be/acpype/). Native protein and complexes were simulated with a cubic box and SPC216 water-filled model. 31 Some solvent molecules were added to each system, respectively. We used Na+ or K+ to neutralize the system charge, and 18 Na+ were added. The energy minimization was done by using the 100,000 steepest descent method with a Fourier grid. All MD simulations were run with periodic boundary conditions. The Van der Waals forces were set in a cut-off of 1.0 Å while the Particle–Mesh Ewald method was used with a 1.0 nm cut-off Particle-Mesh-Ewald. The frequency to update the neighbor list was 10.
The accomplishment of MD simulation should be in at least four steps. First and foremost, the entire system was minimized employing the steepest descent followed by conjugate gradient methods. In the second step for equilibration, heavy atoms were restrained using a force constant of 100 kJ/mol nm, and the solvent and ions were allowed to evolve, which was realized after minimization and molecular dynamics in both Canonical Ensemble (NVT) and Isothermal-Isobaric Ensemble (NPT) ensembles for 100 ps, respectively. Then, the temperature of the system was increased, and the velocities were reassigned based on the Maxwell–Boltzmann distribution so as to achieve equilibrium geometry at 298°K and 1 atom. Temperature coupling was set to 0.1 ps and pressure coupling to 2 ps. Besides, we used Berendsen algorithm for the thermostat and barostat during the equilibration step and constrain all bonds through the linear constraint solver (LINCS) algorithm.
The final MD step for each of the equilibrated Protein-Ligand (PL) systems was performed under the NVT and NPT ensembles for a 100 ns time scale with time steps of 2 fs and temperature at 298°K. 32 All bond lengths were also constrained by LINCS algorithm to constrain the lengths of the hydrogen-containing bonds.
MM-PBSA binding free energy calculation
MM-PBSA was used for calculating the binding free energy calculations of ligand–NMDAR complexes. Here, gmx_MMPBSA was used to calculate binding free energy by integrating MD simulation data. 33 The binding free energy for a complex can be described as:
Here, ΔGbind, ΔGcomplex, ΔGprotein, and Gligand denoted the binding free energy, the free energy of the ligand–protein complex, total energy of protein in the solvent, and total energy of ligand in the solvent. Configurations for each complex in the last 10 ns simulation were employed for further MM-PBSA calculations. 34
Glide XP docking for the other 46 protein sites with CNP0099440 and ifenprodil
All targeted proteins were downloaded from RCSB-PDB and then prepared in Maestro with the same parameters. In addition, a 12 Å-binding pocket was generated for each protein. Both CNP0099440 and ifenprodil were docked with 46 proteins in Glide XP.
Results
Identification of lead compounds through pharmacophore-based virtual screening and ligand docking
In this study, a subset of natural compounds (n = 81,366) was downloaded from six databases (COCONUT, TIPdb, PAMDB, CMNPD, YMDB, and NPAtlas) for virtual screening against GluN2B NMDAR. All 2293 compounds were initially identified, according to matching four out of five pharmacophore features. The top 200 compounds were selected according to the initial SP docking scores. The top score has achieved −14.894 for compound NPA002187 through Glide XP docking while ifenprodil was −10.178, setting as a positive control. What’s more, CNP0099440 was also higher than ifenprodil achieving a score of −11.666 (Supplemental Table S1). The top 100 compounds were selected according to Glide XP docking score for further BatchDTA analyses to predict their binding affinity. Affinity scores of all 100 candidates were shown in the box plot, which contained the maximum of 8.17406, minimum of 5.452629, upper quartile value of 6.974377, lower quartile value of 6.47933, and median value of 6.68164 (Figure 1(B)). The concrete statistic of binding affinity scores for each compound could be found in Supplemental Table S2. The top six compounds all had the same Tot Q of one, which meant they carried one positive charge. All the top 50 compounds in Supplemental Table S2 showed high affinity scores more than their median value of 6.68164. Both two compounds CNP0099440 and NPA003104 even had gained a value more than 8.00000, indicating a fantastic combined trend between ligands and target protein.
Assessment of drug-likeness through physicochemical property analysis
The control of physicochemical properties was beneficial in identifying lead compounds of candidate drug quality. The top 50 screened compounds were performed physicochemical property analysis according to 10 principal descriptors (MW, Frac.Csp3, HBA, HBD, nRING, nRot, MR, TPSA, logP, and logS). According to the standard of H-ADMET, a good drug should have an MW <500 and >150 Da, a TPSA <140 Å2, a logP <5.6 and >−0.4, a logS <0 and >−6 mol/L, an MR <130 and >40 m3/mol, a nRING <6, an HBA <10, an HBD <5, an nRot <10, and an Frac.Csp3 <1 and >0.25. As shown in Figure 2, 15 compounds and ifenprodil matched all the criteria. Supplemental Table S3 shows the concrete parameters of these 10 principal descriptors. The water solubility (logS) represents the solubility of a compound in water. The higher the value is, the better the water solubility can be. When the water solubility is less than −6, the compound is considered to have unsatisfactory water solubility. According to Supplemental Table S3, the value of each compound is larger than −6. What’s more, the logS value of CNP0099440 is −4.046 better than ifenprodil (−4.272), which means CNP0099440 can be more easily absorbed by human body and less off-target than ifenprodil.
Figure 2.
The bioavailability radar of screened 15 compounds and ifenprodil. The yellow area represented the optimal range for each property, and the red area represented the minimal range for each property (MW: 150–500 g/mol, TPSA: 0–140 Å2, logP: 0.4–5.6 mol/mol, logS: −6 to 0 mol/L, MR: 40–130 m3/mol, nRING: 0–6, HBA: 0–10, HBD: 0–5, nRot: 0–10, and Frac.Csp3: 0.25–1). The optimal range for each property was between the yellow and red areas.
MD simulation analysis
The structure of the 15 screened ligand–NMDAR complexes and GluN1/GluN2B NMDAR protein was, respectively, employed for 100 ns MD simulation for predicting the dynamic changes during ligand–protein interaction and their natural stability. The binding free energy was calculated via gmx_MMPBSA tool. As shown in Table 1 and Figure 3, ΔGgas was divided into ΔGvdw and ΔGele while ΔGsolv(kcal/mol) was composed of ΔEpb, ΔEnpolar, and ΔEdisper. ΔTotal was the sum of ΔGgas and ΔGsolv. The 15 compounds and ifenprodil were classified into three groups according to Tot Q. Tot Q in group 1 was equal to 1, which means each compound carried one positive charge, while Tot Q was equal to 0 in group 2, which represents electroneutral compounds. The Tot Q was equal to −1 in the third group, referring to each compound with one negative charge. According to the ΔTotal scores of the 15 compounds ranked from high to low in each group, the binding free energy of CNP0099440 achieved −22.18 kcal/mol was the top one, higher than the initial ligand ifenprodil. Interestingly, the top five compounds were all carried positive charges from group 1. Only the ΔGgas and ΔGsolv of NPA023404 in group 3 exhibited the opposite numeric values from the others. It may due to the mutually exclusive resistance between protein and NPA023404. Furthermore, the absolute value of ΔGele and ΔEpb in group 1 and group 3 remarkably exceeded group 2, which may be triggered by electrostatic interaction force between ligands and protein. Based on these analyses, we concluded that the target pocket with negative charge tended to bind positive charge molecules. According to MM-PBSA results, the top five complexes as well as ifenprodil–NMDAR complex and the empty protein were selected for further analysis in detail, involving protein–ligand analysis, RMSD, root-mean-square fluctuation (RMSF), radius of gyration (Rg), SASA, H-bond, principal component analysis (PCA), and Gibbs-free energy landscape, respectively.
Table 1.
Binding free energies of ifenprodil and the top 15 screened compounds. The stars represent for the potential five screened antagonists.
| Group | S. No. | Compound | Database | Tot Q | ΔGgas (kcal/mol) | ΔGsolv (kcal/mol) | ΔTotal (kcal/mol) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔGvdw | ΔGele | ΔEpb | ΔEnpolar | ΔEdisper | ΔGgas | ΔGsolv | ΔTotal | |||||
| 1 | 1 | CNP0099440✶ | COCONUT | 1 | −52.53 | −349.37 | 357.64 | −35.36 | 57.44 | −401.9 | 379.72 | −22.18 |
| 2 | Ifenprodil | – | 1 | −49.76 | −352.76 | 358.01 | −35.9 | 60.28 | −402.52 | 382.39 | −20.12 | |
| 3 | CNP0180570✶ | COCONUT | 1 | −51.15 | −340.59 | 350.36 | −33.57 | 57.6 | −391.74 | 374.39 | −17.35 | |
| 4 | CNP0075682✶ | COCONUT | 1 | −51.73 | −339.25 | 351.9 | −36.57 | 63.31 | −390.98 | 378.64 | −12.34 | |
| 5 | TIP007089✶ | TIPdb | 1 | −41.48 | −324.77 | 333.09 | −28.17 | 49.58 | −366.25 | 354.5 | −11.75 | |
| 6 | NPA024428✶ | NPAtlas | 1 | −46.7 | −356.29 | 364.77 | −33.78 | 60.52 | −402.99 | 391.51 | −11.48 | |
| 7 | CNP0378842 | COCONUT | 1 | −46.21 | −336.16 | 349.43 | −32.26 | 53.82 | −382.37 | 370.99 | −11.38 | |
| 8 | NPA003104 | NPAtlas | 1 | −34.41 | −321.3 | 325.41 | −23.22 | 43.57 | −355.71 | 345.76 | −9.95 | |
| 9 | CNP0332516 | COCONUT | 1 | −48.18 | −345.7 | 358.85 | −35.1 | 60.55 | −393.88 | 384.3 | −9.58 | |
| 10 | CNP0383618 | COCONUT | 1 | −47.87 | −376.24 | 389.79 | −36.17 | 64.46 | −424.11 | 418.08 | −6.31 | |
| 11 | TIP003185 | TIPdb | 1 | −37.87 | −317.68 | 332.97 | −29.18 | 51.62 | −355.55 | 355.41 | −0.13 | |
| 12 | NPA018669 | NPAtlas | 1 | −41.43 | −407.35 | 428.56 | −30.66 | 54.23 | −448.78 | 452.13 | 3.34 | |
| 2 | 13 | TIP012316 | TIPdb | 0 | −45.44 | −44.11 | 60.61 | −35.66 | 62.28 | −89.55 | 87.23 | −2.32 |
| 14 | NPA016262 | NPAtlas | 0 | −42.33 | −8.12 | 26.31 | −32.19 | 55.79 | −50.45 | 49.91 | −0.55 | |
| 15 | NPA015772 | NPAtlas | 0 | −44.94 | −22.29 | 46.52 | −32.13 | 59.96 | −67.23 | 74.35 | 7.13 | |
| 3 | 16 | NPA023404 | NPAtlas | −1 | −44.02 | 201.69 | −170.56 | −30.85 | 56.42 | 157.67 | −144.99 | 12.69 |
Figure 3.
The binding free energy of six ligand–protein complexes, namely GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428.
Protein–ligand analysis and visualization of the five screened candidates and ifenprodil
The lowest energy of NMDAR–ligand conformation from the last stable 10 ns of simulation was extracted and visualized in Maestro. The interacting amino acid residues of top five compounds and ifenprodil were displayed in Table 2. As shown in Figure 4(B), ifenprodil binds at the phenylethanolamine-binding site of NMDAR, a narrow and elongated location in an extended conformation. One end of this site nestled the benzylpiperidine moiety of ifenprodil; buried in the hydrophobic interface of the upper lobes of the GluN1 and GluN2B N-terminal domains (NTDs); and capped by Phe114, Ile82 (GluN2B), and Ala75, Pro106 (GluN1). The other end of the site hosted the phenol moiety, partially exposing to solvent, and interacted with receptors via polar and hydrophobic interactions. The distal hydroxyl group of the phenol moiety interacts with Glu236 from the lower lobe of GluN2B, whereas the aromatic ring interacts with a cluster of hydrophobic residues, including GluN1-Leu135, GluN2B-Phe176, and GluN2B-Pro177. Thus, the residues of GluN1/GluN2B NMDAR such as Ile82, Glu110, Phe114, Phe176, Pro177, and Glu236 (GluN2B) were reportedly essential for protein activity. In Figure 4, two-dimensional (2D) sketchers vividly depicted the protein–ligand interaction of the five screened compound and ifenprodil. The top five molecules and ifenprodil bound with NMDAR in a noncovalent interaction, including electrostatic interactions, π effects, Van der Waals interactions, and hydrophobic effects. CNP0180570 was predicted to bind with NMDAR at residues Thr110, Ala107, Gln110, and Asp113 by four conventional hydrogen bonds with a distance of 4.9, 2.7, 5.8, and 2.7 Å, respectively. Furthermore, amino acid residue Tyr109 contributed to cation–π interaction among compounds CNP0099440, CNP0180570, CNP0075682, and TIP007089 with a distance of 4.3, 4.2, 4.0, and 3.9 Å, respectively. Amino acid residue Phe114 contributed to π–π stacking interaction both in compound ifenprodil and in compound CNP0180570 with the same distance of 5.4 Å. As for compound CNP0075682, residues Ser132 in Chain A and Gln110 and Ala135 in Chain B contributed to NMDAR-CNP0075682 interaction by forming three stable H-bonds. Residue Tyr109 also formed a cation–π bonding so that the interaction can be further strengthened. TIP007089 and NPA024428 formed three and four H-bonds, respectively. However, no π–π stacking and cation–π interaction were found in NPA024428, and only Tyr109 was predicted to make contribution to cation–π interaction in complex TIP007089-NMDAR. Residue Tyr109, as a core residue, participated in all ligand–protein interaction except ifenprodil and NPA024428. CNP0099440 with optimal binding free energy (−22.18 kcal/mol) interacted with NMDAR at residues Ala107, Gln110, and Asp113 by hydrogen-bonding interaction and at residue Tyr109 by cation–π interaction.
Table 2.
The H-bond interaction, cation–π interaction, and π–π stacking interactions between amino acid residues and their distances (Å).
| S. No. | Compound | Number of total hydrogen bonds | H-bond interaction between residues and their distances (Å) | Cation–π interaction between residues and their distances (Å) | π–π stacking interactions between residues and their distances (Å) | |
|---|---|---|---|---|---|---|
| Chain A | Chain B | |||||
| 1 | Ifenprodil | 3 | Ser132 (2.8) | Gln110 (2.8), Glu236 (2.9) | – | B: Phe114 (5.4) |
| 2 | CNP0099440 | 3 | – | Ala107 (3.3), Gln110 (4.9), Asp113 (2.6) | A: Tyr109 (4.3) | – |
| 3 | CNP0180570 | 4 | Thr110 (4.9) | Ala107 (2.7), Gln110 (5.8), Asp113 (2.7) | A: Tyr109 (4.2) | B: Phe114 (5.4) |
| 4 | CNP0075682 | 3 | Ser132 (2.8) | Gln110 (2.6), Ala135 (2.7) | A: Tyr109 (4.0) | – |
| 5 | TIP007089 | 3 | Thr110 (4.7) | Gln110 (3.0), Asp113 (4.4) | A: Tyr109 (3.9) | – |
| 6 | NPA024428 | 4 | Ile133 (2.8), a Arg115 (2.7, 3.4) | Gln110 (2.7) | – | – |
Occurrence of two hydrogen bonds.
Figure 4.
Two-dimensional sketchers and their distances of protein–ligand interactions of top five screened natural compounds as well as ifenprodil with the key amino acid residues of GluN1/GluN2B NMDAR: (A) GluN1/GluN2B-whole complexes; (B) GluN1/GluN2B-ifenprodil; (C) GluN1/GluN2B-CNP0099440; (D) GluN1/GluN2B-CNP0180570; (E) GluN1/GluN2B-CNP0075682; (F) GluN1/GluN2B-TIP007089; and (G) GluN1/GluN2B-NPA024428.
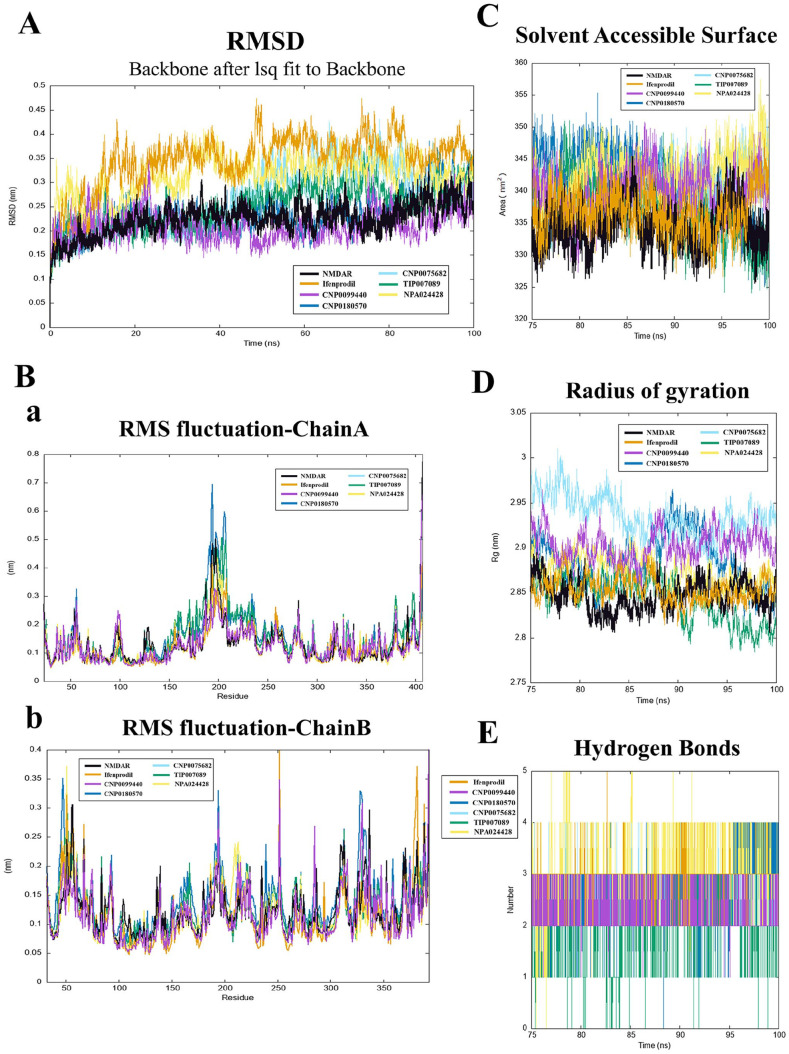
Assessment of structural stability using RMSD
The structure of complexes and an empty protein NMDAR all have been employed for further 100 ns MD simulation to predict the dynamic changes during ligand–protein interaction and their natural stability, respectively. The RMSD metric value was used to describe the spatial structural variation between redocking and co-crystallized ligand–protein complex structures. After a 25 ns of simulation, the RMSD values were 0.22, 0.35, 0.21, 0.24, 0.27, 0.25, 0.39, and 0.31 nm for GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B NMDAR-NPA024428, respectively. The lowest RMSD value indicated the best fit between ligand and NMDARs’ active site. These data suggested that GluN1/GluN2B NMDAR-CNP0099440 was a relatively stable complex compared with the others (Figure 5(A)). Because each GluN1/GluN2B NMDAR compound complex demonstrated stability after the 75 ns simulation, we performed further evaluations on each for last 25 ns trajectory (Figure 5(A)).
Figure 5.
(A) RMSD values for the GluN1/GluN2B-compound complexes; (B) RMSF values for the GluN1/GluN2B-compound complexes: (a) RMSF values for the GluN1-compound complexes over the final 25 ns of the simulations, and (b) RMSF values for the GluN2B-compound complexes over the final 25 ns of the simulations; (C) Rg values for the final 25 ns of the simulations; (D) SASA values for the final 25 ns of the simulations. Black, orange, purple, deep blue, light blue, green, and yellow colors represent GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428, respectively; (E) the number of hydrogen bonds in each respective complex according to data from the final 25 ns trajectory. Orange, purple, deep blue, light blue, green, and yellow colors represent GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428, respectively.
Assessment of structural flexibility using RMSF
The RMSF value of each system was employed to observe the conformational changes of the protein during MD simulations and performed to better understand the displacement and stability of each residue in the trajectory. In Chain A, as the region of GluN1, the evaluation of RMSF values were 0.132, 0.123, 0.127, 0.153, 0.138, 0.155, and 0.123 nm for GluN1, GluN1-ifenprodil, GluN1-CNP0099440, GluN1-CNP0180570, GluN1-CNP0075682, GluN1-TIP007089, and GluN1-NPA024428, respectively. What’ more, in Chain B, as the region of GluN2B, the evaluation of RMSF values were 0.123, 0.116, 0.116, 0.142, 0.117, 0.125, and 0.119 nm for GluN2B, GluN2B-ifenprodil, GluN2B-CNP0099440, GluN2B-CNP0180570, GluN2B-CNP0075682, GluN2B-TIP007089, and GluN2B-NPA024428, respectively (Figure 5(B)). Higher RMSF values were due to alterations in structural geometry caused by ligand binding. Minimal fluctuations in Chain A were observed in GluN1-ifenprodil, GluN1-CNP0075682, and GluN1-NPA024428 complexes.
Assessment of complex compactness through Rg analysis
The Rg was used to assess the compactness of ligand–protein complex and conformational changes throughout the MD simulation. Rg values of 2.847, 2.854, 2.902, 2.886, 2.936, 2.844, and 2.874 nm were revealed for GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428, respectively (Figure 5(C)). The lower value of Rg indicated the more compact binding of complex. Therefore, GluN1/GluN2B-TIP007089 exhibited a more compact structure than the others.
Assessment of complex compactness through SASA analysis
To identify changes in the solvent-accessible regions of the complexes, SASA values during the course of the final 25 ns of the simulation were measured. The values of 334.459, 336.810, 339.872, 341.566, 340.598, 337.188, and 342.794 nm2 were revealed, respectively, for GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428 (Figure 5(D)). The results showed minimal changes after binding with each compound. It may because of the relatively compact structure of the empty protein.
Interaction analysis through hydrogen bonding
Hydrogen bonding is an essential bond for stabilizing ligand–protein interactions. The average number of hydrogen bonds for complexes GluN1/GluN2B-CNP0099440 and GluN1/GluN2B-TIP007089 over the final 25 ns of the simulations was 1–3 and 0–3, respectively, while that for GluN1/GluN2B-CNP0180570 and GluN1/GluN2B-CNP0075682 was 0–4 and 1–4, respectively. We surprisedly found that both GluN1/GluN2B-ifenprodil and GluN1/GluN2B-NPA024428 could form at most five hydrogen bonds (Figure 5(E)). Our study revealed that all these compounds interacted with GluN1/GluN2B NMDAR with stable conformations.
PCA
In PCA, the sum of the eigenvalues described the overall flexibility of the molecular motion. The first five eigenvalues were calculated to determine the percentage in structural movement from the final 25 ns trajectories. The first five eigenvectors accounted for 73.29%, 68.16%, 66.96%, 80.08%, 77.42%, 81.22%, and 67.76% of the motions for GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428, respectively (Figure 6(A)). In addition, a 2D plot for assessing protein dynamics after compound binding was generated to show the overall stability of GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, and GluN1/GluN2B-NPA024428 (Figure 6(B)), indicating CNP0099440 and NPA024428 as possible lead compounds for further evaluation as subtype-selective NMDAR inhibitors.
Figure 6.

Principal component analysis (A) eigenvalues used for PCA; (B) first two PCs depicted GluN1/GluN2B NMDAR motion in space for all the systems. Black, orange, purple, deep blue, light blue, green, and yellow colors represent GluN1/GluN2B, GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428, respectively.
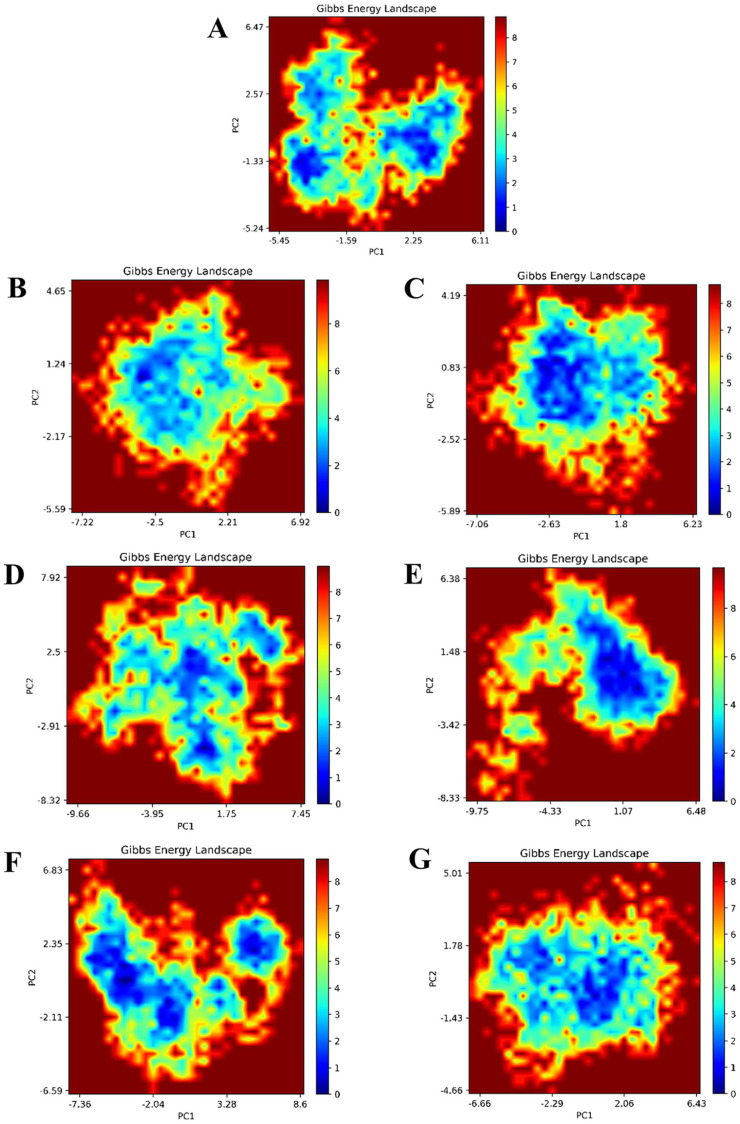
Gibbs-free energy landscape of ligand–protein complexes
The Gibbs-free energy landscape of protein was calculated according to the first two principal components (PC1 and PC2). Figure 7 showed the color-coded and plotted image generated for GluN1/GluN2B NMDAR and each complex. Compared with the Gibbs-free energy of empty GluN1/GluN2B NMDAR (7.874 kJ/mol) and GluN1/GluN2B-ifenprodil (8.851 kJ/mol), lower free energy values were observed for GluN1/GluN2B-CNP0099440 (7.721 kJ/mol) and GluN1/GluN2B-NPA024428 (7.721 kJ/mol), suggesting that these two complexes presented an outstanding overall thermodynamic stability than ifenprodil.
Figure 7.
The Gibbs-free energy landscape of six complexes and empty protein: (A) GluN1/GluN2B; (B) GluN1/GluN2B-ifenprodil; (C) GluN1/GluN2B-CNP0099440; (D) GluN1/GluN2B-CNP0180570; (E) GluN1/GluN2B-CNP0075682; (F) GluN1/GluN2B-TIP007089; and (G) GluN1/GluN2B-NPA024428.
Residual binding energy analysis of complexes
Residual binding energy analysis was performed to identify residues key to ligand–protein interaction. As shown in Table 3, all catalytic amino acid residues within 5 Å in the active site contributed significantly to the combination of ifenprodil, CNP0099440, CNP0180570, CNP0075682, TIP007089, and NPA024428, respectively. Figure 8 showed residues (within 5 Å) energy contributions to the formation of complexes while Figure 9 showed the common residues of the previous ifenprodil-binding pocket, namely Tyr109, Phe113, Arg115, and Ile113 in the region of GluN1 (Chain A) and Ala107, Gln110, Ile111, and Phe114 in GluN2B NTDs (Chain B). The most significant contributions to the binding free energy come from Tyr109 for CNP0180570, Ile133 for NPA024428, and Gln110 for CNP0075682. However, residue Arg115 mainly restricted the binding of CNP0099440, CNP0180570, CNP0075682, and NPA024428, especially ifenprodil. More contacts were observed in relation to CNP0099440-NMDAR binding, suggesting that CNP0099440 was the most possible lead compound of NMDAR antagonist.
Table 3.
All residues within 5 Å contribution to binding in kcal/mol.
| S. No. | Compound | All residues within 5 Å contribution to binding in kcal/mol | |
|---|---|---|---|
| Chain A | Chain B | ||
| 1 | CNP0099440 | Tyr109 (−2.8), Gly112 (0.51), Phe113 (−1.1), Arg115 (2.34), Ile133 (−1.05), His134 (−0.52) | Ala107 (−1.94), Ile108 (−0.56), Gln110 (−3.3), Ile111 (−1.91), Asp113 (−3.28), Phe114 (−0.85), Met134 (−1.01), Ala135 (1.34), Asp138 (−0.79) |
| 2 | Ifenprodil | Tyr109 (−2.23), Gly112 (0.74), Phe113 (−1.37), Arg115 (3.67), Lys131 (0.53), Ser132 (−1.91), Leu135 (−0.54) | Glu106 (−1.86), Ala107 (−0.57), Gln110 (−1.83), Ile111 (−1.73), Tyr175 (−0.53), Phe176 (−0.96), Pro177 (−0.62), Glu236 (−3.03) |
| 3 | CNP0180570 | Pro106 (−0.67), Ser108 (−0.61), Tyr109 (−4.22), Thr110 (−2.04), Gly112 (0.94), Phe113 (−1.24), Arg115 (2.79), Ile133 (−1.56) | Ala107 (−2.54), Ile108 (−0.5), Gln110 (−2.7), Ile111 (−2.13), Phe114 (−1.03), Asp138 (−0.61) |
| 4 | CNP0075682 | Tyr109 (−3.72), Gly112 (0.55), Phe113 (−1.03), Arg115 (2.56), Asp130 (−0.66), Ser132 (−0.87), Ile133 (−1.37) | Gln110 (−3.82), Ile111 (−1.28), Phe114 (−1.01), Met134 (−0.63), Ala135 (−1.64), Asp136 (−0.66), Pro177 (−1.58) |
| 5 | TIP007089 | Pro106 (−0.78), Tyr109 (−2.81), Thr110 (−2.7), Ile133 (−0.52) | Ala107 (−0.52), Ile111 (−1.96), Asp113 (−1.26), Phe114 (−1.29), Lys137 (0.6) |
| 6 | NPA024428 | Tyr109 (−2.52), Phe113 (−0.85), Arg115 (0.6), Ile133 (−3.98) | Gln110 (−1.23), Ile111 (−2.09), Phe114 (−0.67) |
The bonded represents common residue (within 5 Å) contributions to the binding.
Figure 8.
Decomposed residue (within 5 Å) energy contributions to the formation of six NMDAR complexes, namely GluN1/GluN2B-ifenprodil, GluN1/GluN2B-CNP0099440, GluN1/GluN2B-CNP0180570, GluN1/GluN2B-CNP0075682, GluN1/GluN2B-TIP007089, and GluN1/GluN2B-NPA024428.
Figure 9.
Depicting the common amino acid residues of NMDAR contributing to the binding with novel screened natural compounds and ifenprodil. Orange, yellow, light green, brown, light brown, and deep green colors represent NMDAR-ifenprodil, NMDAR-CNP0099440, NMDAR-CNP0180570, NMDAR-CNP0075682, NMDAR-TIP007089, and NMDAR-NPA024428, respectively.
Assessment of off-target activity against other 46 sites
As shown in Table 4, CNP0099440 did not exhibit any significant affinity (docking score <−10) for any of the 46 binding sites, especially 5-HT2A and 5-HT2C receptors. Structures of 5-HT2C-CNP0099440, 5-HT2C-ifenprodil, 5-HT2A-CNP0099440, and 5-HT2A-ifenprodil complexes were shown in Figure 10 with docking scores of −8.813, −9.425, −8.925, and −8.887, respectively. This study provided evidence that CNP0099440 has a low potential for off-target activity.
Table 4.
Docking scores for 46 binding sites.
| Site | PDB ID | Docking score for CNP0099440 | Docking score for ifenprodil |
|---|---|---|---|
| NR2A | 2A5S | −2.819 | −2.655 |
| A1 | 4W7H | −2.779 | −4.504 |
| A3 | 7DVL | −5.409 | −4.948 |
| AT1 | 6YV1 | −3.226 | −5.147 |
| β1 | 1TG7 | −4.06 | −4.147 |
| BZD centrol | 6TIV | −2.729 | −3.889 |
| CCR1 | 7VL8 | −4.098 | −3.177 |
| CCKA | 5IDJ | −2.601 | −4.319 |
| CCKB | 7F8W | −4.142 | −3.769 |
| CGRP | 7N7P | −5.423 | −5.32 |
| D1 | 1FC6 | −5.319 | −5.641 |
| ETA | 7UJB | −3.977 | −3.41 |
| ETB | 1QTF | −2.637 | −5.294 |
| GABA | 6DWO | −5.968 | −6.362 |
| GABA-β3 | 4COF | −3.108 | −5.733 |
| GAL1 | 7WQ3 | −4.464 | −2.872 |
| GAL2 | 7XJL | −2.871 | −4.414 |
| H1 | 4H8K | −5.42 | −4.667 |
| H2 | 3PUF | −3.102 | −3.413 |
| PGDF | 4QCI | −3.827 | −3.342 |
| 5-HT2C | 6BQH | −8.813 | −9.425 |
| 5-HT5a | 6A93 | −8.925 | −8.887 |
| M1 | 1EA3 | −5.227 | −4.62 |
| M2 | 6BKK | −3.013 | −2.121 |
| M3 | 1MKF | −6.19 | −6.487 |
| M4 | 6KP6 | −9.137 | −10.936 |
| M5 | 6Z2B | −3.712 | −3.641 |
| NK1 | 1GMN | −3.361 | −2.745 |
| NK2 | 3HN4 | −3.775 | −3.933 |
| hERG | 7CN0 | −5.320 | −5.323 |
| KV channel | 1SES | −5.139 | −6.458 |
| SKCa channel | 2PNV | −2.401 | −3.575 |
| V1-ATP | 3A5C | −4.217 | −4.580 |
| VPAC1 | 8E3Y | −3.046 | −1.726 |
| Sigma | 1KU2 | −2.735 | −4.864 |
| Na+ channel | 2AHY | −6.613 | −8.795 |
| 5-HT3 | 5AIN | −1.127 | −4.618 |
| 5-HT6 | 7XTB | −4.440 | −2.948 |
| 5-HT7 | 7XTC | −4.803 | −6.980 |
| CXCR2 | 6KVF | −3.885 | −4.540 |
| Dopamine transporter | 4XP1 | −7.842 | −9.584 |
Figure 10.
(A) The structure of 5-HT2C-ligand complexes: (a) the structure of 5-HT2C-CNP0099440 complex, and (b) the structure of 5-HT2C-ifenprodil complex; (B) the structure of 5-HT2A-ligand complexes: (a) the structure of 5-HT2A-CNP0099440 complex, and (b) the structure of 5-HT2A-ifenprodil complexes.
Discussion
NMDARs, members of the iGluR family, have become attractive targets for the treatment of NDDs such as depression, epilepsy, AD, and Parkinson’s disease among others. They are heterotetrameric complexes with multiple subtypes. In the central nervous system of adult, particularly in the hippocampus and cortex, GluN2B are the predominant subunits, indicating their central roles in synaptic function and plasticity. 35 Hence, GluN2B/NMDAR antagonists have shown their clinical effectiveness in number of NDDs. However, the available GluN2B-selective NMDAR antagonist, like ifenprodil, suffers from poor oral bioavailability and off-target activities. Therefore, identification of novel GluN2B/NMDAR antagonists is needed to manage the NDDs. Natural compounds have made great contributions in the screening of lead compounds.36,37 In recent years, with the development of protein structural biology and computational methods, it is easier to understand the interaction between protein and ligand. Therefore, high-quality lead compounds are identified more efficiently. In this study, a novel virtual screening process was established. Multiple computational approaches were employed to the identification of possible natural lead compounds via pharmacophore-based virtual screening, molecular docking, BatchDTA, HelixADMET, molecular dynamics, MM-PBSA followed by off-target analysis. From the pharmacophore-based virtual screening, we selected 2293 natural compounds. Furthermore, molecular docking and protein–ligand affinity analysis of the top 100 compounds. Then, ADMET, 100 ns MD simulation analysis, and MM-PBSA calculations of the top 15 compounds and complexes were performed. The results of ADMET physicochemical properties prediction suggested that the CNP0099440 demonstrated good drug-like behaviors. Several important structural parameters were further calculated, including RMSD, RMSF, Rg, SASA, hydrogen bonds, PCA, and Gibbs-free energy.38–40 Oral availability, drug selectivity, and metabolization stability were related to H-bonds. 41 Hence, H-bonds were calculated. These results indicated that the GluN2B/NMDAR-CNP0099440 complex interacted with essential catalytic residues of the binding site, and more stable than GluN2B/NMDAR–ifenprodil complex. What’s more, the Gibbs-free energy landscape analysis was performed. Furthermore, MM-PBSA binding free energy and residual binding energy were calculated to assess the binding affinities of complexes. Low-binding energy corresponds to the high-binding affinity of protein–ligand complexes. MM-PBSA analysis showed that CNP0099440 was better than ifenprodil. Compared with Tables 2 with 3, we surprisedly found that the closer the distance between amino acid residues in each complex is, the higher binding energy it has. For instances, the amino acid residue Asp115 mainly contributed to the binding in CNP0099440-NMDAR complex with a high-binding energy of −3.28 kcal/mol (Table 3) and the distance was only 2.6 Å. What’s more, the contribution value to binding of amino acid residue Glu236 was −3.03 kcal/mol with a distance within 3 Å. Simultaneously, amino acid residue Asp113 contributed less to TIP007089-NMDAR complex with only a value of −1.26 kcal/mol and the distance (4.4 Å) was a bit far.
Besides, target specificity analysis showed that CNP0099440 selectively inhibited GluN2B/NMDAR, which means the side effects caused by ifenprodil could be avoided. This study culminated to discovery of CNP0099440, a potential GluN2B-selective NMDAR natural antagonist. CNP0099440 interacted with GluN2B/NMDAR and formed three conventional hydrogen bonds at position Ala107, Gln110, and Asp113 and cation–π bonding via Tyr109. However, though virtual screening has achieved great progress in saving much time and cost on developing novel drugs and the algorithm is also improving and becoming more and more accurate in the course of these years, we still need conducting experiments and clinical trials before putting it into clinical use. Because the whole algorithm system remains to be improved, CNP0099440 was chemically synthesized to further evaluate via the in vitro and in vivo experimental techniques. To increase persuasion and accuracy of the conclusion, animal assays, biochemical assays, and cell assays are planned to carry out in the near future.
NDDs are serious problem for our society in global, and it is necessary to look for possible therapeutic drugs. The GluN2B/NMDAR is an essential glutamate-gated ion channel protein involved in the pathology of NDDs. In view of the clinical benefits brought by natural compounds, we employed computational approaches to identify novel nature-originated GluN2B-selective NMDAR antagonist which can greatly save development costs and avoid wasting unnecessary time. Nowadays, more and more researchers use deep learning methods to develop novel drugs and make great achievements.42–44 Besides, the whole virtual screening process can be integrated into a novel algorithm to simplify the steps of calculation in the future. The accuracy of the final result can also be further improved. Here, a new idea was provided for future development in artificial intelligence. The result can be concluded that we have successfully identified one possible lead compound CNP0099440 that represents superior inhibition against GluN1/GluN2B NMDAR and better selectivity by contrast to ifenprodil. The result of drug-likeness analysis showed a higher logS value of CNP0099440 than ifenprodil, indicating a better solubility in water and oral bioavailability. CNP0099440 was potentially effective drug-like inhibitor for application as NDD therapeutics.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702231220666 for The discovery of subunit-selective GluN1/GluN2B NMDAR antagonist via pharmacophere-based virtual screening by Jialing Tang, Ju Jin, Zhihong Huang, Faliang An, Caiguo Huang and Wenli Jiang in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: JT, FA, contributed to the review of previous literature, carried out the analysis, and interpreted the data. ZH and JJ helped in docking. WJ and CH made substantial contributions to the design of the study and data discussion, and critically commented on the manuscript for scientific content. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Shanghai Sailing Program (grant no. 22YF1458500).
ORCID iD: Jialing Tang  https://orcid.org/0009-0003-6723-9227
https://orcid.org/0009-0003-6723-9227
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Erekat NS. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin Anat 2022;35:65–78 [DOI] [PubMed] [Google Scholar]
- 2. Uddin MS, Al Mamun A, Kabir MT, Ashraf GM, Bin-Jumah MN, Abdel-Daim MM. Multi-target drug candidates for multifactorial Alzheimer’s disease: AChE and NMDAR as molecular targets. Mol Neurobiol 2021;58:281–303 [DOI] [PubMed] [Google Scholar]
- 3. Cummings JL, Tong G, Ballard C. Treatment combinations for Alzheimer’s disease: current and future pharmacotherapy options. J Alzheimers Dis 2019;67:779–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seidel T, Schuetz DA, Garon A, Langer T. The pharmacophore concept and its applications in computer-aided drug design. Prog Chem Org Nat Prod 2019;110:99–141 [DOI] [PubMed] [Google Scholar]
- 5. Ploux E, Freret T, Billard JM. d-serine in physiological and pathological brain aging. Biochim Biophys Acta Proteins Proteom 2021;1869:140542. [DOI] [PubMed] [Google Scholar]
- 6. Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis 2017;57:1041–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 2010;460:525–42 [DOI] [PubMed] [Google Scholar]
- 8. Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain 2018;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 2014;115:157–88 [DOI] [PubMed] [Google Scholar]
- 10. Khan MA, Houck DR, Gross AL, Zhang XL, Cearley C, Madsen TM, Kroes RA, Stanton PK, Burgdorf J, Moskal JR. NYX-2925 is a novel NMDA receptor-specific spirocyclic-beta-lactam that modulates synaptic plasticity processes associated with learning and memory. Int J Neuropsychopharmacol 2018;21:242–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adell A. Brain NMDA receptors in schizophrenia and depression. Biomolecules 2020;10:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA, Jr, Gould TD. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 2018;70:621–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 1993;44:851–9 [PubMed] [Google Scholar]
- 14. Huang LE, Guo SH, Thitiseranee L, Yang Y, Zhou YF, Yao YX. N-methyl D-aspartate receptor subtype 2B antagonist, Ro 25-6981, attenuates neuropathic pain by inhibiting postsynaptic density 95 expression. Sci Rep 2018;8:7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, De Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 2018;46:W296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorokina M, Merseburger P, Rajan K, Yirik MA, Steinbeck C. COCONUT online: collection of Open Natural Products database. J Cheminform 2021;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin YC, Wang CC, Chen IS, Jheng JL, Li JH, Tung CW. TIPdb: a database of anticancer, antiplatelet, and antituberculosis phytochemicals from indigenous plants in Taiwan. Sci World J 2013;2013:736386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moumbock AFA, Gao M, Qaseem A, Li J, Kirchner PA, Ndingkokhar B, Bekono BD, Simoben CV, Babiaka SB, Malange YI, Sauter F, Zierep P, Ntie-Kang F, Gunther S. StreptomeDB 3.0: an updated compendium of streptomycetes natural products. Nucleic Acids Res 2021;49:D600–04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang W, Brewer LK, Jones JW, Nguyen AT, Marcu A, Wishart DS, Oglesby-Sherrouse AG, Kane MA, Wilks A. PAMDB: a comprehensive Pseudomonas aeruginosa metabolome database. Nucleic Acids Res 2018;46:D575–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyu C, Chen T, Qiang B, Liu N, Wang H, Zhang L, Liu Z. CMNPD: a comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res 2021;49:D509–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramirez-Gaona M, Marcu A, Pon A, Guo AC, Sajed T, Wishart NA, Karu N, Djoumbou Feunang Y, Arndt D, Wishart DS. YMDB 2.0: a significantly expanded version of the yeast metabolome database. Nucleic Acids Res 2017;45:D440–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kakarala KK, Jamil K, Devaraji V. Structure and putative signaling mechanism of Protease activated receptor 2 (PAR2) – a promising target for breast cancer. J Mol Graph Model 2014;53:179–99 [DOI] [PubMed] [Google Scholar]
- 23. Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, Wang L, Lupyan D, Dahlgren MK, Knight JL, Kaus JW, Cerutti DS, Krilov G, Jorgensen WL, Abel R, Friesner RA. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput 2016;12:281–96 [DOI] [PubMed] [Google Scholar]
- 24. Repasky MP, Shelley M, Friesner RA. Flexible ligand docking with Glide. Curr Protoc Bioinformatics 2007; Chapter 8:Unit 8.12 [DOI] [PubMed] [Google Scholar]
- 25. Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, Xu C, Rhee K, Chen T, Zhang H, Palakurthi S, Jang J, Lelais G, DiDonato M, Bursulaya B, Michellys PY, Epple R, Marsilje TH, McNeill M, Lu W, Harris J, Bender S, Wong KK, Janne PA, Eck MJ. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang S, Jiang M, Wang S, Wang X, Wei Z, Li Z. SAG-DTA: prediction of drug-target affinity using self-attention graph network. Int J Mol Sci 2021;22:8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo H, Xiang Y, Fang X, Lin W, Wang F, Wu H, Wang H. BatchDTA: implicit batch alignment enhances deep learning-based drug-target affinity estimation. Brief Bioinform 2022;23:bbac260 [DOI] [PubMed] [Google Scholar]
- 28. Ferreira LLG, Andricopulo AD. ADMET modeling approaches in drug discovery. Drug Discov Today 2019;24:1157–65 [DOI] [PubMed] [Google Scholar]
- 29. Ozturk H, Ozgur A, Ozkirimli E. DeepDTA: deep drug-target binding affinity prediction. Bioinformatics 2018;34:i821–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pronk S, Pall S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013;29:845–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lauria A, Ippolito M, Almerico AM. Molecular dynamics studies on HIV-1 protease: a comparison of the flap motions between wild type protease and the M46I/G51D double mutant. J Mol Model 2007;13:1151–6 [DOI] [PubMed] [Google Scholar]
- 32. Cuendet MA. The Jarzynski identity derived from general Hamiltonian or non-Hamiltonian dynamics reproducing NVT or NPT ensembles. J Chem Phys 2006;125:144109. [DOI] [PubMed] [Google Scholar]
- 33. Valdes-Tresanco MS, Valdes-Tresanco ME, Valiente PA, Moreno E. gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J Chem Theory Comput 2021;17:6281–91 [DOI] [PubMed] [Google Scholar]
- 34. Miller BR, 3rd, McGee TD, Jr, Swails JM, Homeyer N, Gohlke H, Roitberg AE. MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theory Comput 2012;8:3314–21 [DOI] [PubMed] [Google Scholar]
- 35. Liu W, Jiang X, Zu Y, Yang Y, Liu Y, Sun X, Xu Z, Ding H, Zhao Q. A comprehensive description of GluN2B-selective N-methyl-D-aspartate (NMDA) receptor antagonists. Eur J Med Chem 2020;200:112447. [DOI] [PubMed] [Google Scholar]
- 36. Bu M, Yang BB, Hu L. Natural endoperoxides as drug lead compounds. Curr Med Chem 2016;23:383–405 [DOI] [PubMed] [Google Scholar]
- 37. Bizzarri M, Giuliani A, Monti N, Verna R, Pensotti A, Cucina A. Rediscovery of natural compounds acting via multitarget recognition and noncanonical pharmacodynamical actions. Drug Discov Today 2020;25:920–7 [DOI] [PubMed] [Google Scholar]
- 38. Pathak RK, Gupta A, Shukla R, Baunthiyal M. Identification of new drug-like compounds from millets as Xanthine oxidoreductase inhibitors for treatment of Hyperuricemia: a molecular docking and simulation study. Comput Biol Chem 2018;76:32–41 [DOI] [PubMed] [Google Scholar]
- 39. Rai SK, Pathak RK, Singh DB, Bhatt A, Baunthiyal M. Chemo-informatics guided study of natural inhibitors targeting rho GTPase: a lead for treatment of glaucoma. In Silico Pharmacol 2021;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shukla R, Singh TR. Identification of small molecules against cyclin dependent kinase-5 using chemoinformatics approach for Alzheimer’s disease and other tauopathies. J Biomol Struct Dyn 2022;40:2815–27 [DOI] [PubMed] [Google Scholar]
- 41. Yadav DK, Khan F, Negi AS. Pharmacophore modeling, molecular docking, QSAR, and in silico ADMET studies of gallic acid derivatives for immunomodulatory activity. J Mol Model 2012;18:2513–25 [DOI] [PubMed] [Google Scholar]
- 42. Yang C, Chen EA, Zhang Y. Protein-ligand docking in the machine-learning era. Molecules 2022;27:4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clyde A. Ultrahigh throughput protein-ligand docking with deep learning. Methods Mol Biol 2022;2390:301–19 [DOI] [PubMed] [Google Scholar]
- 44. Pathak RK, Seo YJ, Kim JM. Structural insights into inhibition of PRRSV Nsp4 revealed by structure-based virtual screening, molecular dynamics, and MM-PBSA studies. J Biol Eng 2022;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702231220666 for The discovery of subunit-selective GluN1/GluN2B NMDAR antagonist via pharmacophere-based virtual screening by Jialing Tang, Ju Jin, Zhihong Huang, Faliang An, Caiguo Huang and Wenli Jiang in Experimental Biology and Medicine