Abstract
Simple Summary
Using antibiotics as growth promoters or antimicrobials is a potential health hazard. Garlic (Allium sativum L.) has been extensively used in several aspects of poultry production systems. Therefore, this review article discusses the impact of using garlic as a feed additive on the performance, immunity, gut health, anti-oxidant status, blood parameters, and intestinal microbiota of poultry. Garlic feeding has been regarded as a potential antibiotic-alternative feed additive due to its great benefits to the health of poultry.
Abstract
The use of antibiotics as growth promoters or for the prevention of some poultry diseases has faced global concern and serious criticism. Their addition to poultry feed has shown hazardous effects, including the development of antimicrobial resistance and a potentially harmful effect on human health. To eliminate these threats, there is increasing interest in natural alternatives. Plant derivatives such as garlic (Allium sativum L.) and its derivatives are presently extensively used in the poultry production system. The dietary supplementation of broilers and layers with garlic induced improvement in the production parameters, carcass quality, and intestinal integrity. The modulation of the immune response against some important viral diseases has resulted from the supplementation of poultry with garlic. Moreover, garlic has been shown to modulate gut health through antibacterial and antiparasitic activities. Treatment with garlic can also mitigate oxidative stress and reduce free-radical production. The reduction of cholesterol levels and improvement of some liver and blood parameters were also reported following the dietary inoculation of garlic. This review was designed to investigate the influence of garlic as a dietary additive on the performance, immunity, gut health, anti-oxidant status, blood parameters, and intestinal microbiota of poultry.
Keywords: Allium sativum, meat and egg production, antibodies, anti-oxidant enzymes, cholesterol
1. Introduction
As a result of the worldwide ban on antibiotic growth promoters, attention has turned toward finding alternatives without resistance or residues [1]. Through a global trend to go back to nature, the World Health Organization has encouraged the use of natural phytogenic substances to replace or reduce the use of antibiotic growth promoters. Phytobiotics, or phytogenics, are plant derivatives that have been used as feed additives to improve the health and performance of animals [2]. Over the past decade, this safe source of active ingredients has been regarded as an attractive research subject and has shown promising results [3]. Herbal plants possess multiple therapeutic properties and different effects.
Garlic (Allium sativum) is a perennial bulb-producing plant that belongs to the genus Allium in the family Liliaceae. Since antiquity, garlic has been grown on a large scale in all countries and has been widely used as a feed additive and growth promoter [4]. It has a specific smell and taste, as well as therapeutic properties in alternative medicine [5].
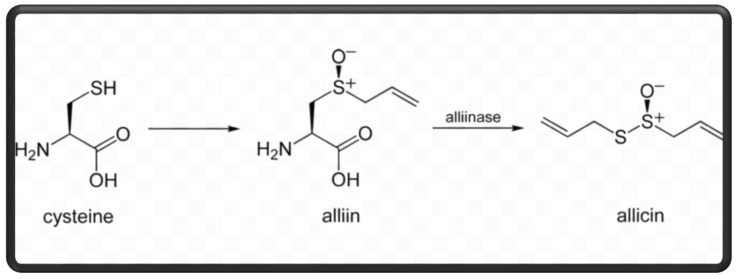
Garlic is estimated to contain different bioactive compounds, including organosulfur compounds diallyl thiosulfonate (allicin), diallyl sulfides, diallyl disulfide, diallyl trisulfide, and S-allyl-cysteine sulfoxide (alliin), saponins, phenols (β-resorcylic acid, pyrogallol, gallic acid, rutin, protocatechuic acid, and quercetin), amino acids, polysaccharides (fructose, glucose, and galactose), essential oils, vitamins (ascorbic acid, ribofavin, niacin, thiamine, and folic acid), minerals (germanium, selenium, phosphates, calcium, and iron), and enzymes [6,7,8,9]. The chemical structure of garlic is illustrated in Figure 1. The allin and alliinase enzymes collaborate to produce allicin [10], which is released from its precursor form when garlic bulbs are crushed or destroyed in digestion. Allicin, or daily thiosolphinic acid, is an active inhibitory principle of garlic [11]. Moreover, allicin ingredients can decompose, forming many volatile organosulfur compounds with bioactivities [12]. The nutritional value of raw garlic is represented in Table 1. However, little information exists on the effects of garlic products on nutrient utilization in poultry.
Figure 1.
Chemical structure of garlic.
Table 1.
The nutritional value of raw garlic/100 g.
| Component | Importance |
|---|---|
| Carbohydrates (33.06 g) | Important for energy, immunity, disease prevention, and blood clotting |
| Protein (6.36 g) | Development of body tissues |
| Fiber (2.1 g) | Shortens the stagnant time in the gut |
| Sugar (1 g) | Important for energy, immunity, disease prevention, and blood clotting |
| Fats (0.5 g) | Formation of cell membranes |
| Vitamin B3 (0.7 g) | Formation of coenzyme NAD |
| Vitamin B5 (0.6 g) | Formation of coenzymes of amino acid metabolism |
| Vitamin B2 (0.1 g) | Formation of coenzyme FAD |
| Vitamin B1 (0.2 mg) | Carbohydrate metabolism and synthesis of acetylcholine |
| Vitamin B6 (1.2 mg) | Formation of coenzymes in different reactions |
| Vitamin C (31.2 mg) | Protein synthesis |
| Vitamin B9 (3 µg) | Synthesis of DNA |
| Calcium (181 mg) | Formation of bone and coagulation process |
| Phosphorus (153 mg) | Formation of lipids, proteins, sugars, and nucleic acid |
| Magnesium (25 mg) | Cofactor for kinase and decarboxylase |
| Sodium (17 mg) | Formation of membrane |
| Zinc (1.16 mg) | Cofactor for some enzymes |
| Selenium (14.2 µg) | Cofactor for glutathione coagulase |
| Sulfur (16%) | Antimicrobial |
Garlic contains more than 200 chemical substances that are used for the prevention and treatment of cardiovascular disease [13], as well as anti-oxidants [14], antimicrobial [15,16], anti-inflammatory [17], anti-atherosclerotic, anti-thrombotic, anti-hypertensive, anti-diabetic, anti-cancer, and hypoglycemic properties [16,18,19]. The most important immune-modulating compounds in garlic are polysaccharides. The metabolism of fungi can be interrupted by garlic oil through the production of key genes represented in oxidative phosphorylation, cell cycle, and the processing of protein in the endoplasmic reticulum. Moreover, fungal growth could be hindered via the penetration of garlic oil into cells, causing the destruction and escape of cytoplasm and macromolecules [20].
Garlic could be given to poultry in the form of powder, aqueous extract, essential oil, and other commercial products either in the feed or in the drinking water. Dietary feeding of poultry on garlic resulted in enhancement in growth performance, gut health, dressing yield, and production cost [21,22,23], modulation of immunity and blood parameters [14], prevention of bacterial and parasitic infections [24,25], and mitigation of heat stress [26]. The addition of garlic to the broilers’ feed has no negative influence because it does not leave any residue, and the birds’ manure does not contaminate the environment. Therefore, products from garlic-consuming animals are safe for consumption.
The objectives of this review article were to investigate the findings on the influence of garlic as a dietary additive on the performance, immunity, gut health, anti-oxidant status, blood parameters, and intestinal microbiota of poultry.
2. The Different Influences of Dietary Garlic on Poultry Health
2.1. Production Parameters
2.1.1. Performance
The different effects of dietary garlic on the production performance parameters of broilers and layers are presented in Table 2 and Table 3 [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. Inoculation of garlic in the diets of birds could enhance the production performance parameters, including feed intake (FI), body weight (BW), body weight gain (BWG), and feed conversion ratio (FCR) [57]. The mechanism by which the garlic powder can improve these parameters could be related to the presence of several organosulfur components, including allicin, alliin, ajoene, dithiin, diallyl sulfide, and S-allyl cysteine [58]. Similarly, the study of Ross et al. [59] demonstrated that the antibacterial compound dialkyl polysulfide in garlic plays a central role axial role in improving the BWG in broilers. A combined diet containing garlic and turmeric (10 g/kg each) reduced the pH of the digestive tract and enhanced apparent and digestible metabolized energy in the ileum of broiler chickens [60]. Moreover, garlic might increase the performance of pancreatic enzymes, which creates a good environment for nutrient digestion and absorption [14].
Table 2.
The different effects of dietary garlic on the production performance parameters of broilers.
| Dose/Route | Effects | Reference |
|---|---|---|
| Garlic paste (3.8%), solvent fractions, or garlic oil equal to this quantity in feed | No effect on FI | [27] |
| Garlic 0, 0.01, 0.1 or 1% in meal | No improvement in the performance | [28] |
| Garlic powder 0.2% and 0.4% of feed | No effects on BWG, FI, FCR, carcass cuts, and visceral organs | [29] |
| Garlic at 1 kg/ton feed | Enhanced carcass yield | [30] |
| Garlic 1, 3, and 5% and 3% garlic powder + 200 IU of α-tocopherol/kg of feed | No influence on performance Increased crude protein Decreased crude fat contents of carcass, the pH, and thiobarbituric acid reactive substances of meat |
[31] |
| Garlic 0.5%, 1.0%, and 3% | Decreased heart weight | [32] |
| Garlic powder 0.5% of feed | Increased live BWG | [33] |
| Garlic powder 3% and 5% of diet | Increased breast weight (3%) Low BW (5%) |
[34] |
| A mixture of ginger and garlic (1:1 ratio) 50 mL/L of the drinking water | Improved BW, BWG, FI, and FCR | [35] |
| Garlic bulb 5, 10, or 15 g/kg meal | Decreased BW (high dose and standard temperature) No effect on the FCR |
[36] |
| The 5 g/kg garlic powder + 1 g/kg black pepper powder and 10 g/kg garlic powder + 2 g/kg black pepper powder | Improved WG and FCR | [37] |
| Fresh garlic paste 0.2, 0.4, 0.6, and 0.8%/L of drinking water | No effect on BWG or FCR Decreased mortality |
[38] |
| Garlic powder 3% in diet and a mixture of garlic powder 1.5% plus turmeric powder 0.25% | Improved BWG, FI, FCR, performance index, and protein efficiency ratio | [39] |
| Garlic paste 0.25% and 0.50% with basal diet | Improved BWG, FCR, and livability No influence on carcass attributes |
[40] |
| Garlic 5 g/kg feed, black cumin 5 g/kg, or their combination | No difference in BWG, FI, FCR, and relative organ weights | [41] |
| A basal diet plus 0.25, 0.50, and 0.75 g garlic powder/kg diet | Increased BW and BWG at 21 and 42 days of age High length and average width of small intestine |
[14] |
| Garlic essential oil (200 mg/kg diet) alone/or in combination with lemon essential oil (200 mg/kg diet) under heat stress | Enhancement in BW, FCR, carcass dressing, and increasing the digestive enzymes Decreasing mortality rate and abdominal fat content |
[26] |
| Garlic powder 3% of feed | Improved BWG and final BW | [42] |
Table 3.
The different effects of dietary garlic on the production performance parameters of layers.
| Dose/Route | Effects | Reference |
|---|---|---|
| 1 or 3% garlic meal | Decreased egg yolk cholesterol | [43] |
| Garlic paste (3.8%), solvent fractions, or garlic oil equal to this quantity in feed | No effect on daily FI | [27] |
| Garlic oil 0.02% in meal | No effect on egg production, egg mass, body weight, feed consumption, and feed efficiency | [44] |
| Garlic powder 3% in diet | No differences in the color and flavor of eggs No change in yolk cholesterol concentrations |
[45] |
| Sun-dried garlic paste 0, 2, 4, 6, 8, or 10% of diet | No effect on egg weight, egg mass, feed consumption, and feed efficiency among diets or birds’ strain Increased Yolk weight with increasing levels of dietary garlic Decreased yolk cholesterol concentrations |
[46] |
| Garlic powder 0, 5, 10, and 15 g/kg feed | Decreased yolk weight | [47] |
| Garlic powder 0.5 and 10 g/kg feed | Increased egg weight Decreased egg yolk cholesterol triglyceride No effect on performance or egg albumin index, eggshell index, and egg Haugh unit |
[48] |
| Garlic powder 0, 2, 6, or 8% in feed | Increased egg production | [49] |
| Garlic 2% and fenugreek 2% | No effect on FI, FCR, BW, BWG, egg rate, egg weight, and egg mass Increased yolk weight and color and Haugh units Decreased albumen weight |
[50] |
| Garlic powder 8% in feed | Better egg production No effect on egg mass and egg weight |
[51] |
| Garlic powder 1, 2, and 4% in feed | Increased egg production No effect on egg weight, yolk index, shell weight, shell thickness, yolk weight (1% garlic) Decreased eggshell index and Haugh unit (4% garlic) |
[52] |
| Garlic juice at 0.25, 0.50, and 1% | Improved egg albumin, yolk and shell weight, albumin height, and Haugh unit | [53] |
| Garlic powder 1%, fenugreek 1%, and garlic powder 1% + fenugreek 0.5% | No effect on laying hens’ performance | [54] |
| Garlic 1, 2, and 3% of ration | No effect on BWG, FCR, egg production, egg mass, albumen weight, albumen height, Haugh unit, yolk index, yolk height, egg weight, fertility, hatchability, embryonic mortality, chick weight, and chick visual score, shell thickness, and shell weight An improvement in yolk diameter, yolk weight, chick length, and yolk color |
[55] |
| A mixture of lemon, onion, and garlic juice in portions 1.00, 1.00, and 0.125/L of the drinking water, respectively | Improved FCR Increased number of eggs/hen, percentage of egg production, and egg mass/hen Enhanced yolk color and yolk percentage |
[56] |
The FI of broilers [61] and layers [49] increased by increasing the level of garlic powder inoculation in the diet. This result may be owed to the high content of garlic to aromatic oil that enhances the digestion process.
2.1.2. Intestinal Architecture
The addition of eugenol and garlic tincture could improve intestinal integrity and enhance mucin-producing goblet cell numbers as a defensive response in birds against necrotic enteritis [62]. The inoculation of broilers diets with garlic at concentrations of 0.125, 0.25, 0.5, and 1% significantly increased the villus height and crypt depth and reduced the epithelial thickness and goblet cell numbers in the intestines of birds [63]. Moreover, the highest capacity of crypts and villi in small intestines was detected following the dietary addition of garlic in coccidiosis-infected broilers [64]. Allicin can regenerate and improve the physiological structure of the intestinal epithelium layer and increase the crypt’s depth and villus height, which eventually supports the digestive capacity by increasing nutrient absorption and assimilation. Elongated villi with deep crypts is considered an indication of a vigorous intestine architecture and, consequently, a good digestive capacity and pancreatic enzyme activity. Furthermore, the anti-oxidant characteristics of garlic can enhance overall gut function and improve nitrogen energy utilization [65]. Yang et al. [66] reported that the feeding of broilers on garlic reduces the pH of digesta, which increases the production of volatile fatty acids and the proliferation of beneficial bacteria. The dietary addition of 0.5% garlic efficiently reduced systemic hypertension and the prevalence of ascites but had no negative influences on broiler performance [36]. The inulin component of garlic decreases the digesta pH of birds and increases the volatile fatty acid production, which may help enhance beneficial bacterial colonization [67].
Others showed that garlic supplementation does not affect the feed efficiency or growth performance of broilers and layers [44,46,55,68]. This discrepancy might have resulted from the variances in the experiment duration, birds’ genetic and health conditions, and the form, treatment, and quality of garlic end-product components.
2.2. Immunity
The effect of garlic on the immunity of birds is illustrated in Table 4. It has been found that the different forms of dietary garlic alone or in combination with other aromatic phytobiotics can enhance the immune response in terms of enhancing antibody titers against and increasing the immune organ:body weight ratio [14,26,38].
Table 4.
The effect of dietary garlic on the immune response of poultry.
| Dose/Route | Type of Production | Effects | Reference |
|---|---|---|---|
| Garlic powder 1% or 3% garlic | Broiler chickens | Enhanced antibody production against NDV and leukocyte count | [69] |
| Garlic 10 and 30 g/kg diet | White Leghorn chickens | Enhanced antibodies against NDV, SRBCs, and BA Augmented splenocyte and thymocyte proliferations Reduced CD4+, but increasing CD4: CD8- lymphocyte ratios and WBCs count Increased relative weights of immune organs (spleen, thymus glands, and bursa of Fabricius) |
[70] |
| Garlic 0.5%, 1.0%, and 3% | Broiler chickens | Lower weights of bursa of Fabricius and spleen | [32] |
| Garlic powder 0.1% | Broiler chickens | Improved relative weight of bursa of Fabricius without effect on the spleen weight No effect on NDV vaccine (LaSota) antibody response |
[71] |
| Garlic powder 3% and 5% of diet | Broiler chickens | No influence on bursa of Fabricius and thymus weights Decrease spleen weight |
[34] |
| A mixture of ginger and garlic (1:1 ratio) 50 mL/L of the drinking water | Marshal broiler chickens | Increased total protein, albumin, and globulin | [35] |
| Garlic extract (allicin) 25, 50, 75, or 100 mg/kg diet | Broiler chickens | Increased total protein and albumin concentrations by about 4.7 and 5.9%, respectively (50 mg/kg) No effect on total protein, albumin, or globulin concentrations (25, 75, or 100 mg/kg) |
[72] |
| Fresh garlic paste 0.2, 0.4, 0.6, and 0.8%/L of drinking water | Broiler chickens | Increased antibody titer against NDV | [38] |
| Garlic meal 0.125% of feed | Broiler chickens | Reducing scores of IBDV signs Higher mortality rate High antibody response to IBDV |
[73] |
| Garlic essential oil 0.06 mL/L drinking water | Broiler chickens | Improved immune organ index, IgM, IgG, and IgA | [74] |
| A basal diet plus 0.25, 0.50, and 0.75 g garlic powder/kg diet | Broiler chickens | Increasing total protein, globulin, IgM, and IgG Improved liver and immune-related organ weight |
[14] |
| Garlic essential oil (200 mg/kg diet) alone/or in combination with lemon essential oil (200 mg/kg diet) under heat stress | Broiler chickens | Increasing the relative weight of bursa of Fabricius and the serum antibody titer against NDV No changes in relative weights of spleen and thymus glands, and antibody titer against AIV |
[26] |
The improvement in the immune response may be related to the characteristics of biologically active compounds in essential oils, such as antimicrobial, anti-oxidant, and anti-inflammatory properties, which provide essential nutrients for the development of the immune cells. In addition, promoting the proliferation of lymphocytes in the primary immune organs and improving intestinal integrity could stimulate the production of immunoglobulin (Ig), such as IgG, IgM, and IgA, which is associated with increasing the relative weight of the immune organs. Many immuno-stimulator compounds are present in garlic, including the lectin family, which is known to interact with pathogen recognition receptors on immune cell surfaces [75]. Garlic is one of the impressive conductors of the body’s immune system, which stimulates the immune function by making macrophages or killer cells more active. Moreover, garlic can improve humoral immune cell functions via the enhancement of cytokine production and/or antigen-presenting cell phagocytic capacity [70]. Dorhoi et al. [76] demonstrated that a high dose of garlic extract (200 mg/mL) on a macrophage culture of laying chickens could impair the phagocyte function and inhibit phagocytosis, whereas a low dose (50 mg/mL) increased sheep red blood cells count. Inoculation of garlic extract or its protein fraction increased the destruction in peritoneal macrophages and the engulfment of parasites in Leishmanial major-infected Balb [77].
Polysaccharides of garlic show an immune potentiation mechanism through the regulation of interleukin (IL)-6, IL-10, tumor necrotizing factor-α, and interferon-gamma (INF-γ) expression in RAW 264.7 macrophages. In addition, garlic extract could augment concanavalin A (ConA)-induced splenocytes, thymocyte proliferation, and the gene expression of IL-2 and INF-γ in vitro [78]. Moreover, the addition of garlic extract to a culture augmented the induction of IL-2 and IL-12, INF-γ, and tumor necrosis factor α in stimulated splenocytes [79]. Low concentrations of diallyl trisulfide (3–12.5 mg/mL) of garlic enhanced the proliferative reactions in a culture, while higher concentrations (50 mg/mL) inhibited T-lymphocyte proliferation in mice [80]. Aged garlic extract stimulated the proliferation and increased the activity of T-cells and natural killer cells, as well as enhancing phagocytosis and cytokine release [81,82].
Garlic supplementation increased the relative weights of immune organs, such as the spleen, thymus, and bursa of Fabricius, the white blood cell count, as well as lymphocytes, splenocytes, and thymocyte proliferation [70]. In addition, the titers of antibodies against Newcastle disease virus (NDV), sheep red blood cell count, and Brucella abortus (BA) have been increased following the administration of garlic in laying chickens [70].
It has been demonstrated that the anti-oxidative stress of garlic is a potential factor that enhances the immune response [83]. Supplementation with garlic extract at 4 and 8 mg/mL revealed that macrophages may display antimicrobial activity and enhance the production of reactive oxygen species.
2.3. Gut Health
2.3.1. Antibacterial
Garlic oil is regarded as a major antibacterial component that disturbs both the structure and metabolism of bacterial cells. The strong antimicrobial effects of garlic have been reported [40,58,84,85]. It has been reported that garlic extracts exert a differential inhibition between beneficial intestinal microflora and potentially harmful enterobacteria [86]. Garlic could reduce the number of gut-pathogenic bacterial populations such as Escherichia coli (E. coli). Garlic showed an ability to inhibit E. coli 10 times greater than that seen in Lactobacillus casei [87]. Rahimi et al. [71] demonstrated that E. coli count was significantly reduced in the digesta of ileo-cecum of broiler chickens supplemented by a blend of garlic, thyme, and coneflower. Recently, Elbaz et al. [23] found that garlic treatment could reduce the ileal enumeration of E. coli and total coliform but increase the Lactobacillus count. In addition, the positive influences of eugenol and garlic mixture on broiler performance and intestinal health status under necrotic enteritis conditions have been reported [85]. Microencapsulated eugenol and garlic tincture modulated the microbiota balance by inhibiting pathogenic growth while promoting beneficial microbial growth, as well as reducing the severity of the intestinal lesions of broilers with necrotic enteritis [62]. The antimicrobial and bacteriostatic properties of garlic extract are associated with the presence of an allicin-active compound [15]. Allicin exhibited a bacteriostatic effect on some vancomycin-resistant enterococci. In addition, allicin displays SH group reactivity on cysteine residues, causing deactivation and suppression of specific thiol-containing enzymes in pathogens [88]. This reaction induced deactivation and suppression of specific thiol-containing enzymes in pathogens [24,89]. Garlic is a nucleophilic agent that can counteract the impact of electrophilic substances on micro-organisms [90].
2.3.2. Antiparasitic
The in vitro and in vivo anticoccidial activities of different processed extract forms of garlic have been documented [91,92,93,94,95,96,97,98,99]. The study of Ali et al. [100] found that supplementing coccidiosis-infected broiler chickens with garlic at 15 g/kg feed reduced the oocyst shedding and lesion score but improved the histopathology of the small intestines. In the same context, continuous feeding of Eimeria tenella-infected broiler chickens on natural garlic essential oil (0.06 mL/L drinking water) significantly reduced the clinical signs, cecal lesion score, and the oocyst shedding but increased the weight of diseased chickens and effectively improved the intestinal functions [74]. In comparison with ginger oil, garlic oil (150 μL/100 mL) showed superior efficacy against the Eimeria species infection of quails in terms of improved activity level with better health, increased feed intake, and complete recovery from oocysts on day 15 post-infection [101].
Allen et al. [102] reported that the anti-oxidant properties of garlic cause oxidative stress against parasites and neutralize oxygen-reactive species. Furthermore, Pourali et al. [103] have attributed the anticoccidial activity of garlic to its immunomodulatory activity. Similarly, Kim et al. [104] revealed enhanced protection from Eimeria acervulina infection in chickens after feeding on garlic metabolites [104]. Propyl thiosulfinate oxide and propyl thiosulfinate active ingredients of garlic reduced fecal oocyst shedding and enhanced the antibody response against coccidial infection [104]. Likewise, the aqueous garlic extract is rich in phenols, flavonoids, and varying sulfur compounds [16]. The phenolic compounds change the permeability of the cytoplasmic membrane to many cations, inhibit the physiological functions, and, consequently, result in the loss of membrane potential, allowing vital cellular substances to leak out, protein and ATP production to be inhibited, and cellular death to occur [105].
Allicin induces changes in the intestinal microbiota, exerts an anti-oxidant effect on Eimeria oocysts, and stimulates immunity by enhancing the antibody response, which directly destructs sporozoites [57,106]. Additionally, the phenolic component in garlic acts on the cytoplasmic membrane of Eimeria species and makes changes in their cation permeability, leading to the death of Eimeria [107]. Moreover, allicin interrelates with the cytoplasmic membranes of the intestine, changes the permeability of cations, disturbs the internal vital processes of cells, and, finally, causes the death of the parasite [108]. The capability of allicin and alcoholic garlic extract to inactivate the oocysts of Eimeria tenella makes them preferable to chemical disinfectants [25]. Eimeria oocysts sporulated in allicin-containing media exhibited the lowest post mortem lesion score and oocyst count shedding when compared with oocysts sporulated in alcoholic garlic extract and potassium hydroxide [25]. Doses of 360 mg/mL garlic extracts and 180 mg/mL allicin significantly reduced oocyst numbers by 73.5 and 88.3%, respectively [25].
Moreover, garlic crude extract showed great activity against worms and protozoon parasites Cryptosporidium spp. in different animal models [109,110,111].
2.4. Anti-Oxidant Status
Garlic exhibited strong anti-oxidant activity in birds (Table 5) [112,113,114,115,116,117,118,119]. Phenols and saponins, which are components of garlic, have strong anti-oxidant effects. For instance, saponins could inhibit the growth and DNA destruction induced by H2O2. Consequently, protected mouse-derived myoblasts were able to scavenge intracellular reactive oxygen species [120]. The imbalance between the oxidation and reduction in the host’s cells induces significant destruction of them with subsequent oxidative stress. However, the anti-oxidant enzymes can prevent the free radicals from attacking cell membranes [121]. Essential oils, present in different aromatic plants, contain several natural anti-oxidants [14]. Garlic and/or garlic tocopherol induced a much higher anti-oxidant effect by reducing the production of free radicals [14,46], especially in birds under heat-stress conditions [26].
Table 5.
The effect of dietary garlic on the anti-oxidant status and blood parameters of poultry.
| Dose/Route | Type of Production | Effects | Reference |
|---|---|---|---|
| Garlic paste (3.8%), solvent fractions, or garlic oil equal to this quantity in feed | Broiler chickens Leghorn laying pullets |
Decreasing serum cholesterol by 18 and 23% in broilers and Leghorn pullets, respectively | [27] |
| Garlic oil 0.02% in meal | Babcock B-300 strain of laying hens | No effect on serum cholesterol | [44] |
| Garlic 2% in feed | Broiler chickens | Lowering in hepatic cholesterol concentrations | [112] |
| Garlic 3% in meal | Broiler chickens | Decreased cholesterol in plasma and breast and thigh muscles | [113] |
| Garlic powder 3% in diet | Laying hens | No change in serum cholesterol concentrations | [54] |
| Sun-dried garlic paste 0, 2, 4, 6, 8, or 10% of diet | Hisex Brown, Isa Brown, Lohmann, Starcross, Babcock, and Starcross-579 strains of laying hens | Decreased serum cholesterol concentrations | [46] |
| Garlic 0, 1, 3, or 5% in meal | Laying hens | No change in HDL level | [114] |
| Garlic powder 0.5 and 10 g/kg feed | Laying hens | Decreased serum triglyceride | [48] |
| Garlic 2% and fenugreek 2% | Lohmann Brown laying hens | Increased HDL Reduced serum cholesterol and LDL |
[50] |
| Garlic powder 1% or 3% garlic | Broiler chickens | No effect on leukocyte count | [69] |
| Garlic powder 10 and 20 g kg−1 | Laying hens | Reduced total cholesterol, triglyceride, LDL, and HDL | [115] |
| Garlic powder 5–20 g kg−1 | Broiler chickens | Decreased plasma LDL cholesterol No effect on HDL cholesterol | [116] |
| Fermented garlic powder 3% in diet | Laying hens | Decreased serum cholesterol | [117] |
| Garlic powder 1, 2, and 4% in feed | Laying hens | Increased plasma HDL and LDL (1, 2, and 4%). | [52] |
| Garlic 1, 3, and 5% and 3% garlic powder + 200 IU of α-tocopherol/kg of feed | Broiler chickens | Reduced the total and LDL levels Increased HDL levels |
[31] |
| A mixture of garlic and thyme powder 0.1 and 0.2 g kg−1 | Laying hens | No effect on cholesterol, triglyceride, HDL, and LDL | [118] |
| Garlic powder 0.1% | Broiler chickens | Decreased triglycerides, total cholesterol, and LDL Increased HDL |
[71] |
| Garlic powder at 0.2% and 0.4% of feed | Cobb broiler chickens | Reduced triglycerides, cholesterol, and LDL Increased HDL |
[119] |
| Garlic powder 3% and 5% of diet | Broiler chickens | Decrease spleen weight, RBCs, WBCs, and packed cells volume | [34] |
| Garlic powder 1%, fenugreek 1%, and garlic powder 1% + fenugreek 0.5% Garlic and fenugreek 2% |
Laying hens | Decreased LDL Beneficial effects on cholesterol metabolism |
[54] |
| A mixture of ginger and garlic (1:1 ratio) 50 mL/L of the drinking water | Marshal broiler chickens | Increased hemoglobin, packed cell volume, WBCs, RBCs, total protein, albumin, and globulin Decreased cholesterol |
[35] |
| A mixture of lemon, onion, and garlic juice in portions of 1.00, 1.00, and 0.125/liter of drinking water, respectively | Bovan Brown layer chickens | Decreasing total plasma cholesterol content, GPT, GOT, and creatinine | [56] |
| Garlic 5 g/kg feed, black cumin 5 g/kg, or their combination | Ross-308 broiler chickens | Increasing total protein Reduced GOT |
[41] |
| Probiotic, citric acid, and garlic supplemented with 0.5 g kg−1 multi-strain probiotic mixture, citric acid, and garlic powder, respectively. Probiotic-citric and probiotic-garlic groups treated with 0.5 g kg−1 multi-strain probiotic mixture and 0.5 g kg−1 citric acid and garlic powder, respectively, while citric-garlic group fed diet with 0.5 g kg−1 of citric acid and garlic powder. | Broiler chickens | Decreased cholesterol, triglycerides, and LDL Elevated HDL |
[23] |
| A basal diet plus 0.25, 0.50, and 0.75 g garlic powder/kg diet | Broiler chickens | Increasing RBCs, hemoglobin HDL, SOD, and total anti-oxidant capacity Decreasing total cholesterol, LDL, GOT, and AMD |
[14] |
| Garlic essential oil (200 mg/kg diet) alone/or in combination with lemon essential oil (200 mg/kg diet) under heat stress | Broiler chickens | Reducing MDA, triglycerides, cholesterol, and LDL Increasing HDL, SOD, and GPx |
[26] |
Decreased actions of hydroxymethylglutaryl coenzyme A reductase, cholesterol 7 α-hydroxylase, and fatty acid synthetase have been demonstrated after the administration of garlic powder polar fractions (garlic equivalent to 1, 2, 4, 6, and 8% fresh garlic paste) [122]. The diallyl polysulfides from an aged garlic extract could protect the cell membranes from lipid peroxidation [123]. Moreover, essential oils present in garlic and other plants can remove oxygen free radicals by reducing the level of malondialdehyde (MDA) and enhancing the levels of superoxide dismutase (SOD) and glutathione peroxidase (GPx) [103,124,125].
2.5. Blood Parameters
The influence of the dietary addition of garlic on the different blood parameters of poultry is shown in Table 5.
Many studies showed the hypocholesteric effect of garlic in broilers and layers [14,26,54,56,117]. Garlic-containing enzymes may have a role in regulating the metabolism of lipids and enhancing enzyme activities that stimulate biliary cholesterol secretion and lower the fractional absorption of dietary cholesterol [22]. Moreover, the inhibition of acetyl CoA synthetase and 3-hydroxyl-3-methylglutaryl-CoA reductase enzymes, which are required for cholesterogenesis and the biosynthesis of fatty acids, can reduce the blood cholesterol level [126]. Similarly, the potential effect of garlic on the lipid metabolism in layers may be related to the reduction of lipogenic and cholesterogenic-depressing effects of some hepatic enzymes, such as fatty acid synthase, glucose 6 phosphatase dehydrogenase, and malic enzyme, and consequently, the mechanism of hypocholesterol and hypolipid syntheses [53]. Lower serum and liver cholesterol [122] inhibits bacterial growth [127], reduces platelet formation, and decreases oxidative stress [123].
Garlic oil could improve the anti-oxidant enzyme activities in the liver, inhibit 1,3-dichloro-2-propanol metabolic activation, and reduce hepatic apoptosis, thus protecting against liver damage [128]. In addition, organosulfur compounds in garlic could treat liver damage by decreasing the release of hepatic pro-inflammatory cytokines and enhancing anti-oxidant activity by suppressing cytochrome P450 2E1 expression [129,130].
Additionally, the effect of garlic on hematological parameters such as red blood cell (RBC) and white blood cell (WBC) counts, and hemoglobin and packed cell volume have been reported [14,34,35]. The hemolytic bioactives and their metabolites in garlic can be the main causes of these effects. Increasing erythrocyte count with garlic supplementation could be due to the synthesis of RBCs following the formation and secretion of renal erythropoietin [131]. Moreover, the addition of garlic extract to the diets of laying hens could improve the uptake of splenic RBCs [76].
2.6. Intestinal Health and Microbiota
Garlic powder, garlic meal, and garlic derivatives have improved intestinal health status, which may contribute to the improved intestinal morphology of treated broilers [132,133]. The addition of garlic powder successfully reversed the damaged intestinal morphology of lipopolysaccharide-challenged broilers in terms of increased villus height, intestinal health, and growth efficiency [134].
The intestinal microbiota in broiler chickens plays a key role in the health and growth of birds [135]. There is little and contentious information regarding the effects of garlic derivatives on broiler intestinal microbiota. Nevertheless, garlic and garlic products have been found to be effective against several pathogenic bacteria causing enteritis [136]. The garlic derivative propyl-propane thiosulfonate showed antimicrobial activity against enterobacteria, E. coli, Salmonella spp., and Campylobacter jejuni. It has been shown that propyl-propane thiosulfonate can modulate the intestinal microbiota composition and improve the nutrient digestibility of growing broilers [137]. Moreover, a significant reduction in Clostridium coccoides/Eubacterium rectale, and Clostridium leptum log10 number of copies, while increasing in bacteroides and total bacterial contents, were observed in ileum following feeding on 11.3% propyl-propane thiosulfonate [137].
Exposure of broiler chickens to 0.5% A. hookeri leaf resulted in differences in the abundance of gut microbiota genera compared to diets containing 0.3% [138]. A diet containing 0.5% A. hookeri leaf reduced the profusion of Eubacterium nodatum, Marvinbryantia, Oscillospira, and Gelria [138]. This effect may be related to the abundance of pharmacologically active components in garlic, such as organosulfur, polyphenols, and allicin, that are known to affect the gut microbiota by enhancing or suppressing bacterial configuration [139]. A high percentage (~90%) of absorbable polyphenols are digested in the intestine by microbiota rather than the digestible enzymes [140]. Moreover, allicin is an organosulfur compound used against various bacterial pathogens, including Staphylococcus and Pseudomonas [141]. The antibacterial activity of allicin is related to the chemical interaction with thiol groups in enzymes. These enzymes are important for the metabolic activities of cysteine proteinase, which influences bacterial virulence and the antibacterial effect [142].
3. Conclusions
The supplementation of garlic to broiler and layer poultry species mostly shows improvement in performance and production efficiency, enhancing the immune response, maintaining gut health, reducing exudative stress, and modulating many important blood parameters. However, the different modes of action of garlic are indefinite. Therefore, further studies should focus on establishing the mechanisms of actions of garlic and its derivatives.
Abbreviations
| AIV | Avian influenza virus |
| BW | Body weight |
| BWG | Body weight gain |
| BA | Brucella abortus |
| CD | Cluster of differentiation |
| ConA | Concanavalin A |
| E. coli | Escherichia coli |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| GOT | Glutamic-oxaloacetic transaminase |
| GPx | Glutathione peroxidase |
| GPT | Glutamic-pyruvic transaminase |
| HDL | High-density lipoprotein |
| Ig | Immunoglobulin |
| IBD | Infectious bursal disease virus |
| INF-γ | Interferon-gamma |
| IL | Interleukin |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| NDV | Newcastle disease virus |
| RBCs | Red blood cells |
| SOD | Superoxide dismutase |
| WBCs | White blood cells |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All prepared data are presented in the present article.
Conflicts of Interest
The author provides the publisher a copyright license, granting the publisher the exclusive right to publish and sell the study findings in whole or in part in any language. The author has no relevant financial or non-financial interests to disclose.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Saleha A.A., Myaing T.T., Ganapathy K.K., Zulkifli I., Raha R., Arifah K. Possible effect of antibiotic-supplemented feed and environment on the occurrence of multiple antibiotic resistant Escherichia coli in chickens. Int. J. Poult. Sci. 2009;8:28–31. doi: 10.3923/ijps.2009.28.31. [DOI] [Google Scholar]
- 2.Abd El-Ghany W.A. Phytobiotics in poultry industry as growth promoters, antimicrobials and immunomodulators—A review. J. World’s Poult. Res. 2020;10:571–579. doi: 10.36380/jwpr.2020.65. [DOI] [Google Scholar]
- 3.Navidshad B., Darabighane B., Malecky M. Garlic: An alternative to antibiotics in poultry production, a review. Iran. J. Appl. Anim. Sci. 2018;8:9–17. [Google Scholar]
- 4.Ur Rahman S., Khan S., Chand N., Sadique U., Khan R.U. In vivo effects of Allium cepa L. on the selected gut microflora and intestinal histomorphology in broiler. Acta Histochem. 2017;119:446–450. doi: 10.1016/j.acthis.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Ezeorba T.P.C., Chukwudozie K.I., Ezema C.A., Anaduaka E.G., Nweze E.J., Okeke E.S. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol. Res. Mod. Chin. Med. 2022;3:100075. doi: 10.1016/j.prmcm.2022.100075. [DOI] [Google Scholar]
- 6.Najda A., Błaszczyk L., Winiarczyk K., Dyduch J., Tchórzewska D. Comparative studies of nutritional and health-enhancing properties in the “garlic-like” plant Allium ampeloprasum var. ampeloprasum (GHG-L) and A. sativum. Sci. Hortic. 2016;201:247–255. doi: 10.1016/j.scienta.2016.01.044. [DOI] [Google Scholar]
- 7.Ozma M.A., Abbasi A., Ahangarzadeh Rezaee M., Hosseini H., Hosseinzadeh N., Sabahi S., Noori S.M.A., Sepordeh S., Khodadadi E., Lahouty M., et al. A critical review on the nutritional and medicinal profiles of garlic’s (A. sativum L.) bioactive compounds. Food Rev. Int. 2022;39:6324–6361. doi: 10.1080/87559129.2022.2100417. [DOI] [Google Scholar]
- 8.Szychowski K.A., Rybczynska-Tkaczyk K., Gawel-Beben K., Swieca M., Karas M., Jakubczyk A., Matysiak M., Binduga U.E., Gminski J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018;68:73–81. doi: 10.1515/pjfns-2017-0005. [DOI] [Google Scholar]
- 9.Shang A., Cao S.Y., Xu X.Y., Gan R.Y., Tang G.Y., Corke H., Mavumengwana V., Li H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.) Foods. 2019;5:246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puvača N., Kostadinović L., Ljubojević D., Lukač D., Lević J., Popović S., Novakov N., Vidović B., Đuragić O. Effect of garlic, black pepper and hot red pepper on productive performances and blood lipid profile of broiler chickens. Eur. Poult. Sci. 2015;79:1–13. doi: 10.1399/eps.2015.73. [DOI] [Google Scholar]
- 11.Saleem Z.M., Al-Delaimy K.S. Inhibition of Bacillus aurous by garlic extracts. J. Food Prot. 1982;45:1007–1009. doi: 10.4315/0362-028X-45.11.1007. [DOI] [PubMed] [Google Scholar]
- 12.Chang K.J., Cheong S.H. Volatile organosulfur and nutrient compounds from garlic by cultivating areas and processing methods. Fed. Am. Soc. Exp. Biol. J. 2008;22:1108–1112. doi: 10.1096/fasebj.22.1_supplement.1108.2. [DOI] [Google Scholar]
- 13.Bordia A. Effect of garlic on blood lipids in particles with coronary heart disease. Am. J. Clin. Nutr. 1981;34:2100–2103. doi: 10.1093/ajcn/34.10.2100. [DOI] [PubMed] [Google Scholar]
- 14.Ismail I.E., Alagawany M., Taha A.E., Puvača N., Laudadio V., Tufarelli V. Effect of dietary supplementation of garlic powder and phenyl acetic acid on productive performance, blood haematology, immunity and antioxidant status of broiler chickens. Anim. Biosci. 2021;34:363. doi: 10.5713/ajas.20.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivam G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 2001;131:1106S–1108S. doi: 10.1093/jn/131.3.1106S. [DOI] [PubMed] [Google Scholar]
- 16.Jang H.J., Lee H.J., Yoon D.K., Ji D.S., Kim J.H., Lee C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018;27:219–225. doi: 10.1007/s10068-017-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morioka N., Sze L.L., Morton D.L., Irie R.F. A protein fraction from aged garlic extract enhances cytotoxicity and proliferation of human lymphocytes mediated by interleukin-2 and concanavalin A. Cancer Immunol. Immunother. 1993;37:316–322. doi: 10.1007/BF01518454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal K.C. Therapeutic actions of garlic constituents. Med. Res. Rev. 1996;16:111–124. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Ariga T., Seki T. Antithrombotic and anticancer effects of garlic derived sulfur compounds. BioFactors. 2006;26:93–103. doi: 10.1002/biof.5520260201. [DOI] [PubMed] [Google Scholar]
- 20.Li W.R., Shi Q.S., Dai H.Q., Liang Q., Xie X.B., Huang X.M., Zhao G.Z., Zhang L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016;6:22805. doi: 10.1038/srep22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makwana R.B., Raval A.P., Chauhan H.D., Kulkarni R.C., Srivastava A.K., Bhagwat S.R., Rajgor B.B. Effects of garlic (Allium sativum) supplementation on growth performance, carcass characteristics and economics of broilers. J. Anim. Res. 2015;5:843–848. doi: 10.5958/2277-940X.2015.00140.0. [DOI] [Google Scholar]
- 22.Al-Massad M., Al-Ramamneh D., AL-Sharafat A., Abdelqader A., Hussain N. Effect of using garlic on the economical and physiological characteristics of broiler chickens. Russ. Agric. Sci. 2018;44:276–281. doi: 10.3103/S1068367418030096. [DOI] [Google Scholar]
- 23.Elbaz A.M., Ibrahim N.S., Shehata A.M., Mohamed N.G., Abdel-Moneim A.M. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021;53:115. doi: 10.1007/s11250-021-02554-0. [DOI] [PubMed] [Google Scholar]
- 24.Bhatwalkar S.B., Mondal R., Krishna S.B.N., Adam J.K., Govender P., Anupam R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum) Front. Microbiol. 2021;12:613077. doi: 10.3389/fmicb.2021.613077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abd-ELrahman S.M., Mohamed S.A.-A., Mohamed S.E., El-Khadragy M.F., Dyab A.K., Hamad N., Safwat M.M., Nasr A.A.E., Alkhaldi A.A.M., Gareh A., et al. Comparative effect of allicin and alcoholic garlic extract on the morphology and infectivity of Eimeria tenella oocysts in chickens. Animals. 2022;12:3185. doi: 10.3390/ani12223185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbaz A.M., Ashmawy E.S., Salama A.A., Abdel-Moneim A.E., Badri F.B., Thabet H.A. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 2022;18:430. doi: 10.1186/s12917-022-03530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi A.A., Din Z.Z., Abuirmeileh N., Burger W.C., Ahmad Y., Elson C.E. Suppression of avian hepatic lipid metabolism by solvent extracts of garlic: Impact on serum lipids. J. Nutr. 1983;113:1746–1755. doi: 10.1093/jn/113.9.1746. [DOI] [PubMed] [Google Scholar]
- 28.Horton G.M.J., Fennell M.J., Prasad B.M. Effects of dietary garlic (Allium sativum) on performance, carcass composition and blood chemistry changes in broiler chickens. Can. J. Anim. Sci. 1991;71:939–942. doi: 10.4141/cjas91-113. [DOI] [Google Scholar]
- 29.Tollba A.A.H., Hassan M.S.H. Using some natural additives to improve physiological and productive performance of broiler chicks under high temperature conditions. 2. Black cumin (Niglla sativa) or garlic (Allium sativum) Egypt. Poult. Sci. J. 2003;23:327–340. [Google Scholar]
- 30.Ashayerizadeh O., Dastar B., Shargh M.S., Ashayerizadeh A., Rahmatnejad E., Hossaini S.M.R. Use of garlic (Allium sativum), black cumin seeds (Nigella sativa L.) and wild mint (Mentha longifolia) in broiler chickens diets. J. Anim. Vet. Adv. 2009;8:1860–1863. [Google Scholar]
- 31.Choi H., Park W.Y., Kim Y.J. Effects of dietary garlic powder and α-tocopherol supplementation on performance, serum cholesterol levels, and meat quality of chicken. Poult. Sci. 2010;89:1724–1731. doi: 10.3382/ps.2009-00052. [DOI] [PubMed] [Google Scholar]
- 32.Raeesi M., Hoseini-Aliabad S.A., Roofchaee A., Zare Shahneh A., Pirali S. Effect of periodically use of garlic (Allium sativum) powder on performance and carcass characteristics in broiler chickens. World Acad. Sci. Eng. Technol. 2010;4:1388–1394. [Google Scholar]
- 33.Suriya R., Zulkifli I., Alimon A.R. The effect of dietary inclusion of herbs as growth promoter in broiler chickens. J. Anim. Vet. Adv. 2012;11:346–350. doi: 10.3923/javaa.2012.346.350. [DOI] [Google Scholar]
- 34.Elagib H.A.A., El-Amin W.I.A., Malik H.E.E. Effect of dietary garlic (Allium sativum) supplementation as a feed additive on broiler performance and blood profile. J. Anim. Sci. Adv. 2013;3:58–64. [Google Scholar]
- 35.Oleforuh-Okoleh V.U., Harrie M., Solomon N.F., Olorunleke O., Joesph U. Evaluation of growth performance, hematological and serum biochemical response of broiler chickens to aqueous extract of ginger and garlic. J. Agric. Sci. 2015;7:167–173. doi: 10.5539/jas.v7n4p167. [DOI] [Google Scholar]
- 36.Varmaghany S., Torshizi M.A.K., Rahimi S., Lotfollahian H., Hassanzadeh M. The effects of increasing levels of dietary garlic bulb on growth performance, systolic blood pressure, hematology, and ascites syndrome in broiler chickens. Poult. Sci. 2015;94:1812–1820. doi: 10.3382/ps/pev148. [DOI] [PubMed] [Google Scholar]
- 37.Kirubakaran A., Moorthy M., Chitra R., Prabakar G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet. World. 2016;9:470. doi: 10.14202/vetworld.2016.470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautam G., Nabaraj Shishir B. Effect of Allium sativum on immune status against Newcastle disease virus and the productive performance of broiler chickens. J. Poult. Sci. 2017;16:515–521. doi: 10.3923/ijps.2017.515.521. [DOI] [Google Scholar]
- 39.Ratika K., Singh R.J., Singh R.K., Singh M. Weekly assessment of growth performance of broilers fed a diet supplemented with garlic and turmeric powder and their combination. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:1373–1381. doi: 10.20546/ijcmas.2018.702.166. [DOI] [Google Scholar]
- 40.Sangilimadan K., Churchil R.R., Premavalli K., Omprakash A.V. Effect of garlic (Allium sativum) on production performances and carcass traits of Nandanam broiler-2. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:2531–2538. doi: 10.20546/ijcmas.2019.804.295. [DOI] [Google Scholar]
- 41.Aydogan I., Yildirim E., Kurum A., Bolat D., Cinar M., Basalan M., Yigit A. The effect of dietary garlic (Allium Sativum), black cumin (Nigella sativa) and their combination on performance, intestine morphometry, serum biochemistry and antioxidant status of broiler chickens. Braz. J. Poult. Sci. 2020;22:eRBCA-2020-1317. doi: 10.1590/1806-9061-2020-1317. [DOI] [Google Scholar]
- 42.Tanti A., Retnani Y., Soesanto I.R.H. Effect of supplementation garlic (Allium sativum) by various processing on performances of broiler. IOP Conf. Ser. Earth Environ. Sci. 2022;1020:012015. doi: 10.1088/1755-1315/1020/1/012015. [DOI] [Google Scholar]
- 43.Sharma R.K., Singh R.A., Pal R.N., Aggarwal C.K. Cholesterol contents of chicken eggs as affected by feeding garlic, sarpagandha and nicotinic acid. Haryana Agric. Univ. J. Res. 1979;9:263–265. [Google Scholar]
- 44.Reddy R.V., Lightest S.F., Maurice D.V. Effect of feeding garlic oil on performance and egg yolk cholesterol concentration. Poult Sci. 1991;70:2006–2009. doi: 10.3382/ps.0702006. [DOI] [Google Scholar]
- 45.Birrenkott G., Brockenfelt G.E., Owens M., Halpin E. Yolk and blood cholesterol levels and organoleptic assessment of eggs from hens fed a garlic-supplemented diet. Poult. Sci. 2000;79:75–82. [Google Scholar]
- 46.Chowdhury S.R., Chowdhury S.D., Smith T.K. Effects of dietary garlic on cholesterol metabolism in laying hens. Poult. Sci. 2002;81:1856–1862. doi: 10.1093/ps/81.12.1856. [DOI] [PubMed] [Google Scholar]
- 47.Mottaghitalab M., Taraz Z. Effects of garlic (Allium sativum) on egg yolk and blood serum cholesterol in Aryan breed laying hens. Br. Poult. Sci. 2002;43:S42–S43. [Google Scholar]
- 48.Yalcin S., Onbasilar E.E., Reisli Z., Yalcin S. Effects of garlic powder on the performance, egg traits and blood parameters of laying hens. J. Sci. Food Agric. 2006;86:1336–1339. doi: 10.1002/jsfa.2515. [DOI] [Google Scholar]
- 49.Khan S.H., Sardar R., Anjum M.A. Effects of dietary garlic on performance and serum and egg cholesterol concentration in laying hens. Asian Aust. J. Anim. Sci. 2007;21:22–27. doi: 10.3923/ajpsaj.2007.22.27. [DOI] [Google Scholar]
- 50.Saffa H.M. Effect of dietary garlic or fenugreek on cholesterol metabolism in lying hens. Egypt. J. Poult. Sci. 2007;27:1207–1221. [Google Scholar]
- 51.Khan Q.S.H., Hassan S., Sardar R., Anjum M.A. Effects of dietary garlic on cholesterol concentration in native Desi laying hens. Am. J. Food Technol. 2008;3:207–213. [Google Scholar]
- 52.Canogullari S., Baylan M., Erdogan Z., Duzguner V., Kucukgul A. The effects of dietary garlic powder on performance, egg yolk and serum cholesterol concentrations in laying quails. Czech. J. Anim. Sci. 2010;55:286–293. doi: 10.17221/126/2009-CJAS. [DOI] [Google Scholar]
- 53.Mahmoud K.Z., Saad M., Gharaibeh H., Zakaria A., Amer M. Garlic (Allium sativum) supplementation: Influence on egg production, quality and yolk cholesterol level in laying hens. Asian Australs. J. Anim. Sci. 2010;23:1503–1509. doi: 10.5713/ajas.2010.10124. [DOI] [Google Scholar]
- 54.Motamedi S.M., Taklimi S.M. Investigating the effect of fenugreek seed powder and garlic powder in the diet on immune response of commercial laying hens’ egg. Indian J. Sci. Res. 2014;3:277–283. [Google Scholar]
- 55.Asrat M., Zeryehun T., Amha N., Urge M. Effects of supplementation of different levels of garlic (Allium sativum) on egg production, egg quality and hatchability of White Leghorn chicken. Livest. Res. Rural Dev. 2018;30:37. [Google Scholar]
- 56.Omer H.A., El-Mallah G.M., Abdel-Magid S.S., Bassuony N.I., Ahmed S.M., El-Ghamry A.K.A. Impact of adding natural bioactive mixture composed of lemon, onion, and garlic juice at different levels on productive performance, egg quality, and some blood parameters of commercial laying hens. Bull. Natl. Res. Cent. 2019;43:137. doi: 10.1186/s42269-019-0160-4. [DOI] [Google Scholar]
- 57.Khan R.U., Nikousefat Z., Tufarelli V., Naz S., Javdani M., Laudadio V. Garlic (Allium sativum) supplementation in poultry diets: Effect on production and physiology. Worlds Poult. Sci. J. 2012;68:417–424. doi: 10.1017/S0043933912000530. [DOI] [Google Scholar]
- 58.Ziarlarimi A., Irani M., Gharahveysi S., Rahmani Z. Investigation of antibacterial effects of garlic (Allium sativum), mint (Menthe spp.) and onion (Allium cepa) herbal extracts on Escherichia coli isolated from broiler chickens. Afr. J. Biotechnol. 2011;10:10320–10322. doi: 10.5897/AJB10.2513. [DOI] [Google Scholar]
- 59.Ross Z.M., O’Gara E.A., Hill D.J., Sleightholme H.V., Maslin D.J. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl. Environ. Microbiol. 2001;67:475–480. doi: 10.1128/AEM.67.1.475-480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olukosi O.A., Dono N.D. Modification of digesta pH and intestinal morphology with the use of benzoic acid or phytobiotics and the Effects on broiler chicken growth performance and energy and nutrient utilization. J. Anim. Sci. 2014;92:3945–3953. doi: 10.2527/jas.2013-6368. [DOI] [PubMed] [Google Scholar]
- 61.Nusairat B.M. Master’s Thesis. Jordan University of Science and Technology; Irbi, Jordan: 2007. Dietary Supplementation of Garlic (Allium sativum): Influence on Performance Parameters, Meat Quality and Humoral Immune Response in Broiler Chicks. [Google Scholar]
- 62.Kumar A., Kheravii S.K., Ionescu C., Blanchard A., Barekatain R., Bajagai Y.S., Wu S.B. A microencapsulated mixture of eugenol and garlic tincture supplementation mitigates the effect of necrotic enteritis on intestinal integrity and increases goblet cells in broilers. Microorganisms. 2021;9:1451. doi: 10.3390/microorganisms9071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adibmoradi M., Navidshad B., Seifdavati J., Royan M. Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J. Poult. Sci. 2006;43:378–383. doi: 10.2141/jpsa.43.378. [DOI] [Google Scholar]
- 64.Gotep J.G., Tanko J.T., Forcados G.E., Muraina I.A., Ozele N., Dogonyaro B.B., Oladipo O.O., Makoshi M.S., Akanbi O.B., Kinjir H., et al. Therapeutic and safety evaluation of combined aqueous extracts of Azadirachta indica and Khaya senegalensis in chickens experimentally infected with Eimeria oocysts. J. Parasitol. Res. 2016;2016:4692424. doi: 10.1155/2016/4692424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halliwell B., Zhao K., Whiteman M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y., Iji P.A., Choct M. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 2009;65:97–114. doi: 10.1017/S0043933909000087. [DOI] [Google Scholar]
- 67.Grajek W., Olejnik A., Sip A. Probiotics, prebiotics and antioxidants as functional foods. Acta Biochim. Pol. 2005;52:665–671. doi: 10.18388/abp.2005_3428. [DOI] [PubMed] [Google Scholar]
- 68.Yalcin S., Onbaşilar Ĺ., Şehu A., Yalcin S. The effects of dietary garlic powder on the performance, egg traits and blood serum cholesterol of laying quails. Asian-Australas. J. Anim. Sci. 2007;20:944–947. doi: 10.5713/ajas.2007.944. [DOI] [Google Scholar]
- 69.Jafari R., Razi M., Ghorbanpoor M., Marashian S. Effect of dietary garlic on immune response of broiler chicks to live Newcastle disease vaccine. Pak. J. Biol. Sci. 2008;11:1848–1851. doi: 10.3923/pjbs.2008.1848.1851. [DOI] [PubMed] [Google Scholar]
- 70.Hanieh H., Narabara K., Piao M., Gerile C., Abe A., Kondo Y. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim. Sci. J. 2010;81:673–680. doi: 10.1111/j.1740-0929.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 71.Rahimi S., Teymouri Zadeh Z., Karimi Torshizi M.A., Omidbaigi R., Rokni H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Technol. 2011;13:527–539. [Google Scholar]
- 72.El-katcha M.I., Soltan M.A., Sharaf M.M., Hasen A. Growth performance, immune response, blood serum parameters, nutrient digestibility and carcass traits of broiler chicken as affected by dietary supplementation of garlic extract (allicin) Alex. J. Vet. Sci. 2016;49:50–54. doi: 10.5455/ajvs.219261. [DOI] [Google Scholar]
- 73.Oladele O., Esan O., Akpan I., Enibe F. Garlic feed inclusion and susceptibility of broiler chickens to infectious bursal disease. J. Adv. Vet. Anim. Res. 2018;5:275–281. doi: 10.5455/javar.2018.e276. [DOI] [Google Scholar]
- 74.Chang L.Y., Di K.Q., Xu J., Chen Y.F., Xi J.Z., Wang D.H., Hao E.Y., Xu L.J., Chen H., Zhou R.Y. Effect of natural garlic essential oil on chickens with artificially infected E. tenella. Veter. Parasitol. 2021;300:109614. doi: 10.1016/j.vetpar.2021.109614. [DOI] [PubMed] [Google Scholar]
- 75.Percival S.S. Aged garlic extract modifies human immunity. J. Nutr. 2016;146:433S–436S. doi: 10.3945/jn.115.210427. [DOI] [PubMed] [Google Scholar]
- 76.Dorhoi A., Dobrean V., Zahan M., Virag P. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother. Res. 2006;20:352–358. doi: 10.1002/ptr.1859. [DOI] [PubMed] [Google Scholar]
- 77.Ghazanfari T., Hassan Z., Ebtekar M., Ahamdiani A., Naderi G., Azar A. Garlic induces a shift in cytokine pattern in Leishmania major-infected Balb/c mice. Scand. J. Immunol. 2000;52:491–496. doi: 10.1046/j.1365-3083.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- 78.Hanieh H., Narabara K., Tanaka Y., Gu Z., Abe A., Kondo Y. Immunomodulatory effects of Alliums and Ipomoea batata extracts on lymphocytes and macrophages functions in White Leghorn chickens: In vitro study. Anim. Sci. J. 2012;83:68–76. doi: 10.1111/j.1740-0929.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- 79.Kyo E., Uda N., Ushijima M., Kasuga S., Itakura Y. Prevention of psychological stress-induced immune suppression by aged garlic extract. Phytomedicine. 1999;6:325–330. doi: 10.1016/S0944-7113(99)80053-1. [DOI] [PubMed] [Google Scholar]
- 80.Feng Z., Zhang G., Hao T., Zhou B., Zhang H., Jiang Z. Effect of diallyl trisulfide on the activation of T cell and macrophage-mediated cytotoxicity. J. Tongii Med. Univ. 1994;14:142–147. doi: 10.1007/bf02886794. [DOI] [PubMed] [Google Scholar]
- 81.Nantz M.P., Rowe C.A., Muller C.E., Creasy R.A., Stanika J.M., Percival S.S. Supplementation with aged garlic improves both NK and –T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind placebo-controlled nutrition intervention. Clin. Nutr. 2012;31:337–344. doi: 10.1016/j.clnu.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Arreola R., Quintero-Fabián S., López-Roa R.I., Flores-Gutiérrez E.O., Reyes-Grajeda J.P., Carrera-Quintanar L. Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015;2015:401630. doi: 10.1155/2015/401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Costantini D., Møller A. Does immune response cause oxidative stress in birds? A meta-analysis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;153:339–344. doi: 10.1016/j.cbpa.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Karangiya V., Savsani H., Patil S.S., Garg D., Murthy K., Ribadiya N., Vekariya S. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Veter. World. 2016;9:245. doi: 10.14202/vetworld.2016.245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pirgozliev V., Rose S., Catherine I., Blanchard A. Phytogenic Feed Additives Can Alleviate the Negative Impact of Necrotic Enteritis in Broilers; Proceedings of the 6th International Conference Poultry Intestinal Health; Rome, Italy. 3–5 April 2019. [Google Scholar]
- 86.Rees L.P., Minney S.F., Plummer N.T., Slater J.H., Skyrme D.A. A quantitative assessment of the antimicrobial activity of garlic (Allium sativum) World J. Microbiol. Biotechnol. 1993;9:303–307. doi: 10.1007/BF00383068. [DOI] [PubMed] [Google Scholar]
- 87.Skyrme D.A. Ph.D. Thesis. Cardiff University; Cardiff, UK: 1997. The Antimicrobial Activity of Allium sativum. [Google Scholar]
- 88.Elmahallawy E.K., Fehaid A., El-Shewehy D.M., Ramez A.M., Alkhaldi A.A., Mady R., Nasr N.E., Arafat N., Hassanen E.A., Alsharif K.F., et al. S-methylcysteine ameliorates the intestinal damage induced by Eimeria tenella infection via targeting oxidative stress and inflammatory modulators. Front. Veter. Sci. 2022;8:754991. doi: 10.3389/fvets.2021.754991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Müller A., Eller J., Albrecht F., Prochnow P., Kuhlmann K., Bandow J.E., Slusarenko A.J., Leichert L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016;291:11477–11490. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Becerra-Torres S., Soria Fregozo C., Jaramillo-Juarez F., Moreno-Hernandez-Duque J. Allium sativum aqueous extract prevents potassium dichromateinduced nephrotoxicity and lipid oxidation in rats. J. Pharm. Pharmac. Res. 2014;2:45–52. doi: 10.56499/jppres14.020_2.2.45. [DOI] [Google Scholar]
- 91.Abou-Elkhair R., Gaafar K.M., Elbahy N.M., Helal M.A., Mahboub H.D., Sameh G. Bioactive effect of dietary supplementation with essential oils blend of oregano, thyme and garlic oils on performance of broilers infected with Eimeria species. Global Veter. 2014;13:977–985. doi: 10.5829/idosi.gv.2014.13.06.9137. [DOI] [Google Scholar]
- 92.El-Khtam A., Shata A., El-Hewaity M.H. Efficacy of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria spp. in broilers. Int. J. Bas. Appl. Sci. 2014;3:349–356. doi: 10.14419/ijbas.v3i3.3142. [DOI] [Google Scholar]
- 93.El Dakroury M.F., Reda S.F., Baz G.M. A study of the anticoccidial effects of clopidol and garlic powder on Eimeria-infected broilers. Assiut. Veter. Med. J. 2016;62:84–89. [Google Scholar]
- 94.Kefyalew D., Sibhat B., Cheru H. Anticoccidial effect of garlic on leghorn chickens. Biomed. Nurs. 2018;4:70–74. [Google Scholar]
- 95.Udo E.J., Abba A.M. Comparative study of in-vitro anticoccidial efficacy of Allium sativum and Carica papaya. J. Zool. Res. 2018;2:10–14. doi: 10.22259/2637-5575.0202002. [DOI] [Google Scholar]
- 96.Al-Shaibani I.R.M., Al-Khadher A.M.A., AlHibah A.Z.H. Anticoccidial activity of Allium sativum and Punica granatum against experimentally induced Eimeria tenella infection in broiler chickens. Asian J. Res. Anim. Vet. Sci. 2020;5:20–29. doi: 10.13140/RG.2.2.17167.20642. [DOI] [Google Scholar]
- 97.Sidiropoulou E., Skoufos I., Marugan-Hernandez V., Giannenas I., Bonos E., Aguiar-Martins K., Lazari D., Blake D.P., Tzora A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Veter. Sci. 2020;7:420. doi: 10.3389/fvets.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hussein S.H., Soad M., Mervat A.A., Gehan N.A., Rania A.A. Comparative biochemical and pathological studies between toltrazuril and garlic supplementation in chickens experimentally infected with coccidiosis. Egypt. J. Anim. Health. 2021;1:65–80. doi: 10.21608/ejah.2021.134362. [DOI] [Google Scholar]
- 99.Adjei-Mensah B., Atuahene C.C. Avian coccidiosis and anticoccidial potential of garlic (Allium sativum L.) in broiler production: A review. J. Appl. Poult. Res. 2023;32:100314. doi: 10.1016/j.japr.2022.100314. [DOI] [Google Scholar]
- 100.Ali M., Chand N., Khan R.U., Naz S., Gul S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019;47:79–84. doi: 10.1080/09712119.2019.1573731. [DOI] [Google Scholar]
- 101.Asghar M., Durrani U.F., Hussain R., Matloob K., Mahmood A.K., Anees M., Oneeb M. Comparative efficacy of amprolium, garlic oil (A. sativum) and ginger oil (Zingiber officinale) against coccidiosis in common quail (Coturnix coturnix) J. Hellen. Veter. Med. Soc. 2020;71:2273–2278. doi: 10.12681/jhvms.25072. [DOI] [Google Scholar]
- 102.Allen P.C., Danforth H.D., Augustine P.C. Diet modulation of avian coccidiosis. Int. J. Parasitol. 1998;28:1131–1140. doi: 10.1016/S0020-7519(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 103.Pourali M., Kermanshahi H., Golian A., Razmi G.R., Soukhtanloo M. Antioxidant and anticoccidial effects of garlic powder and sulfur amino acids on Eimeria infected and uninfected broiler chickens. Iran. J. Veter. Res. 2014;15:227–232. doi: 10.22099/ijvr.2014.2531. [DOI] [Google Scholar]
- 104.Kim D.K., Lillehoj H.S., Lee S.H., Lillehoj E.P., Bravo D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013;109:76–88. doi: 10.1017/S0007114512000530. [DOI] [PubMed] [Google Scholar]
- 105.Christaki E., Florou-Paneri P., Giannenas I., Papazahariadou M., Botsoglou N.A., Spais A.B. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim. Res. 2004;53:137–144. doi: 10.1051/animres:2004006. [DOI] [Google Scholar]
- 106.Jamil M., Aleem M.T., Shaukat A., Khan A., Mohsin M., Rehman T.U., Abbas R.Z., Saleemi M.K., Khatoon A., Babar W., et al. Medicinal plants as an alternative to control poultry parasitic diseases. Life. 2022;12:449. doi: 10.3390/life12030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanweer A.J., Saddique U., Bailey C.A., Khan R.U. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 2014;113:2951–2960. doi: 10.1007/s00436-014-3957-y. [DOI] [PubMed] [Google Scholar]
- 108.El-Saber Batiha G., Magdy Beshbishy A., Wasef L.G., Elewa Y.H., Al-Sagan A.A., Abd El-Hack M.E., Taha A.E., M. AbdElhakim Y., Prasad Devkota H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients. 2020;12:872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ayaz E., Turel I., Gul A., Yilmaz O. Evaluation of the anthelmentic activity of garlic (Allium sativum) in mice naturally infected with Aspiculuris tetraptera. Rec. Pat. Antiinfect. Drug Discov. 2008;3:149–152. doi: 10.2174/157489108784746605. [DOI] [PubMed] [Google Scholar]
- 110.Dkhil M.A., Abdel-Baki A.S., Wunderlich F., Sies H., AlQuraishy S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Veter. Parasitol. 2011;175:66–72. doi: 10.1016/j.vetpar.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 111.Sadek H.A., Abdel-Rahman S.M., Bakir H.Y., Arafa M.I., Ahmed A.A., Gaber M.M. The potential convention of garlic and black seed different extracts as an effective treatment of Cryptosporidium spp.: An experimental study. J. Egypt. Soc. Parasitol. 2020;50:613–621. doi: 10.21608/jesp.2020.131094. [DOI] [Google Scholar]
- 112.Sklan D., Berner Y.N., Rabinowitch H.D. The effect of dietary onion and garlic on hepatic lipid concentrations and activity of antioxidative enzymes in chicks. J. Nutr. Biochem. 1992;3:322–325. doi: 10.1016/0955-2863(92)90022-B. [DOI] [Google Scholar]
- 113.Konjufca V.H., Pesti G.M., Bakalli R.I. Modulation of cholesterol levels in broiler meat by dietary garlic and copper. Poult. Sci. 1997;76:1264–1271. doi: 10.1093/ps/76.9.1264. [DOI] [PubMed] [Google Scholar]
- 114.Lim K.S., You S.J., An H.K., Kang C.W. Effects of dietary garlic powder and copper on cholesterol content and quality characteristics of chicken eggs. Asian-Australas. J. Anim. Sci. 2006;19:582–586. doi: 10.5713/ajas.2006.582. [DOI] [Google Scholar]
- 115.Azeke M., Ekpo K.E. Egg yolk cholesterol lowering effects of garlic and tea. J. Biol. Sci. 2008;8:456–460. doi: 10.3923/jbs.2008.456.460. [DOI] [Google Scholar]
- 116.Kim Y.J., Jin S.K., Yang H.S. Effect of dietary garlic bulb and husk on the physicochemical properties of chicken meat. Poult. Sci. 2009;88:398–405. doi: 10.3382/ps.2008-00179. [DOI] [PubMed] [Google Scholar]
- 117.Ao X., Yoo J.S., Lee J.H., Jang H.D., Wang J.P., Zhou T.X., Kim I.H. Effects of fermented garlic powder on production performance, egg quality, blood profiles and fatty acids composition of egg yolk in laying hens. Asian Australs. J. Anim. Sci. 2010;23:786–791. doi: 10.5713/ajas.2010.90543. [DOI] [Google Scholar]
- 118.Ghasemi R., Zarei M., Torki M. Adding medicinal herbs including garlic (Allium sativum) and thyme (Thymus vulgaris) to diet of laying hens and evaluating productive performance and egg quality characteristics. Am. J. Anim. Veter. Sci. 2010;5:151–154. doi: 10.3844/ajavsp.2010.151.154. [DOI] [Google Scholar]
- 119.Issa K.J., Omar J.M.A. Effect of garlic powder on performance and lipid profile of broilers. Open J. Anim. Sci. 2012;2:62–68. doi: 10.4236/ojas.2012.22010. [DOI] [Google Scholar]
- 120.Kang J.S., Kim S.O., Kim G.Y., Hwang H.J., Kim B.W., Chang Y.C., Kim W.J., Kim C.M., Yoo Y.H., Choi Y.H. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int. J. Mol. Med. 2016;37:149–156. doi: 10.3892/ijmm.2015.2398. [DOI] [PubMed] [Google Scholar]
- 121.Ojo O.O., Kabutu F.R., Bello M., Babayo U. Inhibition of paracetamol-induced oxidative stress in rats by extracts of lemongrass (Cymbropogon citratus) and green tea (Camellia sinensis) in rats. Afr. J. Biotechnol. 2006;5:1227–1232. [Google Scholar]
- 122.Qureshi A.A., Abuirmeileh N., Din Z.Z., Elson C.E., Burger W.C. Inhibition of cholesterol and fatty acid biosynthesis in liver enzymes and chicken. Hepatocytes by polar fractions of garlic. Lipids. 1983;18:343–348. doi: 10.1007/BF02537229. [DOI] [PubMed] [Google Scholar]
- 123.Horie T., Awazu S., Itakura Y., Fuwa T. Identified diallyl polysulfides from an aged garlic extract which protects the membranes from lipid peroxidation. Planta Med. 1992;58:468–469. doi: 10.1055/s-2006-961517. [DOI] [PubMed] [Google Scholar]
- 124.Chen C.H., Chan H.C., Chu Y.T., Ho H.Y., Chen P.Y., Lee T.H., Lee C.K. Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules. 2009;14:2947–2958. doi: 10.3390/molecules14082947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin W.C., Lee M.T., Chang S.C., Chang Y.L., Shih C.H., Yu B., Lee T.T. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult. Sci. 2017;96:1191–1203. doi: 10.3382/ps/pew350. [DOI] [PubMed] [Google Scholar]
- 126.Pourakbari M., Seidavi A., Asadpour L., Martínez A. Probiotic level effects on growth performance, carcass traits, blood parameters, cecal microbiota, and immune response of broilers. An. Acad. Bras. Ciênc. 2016;88:1011–1021. doi: 10.1590/0001-3765201620150071. [DOI] [PubMed] [Google Scholar]
- 127.Cavallito C.J., Bailey J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944;66:1950–1951. doi: 10.1021/ja01239a048. [DOI] [Google Scholar]
- 128.Ko J.W., Park S.H., Lee I.C., Lee S.M., Shin I.S., Kang S.S., Moon C., Kim S.H., Heo J.D., Kim J.C. Protective effects of garlic oil against 1,3-dichloro-2-propanol-induced hepatotoxicity: Role of CYP2E1 and MAPKs. Mol. Cell. Toxicol. 2016;12:185–195. doi: 10.1007/s13273-016-0023-0. [DOI] [Google Scholar]
- 129.Guan M.J., Zhao N., Xie K.Q., Zeng T. Hepatoprotective effects of garlic against ethanol-induced liver injury: A mini-review. Food Chem. Toxicol. 2018;111:467–473. doi: 10.1016/j.fct.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 130.Lai Y.S., Chen W.C., Ho C.T., Lu K.H., Lin S.H., Tseng H.C., Lin S.Y., Sheen L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014;62:5897–5906. doi: 10.1021/jf500803c. [DOI] [PubMed] [Google Scholar]
- 131.Rehman Z.U., Munir M.T. Effect of garlic on the health and performance of broilers. Veterinaria. 2015;3:32–39. [Google Scholar]
- 132.Peinado M.J., Ruiz R., Echávarri A., Rubio L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012;91:2148–2157. doi: 10.3382/ps.2012-02280. [DOI] [PubMed] [Google Scholar]
- 133.Singh J., Sharma M., Singh N., Kaur P., Sethi A., Sikka S. Effect of sun dried whole bulb garlic powder on nutrient utilization, blood parameters, duodenum morphology and faecal microbial load in broiler chickens. Indian J. Anim. Sci. 2017;87:195–198. doi: 10.56093/ijans.v87i2.67722. [DOI] [Google Scholar]
- 134.Zhang R., Liu J., Liu Y., Wu Y., Xu Y., Feng J. Dietary garlic powder alleviates lipopolysaccharide-induced inflammatory response and oxidative stress through regulating the immunity and intestinal barrier function in broilers. Animals. 2022;12:2281. doi: 10.3390/ani12172281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bjerrum L., Engberg R.M., Leser T.D., Jensen B.B., Finster K., Pedersen K. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poult. Sci. 2006;85:1151–1164. doi: 10.1093/ps/85.7.1151. [DOI] [PubMed] [Google Scholar]
- 136.Amagase H., Petesch B.L., Matsuura H., Kasuga S., Itakura Y. Intake of garlic and its bioactive components. J. Nutr. 2001;131:955–962. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 137.Peinado M.J., Ruiz R., Echávarri A., Aranda-Olmedo I., Rubio L.A. Garlic derivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed Sci. Technol. 2013;181:87–92. doi: 10.1016/j.anifeedsci.2013.03.001. [DOI] [Google Scholar]
- 138.Lee S.H., Bang S., Jang H.H., Lee E.B., Kim B.S., Kim S.H., Kang S.H., Lee K.W., Kim D.W., Kim J.B., et al. Effects of Allium hookeri on gut microbiome related to growth performance in young broiler chickens. PLoS ONE. 2020;15:e0226833. doi: 10.1371/journal.pone.0226833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gutmorphology in broiler chicks. Poult Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- 140.Edwards C., Havlik J., Cong W., Mullen W., Preston T., Morrison D.J., Combet E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017;42:356–360. doi: 10.1111/nbu.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carbonero F., Benefiel A.C., Alizadeh-Ghamsari A.H., Gaskins H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reiter J., Levina N., van der Linden M., Gruhlke M., Martin C., Slusarenko A.J. Diallylthiosulfinate (allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules. 2017;22:1711. doi: 10.3390/molecules22101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All prepared data are presented in the present article.