Abstract
Utilization of heme-hemopexin as a source of heme by Haemophilus influenzae type b is dependent on expression by this bacterium of the 100-kDa HxuA protein, which is both present on the bacterial cell surface and released into the culture supernatant (L. D. Cope, R. Yogev, U. Muller-Eberhard, and E. J. Hansen, J. Bacteriol. 177:2644–2653, 1995). Radioimmunoprecipitation analysis showed that the soluble HxuA protein present in H. influenzae type b culture supernatant bound heme-hemopexin complexes in solution. An isogenic H. influenzae type b hxuA mutant was unable to utilize soluble heme-hemopexin complexes for growth in vitro unless soluble HxuA protein was provided exogenously. Soluble HxuA protein secreted by a nontypeable H. influenzae strain also allowed growth of this H. influenzae type b hxuA mutant. These results indicated that the heme present in heme-hemopexin complexes is rendered accessible to H. influenzae when these complexes are bound by the soluble HxuA protein.
It has been known for many years that all Haemophilus influenzae organisms have an absolute requirement for heme for aerobic growth because they lack the ability to convert δ-aminolevulinic acid to protoporphyrin IX, the immediate biosynthetic precursor of heme (13, 48). Nucleotide sequence analysis of the H. influenzae Rd genome (10) has provided new insights which reveal that most of the pathway for conversion of δ-aminolevulinic acid to heme has been ablated in H. influenzae (45). The only relevant biosynthetic reaction that H. influenzae can accomplish is the ferrochelatase-mediated (and reversible) insertion of ferrous iron into protoporphyrin IX to yield heme (13, 33). Therefore, H. influenzae has evolved or acquired mechanisms for the binding and transport of heme because aerobic growth and acquisition of heme by H. influenzae are absolutely codependent (9).
When the bacterium is grown in vitro, free heme satisfies the porphyrin requirements (13) and, in part, the iron requirements (6) of H. influenzae. In vivo, however, free heme is not available because free heme is toxic and the body has evolved highly specific mechanisms for complexing this tetrapyrrole molecule analogous to those used to restrict the availability of free iron (i.e., the use of lactoferrin and transferrin). The abundant serum proteins albumin and hemopexin bind heme avidly, with Kd values of 10−8 and 10−13 M, respectively (25, 39). With the exception of that in hemolytic states, all circulating heme is complexed to hemopexin because this glycoprotein has a much greater affinity for heme than does albumin (25). In addition, much of the body’s heme is present in the form of hemoglobin. Recognition of this fact has resulted in considerable interest in the heme acquisition systems utilized by bacterial pathogens (3, 7, 8, 18–20, 26–28, 31, 32, 35–37, 40–43, 46).
Previous studies from our laboratory and others have shown that there are distinct mechanisms for binding the free and protein-bound forms of heme to the H. influenzae cell surface (4, 5, 11, 17, 23, 24, 34, 44). An H. influenzae type b (Hib) chromosomal locus, hxuCBA, has been shown to be necessary for utilization of heme bound to the human serum protein hemopexin (5, 17). The last gene in this locus, hxuA, encodes a 100-kDa protein that can be found on the H. influenzae cell surface and is also secreted into culture supernatants (CS) (4). This HxuA protein is expressed by the majority of H. influenzae strains and, when it is immobilized on nitrocellulose, has been shown to bind heme-hemopexin (4). Lack of this protein rendered an isogenic Hib hxuA mutant unable to use heme-hemopexin as the sole source of heme for growth (17). The exact manner by which HxuA allows H. influenzae to acquire heme from heme-hemopexin is still unknown, and it had not been determined whether soluble HxuA is functional. The fact that soluble HxuA protein was present in CS (4) prompted us to determine whether HxuA is functional in solution and, if so, whether binding of soluble HxuA to heme-hemopexin complexes results in the complexed heme being made available to H. influenzae.
The Hib strains DL26, DL42, DL42.107d, DL42.61, and 760705 and the nontypeable H. influenzae (NTHI) strain N182 used in this study have been described previously (4, 5, 16) and are listed in Table 1 together with their relevant phenotypic characteristics. Escherichia coli RR1 has also been described previously (38). Hib and NTHI strains were routinely grown on brain heart infusion (BHI) agar (Difco Laboratories, Detroit, Mich.) containing Levinthal’s base (BHIs) (1) at 37°C in an atmosphere of 5% CO2–95% air. E. coli strains were grown in Luria-Bertani medium (38) containing tetracycline (15 μg/ml). Plasmids used in this study are also listed in Table 1. Human apo-hemopexin was prepared as described previously (21), with the final purification step involving chromatography on Q-Sepharose Fast-Flow in 20 mM Tris-HCl (pH 8.0) with a gradient of up to 8.5 M NaCl in the same buffer. Heme-hemopexin was prepared by mixing a 1:1 solution of hemin in dimethyl sulfoxide with apo-hemopexin, followed by removal of the unbound hemin by chromatography on Whatman DE 52 cellulose in phosphate-buffered saline (PBS).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description or phenotype | Reference(s) or source |
|---|---|---|
| H. influenzae strains | ||
| DL26 | Wild-type Hib strain that expresses the HxuA protein and can utilize heme-hemopexin | 16 |
| DL42 | Wild-type Hib strain that expresses the HxuA protein and can utilize heme-hemopexin | 4, 5 |
| DL42.107d | DL42 mutant with a NotI linker inserted into the hxuA structural gene; this strain does not express HxuA and cannot utilize heme-hemopexin | 17 |
| DL42.61 | DL42.107d with a cat cartridge inserted into the NotI site within the hxuA gene | 5 |
| 760705 | Wild-type Hib strain that does not express the HxuA protein and does not utilize heme-hemopexin | 4, 47 |
| N182 | Wild-type NTHI strain that expresses the HxuA protein and can utilize heme-hemopexin | 4 |
| E. coli strains | ||
| RR1 | Host strain for recombinant plasmids | 38 |
| RR1(pBR322) | Recombinant strain that does not release HxuA into the CS | 38 |
| RR1(pHX1-6) | Recombinant strain that releases HxuA into the CS | This study |
| Plasmids | ||
| pBR322 | Cloning vector, Ampr Tetr | 38 |
| pHX1-6 | pBR322 with a 13.6-kb insert of H. influenzae DL42 chromosomal DNA containing the hxuCBA gene cluster | 5, 17 |
To prepare heme-free culture supernatant containing soluble H. influenzae proteins, including HxuA, H. influenzae strains grown overnight on BHIs agar were inoculated into 50 ml of BHI broth containing only NAD (10 μg/ml). This inoculum yielded a reading of 30 to 40 Klett units (Klett-Summerson colorimeter; Klett Manufacturing, New York, N.Y.). These cultures were incubated with agitation at 37°C for 4 h, until they had attained a cell density that yielded a reading of 160 Klett units (approximately 2 × 109 to 3 × 109 CFU/ml). It should be noted here that H. influenzae cells grown in the presence of large amounts of a heme source (e.g., on BHIs agar plates) apparently sequester heme quite effectively and can then proceed to grow in heme-free medium for several generations. The cultures were then centrifuged at 27,000 × g for 15 min at 4°C; the supernatant was drawn off and centrifuged at 254,000 × g for 1 h at 4°C to remove membrane fragments. The resultant supernatant was concentrated 83-fold by the use of Centriprep-30 concentrators (Amicon, Beverly, Mass.) and filter sterilized. These concentrated CS were used immediately in the feeding experiments described below or in the radioimmunoprecipitation (RIP) experiments. CS from recombinant E. coli strains were prepared similarly with bacteria grown in Luria-Bertani medium.
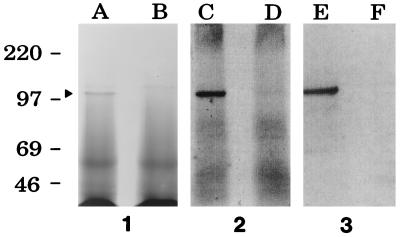
Proteins present in CS from the wild-type Hib strain DL42 and the isogenic hxuA mutant DL42.61 were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two high-molecular-mass proteins with apparent molecular masses of approximately 100 and 115 kDa were detectable by Coomassie blue staining in CS from this wild-type Hib strain (Fig. 1, panel 1, lane A); only the 115-kDa protein, which was probably immunoglobulin A (IgA) protease (2, 14), was present in the CS of the hxuA mutant (Fig. 1, panel 1, lane B). Western blot analysis and autoradiography were performed with radioiodinated goat anti-mouse IgG as the secondary antibody to detect both mouse monoclonal and rat polyclonal IgG antibodies bound to Hib antigens. The Western blot analysis confirmed that the 100-kDa protein was HxuA; this 100-kDa antigen bound the Hib DL42 HxuA-specific monoclonal antibody 4C11 (17) (Fig. 1, panel 3, lane E). As expected, this same monoclonal antibody did not bind to any CS proteins expressed by the hxuA mutant (Fig. 1, panel 3, lane F). Antibodies present in rat antiserum raised against Hib DL26 CS proteins (16) readily bound to HxuA (Fig. 1, panel 2, lane C) and did not bind detectably to other soluble antigens in the CS. This polyclonal CS antiserum was unreactive with CS antigens from the hxuA mutant (Fig. 1, panel 2, lane D).
FIG. 1.
Detection of the 100-kDa HxuA protein in CS from wild-type and mutant Hib strains. Proteins present in CS from the wild-type Hib strain DL42 (lanes A, C, and E) and from the Hib hxuA mutant strain DL42.61 (lanes B, D, and F) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and either stained with Coomassie blue (panel 1) or transferred to nitrocellulose for Western blot analysis with rat antiserum raised against CS from Hib strain DL26 (panel 2) or with the Hib DL42 HxuA-specific monoclonal antibody 4C11 (panel 3). The arrowhead to the left of lane A indicates the position of the HxuA protein. Molecular mass position markers (in kilodaltons) are present on the left side of the figure.
We used this rat polyclonal antiserum to CS proteins in RIP analysis to determine whether soluble HxuA bound heme-hemopexin, working upon the assumption that HxuA-directed antibodies in this antiserum bind HxuA-heme-hemopexin complexes. Radioiodinated heme-hemopexin (106 cpm) was combined with 25 μl of CS and 250 μl of pH 7.3 PBS containing 0.05% (vol/vol) Tween 20 (T) and 0.1% (wt/vol) bovine serum albumin (BSA) in a 1.5-ml microcentrifuge tube. This solution was incubated at room temperature with gentle agitation for 90 min. The antiserum (100 μl) raised against Hib strain DL26 CS proteins or control serum (100 μl) from normal rats was added, and the solution was incubated for an additional hour at room temperature, followed by chilling in crushed ice. Next, a 100-μl portion of a 10% (vol/vol) suspension of protein A-bearing staphylococci (15) in PBS-T-BSA was added to the tube, which was then incubated with gentle agitation for 1 h at 4°C. The antigen-antibody-staphylococcus complexes were then washed four times with PBS-T-BSA by centrifugation and resuspension, and the final washed pellet was resuspended in 150 μl of digestion buffer (15). This suspension was transferred to a 12- by 75-mm glass culture tube, heated at 100°C for 5 min to disrupt the antigen-antibody complexes, and allowed to cool to room temperature. The staphylococci were removed by centrifugation and the radioactivity (radioiodinated heme-hemopexin) in the supernatant was measured by use of a gamma counter.
The rat polyclonal CS antiserum immunoprecipitated much more radioiodinated heme-hemopexin (i.e., in the form of HxuA–heme-hemopexin complexes) than did control serum when CS from the wild-type Hib strain DL42 was used in the RIP (Table 2). This same CS antiserum did not immunoprecipitate more radioiodinated heme-hemopexin than did control serum when CS from the hxuA mutant DL42.107d (which does not express HxuA) was used in the RIP. The latter result indicated that when soluble HxuA was not present in the CS, the amount of radioiodinated heme-hemopexin immunoprecipitated by the CS antiserum was no greater than that immunoprecipitated by the control serum.
TABLE 2.
RIP-based detection of the binding of soluble HxuA to heme-hemopexin complexes
| Source of soluble CS proteins |
125I-heme-hemopexin immuno- precipitated (cpm) by:
|
|
|---|---|---|
| Antiserum to CS proteinsa | Control serumb | |
| Hib DL42 | 206,892 | 21,892 |
| Hib DL42.107d | 15,208 | 21,880 |
| E. coli RR1(pBR322) | 7,906 | 20,306 |
| E. coli RR1(pHX1-6) | 177,312 | 19,766 |
Rat antiserum raised against soluble CS proteins from Hib strain DL26 (16). The HxuA proteins expressed by Hib strains DL26 and DL42 are antigenically cross-reactive.
Control serum from unimmunized rats.
To confirm that HxuA-directed antibodies in the CS antiserum were actually immunoprecipitating soluble HxuA complexed with heme-hemopexin, additional RIP analysis was performed with CS from E. coli strains containing either the plasmid vector pBR322 or the recombinant plasmid pHX1-6 with the Hib DL42 hxuCBA gene cluster (17). The latter recombinant E. coli strain released soluble HxuA protein into the CS (4). Again, the antiserum against CS proteins immunoprecipitated a much greater amount of radioiodinated heme-hemopexin than did the control serum when both sera were tested against CS containing recombinant HxuA protein [i.e., CS from E. coli RR1(pHX1-6)] (Table 2). When the E. coli CS lacked HxuA protein [i.e., CS from E. coli RR1(pBR322)], the amount of radioiodinated heme-hemopexin immunoprecipitated by the CS antiserum was no greater than that immunoprecipitated by the control serum. Taken together, the RIP results indicated that soluble HxuA can bind heme-hemopexin complexes in solution.
We next performed a more stringent test designed to determine whether soluble HxuA can facilitate the acquisition of heme from heme-hemopexin by H. influenzae. This set of experiments initially involved measurement of the growth responses of the isogenic Hib DL42.61 hxuA mutant (i.e., a mutant that cannot utilize heme-hemopexin) (5) to the addition of various heme sources. Assessment of the ability of H. influenzae strains to utilize soluble complexes of HxuA bound to heme-hemopexin was accomplished with broth culture. Several H. influenzae colonies from a BHIs agar plate incubated overnight were inoculated into a 16- by 150-mm culture tube containing 10 ml of BHI supplemented with only NAD (10 μg/ml) (basal medium) which was then placed in an ice-water slurry in a large bucket. The bucket was placed in an environmental room at 37°C for approximately 16 h; the use of the ice slurry prevented growth from starting for several hours. The next morning, a 1-ml portion of this culture was inoculated into 10 ml of the basal medium in a 500-ml culture flask, which was then incubated at 37°C with agitation for 4 to 6 h; the incubation was necessary to eliminate all heme stores from the H. influenzae cells. The culture was then diluted 1:2,000 in this same medium, and 13 μl of this diluted suspension, containing 103 CFU, was inoculated into a 12- by 75-mm culture tube containing 1.3 ml of broth medium supplemented with various compounds.
Five different media were tested in these experiments: (i) 1.3 ml of basal medium (negative control), (ii) 1.3 ml of basal medium containing heme (10 μg/ml) (positive control), (iii) 1.3 ml of basal medium containing heme-hemopexin (10 μg/ml), (iv) 1.0 ml of basal medium and 0.3 ml of CS (i.e., source of HxuA), and (v) 1.0 ml of basal medium containing 0.3 ml of CS and heme-hemopexin (10 μg/ml). The final concentration of HxuA protein in the last two media was 3 μg/ml. These cultures were incubated at 37°C in a stationary water bath, and duplicate 100-μl samples of each culture were removed at the start of the experiment and plated onto BHIs agar. Additional 10- or 100-μl duplicate samples were removed from each culture at 2-h intervals for the duration of the experiment and either plated directly onto BHIs agar or diluted in BHIs before being plated.
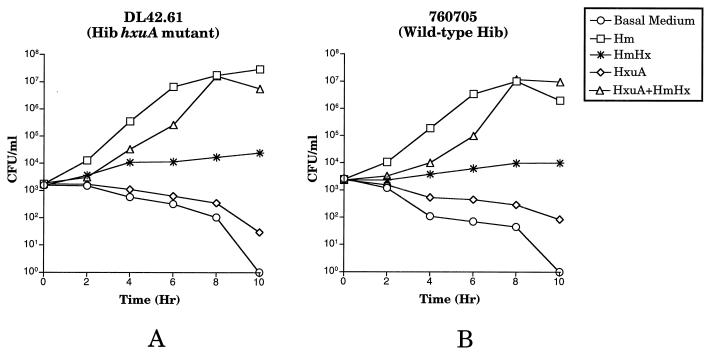
Heme-starved cells of the wild-type Hib strain DL42 were unable to grow when they were inoculated into basal medium alone and also could not grow in basal medium supplemented only with HxuA (Fig. 2A). The wild-type Hib strain utilized free heme for growth and also grew readily when it was supplemented with heme-hemopexin in the presence or absence of exogenous HxuA (Fig. 2A). Growth of the wild-type Hib strain with heme-hemopexin in the absence of exogenous HxuA was expected because this Hib strain expresses its own HxuA protein (4). Also as expected, heme-starved cells of the hxuA mutant DL42.61 grew when they were inoculated into basal medium containing free heme and did not grow in its absence (Fig. 2B). When it was supplemented with HxuA only, the hxuA mutant was unable to grow, and when it was supplemented with heme-hemopexin only, the hxuA mutant did not grow (Fig. 2B). However, when exogenous soluble HxuA was present together with heme-hemopexin, the hxuA mutant was able to grow and, after 10 h, increase its numbers nearly 3 orders of magnitude above the number of viable mutant cells present in the presence of heme-hemopexin alone (Fig. 2B).
FIG. 2.
Effect of exogenous Hib HxuA on the ability of wild-type and mutant Hib strains to utilize heme-hemopexin. Heme-starved cells of the wild-type strain DL42 (A) and the hxuA mutant DL42.61 (B) were inoculated into BHI-NAD broth (basal medium) supplemented with the following compounds at the concentrations listed in the text: no supplement (negative control) (circles), heme (Hm) (positive control) (squares), heme-hemopexin (HmHx) (asterisks), Hib DL42 CS (i.e., HxuA) (diamonds), and Hib DL42 CS (i.e., HxuA) and heme-hemopexin (triangles). Growth was monitored by plating samples from each culture on BHIs agar plates at timed intervals. The data points in each graph are the means of results from two separate experiments; error bars are included.
These results indicated that the exogenously supplied, soluble Hib HxuA protein bound heme-hemopexin complexes and allowed utilization of this heme by the Hib hxuA mutant. We next investigated whether exogenously supplied HxuA protein that was produced by an NTHI strain would allow Hib strains to utilize heme-hemopexin. NTHI strains have previously been shown to secrete soluble HxuA protein into CS in proportionally greater quantities than do Hib strains, and the amino acid sequences of Hib and NTHI HxuA proteins are not identical (4). Two different Hib strains were used in these experiments: the Hib hxuA mutant and the wild-type Hib strain 760705, which was previously shown to be unable to utilize heme-hemopexin and to lack expression of an HxuA protein (4). CS (i.e., the source of HxuA) from wild-type NTHI N182 was prepared as described above. When it was supplemented with NTHI HxuA, the Hib hxuA mutant was able to utilize heme-hemopexin for growth (Fig. 3A). As with the results obtained with Hib HxuA (Fig. 2B), at the end of this experiment, there was at least 100-fold more CFU in the cultures containing both heme-hemopexin and NTHI HxuA than in the culture containing only heme-hemopexin (Fig. 3A). Finally, the wild-type Hib strain 760705, which was unable to grow in the presence of heme-hemopexin alone, grew readily when both heme-hemopexin and NTHI HxuA were present (Fig. 3B).
FIG. 3.
Effect of exogenous NTHI HxuA on the ability of wild-type and mutant Hib strains to utilize heme-hemopexin. Heme-starved cells of the Hib hxuA mutant DL42.61 (A) and the wild-type Hib strain 760705 (B) were inoculated into BHI-NAD broth (basal medium) supplemented with the following compounds: no supplement (negative control) (circles), heme (Hm) (positive control) (squares), heme-hemopexin (HmHx) (asterisks), NTHI N182 CS (i.e., HxuA) (diamonds), and NTHI N182 CS (i.e., HxuA) and heme-hemopexin (triangles). Growth was monitored by plating samples from each culture on BHIs agar plates at timed intervals.
The fact that the hxuA mutant (Fig. 2B and 3A) and the wild-type strain 760705 (Fig. 3B) did not die off rapidly in the presence of heme-hemopexin alone can probably be attributed to the presence of tiny amounts of free heme present in the heme-hemopexin preparation. In fact, we have previously shown that H. influenzae can grow in BHI-NAD broth containing heme at a concentration of less than 100 ng/ml (5). Alternatively, it has been proposed that H. influenzae may possess a means for obtaining heme from heme-hemopexin complexes that does not involve HxuA (50). One laboratory has reported that the HxuA protein is not essential for the utilization of heme-hemopexin by H. influenzae (49, 50). With the wild-type Hib strain 760705 (which is unable to express the HxuA protein [4]), three H. influenzae proteins that apparently bound heme-hemopexin in affinity chromatography experiments were identified (50). However, in our hands, Hib 760705 could not utilize heme-hemopexin as a source of heme for growth on solidified medium (4). Because the studies from the other laboratory (50) involved only affinity assays and no mutant analysis was reported, it is difficult to determine the relevance of this possible alternative uptake system. Regardless, if an alternative, HxuA-independent mechanism does exist for utilization of heme-hemopexin by H. influenzae, it can be inferred from the present experiments (Fig. 3) that this alternative method is rather inefficient relative to that involving HxuA.
Taken together, these results indicate not only that the soluble HxuA protein can bind heme-hemopexin complexes but also that these HxuA–heme-hemopexin complexes are then made available to H. influenzae cells for utilization of the heme moiety. To the best of our knowledge, this is the first example of a secreted protein, expressed by a pathogen, that has been shown to interact with a heme-binding serum protein for a functional purpose. However, free heme and the heme in hemoglobin are bound by the soluble, extracellular HasA protein from Serratia marcescens (30). The 19-kDa HasA protein (22) is necessary for the utilization of heme iron by S. marcescens (30) and belongs to the family of secreted proteins that lack an N-terminal signal peptide (29). HasA has been shown to be secreted by an ABC transporter (29). In addition, it was recent ly found that HasA functions synergistically with HasR, an iron-regulated 98-kDa outer membrane protein from S. marcescens, to facilitate heme acquisition by an E. coli hemA mutant (12). Whether the extracellular HasA protein can bind the heme contained in serum-derived heme carrier proteins, including heme-hemopexin and hemoglobin-haptoglobin, remains to be determined.
The fact that soluble HxuA can bind heme-hemopexin and make it available to the H. influenzae hxuA mutant for utilization of the heme moiety allows us to hypothesize the existence of an H. influenzae surface receptor for the HxuA–heme-hemopexin complex. Moreover, this receptor cannot be an outer-membrane-bound form of the HxuA protein because the hxuA mutant can utilize HxuA–heme-hemopexin complexes. One possible candidate for this receptor is the HxuC outer membrane protein, which has been shown to be essential for the utilization of low levels of free heme by Hib DL42 (5). The apparent polar nature of the hxuC mutation in the hxuC mutant constructed in a previous study by our laboratory (5) precluded direct investigation of this possibility. Identification of a possible receptor for HxuA–heme-hemopexin complexes will be the subject of future research efforts in our laboratory.
Acknowledgments
This study was supported by U.S. Public Health Service grant AI17621 to E.J.H.
We thank Ursula Muller-Eberhard for her interest in these studies and for very helpful discussions.
REFERENCES
- 1.Alexander H E. The Haemophilus group. In: Dubos R J, Hirsch J G, editors. Bacterial and mycotic infections of man. J. B. Philadelphia, Pa: Lippincott Co.; 1965. pp. 724–741. [Google Scholar]
- 2.Bricker J, Mulks M H, Plaut A G, Moxon E R, Wright A. IgA1 proteases of Haemophilus influenzae: cloning and characterization in Escherichia coli K-12. Proc Natl Acad Sci USA. 1983;80:2681–2685. doi: 10.1073/pnas.80.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C-J, Sparling P F, Lewis L A, Dyer D W, Elkins C. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun. 1996;64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cope L D, Thomas S E, Latimer J L, Slaughter C A, Muller-Eberhard U, Hansen E J. The 100 kDa heme:hemopexin-binding protein of Haemophilus influenzae: structure and localization. Mol Microbiol. 1994;13:863–873. doi: 10.1111/j.1365-2958.1994.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 5.Cope L D, Yogev R, Muller-Eberhard U, Hansen E J. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzae type b. J Bacteriol. 1995;177:2644–2653. doi: 10.1128/jb.177.10.2644-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulton J W, Pang J C S. Transport of hemin by Haemophilus influenzae type b. Curr Microbiol. 1983;9:93–98. [Google Scholar]
- 7.Daskaleros P A, Stoebner J A, Payne S M. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect Immun. 1991;59:2706–2711. doi: 10.1128/iai.59.8.2706-2711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkins C, Chen C-J, Thomas C E. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect Immun. 1995;63:2194–2200. doi: 10.1128/iai.63.6.2194-2200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans N M, Smith D D, Wicken A J. Haemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae and Haemophilus parainfluenzae. J Med Microbiol. 1974;7:359–365. doi: 10.1099/00222615-7-3-359. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback R C, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Frichman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Frangipane M E, Morton D J, Wooten J A, Pozsgay J M, Stull T L. Binding of human hemoglobin by Haemophilus influenzae. FEMS Microbiol Lett. 1994;118:243–248. doi: 10.1111/j.1574-6968.1994.tb06835.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghigo J-M, Letoffe S, Wandersman C. A new type of hemophore-dependant heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J Bacteriol. 1997;179:3572–3579. doi: 10.1128/jb.179.11.3572-3579.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granick S, Gilder H. The porphyrin requirements of Haemophilus influenzae and some functions of the vinyl and propionic acid side chains of heme. J Gen Physiol. 1946;30:1–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy F J, Plaut A, Wright A. Haemophilus influenzae immunoglobulin A1 protease genes: cloning by plasmid integration-excision, comparative analyses, and localization of secretion determinants. J Bacteriol. 1987;169:4442–4450. doi: 10.1128/jb.169.10.4442-4450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulig P A, McCracken G H, Jr, Frisch C F, Johnston K H, Hansen E J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982;37:82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulig P A, McCracken G H, Jr, Holmans P L, Hansen E J. Immunogenic proteins in cell-free culture supernatants of Haemophilus influenzae type b. Infect Immun. 1984;44:41–48. doi: 10.1128/iai.44.1.41-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson M S, Pelzel S E, Latimer J L, Muller-Eberhard U, Hansen E J. Identification of a genetic locus of Haemophilus influenzae type b necessary for the binding and utilization of heme bound to human hemopexin. Proc Natl Acad Sci USA. 1992;89:1973–1977. doi: 10.1073/pnas.89.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson D P, Payne S M. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J Bacteriol. 1994;176:3269–3277. doi: 10.1128/jb.176.11.3269-3277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinnebusch B J, Perry R D, Schwan T G. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague of fleas. Science. 1996;273:367–370. doi: 10.1126/science.273.5273.367. [DOI] [PubMed] [Google Scholar]
- 20.Hornung J M, Jones H A, Perry R D. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem-protein complexes as iron sources. Mol Microbiol. 1996;20:725–739. doi: 10.1111/j.1365-2958.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 21.Hrkal Z, Cabart P, Kalousek I. Isolation of human haemopexin in apo-form by chromatography on S-Sepharose fast flow and blue Sepharose CL-6B. Biomed Chromatogr. 1992;6:212–214. doi: 10.1002/bmc.1130060412. [DOI] [PubMed] [Google Scholar]
- 22.Izadi N, Henry Y, Goldberg M E, Wandersman C, Delepierre M, Lecroisey A. Purification and characterization of an extracellular heme-binding protein, HasA, involved in heme iron acquisition. Biochemistry. 1997;36:7050–7057. doi: 10.1021/bi962577s. [DOI] [PubMed] [Google Scholar]
- 23.Jarosik G P, Sanders J D, Cope L D, Muller-Eberhard U, Hansen E J. A functional tonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Ren Z, Pozsgay J M, Elkins C, Whitby P W, Morton D J, Stull T L. Cloning of a DNA fragment encoding a heme-repressible hemoglobin-binding outer membrane protein from Haemophilus influenzae. Infect Immun. 1996;64:3134–3141. doi: 10.1128/iai.64.8.3134-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koskelo P, Muller-Eberhard U. Interaction of porphyrins with proteins. Semin Hematol. 1977;14:221–226. [PubMed] [Google Scholar]
- 26.Lee B C. Isolation of an outer membrane hemin-binding protein of Haemophilus influenzae type b. Infect Immun. 1992;60:810–816. doi: 10.1128/iai.60.3.810-816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B C. Isolation and characterization of the haemin-binding proteins from Neisseria meningitidis. Microbiology. 1994;140:1473–1480. doi: 10.1099/00221287-140-6-1473. [DOI] [PubMed] [Google Scholar]
- 28.Lee B C. Quelling the red menace: haem capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 29.Letoffe S, Ghigo J-M, Wandersman C. Secretion of the Serratia marcescens HasA protein by an ABC transporter. J Bacteriol. 1994;176:5372–5377. doi: 10.1128/jb.176.17.5372-5377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letoffe S, Wandersman C. Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc Natl Acad Sci USA. 1994;91:9876–9880. doi: 10.1073/pnas.91.21.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis L A, Dyer D W. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J Bacteriol. 1995;177:1299–1306. doi: 10.1128/jb.177.5.1299-1306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis L A, Gray E, Wang Y-P, Roe B A, Dyer D W. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 33.Loeb M R. Ferrochelatase activity and protoporphyrin IX utilization in Haemophilus influenzae. J Bacteriol. 1995;177:3613–3615. doi: 10.1128/jb.177.12.3613-3615.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maciver I, Latimer J L, Liem H H, Muller-Eberhard U, Hrkal Z, Hansen E J. Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infect Immun. 1996;64:3703–3712. doi: 10.1128/iai.64.9.3703-3712.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto B R, Verweij-van Vught A M J J, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 37.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Seery V L, Muller-Eberhard U. Binding of porphyrins to rabbit hemopexin and albumin. J Biol Chem. 1973;248:3796–3800. [PubMed] [Google Scholar]
- 40.Stojiljkovic I, Hantke K. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in Gram-negative bacteria. EMBO J. 1992;11:4359–4367. doi: 10.1002/j.1460-2075.1992.tb05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 42.Stojiljkovic I, Larson J, Hwa V, Anic S, So M. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stojiljkovic I, Srinivasan N. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J Bacteriol. 1997;179:805–812. doi: 10.1128/jb.179.3.805-812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stull T L. Protein sources of heme for Haemophilus influenzae. Infect Immun. 1987;55:148–153. doi: 10.1128/iai.55.1.148-153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatusov R L, Mushegian A R, Bork P, Brown N P, Hayes W S, Borodovsky M, Rudd K E, Koonin E V. Metabolism and evolution of Haemophilus influenzae deduced from a whole-genome comparison with Escherichia coli. Curr Biol. 1996;6:279–291. doi: 10.1016/s0960-9822(02)00478-5. [DOI] [PubMed] [Google Scholar]
- 46.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 47.van Alphen L, Klein M H, Geelen-van der Broek L, Riemens T, Eijk P P, Kamerling J P. Biochemical characterization and worldwide distribution of serologically distinct lipopolysaccharides of Haemophilus influenzae type b. J Infect Dis. 1990;162:659–663. doi: 10.1093/infdis/162.3.659. [DOI] [PubMed] [Google Scholar]
- 48.White D C, Granick S. Hemin biosynthesis in Haemophilus. J Bacteriol. 1963;85:842–850. doi: 10.1128/jb.85.4.842-850.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong J C Y, Holland J, Parsons T, Smith A, Williams P. Identification and characterization of an iron-regulated hemopexin receptor in Haemophilus influenzae type b. Infect Immun. 1994;62:48–59. doi: 10.1128/iai.62.1.48-59.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong J C Y, Patel R, Kendall D, Whitby P W, Smith A, Holland J, Williams P. Affinity, conservation, and surface exposure of hemopexin-binding proteins in Haemophilus influenzae. Infect Immun. 1995;63:2327–2333. doi: 10.1128/iai.63.6.2327-2333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]