Keywords: fibroblast, IPF, lung, MARCH8, TGF-β
Abstract
Interstitial lung diseases can result in poor patient outcomes, especially in idiopathic pulmonary fibrosis (IPF), a severe interstitial lung disease with unknown causes. The lack of treatment options requires further understanding of the pathological process/mediators. Membrane-associated RING-CH 8 (MARCH8) has been implicated in immune function regulation and inflammation, however, its role in the development of pulmonary fibrosis and particularly the fibroblast to myofibroblast transition (FMT) remains a gap in existing knowledge. In this study, we demonstrated decreased MARCH8 expression in patients with IPF compared with non-PF controls and in bleomycin-induced PF. TGF-β dose- and time-dependently decreased MARCH8 expression in normal and IPF human lung fibroblast (HLFs), along with induction of FMT markers α-SMA, collagen type I (Col-1), and fibronectin (FN). Interestingly, overexpression of MARCH8 significantly suppressed TGF-β-induced expression of α-SMA, Col-1, and FN. By contrast, the knockdown of MARCH8 using siRNA upregulated basal expression of α-SMA/Col-1/FN. Moreover, MARCH8 knockdown enhanced TGF-β-induced FMT marker expression. These data clearly show that MARCH8 is a critical “brake” for FMT and potentially affects PF. We further found that TGF-β suppressed MARCH8 mRNA expression and the proteasome inhibitor MG132 failed to block MARCH8 decrease induced by TGF-β. Conversely, TGF-β decreases mRNA levels of MARCH8 in a dose- and time-dependent manner, suggesting the transcriptional regulation of MARCH8 by TGF-β. Mechanistically, MARCH8 overexpression suppressed TGF-β-induced Smad2/3 phosphorylation, which may account for the observed effects. Taken together, this study demonstrated an unrecognized role of MARCH8 in negatively regulating FMT and profibrogenic responses relevant to interstitial lung diseases.
NEW & NOTEWORTHY MARCH8 is an important modulator of inflammation, immunity, and other cellular processes. We found that MARCH8 expression is downregulated in the lungs of patients with idiopathic pulmonary fibrosis (IPF) and experimental models of pulmonary fibrosis. Furthermore, TGF-β1 decreases MARCH8 transcriptionally in human lung fibroblasts (HLFs). MARCH8 overexpression blunts TGF-β1-induced fibroblast to myofibroblast transition while knockdown of MARCH8 drives this profibrotic change in HLFs. The findings support further exploration of MARCH8 as a novel target in IPF.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is the most frequent among interstitial lung diseases and affects ∼3 million people worldwide (1). Although the etiology of IPF remains unknown, many risk factors that contributed to the increased risk of IPF have been identified. Among them include male gender, smoking, and occupational or environmental exposure to dust and pesticides (2). Those affected by IPF experience a median survival of 3–5 yr after diagnosis (3), largely due to the progressive and irreversible feature of this disease and a lack of curative therapeutics. Currently, there is an increasing prevalence of IPF in both the developed and the developing countries (4). The identification of novel targets is therefore an urgent need for developing effective therapeutic strategies.
The pathology of IPF is complex and remains incompletely understood. It involves the injury to the alveolar epithelial cells especially type II cells, inflammatory infiltration, coagulation activation and thrombogenesis, fibroblast proliferation and differentiation, and excessive extracellular matrix production. (5) Among those cell types affected, fibroblasts are of particular interest and are under intense investigation in the context of IPF. For example, activated lung fibroblasts not only proliferate but also undergo a transition from fibroblasts to myofibroblasts (FMT). The latter are critical for the development of pulmonary fibrosis through multiple mechanisms, including extracellular matrix remodeling and the production of cytokines/chemokines to provoke immune responses, among others (6). Work from others and our team have shown multiple layers of regulation on FMT, including the canonical TGF-β/Smad pathway, and PI3K/AKT/GSK3β, NF-κB, p38, RHO/Rac, and β-catenin signaling (7–11). Instead of blocking these pathways, the identification of downstream mediators might provide novel insights for the treatment of this disease.
MARCH8 is a member of the membrane-associated RING-CH-type finger (MARCH) family of E3 ubiquitin ligases that consist of eleven members. It was first identified as a protein ligase, a cellular modulator of immune recognition (c-MIR) since it targets immune recognition-related molecules for degradation. Increasing evidence suggests that MARCH8 has a broad spectrum of targets on membrane-bound proteins, including MHCII, CD86, transferrin receptor, IL-1 receptor accessory protein, DR4, E-cadherin, and, more recently, PD-L1 (12–18). Due to such roles, MARCH8 has been implicated in regulating diverse pathological processes, including apoptosis, inflammation, metabolism, etc. Another vital role of MARCH8 lies in modulating viral infection. The list of viruses that are affected by MARCH8 is expanding, in addition to HIV, HCV, dengue virus, Zika virus, and influenza A virus (19–21). A recent republication has also implicated MARCH8 in the downregulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the culprit for the COVID-19 pandemic (22). However, whether MARCH8 is involved in pulmonary fibrosis, especially the TGF-β signaling, remains a key knowledge gap and was explored in the present study.
In the present study, we reported a previously unrecognized role of MARCH8 as a “brake” for suppressing FMT that must be “released” for the successful differentiation of fibroblasts to myofibroblasts. Downregulation of MARCH8 was observed in lung tissues from patients with IPF and murine models of pulmonary fibrosis. These findings provide novel insights into the precise regulation of FMT and may infer future therapeutic treatment development.
MATERIALS AND METHODS
Reagents and Antibodies
Recombinant human transforming growth factor β1 (noted as TGF-β if not specified, 7754-BH-025/CF) was purchased from R&D Systems (Minneapolis, MN). Small molecular inhibitors including actinomycin D (Act D, HY-17559), LY294002 (HY-10108), MG132 (HY-13259), SIS3 (HY-13013), SB230580 (HY-10256), and U0126 (HY-12031A) were purchased from MedChemExpress (Monmouth Junction, NJ). MARCH8 (14119-1-AP) and GAPDH (clone 1E6D9) antibodies were purchased from Proteintech Group, Inc (Rosemont, IL). Other primary antibodies used in this study include α-SMA (clone 1A4), β-actin (A1978), and actin (A2066) from Sigma (St. Louis, MO); collagen type I (1310-08) from SouthernBiotech (Birmingham, AL); fibronectin (ab2413) from Abcam (Waltham, MA); and phosphor-Smad3 (p-Smad3, 9520S), p-Smad2 (3108S), Smad3 (9523S), and Smad2 (5339S) from Cell Signaling Technology (Danvers, MA). These antibodies have been validated by the vendor and published previously (7, 8, 23–25).
Human Lung Tissues
The lung specimens from patients with IPF and histologically normal control donors were provided by Dr. Steven Huang at the University of Michigan (Ann Arbor, MI). These biospecimens were granted under a Material Transfer Agreement (MTA). Lung tissue sections from three normal donors and three patients with IPF were provided. The normal lung tissues were obtained from deceased donors provided by Gift of Life Michigan. The patients were clinically diagnosed with IPF by an experienced physician based on clinical features and chest radiography or chest computed tomography (CT) scan. The pathology was verified as usual interstitial pneumonitis (UIP) for all patients with IPF by an experienced pathologist. Written informed consent was obtained from all the subjects. The collection of lung tissues was carried out in accordance with relevant guidelines, and the research protocols were approved by the Michigan Medicine Institutional Review Board (IRB).
Animal Models of Pulmonary Fibrosis
The mouse lung tissues used in this study were collected from experimental pulmonary fibrosis models as reported previously (7, 9). C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor ME). Briefly, male C57BL/6 mice (12–16 wk old) were randomly divided into two groups (n = 5/group). Treatment group mice were instilled once with 1 U/kg bleomycin (Teva) in a volume of ∼40 µL/mouse intratracheally and control group mice received 0.9% saline in the same manner. The mice were monitored and maintained for three weeks following bleomycin treatment (7). The archived mouse lung tissues from the TGF-β1 adenovirus-induced pulmonary fibrosis were reported previously (9). All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at Tyler and performed according to the relevant guidelines.
Immunohistochemical Staining and Collagen Staining
The formalin-fixed and paraffin-embedded (FFPE) lung specimens were used for the immunohistochemical (IHC) staining of MARCH8. Briefly, these FFPE slides were routinely de-waxed and hydrated. The endogenous peroxidase activity was quenched by incubation of the slides with 3% H2O2 in methanol for 15 min, followed by antigen retrieval with EDTA buffer. The primary antibody against MARCH8 (1:100) was applied to these slides (4°C, overnight) immediately following blocking with 10% goat serum for 1 h at room temperature. The next day, the slides were incubated with a secondary antibody (1:100 dilution) for 1 h at room temperature. After washing, these slides were then stained with DAB according to the ABC method. The collagen expression in human and animal lung tissues was determined by the Masson’s Trichrome staining, according to the manufacturer’s instructions and as previously reported (7, 8). The images were taken using a Nikon NiU microscope.
Cell Culture and Treatment
The primary human lung fibroblasts (HLFs) from normal donors and patients with IPF were provided by Dr. Steven Huang at the University of Michigan under an MTA and used as previously reported (24). Briefly, these cells were cultured in a DMEM medium supplemented with 10% FBS and penicillin/streptomycin (100 U/mL). Cells were cultured in a humidified incubator supplied with 5% CO2. The cells were starved overnight in a serum-free medium before treatment with TGF-β. A passage number of less than 10 was used for the experiments.
Plasmids and Transfection
Expression plasmids including HA-vector control (pC-HA) and the human full-length MARCH8 (pC-HA-MARCH8) (21) were provided by Dr. Kenzo Tokunaga (National Institutes of Infectious Disease, Tokyo, Japan) under an MTA. HLFs were seeded onto 6-cm dishes to reach ∼80% confluency before transfection with control empty vector or MARCH8 using the transfection reagent jetPRIME from Polyplus-transfection (New York, NY) as previously reported (17).
Knockdown of MARCH8 Using Small Interfering RNA
Cells were seeded on 6-cm dishes to reach ∼60% confluency before transfection with scramble control (Cat. No. SIC001, Sigma) or siRNA targeting human MARCH8 (siMARCH8, Cat. No. SASI_Hs01_00064950, Sigma). The transfection reagent jetPRIME was used and transfection was conducted similarly as previously reported (7).
Western Blotting
The whole cell lysates were collected from cells for Western blotting analysis. The RIPA buffer containing proteinase and phosphatase inhibitors (1:100, Thermo Fisher Scientific) was used to lyse the cells. The method was similar as previously reported (23, 24). Briefly, 10–15 µg of total protein was denatured in SDS sample buffer by heating at 95°C for 10 min and then resolved by SDS-PAGE, followed by transfer to PVDF membrane. The membranes were then blocked with 5% BSA (for collagen type 1 detection) or 5% skim milk in phosphate-buffered saline with Tween 20 (PBST) (for all other proteins) for 1 h, at room temperature. For the detection of collagen, a secondary streptavidin antibody (016–030-084, 1:3,000) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Other species-specific secondary antibodies (1:3,000) were purchased from Thermo Fisher Scientific (Waltham, MA). Primary antibodies and their dilution factors: MARCH8 (1:1,000), GAPDH (1:8,000), α-SMA (1:3,000), β-actin (1:3,000), Actin (1:3,000), collagen type I (1:2,000), fibronectin (1:1,000), phosphor-Smad3 (1:300), p-Smad2 (1:500), Smad3 (1:1,000), and Smad2 (1:1,000). The protein quantification was done by densitometric analysis using NIH ImageJ software, the band intensity was first normalized to the corresponding loading control, the control group value was set as 1, and the fold changes in treatment groups were generated and plotted.
Mouse lung tissues were collected from the whole left lung lobe, minced into small pieces, and homogenized. After washing with cold PBS twice, they were pelleted by centrifuge at 500 g for 5 min at 4°C. The homogenized lung tissues were briefly sonicated on ice (3 cycles, 10-s on/10-s off, 30% amplitude). Then the tissues were lysed with RIPA buffer containing proteinase and phosphatase inhibitors, as that used for cells. Total protein concentration was determined through the bicinchoninic acid (BCA) assay using a Tecan Infinite M Plex microplate reader. Equal amounts of total proteins from lung tissues (20 μg) were loaded for the detection of MARCH8 (1:200) with Actin (1:1,000) as the loading control.
Quantitative Real-Time PCR Analysis
Total RNA was extracted using a TRIzol reagent following the manufacturer’s guide. The total RNA (1 µg) from each treatment was then reversely transcribed into cDNA template using a reverse transcription kit from BioRad (Hercules, CA). SYBR green reagent was used for quantitative real-time PCR (qPCR) analysis of the target gene MARCH8 with GAPDH as an internal control. The primers have been reported previously (17, 26) and were listed in Supplemental Table S1.
Cycloheximide Chase Assay
The cycloheximide (CHX) chase assay was used to compare protein degradation rates as reported previously (27). Serum-starved primary HLFs were treated with PBS or TGF-β (5 ng/mL) overnight, then the cells were given 10 μg/mL CHX, followed by whole cell lysates harvest at various times for Western blotting analysis of MARCH8. Band intensities were quantified using the NIH ImageJ software. For each group, the levels of MARCH8 protein after adjusting by internal reference GAPDH were presented as a percentage of the levels at 0-time post-CHX treatment.
Statistical Analysis
The data were presented as means ± SD. The comparisons between the two groups were performed using an unpaired Student’s t test. Comparisons among different groups were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test. The statistical tests were done with the GraphPad Prism 9 software. A P value of less than 0.05 was considered statistically significant.
RESULTS
MARCH8 Expression is Downregulated in Patients with IPF
We first determined whether MARCH8 expression is altered in patients with IPF. Representative Masson’s Trichrome staining showed that IPF lungs exhibit a dramatic deposition of collagen in contrast to the minimal collagen staining in the histologically normal donors, n = 3 donors/group. (Fig. 1A). Immunohistochemical (IHC) staining demonstrated a markedly decreased expression of MARCH8 in the lungs of patients with IPF compared with that of controls (Fig. 1B). Quantification analysis using the reciprocal intensity method (8, 28, 29) showed significantly decreased MARCH8 expression in patients with IPF (Fig. 1C). The data suggest that downregulation of MARCH8 might be involved in the development of IPF.
Figure 1.

MARCH8 expression is downregulated in patients with idiopathic pulmonary fibrosis (IPF). A: representative Masson’s trichrome staining of lung tissues from histologically normal donors and patients with IPF showing increased collagen deposition (blue) in patients with IPF compared with that of controls. B: immunohistochemical (IHC) staining of MARCH8 showed that compared with the notable expression of MARCH8 in the control lung tissue, MARCH8 expression was much decreased. Scale bars represent 100 μm, images were taken at the same magnification. Boxes show enlarged views of the areas of interest. C: reciprocal intensity method was used for the quantification of MARCH8 staining as in B from 10 views per slide/donor, n = 3 donors/group; a.u., arbitrary units. *P < 0.05 compared with normal control, tested by unpaired Student’s t test.
MARCH8 Expression is Elevated in Bleomycin and TGF-β Induced Pulmonary Fibrosis
We further examined the expression of MARCH8 in the bleomycin model, a classical model of experimental PF (30). In accord with the finding in human patients with IPF, MARCH8 expression was found notably decreased in the lung tissues of mice challenged with bleomycin for 21 days compared with that of the saline control group (Fig. 2A). Quantification using the reciprocal method demonstrated significantly lower levels of lung MARCH8 in the bleomycin group compared with that in the saline control (Fig. 2B). We further confirmed the findings using another pulmonary fibrosis model induced by intratracheal TGF-β1 adenovirus administration (14 days) (9). Consistently, while MARCH8 expression in the lungs of mice in the control adenovirus group was remarkable, its expression in the TGF-β adenovirus-treated mice was notably and significantly blunted (Fig. 2, C and D). Furthermore, the protein expression of MARCH8 was found to be significantly decreased in the lung tissues of mice challenged with bleomycin compared with that of saline control (Fig. 2, E and F). Together, these data suggest that the downregulation of MARCH8 might be a common pathological event in the development of pulmonary fibrosis, thus providing an opportunity for intervention.
Figure 2.
MARCH8 expression is decreased in preclinical models of pulmonary fibrosis. A: representative immunohistochemical (IHC) staining of MARCH8 in the lungs of C57BL/6 mice received intratracheal instillation of saline or bleomycin for 21 days. Black boxes indicate the enlarged view of areas of interest. Bar represents 100 µm. B: reciprocal intensity method was used for the quantification of MARCH8 as in a form of 10 views per slide/mouse, n = 5 mice/group. *P < 0.05 compared with saline group, tested by unpaired Student’s t test; a.u., arbitrary units. C: representative IHC images of the lung tissues from C57BL/6 mice administered adenoviral control (control) or TGF-β1 adenovirus (TGF-β) intratracheally for 14 days. Black boxes indicate the enlarged view of areas of interest. Bar represents 100 µm. D: reciprocal intensity method was used for the quantification of MARCH8 as in C from 10 views per slide/mouse, n = 5 mice/group. *P < 0.05 compared with adenoviral control group, tested by unpaired Student’s t test; a.u., arbitrary units. E: lung tissues were collected from saline and Bleomycin treated mice for Western blot analysis of MARCH8. Actin was used as the loading control. n = 4 mice/group. F: ImageJ was used to quantify the band density of MARCH8 as shown in E and adjusted to the corresponding loading control. The relative values were used to generate the graph. *P < 0.05 compared with saline group, tested by unpaired Student’s t test. n = 4 mice/group.
TGF-β Suppressed MARCH8 Expression along with FMT Induction
Lung fibroblasts are a crucial cell type that contribute to the development of IPF through proliferation and differentiation into myofibroblasts. To determine whether MARCH8 is involved in FMT, we first tested if the expression of MARCH8 is altered in primary HLFs. We found that TGF-β suppressed MARCH8 expression in a dose-dependent manner in both primary normal (Fig. 3A) and IPF HLFs (Fig. 3C) along with the induction of α-SMA, Col-1, and FN. Reproducible results were observed, and a significant decrease in MARCH8 was observed (Fig. 3, B and D). Time-dependent reduction of MARCH8 was also found in both the primary normal (Fig. 3E) and IPF (Fig. 3F) HLFs along with induction of FMT markers. These findings were confirmed with an additional line of primary normal HLFs (Supplemental Fig. S1). Together, these data indicate that MARCH8 is suppressed by TGF-β during the process of FMT, suggesting a potential negative regulation of MARCH8 with regards to FMT.
Figure 3.
MARCH8 expression was decreased by transforming growth factor-beta (TGF-β) in primary normal (A, B, E) and idiopathic pulmonary fibrosis (IPF; C, D, F) human lung fibroblasts (HLFs). A: serum-starved primary normal HLFs were treated with different doses of TGF-β for 24 h, followed by Western blotting (WB) analysis of MARCH8 and fibroblast to myofibroblast transition (FMT) markers including alpha-smooth muscle actin (α-SMA), fibronectin (FN), and collagen type I (Col-1). GAPDH was used as the loading control. B: quantification of A based on 3 independent experiments. C: serum-starved primary IPF HLFs were treated with different doses of TGF-β, followed by WB analysis of MARCH8 and FMT markers. D: the quantification of C based on 3 independent experiments. E and F: serum-starved primary normal (E) and IPF (F) HLFs were treated with 5 ng/mL TGF-β for different times as indicated, followed by WB analysis of given proteins. NIH ImageJ software was used to quantify the band intensity. *P < 0.05 compared with vehicle control, n = 3 independent experiments. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons.
Overexpression of MARCH8 Attenuates TGF-β-Induced FMT
Next, we sought to answer the question of whether MARCH8 downregulation is required in TGF-β-induced FMT. We transfected normal HLFs with control vector or MARCH8 overexpression plasmids, followed by treatment with TGF-β (5 ng/mL) for an additional 24 h. Intriguingly, MARCH8 overexpression successfully blocked TGF-β-induced expression of those FMT markers α-SMA, Col-1, and FN (Fig. 4B). Independently repeated experiments showed consistent findings (Fig. 4C) and significant differences in FMT markers were found in MARCH8 overexpression group compared with control vector group that received TGF-β treatment. Similar effects were found using an additional line of primary normal HLFs (Supplemental Fig. S2). The results support an essential and negative role of MARCH8 in FMT.
Figure 4.
Overexpression of MARCH8 attenuates transforming growth factor-beta (TGF-β)-induced fibroblast to myofibroblast transition (FMT) in primary normal human lung fibroblasts (HLFs). A: primary HLFs were transfected with empty vector control (EV) or MARCH8 plasmid for 48 h and MARCH8 was detected by Western blotting (WB) with GAPDH as the loading control. B: primary HLFs were transfected with EV or MARCH8 plasmid, followed by treatment with TGF-β (5 ng/mL) for an additional 24 h. Whole cell lysates were collected for WB analysis of MARCH8 and FMT markers (α-SMA, Col-1, and FN). GAPDH was used as the loading control. C: quantification of MARCH8 and FMT markers as in B, based on three independent experiments. NIH ImageJ software was used to quantify the band intensity. *P < 0.05 vs. control EV group; #P < 0.05 vs. TGF-β-treated EV control, n = 3 independent experiments. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons. α-SMA, alpha-smooth muscle actin; Col-1, collagen 1A1; FN, fibronectin.
Knockdown of MARCH8 Enhances TGF-β-Induced FMT Marker Expression
Knowing that the ectopic MARCH8 expression blocks TGF-β-induced FMT, we are interested in whether it is also true and vice versa. Indeed, we found that MARCH8 knockdown using siRNA induces the expression of FMT markers including α-SMA, Col-1, and FN in primary normal HLFs (Fig. 5A) and a significant difference was observed based on three independent experiments (Fig. 5B). Furthermore, knockdown of MARCH8 was found to enhance TGF-β-induced FMT markers expression (Fig. 5C) and significant increase of these FMT markers was found in MARCH8 knockdown plus TGF-β treatment group compared with TGF-β treatment alone group (Fig. 5D). Similar results were found using an additional line of primary normal HLFs (Supplemental Fig. S3). These data provide proof of concept that MARCH8 downregulation is not only required but also a driver for FMT.
Figure 5.
Inhibition of MARCH8 promotes fibroblast to myofibroblast transition (FMT) in primary normal human lung fibroblasts (HLFs). A: primary normal HLFs were transfected with scramble or siRNA targeting MARCH8 (siMARCH8) and whole cell lysates were collected 48 h after transfection for Western blotting (WB) analysis of MARCH8 and FMT markers (α-SMA, Col-1, and FN). β-actin was used as the loading control. B: quantification of MARCH8 and FMT markers as in A, based on three independent experiments. *P < 0.05 vs. scramble group based on three independent experiments tested by unpaired Student’s t test. C: primary normal HLFs were transfected with scramble or siMARCH8, followed by treatment with TGF-β (5 ng/mL) for an additional 24 h. Whole cell lysates were collected for WB analysis of MARCH8 and FMT markers. D: quantification of MARCH8 and FMT markers as in C, based on three independent experiments. NIH ImageJ software was used to quantify the band intensity. *P < 0.05 vs. scramble control; #P < 0.05 vs. TGF-β-treated scramble group, n = 3 independent experiments. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons. α-SMA, alpha-smooth muscle actin; Col-1, collagen 1A1; FN, fibronectin.
MARCH8 Overexpression Attenuates Smad Signaling in Primary Normal HLFs
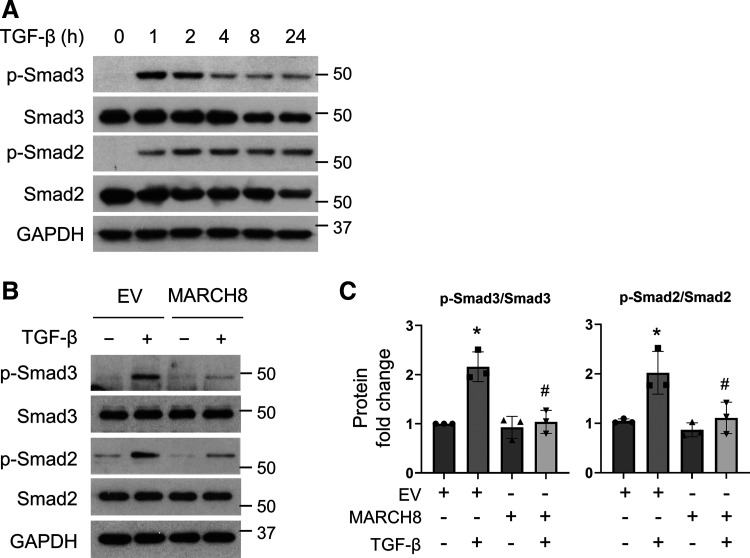
To answer the question how MARCH8 is implicated in TGF-β-induced FMT, we tested whether MARCH8 affects the Smad3/2 signaling, which is critical for driving FMT (31–33). Not surprisingly, Smad3 and Smad2 phosphorylation was induced rapidly as early as 2 h post-TGF-β treatment, which prolongs up to 24 h, as tested (Fig. 6A). Intriguingly, we found that ectopic MARCH8 expression attenuates the activation of Smad3 and Smad2 induced by TGF-β treatment, as shown by inhibited Smad3 and Smad2 phosphorylation (Fig. 6, B and C). Similar effects were observed using an additional line of primary normal HLFs (Supplemental Fig. S4). The results suggest that MARCH8 interferes with Smad signaling to block TGF-β-induced FMT.
Figure 6.
Overexpression of MARCH8 inhibits the activation of Smad3 and Smad2 induced by transforming growth factor-beta (TGF-β). A: serum-starved primary normal human lung fibroblasts (HLFs) were treated with TGF-β (5 ng/mL), and whole cell lysates were collected at various time points to detect phosphor-Smad3 (p-Smad3) and p-Smad2 by Western blotting (WB). GAPDH was used as the loading control. B: primary normal HLFs were transfected with control empty vector (EV) or MARCH8 plasmid (MARCH8), followed by treatment with TGF-β (5 ng/mL) for an additional 2 h. Whole cell lysates were collected for WB analysis of p-Smad3, Smad3, p-Smad2, and Smad2. GAPDH was used as the loading control. C: quantification of the ratios of p-Smad3/Smad3 and p-Smad2/Smad2 as in B, based on three independent experiments. NIH ImageJ software was used to quantify the band intensity. *P < 0.05 vs. EV control group; #P < 0.05 vs. TGF-β-treated EV group, n = 3 independent experiments. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons.
Transcriptional Regulation of MARCH8 by TGF-β
To determine how TGF-β reduces MARCH8 expression in HLFs, we conducted qPCR analysis of mRNA expression of MARCH8 in primary normal HLFs treated with TGF-β. We found that TGF-β dose and time-dependently suppressed MARCH8 mRNA expression (Fig. 7, A and B). Furthermore, we pretreated the primary normal HLFs with the transcriptional inhibitor actinomycin D (Act D) followed by TGF-β treatment (5 ng/mL) for an additional 16 h. We found that Act D successfully blocked TGF-β-induced downregulation of MARCH8 (Fig. 7, C and D), confirming transcriptional repression of MARCH8 by TGF-β. Similar results were found using an additional line of primary normal HLFs (Supplemental Fig. S5). In contrast, the proteasome inhibitor MG132 failed to block TGF-β-induced MARCH8 reduction (Fig. 7, E and F), excluding the proteasome-dependent degradation of MARCH8 by TGF-β in HLFs. The CHX chase assay supports this notion since treatment with TGF-β did not alter protein degradation rates of MARCH8 in primary normal HLFs (Fig. 7, G and H).
Figure 7.
Transforming growth factor-beta (TGF-β) downregulates MARCH8 at the transcriptional level. A-B, qPCR analysis showed that MARCH8 mRNA was reduced by TGF-β in dose-dependent (A, 12 h) and time-dependent (B, 5 ng/mL) manners in primary normal human lung fibroblasts (HLFs). GAPDH was used as the internal control, n = 3 replicates. *P < 0.05 vs. vehicle control group. C: primary normal HLFs were pretreated with actinomycin D (Act D, 1 μg/mL) for 30 min, followed by TGF-β treatment (5 ng/mL, 16 h) for Western blotting (WB) analysis of MARCH8. GAPDH was the loading control. D: the quantification of C based on three independent experiments. *P < 0.05. NS, not significant. E: primary normal HLFs were pretreated with the proteasome inhibitor MG132 (5 μM) for 30 min, followed by TGF-β treatment (5 ng/mL, 16 h) for WB analysis of MARCH8. F: the quantification of E based on three independent experiments. *P < 0.05 based on three independent experiments. G: primary normal HLFs were treated with TGF-β (5 ng/mL, 16 h), followed by 10 μg/mL cycloheximide (CHX) treatment for the indicated times. Whole cell lysates were collected for WB analysis of MARCH8 degradation. H: MARCH8 levels were plotted relative to those at time 0 of CHX treatment (G) after being quantified by NIH ImageJ software and normalized to Actin. Solid line indicates control group and dash line indicates TGF-β group. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons.
TGF-β Downregulation of MARCH8 is p38 MAPK Pathway Dependent
Lastly, we determined which pathway is involved in the downregulation of MARCH8 caused by TGF-β. Different pathways inhibitors were used to pre-treat the primary normal HLFs, followed by TGF-β treatment for an additional 16 h. The results showed that among those pathway inhibitors tested, only p38 MAPK inhibitor SB203580 abolished the TGF-β-induced decrease of MARCH8 in primary normal HLFs (Fig. 8, A and B and Supplemental Fig. S6A). Further experiments confirmed that SB203580 alleviates the downregulation of MARCH8 by TGF-β in a dose-dependent manner in primary normal HLFs (Fig. 8, C and D and Supplemental Fig. S6B). Together, these data indicate that the p38 MAPK pathway is involved in the downregulation of MARCH8 by TGF-β.
Figure 8.
MARCH8 downregulation by transforming growth factor-beta (TGF-β) is p38 MAPK pathway dependent. A: primary normal HLFs were pretreated with various inhibitors (10 μM) of Smad3 (SIS3), AKT (Ly294002), ERK (U0126), JNK (SP600125), and p38 MAPK (SB203580) for 30 min, followed by TGF-β treatment (5 ng/mL) for an additional 16 h. Whole cell lysates were collected for Western blotting (WB) analysis of MARCH8 with GAPDH as a loading control. B: the quantification of MARCH8 as in A, based on three independent experiments. *P < 0.05 vs. TGF-β alone group, n = 3 independent experiments. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons. C: primary normal human lung fibroblasts (HLFs) were pretreated with various concentrations of SB203580 for 30 min, followed by treatment with 5 ng/mL TGF-β for an additional 16 h. Whole cell lysates were collected for WB analysis of MARCH8 with GAPDH as a loading control. D: the quantification of MARCH8 as shown in C, based on three independent experiments. NIH ImageJ software was used to quantify the band intensity. *P < 0.05 vs. TGF-β alone group, n = 3 independent experiments. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons.
DISCUSSION
In the current study, we identify MARCH8 as a novel regulator of myofibroblast differentiation. MARCH8 expression is found to be downregulated in IPF lung tissues and murine models of pulmonary fibrosis. We show that TGF-β downregulates MARCH8, which is a prerequisite for successful myofibroblast differentiation. Conversely, suppression of MARCH8 enhances TGF-β-induced myofibroblast differentiation. These findings demonstrate a new layer of control of FMT by MARCH8 and provide novel insights into the pathogenesis of pulmonary fibrosis.
In this study, we demonstrated for the first time the downregulation of MARCH8 in the lungs of patients with IPF as well as in the fibrotic lung tissues of mice challenged with bleomycin or active TGF-β1 (Figs. 1 and 2). Of note is the lower expression of MARCH8 in those fibrotic foci of human and murine lungs compared with the corresponding nonfibrotic controls. These observations show an inverse relationship between MARCH8 expression and pulmonary fibrosis and provide proof of concept that MARCH8 downregulation might be a common and critical event in the pathogenesis of pulmonary fibrosis.
Myofibroblasts constitute the major cell population in the fibrotic foci. Myofibroblast differentiation from fibroblast (FMT) represents an important source for the pool of myofibroblasts. Inhibition of the FMT process has been shown to block or reverse the progression of pulmonary fibrosis by us and others (9, 34). For example, inhibition of GSK3β with the pharmaceutic inhibitor 9-ING-41 reverses FMT induced by TGF-β in vitro and the progression of bleomycin-induced pulmonary fibrosis in C56BL/6 mice (9). TGF-β plays a central role in driving FMT although the precise mechanisms remain incompletely understood. Due to the lower expression of MARCH8 in the fibrotic lungs, we speculate that MARCH8 is likely playing a role in the FMT process. Indeed, we demonstrated the downregulation of MARCH8 by TGF-β in primary HLFs from normal and IPF donors (Fig. 1 and Supplemental Fig. S1). These data are consistent with the findings in animal models and human patients with IPF and support further investigation of MARCH8 in the context of FMT. Generally, TNF-α as a proinflammatory cytokine has been considered to antagonize the profibrotic responses induced by TGF-β although cellular context-dependent effects exist (35–38). Interestingly, a previous study demonstrated that TNF-α induces MARCH8 expression in neurons (39). In this study, we initially identified a negative role of MARCH8 in regulating TGF-β signaling during FMT. In view of this, it would be interesting to test the dynamics of MARCH8 in the progression of pulmonary fibrosis.
Ectopic expression of MARCH8 in primary HLFs effectively attenuates TGF-β-induced FMT (Fig. 4). Conversely, inhibition of MARCH8 using siRNA induces FMT and enhances TGF-β-induced FMT (Fig. 5 and Supplemental Fig. S2). These results suggest that the downregulation of MARCH8 expression contributes to myofibroblast differentiation. Therefore, targeting MARCH8 might eventually emerge as a candidate target for the treatment of pulmonary fibrosis. Due to a lack of agonists and pharmaceutical inhibitors of MARCH8, the utilization of MARCH8 transgenic mouse model and knockout model will provide essential in vivo evidence for targeting MARCH8 in the treatment or prevention of PF. Currently, MARCH8 deficient mice have been generated from several groups. A couple of studies that involve the use of MARCH8 deficient mice mainly focus on viral infection. For instance, MARCH8 deficient mice were shown to be more susceptible to influenza A virus infection (20) but less susceptible to infection caused by herpes simplex virus 1 (40). The underlying mechanisms are attributed to the degradation of viral envelop proteins important for infection and the modulation of innate responses by MARCH8. On the other hand, virus infection is a risk factor for IPF (41, 42). Therefore, MARCH8 may play a role in virus infection-related pulmonary fibrosis, and further investigation is deserved.
Currently, little, if any, is known about the regulation of MARCH8 by TGF-β. In primary HLFs, we show that TGF-β reduces MARCH8 levels at the transcriptional level in a dose- and time-dependent manner (Fig. 3 and Supplemental Fig. S1). Such inhibition was abolished by the pretreatment with a transcription inhibitor Act D but not by the inhibition of proteasome with MG132. In accord, protein stability was not affected by treatment with TGF-β (Fig. 7 and Supplemental Fig. S3). A brief screening with different pathway inhibitors identifies p38 MAPK pathway as a critical mediator of the downregulation of MARCH8 by TGF-β, while largely sparing other pathways including Smad3, AKT, ERK, or the JNK (Fig. 8 and Supplemental Fig. S4). Further study is needed to explore the detailed mechanisms underlying TGF-β induced MARCH8 downregulation.
There are several limitations of the current study. The sample size is small for the comparison of MARCH8 expression between patients with IPF and controls and these two groups of donors are not matched for gender or age. A future study with a large sample size that can adjust for covariates is warranted to determine whether MARCH8 can serve as a marker of IPF. MARCH8 expression has been found in macrophages and dendritic cells (21) that affects viral infection. In addition to fibroblast-derived MARCH8 as reported in this study, the potential implication of MARCH8 expression in other cell types (e.g., macrophage) in the context of pulmonary fibrosis needs to be addressed in future studies. Further investigation using conditional MARCH8 knock-in and knockout mice models is warranted to answer this question. More in-depth mechanistic exploration is needed to elucidate how MARCH8 mediates FMT process, e.g., how MARCH8 affects Smad3/2 phosphorylation and whether other mechanisms are also involved.
In summary, this study reported a previously unrecognized role of MARCH8 in the pathogenesis of PF. MARCH8 seems to act as a “brake” on FMT, which must be released for successful fibroblast differentiation. Findings in this study provide novel insights on the regulation of FMT and suggest MARCH8 may thereby contribute to the pathogenesis of pulmonary fibrosis.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
All supplemental data are available at https://doi.org/10.6084/m9.figshare.21777344.
GRANTS
This work was supported by the National Institutes of Health R56HL163554 (to G.Q.) and R00HL141583 (to X.G.), and the University of Texas Health Science Center at Tyler Seed Grant (to G.Q.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.G. and G.Q. conceived and designed research; X.G., O.A., A.M.O., V.M., C.S., and J.A. performed experiments; X.G. and G.Q. analyzed data; X.G. and G.Q. interpreted results of experiments; X.G. and G.Q. prepared figures; X.G. and G.Q. drafted manuscript; X.G., S.H., T.A.T., S.I., and G.Q. edited and revised manuscript; X.G., O.A., A.M.O., V.M., C.S., J.A., S.H., T.A.T., S.I., and G.Q. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract created with BioRender and published with permission.
REFERENCES
- 1. Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 3: 17074, 2017. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 2. Park Y, Ahn C, Kim TH. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses. Sci Rep 11: 4318, 2021. doi: 10.1038/s41598-021-81591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauleda J, Núñez B, Sala E, Soriano JB. Idiopathic pulmonary fibrosis: epidemiology, natural history, phenotypes. Med Sci (Basel) 6: 110, 2018. doi: 10.3390/medsci6040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179, 2014. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc 5: 334–337, 2008. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo X, Sunil C, Adeyanju O, Parker A, Huang S, Ikebe M, Tucker TA, Idell S, Qian G. PD-L1 mediates lung fibroblast to myofibroblast transition through Smad3 and β-catenin signaling pathways. Sci Rep 12: 3053, 2022. doi: 10.1038/s41598-022-07044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo X, Adeyanju O, Sunil C, Mandlem V, Olajuyin A, Huang S, Chen SY, Idell S, Tucker TA, Qian G. DOCK2 contributes to pulmonary fibrosis by promoting lung fibroblast to myofibroblast transition. Am J Physiol Cell Physiol 323: C133–C144, 2022. doi: 10.1152/ajpcell.00067.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeffers A, Qin W, Owens S, Koenig KB, Komatsu S, Giles FJ, Schmitt DM, Idell S, Tucker TA. Glycogen synthase kinase-3β inhibition with 9-ING-41 attenuates the progression of pulmonary fibrosis. Sci Rep 9: 18925, 2019. doi: 10.1038/s41598-019-55176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 58: 13–19, 2014. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 11. Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep 2: 787–792, 2014. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Rohrscheidt J, Petrozziello E, Nedjic J, Federle C, Krzyzak L, Ploegh HL, Ishido S, Steinkasserer A, Klein L. Thymic CD4 T cell selection requires attenuation of March8-mediated MHCII turnover in cortical epithelial cells through CD83. J Exp Med 213: 1685–1694, 2016. doi: 10.1084/jem.20160316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen R, Li M, Zhang Y, Zhou Q, Shu HB. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1β-induced NF-κB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc Natl Acad Sci USA 109: 14128–14133, 2012. doi: 10.1073/pnas.1205246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van de Kooij B, Verbrugge I, de Vries E, Gijsen M, Montserrat V, Maas C, Neefjes J, Borst J. Ubiquitination by the membrane-associated RING-CH-8 (MARCH-8) ligase controls steady-state cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptor 1. J Biol Chem 288: 6617–6628, 2013. doi: 10.1074/jbc.M112.448209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujita H, Iwabu Y, Tokunaga K, Tanaka Y. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J Cell Sci 126: 2798–2809, 2013. doi: 10.1242/jcs.119909. [DOI] [PubMed] [Google Scholar]
- 16. Kim MH, Rebbert ML, Ro H, Won M, Dawid IB. Cell adhesion in zebrafish embryos is modulated by March 8. PLoS One 9: e94873, 2014. doi: 10.1371/journal.pone.0094873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qian G, Guo J, Vallega KA, Hu C, Chen Z, Deng Y, Wang Q, Fan S, Ramalingam SS, Owonikoko TK, Wei W, Sun SY. Membrane-associated RING-CH 8 functions as a novel PD-L1 E3 ligase to mediate PD-L1 degradation induced by EGFR inhibitors. Mol Cancer Res 19: 1622–1634, 2021. doi: 10.1158/1541-7786.MCR-21-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tze LE, Horikawa K, Domaschenz H, Howard DR, Roots CM, Rigby RJ, Way DA, Ohmura-Hoshino M, Ishido S, Andoniou CE, Degli-Esposti MA, Goodnow CC. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J Exp Med 208: 149–165, 2011. doi: 10.1084/jem.20092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar S, Barouch-Bentov R, Xiao F, Schor S, Pu S, Biquand E, Lu A, Lindenbach BD, Jacob Y, Demeret C, Einav S. MARCH8 ubiquitinates the hepatitis c virus nonstructural 2 protein and mediates viral envelopment. Cell Rep 26: 1800–1814.e5, 2019. doi: 10.1016/j.celrep.2019.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Xu F, Ren L, Zhao F, Huang Y, Wei L, Wang Y, Wang C, Fan Z, Mei S, Song J, Zhao Z, Cen S, Liang C, Wang J, Guo F. MARCH8 inhibits influenza A virus infection by targeting viral M2 protein for ubiquitination-dependent degradation in lysosomes. Nat Commun 12: 4427, 2021. doi: 10.1038/s41467-021-24724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tada T, Zhang Y, Koyama T, Tobiume M, Tsunetsugu-Yokota Y, Yamaoka S, Fujita H, Tokunaga K. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins. Nat Med 21: 1502–1507, 2015. doi: 10.1038/nm.3956. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Ozono S, Tada T, Tobiume M, Kameoka M, Kishigami S, Fujita H, Tokunaga K. MARCH8 targets cytoplasmic lysine residues of various viral envelope glycoproteins. Microbiol Spectr 10: e0061821, 2022. doi: 10.1128/spectrum.00618-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qian G, Adeyanju O, Roy S, Sunil C, Jeffers A, Guo X, Ikebe M, Idell S, Tucker TA. DOCK2 promotes pleural fibrosis by modulating mesothelial to mesenchymal transition. Am J Respir Cell Mol Biol 66: 171–182, 2021. doi: 10.1165/rcmb.2021-0175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian G, Adeyanju O, Sunil C, Huang SK, Chen SY, Tucker TA, Idell S, Guo X. Dedicator of cytokinesis 2 (DOCK2) deficiency attenuates lung injury associated with chronic high-fat and high-fructose diet-induced obesity. Am J Pathol 192: 226–238, 2022. doi: 10.1016/j.ajpath.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qin W, Jeffers A, Owens S, Chauhan P, Komatsu S, Qian G, Guo X, Ikebe M, Idell S, Tucker TA. NOX1 promotes mesothelial-mesenchymal transition through modulation of reactive oxygen species-mediated signaling. Am J Respir Cell Mol Biol 64: 492–503, 2021. doi: 10.1165/rcmb.2020-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qian G, Yao W, Zhang S, Bajpai R, Hall WD, Shanmugam M, Lonial S, Sun SY. Co-inhibition of BET and proteasome enhances ER stress and Bim-dependent apoptosis with augmented cancer therapeutic efficacy. Cancer Lett 435: 44–54, 2018. doi: 10.1016/j.canlet.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 27. Zang H, Qian G, Arbiser J, Owonikoko TK, Ramalingam SS, Fan S, Sun SY. Overcoming acquired resistance of EGFR-mutant NSCLC cells to the third generation EGFR inhibitor, osimertinib, with the natural product honokiol. Mol Oncol 14: 882–895, 2020. doi: 10.1002/1878-0261.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen DH, Zhou T, Shu J, Mao JH. Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes 2, 2013. doi: 10.1038/protex.2013.097. [DOI] [Google Scholar]
- 29. Girard RA, Chauhan PS, Tucker TA, Allen T, Kaur J, Jeffers A, Koenig K, Florova G, Komissarov AA, Gaidenko TA, Chamiso MB, Fowler J, Morris DE, Sarva K, Singh KP, Idell S, Idell RD. Increased expression of plasminogen activator inhibitor-1 (PAI-1) is associated with depression and depressive phenotype in C57Bl/6J mice. Exp Brain Res 237: 3419–3430, 2019. doi: 10.1007/s00221-019-05682-0. [DOI] [PubMed] [Google Scholar]
- 30. Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol 1627: 27–42, 2017. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 31. Gu L, Zhu YJ, Yang X, Guo ZJ, Xu WB, Tian XL. Effect of TGF-beta/Smad signaling pathway on lung myofibroblast differentiation. Acta Pharmacol Sin 28: 382–391, 2007. doi: 10.1111/j.1745-7254.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 32. Harris WT, Kelly DR, Zhou Y, Wang D, MacEwen M, Hagood JS, Clancy JP, Ambalavanan N, Sorscher EJ. Myofibroblast differentiation and enhanced TGF-B signaling in cystic fibrosis lung disease. PLoS One 8: e70196, 2013. [Erratum in PLoS One8: 2013]. doi: 10.1371/journal.pone.0070196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ard S, Reed EB, Smolyaninova LV, Orlov SN, Mutlu GM, Guzy RD, Dulin NO. Sustained Smad2 phosphorylation is required for myofibroblast transformation in response to TGF-β. Am J Respir Cell Mol Biol 60: 367–369, 2019. doi: 10.1165/rcmb.2018-0252LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bollong MJ, Yang B, Vergani N, Beyer BA, Chin EN, Zambaldo C, Wang D, Chatterjee AK, Lairson LL, Schultz PG. Small molecule-mediated inhibition of myofibroblast transdifferentiation for the treatment of fibrosis. Proc Natl Acad Sci USA 114: 4679–4684, 2017. doi: 10.1073/pnas.1702750114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu ZW, Zhang YM, Zhang LY, Zhou T, Li YY, Zhou GC, Miao ZM, Shang M, He JP, Ding N, Liu YQ. Duality of interactions between TGF-β and TNF-α during tumor formation. Front Immunol 12: 810286, 2021. doi: 10.3389/fimmu.2021.810286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Distler JH, Schett G, Gay S, Distler O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheum 58: 2228–2235, 2008. doi: 10.1002/art.23645. [DOI] [PubMed] [Google Scholar]
- 37. Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal 16: 873–880, 2004. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 38. Guo X, Sunil C, Qian G. Obesity and the development of lung fibrosis. Front Pharmacol 12: 812166, 2021. doi: 10.3389/fphar.2021.812166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo S, Zhang Y, Wei C, Shi L, Feng Y. The E3 ubiquitin ligase MARCH8 regulates TNF-α-induced apoptosis in hippocampal neurons by targeting myosin light chain 2 for degradation. Anat Rec (Hoboken) 302: 2271–2278, 2019. doi: 10.1002/ar.24238. [DOI] [PubMed] [Google Scholar]
- 40. Yang X, Shi C, Li H, Shen S, Su C, Yin H. MARCH8 attenuates cGAS-mediated innate immune responses through ubiquitylation. Sci Signal 15: eabk3067, 2022. doi: 10.1126/scisignal.abk3067. [DOI] [PubMed] [Google Scholar]
- 41. Moore BB, Moore TA. Viruses in idiopathic pulmonary fibrosis. Etiology and exacerbation. Ann Am Thorac Soc 12, Suppl 2: S186–S192, 2015. doi: 10.1513/AnnalsATS.201502-088AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang WJ, Tang XX. Virus infection induced pulmonary fibrosis. J Transl Med 19: 496, 2021. doi: 10.1186/s12967-021-03159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All supplemental data are available at https://doi.org/10.6084/m9.figshare.21777344.
Data Availability Statement
Data will be made available upon reasonable request.