Abstract
Simple Summary
Breast cancer (BC) is a heterogeneous disease classified into different subtypes presenting several treatment challenges, especially in more advanced cases arising from triple negative breast cancer. NORAD is a long non-coding RNA (lncRNA) activated by DNA damage, with an impacting role in the repair process of DNA insults. This lncRNA is differentially expressed in BC subtypes, participating in cancer initiation and progression, by interacting with an extended range of signaling partners. Here, we review the network of NORAD molecular interactions with relevance, as well as NORAD’s potential as a prognostic, predictive and target for BC treatment.
Abstract
Long non-coding RNA activated by DNA damage (NORAD) has recently been associated with pathologic mechanisms underlying cancer progression. Due to NORAD’s extended range of interacting partners, there has been contradictory data on its oncogenic or tumor suppressor roles in BC. This review will summarize the function of NORAD in different BC subtypes and how NORAD impacts crucial signaling pathways in this pathology. Through the preferential binding to pumilio (PUM) proteins PUM1 and PUM2, NORAD has been shown to be involved in the control of cell cycle, angiogenesis, mitosis, DNA replication and transcription and protein translation. More recently, NORAD has been associated with PUM-independent roles, accomplished by interacting with other ncRNAs, mRNAs and proteins. The intricate network of NORAD-mediated signaling pathways may provide insights into the potential design of novel unexplored strategies to overcome chemotherapy resistance in BC treatment.
Keywords: lncRNAs, NORAD, pumilio, breast cancer, chemotherapy resistance
1. Introduction
Long non-coding RNAs (lncRNAs) are a class of non-protein coding RNAs longer than 200 nucleotides in length and with limited or no detectable open reading frame (ORF) [1,2]. Despite being considered for decades as “junk RNA” [3], they were found to control transcriptional gene expression, translational and post-translational events [4]. The mechanism behind lncRNA function spans from the modulation of chromatin conformation, interaction with transcription factors, binding to RNA-binding proteins (RBPs) and messenger RNAs (mRNAs) or even acting as competitive endogenous RNA (ceRNA) by sponging microRNAs (miRNAs) [5,6]. Hence, they participate in several biological processes, namely in alternative splicing, epigenetic regulation, RNA decay and protein translation [7]. Most lncRNAs are transcribed by RNA polymerase II, capped at their 5′ end and then spliced and polyadenylated at their 3′ end, similar to mRNA [8,9]. They are generally expressed at low levels, tissue-specific and poorly conserved, and have been shown to influence physiological and pathological conditions, in particular neurological disorders, aging and cancer [6].
Long non-coding RNA activated by DNA damage (NORAD) was first discovered by Lee et al., when exploring the role of lncRNAs in genomic stability regulation [10]. NORAD is a 5.3 kb, highly conserved lncRNA, localized on chromosome 20 (20q11.23) and located in the cytoplasm, accumulating in the nucleus during replication, stress and DNA damage [1,11]. Various studies link NORAD to genomic stability as, in its absence, cells acquire a chromosomal instability (CIN) phenotype and aneuploidy [12]. NORAD’s main mechanism of action is binding to pumilio (PUM) proteins. These proteins repress several mRNA transcripts involved in germline homeostasis, cell cycle control and neuronal activity and function that are required for adequate mitosis, DNA repair and replication [1,10]. Mechanistically, NORAD sequesters PUM proteins, preventing their mRNA targets’ inhibition and leading to chromosomal stability maintenance [10,13]. Apart from PUM proteins, recent advances have highlighted other NORAD-interacting partners [1,11], such as proteins involved in different signaling pathways, particularly STAT, TGF-β, Akt/mTOR and PI3K/Akt pathway and miRNAs [14].
Due to NORAD’s extensive network of binding partners, it has been associated with different pathological conditions including cardiovascular, cerebrovascular and degenerative diseases [15,16,17], but mostly with cancer [1]. Studies suggest that NORAD is dysregulated in numerous cancers, including breast, renal, gastric, bladder, pancreatic, ovarian, cervical, prostate, lung and endometrial cancer [18]. Most of the studies describe NORAD as being overexpressed, leading to cancer cell proliferation, invasion and metastatic behavior [18]. In addition, this lncRNA has been associated with resistance to chemotherapeutic agents used in clinical practice, such as 5-fluorouracil in colorectal cancer, gemcitabine in bladder cancer and doxorubicin in neuroblastoma [19,20,21].
Breast cancer (BC) is the most diagnosed cancer worldwide [22] and represents one of the malignancies in which NORAD expression is altered. BC can be classified into luminal A and B (LumA and LumB, respectively), human epidermal growth factor receptor 2 (HER2)-enriched and triple negative breast cancer (TNBC), according to the expression (or absence) of estrogen, progesterone and HER2 receptors and the level of Ki67 [23]. TNBC can be further stratified into more specific and intrinsic subtypes, such as the basal-like (BL) subtype [24]. BC treatment is decided according to the subtype, and the most common treatments are surgery, radiation, chemotherapy, hormonal therapy (for tumors that express estrogen and progesterone receptors), targeted therapy (especially directed to HER2 in HER2-enriched BC) and immunotherapy [25,26,27]. TNBC, however, has less targeted treatment options and is less responsive to chemotherapy [25,27], with a high number of patients presenting recurrence and metastasis [28]. In the last decades, more personalized and targeted molecular treatments have been developed, such as inhibitors of poly(ADP-ribose) polymerase (PARP), cyclin-dependent kinases (CDK) 4 and 6 and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB)/mammalian target of rapamycin (mTOR) pathway [29].
The role of NORAD in BC progression remains controversial [30]. NORAD is predominantly associated with oncogenic functions, displaying high expression in BC tumors, human cell lines and in the peripheral blood of BC patients [31]. This lncRNA is associated with increased cell proliferation, invasion and migration, tumor growth and size, worst clinical stage, histological grade and lymph node metastasis (LNM), leading to poor prognosis and reduced disease-free survival (DFS) in BC patients [30,31,32,33,34]. It is also correlated positively with metastasis and stemness and negatively associated with DNA repair and inflammation [31]. Additionally, NORAD-silenced cells present reduced invasion, migration, cell viability and colony formation [34], and xenograft BC mouse models established with NORAD-silenced cells present development of smaller tumors [35]. NORAD is also significantly upregulated in BC-derived exosomes, associated with increased m6A methylation [36,37]. In other studies, NORAD is considered a tumor suppressor as its expression levels are lower in BC tissues and cancer cells compared to normal conditions, leading to increased cell proliferation, migration and invasion, LNM development and poor prognosis [38,39]. In this context, NORAD overexpression (OE) in human BC cell lines leads to reduced migration and invasion, while NORAD silencing has the opposite effect [40]. NORAD is described, on one hand, to be more expressed in the LumA subtype as compared to BL [41,42], with the lowest levels in BL [39], and, related to luminal subtypes [31], and, on the other hand, to be more expressed in TNBC compared to LumA [43]. The studies agree, however, that NORAD is differentially expressed in distinct BC subtypes and is related to patient survival in the BL subtype [42,43].
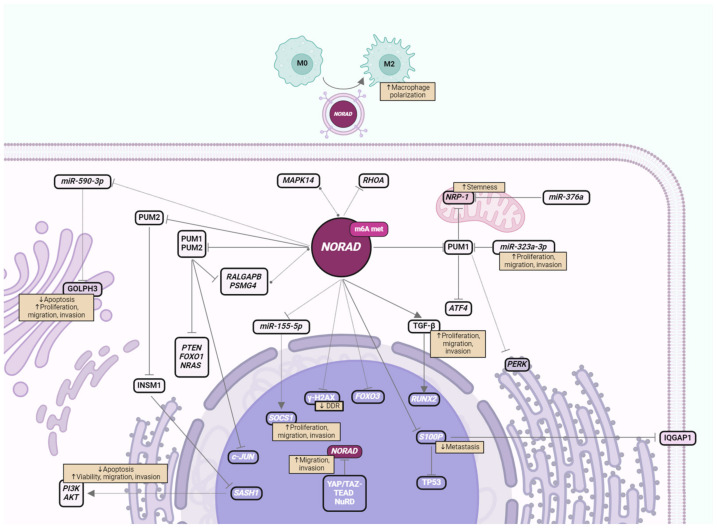
There are several signaling pathways with relevance for BC progression that are affected by NORAD expression [14]. Understanding the impact and context-dependent interactions of NORAD in crucial signaling pathways may highlight NORAD as a relevant therapeutic option to treat BC and overcome therapy resistance. In this review, we will summarize NORAD interactions and their relevance in BC progression and treatment. This includes NORAD interactions that are well established in BC, both involving PUM proteins and ncRNA sponging, and also other interactions shown to be affected by NORAD where the exact mechanism is not yet well understood (Figure 1 and Table 1).
Figure 1.
Schematic of NORAD molecular interacting partners in BC, emphasizing cellular localization, and impact on cancer progression. Solid lines indicate direct binding, dotted lines indicate indirect, undescribed or untested experimentally binding mechanism in BC, where circular ends refer to co-expression, cut ends to repression and pointed ends to promotion of expression. The impact on BC progression is also described, specifically how these interactions are reflected in cellular viability, proliferation, migration, invasion, apoptosis, metastatic capacity and stemness. In the nucleus, YAP/TAZ-TEAD and NuRD repress NORAD transcription, increasing migration and invasion of BC cells. NORAD also affects histone γH2AX expression and the consequent DNA damage response (DDR). In the cytoplasm, NORAD has increased expression and m6A methylation and is consequently secreted in exosomes to influence other cell types such as macrophage polarization into M2 protumoral phenotype. NORAD sequesters PUM1 and PUM2, leading to repression of c-JUN transcription and decreased levels of PTEN, FOXO1, NRAS, RALGAPB and PSMG4 transcripts in the cytoplasm, where the latter two co-express with NORAD. MiR-323a-3p also binds PUM1 along with NORAD, resulting in increased NRP-1 expression in the mitochondria, which is in turn repressed by miR-376a. eIF2 downstream effectors PERK on the rough endoplasmic reticulum and ATF4 are consequently induced. PUM2, sequestered by NORAD, also represses INSM1, which represses SASH1 transcription, lowering PI3K and AKT levels. Independently of PUM, in the cytoplasm, NORAD represses RHOA, miR-155-5p (inducing nuclear SOCS1 expression) and miR-590-3P (decreasing Golgi apparatus GOLPH3), induces TGF-β (increasing nuclear RUNX2) and co-expresses with MAPK14. NORAD also represses S100P transcription, decreasing S100P binding to TP53 and IQGAP1 proteins, and the amount of IQGAP1 in the membrane. Subcellular localization was based on UniProt data [44]. Created with BioRender.com.
Table 1.
Target genes, proteins and pathways affected by NORAD expression in breast cancer. N/A stands for non-applicable.
| Targets | In Vitro | In Vivo | Mechanism of ACTION | BC Impact | References |
|---|---|---|---|---|---|
| PUM/PSMG4 | N/A | Human bioinformatic database (TCGA) and tumors | NORAD targets PUM, PUM targets PSMG4, and NORAD co-expresses with PSMG4 | Lower DFS in BL | [45] |
| PUM2/ miR-376a/ NRP-1 |
Human cell lines (MCF-7, T47D, MDA-MB-231, MDA-MB-453, HMEpC) | Human tumors | NORAD targets PUM; PUM2 and miR-376a competitive-bind to NRP-1 | Higher cell stemness | [46] |
| PUM/ RALGAPB |
N/A | Human bioinformatic databases (TCGA, cBioPortal) | NORAD co-expresses with RALGAPB and PUM targets RALGAPB | Worse prognosis and poor OS in LumA; subtype biomarker | [41] |
| PUM/ c-JUN, FOXO1, NRAS, PTEN |
Human cell lines (SK-BR-3, MDA-MB-231, CAL51, BT-20, BT-549) | Human bioinformatic database (GEO) and tumors | NORAD targets PUM; PUM targets c-JUN, FOXO1, NRAS and PTEN | Lower cell proliferation and invasion | [47] |
| PUM1/ eIF2/ PERK/ ATF4 |
Human cell lines (MCF10A, MCF-7, MDA-MB-231, MDA-MB-468, MDA-MB-453, T47D) | Human tumors and cancer xenograft mouse models | NORAD targets PUM; PUM1 targets eIF2/PERK/ATF4 | Suppression of tumor growth; lower cell viability, proliferation, migration and invasion | [30] |
| S100P | Human cell lines (293FT, MDA-MB-231, Hs578T, T47D, ZR75) | Human bioinformatic databases (TCGA, GEO, PROGgeneV2) and tumors, cancer mouse models | NORAD binds S100P, preventing its binding to TP53 and IQGAP1 | Suppression of migration, invasion and metastasis | [39] |
| PUM2/ INSM1/ SASH1/ PI3K/AKT |
Human cell lines (MCF-10A, MCF-7, MDA-MB-231) | Human tumors and cancer xenograft mouse models | NORAD targets PUM; PUM2 targets INSM1, decreasing SASH1 repression and inhibiting PI3K/AKT | Lower cell viability, migration, invasion and tumor growth and reduced apoptosis | [48] |
| MAPK14 | N/A | Human bioinformatic databases (HGNC, lncBase v2, Expression Atlas, Co-lncRNA) and tumors | NORAD co-expresses with MAPK14 | Biomarker | [49] |
| miR-155-5p and SOCS1 | Human cell lines (HCC70, MCF-7, SKBR-3 and T-47D) | N/A | NORAD targets miR-155-5p, preventing its binding to SOCS1 | Lower cell proliferation and invasion | [38] |
| miR-590-3p and GOLPH3 | Human cell lines (MCF-7, MDA-MB-231, T47D, BT-549) | Human tumors | NORAD targets miR-590-3p, preventing the degradation of GOLPH3 | Higher cell proliferation, invasion and migration and lower apoptosis | [32] |
| miRNAs/FOXO3 and RHOA | N/A | Human bioinformatic database (GEO) | NORAD interacts with miR-183, miR-182, miR-7, miR-149, miR-200c, miR-101 and miR-342, regulates FOXO3 and RHOA | Biomarker | [50,51] |
| γ-H2AX | Human cell lines (MCF-7, MDA-MB-231, MDA-MB-436, MDA-MB-468) | N/A | NORAD recruits DDR proteins that repair damage through the phosphorylation of H2AX | Lower cell proliferation and invasion | [52] |
| TGF-β/ RUNX2 |
Human cell lines (MDA-MB-231 and MCF-7) | Human tumors and cancer mouse models | NORAD depletion decreases TGF-β protein expression | Higher cell proliferation, invasion and migration and worse prognosis | [35] |
| Immune cells | Human cell lines (MCF-10A, MDA-MB-231) | Human bioinformatic database (TCGA) and tumors | NORAD higher in low CD8 T-cell count; the promotion of malignant M2 macrophage polarization by exosome internalization | Poorer prognosis, higher tumor progression |
[36,53] |
2. Impact of NORAD in BC Signaling Pathways
2.1. PUM Proteins and Target Genes
PUM proteins are RBPs from the highly conserved Puf family. In mammals, the two canonical cytoplasmic PUM proteins are pumilio RNA binding family member 1 and 2 (PUM1 and PUM2, respectively). PUM proteins bind specifically and with great affinity to the conserved motifs of Pumilio Recognition/Response Element (PRE) found in the 3′ Untranslated Region (UTR) of their target genes, and they post-transcriptionally regulate mRNA degradation and repress protein translation [54,55]. In some cases, PUMs can act in translation to prevent their target’s ubiquitination and increase protein stability [56]. Some PUM target genes, including PARP1, minichromosome maintenance complex component 4 (MCM4), the structural maintenance of chromosomes 1A (SMC1A) and centromere protein J (CENPJ), regulate important biological functions, such as DNA repair and replication, cell cycle and mitosis. NORAD was discovered and first described in the human colorectal cancer cell line HCT116 where in silico assays revealed repetitive sequences containing PREs, allowing for PUM1 and PUM2 binding [10]. After DNA damage induction, NORAD co-localizes with PUM in NORAD–PUM (NP) bodies in the cytoplasm where NORAD negatively regulates cytoplasmic PUM proteins in phase-separated condensates as ribonucleoprotein (RNP) granules. NORAD’s high expression and the presence of multiple PREs allows for complete and competitive PUM recruitment and the subsequent maintenance of genome stability [57]. PUM expression and its impact on BC is also being debated. Some studies report PUM1 to be one of the most differently expressed and methylated genes in BC [58] and PUM2 to have higher expression in tumors as in TNBC, where it negatively correlates with BC patient overall survival (OS) and relapse-free survival (RFS) [46]. Other studies report lower PUM2 expression in LumA and TNBC tumors compared to normal tissues and that PUM2 silencing increases cell viability, migration and invasion in cancer cells lines, while its OE produces the opposite effect [48]. Slight variations in the expression or availability of PUM proteins are sufficient to lead to CIN. In this context, the absence of NORAD leads to the release and hyperactivation of PUM proteins and the appearance of deleterious effects, such as accelerating premature aging in mice [12]. NORAD can sequester a significant fraction of PUM proteins, negatively regulating their capacity to repress target mRNAs [10]. In this line of thought, several PUM targets and their implications in BC progression will be further described below (summarized in Figure 1 and Table 1).
Ral GTPase activating protein non-catalytic subunit beta (RALGAPB) participates in the regulation of mitosis, and its dysregulation is associated with genomic instability [59]. In some cancers, such as pancreatic ductal adenocarcinoma (PDAC) and oral squamous cell carcinoma (OSCC), RALGAPB depletion has been reported to promote invasion, migration, tumor growth and metastasis by increasing transforming growth factor beta 1 (TGFB1) signaling and decreasing c-Jun N-terminal kinase activity [60,61] and mTORC1-dependent pancreatic tumor cell invasion [62,63]. Based on The Cancer Genome Atlas (TCGA) RNA-seq data on BC tissues and clinical data from the cBioPortal platform, PUM-binding lncRNAs were selected and evaluated in each BC subtype. Interestingly, RALGAPB was revealed to co-express with NORAD in all analyzed BC subtypes. The high expression of both NORAD and RALGAPB was associated with worse prognosis and poorer OS in LumA subtype. Moreover, both genes (combined or separately) show biomarker potential to discriminate BL and LumA from non-tumoral and BL from LumA, supporting NORAD as the most relevant lncRNA with PUM binding sites in BC and the molecular axis where NORAD, PUM and RALGAPB participate as a potential target for novel BC targeting strategies [41].
Neuropilin 1 (NRP-1) transcript and protein levels were associated with BC progression, with increased levels in BC cell lines [64], higher expression in TNBC compared to LumB [65], poorer BC prognosis [66] and higher treatment resistance [67]. In BC, miR-376a was reported to have decreased expression in circulation [68], tumors [69] and several cell lines, and it is positively associated with OS. Indeed, miR-376a OE suppressed BC cell proliferation, migration and invasion and increased apoptosis, through direct binding to NRP-1 [70]. PUM2 knockdown (KD) in MDA-MB-231 and MDA-MB-453 cell lines showed the attenuation of stemness properties, such as decreased expression of aldehyde dehydrogenase 1 (ALDH1) family member A1 and Nanog homeobox (NANOG) proteins, lower ALDH1 activity and decreased spheroid formation capacity. Bioinformatic analysis and luciferase assays revealed that both PUM2 and miR-376a bind to the 3′UTR region of NRP-1. Mechanistically, PUM2 and miR-376a compete for NRP-1 binding, with PUM2 promoting BC stemness and miR-376a attenuating it. PUM2 can then induce the expression of NRP-1 by binding its mRNA and thus regulate BC progression [46].
Differential alternative polyadenylation (APA) was previously reported to be altered in BC tumors [71], and increased expression of polyadenylation components, like cleavage stimulation factor subunit 3 (CSTF3), was detected in TNBC cell lines. Several mRNAs with different prevalence of 3′ UTR isoforms, such as shortened and lengthened 3′UTR regions, were detected in BC tumors. It was found that PRE is the most frequently lost motif in shortened 3′UTRs in BC, but also the most often gained through APA. This suggests that PRE-containing RNAs are frequently altered by APA. Moreover, BL and TNBC tumors present more extensive and exclusive patterns of APA than LumA and LumB tumors. Gene Ontology (GO) analysis of the APA-exclusive alterations in TNBC tumors showed that the transcripts are related to the negative regulation of apoptosis, kinase activity and nucleotide binding. For instance, forkhead box O1 (FOXO1), a tumor suppressor transcription factor from the FOXO family group, showed extended 3′ UTR, whereas the tumor suppressor phosphatase and tensin homolog (PTEN), the proto-oncogene Neuroblastoma RAS viral oncogene homolog (NRAS) and the Jun proto-oncogene (c-JUN) showed recurrent 3′ UTR shortening, the latter two being the most recurring alterations. Overall, this study suggests that the dysregulated expression of PTEN, NRAS, c-JUN and FOXO1 in BC relies on increased or decreased PRE-bound PUM-regulation [47], with PUM playing an important part in regulating relevant cancer-related signaling pathways.
MiR-323a-3p is a miRNA related to tumor resistance, with decreased expression in BC tissues and cell lines and tumor suppressor roles in neuroblastoma [72] and esophageal squamous cell carcinoma (ESCC) [73]. The downregulation of miR-323a-3p in BC cell lines results in increased viability, migration and invasion and the opposite upon miR-323a-3p OE. Bioinformatics and experimental assays such as RNA pulldown uncovered NORAD and miR-323a-3p binding. Moreover, NORAD expression directly influences miR-323a-3p levels, and a decrease in miR-323a-3p expression promotes NORAD-induced aggressive behavior in MDA-MB-453 cells. Bioinformatic database (Targetscan, DIANA and Starbase) analysis and RNA pulldown assays revealed that PUM1, which displays increased levels in BC tumors and cell lines, binds to miR-323a-3p. Indeed, NORAD OE impacts PUM1 expression, and PUM1 depletion reverses the proliferation, migration and invasion capacities induced by upregulated NORAD, while miR-323a-3p negatively regulates PUM1 levels [30]. In this study, it was shown that both NORAD and miR-323a-3p can influence PUM1 and eukaryotic translation initiation factor 2 alpha kinase 3 (PERK)/eukaryotic initiating factor 2 (eIF2)/activating transcription factor 4 (ATF4) PERK/eIF2/ATF4 signaling pathway as NORAD OE decreases p-PERK, p-eIF2 and ATF4 protein levels. In vivo xenograft mouse models established with NORAD-depleted or miR-323a-3p-overexpressing BC cell lines reveal reduced size and weight of xenograft tumors and increased apoptosis as measured by TUNEL assay. Immunohistochemistry analysis of xenografts’ tumor sections confirmed that in vivo NORAD inhibition results in increased miR-323a-3p and p-PERK and decreased PUM1 levels. In sum, NORAD inhibition or miR-323a-3p OE can decrease BC cell malignant behavior by inhibiting PUM1 and activating the downstream eIF2 signaling pathway [30].
A study using transcriptomics analysis from invasive breast carcinoma surgical tissue samples revealed the downregulation of NORAD in BL when compared to the LumA subtype. Survival analysis did not render any significant differences, but higher levels of NORAD were associated with lower DFS only in BL patients. Despite that, NORAD promoted accessibility, as measured using ATAC-seq, whereas methylation, from genome-wide methylation studies, was not significantly altered between the BL and LumA subtypes. Transcriptomic analysis from TCGA highlights NORAD as the central regulator for regulon reconstruction, revealing a network of co-expression with genes potentially modulated by NORAD, some of them being PUM target genes, such as the proteasome assembly chaperone 4 (PSMG4) [45], a proteasome assembly chaperone protein upregulated in lung neoplastic cells and correlated with poor prognosis [74]. NORAD regulon showed a positive activity in ER+ and PR+ tumors but was inactive in BL tumor samples. Moreover, molecular signatures and GO analysis did not reveal any significant terms between the networks of BL and LumA tumor samples, but the pathways observed were closely linked to luminal epithelial cell transformation, including BMP and ALK1 signaling. NORAD is thus differently expressed in BC subtypes and participates in a complex regulatory network alongside many PUM target genes [42].
Secretory carrier membrane protein 1 (SCAMP1) is a lncRNA that promotes cancer progression through cell viability and invasion [75]. The SCAMP1 variant 2 (SCAMP1-TV2) shows increased expression in BC tumors from both LumA and TNBC subtypes and in several human BC cell lines, where SCAMP1-TV2 silencing promotes decreased levels of PI3K and AKT, both phosphorylated and unphosphorylated forms. Evidence suggests that SCAMP1-TV2 binds PUM2, which in turn targets INSM transcriptional repressor 1 (INSM1), which is able to inhibit SAM and SH3 domain containing 1 (SASH1), which can finally influence PI3K/AKT signaling [48]. INSM1 is a protein that regulates MYC proto-oncogene (c-Myc) and promotes BC carcinogenesis [76]. INSM1 expression is increased in human BC, and it has been proposed as a prognostic neuroendocrine marker for LumB [77,78,79]. In this study, INSM1 OE promoted increased MDA-MB-231 and MCF-7 BC cell viability, migration and invasion and decreased apoptosis. Moreover, it reversed the BC inhibitory effects of PUM2 OE and was accompanied by decreased expression of SASH1, a protein with tumor suppressor activity in TNBC involved in the toll-like receptor 4 (TLR4) signaling pathway [80,81,82,83]. Additionally, SASH1 OE decreased BC cell viability, migration and invasion and PI3K and AKT levels, while it increased apoptosis. In vivo tumor xenograft mice models established by the inoculation of MCF-7 or MDA-MB-231 cell lines with several combinations of SCAMP1-TV2 and PUM2 expression revealed that the simultaneous silencing of SCAMP1-TV2 and PUM2 OE renders the highest inhibition of xenograft tumor growth [48]. PUM2 proves, yet again, its importance and broad range of targets and its ability to influence cancer-related signaling pathways.
2.2. NORAD-Regulated Signaling Pathways via ncRNA Sponging
There are various classes of ncRNAs, namely, transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small RNAs (sRNAs) and lncRNAs [6]. ncRNAs can create complex networks by interacting with each other, affecting cancer cell fate and survival through different mechanisms, being considered promising diagnostic, prognostic biomarkers and therapeutic targets in cancer [84]. In particular, lncRNAs are the most predominant and diverse class among all ncRNAs [6]. They can interact with different biological molecules, such as DNA, RNA, including other ncRNAs, and proteins [84]. On the other hand, miRNAs can regulate gene expression by cleaving RNA or repressing the translation of their mRNA targets, thus regulating several biological processes such as cell cycle progression, proliferation, apoptosis and development [6]. LncRNAs can, however, act as ceRNAs by binding to miRNAs and suppress their targeting of mRNAs [85]. Next, we will describe examples of ncRNAs regulated by NORAD with an impact on BC progression (summarized in Figure 1 and Table 1). The impact of miR-323a-3p, a NORAD-binding miRNA, was previously discussed in the context of PUM target genes (see Section 2.1).
The upregulation of miR-155-5p has been associated with the malignant behavior of BC cells. MiR-155-5p is implicated in BC by targeting suppressor of cytokine signaling 1 (SOCS1), a key regulator of cell proliferation and apoptosis that plays a crucial role in the degradation of ubiquitination substrates. Notably, SOCS1 acts as a tumor suppressor by facilitating the degradation of oncoproteins, inhibiting cell proliferation and apoptosis [86]. The reduced expression of SOCS1 is linked to poor prognosis in BC patients, leading to lower OS rate as compared to high-SOCS1-expression patients. In the human HCC70 BC cell line, NORAD seems to work as a tumor suppressor through its capability to sponge miR155-5p, which leads to the positive regulation of SOCS1 and a reduction in cell proliferation, migration and invasion behavior in vitro, affecting overall BC progression [38].
MiR-590-3p has been described as a tumor suppressor in several cancers [87,88,89]. In BC cells, miR-590-3p OE is associated with the inhibition of proliferation and higher apoptosis [87]. Moreover, miR-590-3p inhibits Golgi phosphoprotein 3 (GOLPH3), a protein associated with a poor prognosis and chemoresistance in BC patients [90], suggesting that miR-590-3p can regulate BC progression through the regulation of GOLPH3. Mechanistically, the lncRNA NORAD can function as a sponge to miR-590-3p, negatively regulating its expression and oncogenic function in the context of BC. The depletion of NORAD or miR-590-3p OE resulted in decreased MCF-7 and MDA-MB-231 BC cell proliferation, invasion and migration in vitro, with a concomitant decrease in GOLPH3 protein levels, indicating that NORAD might be involved in BC pathophysiology by mediating the miR-590-3p/GOLPH3 signaling axis [32].
A study analyzing the differently expressed transcripts between normal and TNBC, HER2+, LumA and LumB tumors predicted that NORAD could promote the occurrence and development of BC tumors. It proposes that NORAD accomplishes this by interacting with other ncRNAs like metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and sponging several miRNAs, including miR-183, miR-182, miR-7, miR-149, miR-200c, miR-101 and miR-342. In turn, these miRNAs can regulate the expression of key signaling pathways, as forkhead box O3 (FOXO3) and ras homolog family member A (RHOA) [50]. The reduced expression of both FOXO3 and RHOA is associated with clinical outcomes in BC, namely, metastasis, BC cell proliferation and tumorigenesis [91,92,93]. In this context, NORAD levels also correlate with RHOA and RAD51 antisense RNA 1 (RAD51-AS1) expression. NORAD is significantly increased in BC tumors compared to adjacent normal tissue, presenting a great specificity value for segregation between BC and non-tumoral tissues [51].
2.3. Protein- and mRNA-Mediated Regulation of Signaling Pathways by NORAD
The transforming growth factor β (TGF-β), mitogen-activated protein kinase (MAPK) and the response to DNA damage are major signaling pathways in BC. NORAD was shown to regulate these pathways through the differential interaction with numerous mRNA and protein partners. In particular, the MAPK14, a member of the MAPK family, has been described to promote BC tumor progression [94,95,96]. Although there was no significant difference in either NORAD or MAPK14 levels between tumors and adjacent normal tissue, NORAD was shown to be significantly correlated with MAPK14 expression in BC tumors [49]. Hereafter, we will describe other NORAD interactions that can play crucial roles in BC (Figure 1 and Table 1).
TGF-β is a highly conserved family whose signaling is involved in different cellular processes such as cell growth, proliferation, migration and differentiation [97,98]. TGF-β signaling can either suppress or induce tumor progression, as it promotes cell cycle arrest and apoptosis in early BC stages, whereas in advanced stages, it favors cell motility, invasion and epithelial-to-mesenchymal transition (EMT) [99]. A study by Zhou et al. revealed that the upregulated expression of NORAD in human BC cells and patient tumors is associated with increased cell proliferation, migration and invasion in vitro and worse patient survival, by influencing the TGF-β signaling pathway. Silencing NORAD expression in BC cell lines leads to decreased TGF-β protein expression and the downregulation of its downstream effectors, such as SMAD family member 2 (Smad2) and RUNX family transcription factor 2 (RUNX2). In this way, NORAD promotes BC progression by regulating the TGF-β signaling pathway [35], highlighting the potential control of NORAD as a key tumor-suppressive event in BC.
In the context of BC therapy, the treatment of the TNBC MDA-MB-231 human cell line with doxorubicin triggers sustained DNA damage signals via H2A.X variant histone (H2AX) phosphorylation. Double-strand break amplification culminates in the recruitment of DNA damage signaling and repair proteins, such as BRCA1 DNA repair-associated protein (BRCA1) and tumor protein TP53 binding protein 1 (53BP1), to the damaged sites [52,99,100]. In the absence of NORAD, cells persist in signaling DNA damage via H2AX phosphorylation which may stem from an aberration either downstream or upstream of NORAD. Upon NORAD depletion, MDA-MB-231 cells show decreased levels of PARP1, impairing the DNA damage repair [52]. Noteworthy, PARP inhibitors are currently employed in treating advanced-stage metastatic BC particularly in cases with germline mutations in BRCA1 or BRCA2 genes, frequently associated with the TNBC subtype [100].
The yes-associated protein (YAP)/WW domain containing the transcription regulator 1 (TAZ)–TEA domain transcription factor (TEAD) complex is shown to be inversely correlated with NORAD expression in breast-invasive carcinoma in TCGA [39]. TEAD3 and TEAD4 are the anchor proteins of this complex, which are modulated by the Hippo signaling pathway, controlling cell growth and cancer progression [101]. TEAD4 was found to bind the NORAD promoter in the 5′ regulatory region of NORAD and silencing of TEAD1/3/4 resulted in increased NORAD expression in the human TNBC Hs578T cell line [39]. YAP, TAZ and the NuRD-repressive complex [102] and other components, including metastasis-associated protein (MTA1) and chromodomain helicase DNA binding protein 4 (CHD4), were all recruited to that same region of NORAD promoter. Furthermore, silencing MTA1 and CHD4 led to further NORAD upregulation, confirming that YAP/TAZ and NuRD repress NORAD transcription. On the other hand, NORAD repression by the YAP/TAZ pathway contributes to the YAP/TAZ-mediated promotion of migration and invasion in the BC-mutated cell line Hs578 YAP 8SA [39], where YAP is inactive and cannot be phosphorylated [100,103]. NORAD silencing in the human ZR75 luminal BC cell line increased S100P association with the IQ motif containing GTPase activating protein 1 (IQGAP1) and TP53 proteins, while NORAD OE attenuated this interaction. In the human TNBC MDA-MB-231 cell line, the specific binding of S100P protein and NORAD was observed, with S100P OE reversing NORAD OE and S100P silencing counteracting NORAD depletion. A similar relationship was observed in vivo, where MDA-MB-231 NORAD-overexpressing cells, upon tail vein i.p. injection, formed fewer lung metastatic nodules compared to control or NORAD/S100P double KD cells. In this context, although NORAD is shown to be transcriptionally repressed by YAP/TAZ-TEAD, NORAD also sponges S100P to inhibit metastasis [39].
2.4. NORAD-Regulated Cytokines and Immune Cells
The tumor microenvironment (TME) plays a major role in BC progression and therapy response [104]. In particular, CD8 T immune cells are crucial in anticancer immune response [105], where a higher amount of CD8 T-infiltrating lymphocytes (TILs) predicts a better immunotherapy response [106] and high levels of CD8 T-cells in samples correlate with better BC prognosis [53]. NORAD expression in BC tissues is also proven to be correlated with the TME, immune infiltration and expression of immune checkpoint inhibitors [31]. The impact of NORAD in immune cell regulation during BC progression and in the therapy response will be highlighted below.
A study using data from TCGA, which divided BC samples into high and low CD8 T-cell numbers, revealed that NORAD expression was elevated in the low CD8 T-cell group and high-risk BC samples, with smaller OS rate. Moreover, NORAD was negatively correlated with the presence of CD8 T-cells, cytotoxic lymphocytes and T-cells in the tumor, while it was positively associated with the levels of fibroblasts, endothelial cells and neutrophils. NORAD expression was also negatively related to immune checkpoint genes such as lymphocyte-activating 3 (LAG3), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and programmed cell death 1 (PDCD1) [53]. NORAD co-expresses with several targets of immune regulation signaling pathways such as cytokines and interleukins (ILs), as TGF-β, IL-3, IL-4 and Type I Interferon [36]. These data show a connection between NORAD expression and immune cell regulation in BC, including CD8 T-cell numbers, which can potentially be modulated to improve therapy response.
In BC, NORAD expression was found to be preferentially related to macrophage regulation, which shows a preferential upregulation of M2-polarized protumoral CD206-expressing macrophages, in comparison with M1-polarized antitumoral CD68-expressing macrophages. A study revealed that macrophage polarization can be directed by TNBC cell line-derived exosome internalization. In comparison to macrophages incubated with exosomes derived from normal breast epithelium MCF-10A cells and NORAD-depleted MDA-MB-231 cells, MDA-MB-231-derived exosome co-culture with non-polarized macrophages resulted in higher levels of NORAD and expression of M2 markers (CD163; mannose receptor C type 2, MRC2; Arginase 1, Arg1) and lower expression of M1 markers (CD80; C-C motif chemokine ligand 2, MCP-1; nitric oxide synthase 2 iNOS). Moreover, macrophages previously incubated with NORAD-depleted MDA-MB-231-derived exosomes, when co-cultured with MDA-MB-231 cells, promoted several effects in the BC cells, including decreased expression of NORAD, reduced proliferation, migration and invasion and increased apoptosis. Moreover, silencing NORAD in macrophages decreased the expression of TGFB1 and phosphorylated Smad2 and 3, potentially through miR-92b-3p, that binds both NORAD and TGFB1. These results show that NORAD can contribute to the activation of macrophages that promote malignant behavior in BC cells [36].
3. Potential Implication of NORAD in BC Therapies
As previously discussed, elevated NORAD levels have mostly been associated with BC aggressiveness and poor RFS in patients. Conversely, NORAD KD has shown inhibitory effects on BC cell viability and migration in vitro [30] and in vivo cancer progression [35]. Considering the association between NORAD and genomic instability, together with the paradoxical role of CIN in tumor progression, Alves-Vale et al. explored the possibility of simultaneously targeting NORAD together with cytotoxic drugs in BC treatment. Proteome analysis by liquid chromatography–tandem mass spectrometry (LC/MS-MS) revealed that NORAD KD in TNBC cells produced a significant alteration in the modulation of proteins associated with DNA repair, chromatin remodeling and epigenetic regulation. This suggests a potential impact on sensitivity to DNA-damaged agents such as doxorubicin. Of note, a significant decrease in the levels of minichromosome maintenance complex component 6 (MCM6), a critical player in DNA replication initiation, and Aly/REF export factor (ALYREF), a known interactor of NORAD associated with poor survival in BC patients, indicate a potential influence on cancer cell survival and therapy response [43]. Moreover, combinatorial NORAD/PARP1 silencing in the presence of doxorubicin in MDA-MB-231 cells had a synergistic effect on the abnormal accumulation of phosphorylated H2AX (γH2AX), a marker of DNA damage. These observations suggest that NORAD might confer BC resistance to chemotherapeutic agents, further impacting sensitivity to treatment [43]. Similar to NORAD, the lncRNA H19 has been implicated in BC chemoresistance, and H19 upregulation in doxorubicin-resistant BC cells correlates with decreased sensitivity to chemotherapy. Silencing H19 expression has been shown to sensitize doxorubicin-resistant MCF-7 cells to chemotherapy, indicating a potential similar function of NORAD and highlighting lncRNA targeting in sensitizing BC therapy-resistant cells [107].
NORAD has the potential to significantly enhance the effectiveness of chemotherapy, presenting an opportunity to improve treatment outcomes [43]. Radiotherapy is often used in BC patients following surgery and systemic chemotherapy [108]. It exerts its action by inducing mainly DNA double-stranded breaks (DSBs), promoting cell apoptosis and inhibiting cell cycle progression [109]. Thus, the DNA damage machinery has a key role in resistance to radiotherapy. DNA damage can be repaired by homologous repair (HR) and non-homologous end joining (NHEJ), and alterations in these pathways can lead to radiotherapy-resistant tumors [110]. In ESCC, NORAD depletion can be used in combination with radiotherapy for sensitizing radiotherapy-resistant ESCC cells in a colony formation assay, suggesting that NORAD can be an effective target to enhance the cytotoxic effects of radiation in ESCC patients [111]. Efforts in the development of combined approaches using immunotherapy in cancer treatment have been rising in recent years. Sun et al. 2021 conducted a study where the inhibition of DNA repair machinery was used to increase the immune checkpoint therapy efficiency combined with radiotherapy in ESCC cells. Using an in vivo xenograft model with NORAD-depleted KYSE-150 cells, the authors showed that increased NORAD expression was correlated with ESCC cells’ radio-resistance and that a combination of radiation with antiprogrammed death-1 (PD-1) antibodies was able to decrease tumor growth in NORAD-depleted tumor-bearing mice. These efforts highlight the potential of targeting NORAD in combination with radiotherapy in promoting a more efficient response to immunotherapy in cancer treatment [112]. Although no studies combining NORAD depletion with radiotherapy and immunotherapy in BC patients have been conducted, these data highlight the potential use of NORAD in sensitizing BC patients to combined therapies, such as radiotherapy [111]. Based on the reported studies, we envision that several currently available therapies could potentially benefit from NORAD depletion (summarized in Table 2). The impairment of the DNA damage response machinery is a key point in all the strategies, with a more direct impact on therapies using PARP inhibitors. When targeting the FOXO1 pathway, NORAD KD has the potential to synergize with the expected molecular outcomes of the therapy. NORAD KD is also associated with increased sensitivity to several therapies, which opens the possibility of improving tumor response to treatments with, for instance, PI3K/AKT/mTOR (PAM) inhibitors in combination with doxorubicin.
Table 2.
Examples of BC therapies that might benefit from NORAD depletion.
| Therapy | BC Application | Mechanism of Action | Barriers to Therapy Response | Potential Impact of NORAD Depletion | Impact of NORAD Depletion in Therapy Response | References |
|---|---|---|---|---|---|---|
| PARP inhibitors | BRCA mutations | Impairment of SSB repair | Restoration of HR | Improved PARP downregulation and impairment of DDR | Inhibition of tumor cell growth and proliferation | [52] |
| DNA damage-inducing chemotherapy | First-line therapy | DNA damage leads to apoptosis and inhibition of proliferation | DNA damage repair and resistance to therapy | Potential synergistic effect on FOXO1 downregulation | Reinforcement of apoptosis and inhibition of proliferation | [113] |
| FOXO1 inhibitor (AS1842856) | BL tumors | FOXO1 pathway inhibition | Inhibitor does not bind to the phosphorylated form of FOXO1 | Potential synergistic effect on downregulating FOXO1 and its phosphorylated form | Reinforcement of apoptosis and inhibition of proliferation | [114] |
| PAM inhibitors combined with CDK4/6 inhibitors | ER+ tumors | PAM downregulation leads to the diminished capability of BC to acquire resistance to endocrine therapy | Acquired resistance to endocrine therapy | mTOR inhibition | Improved sensitization of tumor cells to endocrine therapy | [115] |
| PAM inhibitors combined with anti-HER2 antibodies | HER2+ tumors | PAM downregulation sensitizes to anti-HER2 antibodies | Acquired resistance to HER2 antibodies | Synergistic effect on downregulating PAM | Improved sensitization of tumor cells to HER2 antibodies | [115] |
| Doxorubicin | First-line therapy | DNA DSB and activation of RhoA/MLC pathway | Promotes migration and invasion via RhoA/MLC pathway | Impairment of DNA damage repair machinery | Decreased tumor cell survival and inhibition of migration and invasion | [116] |
DDR—DNA damage repair; DSB—double-strand break; HR—homologous repair; SSB—single-strand break.
4. Conclusions and Future Perspectives
The lncRNA NORAD has been associated with the progression of several cancers. Compelling evidence suggests that NORAD is implicated in a myriad of signaling pathways relevant for BC, such as TGF-β, PI3K/AKT and FOXO1. Its dysregulation has been observed in a spectrum of cancers, particularly in BC, where its OE is mostly associated with increased proliferation, invasion, metastasis, resistance to chemotherapy, poor patient prognosis and OS. By sequestering PUM1 and PUM2, NORAD indirectly regulates the expression of PUM targets. In this line of thought, all the described effects of PUM in BC can be indirectly regulated by NORAD, increasing its therapeutic potential. Due to its predominant oncogenic role, it would be especially relevant to test the potential of targeting NORAD in a neoadjuvant therapy setting to sensitize and potentiate BC treatment.
Although some studies discriminated among BC subtypes, the majority only analyzed bulk BC samples, many with small sample sizes. As lncRNAs present a tissue-specific expression and NORAD has been shown to act differently according to the BC subtype, it would be important to further discern the role of NORAD in the different BC subtypes more accurately. In addition, the majority of conducted studies of NORAD and PUM targets in BC were performed using 2D cell line models, disregarding 3D cell–cell and cell–matrix interactions of cancer, stroma and immune cells in the tumor microenvironment, of outmost importance for cancer progression. Thus, it would be important to deepen our knowledge of NORAD in BC in more complex models that better mimic the native BC tumor.
The synergy observed between NORAD depletion and chemotherapeutic agents suggests a promising path for enhancing BC treatment outcomes (Table 2). The involvement of NORAD in radio-resistance, as observed in ESCC, opens possibilities for its exploitation in combination with radiotherapy for BC treatment. Several immune checkpoint inhibitors, such as CTLA4, PD-1 and PD-L1 have received approval from the FDA for treating solid tumors, including BC. The correlation between the number of TILs and favorable prognoses in HER2+ and TNBC is significant, with a potential to decrease the risk of relapse and death to 15–25% [117]. The impact of NORAD on immune regulation and correlation with immune checkpoint markers and its ability to influence the response to immunotherapy highlight its potential role in enhancing the efficacy of immunotherapy in BC. We suggest that NORAD depletion may improve immunotherapy results through different mechanisms. By disrupting its role in maintaining genomic stability, NORAD KD may induce increased genomic instability, leading to the generation of more neoantigens that enhance tumor immunogenicity [118]. This heightened immunogenicity could render the tumor more susceptible to immunotherapeutic interventions, particularly those targeting neoantigens recognized by the immune system. Furthermore, NORAD KD may enhance immunogenic cell death, a process triggered by DNA damage, potentially increasing the release of danger signals that attract immune cells to the tumor site [119], thereby improving the effectiveness of immunotherapies. Moreover, reduced NORAD levels may sensitize cancer cells to immunotherapeutic interventions, particularly immune checkpoint inhibitors, by modifying cellular processes that contribute to immune evasion [120].
The future of NORAD in BC therapies holds promise, emphasizing the need for continued research to unravel its intricate mechanisms. As we navigate the complexities of BC treatment, NORAD emerges as a potential biomarker by distinguishing BC subtypes to better assist clinical decision, being a potential neoadjuvant therapeutic target, as its silencing allows for the sensitization of BC cells to chemotherapy, and being a key player in shaping the landscape of personalized and targeted interventions against BC. As NORAD interacts with RNAs, coding and non-coding and proteins but most of the studies are performed exploring PUM in BC, it would be interesting to further explore the vast array and relevance of NORAD in other signaling pathways relevant to BC progression. The ongoing exploration of the role of NORAD in BC will open new possibilities for improved patient outcomes and a deeper understanding of the molecular intricacies concerning BC progression and treatment.
Acknowledgments
We thank members of the Bruno Bernardes de Jesus and Sandrina Nóbrega-Pereira laboratories for insightful discussions and advice.
Author Contributions
S.N.-P. and B.B.d.J. contributed to manuscript conceptualization and review. A.M.C., C.T.-M. and H.F.E.-A. contributed to the original draft preparation, literature review and writing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by Fundação para a Ciência e Tecnologia (FCT) and FEDER (10.54499/EXPL/BIA-CEL/0358/2021, 2022.01199.PTDC 10.54499/2022.01199.PTDC and LISBOA-01-0145-FEDER-007391) and Liga Portuguesa Contra o Cancro—Bolsa Dr. Dário Cruz 2023. S.N.-P. received funding from the FCT CEECIND Program (10.54499/2020.00355.CEECIND/CP1589/CT0024). AMC is a recipient of an individual FCT predoctoral fellowship (2023.03956.BD).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yang Z., Zhao Y., Lin G., Zhou X., Jiang X., Zhao H. Noncoding RNA activated by DNA damage (NORAD): Biologic function and mechanisms in human cancers. Clin. Chim. Acta. 2019;489:5–9. doi: 10.1016/j.cca.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A., et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023;24:430–447. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamantopoulos M.A., Tsiakanikas P., Scorilas A. Non-coding RNAs: The riddle of the transcriptome and their perspectives in cancer. Ann. Transl. Med. 2018;6:241. doi: 10.21037/atm.2018.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karakas D., Ozpolat B. The Role of LncRNAs in Translation. Noncoding RNA. 2021;7:16. doi: 10.3390/ncrna7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh C.Y., Hsieh Y.H., Chu H.P. Functions and properties of nuclear lncRNAs-from systematically mapping the interactomes of lncRNAs. J. Biomed. Sci. 2020;27:44. doi: 10.1186/s12929-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun M., Kraus W.L. From Discovery to Function: The Expanding Roles of Long NonCoding RNAs in Physiology and Disease. Endocr. Rev. 2015;36:25–64. doi: 10.1210/er.2014-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L., Bajic V.B., Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:924–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park E.G., Pyo S.J., Cui Y., Yoon S.H., Nam J.W. Tumor immune microenvironment lncRNAs. Brief. Bioinform. 2022;23:bbab504. doi: 10.1093/bib/bbab504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 10.Lee S., Kopp F., Chang T.C., Sataluri A., Chen B., Sivakumar S., Yu H., Xie Y., Mendell J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munschauer M., Nguyen C.T., Sirokman K., Hartigan C.R., Hogstrom L., Engreitz J.M., Ulirsch J.C., Fulco C.P., Subramanian V., Chen J., et al. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature. 2018;561:132–136. doi: 10.1038/s41586-018-0453-z. [DOI] [PubMed] [Google Scholar]
- 12.Kopp F., Elguindy M.M., Yalvac M.E., Zhang H., Chen B., Gillett F.A., Lee S., Sivakumar S., Yu H., Xie Y., et al. PUMILIO hyperactivity drives premature aging of Norad-deficient mice. Elife. 2019;8:e42650. doi: 10.7554/eLife.42650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elguindy M.M., Kopp F., Goodarzi M., Rehfeld F., Thomas A., Chang T.C., Mendell J.T. PUMILIO, but not RBMX, binding is required for regulation of genomic stability by noncoding, RNA NORAD. Elife. 2019;8:e48625. doi: 10.7554/eLife.48625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghafouri-Fard S., Azimi T., Hussen B.M., Abak A., Taheri M., Dilmaghani N.A. Non-coding RNA Activated by DNA Damage: Review of Its Roles in the Carcinogenesis. Front. Cell Dev. Biol. 2021;9:714787. doi: 10.3389/fcell.2021.714787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kai H., Wu Q., Yin R., Tang X., Shi H., Wang T., Zhang M., Pan C. LncRNA NORAD Promotes Vascular Endothelial Cell Injury and Atherosclerosis Through Suppressing VEGF Gene Transcription via Enhancing H3K9 Deacetylation by Recruiting HDAC6. Front. Cell Dev. Biol. 2021;9:701628. doi: 10.3389/fcell.2021.701628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D., Li L., Xu J., Luo W., Pan L., Niu Q., Wei D. Upregulated lncRNA NORAD can diagnose acute cerebral ischemic stroke patients and predict poor prognosis. Folia Neuropathol. 2023;61:105–110. doi: 10.5114/fn.2022.121478. [DOI] [PubMed] [Google Scholar]
- 17.Song Q., Geng Y., Li Y., Wang L., Qin J. Long noncoding RNA NORAD regulates MPP+-induced Parkinson’s disease model cells. J. Chem. Neuroanat. 2019;101:101668. doi: 10.1016/j.jchemneu.2019.101668. [DOI] [PubMed] [Google Scholar]
- 18.Soghli N., Yousefi T., Abolghasemi M., Qujeq D. NORAD, a critical long non-coding RNA in human cancers. Life Sci. 2021;264:118665. doi: 10.1016/j.lfs.2020.118665. [DOI] [PubMed] [Google Scholar]
- 19.Wang B., Xu L., Zhang J., Cheng X., Xu Q., Wang J., Mao F. LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed. Pharmacother. 2020;129:110268. doi: 10.1016/j.biopha.2020.110268. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Zhang G., Li J., Gong R., Wang Y., Qin Y., Ping Q., Hu L. Long noncoding RNA NORAD acts as a ceRNA mediates gemcitabine resistance in bladder cancer by sponging miR-155–5p to regulate WEE1 expression. Pathol. Res. Pract. 2021;228:153676. doi: 10.1016/j.prp.2021.153676. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Wu H., Zhang Y., Xiao X., Chu F., Zhang L. Induction of lncRNA NORAD accounts for hypoxia-induced chemoresistance and vasculogenic mimicry in colorectal cancer by sponging the miR-495-3p/hypoxia-inducible factor-1α (HIF-1α) Bioengineered. 2022;13:950–962. doi: 10.1080/21655979.2021.2015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 23.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann B.D., Pietenpol J.A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast. 2015;24:S36–S40. doi: 10.1016/j.breast.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waks A.G., Winer E.P. Breast Cancer Treatment. JAMA. 2019;321:288. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 26.Burstein H.J., Lacchetti C., Anderson H., Buchholz T.A., Davidson N.E., Gelmon K.A., Giordano S.H., Hudis C.A., Solky A.J., Stearns V., et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019;37:423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 27.Breast Cancer Treatment Options-National Breast Cancer Foundation [Internet] 2020. [(accessed on 6 September 2023)]. Available online: https://www.nationalbreastcancer.org/breast-cancer-treatment/
- 28.Tong C.W.S., Wu M., Cho W.C.S., To K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018;8:227. doi: 10.3389/fonc.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Škubník J., Pavlíčková V., Ruml T., Rimpelová S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants. 2021;10:569. doi: 10.3390/plants10030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi P., Zhang J., Li X., Li W., Li H., Fu P. Long non-coding RNA NORAD inhibition upregulates microRNA-323a-3p to suppress tumorigenesis and development of breast cancer through the PUM1/eIF2 axis. Cell Cycle. 2021;20:1295–1307. doi: 10.1080/15384101.2021.1934627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Fan X., Hong J., Yang E., Xuan C., Fang H., Ding X. Diagnostic implications of lncRNA NORAD in breast cancer. Sci. Rep. 2023;13:20426. doi: 10.1038/s41598-023-47434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan Q., Qu F., Yang W., Chen N. Effect of LINC00657 on Apoptosis of Breast Cancer Cells by Regulating miR-590-3p. Cancer Manag. Res. 2020;12:4561–4571. doi: 10.2147/CMAR.S249576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidari R., Akbariqomi M., Asgari Y., Ebrahimi D., Alinejad-Rokny H. A systematic review of long non-coding RNAs with a potential role in breast cancer. Mutat. Res./Rev. Mutat. Res. 2021;787:108375. doi: 10.1016/j.mrrev.2021.108375. [DOI] [PubMed] [Google Scholar]
- 34.Liao S.A., Guan J., Mo H., He J.L., Zhan X.L. lncRNA LSINCT5 Regulates miR-20a-5p/XIAP to Inhibit the Growth and Metastasis of Osteosarcoma Cells. Onco Targets Ther. 2020;13:8209–8221. doi: 10.2147/OTT.S251843. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Zhou K., Ou Q., Wang G., Zhang W., Hao Y., Li W. High long non-coding RNA NORAD expression predicts poor prognosis and promotes breast cancer progression by regulating TGF-β pathway. Cancer Cell Int. 2019;19:63. doi: 10.1186/s12935-019-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Zhou Y., Wu M., Yuan Y., Wu W. m6A Modification Mediates Exosomal LINC00657 to Trigger Breast Cancer Progression Via Inducing Macrophage M2 Polarization. Clin. Breast Cancer. 2023;23:546–560. doi: 10.1016/j.clbc.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Yi D., Xu F., Wang R., Jiang C., Qin J., Lee Y.H., Shi X., Sang J. Deciphering the map of METTL14-mediated lncRNA m6A modification at the transcriptome-wide level in breast cancer. J. Clin. Lab. Anal. 2022;36:e24754. doi: 10.1002/jcla.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W., Zhou X., Li Y., Jiang H., Chen A. Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/SOCS1 Axis. J. Breast Cancer. 2021;24:330. doi: 10.4048/jbc.2021.24.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan B.S., Yang M.C., Singh S., Chou Y.C., Chen H.Y., Wang M.Y., Wang Y.C., Chen R.H. LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene. 2019;38:5612–5626. doi: 10.1038/s41388-019-0812-8. [DOI] [PubMed] [Google Scholar]
- 40.Wu H.C., Lin Y.C., Liu C.H., Chung H.C., Wang Y.T., Lin Y.W., Ma H.I., Tu P.H., Lawler S.E., Chen R.H. USP11 regulates PML stability to control Notch-induced malignancy in brain tumours. Nat. Commun. 2014;5:3214. doi: 10.1038/ncomms4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller C.S.M., Giner I.S., Zambalde É.P., Carvalho T.M., Ribeiro E.M.d.S.F., Carvalho de Oliveira J., Mathias C., Gradia D.F. The Potential of NORAD–PUMILIO–RALGAPB Regulatory Axis as a Biomarker in Breast Cancer. Noncoding RNA. 2022;8:76. doi: 10.3390/ncrna8060076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathias C., Pedroso G.A., Pabst F.R., de Lima R.S., Kuroda F., Cavalli I.J., de Oliveira J.C., Ribeiro E.M.d.S.F., Gradia D.F. So alike yet so different. Differential expression of the long non-coding RNAs NORAD and HCG11 in breast cancer subtypes. Genet. Mol. Biol. 2021;44:e20200153. doi: 10.1590/1678-4685-gmb-2020-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alves-Vale C., Capela A.M., Tavares-Marcos C., Domingues-Silva B., Pereira B., Santos F., Gomes C.P., Espadas G., Vitorino R., Sabidó E., et al. Expression of NORAD correlates with breast cancer aggressiveness and protects breast cancer cells from chemotherapy. Mol. Ther. Nucleic Acids. 2023;33:910–924. doi: 10.1016/j.omtn.2023.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateman A., Martin M.J., Orchard S., Magrane M., Ahmad S., Alpi E., Bowler-Barnett E.H., Britto R., Bye-A-Jee H., Cukura A., et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023;51:D523–D531. doi: 10.1093/nar/gkac1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.PSMG4 Gene-GeneCards|PSMG4 Protein|PSMG4 Antibody [Internet] [(accessed on 18 December 2023)]. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PSMG4.
- 46.Zhang L., Chen Y., Li C., Liu J., Ren H., Li L., Zheng X., Wang H., Han Z. RNA binding protein PUM2 promotes the stemness of breast cancer cells via competitively binding to neuropilin-1 (NRP-1) mRNA with miR-376a. Biomed. Pharmacother. 2019;114:108772. doi: 10.1016/j.biopha.2019.108772. [DOI] [PubMed] [Google Scholar]
- 47.Miles W.O., Lembo A., Volorio A., Brachtel E., Tian B., Sgroi D., Provero P., Dyson N. Alternative Polyadenylation in Triple-Negative Breast Tumors Allows NRAS and c-JUN to Bypass PUMILIO Posttranscriptional Regulation. Cancer Res. 2016;76:7231–7241. doi: 10.1158/0008-5472.CAN-16-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao W., Ma J., Zheng J., Liu X., Liu Y., Ruan X., Shen S., Shao L., Chen J., Xue Y. Silencing SCAMP1-TV2 Inhibited the Malignant Biological Behaviors of Breast Cancer Cells by Interaction with PUM2 to Facilitate INSM1 mRNA Degradation. Front. Oncol. 2020;10:613. doi: 10.3389/fonc.2020.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dashti S., Taherian-Esfahani Z., Kholghi-Oskooei V., Noroozi R., Arsang-Jang S., Ghafouri-Fard S., Taheri M. In silico identification of MAPK14-related lncRNAs and assessment of their expression in breast cancer samples. Sci. Rep. 2020;10:8316. doi: 10.1038/s41598-020-65421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassani B., Mollanoori H., Pouresmaeili F., Asgari Y., Ghafouri-Fard S. Constructing mRNA, miRNA, circRNA and lncRNA regulatory network by Analysis of microarray data in breast cancer. Gene Rep. 2022;26:101510. doi: 10.1016/j.genrep.2022.101510. [DOI] [Google Scholar]
- 51.Nicknam A., Khojasteh Pour S., Hashemnejad M.A., Hussen B.M., Safarzadeh A., Eslami S., Taheri M., Ghafouri-Fard S., Jamali E. Expression analysis of Rho GTPase-related lncRNAs in breast cancer. Pathol. Res. Pract. 2023;244:154429. doi: 10.1016/j.prp.2023.154429. [DOI] [PubMed] [Google Scholar]
- 52.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., et al. Adjuvant Olaparib for Patients with BRCA1-or BRCA2 -Mutated Breast Cancer. N. Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z., Feng R., Kahlert U.D., Chen Z., Torres-dela Roche L.A., Soliman A., Miao C., De Wilde R.L., Shi W. Construction of ceRNA Networks Associated with CD8 T Cells in Breast Cancer. Front. Oncol. 2022;12:883197. doi: 10.3389/fonc.2022.883197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arvola R.M., Chang C.T., Buytendorp J.P., Levdansky Y., Valkov E., Freddolino P.L., Goldstrohm A.C. Unique repression domains of Pumilio utilize deadenylation and decapping factors to accelerate destruction of target mRNAs. Nucleic Acids Res. 2020;48:1843–1871. doi: 10.1093/nar/gkz1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstrohm A.C., Hall T.M.T., McKenney K.M. Post-transcriptional Regulatory Functions of Mammalian Pumilio Proteins. Trends Genet. 2018;34:972–990. doi: 10.1016/j.tig.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y.H., Wu C.C., Chou C.K., Huang C.Y.F. A Translational Regulator, PUM2, Promotes Both Protein Stability and Kinase Activity of Aurora-A. Meurs EF, editor. PLoS ONE. 2011;6:e19718. doi: 10.1371/journal.pone.0019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elguindy M.M., Mendell J.T. NORAD-induced Pumilio phase separation is required for genome stability. Nature. 2021;595:303–308. doi: 10.1038/s41586-021-03633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sindi S., Hamdi N., Hassan S., Ganash M., Alharbi M., Alburae N., Azhari S., Alkhayyat S., Linjawi A., Alkhatabi H., et al. Promoter Methylation-Regulated Differentially Expressed Genes in Breast Cancer. Breast Cancer Targets Ther. 2023;15:435–450. doi: 10.2147/BCTT.S408711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Personnic N., Lakisic G., Gouin E., Rousseau A., Gautreau A., Cossart P., Bierne H. A role for Ral GTPase-activating protein subunit β in mitotic regulation. FEBS J. 2014;281:2977–2989. doi: 10.1111/febs.12836. [DOI] [PubMed] [Google Scholar]
- 60.Cao M., Li X., Trinh D.A., Yoshimachi S., Goto K., Sakata N., Ishida M., Ohtsuka H., Unno M., Wang Y., et al. Ral GTPase promotes metastasis of pancreatic ductal adenocarcinoma via elevation of TGF-β1 production. J. Biol. Chem. 2023;299:104754. doi: 10.1016/j.jbc.2023.104754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshimachi S., Shirakawa R., Cao M., Trinh D.A., Gao P., Sakata N., Miyazaki K., Goto K., Miura T., Ariake K., et al. Ral GTPase–activating protein regulates the malignancy of pancreatic ductal adenocarcinoma. Cancer Sci. 2021;112:3064–3073. doi: 10.1111/cas.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin T.D., Chen X.W., Kaplan R.E.W., Saltiel A.R., Walker C.L., Reiner D.J., Der C.J. Ral and Rheb GTPase Activating Proteins Integrate mTOR and GTPase Signaling in Aging, Autophagy, and Tumor Cell Invasion. Mol. Cell. 2014;53:209–220. doi: 10.1016/j.molcel.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao P., Liu S., Yoshida R., Shi C.Y., Yoshimachi S., Sakata N., Goto K., Kimura T., Shirakawa R., Nakayama H., et al. Ral GTPase Activation by Downregulation of RalGAP Enhances Oral Squamous Cell Carcinoma Progression. J. Dent. Res. 2019;98:1011–1019. doi: 10.1177/0022034519860828. [DOI] [PubMed] [Google Scholar]
- 64.Han Z., Jiang G., Zhang Y., Xu J., Chen C., Zhang L., Xu Z., Du X. Effects of RNA interference-mediated NRP-1 silencing on the proliferation and apoptosis of breast cancer cells. Mol. Med. Rep. 2015;12:513–519. doi: 10.3892/mmr.2015.3405. [DOI] [PubMed] [Google Scholar]
- 65.Naik A., Al-Zeheimi N., Bakheit C.S., Al Riyami M., Al Jarrah A., Al Moundhri M.S., Al Habsi Z., Basheer M., Adham S.A. Neuropilin-1 Associated Molecules in the Blood Distinguish Poor Prognosis Breast Cancer: A Cross-Sectional Study. Sci. Rep. 2017;7:3301. doi: 10.1038/s41598-017-03280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rachner T.D., Kasimir-Bauer S., Goebel A., Erdmann K., Hoffmann O., Rauner M., Hofbauer L.C., Kimmig R., Bittner A.K. Soluble Neuropilin-1 is an independent marker of poor prognosis in early breast cancer. J. Cancer Res. Clin. Oncol. 2021;147:2233–2238. doi: 10.1007/s00432-021-03635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Zeheimi N., Adham S.A. Modeling Neoadjuvant chemotherapy resistance in vitro increased NRP-1 and HER2 expression and converted MCF7 breast cancer subtype. Br. J. Pharmacol. 2020;177:2024–2041. doi: 10.1111/bph.14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuk K., Zucknick M., Madhavan D., Schott S., Golatta M., Heil J., Marmé F., Turchinovich A., Sinn P., Sohn C., et al. Plasma MicroRNA Panel for Minimally Invasive Detection of Breast Cancer. PLoS ONE. 2013;8:e76729. doi: 10.1371/journal.pone.0076729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holý P., Brynychová V., Šeborová K., Haničinec V., Koževnikovová R., Trnková M., Vrána D., Gatěk J., Kopečková K., Mrhalová M., et al. Integrative analysis of mRNA and miRNA expression profiles and somatic variants in oxysterol signaling in early-stage luminal breast cancer. Mol. Oncol. 2023;17:2074–2089. doi: 10.1002/1878-0261.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L., Chen Y., Wang H., Zheng X., Li C., Han Z. miR-376a inhibits breast cancer cell progression by targeting neuropilin-1, N.R. Onco Targets Ther. 2018;11:5293–5302. doi: 10.2147/OTT.S173416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lembo A., Di Cunto F., Provero P. Shortening of 3′UTRs Correlates with Poor Prognosis in Breast and Lung Cancer. PLoS ONE. 2012;7:e31129. doi: 10.1371/journal.pone.0031129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhavsar S.P., Olsen L., Løkke C., Koster J., Flægstad T., Einvik C. Hsa-miR-323a-3p functions as a tumor suppressor and targets STAT3 in neuroblastoma cells. Front. Pediatr. 2023;11:1098999. doi: 10.3389/fped.2023.1098999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Men Y., Zhai Y., Wu L., Liu L., Zhang W., Jiang W., Bi N., Song Y., Hui Z., Wang L. MiR-323a-3p acts as a tumor suppressor by suppressing FMR1 and predicts better esophageal squamous cell carcinoma outcome. Cancer Cell Int. 2022;22:140. doi: 10.1186/s12935-022-02541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xuan D.T.M., Yeh I.J., Su C.Y., Liu H.L., Ta H.D.K., Anuraga G., Chiao C.C., Wang C.Y., Yen M.C. Prognostic and Immune Infiltration Value of Proteasome Assembly Chaperone (PSMG) Family Genes in Lung Adenocarcinoma. Int. J. Med. Sci. 2023;20:87–101. doi: 10.7150/ijms.78590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li R., Chen Z., Zhou Y., Maimaitirexiati G., Yan Q., Li Y., Maimaitiyimin A., Zhou C., Ren J., Liu C., et al. LncRNA SCAMP1 disrupts the balance between miR-26a-5p and ZEB2 to promote osteosarcoma cell viability and invasion. Front. Oncol. 2022;12:967000. doi: 10.3389/fonc.2022.967000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan Y., Sun Y., Liu Z., Zhang Y., Cao M., Wang W., Tao J., Yao Y. INSM1 promotes breast carcinogenesis by regulating, C.-M.Y.C. Am. J. Cancer Res. 2023;13:3500–3516. [PMC free article] [PubMed] [Google Scholar]
- 77.Razvi H., Tsang J.Y., Poon I.K., Chan S.K., Cheung S.Y., Shea K.H., Tse G.M. INSM1 is a novel prognostic neuroendocrine marker for luminal B breast cancer. Pathology. 2021;53:170–178. doi: 10.1016/j.pathol.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhong E., Pareja F., Hanna M.G., Jungbluth A.A., Rekhtman N., Brogi E. Expression of novel neuroendocrine markers in breast carcinomas: A study of INSM1, ASCL1, and POU2F3. Hum. Pathol. 2022;127:102–111. doi: 10.1016/j.humpath.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metovic J., Castellano I., Marinelli E., Osella-Abate S., Sapino A., Cassoni P., Papotti M. INSM1 Expression in Breast Neoplasms with Neuroedocrine Features. Endocr. Pathol. 2021;32:452–460. doi: 10.1007/s12022-021-09682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang K., Liu P., Xu H., Liang D., Fang K., Du S., Cheng W., Ye L., Liu T., Zhang X., et al. SASH1 suppresses triple-negative breast cancer cell invasion through YAP-ARHGAP42-actin axis. Oncogene. 2020;39:5015–5030. doi: 10.1038/s41388-020-1356-7. [DOI] [PubMed] [Google Scholar]
- 81.Zeller C., Hinzmann B., Seitz S., Prokoph H., Burkhard-Goettges E., Fischer J., Jandrig B., Schwarz L.E., Rosenthal A., Scherneck S. SASH1: A candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene. 2003;22:2972–2983. doi: 10.1038/sj.onc.1206474. [DOI] [PubMed] [Google Scholar]
- 82.Martini M., Gnann A., Scheikl D., Holzmann B., Janssen K.P. The candidate tumor suppressor SASH1 interacts with the actin cytoskeleton and stimulates cell–matrix adhesion. Int. J. Biochem. Cell Biol. 2011;43:1630–1640. doi: 10.1016/j.biocel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 83.Dauphinee S.M., Clayton A., Hussainkhel A., Yang C., Park Y.J., Fuller M.E., Blonder J., Veenstra T.D., Karsan A. SASH1 Is a Scaffold Molecule in Endothelial TLR4 Signaling. J. Immunol. 2013;191:892–901. doi: 10.4049/jimmunol.1200583. [DOI] [PubMed] [Google Scholar]
- 84.Grillone K., Riillo C., Scionti F., Rocca R., Tradigo G., Guzzi P.H., Alcaro S., Di Martino M.T., Tagliaferri P., Tassone P. Non-coding RNAs in cancer: Platforms and strategies for investigating the genomic “dark matter”. J. Exp. Clin. Cancer Res. 2020;39:117. doi: 10.1186/s13046-020-01622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamura S., Imai-Sumida M., Tanaka Y., Dahiya R. Interaction and cross-talk between non-coding RNAs. Cell. Mol. Life Sci. 2018;75:467–484. doi: 10.1007/s00018-017-2626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ying J., Qiu X., Lu Y., Zhang M. SOCS1 and its Potential Clinical Role in Tumor. Pathol. Oncol. Res. 2019;25:1295–1301. doi: 10.1007/s12253-019-00612-5. [DOI] [PubMed] [Google Scholar]
- 87.Rohini M., Gokulnath M., Miranda P.J., Selvamurugan N. miR-590–3p inhibits proliferation and promotes apoptosis by targeting activating transcription factor 3 in human breast cancer cells. Biochimie. 2018;154:10–18. doi: 10.1016/j.biochi.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 88.Youssef A.I., Khaled G.M., Amleh A. Functional role and epithelial to mesenchymal transition of the miR-590-3p/MDM2 axis in hepatocellular carcinoma. BMC Cancer. 2023;23:396. doi: 10.1186/s12885-023-10861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salem M., Shan Y., Bernaudo S., Peng C. miR-590-3p Targets Cyclin G2 and FOXO3 to Promote Ovarian Cancer Cell Proliferation, Invasion, and Spheroid Formation. Int. J. Mol. Sci. 2019;20:1810. doi: 10.3390/ijms20081810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang S., Pan H., Wei W., Yang H., Liu J., Yang R. GOLPH3: A novel biomarker that correlates with poor survival and resistance to chemotherapy in breast cancer. Oncotarget. 2017;8:105155–105169. doi: 10.18632/oncotarget.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalpana G., Figy C., Yeung M., Yeung K.C. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 2019;9:16351. doi: 10.1038/s41598-019-52746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pellegrino M., Rizza P., Donà A., Nigro A., Ricci E., Fiorillo M., Perrotta I., Lanzino M., Giordano C., Bonofiglio D., et al. FoxO3a as a Positive Prognostic Marker and a Therapeutic Target in Tamoxifen-Resistant Breast Cancer. Cancers. 2019;11:1858. doi: 10.3390/cancers11121858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou Y., Tsai W.B., Cheng C.J., Hsu C., Chung Y.M., Li P.C., Lin S.H., Hu M.C.T. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:R21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H., Wang Y., Liu C., Li W., Zhou F., Wang X., Zheng J. The Apolipoprotein C1 is involved in breast cancer progression via EMT and MAPK/JNK pathway. Pathol. Res. Pract. 2022;229:153746. doi: 10.1016/j.prp.2021.153746. [DOI] [PubMed] [Google Scholar]
- 95.Xie S.Y., Shi D.B., Ouyang Y., Lin F., Chen X.Y., Jiang T.C., Xia W., Guo L., Lin H.X. SHMT2 promotes tumor growth through VEGF and MAPK signaling pathway in breast cancer. Am. J. Cancer Res. 2022;12:3405–3421. [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang W., Wang X., Zhang C., Xue L., Yang L. Expression and clinical significance of MAPK and EGFR in triple-negative breast cancer. Oncol. Lett. 2020;19:1842–1848. doi: 10.3892/ol.2020.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tzavlaki K., Moustakas A. TGF-β Signaling. Biomolecules. 2020;10:487. doi: 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hussen B.M., Hidayat H.J., Abdullah S.R., Mohamadtahr S., Rasul M.F., Samsami M., Taheri M. Role of long non-coding RNAs and TGF-β signaling in the regulation of breast cancer pathogenesis and therapeutic targets. Cytokine. 2023;170:156351. doi: 10.1016/j.cyto.2023.156351. [DOI] [PubMed] [Google Scholar]
- 99.Kawasaki N., Miwa T., Hokari S., Sakurai T., Ohmori K., Miyauchi K., Miyazono K., Koinuma D. Long noncoding RNA NORAD regulates transforming growth factor-β signaling and epithelial-to-mesenchymal transition-like phenotype. Cancer Sci. 2018;109:2211–2220. doi: 10.1111/cas.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ortega Á., Vera I., Diaz M., Navarro C., Rojas M., Torres W., Parra H., Salazar J., De Sanctis J., Bermúdez V. The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises. Int. J. Mol. Sci. 2021;23:430. doi: 10.3390/ijms23010430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hillmer R.E., Link B.A. The Roles of Hippo Signaling Transducers Yap and Taz in Chromatin Remodeling. Cells. 2019;8:502. doi: 10.3390/cells8050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das A., Fischer R.S., Pan D., Waterman C.M. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J. Biol. Chem. 2016;291:6096–6110. doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Von der Lippe Gythfeldt H., Lien T., Tekpli X., Silwal-Pandit L., Borgen E., Garred Ø., Skjerven H., Schlichting E., Lundgren S., Wist E., et al. Immune phenotype of tumor microenvironment predicts response to bevacizumab in neoadjuvant treatment of ER-positive breast cancer. Int. J. Cancer. 2020;147:2515–2525. doi: 10.1002/ijc.33108. [DOI] [PubMed] [Google Scholar]
- 105.Raskov H., Orhan A., Christensen J.P., Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Z., Wang M., De Wilde R.L., Feng R., Su M., Torres-de la Roche L.A., Shi W. A Machine Learning Model to Predict the Triple Negative Breast Cancer Immune Subtype. Front. Immunol. 2021;12:749459. doi: 10.3389/fimmu.2021.749459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kallen A.N., Zhou X.B., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J.S., Zhang H., et al. The Imprinted H19 LncRNA Antagonizes Let-7 MicroRNAs. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., Anderson B., Burstein H.J., Chew H., Dang C., et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022;20:691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 109.Dong S., Lyu X., Yuan S., Wang S., Li W., Chen Z., Yu H., Li F., Jiang Q. Oxidative stress: A critical hint in ionizing radiation induced pyroptosis. Radiat. Med. Prot. 2020;1:179–185. doi: 10.1016/j.radmp.2020.10.001. [DOI] [Google Scholar]
- 110.Huang R., Zhou P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021;6:254. doi: 10.1038/s41392-021-00648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun Y., Wang J., Pan S., Yang T., Sun X., Wang Y., Shi X., Zhao X., Guo J., Zhang X. LINC00657 played oncogenic roles in esophageal squamous cell carcinoma by targeting miR-615-3p and JunB. Biomed. Pharmacother. 2018;108:316–324. doi: 10.1016/j.biopha.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Sun Y., Wang J., Ma Y., Li J., Sun X., Zhao X., Shi X., Hu Y., Qu F., Zhang X. Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. J. Exp. Clin. Cancer Res. 2021;40:306. doi: 10.1186/s13046-021-02084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cui Q., Sun J., Yuan J., Li J., Yang C., Du G., Zhou C., Qiu P., Gao J., Zhang Y., et al. DNA damage chemotherapeutic drugs suppress basal-like breast cancer growth by down-regulating the transcription of the FOXO1-KLF5 axis. Genes Dis. 2024;11:91–94. doi: 10.1016/j.gendis.2023.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flores D., Lopez A., Udawant S., Gunn B., Keniry M. The FOXO1 inhibitor AS1842856 triggers apoptosis in glioblastoma multiforme and basal-like breast cancer cells. FEBS Open Bio. 2023;13:352–362. doi: 10.1002/2211-5463.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu K., Wu Y., He P., Fan Y., Zhong X., Zheng H., Luo T. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells. 2022;11:2508. doi: 10.3390/cells11162508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu C.L., Chen M.J., Lin J.C., Lin C.H., Huang W.C., Cheng S.P., Chen S.N., Chang Y.C. Doxorubicin Promotes Migration and Invasion of Breast Cancer Cells through the Upregulation of the RhoA/MLC Pathway. J. Breast Cancer. 2019;22:185. doi: 10.4048/jbc.2019.22.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barzaman K., Moradi-Kalbolandi S., Hosseinzadeh A., Kazemi M.H., Khorramdelazad H., Safari E., Farahmand L. Breast cancer immunotherapy: Current and novel approaches. Int. Immunopharmacol. 2021;98:107886. doi: 10.1016/j.intimp.2021.107886. [DOI] [PubMed] [Google Scholar]
- 118.Morisaki T., Kubo M., Umebayashi M., Yew P.Y., Yoshimura S., Park J.H., Kiyotani K., Kai M., Yamada M., Oda Y., et al. Neoantigens elicit T cell responses in breast cancer. Sci. Rep. 2021;11:13590. doi: 10.1038/s41598-021-91358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barros E.M., McIntosh S.A., Savage K.I. The DNA damage induced immune response: Implications for cancer therapy. DNA Repair. 2022;120:103409. doi: 10.1016/j.dnarep.2022.103409. [DOI] [PubMed] [Google Scholar]
- 120.George A., Sahin I., Carneiro B.A., Dizon D.S., Safran H.P., El-Deiry W.S. Strategies to sensitize cancer cells to immunotherapy. Hum. Vaccin. Immunother. 2021;17:2595–2601. doi: 10.1080/21645515.2021.1891817. [DOI] [PMC free article] [PubMed] [Google Scholar]